Abstract
Studies in our laboratory have demonstrated that subchronic 2,3,7,8,-tetrachlorodibenzo-p-dioxin (TCDD) exposure of adult mice results in hypertension, cardiac hypertrophy, and reduced nitric oxide (NO)-mediated vasodilation. Moreover, increased superoxide anion production was observed in cardiovascular organs of TCDD-exposed mice and this increase contributed to the reduced NO-mediated vasodilation. Since cytochrome P4501A1 (CYP1A1) can contribute to some TCDD-induced toxicity, we tested the hypothesis that TCDD increases reactive oxygen species (ROS) in endothelial cells by the induction of CYP1A1. A concentration-response to 24 h TCDD exposure (10 pM-10 nM) was performed in confluent primary human aortic endothelial cells (HAECs). Oxidant-sensitive fluorescent probes dihydroethidium (DHE) and 2’,7’-dichlorofluorescin diacetate (DCFH-DA), were used to measure superoxide anion, and hydrogen peroxide and hydroxyl radical, respectively. NO was also measured using the fluorescent probe diaminofluorescein-2 diacetate (DAF-2DA). These assessments were conducted in HAECs transfected with siRNA targeting the aryl hydrocarbon receptor (AhR), CYP1A1, or CYP1B1. TCDD concentration-dependently increased CYP1A1 and CYP1B1 mRNA, protein, and enzyme activity. Moreover, 1 nM TCDD maximally increased DHE (Cont=1.0±0.3; TCDD=5.1±1.0; p=0.002) and DCFH-DA (Cont=1.0±0.2; TCDD=4.1±0.5; p=0.002) fluorescence and maximally decreased DAF-2DA fluorescence (Cont=1.0±0.4; TCDD=0.68±0.1). siRNA targeting AhR and CYP1A1 significantly decreased TCDD-induced DHE (siAhR: Cont=1.0±0.1; TCDD=1.3±0.2; p=0.093) (siCYP1A1: Cont=1.0±0.1; TCDD=1.1±0.1; p=0.454) and DCFH-DA (siAhR: Cont=1.0±0.2; TCDD=1.3±0.3; p=0.370) (siCYP1A1: Cont=1.0±0.1; TCDD=1.3±0.2; p=0.114) fluorescence and increased DAF-2DA fluorescence (siAhR: Cont=1.00±0.03; TCDD=0.97±0.03; p=0.481) (siCYP1A1: Cont=1.00±0.03; TCDD=0.92±0.03; p=0.034), while siRNA targeting CYP1B1 did not. These data suggest that TCDD-induced increase in ROS is AhR dependent and may be mediated, in part, by CYP1A1 induction.
Keywords: aryl hydrocarbon receptor, cytochrome P4501A1, reactive oxygen species, TCDD, nitric oxide, endothelial cells
Introduction
Many studies have demonstrated that the vasculature is a target of toxicity mediated by persistent aryl hydrocarbon receptor (AhR) agonists, such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) with effects described during early development as well as in adulthood (Ivnitski-Steele et al., 2005a;Kopf et al., 2008). For example, during fish embryo development TCDD reduces blood flow and circulatory function, and these effects are associated with subcutaneous hemorrhage and pericardial edema (Henry et al., 1997;Spitsbergen et al., 1991). Studies have shown that these changes are associated with increases in vascular endothelial cell permeability and are preceded by an inhibition of vasculogenesis and remodeling of the common cardinal vein (Bello et al., 2004;Guiney et al., 2000). In the developing avian embryo, TCDD induces a dilated cardiomyopathy that is associated with pericardial effusion, subcutaneous edema, and hemorrhage (Walker and Catron, 2000). Studies have further demonstrated that TCDD inhibits coronary vascularization of the developing avian heart as a result of inhibiting coronary endothelial cell proliferation and migration (Ivnitski-Steele and Walker, 2003;Ivnitski-Steele et al., 2005b).
The effects of AhR agonists on the adult vasculature are less well studied, but a few reports suggest that it is also a target of toxicity. Studies conducted by the National Toxicology Program reveal that chronic exposure of rats for 2 years to persistent AhR agonists induces arteriolar inflammation and remodeling in mesenteric and pancreatic vascular beds (Jokinen et al., 2003;Yoshizawa et al., 2007). TCDD exposure also suppresses vascular remodeling of the placenta in rats (Ishimura et al., 2006) and reduces endothelial-dependent vascular relaxation in mice; the latter of which is associated with the development of hypertension (Kopf et al., 2008).
One mechanism that has been proposed to mediate TCDD’s effects on the vasculature, at least in part, is the induction of cytochrome P4501A1 (CYP1A1) and the production of reactive oxygen species (ROS). Exposure of fish, birds, and mammals to persistent AhR agonists results in robust and sustained induction of CYP1A1 in vascular endothelial cells (Garrick et al., 2006;Guiney et al., 1997;Schlezinger et al., 2000;Smolowitz et al., 1991). Furthermore, the overexpression of CYP1A1 is associated with the production of ROS, including superoxide anion and hydrogen peroxide (H2O2) (Zangar et al., 2004). In some fish studies, inhibitors of CYPs or CYP1A antisense morpholinos protect against TCDD-induced circulatory failure, edema, and mortality (Cantrell et al., 1996;Dong et al., 2002;Teraoka et al., 2003). Further, in one study pretreatment with an antioxidant prevents a reduction in blood flow in the zebrafish embryo (Dong et al., 2002). The results of other studies, however, have been contradictory with neither antioxidants nor CYP1A antisense morpholinos being protective (Carney et al., 2004;Hornung et al., 1999). Thus, the contribution of CYP1A induction and ROS production to TCDD-induced vascular toxicity during development remains unclear.
In the adult mouse vasculature, TCDD-induced hypertension and endothelial dysfunction are associated with significant increases in CYP1A1 and superoxide anion in cardiovascular tissues, including heart, kidney, and aorta (Kopf et al., 2008). Further, the superoxide dismutase mimetic, tempol, prevents the endothelial dysfunction, suggesting that increased vascular superoxide anion may scavenge the endothelial-dependent vasorelaxation mediator, nitric oxide (NO), and contribute to the loss of vascular relaxation as has been described in many other models of hypertension (reviewed in Simonsen et al., 2009;Wilcox and Pearlman., 2008). Thus, in these studies we sought to determine whether TCDD exposure of aortic endothelial cells increased ROS production, whether the increased ROS could reduce NO release, and whether the AhR and induction of CYP1A1 and/or CYP1B1 were required to mediate these changes.
Materials and Methods
Chemicals
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) was a generous gift from Dr. Richard E. Peterson (University of Wisconsin-Madison). Endothelial cell Lipofectamine 2000 and Opti-MEM were purchased from Invitrogen (Carlsbad, CA). RIPA lysis buffer and goat polyclonal α-actin antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal α-CYP1A1 antisera were purchased from Millipore (Billireca, MA). Rabbit polyclonal α-CYP1B1 antisera were a kind gift from Craig Marcus, Ph.D. (Oregon State University). Secondary antibodies were purchased from SouthernBiotech (Birmingham, AL). Dihydroethidium (DHE), 2’,7’-dichlorofluorescein diacetate (DCFH-DA), anhydrous dimethyl sulfoxide (DMSO), all components of Krebs-Ringer buffer, acetylcholine (ACh), all components of high salt TNE buffer, and bisbenzimide H 33258 were purchased from Sigma-Aldrich (St. Louis, MO). Diaminoflurescein-2 diacetate (DAF-2DA) was purchased from Cell Technology, Inc. (Mountain View, CA).
Cell culture and siRNA transfection
Primary human aortic endothelial cells (HAECs) were purchased from Lonza and restricted to use between passages 1–4. Cells were grown according to manufacturer’s protocol in EGM-2 media (Lonza) at 37°C, 5% CO2. Confluent cells were exposed to vehicle control (0.1% DMSO) or TCDD in fresh EGM-2 media for 24 h. The cell system was characterized using a concentration-response to TCDD with concentrations of 10 pM, 100 pM, 1 nM, and 10 nM TCDD.
siRNA targeting AhR, CYP1A1, and CYP1B1 mRNA (Dharmacon, Chicago, IL) was used to determine the role of each in ROS production. Cells were transfected with siRNA using Lipofectamine 2000 according to the manufacturer’s protocol (Invitrogen). Cells were cultured in antibiotic-free EGM-2 media 24 h prior to transfection. The cells were then incubated in antibiotic-free EGM-2 media containing lipofectamine-siRNA complexes at final concentration of 0.5% lipofectamine and 33 nM siRNA for 24 h. siGENOME RISC-Free Control siRNA was used as the control. The cells were then rinsed with fresh media, and then exposed to vehicle control (0.1% DMSO) or 1 nM TCDD in fresh EGM-2 media containing antibiotics for 24 h.
