Abstract
Background
Maternal nutrition during pregnancy has been linked with fetal brain development and psychopathology in the offspring. We examined for associations of maternal folate status and dietary intake during pregnancy with brain growth and childhood behavioural difficulties in the offspring.
Methods
In a prospective cohort study, maternal red blood cell folate (RCF) was measured at 14 weeks of pregnancy and total folate intake (TFI) from food and supplements was assessed in early and late pregnancy. The offspring’s head circumference and body weight were measured at birth and in infancy, and 100 mothers reported on children’s behavioural difficulties at a mean age of 8.75 years using the Strengths and Difficulties Questionnaire.
Results
Lower maternal RCF and TFI in early pregnancy were associated with higher childhood hyperactivity (RCF: beta = −.24; p = .013; TFI: beta = −.24; p = .022) and peer problems scores (RCF: beta = −.28; p = .004; TFI: beta = −.28; p = .009) in the offspring. Maternal gestational RCF was positively associated with head circumference at birth (adjusted for gestational age), and mediation analyses showed significant inverse indirect associations of RCF with hyperactivity/inattention and peer problems via fetal brain growth. Adjustment for mother’s smoking and drinking alcohol during pregnancy did not change the results.
Conclusions
Although the associations are small and residual confounding is possible, our data provide preliminary support for the hypothesis that lower folate status in early pregnancy might impair fetal brain development and affect hyperactivity/inattention and peer problems in childhood.
Keywords: fetal programming, folate, behavioural difficulties, fetal brain growth, hyperactivity, peer problems
The concept of developmental programming states that adverse environmental events during sensitive periods of organ development trigger plastic responses that result in long lasting functional alterations and influence risk for disease later in life (Barker, 2004). Adverse fetal environments shape fetal growth trajectories, and small size at birth adjusted for gestational age provides an indicator of prenatal adversity. There is accumulating evidence that lower fetal growth within the normal range is associated with childhood behavioural difficulties in the offspring, particularly hyperactivity/inattention, conduct problems and total behavioural difficulties (Schlotz & Phillips, 2009). As head circumference is an indicator of brain volume (Bartholomeusz, Courchesne, & Karns, 2002) and fetal head growth reflects the development of the fetal brain, head circumference at birth might be used to examine whether prenatal adversity influences later behaviour through altered brain growth. This is supported by previous findings that head circumference at birth was a better predictor of hyperactivity/inattention and total behavioural difficulties in childhood than birth weight (Lahti, et al., 2006; Schlotz, Jones, Godfrey, & Phillips, 2008).
Although these results suggest a lasting effect of an adverse prenatal environment they do not indicate which specific factors were involved in producing the adverse fetal environment. Fetal size at birth is the result of a combination of many determinants, for example nutrition, smoking, drug use or psychosocial stress of the mother during pregnancy, as well as genetic factors (Schlotz & Phillips, 2009). Therefore, examining the effects of specific exposures during pregnancy may enhance our understanding of the causal pathways that link prenatal adversity with maladjusted behaviour, and it might point towards interventions that could have long-term benefits for the child’s health later in life.
Amongst the potentially risk-inducing prenatal exposures, nutrition of the mother during pregnancy is of particular interest. Maternal nutrition affects fetal brain development (Georgieff, 2007), and famine during gestation as well as availability of specific micronutrients have been shown to be linked with maladjusted behaviour of the offspring later in life (A. S. Brown, Susser, Lin, Neugebauer, & Gorman, 1995; Colombo, et al., 2004; Gale, et al., 2008; Hibbeln, et al., 2007; Neugebauer, Hoek, & Susser, 1999; Parsons, Zhou, Spurrier, & Makrides, 2008; Zhou, Gibson, Crowther, Baghurst, & Makrides, 2006). Folate is a micronutrient involved in various physiological pathways and essential for fetal growth and development due to its role in DNA synthesis and cell replication (Scholl & Johnson, 2000; Tamura & Picciano, 2006). Folate deficiency during gestation increases the risk of neural tube defects in humans, highlighting its importance in neural development (Pitkin, 2007). However, it is less clear whether fetal brain development and later behavioural adjustment is affected by the variations in maternal folate status that occur in many populations. In support of this, studies in mice demonstrated that lesser degrees of gestational folate deficiency resulted in a loss of progenitor cells (Craciunescu, et al., 2004), a net reduction of cells in the fetal brain (Xiao, et al., 2005), reduced brain weight (Middaugh, Grover, Blackwell, & Zemp, 1976) and anxiety-related behaviour in the offspring (Ferguson, et al., 2005), suggesting a causal effect of prenatal folate status on neurodevelopment and behavioural functioning later in life. However, potential long lasting effects of variations in folate levels of mothers during pregnancy on childhood behaviour in their offspring have not yet been studied in humans.
The principal aim of this study was to test for a potential developmental programming effect of maternal folate status (measured by red blood cell folate) during pregnancy on childhood behavioural difficulties in the offspring. Given the essential role of folate for fetal growth and neurodevelopment, this association may be mediated by fetal body or head growth. In order to elucidate the origins of associations of fetal growth with behavioural difficulties in childhood, we tested this pathway in a prospective birth cohort.
In summary, we tested the following hypothesis: (i) lower folate status and intake in early pregnancy are associated with childhood behavioural difficulties in the offspring; (ii) these associations are mediated by fetal growth, in particular fetal head growth.
Methods
Participants
The mothers of the children in this study had taken part in an earlier study of nutrition during pregnancy and fetal growth in Caucasian women. Mothers were recruited in early pregnancy at the Princess Anne Maternity Hospital, Southampton UK (Godfrey, et al., 2001). The mothers were included if they were 16 years of age or older, had known menstrual dates, and attended the antenatal booking clinic before 17 weeks of gestation. Seven mother-child pairs were excluded because the child either died in the perinatal period or had major congenital abnormalities. Of the 553 mothers who took part in the original study, those whose addresses were traceable were contacted by mail, and a total of 139 children (69 boys, 70 girls) at a mean age of eight years and nine months (SD = 5.6 months, range: 7.6–9.8) and their mothers took part in this study. The number of subjects recruited for the follow-up was based on practical and statistical considerations with respect to testing hypotheses on associations of fetal growth with physiological responses to psychosocial stress in children using a laboratory stress test, which was the main goal of the study (Jones, et al., 2006).
