Abstract
Stress induced circulating catecholamines are hypothesized to selectively activate adrenergic receptors (ARs) on immunocompetent cells modulating their inflammatory response to trauma or environmental toxins. We characterized changes in expression of a pro-inflammatory cytokine modulated by β-AR activation in human primary and immortalized monocytes that had been simultaneously stimulated with lipopolysaccharide (LPS). Results from cytokine antibody arrays demonstrated that half-maximal effective concentrations of the selective β-AR agonist isoproterenol (Iso) qualitatively increased LPS-mediated expression of the soluble cytokine, interleukin-1β (IL-1β). Semi-quantitative immunoblot techniques confirmed a synergistic increase of IL-1β production in both LPS stimulated THP-1 cells and primary human monocytes co-incubated with Iso. Immunoblot techniques as well as radioligand binding studies were also used to characterize the heterogeneous expression of β1- and β2-AR subtypes on THP-1 cells. β-AR activation is classically associated with generation of cAMP in many tissues and cell types. Therefore, using the method of Schild, we generated Iso concentration-response curves in the presence of fixed subtype-selective β-AR antagonist concentrations to demonstrate that β1-AR activation was exclusively linked with the generation of cAMP in THP-1 cells. Furthermore, use of a selective kinase inhibitor demonstrated that Iso potentiated the expression of soluble IL-1β through activation of cAMP-dependent protein kinase A. Finally, discriminating concentrations of subtype-selective β-AR antagonists revealed that β1-AR stimulation alone accounted for the synergistic production of IL-1β in LPS stimulated monocytes co-incubated with Iso. These results demonstrate a unique synergistic pro-inflammatory response mediated through a β1-AR cAMP-dependent mechanism in LPS challenged monocytic cells.
Keywords: β-Adrenergic Receptors, Interleukin-1β, Monocytes, Receptor Signaling, Catecholamines
INTRODUCTION
During acute infections monocytes are the primary producers of cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor α (TNF-α), which contribute to the microbicide activity of the innate immune system (Cavaillon and Adib-Conquy, 2005). In chronic inflammatory conditions such as atherosclerosis, generation of IL-1β and TNF-α by these same immunocompetent cells induces damage to endothelial cells, which is a major factor in the development of vascular plaques (Loppnow, et al., 2008). Understanding how to regulate monocyte production of IL-1β, TNF-α and other innate cytokines may prove useful in the treatment of sepsis or slowing the progression of cardiovascular diseases caused by atherosclerotic lesions.
Increased sympathetic responses associated with acute infection or tissue injury constitute an important regulatory mechanism that optimizes innate inflammatory responses (Calcagni and Elenkov, 2006). For instance, the sympathetic neurotransmitter norepinephrine has been shown to have a variety of effects on the innate immune response in vivo, including acting as a chemotactic agent for monocytes as well as inhibiting the bacterial endotoxin initiated production of TNF-α (Straub, et al., 2000; van der Poll, et al., 1994). In the same manner, prolonged or inappropriate stimulation of the sympathetic nervous system on innate immune responses can lead to excess inflammation or uncontrolled infection resulting in pathological effects, including toxic shock and tissue damage (Spengler, et al., 1990; Murray, et al., 2000). Therefore, understanding the mechanisms that modulate sympathetic regulation of monocyte function and thereby innate cytokine production is important in the development of therapeutic strategies to selectively regulate the inflammatory response to diseases in which immunocompetent cells of the innate immune system play an important pathological function.
In humans, responses to the endogenous catecholamines norepinephrine and epinephrine are mediated through activation of three independent families of adrenergic receptors (ARs), which include within each group three characterized receptor subtypes (α1a, α1b, α1d, α2a, α2b, α2c, β1, β2, β3; see review by Guimarães and Moura, 2001) Early studies have characterized high β-AR expression levels from an ex vivo preparation of human mononuclear leukocytes (MNL), which comprise a mixed population of monocytes, lymphocytes (adaptive immunocompetent cells) and platelets (Motulsky, et al., 1986). A subsequent investigation utilizing oral administration of subtype-selective β-AR antagonists to block isoproterenol (Iso) induced changes in β-AR density and generation of cAMP documented a β2-AR population in this same MNL preparation (van Tits, et al., 1990). Although an elegant analysis, this investigation did not quantify direct receptor interactions for subtype-selective β-AR competitive antagonists and thus heterogeneous expression of β-AR subtypes cannot be ruled out in this mixed population of immunocompetent cells. Nonetheless, ex vivo and in vitro preparations of human monocytes are considered to solely express the β2-AR subtype, whose activation has further been shown to have “anti-inflammatory” effects resulting in dampening of the innate immune response to infection or injury (Farmer and Pugin, 2000; Mizuno, et al., 2005; van der Poll, et al., 1994). However, complicating the literature are reports of β1-AR subtype expression in preparations of human monocytes or “pro-inflammatory” responses attributed to β2-AR activation, suggesting a pluripotent β2-AR effect in these same cells (Kavelaars, et al., 1997; Szelenyi, et al., 2006; Talmadge, et al., 1993). In this study we tested the hypothesis that pro-inflammatory outcomes of β-AR activation in monocytes are not due to the bi-functional ability of a single receptor, but instead are related to the signaling capacity for a specific β-AR subtype expressed in a heterogeneous receptor population. We explored the dichotomous β-AR inflammatory response in human monocytes that had been simultaneously incubated with the bacterial endotoxin, lipopolysaccharide (LPS). We characterize the expression of both β1- and β2-AR subtypes on human monocytes, which when stimulated concomitantly with LPS and Iso generated a unique synergistic increase in IL-1β production. Using subtype-selective receptor antagonists, we observed that this novel pro-inflammatory response is associated with exclusive activation of the β1-AR subtype and was functionally correlated to the generation of cAMP along with subsequent activation of protein kinase A (PKA). Our results are the first to demonstrate, using classical pharmacological techniques, a “pro-inflammatory” effect of β1-AR activation in human monocytes that have been pathogenically challenged to initiate an inflammatory response.
EXPERIMENTAL
Materials and Methods
Cell Culture
A human monocytic cell line, THP-1 (ATCC, Manassas, VA) was propagated using standard cell culture conditions (37° C/5% CO2) in RPMI 1640 medium with 2 mM L-glutamine adjusted to contain 1.5 g/L sodium bicarbonate, 4.5 g/L glucose and 10 mM HEPES (complete media), supplemented with 10% heat inactivated fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville, GA). Confluent THP-1 cells (106 cells/mL) were washed in serum-free complete medium, allowed to become quiescent for 30 min before pre-incubating with or without AR antagonists or inhibitors of PKA for 60 min prior to addition of the selective β-AR agonist, Iso (Sigma-Aldrich, St. Louis, MO) and/or an inflammatory receptor agonist, LPS (Sigma-Aldrich, St. Louis, MO).
Isolation of Primary Human Monocytes
The lymphocyte layer was obtained from the peripheral blood of healthy adults. Monocytes were separated by centrifugation at 600 × g for 20 min at 22° C using a 30-45-60% Percoll density gradient. The monocyte-enriched fraction was removed from the 30-45% gradient interface, washed and resuspended in complete media containing 10% heat inactivated FBS. 106 cells/mL were washed in serum-free medium and allowed to become quiescent for 30 min prior to the addition of Iso (Sigma-Aldrich, St. Louis, MO) and/or LPS (Sigma-Aldrich, St. Louis, MO).
Cytokine Antibody Arrays
A commercially available cytokine array system was used to simultaneously detect multiple inflammation-related factors generated by THP-1 cells according to the manufacturer's instructions (RayBiotech, Norcross, GA). Briefly, quiescent THP-1 cells were stimulated by LPS in the absence or presence of Iso for 2 hr. Cells were gently pelleted and 1 mL of the supernatant used to cover the membrane containing the arrayed antibodies. After an overnight incubation at 4° C, the membrane was washed and incubated at 22° C for 2 hr with biotin-conjugated antibodies. After washing, membranes were incubated at 22° C for 60 min with diluted horseradish peroxidase-conjugated streptavidin. Bound antibody was visualized by digital photography (UVP, Upland, CA) using ECL imaging solutions from the manufacturer (RayBiotech, Norcross, GA).
Membrane Preparation for Receptor Binding
Crude THP-1 cell membranes were prepared as previously described (Rojanathammanee, et al., 2009). Briefly, suspended cells were transferred to a 50 mL conical tube and twice washed by centrifugation at 1000 × g using cold Hank's balance salt solution (HBSS). The intact cell pellet was resuspended in 10 mL of 0.25 M sucrose containing 10 μg/mL bacitracin, 10 μg/mL benzamidine, 10 μg/mL leupeptin, and 20 μg/mL phenylmethysulfonylfluoride. The cells were disrupted by freezing followed by homogenization of the thawed suspension using 20 strokes from a loose fitting Dounce homogenizer (B) pestle. This mixture was then centrifuged at 1260 × g for 5 min at 4° C. Buffer A (20 mM HEPES, pH 7.5, 1.4 mM EGTA, 12.5 mM MgCl2) was added to the supernatant and centrifuged at 30,000 × g for 15 min at 4° C. The resultant pellet was resuspended in buffer A then centrifuged once more at 30,000 × g for 15 min at 4° C. The final crude membrane pellet was resuspended in buffer A containing 10% glycerol and stored in aliquots at −80° C until used for radioligand binding. Protein concentrations were measured using the method of Bradford (Bradford, 1976).