Gene expression of CYP1A1 and CYP1B1
Total RNA was isolated from HAECs treated with TCDD for 24 h with RNeasy Mini Kit (Qiagen, GmbH, Germany). cDNA was synthesized using iScript Select cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA) with the supplied random primers and 250 ng RNA. PCR amplification was performed using an iCycler (Bio-Rad Laboratories) with a reaction mixture comprised of iQ SYBR Green Supermix (Bio-Rad Laboratories) with 500 µM sense and antisense primers (Table I) and 250 pg cDNA/µL. Cycle threshold data for both the target gene and reference gene, 18S ribosomal RNA, were used to calculate mean normalized expression as previously described (Lund et al., 2005).
Table I.
Real time PCR primer sequence
| Gene | Sense primer | Antisense primer |
|---|---|---|
| 18S | CGGAGGTTCGAAGACGATCAGATA | TTGGTTTCCCGGAAGCTGCC |
| Cytochrome P4501A1 (CYP1A1) |
CACATGCTGACCCTGGGAAAG | GGTGTGGAGCCAATTCGGATC |
| Cytochrome P4501B1 (CYP1B1) |
CGTCATGAGTGCCGTGTGTGTT | GCCCGAACTCTTCGTTGTGG |
CYP1A1 and CYP1B1 protein levels
After 24 h of TCDD exposure, cells were rinsed with ice-cold PBS, scraped in ice-cold RIPA lysis buffer, and transferred to a microcentrifuge tube on ice for 30 min. Samples were centrifuged for 20 min at 15000x g at 4°C and the supernatant was stored at −80°C. The protein concentration was determined by the Bradford assay. Protein samples were denatured by heating at 95°C in SDS loading buffer, resolved by electrophoresis on a 10% SDS-polyacrylamide gel, and transferred to a PVDF membrane. Membranes were probed using either rabbit polyclonal α-CYP1A1 or rabbit polyclonal α-CYP1B1 primary antibodies (1:2000 dilution) and a goat α-rabbit IgM+IgG-HP secondary antibody (1:4000 dilution). Detection was performed using ECL reagent and imaged on a Kodak Image Station 4000MM (Carestream Health, Rochester, NY). Membranes were then stripped using a mild stripping buffer, probed using a goat polyclonal α-actin primary antibody (1:500 dilution) and a donkey α-goat IgG-HRP secondary antibody (1:2000 dilution). Detection was performed as described above.
CYP1A1/CYP1B1 enzymatic activity
After 24 h of TCDD exposure, CYP1A1 and CYP1B1 activity was assessed using a P450-Glo Assay Kit (Promega, Madison, WI) according to the manufacturer’s instructions for the “Nonlytic P450-Glo Assay Using Cultured Cells in Monolayers”. The substrate utilized by this assay, luciferin-6’ chloroethyl ether, is metabolized by both CYP1A1 and CYP1B1. Luminescence was measured using a multi-label counter (Wallac Victor2, Perkin Elmer, Waltham, MA). After the luminescence measurement, fresh Krebs Ringer buffer was placed on the cells and the plates were frozen at −80°C.
Superoxide anion production
A working stock of 10 mM DHE in anhydrous DMSO was freshly prepared immediately prior use. After 24 h of TCDD exposure, cells were rinsed thee times with Krebs Ringer buffer at 37°C (20 mM HEPES, 10 mM dextrose, 127 mM NaCl, 5.5 mM KCl, 1 mM CaCl2, 2 mM MgSO4, pH 7.4) and then incubated in the dark with Krebs Ringer buffer containing 10 µM DHE for 45 min at 37°C, 5% CO2. Fluorescence was measured with an excitation wavelength of 485 nm and an emission wavelength of 595 nm at a gain of 110 using a GENios plate reader (Tecan, Männedorf, Switzerland). After the fluorescence measurement, fresh Krebs Ringer buffer was placed on the cells and the plates were frozen at −80°C.
H2O2 production
A working stock of 20.5 mM DCFH-DA in anhydrous DMSO was freshly prepared immediately prior to the experiment. After 24 h of TCDD exposure, cells were rinsed three times with Krebs Ringer buffer at 37°C and then incubated in the dark with Krebs Ringer buffer containing 20.5 µM DCFH-DA for 45 min at 37°C, 5% CO2. Fluorescence was measured with an excitation wavelength of 485 nm and an emission wavelength of 535 nm at a gain of 110 using a GENios plate reader (Tecan, Männedorf, Switzerland). After the fluorescence measurement, fresh Krebs Ringer buffer was placed on the cells and the plates were frozen at −80°C.
NO production
After 24 h of TCDD exposure, cells were rinsed three times with Krebs Ringer buffer at 37°C and then incubated in the dark with Krebs Ringer buffer containing 20 µM DAF-2DA for 60 min at 37°C, 5% CO2. Cells were rinsed three times with Krebs Ringer buffer at 37°C and then incubated in the dark with Krebs Ringer buffer containing 1 µM (ACh) for 15 min at 37°C, 5% CO2. Fluorescence was measured with an excitation wavelength of 488 nm and an emission wavelength of 515 nm at a gain of 110 using an Infinite M200 plate reader (Tecan, Männedorf, Switzerland). After the fluorescence measurement, fresh Krebs Ringer buffer was placed on the cells and the plates were frozen at −80°C.
Normalization to DNA content
Frozen plates from CYP1A1/CYP1B1 activity, DHE, and DCFH-DA experiments were thawed at room temperature for 24 h. Two volumes of high salt TNE buffer (10 mM Tris-base, 2 M NaCl, 1 mM Na2-EDTA, pH 7.4) containing 18 nM bisbenzimide H 33258 was added to one volume of Krebs Ringer buffer in each well and incubated overnight at RT. Fluorescence was measured with an excitation wavelength of 360 nm and an emission wavelength of 465 nm at a gain of 96 using a GENios plate reader.
Statistical analysis
mRNA expression, protein levels, CYP1A1/CYP1B1 activity, DHE, DCFH-DA, and DAF-2DA TCDD concentration-response experiments were analyzed by one-way ANOVA, with post-hoc Holm-Sidak pairwise comparisons. mRNA expression, protein levels, CYP1A1/CYP1B1 activity, DHE, DCFH-DA, and DAF-2DA experiments using siRNA were analyzed by two-way ANOVA, with post-hoc Holm-Sidak pairwise comparisons. A p-value < 0.05 was considered significant.
Results
CYP1A1 and CYP1B1 induction in HAECs
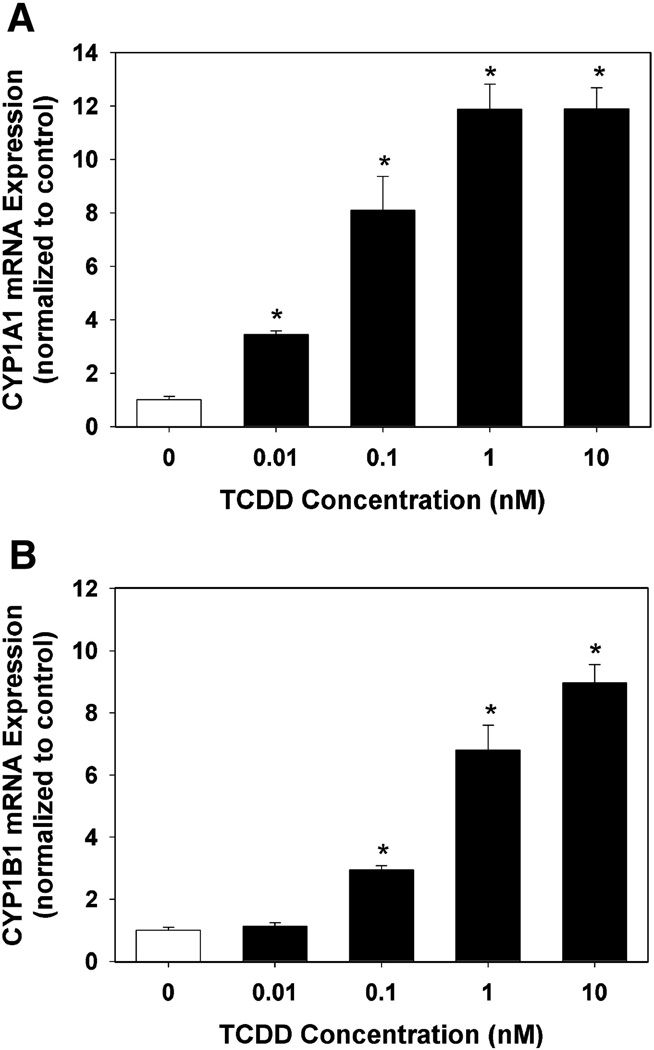
A TCDD concentration-response was performed to determine the level of induction of CYP1A1 and CYP1B1 mRNA expression in endothelial cells. Both CYP1A1 and CYP1B1 mRNA were detectable in the DMSO control wells. CYP1A1 mRNA expression was inducible in a concentration-dependent manner, reaching a maximal induction of 11.9 ± 0.9 fold above vehicle control at a concentration of 1 nM TCDD (Fig. 1A). CYP1B1 mRNA expression was also inducible in a concentration-dependent manner with an induction of 6.8 ± 0.8 fold above vehicle control at a concentration of 1 nM TCDD, with the highest induction of 9.0 ± 0.6 fold above vehicle control observed at a concentration of 10 nM (Fig. 1B).