The Local Research Ethics Committee approved the study and both parents and children gave written informed consent.
Measurements
Blood samples were collected when the women were recruited at their first hospital antenatal clinic visit (median gestational day 95; interquartile range (IQR) 89–103). Folate and iron status in early pregnancy were assessed in the Haematology Laboratory, Southampton General Hospital using radioimmunoassays of red cell folate (RCF) concentration and serum ferritin (Becton Dickinson, Oxford, UK). Serum ferritin was additionally measured in late pregnancy (median gestational day 199; IQR 196–204). Coefficients of variation of the assays were less than 10%.
Besides genetic and metabolic factors, blood folate levels are influenced by dietary intake (McNaughton, Mishra, Stephen, & Wadsworth, 2007), particularly in women who do not take folate supplements (J. E. Brown, et al., 1997). Dietary data were collected for all women recruited to the original study of fetal growth. In early (median gestational day 101; IQR 94–111) and late pregnancy (median gestational day 199; IQR 195–204), a trained research nurse administered a food-frequency questionnaire (FFQ) that assessed the average frequency of consumption of 100 foods or food groups in the preceding three months. The nutrient content of a standard portion of each food was multiplied by its reported frequency of use and supplement use was ascertained in detail, allowing calculation of average total intakes of energy (kcal/day) and folate (TFI; µg/day). Oily fish intake was obtained from the FFQ (portions/week). Estimates of nutrient intake from the FFQ have been validated against those determined from food diaries kept over four days (Robinson, Godfrey, Osmond, Cox, & Barker, 1996). Data on maternal alcohol or tobacco use during pregnancy and on the mother’s highest academic qualification were also collected during these interviews.
At birth and at age 9 months, the infant’s weight was measured using digital scales, and trained fieldworkers recorded neonatal head circumference using standard techniques. Occipito-frontal circumference was measured to the nearest 0.1 cm within 48 hours of birth. The infant’s gestational age at birth was calculated from the date of the last menstrual period and ultrasound data (Godfrey, et al., 2001).
The following behavioural difficulties in childhood were measured using the Strengths and Difficulties Questionnaire (SDQ; Goodman, 1997), completed by the mothers during the follow-up visit at age 8 years: Hyperactivity (being overactive, constantly fidgeting, easily distracted), Emotional Symptoms (being worried, unhappy, nervous; complaining about bodily symptoms), Conduct Problems (having temper tantrums; being disobedient; bullying others, stealing, lying) and Peer Problems (playing alone; being bullied and generally not liked by other children). The SDQ has been successfully validated against similar questionnaires such as the Rutter Scales and the Child Behaviour Checklist as well as against DSM-IV diagnoses (Goodman, 1997, 2001; Goodman & Scott, 1999). In our sample, the SDQ scales showed satisfactory measurement reliability with internal consistencies of α = .81 (Hyperactivity); .69 (Emotional Problems); .66 (Conduct Problems); and .57 (Peer Problems). Boys scored higher on Hyperactivity (p < .001) and Conduct Problems (p = .002) than girls.
Statistical analysis
Control variables
We controlled for maternal smoking and alcohol consumption during pregnancy as these risk factors are associated with poor folate status (Scholl & Johnson, 2000) and an increased risk of behavioural difficulties in the offspring (Linnet, et al., 2003). The analyses were also adjusted for the child’s sex because of known sex differences in behavioural difficulties that might mask folate effects. In order to isolate the effect of folate in the causal pathway via fetal growth, mediation analyses were adjusted for a number of variables that are known to affect fetal growth (see below). Because mother’s educational attainment and dietary patterns of mother and child are linked, we used educational attainment of the mother as a control variable in a later stage of the analyses to adjust for potential effects of postnatal diet. A potential causal pathway via fetal brain or body growth should be specific to prenatal (vs. postnatal) development. We therefore added tests of associations with brain and body growth at 9 months of age (adjusting for gestational age, sex, and head circumference at birth or birth weight). We repeated the analysis of RCF using TFI. While RCF levels were only available from early pregnancy, the FFQ was administered twice, in early and late pregnancy, which presents the opportunity to test if potential associations with TFI were specific to early pregnancy. All models analysing nutritional intake were adjusted for total energy intake.
Transformations
RCF concentrations, serum ferritin concentrations, TFI and iron intake were log-transformed to yield unskewed distributions. Due to non-normal distributions that could not be normalised by standard transformations, the four behavioural outcome variables were recoded into four similar-sized categories based on quartiles, assuming that this ordinal variable is related to a continuous latent variable that indicates the respective behavioural problem dimension.
Hypothesis tests
Ordered logit regression models (Long, 1997) were used to test for associations of behavioural problems with RCF or TFI. Likelihood-ratio tests of the proportionality of odds across categories showed no evidence for violation of the proportional odds assumption for any of the models (all ps > .15). To test for specificity of any folate effect we ran similar models predicting behavioural problems by iron status or energy and oily fish intake (Gale, et al., 2008; Parsons, et al., 2008) and other control variables as described above. Ordinary least squares linear regression models were used to test if maternal RCF and TFI during pregnancy were associated with fetal body and head growth (birth weight and head circumference at birth adjusted for gestational age); all of these models were adjusted for gestational age and sex. Ordered logit regression models were computed to test for associations of body and/or head growth with behavioural difficulties that had shown significant associations with RCF/TFI in the previous analyses (again, likelihood-ratio test showed no evidence for a violation of the proportional odds assumption for any of the models; all ps > .29). Finally, if a behavioural outcome variable showed significant associations with RCF/TFI and with a fetal growth indicator, a mediation model was tested (adjusted for gestational age, sex and mother’s parity, age at conception, alcohol intake and smoking during pregnancy and educational attainment). Direct and indirect effects were estimated by path analysis with ordered categorical outcome variables using Mplus v5 (Muthen & Muthen, 2007, Los Angeles, CA, USA). Stata v9.2 (StataCorp, 2006, College Station, TX, USA) was used for all other statistical analyses. We used an alpha level of p ≤ .05 for all statistical tests.