Radioligand Binding
The radioligand binding protocol used has been previously described (Rojanathammanee, et al., 2009). Briefly, the density of expressed β-ARs on THP-1 cells was determined by saturation binding experiments using the selective β-AR antagonist (–)3-[125I]iodocyanopindolol, (125I-CYP) as the radiolabel (PerkinElmer, Waltham, MA). Crude THP-1 cell membranes were allowed to equilibrate at 37° C with increasing concentrations of 125I-CYP (5-600 pM) in a 0.25 mL total volume of buffer A using 10 μM propranolol to determine non-specific binding. Binding was stopped by filtering the membranes though Whatman GF/C glass fiber filters, followed by 5 × 5 mL washes with cold buffer A to remove unbound drug. Amounts of total and non-specific radiolabel bound to cell membranes were calculated from radioactive counts remaining on the glass fiber filters. From the plotted saturation hyperbola, β-AR density (Bmax) and affinity value (Kd) of 125I-CYP for THP-1 binding sites were calculated using iterative non-linear regression analysis (Motulsky and Ransnas, 1987). Competition binding studies using increasing concentrations of unlabeled AR ligands were performed in the same buffer used for saturation binding experiments. Iterative non-linear regression analysis was again used to determine IC50 concentrations from which affinity values (Ki) of competing AR ligands for specific THP-1 cell binding sites were calculated using the method of Cheng and Prusoff (Cheng and Prusoff, 1973).
Measurement of cAMP
THP-1 cells used for the quantification of cAMP were washed and pre-incubated for 30 min in serum-free complete medium containing 1 mM of 1-methyl-3-isobutylxanthine (IBMX) to inhibit phosphodiesterase (PDE) prior to the addition of Iso. After 30 min of agonist treatment, cells were lysed using 0.1 M HCl and collected for determination of cAMP production according to the manufacturer's protocol (GE Healthcare, Piscataway, NJ). Briefly, 3H-cAMP added to cell lysates was used to compete with endogenous cAMP for binding to a specific cAMP-binding protein provided by the manufacturer. 3H-cAMP levels were then assessed by liquid scintillation and related to endogenously generated cAMP by comparison with known standards.
Schild Regression Analysis
The method of Schild as previously described was used to determine the β-AR subtype facilitating generation of cAMP in THP-1 cells (Hillman, et al., 2005). Briefly, cumulative concentration-response curves for Iso induced cAMP production were generated in THP-1 cells that had been pre-incubated for 30 min with and without subtype-selective β-AR antagonists. Iterative non-linear regression analysis was used to calculate the EC50 concentration of Iso that generated half-maximal amounts of cAMP (GraphPad, La Jolla, CA). Dose-ratios were calculated by dividing the EC50 value in the presence of AR antagonist with the control EC50 value. Schild regressions were constructed by plotting the log of the dose ratio-1 versus the log of the receptor antagonist concentration. Linear regression analyses of these plotted points were used to determine the Schild regression slope (GraphPad, La Jolla, CA). Slopes of the Schild regressions are expressed as the mean ± 95% confidence interval and were only considered different from unity if the 95% confidence interval did not include the value of 1 (Kenakin, 1997). Equilibrium dissociation constant values (Kb) of subtype-selective β-AR antagonists for inhibiting Iso mediated production of cAMP in THP-1 cells were calculated from Schild regression x-intercepts. Experimental differences in Kb values and Schild regression slopes were determined by analysis of covariance with a p < 0.05 level of probability accepted as significant.
Immunoblot Hybridization
After 2 hr of receptor agonist treatment, THP-1 cells were lysed using a modified RIPA buffer (150 mM NaCl, 10 mM Tris, pH 7.2, 0.1% sodium dodecyl sulphate, 1.0% Triton X-100, 1.0% sodium deoxycholate, 5 mM EDTA, 1.0% Sigma protease inhibitor cocktail). Equal amounts of total cell lysates were resolved by SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes. Expression was measured by 4° C overnight immunoblotting with diluted antibodies against human IL-1β (2 μg/mL; R&D Systems, Minneapolis, MN), specific β1, β2, or β3-AR subtypes (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA), actin (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA) or α-tubulin (1:10,000; Santa Cruz Biotechnology, Santa Cruz, CA). After washing, membranes were incubated at 22° C for 90 min with the appropriate diluted horseradish peroxidase-linked secondary antibody (1:5000; Jackson ImmunoResearch, West Grove, PA). Bound antibody was visualized by chemiluminescent imaging (Thermo Fisher Scientific, Rockford, IL) and documented by digital photography (UVP, Upland, CA). Protein concentrations were measured using the method of Bradford (Bradford, 1976).
Enzyme-Linked Immunosorbent Assay (ELISA)
Following the 2 h agonist treatment, an equal number of THP-1 cells were centrifuged at 600 × g for 5 min to pellet cells. The supernatant was then collected and stored at −20° C until use for ELISA. Concentrations of IL-1β in the culture media were determined using the human IL-1β/IL-1F2 Quantikine HS ELISA assay (R&D Systems, Minneapolis, MN) according to manufacturer's instructions.
Statistical Analyses
A Wald-Wolfowitz runs test was used to determine if the data differed significantly from a linear relationship (p < 0.05). For each experiment, the fitted iterative nonlinear regression curve that best represented the data was determined using a partial f test (p < 0.05). Significance among groups was tested using a paired t test or one-way analysis of variance followed by a Newman-Keuls multiple comparison test (p < 0.05). All values are reported as the mean ± S.E. of n individual experimental treatments.
Results
Adrenergic Receptor Modulation of LPS Mediated Inflammatory Response
As a response to stress, increased levels of catecholamines can be found circulating in the plasma or at the synaptic cleft. Therefore, in order to understand the modulatory effects that AR stimulation would have on immune cell inflammatory responses, we challenged THP-1 cells with LPS in the presence and absence of the selective β-AR agonist Iso then examined the expression of inflammatory mediators using a cytokine antibody array. This array contains 39 antibodies specific for known mediators of inflammation immobilized on a membrane support (Table 1). Conditioned non-serum complete media from 5×106 THP-1 cells/mL previously stimulated for 2 h with or without 30 ng/mL LPS in the absence or presence of 0.1 μM Iso, were individually incubated with an arrayed antibody support. Membranes were then processed as described in “Materials and Methods” and analyzed for expression of inflammatory mediators. Results from a representative experiment (n = 3) are shown in Figure 1. Panel A shows the basal expression of proteins released into the media in the absence of receptor agonist stimulation. There was strong basal expression of IL-8 and macrophage inflammatory protein-1β by THP-1 cells that was consistent throughout all treatments. In addition, there was minor yet variable protein expression of granulocyte-colony stimulating factor, IL-1α, IL-6 soluble receptor, soluble tumor necrosis factor receptor II and tissue inhibitor of metalloproteinease-2 (TIMP-2). Under all conditions, the most consistent detection of these weaker expressing proteins was IL-1α and TIMP-2. Panel B shows the expression of secreted proteins from THP-1 cells that had been stimulated with 30 ng/mL of LPS only. There was a reliable qualitative increase in the moderate and weak expression of IL-1β and TNF-α respectively, from these treated cells. All other proteins demonstrated no differences in their expression when compared to control. Panel C shows the THP-1 response to a half-maximal effective concentration (0.1 μM) of the selective β-AR agonist Iso. For these cells there were no differences in the expression pattern when compared to the basal response. Panel D displays the expression pattern of inflammatory proteins secreted in response to 30 ng/mL LPS and 0.1 μM Iso simultaneously. There was a strong expression pattern for soluble IL-1β representing a qualitative increase in the amount of this secreted cytokine when compared to LPS stimulation alone. This effect of β-AR stimulation in the presence of LPS was synergistic because no detectable IL-1β secretion was observed in response to Iso only. Conversely, there was a qualitative loss of LPS mediated TNF-α expression in the presence of Iso when compared to LPS alone, which validated observations previously shown by others (Farmer and Pugin, 2000). These results demonstrate the utility of this cytokine antibody array as a quick and accurate method to simultaneously analyze multiple inflammation-related factors secreted by immunocompetent cells that have been challenged with pro-inflammatory receptor agonists.
TABLE 1.