Fig. 1.
Effect of TCDD exposure of HAECs on CYP1A1 (A) and CYP1B1 (B) mRNA expression. Total RNA was isolated from HAECs following 24 h exposure to DMSO or graded concentrations of TCDD. CYP1A1 and CYP1B1 RNA expression was determined by real-time PCR. Results were normalized to 18s rRNA and expressed relative to DMSO control as mean ± SEM (n=5). *p < 0.05, compared to DMSO control.
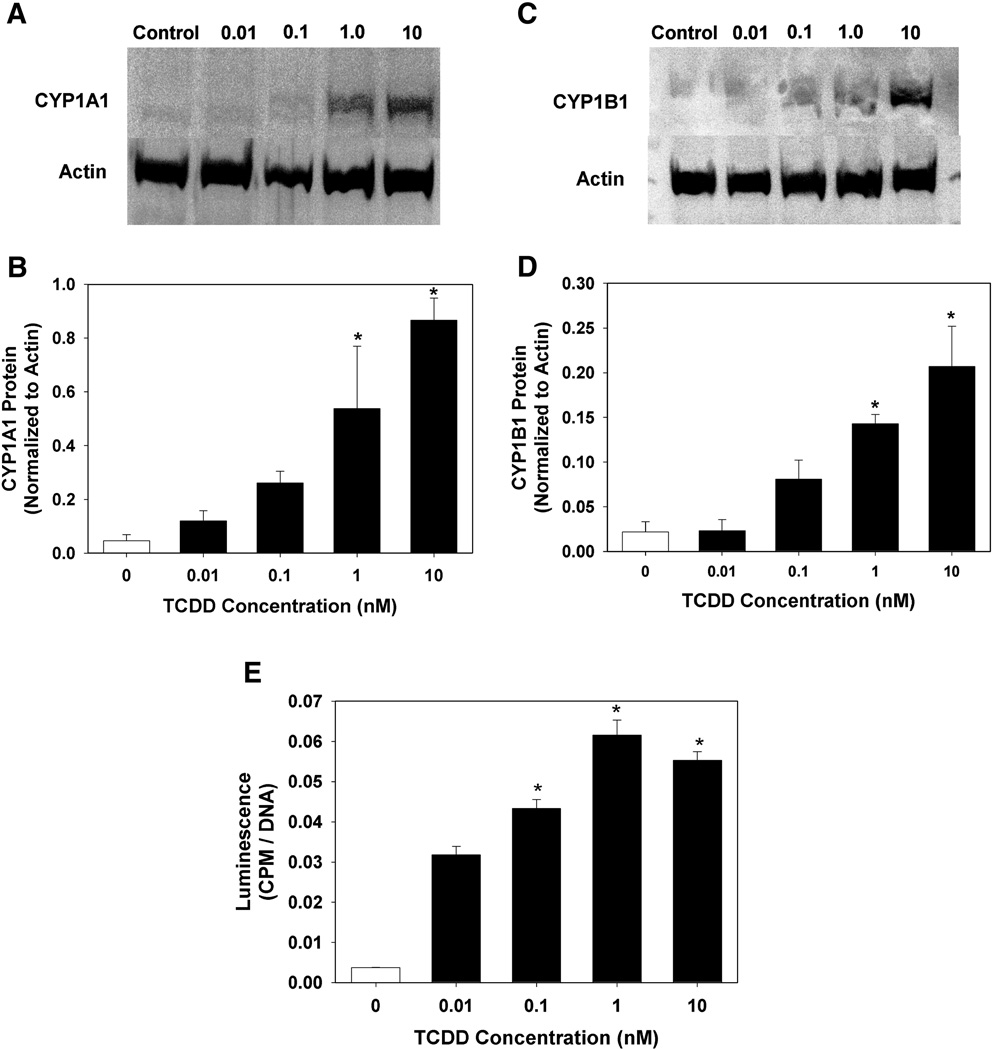
To confirm that the induction of mRNA resulted in increased in the relative protein concentration and enzymatic activity, we examined CYP1A1 and CYP1B1 protein levels by western blot analysis as well as enzymatic activity using a luminogenic substrate that can be metabolized by both enzymes. Protein and activity were detectable in the DMSO control wells, although minimally. Similar to mRNA expression, protein and enzyme activity were inducible in a concentration-dependent manner, with maximal detection at the highest concentration of 10 nM TCDD (Fig. 2A–D) and maximal enzymatic activity at a concentration of 1 nM TCDD (Fig. 2E).
Fig. 2.
Effect of TCDD exposure of HAECs on CYP1A1 (A,B) and CYP1B1 (C,D) protein expression, and CYP1A1/1B1 enzymatic activity (E). Total protein was isolated from HAECs following 24 h exposure to DMSO or graded concentrations of TCDD. The relative protein concentration of CYP1A1 and CYP1B1 was determined by western blot (n=3), while enzymatic activity was determined by P450Glow assay (Promega) (n=5). Protein expression was normalized to β-actin, while enzymatic activity was normalized to DNA content. Results are expressed as mean ± SEM. *p < 0.05, compared to DMSO control.
ROS and NO production
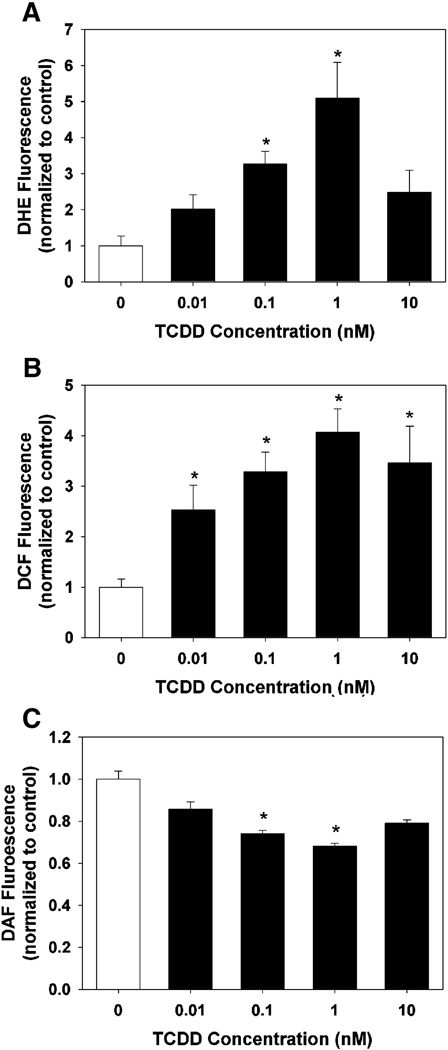
To determine the degree to which TCDD exposure caused an increase in ROS production, a concentration-response was performed using the oxidant-sensitive fluorescent probes DHE and DCFH-DA. DHE is a cell permeable molecule that fluoresces in the blue spectrum. Upon oxidation by superoxide anion to ethidium, the product intercalates into DNA and fluoresces in the red spectrum. TCDD exposure of HAECs resulted in a significant increase in DHE oxidation at 0.1 and 1 nM, but no significant induction at 10 nM (Fig.3A).
Fig. 3.