Results
Sample characteristics
Out of the 139 children that took part in the follow up study, 39 had missing data for mother’s RCF during early pregnancy or behavioural difficulties. Thus, a sub-sample of 100 subjects was studied here. Comparison of this sub-sample with the other 453 in the full cohort from which they were drawn showed no statistically significant differences in birth weight, gestational age, maternal education, drinking alcohol during pregnancy, RCF, TFI in late pregnancy and daily energy intake in early or late pregnancy (all ps > .10). In this sub-sample, fewer mothers reported that they smoked during pregnancy (21% vs 35%; p = .007), mothers were slightly older at conception (mean = 28.2 vs 27.2 years; p = .06), had a higher TFI in early pregnancy (median = 466 vs 413 µg/d; p = .027) and their children’s head circumference at birth tended to be larger (mean = 35.1 vs 34.8 cm; p = .052) than in the rest of the original cohort. Table 1 shows characteristics of children and mothers in the study sample. All median SDQ scale scores in this sample were in the normal range.
Table 1.
Characteristics of children and mothers participating in the study
| All participants (n = 100) | |
|---|---|
| Child’s characteristics | |
| Gestational age (days)a | 280 (11.06) |
| Birth weight (kg)a | 3.47 (0.53) |
| Head circumference at birth (cm)a | 35.14 (1.46) |
| Age at study date (years)a | 8.73 (0.49) |
| SDQ Hyperactivity b | 4 (1–6) |
| SDQ Emotional problems b | 2 (1–3.5) |
| SDQ Conduct problems b | 1 (0–2) |
| SDQ Peer problems b | 1 (0–2) |
| Mother’s characteristics | |
| Red cell folate (RCF) in early pregnancy (µg/L)b | 466 (373.5–588.5) |
| Total folate intake (TFI) (µg/d)b | |
| Early pregnancy | 465.6 (328.5–624.4) |
| Late pregnancy | 323.1 (269.9–410.0) |
| Daily energy intake (kcal/d)a | |
| Early pregnancy | 2470.3 (819.2) |
| Late pregnancy | 2664.5 (785.5) |
| Age at conception (years)a | 28.17 (4.88) |
| Highest academic qualificationc | |
| None | 6 (6%) |
| CSE | 11 (11%) |
| O levels | 30 (30%) |
| A levels | 33 (33%) |
| Higher National Diploma | 8 (8%) |
| Degree | 12 (12%) |
Notes. SDQ: Strengths and Difficulties Questionnaire
M (SD)
Median (IQR)
Frequency (%)
Folate in early pregnancy and behavioural difficulties in the offspring
Lower RCF levels in early pregnancy were associated with higher scores on Hyperactivity and Peer Problems in the offspring, whereas there were no associations with Emotional Problems or Conduct Problems scores (Table 2). The results were stable when models were adjusted for the child’s sex, the mother’s tobacco or alcohol consumption during pregnancy and her educational attainment. In contrast, iron status in early pregnancy was not associated with behavioural problems, and the associations of folate status with Hyperactivity and Peer Problems were stable when iron status was added to the model (both p = .005; data available on request). As red cell folate concentrations were available for early pregnancy only, no associations could be tested for late pregnancy.
Table 2.
Results of ordered regressions of categorised behavioural difficulties in 7–9 year old children on the mother’s red cell folate in early pregnancy, unadjusted and adjusted for potentially confounding variables
| Predictors in the model | Behavioural difficulties | |||
|---|---|---|---|---|
| Hyperactivity | Emotional problems |
Conduct problems |
Peer problems | |
| Red cell folate (µg/L) | ||||
| b | −1.23 | −0.58 | −0.90 | −1.45 |
| (95% CI) | (−2.20; −0.26) | (−1.55; 0.39) | (−1.96; 0.16) | (−2.47; −0.46) |
| β | −.24 | −.12 | −.18 | −.28 |
| P | .013 | .24 | .09 | .004 |
| + Child’s sex | ||||
| b | −1.17 | −0.59 | −0.80 | −1.42 |
| (95% CI) | (−2.18; −0.17) | (−1.57; 0.39) | (−1.88; 0.28) | (−2.43; −0.41) |
| β | −.21 | −.12 | −.15 | −.27 |
| p | .022 | .24 | .15 | .006 |
| + Mother’s smoking and drinking alcohol during pregnancy |
||||
| b | −1.40 | −1.38 | ||
| (95% CI) | (−2.45; −0.35) | (−2.42; −0.33) | ||
| β | −.25 | −.26 | ||
| p | .009 | .010 | ||
| + Mother’s educational attainment | ||||
| b | −1.40 | −1.33 | ||
| (95% CI) | (−2.48; −0.32) | (−2.40; −0.25) | ||
| β | −.25 | −.25 | ||
| p | .011 | .015 | ||
Note. b: Raw coefficient; β: fully standardised coefficient (equivalent to correlation coefficient)
Table 3 shows the results for TFI. Similar to RCF, lower TFI during early pregnancy was associated with higher scores on Hyperactivity and Peer Problems of the offspring in childhood. The associations were stable when TFI was adjusted for sex and maternal educational attainment, tobacco or alcohol consumption and daily energy intake, with effect sizes similar to those for RCF. Iron and oily fish intake in early pregnancy were not related to Hyperactivity or Peer Problems, and adding them to the folate models did not change the results (data available on request). In contrast to folate intake in early pregnancy, Hyperactivity and Peer Problems were unrelated to TFI in late pregnancy (Table 3).
Table 3.