Map of Cytokine Antibody Array
| POS | POS | NEG | NEG | EOTAXIN | EOTAXIN-2 | GCSF | GM-CSF | ICAM-1 | IFN-γ | I-309 | IL-1α |
| POS | POS | NEG | NEG | EOTAXIN | EOTAXIN-2 | GCSF | GM-CSF | ICAM-1 | IFN-γ | I-309 | IL-1α |
| IL-1β | IL-2 | IL-3 | IL-4 | IL-6 | IL-6Sr | IL-7 | IL-8 | IL-10 | IL-11 | IL-12 p40 | IL-12 p70 |
| IL-1β | IL-2 | IL-3 | IL-4 | IL-6 | IL-6Sr | IL-7 | IL-8 | IL-10 | IL-11 | IL-12 p40 | IL-12 p70 |
| IL-13 | IL-15 | IL-16 | IL-17 | IP-10 | MCP-1 | MCP-2 | M-CSF | MIG | MIP-1α | MIP-1β | MIP-1δ |
| IL-13 | IL-15 | IL-16 | IL-17 | IP-10 | MCP-1 | MCP-2 | M-CSF | MIG | MIP-1α | MIP-1β | MIP-1δ |
| RANTES | TGF-β1 | TNF-α | TNF-β | s TNF RI | s TNF RII | PDGR-BB | TIMP-2 | BLANK | BLANK | NEG | POS |
| RANTES | TGF-β1 | TNF-α | TNF-β | s TNF RI | s TNF RII | PDGR-BB | TIMP-2 | BLANK | BLANK | NEG | POS |
Positions on membrane support of antibodies that recognize specific mediators of inflammation. Abbreviations used are POS, positive biotinylated protein control; NEG, negative BSA control; GCSF, granulocyte-colony stimulating factor; GM-CSF, granulocyte-macrophage colony stimulating factor; ICAM, intercellular adhesion molecule; IFN, interferon; IL, interleukin; IL-6Sr, interleukin-6 soluble receptor; IL-12 p40, interleukin-12 p40 subunit; IL-12 p70, interleukin-12 p70 subunit; IP-10, IFN receptor inducible protein 10; MCP, monocyte chemoattractant protein; M-CSF, macrophage-colony stimulating factor, MIG, monokine induced by γ-interferon; MIP, macrophage inflammatory protein; TGF, tissue growth factor; TNF, tissue necrosis factor; s TNF R, soluble tumor necrosis factor receptor; PDGR, platelet-derived growth factor; TIMP, tissue inhibitor of metalloproteinases.
Figure 1.
β-AR modulation of LPS initiated inflammatory mediators. Representative series of antibody array membranes for specific inflammatory cytokines (see Table 1) incubated with conditioned media from THP-1 cells treated with A – complete media only, B – 30 ng/mL LPS, C – 0.1 μM Iso or D – 30 ng/mL LPS plus 0.1 μM Iso as outlined in the “Materials and Methods”. There was a qualitative increase in the amount of soluble IL-1β (dashed lines) along with decreases in TNF-α (solid lines) from cells incubated with Iso plus LPS when compared to LPS alone (n = 3). There were no detectable amounts of soluble IL-1β or TNF-α from membranes incubated with complete media alone or Iso only. In addition, no consequential changes in the levels for other inflammatory cytokines were observed between the different treatment conditions.
In an attempt to better quantitate the observed modulation of LPS mediated IL-1β production by β-AR activation, we probed control and treated THP-1 cell lysates with a monoclonal antibody specific for the human form of IL-1β using immunoblot analysis. Figure 2, Panel A is a representative immunoblot (n = 5) of these studies showing changes for the amount of IL-1β produced in response to individual or combined treatments of 30 ng/mL LPS and 0.1 μM Iso. Detectable increases over basal in the amount of IL-1β produced was observed for each separate treatment. However, there was a considerable quantity of IL-1β produced from THP-1 lysates that were incubated in the presence of both LPS and Iso when compared to each treatment alone. Semi-quantitative analysis demonstrated a significant (p < 0.05) amount of IL-1β produced in the presence of LPS (12.1 ± 3.9 fold) when compared to control. In addition, concurrent incubation with LPS and Iso showed a significant (p < 0.05) increase in the generation of IL-1β (45.2 ± 15.8 fold over basal) when compared to Iso (3.1 ± 0.8 fold over basal) or LPS treatments only (Fig 2, Panel B). The amount of IL-1β produced by the combination of LPS and Iso is far greater than the additive amount of each individual treatment alone. This synergistic effect on IL-1β production by LPS and Iso is similar to the qualitative results shown using the cytokine antibody array in Figure 1. These findings go on to further document a > 4-fold synergistic modulation by β-AR activation on LPS mediated production of IL-1β in THP-1 cells.
Figure 2.
β-AR activation synergistically increases LPS initiated production of IL-1β in THP-1 cells. Panel A, Representative immunoblot for equal amounts of total resolved protein from THP-1 cell lysates individually treated with 30 ng/mL LPS, 0.1 μM Iso or concurrently with both, then subsequently probed with an IL-1β antibody as outlined in the “Materials and Methods”. There was a qualitative increase in pro-IL-1β (33 kDa) production from lysates treated with LPS plus Iso when compared to Iso only or LPS alone. Panel B, Semi-quantitative analysis of all immunoblots (n = 5) showed a significant (p < 0.05) increase in the expression of IL-1β from lysates treated with LPS (12.1 ± 3.9 fold) when compared to basal (*). In addition, there was a significant (p < 0.05) synergistic production of IL-1β from lysates incubated with LPS plus Iso (45.2 ± 15.8 fold over basal) when compared to LPS alone (#). There were no significant differences in the amount of IL-1β generated from lysates treated with Iso (3.1 ± 0.8 fold) when compared to basal. Panel C, Amounts of secreted IL-1β quantified from the media of treated THP-1 cells using ELISA analysis (n = 6). Significant (p < 0.05) increases were found in the amount of IL-1β secreted (16.9 ± 2.6 pg/mL) from cells treated with LPS when compared to control (*). In addition, cells treated with LPS plus Iso demonstrated a synergistic increase in IL-1β (76.3 ± 24.7 pg/mL), which was significantly (p < 0.05) different from amount secreted from LPS only (#). There were no changes in the amount of secreted IL-1β from cells treated with 0.1 μM Iso (4.8 ± 1.4 pg/mL) when compared to control (1.6 ± 0.5 pg/mL).
Monocyte processing of IL-1β into the bioactive 17 kDa secreted form is a result of constitutive caspase-1 activation through a one-time stimulation of TLR4 receptors by LPS (Netea et al., 2009). As a result, we expect that heighted pro-IL-1β generation observed from THP-1 cell lysates treated in the presence of LPS and/or Iso, correlate to the same increase in the secreted form of this cytokine qualitatively shown in Figure 1. To test this hypothesis the amount of secreted IL-1β was quantified from the media of THP-1 cells treated with 30 ng/mL LPS, 0.1 μM Iso or LPS plus Iso, using ELISA analysis (n = 6; Fig 2, Panel C). Similar to results that examined the IL-1β 32 kDa pro-form, there was no significant change in the amount of secreted IL-1β from the media of cells treated with 0.1 μM Iso (4.8 ± 1.4 pg/mL) when compared to control (1.6 ± 0.5 pg/mL). However, there were significant (p < 0.05) increases in the amount of IL-1β secreted (16.9 ± 2.6 pg/mL) from cells treated with LPS alone when compared to control. More importantly, we observed in the media from cells treated with LPS plus Iso, a synergistic increase in IL-1β (76.3 ± 24.7 pg/mL) that was significantly (p < 0.05) different from the amount secreted from LPS only. In addition, differences in the amounts of IL-1β released into the media between LPS alone and LPS plus Iso treatments are again similar to differences observed for pro-IL-1β production in THP-1 cells similarly treated (> 4-fold). These results validate observations for the generation of pro-IL-1β using immunoblot techniques as corresponding to the amounts of bioactive cytokine secreted into the media.
To better understand the physiological relevance of our findings, total cell lysates from primary monocytes isolated from human peripheral blood were treated with 30 ng/mL LPS, 0.1 μM Iso or LPS plus Iso then lysed for immunoblot analysis to probe for the expression of IL-1β. Figure 3, Panel A is a representative immunoblot (n = 3) of these studies showing IL-1β production changes in response to these individual or combined LPS and Iso treatments. Detectable increases over basal in the amount of IL-1β produced was observed for each separate treatment. However, there was a considerable quantity of IL-1β produced from primary human monocyte lysates that were incubated in the presence of both LPS and Iso when compared to each treatment alone. Actin immunoblotting is shown as a loading control and corroborates other results using the method of Bradford to determine equal protein loading. Semi-quantitative analysis demonstrated significant (p < 0.05) IL-1β production in the presence of LPS only (3.4 ± 1.8 fold) when compared to control. In addition, concurrent incubation with LPS and Iso showed a significant (p < 0.05) generation of IL-1β (8.6 ± 0.8 fold over basal) when compared to Iso (0.7 ± 0.2 fold over basal) or LPS treatments alone (Fig 3, Panel B). There were no significant differences in IL-1β production from cells treated with Iso compared to control. Although total amounts of IL-1β generated from primary human monocytes is less than THP-1 cell production, the combination of LPS and Iso produced similar fold increases when contrasted with each individual treatment alone. This comparable response by LPS and Iso in primary human monocytes, demonstrates that THP-1 cells are a valid model system for studying the signal transduction mechanism associated with AR potentiation of LPS-mediated IL-1β production.
Figure 3.
