Abstract
Background
Gallbladder pathology (GBP) is a relatively uncommon, naturally occurring morbidity in both baboons and humans.
Methods
A retrospective analysis was performed on 7,776 necropsy reports over a twenty year period to determine the prevalence of baboon GBP.
Results
Ninety-seven cases of GBP were identified, yielding a twenty year population prevalence of 1.25%. GBP is more common in adult female baboons, occurring with a female to male ratio of nearly 2:1. Among gallbladder pathologies, cholecystitis (35.1%) and cholelithiasis (29.9%) were the most prevalent abnormalities, followed by hyperplasia (16.5%), edema (15.5%), amyloidosis (5.2%), fibrosis (4.1%), necrosis (4.1%, and hemorrhage (1.0%).
Conclusion
Many epidemiologic similarities exist between GBP in baboons and humans suggesting that the baboon may serve as a reliable animal model system for investigating GBP in humans.
Keywords: nonhuman primate, cholelithiasis, bile, cholecystitis, gallstones, Papio
Introduction
Gallbladder pathology (GBP) in humans affects approximately 10% of the adult population aged forty years and older in developed countries [17, 42]. Risk factors for GBP include gender, obesity, pregnancy, weight loss, high cholesterol, family history of GBP, and ethnicity [14, 18, 26–31, 33]. GBP has been linked to a variety of other diseases, including metabolic diseases, liver disease, pancreatic disease, renal disease, and diseases of the small intestine [2, 4, 5, 7, 11, 15, 19, 20, 32, 36]. The primary clinical manifestation of GBP in humans is cholelithiasis, the development of gallstones. In humans, the prevalence of gallstones is higher in females [29, 46], and only 20–30% of gallstones are symptomatic [9].
Gallstones are characterized by stone composition and are classified as either cholesterol, pigment, or mixed gallstones [39]. Cholesterol gallstones are most prevalent in the western hemisphere, account for greater than 80% of human clinical cases, and have been linked with obesity, sudden weight loss, diabetes, and pregnancy [16]. By definition, cholesterol gallstone composition is greater than 50% crystalline cholesterol monohydrate by weight. These crystals form when cholesterol concentrations rise to a level that surpasses bile’s solubilizing capacity. As a result, supersaturation causes cholesterol to nucleate, forming gallstones [16]. Pigment stones are composed of greater than 50% bilirubin calcium salts and are more common in patients with cirrhosis. Bacteria may also play a role in stone pathogenesis through the hydrolyzation of bilirubin glucuronide [16, 39].
Cholecystitis occurs 90% of the time due to the obstruction of the neck of the gallbladder or the cystic duct by gallstones, leading to inflammation of the gallbladder [16]. If obstruction of the pancreatic duct occurs, pancreatitis may result [41].
Baboons (Papio species) have previously been used to investigate mechanisms of GBP, possible drug interventions, and treatment modalities. GBP studies in baboons have examined the composition of gallstones, biliary metabolism, and pregnancy in induced models of GBP [24, 25, 34]. In one study of natural occurring GBP in baboons, investigators demonstrated that the lipid composition of both gallbladder and hepatic bile was identical to that of human bile [25].
To determine the extent to which baboons with spontaneous GBP elicit similar disease profiles to humans, we examined the prevalence of spontaneous GBP as well as concurrent lesions in other tissues in baboons, and compared these to the human literature. In our study, GBP in baboons was diagnosed at necropsy through both gross and histological pathology.
Materials and Methods
Animals
Data was obtained from baboons (Papio spp.) that were part of the colony housed at the Southwest National Primate Research Center (SNPRC) at the Southwest Foundation for Biomedical Research (SFBR), in San Antonio, TX. The baboons were housed in metal and concrete indoor-outdoor cages, outdoor corrals, or individual metal cages. They were fed a commercial primate diet, supplemented with fruits and vegetables, and water was available ad libitum. The baboons were normal members of the breeding colony and not used experimentally. If there was any question that the pathological lesions could be related to experimental use of an animal, that animal was excluded from our study. All animal care and procedures were approved by the SFBR Institutional Animal Care and Use Committee.