Results of ordered regressions of categorised behavioural difficulties in 7–9 year old children on the mother’s total folate intake (food and supplemental) in early pregnancy, unadjusted and adjusted for potentially confounding variables
| Predictors in the model | Early pregnancy | Late pregnancy | ||
|---|---|---|---|---|
| Hyperactivity | Peer problems | Hyperactivity | Peer problems | |
| Total folate intake (µg/d) | ||||
| b | −0.75 | −0.87 | 0.07 | 0.25 |
| (95% CI) | (−1.39; −0.11) | (−1.52; −0.21) | (−0.80; 0.93) | (−0.65; 1.14) |
| β | −.24 | −.28 | .02 | −.05 |
| p | .022 | .009 | .88 | .59 |
| + Daily energy intake | ||||
| b | −0.74 | −1.14 | 0.11 | −0.39 |
| (95% CI) | (−1.41; −0.07) | (−1.87; −0.42) | (−0.94; 1.16) | (−1.52; 0.74) |
| β | −.24 | −.35 | .03 | −.09 |
| p | .032 | .002 | .83 | .50 |
| + Child’s sex | ||||
| b | −0.44 | −1.12 | 0.47 | 0.09 |
| (95% CI) | (−1.12; 0.25) | (−1.86; −0.38) | (−0.62; 1.55) | (−0.89; 1.08) |
| β | −.13 | −.35 | .09 | .02 |
| p | .21 | .003 | .40 | .85 |
| + Mother’s smoking and drinking alcohol during pregnancy |
||||
| b | −1.11 | |||
| (95% CI) | (−1.86; −0.35) | |||
| β | −.34 | |||
| p | .004 | |||
| + Mother’s educational attainment | ||||
| b | −1.09 | |||
| (95% CI) | (−1.87; −0.31) | |||
| β | −.34 | |||
| p | .006 | |||
Note. b: Raw coefficient; β: Fully standardised coefficient (equivalent to correlation coefficient)
Fetal growth
Regression analyses showed a significant association of head circumference at birth adjusted for gestational age and sex, i.e. fetal head growth, with maternal RCF in early pregnancy (b = 0.68; SE = 0.31; β = .17; p = .031) and a trend for a similar association with TFI, adjusted for daily energy intake (b = 0.36; SE = 0.20; β = .15; p = .083), suggesting that maternal folate status in pregnancy might affect fetal head growth. In models that were adjusted for gestational age and sex, RCF explained 2.8% (ΔR2 = .028) and TFI in early pregnancy 1.8% (ΔR2 = .018) of the variance in head circumference. In contrast, no association was found for TFI in late pregnancy (β = −.05; p = .64); RCF and TFI were unrelated to birth weight adjusted for gestational age, i.e. fetal body growth (all p > .15). To test if the associations were specific for prenatal growth, we also tested associations of maternal folate with postnatal growth at 9 months of age. There were no significant associations of gestational RCF or TFI with head growth (p > .73) or body growth (p > .71) in infancy, suggesting that the associations with folate status in early pregnancy was specific to prenatal head growth. Further analyses showed that fetal head growth was associated with high scores on Hyperactivity (b = −0.55; 95% CI: −0.90,−0.19; p = .003) and Peer Problems (b = −0.39; 95% CI: −0.73,−0.05; p = .026) in childhood.
Mediation analyses
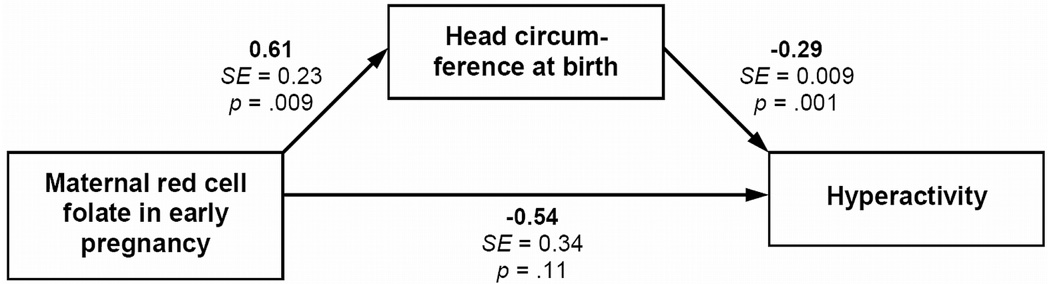
The direct association of RCF in early pregnancy with Hyperactivity decreased when fetal head growth was included in the model (Figure 1). The overall indirect effect of folate on Hyperactivity via head circumference at birth was significant and negative (b = −0.18; SE = 0.09; p = .050). Adjustment for the mother’s educational attainment slightly attenuated the indirect effect of RCF (b = −0.17; SE = 0.09; p = .07). These results suggest that the association of early pregnancy RCF with childhood hyperactivity/inattention in the offspring was mediated by fetal head growth.
Figure 1.
Mediation analysis for effects of maternal red cell folate in early pregnancy on childhood Hyperactivity scores in the offspring via head circumference at birth adjusted for gestational age (i.e., fetal head growth). Model adjusted for gestational age, sex, and mother’s parity, age at conception, alcohol intake and smoking during pregnancy (not shown)
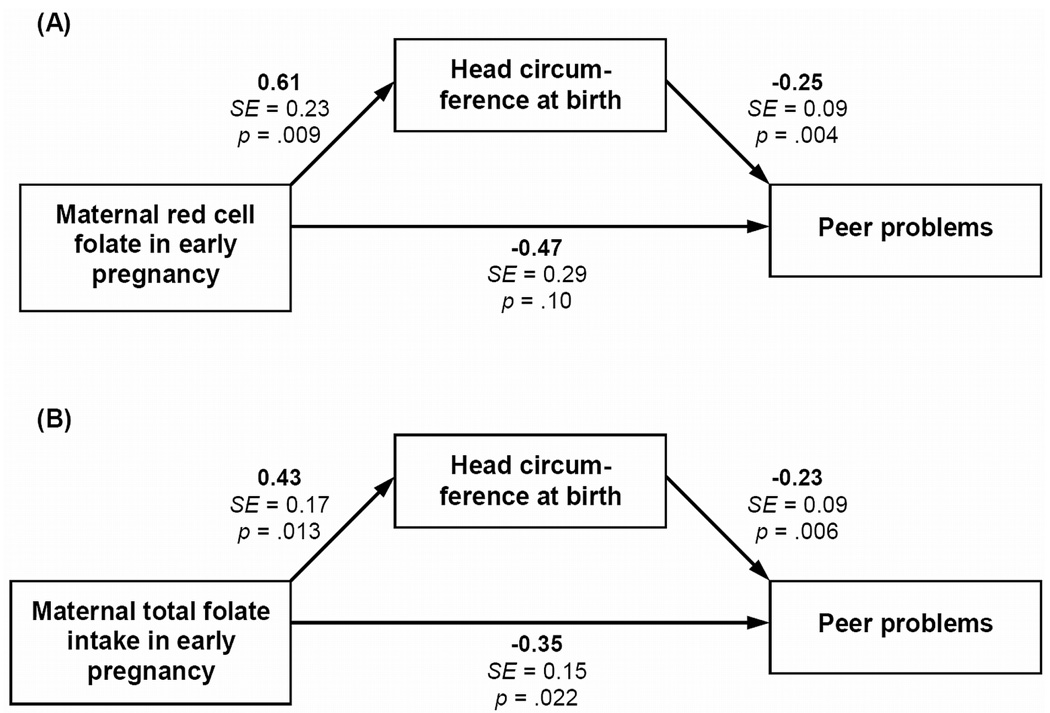
Figure 2 shows the results of the mediation models for Peer Problems. There were highly significant paths from both folate measures in early pregnancy to head circumference at birth, and from head circumference at birth to Peer Problems scores in childhood. However, scores on Peer Problems were still significantly associated with TFI, and a trend (p = .10) remained for RCF. The overall indirect effect of folate on Peer Problems via head circumference at birth was significant and negative for RCF (b = −0.15; SE = 0.07; p = .040), whereas only a trend was found for TFI (b = −0.10; SE = 0.06; p = .07). Adjustment for the mother’s educational attainment slightly attenuated the indirect effect of RCF (b = −0.14; SE = 0.08; p = .06) and TFI (b = −0.10; SE = 0.06; p = .08). These results suggest a partial mediation of the association of early pregnancy folate status and intake on the offspring’s childhood peer problems by fetal head growth.