β-AR activation synergistically increases LPS-mediated increases of IL-1β production in primary human monocytes. Panel A, Representative immunoblot for equal amounts of total resolved protein lysates from peripheral blood monocytes, individually treated with 30 ng/mL LPS, 0.1 μM Iso or concurrently with both, then subsequently probed with an IL-1β antibody as outlined in the “Materials and Methods”. There was a qualitative increase in pro-IL-1β (33 kDa) production from lysates treated with LPS plus Iso when compared to Iso or LPS alone. The 42 kDa actin band is shown as a loading control. Panel B, Semi-quantitative analysis of all immunoblots (n = 3) showed a significant (p < 0.05) increase in the expression of IL-1β from lysates treated with LPS (3.4 ± 1.8 fold) when compared to basal (*). In addition, there was a significant (p < 0.05) synergistic production of IL-1β from lysates incubated with LPS plus Iso (8.6 ± 0.8 fold over basal) when compared to LPS alone (#). There were no significant differences in the amount of IL-1β generated from lysates treated with Iso (0.7 ± 0.2 fold) when compared to basal.
β-Adrenergic Receptor Expression on THP-1 Cell Membranes
It was important to describe the pattern of AR expression in THP-1 cells in order to pharmacologically isolate the subtype(s) of β-AR(s) modulating the LPS mediated inflammatory response. Therefore, THP-1 cell lysates were probed with specific antibodies that selectively identify all three β-AR subtypes potentially expressed on these cells using procedures described in the “Materials and Methods”. These antibodies have been previously used to identify specific β-AR subtypes expressed in hippocampal tissue slices (Jurgens, et al., 2005). Figure 4 shows the image from three independent THP-1 cell lysate preparations that were resolved using SDS-PAGE, immobilized on PVDF membranes, then individually probed with specific antibodies for β1-AR, β2-AR, β3-AR or α-tubulin, respectively. There were qualitative levels of expression observed for both the β1- and β2-AR although protein levels for the β2-AR appeared to be considerably less than that of the β1-AR subtype. The putative β1-AR protein in resolved THP-1 lysates migrated around the expected size of ~65 kDa while the specific β2-AR band traveled slower with an approximate molecular weight twice that of the expected 56 kDa size, possibly representing a β-AR homo- or heterodimer. No signal was observed when the specific β3-AR antibody was used to probe THP-1 cell lysates. α-Tubulin expression was consistent for all three THP-1 cell lysate preparations and is shown as a loading control.
Figure 4.
Immunoblot analysis of β-AR protein expression from THP-1 cell lysates. Independent THP-1 cell lysates preparations (n = 3) were resolved as outlined in the “Materials and Methods” then probed with antibodies that recognize α-tubulin, β1-, β2-, or the β3-AR subtype. In all preparations the putative band for the β1-AR was identified around the predicted size of ~65 kDa while a specific protein band representing the β2-AR subtype migrated at twice the expected 56 kDa size. No specific β3-AR protein band was detected in any monocyte preparation. α-tubulin expression is shown as a loading control.
To support the results generated from immunoblot analysis, radioligand binding assays were performed to quantify the amount and characteristics of the β-AR subtypes expressed on THP-1 cell membranes. Increasing amounts of the iodinated selective β-AR antagonist 125I-CYP specifically labeled a saturable, homogeneous, high affinity binding site on these THP-1 membranes (data not shown). The number of binding sites was determined to be 341 ± 77 fmol/mg protein (n = 6) and the equilibrium dissociation constant (Kd) of 125I-CYP for these binding sites was 30.1 ± 5.1 pM (n = 6), which is similar to the calculated affinity value of this radiochemical when previously used to identify β-AR binding sites on cell membranes (Rojanathammanee, et al., 2009). Competitive binding assays using a variety of subtype-selective AR antagonists were also performed to establish the characteristics of this β-AR population expressed on THP-1 membranes. From these experiments competition curves were generated and used to calculate affinity values for selective and subtype-selective β-AR antagonists (Table 2). When the selective β-AR antagonist, propranolol was used to compete for specific 125I-CYP binding sites a one-site competition curve was modeled to the data with a calculated equilibrium dissociation constant (Ki) of 2.3 ± 0.4 nM (n = 9). This value is similar to the calculated high affinity of propranolol when it was used to identify β1- and β2-AR subtypes in other membrane preparations (Smith and Teitler, 1999) and suggests that the β3-AR, which has a low propranolol affinity, is not expressed in our system. This finding also substantiates our results from saturation binding analysis using the selective radiolabel 125I-CYP, which identified a homogenous population of β-AR binding sites. Subtype-selective β-AR antagonists were also used in competitive binding assays to establish the β-AR subtype expression pattern on THP-1 cells. A two-site model fit best when the subtype-selective β1-AR antagonists, atenolol and CGP 20712A, or the β2-AR subtype-selective antagonist, ICI 118,551 were used for competitive displacement of specific 125I-CYP binding sites on THP-1 cells. From these curves high and low equilibrium dissociation constant values (KiH/KiL) of 270 ± 73/3846 ± 52 nM, 1.6 ± 0.5/1972 ± 137 nM and 1.3 ± 0.4/120 ± 27 nM were calculated for atenolol (n = 6), CGP 20712A (n = 9) and ICI 118,551 (n = 9) respectively. These values correspond to previous reports where these subtype-selective AR antagonists were used to identify β1- and β2-AR in other tissues (Candelore, et al., 1999; Hoffmann, et al., 2004; Smith and Teitler, 1999). However, use of atenolol, CGP 20712A and ICI 118,551 can only discern between β1- or β2- and not β3-AR expression. Therefore, a β3-selective AR antagonist, SR 59230A was included in the pharmacological profile of ligands used for competitive binding assays to determine if β3-AR binding sites were also expressed on THP-1 cells (Table 2). A one-site curve was modeled to the SR 59230A competitive binding data with a calculated Ki of 107 ± 6 nM (n = 7) for these THP-1 binding sites. This Ki does not correspond to previous reports where SR 59230A was used to identify β3-AR subtypes in other preparations (Candelore, et al., 1999) and supports our propranolol results, which suggested a lack of β3-AR expression on THP-1 cells. However, this lower affinity is comparable to calculated values of this ligand for homogeneous β1- or β2-AR binding sites expressed on membranes from stable cell lines (Candelore, et al., 1999). This affinity profile of selective and subtype-selective AR antagonists supports our observations using subtype-selective antibodies which also identified these same membrane proteins and together demonstrates that a heterogeneous population of β1- and β2-AR subtypes is expressed on THP-1 cell membranes.
TABLE 2.
Equilibrium Dissociation Constants of β-AR Antagonist for 125I-CYP Specific Binding Sites on THP-1 Membranes
| Propranolol (9) | Atenolol (6) | CGP 20712A (9) | ICI 118,551 (9) | SR 59230A (7) | |
|---|---|---|---|---|---|
| Ki | 2.3 ± 0.4 | 107 ± 6 | |||
| KiH | 270 ± 73 | 1.6 ± 0.5 | 1.3 ± 0.4 | ||
| KiL | 3846 ± 52 | 1972 ± 137 | 120 ± 27 |
Mean equilibrium dissociation constants (nM) of selective β-AR antagonists for specific 125I-CYP binding sites calculated from individual competitive binding assays (n). For each experiment, the fitted iterative nonlinear regression curve (GraphPad, La Jolla, CA) that best represented the data (i.e., one vs. two-site fit) was determined using a partial f test (p < 0.05).
β-Adrenergic Receptor Subtype Mediating the Generation of cAMP
β-AR activation is characteristically associated with the generation of cAMP in many tissues and cell types. In order to begin characterizing signaling pathways associated with β-AR modulation of LPS inflammatory responses we quantified amounts of cAMP generated in THP-1 cells using Iso as the receptor agonist. Additionally, these assays were performed in the presence of subtype-selective AR antagonists in order to link specific β-AR subtype activation to the production of cAMP. When 106 THP-1 cells were treated as described in the “Materials and Methods” a concentration-dependent increase in the amount of Iso generated cAMP was observed (Fig 5, Panel A). The half-maximal concentration (EC50) of Iso to generate cAMP in control cells was calculated to be 101 ± 17 nM (n = 4) using non-linear regression analysis. In addition, the potency of Iso to initiate this response in THP-1 cells was not altered in the presence of 30 ng/mL LPS (data not shown). This potency of Iso to increase cAMP in monocytes is similar to the EC50 of Iso calculated in other experimental models that measured both intracellular signaling molecules and functional cell responses (Zuscik et al., 1998; Harmon et al., 2005; Hillman et al., 2005). Moreover, this conformity of calculated Iso potencies from different studies justifies the use of 0.1 μM Iso as an effective concentration to elicit functional β-AR responses in the current investigation. Pre-incubation of THP-1 cells with fixed concentrations (100 nM, 300 nM or 1000 nM) of the subtype-selective β2-AR antagonist ICI 118,551, prior to stimulation with increasing concentrations of Iso, demonstrated a parallel rightward shift in the calculated Iso EC50 (264 ± 86 nM, 460 ± 101 nM and 2128 ± 693 nM, respectively; n = 4), which were significantly different (p < 0.05) from control (Fig 5, Panel A). This decreased Iso potency in the presence of a competitive AR antagonist was exploited to calculate an ICI 118,551 affinity value using the method of Schild. A plot of the logarithm dose ratio-1 versus logarithm of the ICI 118,551 concentration was used to generate a straight line employing linear regression analysis (Fig 5, Panel B). The x-intercept of this Schild regression line represents the ICI 118,551 equilibrium dissociation constant (pKb) for the β-AR mediating an increased cAMP production in THP-1 cells. The calculated Kb of 84 ± 15 nM (n = 4; slope = 1.0 ± 0.1) is similar to the ICI 118,551 low affinity binding value (KiL) on THP-1 membranes (Table 2). Moreover, this affinity value is comparable to equilibrium dissociation constants calculated by others when identifying the β1-AR subtype expressed on mammalian cells (Hoffmann, et al., 2004).