Effect of TCDD exposure of HAECs on superoxide production (A), H2O2 and hydroxyl radical production (B), and ACh-stimulated production of NO (C). Following exposure of HAECs to DMSO or graded concentrations of TCDD for 24 h, cells were exposed to (A) DHE for 45 min, (B) DCFH-DA for 45 min, or (C) DAF-DA for 60 min followed by exposure to 1 µM ACh for 15 min, and fluorescence measured. Results are expressed as mean ± SEM (n=6). *p < 0.05, compared to DMSO control.
DCFH-DA is a cell permeable molecule that is deacetylated to the cell impermeable DCFH molecule by cytoplasmic esterases. DCFH can be oxidized by H2O2 or hydroxyl radical to the fluorescent molecule DCF. TCDD exposure of HAECs resulted in a concentration-dependent increase in DCFH oxidation, elevated significantly at all concentrations and maximally by a concentration of 1 nM (Fig. 3B).
To determine the effect of increased ROS on NO production, a concentration-response was performed using the cell permeable NO probe DAF-2DA. DAF-2DA is deacetylated by intracellular esterases. DAF-2 can react with NO, producing the fluorescent DAF-2T molecule (Nakatsubo et al., 1998). Basal levels of NO were not detectable in any wells. Thus, we stimulated eNOS activity by ACh and measured NO production. TCDD exposure reduced Ach-stimulated NO production in a concentration-dependent manner at 0.1 and 1 nM, but had no significant effect at 10 nM, consistent with the increased DHE fluorescence following TCDD exposure (Fig. 3C).
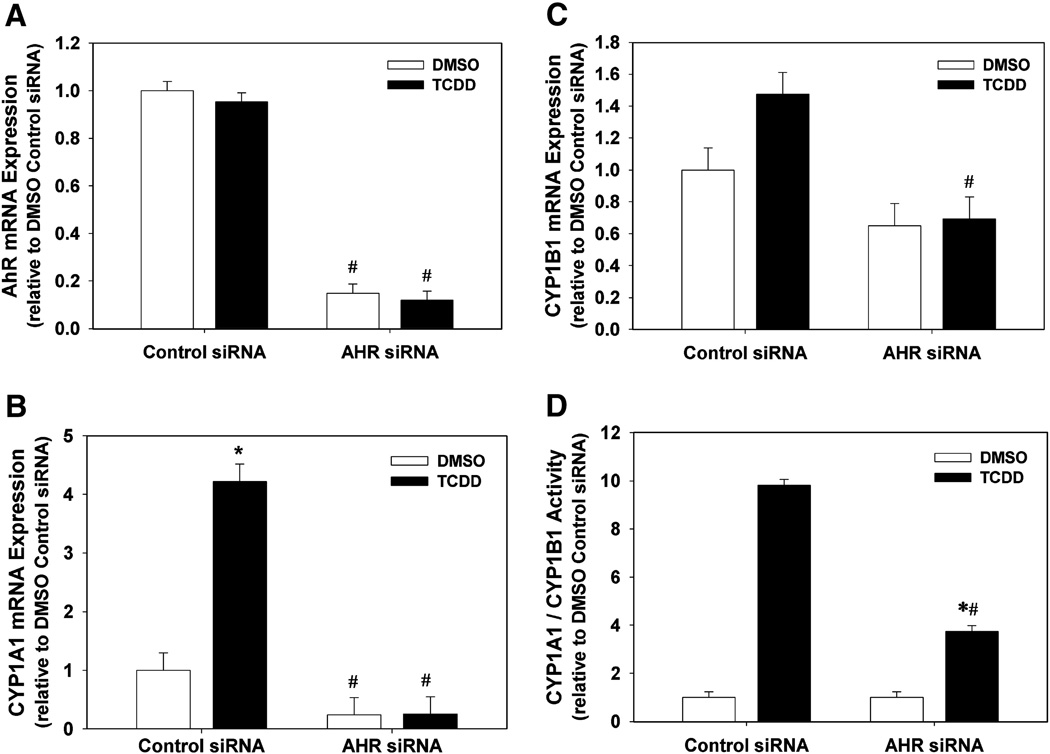
Suppression of AhR, CYP1A1, and CYP1B1 mRNA and CYP1A1/1B1 Activity by AhR siRNA
To determine the effectiveness of our AhR siRNA to reduce AhR expression and activity, we measured mRNA expression of AhR, CYP1A1, and CYP1B1, and CYP1A1/1B1 enzyme activity, following transfection of HAECs with control or AhR siRNA followed by exposure to DMSO or 1 nM TCDD. siRNA targeting AhR significantly reduced AhR mRNA levels in both DMSO and TCDD exposure groups, compared to control siRNA (Fig. 4A). In addition, AhR siRNA suppressed the basal level of CYP1A1 mRNA in the DMSO group and completely prevented the TCDD induction of CYP1A1 (Fig. 4B). While AhR siRNA did not significantly reduce the basal level of CYP1B1 mRNA in the DMSO group, it did significantly reduce the TCDD-induced increase in CYP1B1 mRNA (Fig. 4C). Lastly, AhR siRNA did not significantly reduce the basal level of CYP1A1/1B1 enzyme activity, but did significantly reduce the TCDD-induced level from 9.8 ± 0.3 fold in cells transfected with control siRNA to 3.7 ± 0.3 fold in cells transfected with AhR siRNA (Fig. 4D).
Fig. 4.
Effect of AhR siRNA on AhR mRNA (A), CYP1A1 mRNA (B), CYP1B1 mRNA (C), and CYP1A1/1B1 enzymatic activity (D) in DMSO- and TCDD-treated HAECs. (A–C). Total RNA was isolated 48 h after transfection of HAECs with control siRNA or AhR siRNA and 24 h after exposure to DMSO or 1 nM TCDD (n=3). AhR, CYP1A1, and CYP1B1 RNA expression was determined by real-time PCR. Results were normalized to 18s rRNA and expressed relative to DMSO control (n=3). (D) CYP1A1/1B1 enzymatic activity was determined 48 h after transfection of HAECs with control siRNA or AhR siRNA and 24 h after exposure to DMSO or 1 nM TCDD. Enzymatic activity, normalized to DNA content, was determined by P450Glow assay (Promega) (n=8). Results are expressed as mean ± SEM. *p < 0.05, compared to respective DMSO control. #p < 0.05, compared to respective control siRNA.
It was also notable that the control siRNA significantly reduced TCDD-inducibility of all downstream responses, including CYP1A1 and CYP1B1 mRNA, CYP1A1/1B1 enzymatic activity, and DHE and DCF fluorescence. This is likely a result of the overall transfection conditions, which resulted in 2–3x lower response to TCDD, compared to inducibility of these endpoints in untransfected cells.
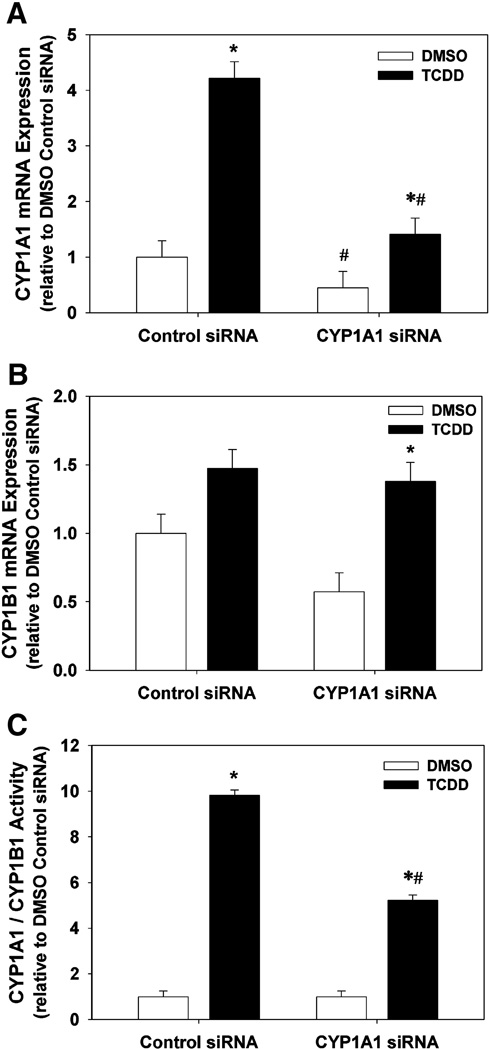
Suppression of TCDD-induced CYP1A1 and CYP1B1 mRNA and activity by CYP1A1 siRNA
In order to determine the effectiveness of CYP1A1 siRNA to reduce TCDD-induced CYP1A1 or CYP1B1 expression and activity, we measured mRNA expression of CYP1A1 and CYP1B1, and CYP1A1/1B1 enzyme activity, following transfection of HAECs with control or CYP1A1 siRNA followed by exposure to DMSO control or 1 nM TCDD. CYP1A1 siRNA suppressed the basal level of CYP1A1 mRNA in the DMSO group and significantly reduced the TCDD induction of CYP1A1 from 4.22 ± 0.30 fold in cells transfected with control siRNA to 1.41 ± 0.30 fold (Fig. 5A). In addition, CYP1A1 siRNA did not significantly alter the expression of CYP1B1 mRNA, but did slightly enhance the TCDD-induced level (Fig. 5B). Lastly, while TCDD exposure induced CYP1A1/1B1 enzymatic activity by 9.8 ± 0.3 fold in cells transfected with control siRNA, CYP1A1 siRNA reduced this induction nearly 50% to 5.2 ± 0.3 fold (Fig. 5C).