Record Review
We have defined our population as the 7,776 baboons necropsied over a twenty year period (1986–2006) at SNPRC. A complete necropsy is performed on each baboon that dies at SNPRC. Since, the death of a given baboon is considered a random event, the 7,776 baboons necropsied serve as a good representation of the living baboon population. Thus, we were able to calculate the prevalence of GBP, without any selection bias.
A complete record search was performed using an internal anatomical pathology database (apath) for all diagnoses of GBP among the population. There were 114 diagnoses of GBP in 97 baboons, which were confirmed via both gross and histological examination of postmortem tissues. Complete medical records including gender, weight, date of birth, date of death, age, and gallbladder pathology were available for all 97 animals and used for the epidemiologic characterization. Lesions were classified as one or more of the following: cholelithiasis, cholecystitis (lymphocytic, neutrophilic, or mixed cell population), hyperplasia, edema, amyloidosis, fibrosis, necrosis, and hemorrhage.
The baboons were separated into one of three age groups: juvenile (0–5 yrs of age), adult (6–19 yrs of age), and geriatric (20+ yrs of age). In order to calculate the percentage of weight lost, the body weight at the time of necropsy was compared to the body weight of the baboon recorded nearest to one year prior to necropsy.
Pathology
A complete necropsy was performed on all baboons after death, including the 97 baboons diagnosed with GBP. For each animal, tissue samples were fixed in 10% neutral buffered formalin, processed conventionally, and embedded in paraffin. Tissue blocks were cut at 4 μm, stained with hematoxylin and eosin (H&E), and evaluated with light microscopy by board- certified veterinary pathologists (GBH or EJD Jr.).
Gallstone Analysis
Gallstones from three baboons were submitted for integrated crystallographic analysis by the Louis C. Herring Company, Orlando, FL, USA.
Statistical Analyses
The prevalence was determined for baboon GBP. Prevalence was defined as the total number of baboons with GBP divided by the total number of baboons necropsied over the twenty year period.
Results
Ninety-seven cases of spontaneously occurring GBP were identified in a retrospective analysis of 7,776 necropsy reports from a population of captive baboons at SFBR over a twenty year period. The overall prevalence of GBP for the colony was 1.25%.
Of the 97 baboons diagnosed with GBP, it was found that GBP was most common in adult baboons (post-maturity, 6–19 years of age), comprising 56.7% of the GBP total, as compared to just 12.4% for juvenile (0–5 years of age) and 30.9% for geriatric baboons (20+ years of age) (Table 1). Furthermore, GBP is more prevalent in female baboons than in males (nearly 2:1); 1.6% of females (67 of 4,157) had GBP compared to only 0.8% of males (30 of 3,619). Twenty-three (23.7%) baboons with GBP experienced significant weight loss (>15% total body weight) within the last year of life.
Table 1.
GBP stratified by age.
| Age | Affected (n) | % of Necropsied baboons with GBP; (% of GBP stratified by age) |
|---|---|---|
| Juvenile (0–5 yrs of age) | 12 | 0.15%; (12.4%) |
| Adult (6–19 yrs of age) | 55 | 0.71%; (56.7%) |
| Geriatric (20+ yrs of age) | 30 | 0.39%; (30.9%) |
The most common GBP (Fig. 1) was cholecystitis, comprising 35.1% of the colony’s GBP (%GBP) and a prevalence (P) of 0.44%, and cholelithiasis (%GBP = 29.9%; P = 0.37%). Additional manifestations of GBP included hyperplasia (%GBP = 16.5%; P = 0.21%), edema (%GBP = 15.5%; P = 0.19%), amyloidosis (%GBP = 5.2%; P = 0.06%), fibrosis (%GBP = 4.1%; P = 0.05%), necrosis (%GBP = 4.1%; P = 0.05%), and hemorrhage (%GBP = 1.0%; P = 0.01%).