Figure 2.
Mediation analysis for effects of maternal folate in early pregnancy on childhood Peer Problems scores in the offspring via head circumference at birth adjusted for gestational age (i.e., fetal head growth): (A) Red cell folate in early pregnancy; (B) Total folate intake in early pregnancy. Both models adjusted for gestational age, sex, and mother’s parity, age at conception, alcohol intake and smoking during pregnancy (not shown). Model B additionally adjusted for mother’s total energy intake in early pregnancy
Discussion
Our findings showed associations between lower maternal folate status and intake in early pregnancy with increased risk of childhood hyperactivity/inattention and peer problems in the offspring. Moreover, these associations were mediated by fetal head growth but not by fetal body growth, and no association with postnatal head growth was found, suggesting a specific pathway via fetal brain growth. To our knowledge, this is the first study in humans to provide evidence for associations of maternal folate with behavioural outcomes in the offspring, and it is the first study to demonstrate a putative pathway via fetal head growth.
Although the results of our mediation models were statistically significant, the amount of variance in fetal growth explained by the maternal folate status in pregnancy was small (2.8%). However, it has to be kept in mind that head growth is a rather crude measure of brain growth. Structural and functional measures of specific brain regions might yield higher effect sizes. Nevertheless, the clinical significance of our findings is limited and needs further exploration. However, our findings are important in that they suggest hypotheses with regards to a potential neurobehavioural pathway that starts during prenatal life and contributes to the development of hyperactivity and peer problems later in life. A weakness of our study is that, although population-based and focussing on healthy infants delivered at term, we were only able to follow up a sub-sample of the offspring of women taking part in a study of maternal nutrition. Compared with the remainder of the cohort, those followed up had mothers that were less likely to smoke and had higher TFI in early pregnancy, but there were no other major differences in maternal or neonatal characteristics. In addition, the distribution of mothers’ highest academic qualification and the offspring’s mean birth weight were similar to those in a recent large UK national cohort study (A levels or over: 53% vs 61%; mean birth weight: 3.47 kg vs 3.42 kg; Kelly, et al., 2009).
Children with attention-deficit/hyperactivity disorder (ADHD) are known to have an increased risk of peer problems (Hoza, 2007). Although the precise mechanisms behind these associations are not known, our data suggest a common vulnerability for these behavioural problems related to maternal folate status in early pregnancy and long-lasting alterations of brain development.
The principal mechanism underlying the potential effect of maternal folate status in early pregnancy on fetal brain growth is based on placental transfer of folate (Antony, 2007). The human brain develops rapidly during early pregnancy, with neurogenesis in most cortical and subcortical structures taking place between 5 and 25 weeks of gestation (Rice & Barone, 2000). Hence, this developmental interval presents a sensitive period for potentially long-lasting effects of adverse environmental exposures on neurodevelopment; findings from the Dutch Hunger Winter study also suggest that nutritional deficiency during early stages of pregnancy has stronger effects than late pregnancy exposure (e.g. Neugebauer, et al., 1999). Marginal folate deficiency during gestation can impair cellular growth and replication in the fetus (Scholl & Johnson, 2000) and has been demonstrated to cause a net reduction of cells in the fetal brain (Xiao, et al., 2005), a loss of progenitor cells in the brain (Craciunescu, et al., 2004)as well as a reduced brain weight (Middaugh, et al., 1976) in mice. Such alterations of brain structure may be long-lasting and may also contribute to behavioural difficulties later in life (e.g. Castellanos, et al., 2002). Epigenetic processes might also be implicated, as nutritional deficiency during early pregnancy can have long-lasting epigenetic consequences (Heijmans, et al., 2008). Folate is involved in CpG methylation and folate levels correlate with genomic DNA methylation status (Nafee, Farrell, Carroll, Fryer, & Ismail, 2008). Such epigenetic changes potentially contribute to symptoms of hyperactivity and inattention (Mill & Petronis, 2008). Developmental alterations in the structure or functioning of the dopaminergic system have been discussed as a potential mechanism underlying associations of fetal brain growth with childhood hyperactivity (Schlotz, et al., 2008). While the development of dopaminergic neurons has been shown to be sensitive to prenatal disruption of neuronal growth (Lavin, Moore, & Grace, 2005), a developmental effect of folate on the dopaminergic system has not been studied.