Figure 5.
Schild regression analysis using the β2-AR antagonist, ICI 118,551. Panel A, Pre-treatment of THP-1 cells with 100 nM (○), 300 nM (■) or 1000 nM (□) of the subtype-selective β2-AR antagonist ICI 118,551 produced parallel rightward shifts of the Iso concentration-response curves that were significantly different (p < 0.05) from control [EC50 = 264 ± 86 nM, 460 ± 101 nM and 2128 ± 693 nM respectively, versus 101 ± 17 nM for control ●; n = 4]. Panel B, Dose ratios calculated from each individual experiment were plotted as a function of the ICI 118,551 concentration and used for Schild regression analysis, providing an estimated Kb value of 84 ± 15 nM (n = 4) with a slope of 1.0 ± 0.1.
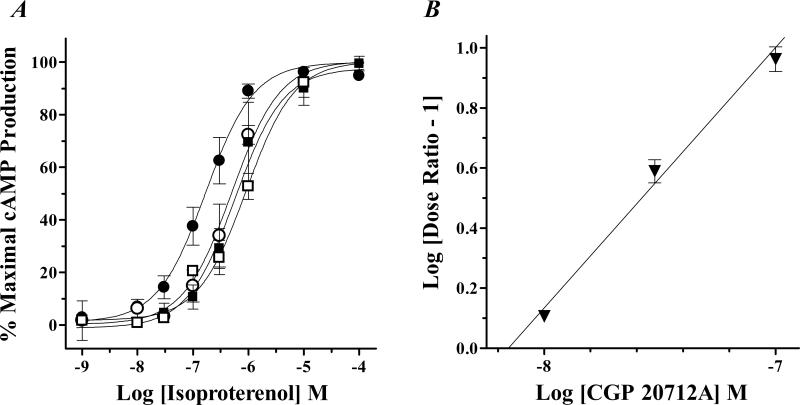
In other experiments, increasingly fixed concentrations (10 nM, 30 nM or 100 nM) of the subtype-selective β1-AR antagonist CGP 20712A were used to generate parallel rightward shifts of the Iso cAMP concentration-response curve in THP-1 cells (EC50 = 499 ± 220 nM, 575 ± 269 nM and 908 ± 556 nM respectively), which were significantly different (p < 0.05) from control (EC50 = 170 ± 48 nM; n=4; Fig 6, Panel A). Agonist dose ratios were again plotted as a function of the competitive receptor antagonist concentration and Schild regression analysis was used to determine an equilibrium dissociation constant of CGP 20712A for inhibiting the β-AR mediated production of cAMP (Fig 6, Panel B). The Kb estimated for CGP 20712A of 8.0 ± 2.0 nM (n = 4; slope = 1.0 ± 0.1) is similar to the estimated high affinity binding value (KiH) of this β1-selective AR antagonist (Table 2). Furthermore, this affinity is comparable to values calculated by others when identifying the β1-AR subtype expressed on mammalian cells (Hoffmann, et al., 2004). Together, this functional description of subtype-selective AR antagonist affinity values for inhibiting Iso initiated signaling demonstrates that β1-AR activation is linked to the generation of cAMP in THP-1 cells.
Figure 6.
Schild regression analysis using the β1-AR antagonist, CGP 20712A. Panel A, Pre-treatment of THP-1 cells with 10 nM (○), 30 nM (■) or 100 nM (□) of the subtype-selective β1-AR antagonist CGP 20712A produced parallel rightward shifts of the Iso concentration-response curves that were significantly different (p < 0.05) from control [EC50 = 499 ± 220 nM, 575 ± 269 nM and 908 ± 556 nM respectively, versus 170 ± 48 nM for control ●; n = 4]. Panel B, Dose ratios calculated from each individual experiment were plotted as a function of the CGP 20712A concentration and used for Schild regression analysis, providing an estimated Kb value of 8.0 ± 2.0 nM (n = 4) with a slope of 1.0 ± 0.1.
cAMP-Dependent Modulation of LPS Mediated Inflammatory Response by β-Adrenergic Receptor Activation
In an attempt to link the generation of cAMP with the β-AR synergistic effect on LPS-mediated production of IL-1β we examined the amount of cytokine formed in the absence and presence of the cAMP dependent-PKA inhibitor, H-89. Figure 7, Panel A is a representative blot of these studies (n = 6) showing changes for the amount of IL-1β produced in response to individual or combined treatments of 30 ng/mL LPS and 0.1 μM Iso with or without 100 nM H-89 pre-incubation. Qualitative increases over basal in the amount of IL-1β was observed for each separate treatment. As previously shown in Figures 2 and 3 there was a synergistic amount of IL-1β produced from monocyte lysates incubated in the presence of both LPS and Iso when compared to each treatment alone. However, this synergistic effect on IL-1β production was reversed to a level similar to that observed with LPS alone after pre-treating THP-1 cells with H-89, suggesting a dependence upon PKA activation by cAMP. The H-89 treatment used in these studies is recognized to block cAMP dependent-PKA responses because previous investigations from our laboratory have documented the inhibition of PKA-dependent phosphorylation for the cAMP responsive element binding (CREB) protein using this same selective concentration of inhibitor (Harmon, et al., 2005). Also in this study as well as in the current investigation, H-89 did not alter responses in the absence of receptor agonist (data not shown). Figure 7, Panel B shows the semi-quantitative analysis of our observations confirming that a significant (p < 0.05) amount of IL-1β was produced in the presence of LPS and Iso (4.6 ± 1.3 fold) when compared to LPS alone (n = 6). In addition, there was a significant decrease (p < 0.05) in the quantity of IL-1β generated from THP-1 lysates pre-incubated with H-89 (1.4±0.1 fold over LPS) when compared to concurrent LPS and Iso only. However, there were no significant differences in the production of IL-1β for Iso only (0.3 ± 0.1 fold over LPS) or LPS and Iso together in the presence of H-89 when compared to LPS alone (Fig 7, Panel B). These results support the idea that a synergistic effect on LPS mediated IL-1β production by β-AR stimulation is contingent upon activation of PKA by cAMP and provides insight into the potential mechanism of this response in human monocytes.
Figure 7.
PKA inhibition reverses the β-AR synergistic increase of IL-1β production with LPS. Panel A, Representative immunoblot for equal amounts of total resolved protein from THP-1 cell lysates individually treated with 30 ng/mL LPS, 0.1 μM Iso or concurrently with both, in the presence or absence of 100 nM H-89, then subsequently probed with an IL-1β antibody as outline in the “Materials and Methods”. There was a qualitative increase in pro-IL-1β (33 kDa) production from lysates treated with LPS plus Iso when compared to Iso only or LPS alone. In addition, there was a qualitative decrease in the quantity of IL-β produced from cells pre-treated with H-89 compared to those incubated with LPS plus Iso, with no differences in IL-1β when compared to LPS alone. Panel B, Semi-quantitative analysis of all immunoblots (n = 6) demonstrated a significant (p < 0.05) increase in the expression of IL-1β from lysates treated with LPS plus Iso (4.6 ± 1.3 fold over LPS) when compared to Iso only (0.3 ± 0.1 fold over LPS) or LPS alone (#). However, there was a significant decrease (p < 0.05) in the quantity of IL-1β from lysates pre-incubated with H-89 then subsequently treated with LPS plus Iso (1.4 ± 0.1 fold over LPS) when compared to LPS plus Iso only (*), with no differences in IL-1β expression compared to cells incubated with LPS alone.