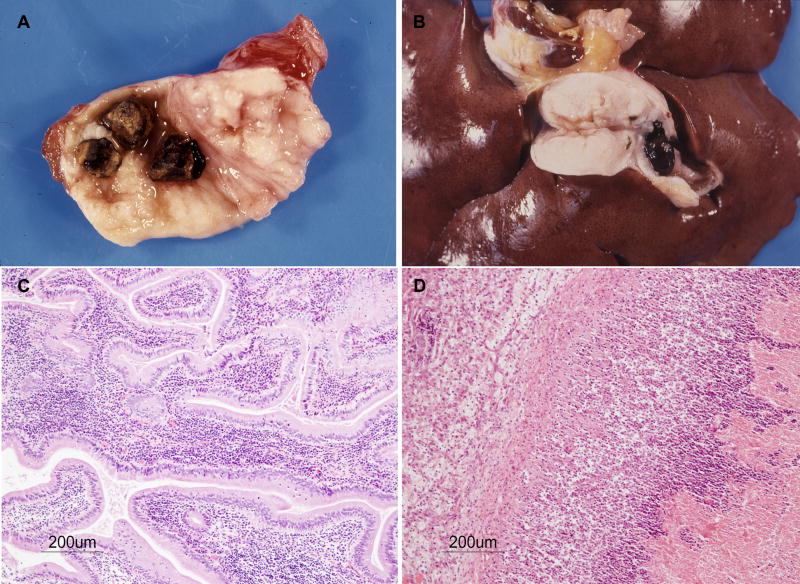
Figure 1.
Baboon GBP. A and B: Gallstones. Note severe edema and fibrosis in A and B, and obstruction of the gallbladder in B. C: Lymphocytic cholecystitis. H&E. D: Neutrophilic cholecystitis and necrosis of the gallbladder. H&E.
Concomitant organ pathologies (Table 2) associated with GBP included liver, kidney, and pancreas pathology, with a population prevalence of 0.63%, 0.54%, and 0.14%, respectively. Of the baboons with concomitant liver disease, 42.9% had hepatitis (a population prevalence of .27%). At 45.2%, nephritis was the most frequent finding in baboons with both GBP and kidney disease (a population prevalence of .24%).
Table 2.
Distribution of naturally occurring GBP in baboons.
| Male (n = 3619) |
Female (n = 4157) |
Total (n = 7776) |
||||
|---|---|---|---|---|---|---|
| GBP Status | n | % | n | % | n | % |
| No GBP | 3,589 | 46.15 | 4,090 | 52.60 | 7,679 | 98.75 |
| GBP | 30 | 0.83 | 67 | 1.61 | 97 | 1.25 |
| GBP + Liver Disease | 17 | 0.47 | 32 | 0.77 | 49 | 0.63 |
| GBP + Kidney Disease | 11 | 0.30 | 31 | 0.75 | 42 | 0.54 |
| GBP + Pancreatic Disease | 2 | 0.06 | 9 | 0.22 | 11 | 0.14 |
Prevalence of GBP within the colony, stratified by gender and by concomitant pathology.
Integrated crystallographic analysis of the gallstones indicated that the calculi from the three baboons evaluated were of the same composition: predominantly cholesterol with small amounts of calcium bicarbonate and calcium carbonate.
Discussion
We conducted a record review of 7,776 baboons that were necropsied over a twenty year period. We found the prevalence of GBP (1.25%) was lower in our captive population of baboons than what is reported in the human population in the U.S. (14.63%) [12]. Many characteristics of baboon GBP are similar to human GBP, such as the majority of cases occurring in females. Like humans, this ratio of females to males is very close to 2:1. Nearly one quarter of the baboons exhibited significant weight loss (>15% body weight) in the year before death. While many factors may have contributed to this weight loss, it has been shown that a significant weight loss can exacerbate GBP in humans [1, 10, 37].