Whereas folate fortification was associated with a decline in the prevalence of neural tube defects in different countries (Heseker, Mason, Selhub, Rosenberg, & Jacques, 2009), a corresponding decline in behavioural difficulties was not reported. However, there is evidence that use of folic acid supplements has not increased in many regions in California and has actually decreased in some ethnic groups (Centers for Disease Control and Prevention (CDC), 2007). Guidelines to women to take folic acid supplements in the periconceptional period are clearly reflected in TFI differences between early and late pregnancy in our study, but it is unclear how this might have affected our results. Folate status might have differing effects at particular stages in pregnancy. However, data from controlled trials or systematic longitudinal observations of effects of mandatory folic acid fortification are needed to better understand associations between prenatal folate and offspring behaviour.
While our data suggest a potentially important role of the mother’s diet during early pregnancy for the development of childhood behavioural difficulties in her offspring, the child’s diet at the date of the behavioural assessment could be a confounding factor in our analyses. Although there is no evidence for an association with peer problems, associations of a child’s nutrition with hyperactive behaviour have been demonstrated (McCann, et al., 2007; Wiles, Northstone, Emmett, & Lewis, 2009). As we do not have data regarding the children’s diets on the date of the behavioural assessment we cannot rule out a possible influence of concurrent dietary factors on our results. This possibility is strengthened by evidence that children’s diets are often similar to those of their mothers (Robinson, et al., 2007); thus the link between maternal folate status in early pregnancy and children’s behaviour might be a reflection of the type of diet mothers were feeding their child currently. However, dietary patterns of younger children are associated with the mother’s educational attainment (Robinson, et al., 2007), and controlling for the mother’s educational attainment did not change the associations of folate status with childhood behavioural difficulties in the offspring and only slightly attenuated the mediation via fetal head growth. In addition, it must kept in mind that educational attainment is a key influence on the mother’s dietary pattern (Robinson, et al., 2004) and thus controlling for this variable might yield an overcorrection.
The origins of behavioural difficulties in childhood are multifaceted and the developmental pathways are complex (Rutter & Sroufe, 2000). As alcohol and tobacco consumption have been reported to be associated with poor folate status (Scholl & Johnson, 2000) and risk for childhood behavioural difficulties in the offspring (Linnet, et al., 2003) we adjusted our models for these factors. However, measures of other important risk factors for behavioural difficulties such as maternal/family history of psychopathology, cognitive ability, psychosocial stress during postnatal development, parenting or home environment were not available. Furthermore, data on behavioural difficulties were only available from mothers’ reports. In addition, the potential role of shared genes in the causal pathway is unclear. Genes may contribute to the associations by influencing the mother’s health behaviour and other parental behaviour as well as the child’s behaviour later in life via gene-environment correlations. However, it is very unlikely that these common genes similarly affect fetal head growth. In addition, there is now increasing evidence of gene x prenatal environment (GxPreE) interactions (e.g. Brookes, et al., 2006; Kahn, Khoury, Nichols, & Lanphear, 2003; see also Schlotz & Phillips, 2009) that contribute to the complexity of potential genetic contributions. In the context of these limitations it is noteworthy that we could not confirm the association of higher maternal fish intake with lower hyperactivity that we found in another cohort (Gale, et al., 2008), or the association of gestational iron supplementation on peer problems reported earlier (Parsons, et al., 2008). The problem of potential residual confounding should be addressed in future studies by using alternative designs. One option would be to follow-up offspring of mothers who participated in randomised controlled trials of folate supplementation during pregnancy.
In summary, these results add to our understanding of the developmental origins of mental health and behaviour by suggesting a potential pathway from a specific fetal environmental factor to childhood hyperactivity and peer problems via prenatal brain development.
Key points
The micronutrient folate is an important factor in fetal neurodevelopment
Potential long term effects of variations in maternal folate status during gestation on brain development and childhood behaviour in the offspring are unknown
Results in this longitudinal study of a birth cohort show associations of maternal folate status in early pregnancy with fetal head growth, as well as with hyperactivity/inattention and peer problems in childhood
Mediation models suggest that the association of maternal folate status in early pregnancy with childhood behavioural problems might be mediated by fetal brain growth (as measured by head circumference)
Acknowledgements
The study was funded by NICHD grant 1 R01 HD41107-01. Subjects were drawn from a cohort study funded by WellBeing and the Medical Research Council. W.S. was supported by German Research Foundation (Deutsche Forschungsgemeinschaft) fellowship grant DFG SCHL 1708/1-1.
Abbreviations
- TFI
Total folate intake
- RCF
Red cell folate
Footnotes
The authors declare no conflict of interest.
References
- Antony AC. In utero physiology: role of folic acid in nutrient delivery and fetal development. American Journal of Clinical Nutrition. 2007;85:598S–603S. doi: 10.1093/ajcn/85.2.598S. [DOI] [PubMed] [Google Scholar]