Fig. 5.
Effect of CYP1A1 siRNA on CYP1A1 mRNA (A), CYP1B1 mRNA (B), and CYP1A1/1B1 enzymatic activity (C) in DMSO- and TCDD-treated HAECs. (A–C). Total RNA was isolated 48 h after transfection of HAECs with control siRNA or CYP1A1 siRNA and 24 h after exposure to DMSO or 1 nM TCDD (n=3). CYP1A1 and CYP1B1 RNA expression was determined by real-time PCR. Results were normalized to 18s rRNA and expressed relative to DMSO control (n=3). (C) CYP1A1/1B1 enzymatic activity was determined 48 h after transfection of HAECs with control siRNA or CYP1A1 siRNA and 24 h after exposure to DMSO or 1 nM TCDD. CYP1A1/1B1 enzymatic activity, normalized to DNA content, was determined by P450Glow assay (Promega) (n=8). All results are expressed as mean ± SEM. *p < 0.05, compared to respective DMSO control. #p < 0.05, compared to respective control siRNA.
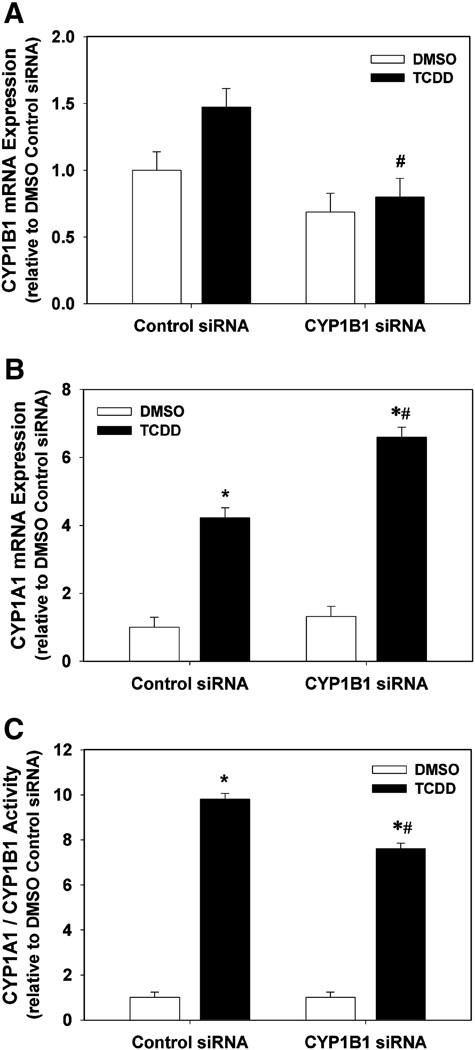
Suppression of TCDD-induced CYP1A1 and CYP1B1 mRNA and activity by CYP1B1 siRNA
In order to determine the effectiveness of CYP1B1 siRNA, we measured mRNA expression of CYP1A1 and CYP1B1, and CYP1A1/1B1 enzyme activity, following transfection of HAECs with control or CYP1B1 siRNA followed by exposure to DMSO control or 1 nM TCDD. CYP1B1 siRNA did not significantly suppress the basal level of CYP1B1 mRNA in the DMSO group; however, it did significantly reduce CYP1B1 mRNA expression in the TCDD treatment group (Fig. 6A). Interestingly, CYP1B1 siRNA significantly increased the induction of CYP1A1 mRNA following TCDD exposure from 4.22 ± 0.30 fold in cells transfected with control siRNA to 6.60 ± 0.30 fold (Fig. 6B). Similar to CYP1A1 siRNA, CYP1B1 siRNA attenuated the TCDD-induced increase in enzymatic activity, but did not completely eliminate it, reducing it from 9.8 ± 0.3 fold in cells transfected with control siRNA to 7.6 ± 0.3 fold (Fig. 6C).
Fig. 6.
Effect of CYP1B1 siRNA on CYP1B1 mRNA (A), CYP1A1 mRNA (B), and CYP1A1/1B1 enzymatic activity (C) in DMSO- and TCDD-treated HAECs. (A–C). Total RNA was isolated 48 h after transfection of HAECs with control siRNA or CYP1B1 siRNA and 24 h after exposure to DMSO or 1 nM TCDD (n=3). CYP1A1 and CYP1B1 RNA expression was determined by real-time PCR. Results were normalized to 18s rRNA and expressed relative to DMSO control (n=3). (C) CYP1A1/1B1 enzymatic activity was determined 48 h after transfection of HAECs with control siRNA or CYP1B1 siRNA and 24 h after exposure to DMSO or 1 nM TCDD. CYP1A1/1B1 enzymatic activity, normalized to DNA content, was determined by P450Glow assay (Promega) (n=8). All results are expressed as mean ± SEM. *p < 0.05, compared to respective DMSO control. #p < 0.05, compared to respective control siRNA.
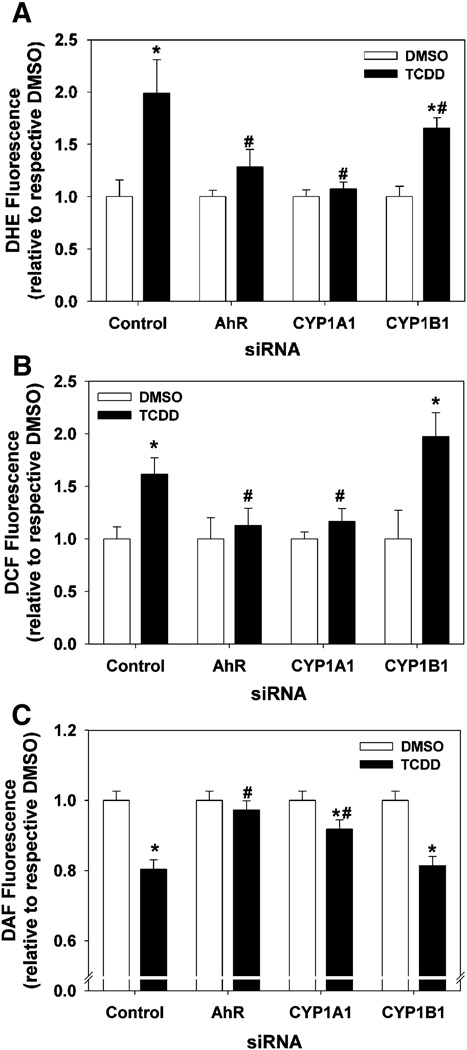
Suppression of TCDD-induced increases in ROS production by siRNA
In order to determine if AhR mediated the observed increase in ROS production and if the induced CYP1A1 or CYP1B1 were involved in this increase, siRNA targeting AhR, CYP1A1, or CYP1B1 were utilized. Transfection of HAECs with siRNA targeting AhR or CYP1A1 completely prevented the TCDD-induced increase in both DHE and DCF fluorescence. However, siRNA targeting CYP1B1 had no effect on the DHE and DCFH oxidation following TCDD exposure (Fig. 7A,B).
Fig. 7.
Effect of AhR, CYP1A1, and CYP1B1 siRNA on superoxide production (A), H2O2 and hydroxyl radical production (B), and ACh-stimulated production of NO (C). Forty-eight hours after transfection of HAECs with control or target siRNA and 24 h after exposure to DMSO or 1 nM TCDD, cells were exposed to (A) DHE for 45 min, (B) DCFH-DA for 45 min, or (C) DAF-DA for 60 min followed by exposure to 1µM ACh for 15 min, and fluorescence measured. All results are expressed as mean ± SEM (n=6). *p < 0.05, compared to DMSO control. #p < 0.05, compared to respective control siRNA.