The most common GBP in baboons and humans are cholelithiasis and cholecystitis. In humans, 95% of GBP is attributed to cholelithiasis [16], but it accounts for only 29.9% of GBP in our baboon colony. The other major GBP is cholecystitis, responsible for approximately 5% of GBP in humans, but 35.1% in baboons.
In humans, GBP is most prevalent from ages 20–74. According to the Sirmione study [3], which evaluated GBP among various age groups in humans, age 40 represents the cut-off between relatively low and high rates of GBP that necessitate cholecystectomies. In fact, the prevalence between ages 40 to 69 was found to be four times higher than that in younger subjects. Likewise, the prevalence in adult baboons was also approximately 4.5 times higher than in juvenile baboons. Thus, we are able to conclude that baboons along with humans have their highest prevalence of GBP in their adult years.
Lastly, each of the gallstones sent for integrated crystallographic analysis was composed predominantly of cholesterol, which is the most prevalent type of gallstone found in humans.
Further studies may examine how risk factors of GBP in humans, such as pregnancy, high-fat diet, and bacterial co-infection, correlate with GBP in baboons. Another avenue of future study is the genetic predisposition to developing GBP, which has been shown in humans [17].
Acknowledgments
We thank Marie Silvia, Michelle Hohmann, and Denise Trejo for their support in necropsy and tissue processing. The authors also wish to thank the clinical pathology and clinical care personnel for their support.
This research was funded in part by National Institutes of Health/National Center for Research Resources (NIH/NCRR) grant P51 RR013986 to the Southwest National Primate Research Center. Nonhuman primates were housed in facilities constructed with support from Research Facilities Improvement Program Grant C06 RR016228 from the NIH/NCRR. This study was supported by the Southwest National Primate Research Center summer student internship program (P51 RR013986).
References
- 1.Andersen T. Liver and gallbladder disease before and after very-low-calorie diets. Am J Clin Nutr. 1992;56:235S–239S. doi: 10.1093/ajcn/56.1.235S. [DOI] [PubMed] [Google Scholar]
- 2.Annese V, Vantrappen G. Gallstones in Crohn’s disease: another hypothesis. Gut. 1994;35:1676. doi: 10.1136/gut.35.11.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbara L, Sama C, Morselli-Labate AM, et al. A Ten Year Incidence of Gallstone Disease. The Sirmione Study. J Hepatol. 1993;18 (Suppl 1):S43. [Google Scholar]
- 4.Bargiggia S, Maconi G, Elli M, Molteni P, Ardizzone S, Parente F, Todaro I, Greco S, Manzionna G, Bianchi Porro G. Sonographic prevalence of liver steatosis and biliary tract stones in patients with inflammatory bowel disease: study of 511 subjects at a single center. J Clin Gastroenterol. 2003;36:417–420. doi: 10.1097/00004836-200305000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Boland L, Folsom A, Rosamond W. Hyperinsulinemia, dyslipidemia, and obesity as risk factors for hospitalized gallbladder disease. A prospective study. Ann Epidemiol. 2002;12:131–140. doi: 10.1016/s1047-2797(01)00260-5. [DOI] [PubMed] [Google Scholar]