β-Adrenergic Receptor Subtype Responsible for Modulation of LPS Mediated Inflammatory Response
Since generation of cAMP in monocytes is coupled to activation of the β1-AR subtype and the β-AR synergistic effects on LPS-mediated IL-1β production is contingent upon PKA activation by cAMP, we utilized discriminating concentrations of subtype-selective β-AR antagonists to link this synergistic response to activation of a specific β-AR subtype. Figure 8, Panel A is a representative blot of these studies (n = 7) showing IL-1β changes in response to individual or combined treatments of 30 ng/mL LPS and 0.1 μM Iso in the absence and presence of subtype-selective β-AR antagonists. Qualitative increases over basal in IL-1β production was observed for each separate treatment. As previously shown in Figures 2, 3 and 7, there was a synergistic amount of IL-1β generated from these monocyte lysates in the presence of both LPS and Iso when compared to each treatment alone. In addition, there were no qualitative changes in this synergistic quantity of IL-1β from cells pre-treated with 30 nM of the β2-AR subtype-selective antagonist, ICI 118,551 when compared to concurrent LPS and Iso incubation only (Fig 8, Panel A). However, this synergistic IL-1β response observed with simultaneous LPS and Iso treatment was reversed to levels qualitatively similar to LPS only after pre-incubation with 300 nM of the β1-AR subtype-selective antagonist, CGP 20712A (Fig 8, Panel A). Incubation of THP-1 cells with ICI 118,551 or CGP 20712A alone showed no qualitative changes in IL-1β generation from basal (data not shown). Figure 8, Panel B shows the semi-quantitative analysis of our observations confirming that a significant (p < 0.05) amount of IL-1β was produced in the presence of LPS and Iso (5.1 ± 1.5 fold over LPS) when compared to Iso only (0.3 ± 0.1 fold over LPS) or LPS alone. As stated previously, this synergistic effect on IL-1β production by LPS and Iso together was observed even after pre-treating THP-1 cells with the β2-AR antagonist, ICI 118,551. Statistically, there was no difference in the production of IL-1β for monocytes treated with LPS plus Iso in the presence of ICI 118,551 (4.4 ± 0.9 fold over LPS) when compared to LPS plus Iso alone (Fig 8, Panel B). This discriminating concentration of ICI 118,551 is approximately 25-fold over the calculated high affinity equilibrium dissociation constant (KiH) of this subtype-selective β-AR antagonist for the β2-AR subtype while still being 4-fold below the low binding affinity (KiL) estimated for the β1-AR subtype expressed on THP-1 cells (Table 2). Theoretically, at this ICI 118,551 concentration nearly all the expressed β2-AR population is being occupied by this subtype-selective receptor antagonist while leaving a majority of the β1-AR subtype population available for activation by Iso. Conversely, pretreatment of THP-1 cells with the β1-AR antagonist, CGP 20712A completely reversed the profile of β-AR subtypes being occupied by a competitive receptor antagonist (Table 2) and eliminated any β-AR synergistic effects on IL-1β production back to levels observed with LPS only. Semi-quantitative analysis showed that there was significant decreases (p < 0.05) in the production of IL-1β for LPS plus Iso in the presence of CGP 20712A (0.8 ± 0.2 fold over LPS) when compared to LPS plus Iso alone (Fig 8, Panel B). However, there were no statistical differences for the generation of IL-1β with LPS plus Iso in the presence of CGP 20712A when compared to LPS alone (Fig 8, Panel B). These results directly associate β1-AR stimulation with the synergistic effect on LPS-initiated IL-1β production in human monocytes.
Figure 8.
Subtype-selective β-AR antagonists identify β-AR population responsible for synergistic production of IL-1β with concurrent LPS treatment. Panel A, Representative immunoblot for equal amounts of total resolved protein from THP-1 cell lysates individually treated with 30 ng/mL LPS, 0.1 μM Iso or concurrently with both, in the presence or absence of 0.03 μM ICI 118,551 or 0.3 μM CGP 20712A, then subsequently probed with an IL-1β antibody as outlined in the “Materials and Methods”. There was a qualitative increase in pro-IL-1β (33 kDa) production from lysates treated with LPS plus Iso when compared to Iso only or LPS alone. Likewise, there was a similar qualitative increase in IL-1β from cells pre-incubated with ICI 118,551 when compared to individual Iso or LPS treatments. Furthermore, there was a qualitative decrease in the generation of IL-β from cells pre-incubated with CGP 20712A when compared to those treated with LPS plus Iso, with no differences in IL-1β amounts compared to LPS alone. Panel B, Semi-quantitative analysis of all immunoblots (n = 7) demonstrated a significant (p < 0.05) increase in IL-1β expression of from lysates treated with LPS plus Iso (5.1 ± 1.5 fold over LPS) or pre-incubated with ICI 118,551 (4.4 ± 0.9 fold over LPS) when compared to Iso only (0.3 ± 0.1 fold over LPS) or LPS alone (#). However, there was a significant decrease (p < 0.05) in the quantity of IL-1β from lysates pre-incubated with CGP 20712A (0.8±0.2 fold fold over LPS) when compared to concurrent LPS and Iso treatment (*), with no IL-1β differences compared with LPS alone.
DISCUSSION
The goal of this study was to characterize the AR mediated modulation of inflammatory responses from immunologically competent cells. We hypothesized that increased catecholamine release in response to stress or trauma would heighten pro-inflammatory components of the innate immune system response through selective activation of specific AR subtypes. For this investigation we used the human monocytic THP-1 cell line as our model system (Tsuchiya, et al., 1980) and examined its response to LPS with and without concomitant β-AR stimulation. A commercially available cytokine antibody array was used to initially identify mediators of inflammation whose expression was modified in the presence of Iso. Using this technique the pro-inflammatory cytokines IL-1β and TNF-α were identified as factors whose secretion was qualitatively changed when cells were stimulated with LPS plus Iso compared with LPS alone. Decreased monocyte TNF-α production after challenge by LPS plus Iso has previously been documented and therefore was not pursued in the current study (Farmer and Pugin, 2000; Szelenyi, et al., 2006). However, these earlier investigations have been used in part to support the concept that β-AR modulations of immune cell functions are “anti-inflammatory”. Conversely, subsequent immunoblot analysis in our study confirmed a significant quantitative increase in the amount of secreted IL-1β from THP-1 cells concomitantly stimulated with LPS and a β-AR agonist when compared to LPS alone, which also was validated using a primary human monocyte preparation. Others have reported a reduction in IL-1β production from LPS-challenged immunocompetent cells in response to AR stimulation (Dello, et al., 2004; van der Poll and Lowry, 1997). However, these studies utilized non-selective endogenous AR agonists in the presence of LPS to modulate immune cell function, which also would stimulate other potentially compensatory AR signaling pathways in these cells. Consequently, our results are the first to document a unique synergistic amplification of soluble IL-1β production in LPS challenged human monocytes simultaneously stimulated with a selective β-AR agonist.
The classical G protein-coupled signal transduction mechanism associated with β-AR stimulation is Gs–mediated generation of cAMP through adenylate cyclase leading to the activation of PKA. In the current investigation, β1-AR potentiation of IL-1β production in LPS stimulated THP-1 cells was inhibited by a selective concentration of the cAMP-dependent PKA inhibitor H-89, whose specificity has been validated in our laboratory (Harmon, et al., 2005). β-AR responses for LPS stimulated monocytes have been documented to signal through generation of cAMP and PKA activation (Farmer and Pugin, 2000; Li, et al., 2003). Moreover, other Gs-coupled receptor systems have previously been shown to regulate LPS challenged immunocompetent cell responses through generation of cAMP (Noda, et al., 2007). However, these investigations report a protective decrease in the production of pro-inflammatory cytokines after receptor activation with LPS. Conversely, increased cytokine production in human monocytes has been described using Iso, but only when phorbol myristyl acetate (PMA) was used as the inflammatory stimulant and not LPS. The dichotomy of β-AR modulation on immune cell inflammatory responses in this study was shown to be dependent upon parallel changes in mitogen activated protein kinase (MAPK) activation (Szelenyi, et al., 2006). Similarly, in a murine macrophage cell model, MAPK activation was shown to increase IL-1β generation through a β2-AR mechanism that was independent of a pro-inflammatory stimulus (Tan et al., 2007). Although in this previous study a differentiated monocytic cell line from a disparate species was utilized, we did not find any increases in IL-1β or other soluble cytokines in response to Iso treatment alone from undifferentiated human monocytes when compared to control. Alternatively, production of inflammatory proteins mediated by LPS has been postulated to be effected by a cell-dependent cAMP-directed compartmentalization of selective signaling molecules (Lee, et al., 2004). A similar mechanism by cAMP specifically associated with the β1-AR potentiated pro-inflammatory effects in LPS stimulated human monocytes could also be occurring and is currently being investigated.
Differences in the β-AR mediated production of cytokines from LPS challenged monocytes can also be attributed to the selective stimulation of specific receptor subtypes. For example, β2-AR activation has been shown to decrease LPS induced production of the pro-inflammatory cytokine IL-18 in human peripheral blood mononuclear cells, contributing to the idea that β-AR stimulation leads to an “anti-inflammatory” response (Mizuno, et al., 2005). Conversely, earlier radioligand binding studies on THP-1 cells have documented a homogeneous β1-AR population linked to TNF-α transcriptional decreases (Talmadge, et al., 1993). However, this investigation utilized AR agonist rank order of potencies to identify characteristics of specific 125I-CYP binding on THP-1 membranes. In addition, data from subtype-selective AR antagonist inhibition of these specific 125I-CYP binding sites were analyzed using only a one-site binding model. This type of biased data analysis potentially overlooks any heterogeneous β-AR expression documented in our contemporary study, whose characteristic properties for subtype-selective β-AR antagonists are associated with the identification of β1- and β2-AR subtypes in other systems (Candelore, et al., 1999; Hoffmann, et al., 2004; Smith and Teitler, 1999). Moreover, the Ki calculated for ICI 118,551 in this previous investigation by Talmadge and others was 46 nM, which is medially between the KiL and KiH values of this same β2-AR selective antagonist calculated not only in our current study but also by others (Hoffmann, et al., 2004; Smith and Teitler, 1999). Consequently, the “anti-inflammatory” decrease in TNF-α transcription described by Talmadge et al., using the selective β-AR agonist Iso could instead be associated with β2-AR activation, which would correlate to ours and others observations of a translational decrease in soluble TNF-α expression (Farmer and Pugin, 2000; Szelenyi, et al., 2006).