Restoration of NO production by siRNA
We examined the ability of siRNA targeting AhR, CYP1A1, or CYP1B1 to prevent the decrease in ACh-stimulated NO production following TCDD exposure. Transfection of HAECs with siRNA targeting AhR prevented the decrease in DAF fluorescence observed following 1 nM TCDD exposure. siRNA targeting CYP1A1 was able to partially restore DAF fluorescence following TCDD exposure, while siRNA targeting CYP1B1 had no effect on DAF fluorescence following TCDD exposure, compared to control siRNA (Fig. 7C).
Discussion
Our results demonstrate that TCDD exposure of endothelial cells induces CYP1A1 and CYP1B1, increases the production of ROS, and decreases ACh-stimulated NO production. Moreover, the ability of AhR siRNA to prevent the TCDD induction of ROS demonstrates that the AhR is required. Furthermore, the ability of CYP1A1 siRNA to significantly attenuate TCDD-induced CYP1A1 expression, CYP1A1/1B1 enzyme activity, and completely prevent the TCDD-induced ROS production suggests that CYP1A1 is a downstream mediator of this ROS.
We observed an induction of CYP1A1 and CYP1B1 mRNA, protein, and enzymatic activity in TCDD-exposed HAECs. Others have shown that CYP1A1 is expressed and inducible in vascular endothelial cells (Farin et al., 1994;Stegeman et al., 1995;Thirman et al., 1994;Toborek et al., 1995), while CYP1B1 is expressed and inducible in vascular smooth muscle cells (Kerzee and Ramos, 2001;Zhao et al., 1998). The results of these previous studies suggested that in the vasculature CYP1A1 is exclusively expressed in endothelial cells, while CYP1B1 is exclusively expressed in smooth muscle cells. However, a recent study has demonstrated both CYP1A1 and CYP1B1 are induced in HAECs, as observed in our study, but only CYP1A1 is induced in primary human umbilical vein endothelial cells (HUVECs) (Eskin et al., 2004). Thus, these data, combined with our results, suggest that expression and the potential for induction of CYP1A1 and CYP1B1 in endothelial cells may be species- and vascular bed-dependent.
TCDD exposure resulted in an increase in ROS production, which was maximal at a concentration of 1 nM. Superoxide anion production was significantly increased at TCDD concentrations of 0.1 and 1 nM, but not at 10 nM. In contrast, the detection of H2O2 and hydroxyl radical by DCF was significantly increased at all TCDD concentrations. It is possible that at the highest concentration of TCDD (10 nM) antioxidants had been induced to dismutate the superoxide anion. One possible antioxidant mediating this effect is NAD(P)H:quinine oxidoreductase 1 (Nqo1). Nqo1 is an effective scavenger of superoxide anion (Siegel et al., 2004) and is considered part of the mouse AhR gene battery (Tijet et al., 2006;Yeager et al., 2009). Further, we have shown that TCDD significantly induces Nqo1 mRNA in the heart of mice (Kopf et al., 2008) and in HUVECs after 24 h exposure (unpublished data).
The TCDD-induced increase in superoxide anion was paralleled by a concentration-dependent decrease in ACh-stimulated NO production. A key mechanism by which NO is reduced by oxidative stress is the reaction between NO and superoxide anion, producing peroxynitrite. Thus, it is consistent that that the 10 nM concentration of TCDD that did not significantly increase superoxide anion production also did not significantly reduce NO production. Further the increase in ROS and decrease in NO by TCDD was prevented by siRNA targeting AhR. These data demonstrate that the increase in ROS following TCDD exposure is AhR-dependent.
One gene that is notably upregulated downstream of AhR activation in endothelial cells and is associated with increased ROS production is CYP1A1. Our data not only show that AhR siRNA prevents both TCDD-induced CYP1A1 expression and ROS production, but go further to establish a cause-and-effect relationship between them, since CYP1A1 siRNA reduces TCDD-induced CYP1A1 expression and prevents TCDD-induced ROS. We found that CYP1A1 siRNA reduced TCDD-induced CYP1A1 expression by 66%, completely prevented the TCDD-induced increase in ROS, and attenuated the TCDD-induced decrease in NO production by 60%. These results suggest that CYP1A1 is an upstream mediator of the increased production of ROS and the decreased production of NO. There is extensive evidence that induced levels of CYP1A1 are capable of producing ROS. Studies of enriched preparations of microsomes of specific human CYP450s reveal that CYP1A1 is prone to the production of superoxide anion and H2O2 (Puntarulo et al., 1998). Furthermore, microsomes from the liver of TCDD-exposed mice produce a greater amount of superoxide, compared to control mice (Shertzer et al., 2004a). Moreover, microsomes from the livers of TCDD-exposed CYP1A1 null mice demonstrate a decrease in the production of H2O2 (superoxide anion was not measured), compared to TCDD-exposed wild type mice, suggesting CYP1A1 may be the primary source of ROS in TCDD-induced microsomes (Shertzer et al., 2004b).
A second gene that is also notably upregulated downstream of AhR activation is CYP1B1. However, in contrast to our results with CYP1A1, siRNA targeting CYP1B1 did not reduce TCDD-induced ROS production or prevent the TCDD-induced decrease in NO, suggesting that CYP1B1 is not a mediator of the ROS in these cells following TCDD exposure. It is possible, however, that any decrease in CYP1B1 production of ROS by CYP1B1 siRNA was masked by an increase in CYP1A1. While CYP1B1 siRNA did not alter the basal expression of CYP1A1 mRNA, it did significantly increase it in the TCDD treatment group. The degree to which this modest increase (1.5x) in CYP1A1 mRNA results in an increase in CYP1A1 protein expression is not known. Despite the increase in CYP1A1 mRNA, the overall impact of CYP1B1 siRNA was a decrease in enzymatic activity, suggesting that CYP1B1 siRNA has a suppressive effect on the total TCDD-induced CYP activity. Nonetheless, to fully answer this question the relative redox coupling efficiencies of CYP1A1 and CYP1B1 would need to be directly compared and the ability of CYP1B1 to produce ROS in the absence of CYP1A1 would need to be determined. Our future studies assessing TCDD-induced ROS production in CYP1A1 null mice will help to address this question. In addition, a recent study suggests that CYP1B1 may reduce the oxidative state of endothelial cells. Retinas of CYP1B1 knockout mice and retinal endothelial cells isolated from CYP1B1 knockout mice exhibit increases in oxidative stress as evidenced by 4-hydroxy-2-nonenal staining and DHE fluorescence, respectively (Tang et al., 2009). Furthermore, treatment of retinal endothelial cells from CYP1B1 knockout mice with antioxidants restores their angiogenic capability. In our studies CYP1B1 siRNA did not reduce basal levels of ROS in control cells or attenuate the ROS production induced by TCDD. Thus, our data do not provide evidence that CYP1B1 has an inherent antioxidant, protective function in HAECs.
It is well established that superoxide anion and H2O2 are common by-products of the P450 catalytic cycle, resulting from inefficient coupling of NADPH consumption to substrate oxidation (Zangar et al., 2004) and this may represent one potential mechanism by which ROS can be produced downstream of CYP1A1 induction. If the activated oxygen is released from the heme iron immediately after the addition of the first electron (Fe2+O2 complex), superoxide will be produced. If the activated oxygen is released from the heme iron after the addition of the second electron (Fe2+OOH complex), H2O2 will be produced (Parkinson, 2001). The degree of coupled NADPH consumption and substrate oxidation varies between CYP450 isoforms, but it is often less than 50% for eukaryotic CYP450s (Gorsky et al., 1984;Gruenke et al., 1995;Kuthan and Ullrich, 1982;Tan et al., 1997).
Another potential downstream mechanism by which induced CYP1A1 can result in increased production of ROS is by the production of quinones, molecules containing a diketone six-carbon ring. A quinone can then be reduced to a semiquinone radical by a one electron transfer from NADPH-cytochome P450 reductase, which results in redox cycling and the production of ROS. For example, CYP1A1 preferentially metabolizes estradiol into 2-hydroxylestradiol and 4-hydroxylestradiol, respectively (Lee et al., 2003;Spink et al., 1994), metabolites that can contribute to cytotoxicity and mutagenicity by the downstream formation of catechol estradiols and subsequent oxidation to semiquinone radicals (Samuni et al., 2003). This potential mechanism is intriguing and deserves further investigation to determine whether endogenous metabolites of CYP1A1 may undergo this metabolic activation.