- 6.Buhman KK, Accad M, Novak S, Choi RS, Wong JS, Hamilton RL, Turley S, Farese RV., Jr Resistance to diet-induced hypercholesterolemia and gallstone formation in ACAT2-deficient mice. Nat Med. 2000;6:1341–1347. doi: 10.1038/82153. [DOI] [PubMed] [Google Scholar]
- 7.Chang TS, Lo SK, Shyr HY, Fang JT, Lee WC, Tai DI, Sheen IS, Lin DY, Chu CM, Liaw YF. Hepatitis C virus infection facilitates gallstone formation. J Gastroenterol Hepatol. 2005;20:1416–1421. doi: 10.1111/j.1440-1746.2005.03915.x. [DOI] [PubMed] [Google Scholar]
- 8.Chow WH, Johansen C, Gridley G, Mellemkjaer L, Olsen JH, Fraumeni JF., Jr Gallstones, cholecystectomy and risk of cancers of the liver, biliary tract and pancreas. Br J Cancer. 1999;79:640–644. doi: 10.1038/sj.bjc.6690101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colecchia A, Sandri L, Bacchi-Reggiani ML, Portincasa P, Palasciano G, Mazzella G, Roda E, Festi D. Is it possible to predict the clinical course of gallstone disease? Usefulness of gallbladder motility evaluation in a clinical setting. Am J Gastroenterol. 2006;101:2576–2581. doi: 10.1111/j.1572-0241.2006.00793.x. [DOI] [PubMed] [Google Scholar]
- 10.Dickerman JL. Gallstone formation associated with weight reduction. J Am Osteopath Assoc. 1990;90:456–458. [PubMed] [Google Scholar]
- 11.Freeman HJ. Hepatobiliary and pancreatic disorders in celiac disease. World J Gastroenterol. 2006;12:1503–1508. doi: 10.3748/wjg.v12.i10.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karaliotas, Broelsch, Habib . Liver and Biliary Tract Surgery- Embryological Anatomy to 3D-Imaging and Transplant Innovations. SpringerWien; New York: [Google Scholar]
- 13.Kim J, Rim B. Cholelithiasis and hepatic adenoma in an adult male baboon (Papio sp) Vet Med Small Anim Clin. 1980;75:257–259. [PubMed] [Google Scholar]
- 14.Ko C, Beresford S, Schulte S, Lee S. Insulin resistance and incident gallbladder disease in pregnancy. Clin Gastroenterol Hepatol. 2008;6:76–81. doi: 10.1016/j.cgh.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kratzer W, Haenle MM, Mason RA, von Tirpitz C, Kaechele V. Prevalence of cholelithiasis in patients with chronic inflammatory bowel disease. World J Gastroenterol. 2005;11:6170–6175. doi: 10.3748/wjg.v11.i39.6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar V, Abbas A, Fausto N. Robbins and Cotran Pathologic Basis of Disease. 7. Philadelphia: Elsevier Inc; 2005. [Google Scholar]
- 17.Lammert F, Sauerbruch T. Mechanisms of disease: the genetic epidemiology of gallbladder stones. Nat Clin Pract Gastroenterol Hepatol. 2005;2:423–433. doi: 10.1038/ncpgasthep0257. [DOI] [PubMed] [Google Scholar]
- 18.Lapidus A, Akerlund J, Einarsson C. Gallbladder bile composition in patients with Crohn’s disease. World J Gastroenterol. 2006;12:70–74. doi: 10.3748/wjg.v12.i1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee HM, Jeffrey RB. Emphysematous pyelonephritis with resultant emphysematous cholecystitis secondary to hematogenous dissemination. Abdom Imaging. 1995;20:169–172. doi: 10.1007/BF00201531. [DOI] [PubMed] [Google Scholar]
- 20.Loria P, Lonardo A, Lombardini S, Carulli L, Verrone A, Ganazzi D, Rudilosso A, D’Amico R, Bertolotti M, Carulli N. Gallstone disease in non-alcoholic fatty liver: prevalence and associated factors. J Gastroenterol Hepatol. 2005;20:1176–1184. doi: 10.1111/j.1440-1746.2005.03924.x. [DOI] [PubMed] [Google Scholar]