Selective stimulation of expressed β1- and β2-AR subtypes generates diverse signaling patterns linked to specific physiological responses that can be attributed to coupling through non-canonical G proteins, localized control of cAMP degradation or second-messenger compartmentalization through formation of signalosomes (Dodge-Kafka, et al., 2006; Houslay, et al., 2007; Xiao, et al., 1999). The classical method of Schild is an established pharmacological technique that can be used to link selective receptor activation to these specific signaling pathways in models that express heterogeneous receptor populations (Hillman, et al., 2005). Correspondingly, we examined whether selective β-AR subtype activation would contribute to the cAMP-dependent, PKA-mediated, synergistic IL-1β response, using this same traditional procedure. Calculated functional equilibrium dissociation constants (Kb) of subtype-selective β-AR antagonists used to competitively inhibit generation of cAMP, strongly correlated to the KiL and KiH for ICI 118,551 and CGP 20712A, respectively, indicating that β1-AR activation mediates cAMP production in our model. Although, this does not rule out any contribution of β2-AR stimulation to the generation of cAMP in our system, it does however suggest that β1-AR subtypes are more efficacious for the Gs signaling pathway in these cells. In support of this assumption, investigations using a sensitive, high dynamic range biosensor to fluorescently measure subtype-selective β-AR mediated cAMP generation from intact cardiac myocytes showed a diffuse cAMP gradient associated with β1-AR activation that was over 2-fold greater than the strictly confined β2-AR response (Nikolaev, et al., 2006). Moreover, measurements of cAMP-dependent effects more distal from the receptor describe an even greater contrast between responses associated with selective β-AR subtype activation. For example, Rochais and others demonstrate an increase in cAMP caused by β1-AR stimulation when monitoring cyclic nucleotide-gated channels in cardiac myocytes as opposed to no detectable effects when β2-AR subtypes were stimulated in these same cells (Rochais, et al., 2006). Likewise, effectively antagonizing only β2-AR subtypes in our system did not change the Iso potentiation of LPS mediated IL-1β production from levels observed in the absence of receptor antagonist. However, adequately blocking β1-AR subtypes while still maintaining the capacity to activate a majority of the remaining β2-ARs expressed on THP-1 cells completely abolished the synergistic LPS plus Iso effect on IL-1β generation to levels observed with LPS alone. If β2-AR subtypes expressed on these monocytes were effectively coupled to increases in cAMP then a similar amplification of IL-1β responding to LPS plus Iso should have been observed in the presence of the subtype-selective β1-AR antagonist, CGP 20712A. However, no β-AR potentiation of IL-1β production was observed for LPS challenged monocytes after pretreatment with a selective concentration of CGP 20712A, substantiating β1-AR activation as mediating a pro-inflammatory cytokine response in these cells.
CONCLUSIONS
In conclusion, simultaneous stimulation of both β-AR subtypes expressed on human monocytes can selectively modulate LPS-induced cytokine production. Previous studies have documented a decreased production of TNF-α transcription from THP-1 cells challenged by LPS in the presence of Iso linking an anti-inflammatory response to stimulation of the β2-AR subtype (Farmer and Pugin, 2000). However, we have characterized a pro-inflammatory effect of β1-AR activation in monocytes concurrently stimulated with LPS that synergistically increases IL-1β secretion through a PKA-dependent mechanism. Therefore, it is only through the use of subtype-selective β-AR ligands that the pro- and anti-inflammatory responses of these independent effects on monocyte cytokine production are realized.
ACKNOWLEDGMENTS
The authors would like to thank Eric Harris for his technical support as well as Deb Kroese and Julie Horn for their administrative assistance.
Abbreviations
- ARs
adrenergic receptors
- Bmax
maximal specific binding of radioligand
- CGP 20712A
[1-(2-((3-carbamoyl-4-hydroxy)phenoxy)ethylamino)-3-(4-(1-methyl-4-trifluoromethyl-2-imidazoylyl)phenoxy)-2-propanol methanesulfonate, 2-hydroxy-5-(2-(hydroxy-3-(4-((1-methyl-4-trifluoromethyl)-1H-imidazol-2-yl)phenoxy)propyl)aminoethoxy)benzamide
- EC50
molar concentration of receptor agonist that produces 50% of its maximal effect
- ELISA
Enzyme-Linked Immunosorbent Assay
- FBS
fetal bovine serum
- H-89
N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide dihydrochloride
- HBSS
Hank's balance salt solution
- IBMX
1-methyl-3-isobutylxanthine
- IC50
molar concentration of competitive ligand that inhibits radioligand binding by 50%
- ICI 118,551
erythro-dl-1-(7-methylindan-4-yloxy)-3-isopropylaminobutan-2-ol
- 125I-CYP
(–)3-[125I]iodocyanopindolol
- IL-1β
interleukin-1β
- Iso
isoproterenol
- Kb
equilibrium dissociation constant of competitive receptor antagonist determined from functional assay
- Kd
equilibrium dissociation constant of radioligand
- Ki
equilibrium dissociation constant of competitive ligand determined from radioligand binding study
- kDa
kilodalton
- LPS
lipopolysaccharide
- MNL
mononuclear leukocytes
- PDE
phosphodiesterase
- PKA
protein kinase A
- PVDF
polyvinylidene difluoride
- THP-1
human monocytic cell line
- TIMP-2
tissue inhibitor of metalloproteinease-2
- TNF-α
tumor necrosis factor α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study was supported by the National Science Foundation [Grant 0235146]; the National Institutes of Health [Grant GM066726]; the National Institutes of Health Centers of Biomedical Research Excellence (COBRE) program [Grant RR016471]; and the North Dakota Experimental Program to Stimulate Competitive Research (EPSCoR) program through the National Science Foundation [Grant EPS-0447679]
The results of this work have been previously presented in part at the Experimental Biology 2009 meeting (Abstract #489).
CONFLICT OF INTEREST STATEMENT
All authors declare that there are no conflicts of interest
REFERENCES
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Calcagni E, Elenkov I. Stress system activity, innate and T helper cytokines, and susceptibility to immune-related diseases. Ann. N.Y. Acad. Sci. 2006;1069:62–76. doi: 10.1196/annals.1351.006. [DOI] [PubMed] [Google Scholar]
- Candelore MR, Deng L, Tota L, Guan XM, Amend A, Liu Y, Newbold R, Cascieri MA, Weber AE. Potent and selective human β3-adrenergic receptor antagonists. J. Pharmacol. Exp. Ther. 1999;290:649–655. [PubMed] [Google Scholar]
- Cavaillon JM, Adib-Conquy M. Monocytes/macrophages and sepsis. Crit. Care Med. 2005;33:S506–S509. doi: 10.1097/01.ccm.0000185502.21012.37. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of which causes 50 percent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Dello RC, Boullerne AI, Gavrilyuk V, Feinstein DL. Inhibition of microglial inflammatory responses by norepinephrine: effects on nitric oxide and interleukin-1β production. J. Neuroinflammation. 2004;1:9. doi: 10.1186/1742-2094-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge-Kafka KL, Langeberg L, Scott JD. Compartmentation of cyclic nucleotide signaling in the heart: the role of A-kinase anchoring proteins. Circ. Res. 2006;98:993–1001. doi: 10.1161/01.RES.0000218273.91741.30. [DOI] [PubMed] [Google Scholar]
- Farmer P, Pugin J. β-Adrenergic agonists exert their “anti-inflammatory” effects in monocytic cells through the IκB/NF-κB pathway. Am. J. Physiol. Lung Cell Mol. Physiol. 2000;279:L675–L682. doi: 10.1152/ajplung.2000.279.4.L675. [DOI] [PubMed] [Google Scholar]
- Guimarães S, Moura D. Vascular adrenoceptors: An update. Pharmacol. Rev. 2001;53:319–356. [PubMed] [Google Scholar]
- Harmon EB, Porter JM, Porter JE. β-Adrenergic receptor activation in immortalized human urothelial cells stimulates inflammatory responses by PKA-independent mechanisms. Cell Commun. Signal. 2005;3:10. doi: 10.1186/1478-811X-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman KL, Doze VA, Porter JE. Functional characterization of the β-adrenergic receptor subtypes expressed by CA1 pyramidal cells in the rat hippocampus. J. Pharmacol. Exp. Ther. 2005;314:561–567. doi: 10.1124/jpet.105.084947. [DOI] [PubMed] [Google Scholar]