In conclusion, this study demonstrates that TCDD exposure results in an increase in ROS production in endothelial cells that is AhR-dependent and downstream of CYP1A1 induction. As others have shown that CYP1A1 contributes to ROS production in isolated liver microsomes (Puntarulo and Cederbaum, 1998;Shertzer et al., 2004b), our results suggest this also occurs in human vascular endothelial cells. Finally, this study provides insight into a potential role of CYP1A1 in TCDD-induced hypertension and endothelial dysfunction in vivo. Future studies will use this cell culture model to investigate the underlying mechanism of CYP1A1-dependent ROS production.
Acknowledgements
This study was supported by a grant from the National Institutes of Health R01 HL078914 to M.K.W. The authors wish to thank Mary T. Walsh for her expert technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Reference List
- 1.Bello SM, Heideman W, Peterson RE. 2,3,7,8-Tetrachlorodibenzo-p-dioxin inhibits regression of the common cardinal vein in developing zebrafish. Toxicol.Sci. 2004;78:258–266. doi: 10.1093/toxsci/kfh065. [DOI] [PubMed] [Google Scholar]
- 2.Cantrell SM, Lutz LH, Tillitt DE, Hannink M. Embryotoxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD): the embryonic vasculature is a physiological target for TCDD-induced DNA damage and apoptotic cell death in Medaka (Orizias latipes) Toxicol.Appl.Pharmacol. 1996;141:23–34. doi: 10.1006/taap.1996.0256. [DOI] [PubMed] [Google Scholar]
- 3.Carney SA, Peterson RE, Heideman W. 2,3,7,8-Tetrachlorodibenzo-pdioxin activation of the aryl hydrocarbon receptor/aryl hydrocarbon receptor nuclear translocator pathway causes developmental toxicity though a CYP1A-independent mechanism in zebrafish. Mol.Pharmacol. 2004;66:512–521. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- 4.Dong W, Teraoka H, Yamazaki K, Tsukiyama S, Imani S, Imagawa T, Stegeman JJ, Peterson RE, Hiraga T. 2,3,7,8-Tetrachlorodibenzo-p-dioxin toxicity in the zebrafish embryo: local circulation failure in the dorsal midbrain is associated with increased apoptosis. Toxicol.Sci. 2002;69:191–201. doi: 10.1093/toxsci/69.1.191. [DOI] [PubMed] [Google Scholar]
- 5.Eskin SG, Turner NA, McIntire LV. Endothelial cell cytochrome P450 1A1 and 1B1: up-regulation by shear stress. Endothelium. 2004;11:1–10. doi: 10.1080/10623320490432434. [DOI] [PubMed] [Google Scholar]
- 6.Farin FM, Pohlman TH, Omiecinski CJ. Expression of cytochrome P450s and microsomal epoxide hydrolase in primary cultures of human umbilical vein endothelial cells. Toxicol.Appl.Pharmacol. 1994;124:1–9. doi: 10.1006/taap.1994.1001. [DOI] [PubMed] [Google Scholar]
- 7.Garrick RA, Woodin BR, Wilson JY, Middlebrooks BL, Stegeman JJ. Cytochrome P4501A is induced in endothelial cell lines from the kidney and lung of the bottlenose dolphin, Tursiops truncatus. Aquat.Toxicol. 2006;76:295–305. doi: 10.1016/j.aquatox.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Gorsky LD, Koop DR, Coon MJ. On the stoichiometry of the oxidase and monooxygenase reactions catalyzed by liver microsomal cytochrome P-450. Products of oxygen reduction. J.Biol.Chem. 1984;259:6812–6817. [PubMed] [Google Scholar]
- 9.Gruenke LD, Konopka K, Cadieu M, Waskell L. The stoichiometry of the cytochrome P-450-catalyzed metabolism of methoxyflurane and benzphetamine in the presence and absence of cytochrome b5. J.Biol.Chem. 1995;270:24707–24718. doi: 10.1074/jbc.270.42.24707. [DOI] [PubMed] [Google Scholar]
- 10.Guiney PD, Smolowitz RM, Peterson RE, Stegeman JJ. Correlation of 2,3,7,8-tetrachlorodibenzo-p-dioxin induction of cytochrome P4501A in vascular endothelium with toxicity in early life stages of lake trout. Toxicol.Appl.Pharmacol. 1997;143:256–273. doi: 10.1006/taap.1996.8051. [DOI] [PubMed] [Google Scholar]
- 11.Guiney PD, Walker MK, Spitsbergen JM, Peterson RE. Hemodynamic dysfunction and cytochrome P4501A mRNA expression induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin during embryonic stages of lake trout development. Toxicol.Appl.Pharmacol. 2000;168:1–14. doi: 10.1006/taap.2000.8999. [DOI] [PubMed] [Google Scholar]
- 12.Henry TR, Spitsbergen JM, Hornung MW, Abnet CC, Peterson RE. Early life stage toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in zebrafish (Danio rerio) Toxicol.Appl.Pharmacol. 1997;142:56–68. doi: 10.1006/taap.1996.8024. [DOI] [PubMed] [Google Scholar]
- 13.Hornung MW, Spitsbergen JM, Peterson RE. 2,3,7,8-Tetrachlorodibenzo-p-dioxin alters cardiovascular and craniofacial development and function in sac fry of rainbow trout (Oncorhynchus mykiss) Toxicol.Sci. 1999;47:40–51. doi: 10.1093/toxsci/47.1.40. [DOI] [PubMed] [Google Scholar]
- 14.Ishimura R, Kawakami T, Ohsako S, Nohara K, Tohyama C. Suppressive effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on vascular remodeling that takes place in the normal labyrinth zone of rat placenta during late gestation. Toxicol.Sci. 2006;91:265–274. doi: 10.1093/toxsci/kfj138. [DOI] [PubMed] [Google Scholar]
- 15.Ivnitski-Steele I, Walker MK. Inhibition of neovascularization by environmental agents. Cardiovasc.Toxicol. 2005a;5:215–226. doi: 10.1385/ct:5:2:215. [DOI] [PubMed] [Google Scholar]
- 16.Ivnitski-Steele ID, Friggens M, Chavez M, Walker MK. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) inhibition of coronary vasculogenesis is mediated, in part, by reduced responsiveness to endogenous angiogenic stimuli, including vascular endothelial growth factor A (VEGF-A) Birth Defects Res.A Clin.Mol.Teratol. 2005b;73:440–446. doi: 10.1002/bdra.20137. [DOI] [PubMed] [Google Scholar]
- 17.Ivnitski-Steele ID, Walker MK. Vascular endothelial growth factor rescues 2,3,7,8-tetrachlorodibenzo-p-dioxin inhibition of coronary vasculogenesis. Birth Defects Res.A Clin.Mol.Teratol. 2003;67:496–503. doi: 10.1002/bdra.10074. [DOI] [PubMed] [Google Scholar]
- 18.Jokinen MP, Walker NJ, Brix AE, Sells DM, Haseman JK, Nyska A. Increase in cardiovascular pathology in female Sprague-Dawley rats following chronic treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin and 3,3',4,4',5-pentachlorobiphenyl. Cardiovasc.Toxicol. 2003;3:299–310. doi: 10.1385/CT:3:4:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kerzee JK, Ramos KS. Constitutive and inducible expression of Cyp1a1 and Cyp1b1 in vascular smooth muscle cells: role of the AhR bHLH/PAS transcription factor. Circ.Res. 2001;89:573–582. doi: 10.1161/hh1901.097083. [DOI] [PubMed] [Google Scholar]
- 20.Kopf PG, Huwe JK, Walker MK. Hypertension, Cardiac Hypertrophy, and Impaired Vascular Relaxation Induced by 2,3,7,8-Tetrachlorodibenzo-p-Dioxin are Associated with Increased Superoxide. Cardiovasc.Toxicol. 2008;8:181–193. doi: 10.1007/s12012-008-9027-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuthan H, Ullrich V. Oxidase and oxygenase function of the microsomal cytochrome P450 monooxygenase system. Eur.J.Biochem. 1982;126:583–588. doi: 10.1111/j.1432-1033.1982.tb06820.x. [DOI] [PubMed] [Google Scholar]
- 22.Lee AJ, Cai MX, Thomas PE, Conney AH, Zhu BT. Characterization of the oxidative metabolites of 17beta-estradiol and estrone formed by 15 selectively expressed human cytochrome p450 isoforms. Endocrinology. 2003;144:3382–3398. doi: 10.1210/en.2003-0192. [DOI] [PubMed] [Google Scholar]