- Barker DJ. The developmental origins of chronic adult disease. Acta Paediatrica. Supplement. 2004;93:26–33. doi: 10.1111/j.1651-2227.2004.tb00236.x. [DOI] [PubMed] [Google Scholar]
- Bartholomeusz HH, Courchesne E, Karns CM. Relationship between head circumference and brain volume in healthy normal toddlers, children, and adults. Neuropediatrics. 2002;33:239–241. doi: 10.1055/s-2002-36735. [DOI] [PubMed] [Google Scholar]
- Brookes KJ, Mill J, Guindalini C, Curran S, Xu X, Knight J, Chen CK, Huang YS, Sethna V, Taylor E, Chen W, Breen G, Asherson P. A common haplotype of the dopamine transporter gene associated with attention-deficit/hyperactivity disorder and interacting with maternal use of alcohol during pregnancy. Archives of General Psychiatry. 2006;63:74–81. doi: 10.1001/archpsyc.63.1.74. [DOI] [PubMed] [Google Scholar]
- Brown AS, Susser ES, Lin SP, Neugebauer R, Gorman JM. Increased risk of affective disorders in males after second trimester prenatal exposure to the Dutch hunger winter of 1944–45. British Journal of Psychiatry. 1995;166:601–606. doi: 10.1192/bjp.166.5.601. [DOI] [PubMed] [Google Scholar]
- Brown JE, Jacobs DR, Jr, Hartman TJ, Barosso GM, Stang JS, Gross MD, Zeuske MA. Predictors of red cell folate level in women attempting pregnancy. JAMA. 1997;277:548–552. doi: 10.1001/jama.1997.03540310046033. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, Blumenthal JD, James RS, Ebens CL, Walter JM, Zijdenbos A, Evans AC, Giedd JN, Rapoport JL. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 2002;288:1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Trends in folic acid supplement intake among women of reproductive age--California, 2002–2006. MMWR. Morbidity and Mortality Weekly Report. 2007;56:1106–1109. [PubMed] [Google Scholar]
- Colombo J, Kannass KN, Shaddy DJ, Kundurthi S, Maikranz JM, Anderson CJ, Blaga OM, Carlson SE. Maternal DHA and the development of attention in infancy and toddlerhood. Child Development. 2004;75:1254–1267. doi: 10.1111/j.1467-8624.2004.00737.x. [DOI] [PubMed] [Google Scholar]
- Craciunescu CN, Brown EC, Mar MH, Albright CD, Nadeau MR, Zeisel SH. Folic acid deficiency during late gestation decreases progenitor cell proliferation and increases apoptosis in fetal mouse brain. Journal of Nutrition. 2004;134:162–166. doi: 10.1093/jn/134.1.162. [DOI] [PubMed] [Google Scholar]
- Ferguson SA, Berry KJ, Hansen DK, Wall KS, White G, Antony AC. Behavioral effects of prenatal folate deficiency in mice. Birth Defects Research. Part A, Clinical and Molecular Teratology. 2005;73:249–252. doi: 10.1002/bdra.20111. [DOI] [PubMed] [Google Scholar]
- Gale CR, Robinson SM, Godfrey KM, Law CM, Schlotz W, O'Callaghan FJ. Oily fish intake during pregnancy - association with lower hyperactivity but not with higher full-scale IQ in offspring. Journal of Child Psychology and Psychiatry. 2008;40:1061–1068. doi: 10.1111/j.1469-7610.2008.01908.x. [DOI] [PubMed] [Google Scholar]
- Georgieff MK. Nutrition and the developing brain: nutrient priorities and measurement. American Journal of Clinical Nutrition. 2007;85:614S–620S. doi: 10.1093/ajcn/85.2.614S. [DOI] [PubMed] [Google Scholar]
- Godfrey K, Walker-Bone K, Robinson S, Taylor P, Shore S, Wheeler T, Cooper C. Neonatal bone mass: influence of parental birthweight, maternal smoking, body composition, and activity during pregnancy. Journal of Bone and Mineral Research. 2001;16:1694–1703. doi: 10.1359/jbmr.2001.16.9.1694. [DOI] [PubMed] [Google Scholar]
- Goodman R. The Strengths and Difficulties Questionnaire: a research note. Journal of Child Psychology and Psychiatry. 1997;38:581–586. doi: 10.1111/j.1469-7610.1997.tb01545.x. [DOI] [PubMed] [Google Scholar]
- Goodman R. Psychometric properties of the strengths and difficulties questionnaire. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:1337–1345. doi: 10.1097/00004583-200111000-00015. [DOI] [PubMed] [Google Scholar]
- Goodman R, Scott S. Comparing the Strengths and Difficulties Questionnaire and the Child Behavior Checklist: is small beautiful? Journal of Abnormal Child Psychology. 1999;27:17–24. doi: 10.1023/a:1022658222914. [DOI] [PubMed] [Google Scholar]
- Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, Slagboom PE, Lumey LH. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:17046–17049. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heseker HB, Mason JB, Selhub J, Rosenberg IH, Jacques PF. Not all cases of neural-tube defect can be prevented by increasing the intake of folic acid. British Journal of Nutrition. 2009;102:173–180. doi: 10.1017/S0007114508149200. [DOI] [PubMed] [Google Scholar]
- Hibbeln JR, Davis JM, Steer C, Emmett P, Rogers I, Williams C, Golding J. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study. Lancet. 2007;369:578–585. doi: 10.1016/S0140-6736(07)60277-3. [DOI] [PubMed] [Google Scholar]
- Hoza B. Peer functioning in children with ADHD. Journal of Pediatric Psychology. 2007;32:655–663. doi: 10.1093/jpepsy/jsm024. [DOI] [PubMed] [Google Scholar]
- Jones A, Godfrey KM, Wood P, Osmond C, Goulden P, Phillips DIW. Fetal growth and the adrenocortical response to psychological stress. Journal of Clinical Endocrinology and Metabolism. 2006;91:1868–1871. doi: 10.1210/jc.2005-2077. [DOI] [PubMed] [Google Scholar]
- Kahn RS, Khoury J, Nichols WC, Lanphear BP. Role of dopamine transporter genotype and maternal prenatal smoking in childhood hyperactive-impulsive, inattentive, and oppositional behaviors. Journal of Pediatrics. 2003;143:104–110. doi: 10.1016/S0022-3476(03)00208-7. [DOI] [PubMed] [Google Scholar]
- Kelly Y, Panico L, Bartley M, Marmot M, Nazroo J, Sacker A. Why does birthweight vary among ethnic groups in the UK? Findings from the Millennium Cohort Study. J Public Health (Oxf) 2009;31:131–137. doi: 10.1093/pubmed/fdn057. [DOI] [PubMed] [Google Scholar]
- Lahti J, Raikkonen K, Kajantie E, Heinonen K, Pesonen AK, Jarvenpaa AL, Strandberg T. Small body size at birth and behavioural symptoms of ADHD in children aged five to six years. Journal of Child Psychology and Psychiatry. 2006;47:1167–1174. doi: 10.1111/j.1469-7610.2006.01661.x. [DOI] [PubMed] [Google Scholar]