- 21.Lowenfels AB, Maisonneuve P, Boyle P, Zatonski WA. Epidemiology of gallbladder cancer. Hepatogastroenterology. 1999;46:1529–1532. [PubMed] [Google Scholar]
- 22.Lowenfels AB, Walker AM, Althaus DP, Townsend G, Domellof L. Gallstone growth, size, and risk of gallbladder cancer: an interracial study. Int J Epidemiol. 1989;18:50–54. doi: 10.1093/ije/18.1.50. [DOI] [PubMed] [Google Scholar]
- 23.Maurer K, Rogers A, Ge Z, Wiese A, Carey M, Fox J. Helicobacter pylori and cholesterol gallstone formation in C57L/J mice: a prospective study. Am J Physiol Gastrointest Liver Physiol. 2006;290:G175–G182. doi: 10.1152/ajpgi.00272.2005. [DOI] [PubMed] [Google Scholar]
- 24.McSherry C, Deitrick J, May P, Niemann W, Morrissey K, Palmer R, Glenn F. Biliary lipid metabolism in the pregnant baboon. Surg Gynecol Obstet. 1977;144:727–733. [PubMed] [Google Scholar]
- 25.McSherry C, Glenn F, Javitt N. Composition of basal and stimulated hepatic bile in baboons, and the formation of cholesterol gallstones. Proc Natl Acad Sci USA. 1971;68:1564–1568. doi: 10.1073/pnas.68.7.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Méndez-Sánchez N, Bahena-Aponte J, Chávez-Tapia N, Motola-Kuba D, Sánchez-Lara K, Ponciano-Radríguez G, Ramos M, Uribe M. Strong association between gallstones and cardiovascular disease. Am J Gastroenterol. 2005;100:827–830. doi: 10.1111/j.1572-0241.2005.41214.x. [DOI] [PubMed] [Google Scholar]
- 27.Méndez-Sánchez N, Chavez-Tapia N, Motola-Kuba D, Sanchez-Lara K, Ponciano-Rodríguez G, Baptista H, Ramos M, Uribe M. Metabolic syndrome as a risk factor for gallstone disease. World J Gastroenterol. 2005;11:1653–1657. doi: 10.3748/wjg.v11.i11.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendez-Sanchez N, Chavez-Tapia N, Uribe M. Pregnancy and gallbladder disease. Ann Hepatol. 2006;5:227–230. [PubMed] [Google Scholar]
- 29.Méndez-Sánchez N, Chávez-Tapia N, Uribe M. Gallbladder disease and obesity. Gac Med Mex. 2004;140(Suppl 2):S59–S66. [PubMed] [Google Scholar]
- 30.Nakeeb A, Comuzzie A, Martin L, Sonnenberg G, Swartz-Basile D, Kissebah A, Pitt H. Gallstones: genetics versus environment. Ann Surg. 2002;235:842–849. doi: 10.1097/00000658-200206000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Novacek G. Gender and gallstone disease. Wien Med Wochenschr. 2006;156:527–533. doi: 10.1007/s10354-006-0346-x. [DOI] [PubMed] [Google Scholar]
- 32.Portincasa P, Moschetta A, Di Ciaula A, Palmieri VO, Milella M, Pastore G, Palasciano G. Changes of gallbladder and gastric dynamics in patients with acute hepatitis A. Eur J Clin Invest. 2001;31:617–622. doi: 10.1046/j.1365-2362.2001.00834.x. [DOI] [PubMed] [Google Scholar]
- 33.Puppala S, Dodd G, Fowler S, Arya R, Schneider J, Farook V, Granato R, Dyer T, Almasy L, Jenkinson C, Diehl A, Stern M, Blangero J, Duggirala R. A genomewide search finds major susceptibility loci for gallbladder disease on chromosome 1 in Mexican Americans. Am J Hum Genet. 2006;78:377–392. doi: 10.1086/500274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Redinger R, Grace D. Cholesterol gallstones and biliary lipid metabolism in the primate. Gastroenterology. 1978;74:201–204. [PubMed] [Google Scholar]