- 23.Lund AK, Peterson SL, Timmins GS, Walker MK. Endothelin-1-mediated increase in reactive oxygen species and NADPH Oxidase activity in hearts of aryl hydrocarbon receptor (AhR) null mice. Toxicol.Sci. 2005;88:265–273. doi: 10.1093/toxsci/kfi284. [DOI] [PubMed] [Google Scholar]
- 24.Nakatsubo N, Kojima H, Kikuchi K, Nagoshi H, Hirata Y, Maeda D, Imai Y, Irimura T, Nagano T. Direct evidence of nitric oxide production from bovine aortic endothelial cells using new fluorescence indicators: diaminofluoresceins. FEBS Lett. 1998;427:263–266. doi: 10.1016/s0014-5793(98)00440-2. [DOI] [PubMed] [Google Scholar]
- 25.Parkinson A. Biotransformation of Xenobiotics. In: Klaassen CD, editor. Casarett & Doull's Toxicology: The Basic Science of Poisons. New York: McGraw-Hill; 2001. pp. 133–224. [Google Scholar]
- 26.Puntarulo S, Cederbaum AI. Production of reactive oxygen species by microsomes enriched in specific human cytochrome P450 enzymes. Free Radic.Biol.Med. 1998;24:1324–1330. doi: 10.1016/s0891-5849(97)00463-2. [DOI] [PubMed] [Google Scholar]
- 27.Samuni AM, Chuang EY, Krishna MC, Stein W, DeGraff W, Russo A, Mitchell JB. Semiquinone radical intermediate in catecholic estrogen-mediated cytotoxicity and mutagenesis: chemoprevention strategies with antioxidants. Proc.Natl.Acad.Sci.U.S.A. 2003;100:5390–5395. doi: 10.1073/pnas.0930078100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlezinger JJ, Stegeman JJ. Dose and inducer-dependent induction of cytochrome P450 1A in endothelia of the eel, including in the swimbladder rete mirabile, a model microvascular structure. Drug Metab Dispos. 2000;28:701–708. [PubMed] [Google Scholar]
- 29.Shertzer HG, Clay CD, Genter MB, Chames MC, Schneider SN, Oakley GG, Nebert DW, Dalton TP. Uncoupling-mediated generation of reactive oxygen by halogenated aromatic hydrocarbons in mouse liver microsomes. Free Radic.Biol.Med. 2004a;36:618–631. doi: 10.1016/j.freeradbiomed.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 30.Shertzer HG, Clay CD, Genter MB, Schneider SN, Nebert DW, Dalton TP. Cyp1a2 protects against reactive oxygen production in mouse liver microsomes. Free Radic.Biol.Med. 2004b;36:605–617. doi: 10.1016/j.freeradbiomed.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 31.Siegel D, Gustafson DL, Dehn DL, Han JY, Boonchoong P, Berliner LJ, Ross D. NAD(P)H:quinone oxidoreductase 1: role as a superoxide scavenger. Mol.Pharmacol. 2004;65:1238–1247. doi: 10.1124/mol.65.5.1238. [DOI] [PubMed] [Google Scholar]
- 32.Simonsen U, Chistensen FH, Buus NH. The effect of tempol on endothelium-dependent vasodilatation and blood pressure. Pharmacol.Ther. 2009;122:109–124. doi: 10.1016/j.pharmthera.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Smolowitz RM, Hahn ME, Stegeman JJ. Immunohistochemical localization of cytochrome P-450IA1 induced by 3,3',4,4'-tetrachlorobiphenyl and by 2,3,7,8-tetrachlorodibenzoafuran in liver and extrahepatic tissues of the teleost Stenotomus chysops (scup) Drug Metab Dispos. 1991;19:113–123. [PubMed] [Google Scholar]
- 34.Spink DC, Hayes CL, Young NR, Chistou M, Sutter TR, Jefcoate CR, Gierthy JF. The effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on estrogen metabolism in MCF-7 breast cancer cells: evidence for induction of a novel 17 beta-estradiol 4-hydroxylase. J.Steroid Biochem.Mol.Biol. 1994;51:251–258. doi: 10.1016/0960-0760(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 35.Spitsbergen JM, Walker MK, Olson JR, Peterson RE. Pathologic alterations in early life stages of lake trout, Salvelinus namaycush, exposed to 2,3,7,8-tetrachlorodibenzo- p -dioxin as fertilized eggs. Aquat.Toxicol. 1991;19:41–72. [Google Scholar]
- 36.Stegeman JJ, Hahn ME, Weisbrod R, Woodin BR, Joy JS, Najibi S, Cohen RA. Induction of cytochrome P4501A1 by aryl hydrocarbon receptor agonists in porcine aorta endothelial cells in culture and cytochrome P4501A1 activity in intact cells. Mol.Pharmacol. 1995;47:296–306. [PubMed] [Google Scholar]
- 37.Tan Y, Patten CJ, Smith T, Yang CS. Competitive interactions between cytochromes P450 2A6 and 2E1 for NADPH-cytochrome P450 oxidoreductase in the microsomal membranes produced by a baculovirus expression system. Arch.Biochem.Biophys. 1997;342:82–91. doi: 10.1006/abbi.1997.9995. [DOI] [PubMed] [Google Scholar]
- 38.Tang Y, Scheef EA, Wang S, Sorenson CM, Marcus CB, Jefcoate CR, Sheibani N. CYP1B1 expression promotes the proangiogenic phenotype of endothelium though decreased intracellular oxidative stress and thombospondin-2 expression. Blood. 2009;113:744–754. doi: 10.1182/blood-2008-03-145219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teraoka H, Dong W, Tsujimoto Y, Iwasa H, Endoh D, Ueno N, Stegeman JJ, Peterson RE, Hiraga T. Induction of cytochrome P450 1A is required for circulation failure and edema by 2,3,7,8-tetrachlorodibenzo-p-dioxin in zebrafish. Biochem.Biophys.Res.Commun. 2003;304:223–228. doi: 10.1016/s0006-291x(03)00576-x. [DOI] [PubMed] [Google Scholar]
- 40.Thirman MJ, Albrecht JH, Krueger MA, Erickson RR, Cherwitz DL, Park SS, Gelboin HV, Holtzman JL. Induction of cytochrome CYPIA1 and formation of toxic metabolites of benzo[a]pyrene by rat aorta: a possible role in atherogenesis. Proc.Natl.Acad.Sci.U.S.A. 1994;91:5397–5401. doi: 10.1073/pnas.91.12.5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tijet N, Boutros PC, Moffat ID, Okey AB, Tuomisto J, Pohjanvirta R. Aryl hydrocarbon receptor regulates distinct dioxin-dependent and dioxin-independent gene batteries. Mol.Pharmacol. 2006;69:140–153. doi: 10.1124/mol.105.018705. [DOI] [PubMed] [Google Scholar]
- 42.Toborek M, Barger SW, Mattson MP, Espandiari P, Robertson LW, Hennig B. Exposure to polychlorinated biphenyls causes endothelial cell dysfunction. J.Biochem.Toxicol. 1995;10:219–226. doi: 10.1002/jbt.2570100406. [DOI] [PubMed] [Google Scholar]
- 43.Walker MK, Catron TF. Characterization of cardiotoxicity induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin and related chemicals during early chick embryo development. Toxicol.Appl.Pharmacol. 2000;167:210–221. doi: 10.1006/taap.2000.8992. [DOI] [PubMed] [Google Scholar]
- 44.Wilcox CS, Pearlman A. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharmacol.Rev. 2008;60:418–469. doi: 10.1124/pr.108.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yeager RL, Reisman SA, Aleksunes LM, Klaassen CD. Introducing the "TCDD-inducible AhR-Nrf2 gene battery". Toxicol.Sci. 2009;111:238–246. doi: 10.1093/toxsci/kfp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshizawa K, Heatherly A, Malarkey DE, Walker NJ, Nyska A. A critical comparison of murine pathology and epidemiological data of TCDD, PCB126, and PeCDF. Toxicol.Pathol. 2007;35:865–879. doi: 10.1080/01926230701618516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zangar RC, Davydov DR, Verma S. Mechanisms that regulate production of reactive oxygen species by cytochrome P450. Toxicol.Appl.Pharmacol. 2004;199:316–331. doi: 10.1016/j.taap.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 48.Zhao W, Parrish AR, Ramos KS. Constitutive and inducible expression of cytochrome P450IA1 and P450IB1 in human vascular endothelial and smooth muscle cells. Vitro Cell Dev.Biol.Anim. 1998;34:671–673. doi: 10.1007/s11626-998-0060-7. [DOI] [PubMed] [Google Scholar]