- Lavin A, Moore HM, Grace AA. Prenatal disruption of neocortical development alters prefrontal cortical neuron responses to dopamine in adult rats. Neuropsychopharmacology. 2005;30:1426–1435. doi: 10.1038/sj.npp.1300696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnet KM, Dalsgaard S, Obel C, Wisborg K, Henriksen TB, Rodriguez A, Kotimaa A, Moilanen I, Thomsen PH, Olsen J, Jarvelin MR. Maternal lifestyle factors in pregnancy risk of attention deficit hyperactivity disorder and associated behaviors: review of the current evidence. American Journal of Psychiatry. 2003;160:1028–1040. doi: 10.1176/appi.ajp.160.6.1028. [DOI] [PubMed] [Google Scholar]
- Long JS. Regression models for categorical and limited dependent variables. Thousand Oaks, CA, USA: Sage; 1997. [Google Scholar]
- McCann D, Barrett A, Cooper A, Crumpler D, Dalen L, Grimshaw K, Kitchin E, Lok K, Porteous L, Prince E, Sonuga-Barke E, Warner JO, Stevenson J. Food additives and hyperactive behaviour in 3-year-old and 8/9-year-old children in the community: a randomised, double-blinded, placebo-controlled trial. Lancet. 2007;370:1560–1567. doi: 10.1016/S0140-6736(07)61306-3. [DOI] [PubMed] [Google Scholar]
- McNaughton SA, Mishra GD, Stephen AM, Wadsworth ME. Dietary patterns throughout adult life are associated with body mass index, waist circumference, blood pressure, and red cell folate. Journal of Nutrition. 2007;137:99–105. doi: 10.1093/jn/137.1.99. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Grover TA, Blackwell LA, Zemp JW. Neurochemical and behavioral effects of diet related perinatal folic acid restriction. Pharmacology, Biochemistry and Behavior. 1976;5:129–134. doi: 10.1016/0091-3057(76)90027-7. [DOI] [PubMed] [Google Scholar]
- Mill J, Petronis A. Pre- and peri-natal environmental risks for attention-deficit hyperactivity disorder (ADHD): the potential role of epigenetic processes in mediating susceptibility. Journal of Child Psychology and Psychiatry. 2008;49:1020–1030. doi: 10.1111/j.1469-7610.2008.01909.x. [DOI] [PubMed] [Google Scholar]
- Nafee TM, Farrell WE, Carroll WD, Fryer AA, Ismail KM. Epigenetic control of fetal gene expression. BJOG. 2008;115:158–168. doi: 10.1111/j.1471-0528.2007.01528.x. [DOI] [PubMed] [Google Scholar]
- Neugebauer R, Hoek HW, Susser E. Prenatal exposure to wartime famine and development of antisocial personality disorder in early adulthood. JAMA. 1999;282:455–462. doi: 10.1001/jama.282.5.455. [DOI] [PubMed] [Google Scholar]
- Parsons AG, Zhou SJ, Spurrier NJ, Makrides M. Effect of iron supplementation during pregnancy on the behaviour of children at early school age: long-term follow-up of a randomised controlled trial. British Journal of Nutrition. 2008;99:1133–1139. doi: 10.1017/S0007114507853359. [DOI] [PubMed] [Google Scholar]
- Pitkin RM. Folate and neural tube defects. American Journal of Clinical Nutrition. 2007;85:285S–288S. doi: 10.1093/ajcn/85.1.285S. [DOI] [PubMed] [Google Scholar]
- Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environmental Health Perspectives. 2000;108 Suppl 3:511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SM, Crozier SR, Borland SE, Hammond J, Barker DJ, Inskip HM. Impact of educational attainment on the quality of young women's diets. European Journal of Clinical Nutrition. 2004;58:1174–1180. doi: 10.1038/sj.ejcn.1601946. [DOI] [PubMed] [Google Scholar]
- Robinson S, Godfrey K, Osmond C, Cox V, Barker D. Evaluation of a food frequency questionnaire used to assess nutrient intakes in pregnant women. European Journal of Clinical Nutrition. 1996;50:302–308. [PubMed] [Google Scholar]
- Robinson S, Marriott L, Poole J, Crozier S, Borland S, Lawrence W, Law C, Godfrey K, Cooper C, Inskip H. Dietary patterns in infancy: the importance of maternal and family influences on feeding practice. British Journal of Nutrition. 2007;98:1029–1037. doi: 10.1017/S0007114507750936. [DOI] [PubMed] [Google Scholar]
- Rutter M, Sroufe LA. Developmental psychopathology: concepts and challenges. Development and Psychopathology. 2000;12:265–296. doi: 10.1017/s0954579400003023. [DOI] [PubMed] [Google Scholar]
- Schlotz W, Jones A, Godfrey KM, Phillips DI. Effortful control mediates associations of fetal growth with hyperactivity and behavioural problems in 7- to 9-year-old children. Journal of Child Psychology and Psychiatry. 2008;49:1228–1236. [PubMed] [Google Scholar]
- Schlotz W, Phillips DIW. Fetal origins of mental health: Evidence and mechanisms. Brain, Behavior, and Immunity. 2009 doi: 10.1016/j.bbi.2009.02.001. doi: 10.1016/j.bbi.2009.1002.1001. [DOI] [PubMed] [Google Scholar]
- Scholl TO, Johnson WG. Folic acid: influence on the outcome of pregnancy. American Journal of Clinical Nutrition. 2000;71:1295S–1303S. doi: 10.1093/ajcn/71.5.1295s. [DOI] [PubMed] [Google Scholar]
- Tamura T, Picciano MF. Folate and human reproduction. American Journal of Clinical Nutrition. 2006;83:993–1016. doi: 10.1093/ajcn/83.5.993. [DOI] [PubMed] [Google Scholar]
- Wiles NJ, Northstone K, Emmett P, Lewis G. 'Junk food' diet and childhood behavioural problems: results from the ALSPAC cohort. European Journal of Clinical Nutrition. 2009;63:491–498. doi: 10.1038/sj.ejcn.1602967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S, Hansen DK, Horsley ET, Tang YS, Khan RA, Stabler SP, Jayaram HN, Antony AC. Maternal folate deficiency results in selective upregulation of folate receptors and heterogeneous nuclear ribonucleoprotein-E1 associated with multiple subtle aberrations in fetal tissues. Birth Defects Research. Part A, Clinical and Molecular Teratology. 2005;73:6–28. doi: 10.1002/bdra.20105. [DOI] [PubMed] [Google Scholar]
- Zhou SJ, Gibson RA, Crowther CA, Baghurst P, Makrides M. Effect of iron supplementation during pregnancy on the intelligence quotient and behavior of children at 4 y of age: long-term follow-up of a randomized controlled trial. American Journal of Clinical Nutrition. 2006;83:1112–1117. doi: 10.1093/ajcn/83.5.1112. [DOI] [PubMed] [Google Scholar]