- Hoffmann C, Leitz MR, Oberdorf-Maass S, Lohse MJ, Klotz KN. Comparative pharmacology of human β-adrenergic receptor subtypes--characterization of stably transfected receptors in CHO cells. Naunyn Schmiedebergs Arch. Pharmacol. 2004;369:151–159. doi: 10.1007/s00210-003-0860-y. [DOI] [PubMed] [Google Scholar]
- Houslay MD, Baillie GS, Maurice DH. cAMP-Specific phosphodiesterase-4 enzymes in the cardiovascular system: A molecular toolbox for generating compartmentalized cAMP signaling. Circ. Res. 2007;100:950–966. doi: 10.1161/01.RES.0000261934.56938.38. [DOI] [PubMed] [Google Scholar]
- Jurgens CW, Rau KE, Knudson CA, King JD, Carr PA, Porter JE, Doze VA. β1 Adrenergic receptor-mediated enhancement of hippocampal CA3 network activity. J. Pharmacol. Exp. Ther. 2005;314:552–560. doi: 10.1124/jpet.105.085332. [DOI] [PubMed] [Google Scholar]
- Kavelaars A, van de Pol M, Zijlstra J, Heijnen CJ. β2-Adrenergic activation enhances interleukin-8 production by human monocytes. J. Neuroimmunol. 1997;77:211–216. doi: 10.1016/s0165-5728(97)00076-3. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Competitive Antagonism. In: Kenakin T, editor. Pharmacologic Analysis of Drug-Receptor Interaction. Lippincott-Raven; Philadelphia: 1997. pp. 331–373. [Google Scholar]
- Lee JH, Del SL, Uhlig S, Porro GA, Whitehead T, Voglis S, Liu M, Slutsky AS, Zhang H. Intercellular adhesion molecule-1 mediates cellular cross-talk between parenchymal and immune cells after lipopolysaccharide neutralization. J. Immunol. 2004;172:608–616. doi: 10.4049/jimmunol.172.1.608. [DOI] [PubMed] [Google Scholar]
- Li CY, Chou TC, Lee CH, Tsai CS, Loh SH, Wong CS. Adrenaline inhibits lipopolysaccharide-induced macrophage inflammatory protein-1α in human monocytes: the role of β-adrenergic receptors. Anesth. Analg. 2003;96:518–523. doi: 10.1097/00000539-200302000-00040. [DOI] [PubMed] [Google Scholar]
- Loppnow H, Werdan K, Buerke M. Vascular cells contribute to atherosclerosis by cytokine- and innate-immunity-related inflammatory mechanisms. Innate Immun. 2008;14:63–87. doi: 10.1177/1753425908091246. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Takahashi HK, Iwagaki H, Katsuno G, Kamurul HA, Ohtani S, Mori S, Yoshino T, Nishibori M, Tanaka N. β2-Adrenergic receptor stimulation inhibits LPS-induced IL-18 and IL-12 production in monocytes. Immunol. Lett. 2005;101:168–172. doi: 10.1016/j.imlet.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Motulsky HJ, Cunningham EM, DeBlasi A, Insel PA. Desensitization and redistribution of β-adrenergic receptors on human mononuclear leukocytes. Am. J. Physiol. 1986;250:E583–E590. doi: 10.1152/ajpendo.1986.250.5.E583. [DOI] [PubMed] [Google Scholar]
- Motulsky HJ, Ransnas LA. Fitting curves to data using nonlinear regression: A practical and nonmathmatical review. FASEB J. 1987;1:365–374. [PubMed] [Google Scholar]
- Murray DR, Prabhu SD, Chandrasekar B. Chronic β-adrenergic stimulation induces myocardial proinflammatory cytokine expression. Circulation. 2000;101:2338–2341. doi: 10.1161/01.cir.101.20.2338. [DOI] [PubMed] [Google Scholar]
- Netea MG, Nold-Petry CA, Nold MF, Joosten LA, Opitz B, van der Meer JH, van de Veerdonk FL, Ferwerda G, Heinhuis B, Devesa I, Funk CJ, Mason RJ, Kullberg BJ, Rubartelli A, van der Meer JW, Dinarello CA. Differential requirement for the activation of the inflammasome for processing and release of IL-1β in monocytes and macrophages. Blood. 2009;113:2324–2335. doi: 10.1182/blood-2008-03-146720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaev VO, Bunemann M, Schmitteckert E, Lohse MJ, Engelhardt S. Cyclic AMP imaging in adult cardiac myocytes reveals far-reaching β1-adrenergic but locally confined β2-adrenergic receptor-mediated signaling. Circ. Res. 2006;99:1084–1091. doi: 10.1161/01.RES.0000250046.69918.d5. [DOI] [PubMed] [Google Scholar]
- Noda M, Kariura Y, Pannasch U, Nishikawa K, Wang L, Seike T, Ifuku M, Kosai Y, Wang B, Nolte C, Aoki S, Kettenmann H, Wada K. Neuroprotective role of bradykinin because of the attenuation of pro-inflammatory cytokine release from activated microglia. J. Neurochem. 2007;101:397–410. doi: 10.1111/j.1471-4159.2006.04339.x. [DOI] [PubMed] [Google Scholar]
- Rochais F, Abi-Gerges A, Horner K, Lefebvre F, Cooper DM, Conti M, Fischmeister R, Vandecasteele G. A specific pattern of phosphodiesterases controls the cAMP signals generated by different Gs-coupled receptors in adult rat ventricular myocytes. Circ. Res. 2006;98:1081–1088. doi: 10.1161/01.RES.0000218493.09370.8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojanathammanee L, Harmon EB, Grisanti LA, Govitrapong P, Ebadi M, Grove BD, Miyagi M, Porter JE. The 27-kDa heat shock protein confers cytoprotective effects through a β2-adrenergic receptor agonist-initiated complex with β-arrestin. Mol. Pharmacol. 2009;75:855–865. doi: 10.1124/mol.108.053397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C, Teitler M. Beta-blocker selectivity at cloned human beta1- and beta2-adrenergic receptors. Cardiovasc. Drugs Ther. 1999;13:123–126. doi: 10.1023/a:1007784109255. [DOI] [PubMed] [Google Scholar]
- Spengler RN, Allen RM, Remick DG, Strieter RM, Kunkel SL. Stimulation of α-adrenergic receptor augments the production of macrophage-derived tumor necrosis factor. J. Immunol. 1990;145:1430–1434. [PubMed] [Google Scholar]
- Straub RH, Mayer M, Kreutz M, Leeb S, Scholmerich J, Falk W. Neurotransmitters of the sympathetic nerve terminal are powerful chemoattractants for monocytes. J. Leukoc. Biol. 2000;67:553–558. doi: 10.1002/jlb.67.4.553. [DOI] [PubMed] [Google Scholar]
- Szelenyi J, Selmeczy Z, Brozik A, Medgyesi D, Magocsi M. Dual β-adrenergic modulation in the immune system: Stimulus-dependent effect of isoproterenol on MAPK activation and inflammatory mediator production in macrophages. Neurochem. Int. 2006;49:94–103. doi: 10.1016/j.neuint.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Talmadge J, Scott R, Castelli P, Newman-Tarr T, Lee J. Molecular pharmacology of the β-adrenergic receptor on THP-1 cells. Int. J. Immunopharmacol. 1993;15:219–228. doi: 10.1016/0192-0561(93)90098-j. [DOI] [PubMed] [Google Scholar]
- Tan KS, Nackley AG, Satterfield K, Maixner W, Diatchenko L, Flood PM. β2 adrenergic receptor activation stimulates pro-inflammatory cytokine production in macrophages via PKA- and NF-κB-independent mechanisms. Cell. Signal. 2007;19:251–260. doi: 10.1016/j.cellsig.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Tsuchiya S, Yamabe M, Yamaguchi Y, Kobayashi Y, Konno T, Tada K. Establishment and characterization of a human acute nomocytic leukemia cell line (THP-1). Int. J. Cancer. 1980;26:171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- van der Poll T, Jansen J, Endert E, Sauerwein HP, van Deventer SJ. Noradrenaline inhibits lipopolysaccharide-induced tumor necrosis factor and interleukin 6 production in human whole blood. Infect. Immun. 1994;62:2046–2050. doi: 10.1128/iai.62.5.2046-2050.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Poll T, Lowry SF. Epinephrine inhibits endotoxin-induced IL-1β production: roles of tumor necrosis factor-α and IL-10. Am. J. Physiol. 1997;273:R1885–R1890. doi: 10.1152/ajpregu.1997.273.6.R1885. [DOI] [PubMed] [Google Scholar]
- van Tits LJ, Michel MC, Grosse-Wilde H, Happel M, Eigler FW, Soliman A, Brodde OE. Catecholamines increase lymphocyte β2-adrenergic receptors via a β2-adrenergic, spleen-dependent process. Am. J. Physiol. 1990;258:E191–E202. doi: 10.1152/ajpendo.1990.258.1.E191. [DOI] [PubMed] [Google Scholar]
- Xiao RP, Avdonin P, Zhou YY, Cheng H, Akhter SA, Eschenhagen T, Lefkowitz RJ, Koch WJ, Lakatta EG. Coupling of β2-adrenoceptor to Gi proteins and its physiological relevance in murine cardiac myocytes. Circ. Res. 1999;84:43–52. doi: 10.1161/01.res.84.1.43. [DOI] [PubMed] [Google Scholar]
- Zuscik MJ, Porter JE, Gaivin R, Perez DM. Identification of a conserved switch residue responsible for selective constitutive activation of the β2-adrenergic receptor. J Biol. Chem. 1998;273:3401–3407. doi: 10.1074/jbc.273.6.3401. [DOI] [PubMed] [Google Scholar]