- 35.Roa I, Ibacache G, Carvallo J, Melo A, Araya J, De Aretxabala X, Figueroa M, Barrientos F, Figueroa C. Microbiological study of gallbladder bile in a high risk zone for gallbladder cancer. Rev Med Chil. 1999;127:1. [PubMed] [Google Scholar]
- 36.Ruhl C, Everhart J. Association of diabetes, serum insulin, and C-peptide with gallbladder disease. Hepatology. 2000;31:299–303. doi: 10.1002/hep.510310206. [DOI] [PubMed] [Google Scholar]
- 37.Shiffman ML, Sugerman HJ, Kellum JM, Brewer WH, Moore EW. Gallstone formation after rapid weight loss: a prospective study in patients undergoing gastric bypass surgery for treatment of morbid obesity. Am J Gastroenterol. 1991;86:1000–1005. [PubMed] [Google Scholar]
- 38.Silva C, Pereira-Lima J, Oliveira A, Guerra J, Marques D, Sarmanho L, Cabral M, Queiroz D. Association of the presence of Helicobacter in gallbladder tissue with cholelithiasis and cholecystitis. J Clin Microbiol. 2003;41:5615–5618. doi: 10.1128/JCM.41.12.5615-5618.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swidsinski A, Lee S. The role of bacteria in gallstone pathogenesis. Front Biosci. 2001;6:E93–E103. doi: 10.2741/swidsinski. [DOI] [PubMed] [Google Scholar]
- 40.Swidsinski A, Ludwig W, Pahlig H, Priem F. Molecular genetic evidence of bacterial colonization of cholesterol gallstones. Gastroenterology. 1995;108:860–864. doi: 10.1016/0016-5085(95)90461-1. [DOI] [PubMed] [Google Scholar]
- 41.Swidsinski A, Schlien P, Pernthaler A, Gottschalk U, Bärlehner E, Decker G, Swidsinski S, Strassburg J, Loening-Baucke V, Hoffmann U, Seehofer D, Hale L, Lochs H. Bacterial biofilm within diseased pancreatic and biliary tracts. Gut. 2005;54:388–395. doi: 10.1136/gut.2004.043059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Volzke H, Baumeister SE, Alte D, Hoffmann W, Schwahn C, Simon P, John U, Lerch MM. Independent risk factors for gallstone formation in a region with high cholelithiasis prevalence. Digestion. 2005;71:97–105. doi: 10.1159/000084525. [DOI] [PubMed] [Google Scholar]
- 43.Wang DQ, Paigen B, Carey MC. Genetic factors at the enterocyte level account for variations in intestinal cholesterol absorption efficiency among inbred strains of mice. J Lipid Res. 2001;42:1820–1830. [PubMed] [Google Scholar]
- 44.Wistuba II, Gazdar AF. Gallbladder cancer: lessons from a rare tumour. Nat Rev Cancer. 2004;4:695–706. doi: 10.1038/nrc1429. [DOI] [PubMed] [Google Scholar]
- 45.Zatonski WA, Lowenfels AB, Boyle P, Maisonneuve P, Bueno de Mesquita HB, Ghadirian P, Jain M, Przewozniak K, Baghurst P, Moerman CJ, Simard A, Howe GR, McMichael AJ, Hsieh CC, Walker AM. Epidemiologic aspects of gallbladder cancer: a case-control study of the SEARCH Program of the International Agency for Research on Cancer. J Natl Cancer Inst. 1997;89:1132–1138. doi: 10.1093/jnci/89.15.1132. [DOI] [PubMed] [Google Scholar]
- 46.Zhu J, Han T, Chen S, Jiang Y, Zhang S. Gallbladder motor function, plasma cholecystokinin and cholecystokinin receptor of gallbladder in cholesterol stone patients. World J Gastroenterol. 2005;11:1685–1689. doi: 10.3748/wjg.v11.i11.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]