Abstract
Whereas the tumor suppressor p53 gene is frequently mutated in most human cancers, this is not the case in human papillomavirus (HPV)-associated cancers, presumably because the viral E6 oncoprotein inactivates the p53 protein. The ability of E6 to transform cells in tissue culture and induce cancers in mice correlates in part with its ability to inactivate p53. In this study, we compared the expression of the HPV16 E6 oncogene to the conditional genetic disruption of p53 in the context of a mouse model for cervical cancer in which estrogen is a critical cofactor. Nearly all of the K14Crep53f/f mice treated with estrogen developed cervical cancer, a stark contrast to its complete absence in like-treated K14E6WTp53f/f mice, indicating that HPV16 E6 must only partially inactivate p53. p53-independent activities of E6 also contributed to carcinogenesis, but in the female reproductive tract, these activities were manifested only in the presence of the HPV16 E7 oncogene. Interestingly, treatment of K14Crep53f/f mice with estrogen also resulted in mammary tumors after only a short latency, many of which were positive for estrogen receptor α. The majority of these mammary tumors were of mixed cell types, suggestive of their originating from a multipotent progenitor. Furthermore, a subset of mammary tumors arising in the estrogen-treated, p53-deficient mammary glands exhibited evidence of an epithelial to mesenchymal transition. These data show the importance of the synergy between estrogen and p53 insufficiency in determining basic properties of carcinogenesis in hormone-responsive tissues, such as the breast and the reproductive tract.
Introduction
Infection with “high-risk” mucosal-tropic or human papilloma-viruses (HPV) is etiologically associated with the development of several human cancers, including cervical cancer (1), the second leading cause of cancer-related deaths among women worldwide (2). HPV16, one of the dozen or so high-risk HPVs, is associated with more than half of all cases of HPV-related cervical cancer (3). Two HPV genes, E6 and E7, are consistently up-regulated in human cervical cancers upon integration of the viral genome into the host chromosome (4, 5). High-risk HPV E6 and E7 are considered potent oncogenes based on their transforming (6) and immortalizing (7) activities in tissue culture systems and their capacities to induce tumors in various animal models (8–11).
High-risk HPV E6 and E7 are multifunctional proteins and are best known for their abilities to bind and inactivate p53 (12, 13) and pRb (14, 15), respectively. We previously investigated the functional contributions of both E6 and E7 in cervical cancer in transgenic mice, which express these genes either individually or in combination. These studies made use of K14E6WT and K14E7WT transgenic mice in which the HPV16 E6 and E7 oncogenes were directed in their expression from the human keratin 14 (K14) promoter, which is specifically active in the basal compartment of stratified epithelia including that of the cervix. Treatment of K14E6WT and K14E6WTE7WT transgenic mice with exogenous estrogen resulted in cervical cancer after 6 months of treatment with estrogen (16). None of the K14E6WT transgenic mice, however, developed high-grade lesions or cancers unless the treatment period was extended to 9 months (17). The ability of E6 to inactivate p53 is thought to be an important contributing factor in cervical carcinogenesis. Consistent with this premise, K14E6I128T transgenic mice, which express a mutant form of the E6 protein hindered in degrading p53 (18, 19), had fewer and smaller cervical cancers (17). This mutant form of E6, however, is also deficient in other activities, including the ability to bind cellular partners that recognize E6 through leucine-rich motifs, including E6AP and E6BP (19). Because p53 is an important tumor suppressor and presumably an important target of E6, it was of interest to investigate further the specific contribution of p53 in cervical cancer.
To determine the role of p53 in cervical carcinogenesis, we treated mice with estrogen for 6 months that had both alleles of p53 conditionally deleted via the Cre-lox system in all stratified epithelia, including the epithelia lining the reproductive tract by using Cre driven by the K14 promoter (K14Cre). We made use of conditional p53-null (Crep53f/f) mice, as mice homozygous for a germ-line deletion of p53 succumb to lymphomas and sarcomas early in life (20) and therefore were not predicted to survive the 6 months of treatment with exogenous estrogen, a required cofactor for cervical cancer in HPV transgenic mice (16, 21). Moreover, use of K14Crep53f/f mice permitted us to determine the importance of p53 inactivation specifically in the relevant epithelial cell type in which HPV infections and associated cancers arise and eliminate any potential influence that inactivation of p53 in the stroma might confer. Finally, the conditional p53 mouse model allowed us to investigate the contributions of p53-dependent and p53-independent activities of HPV E6 in tumorigenesis and whether these activities synergize with those of HPV E7.
Conditional loss of p53 in stratified epithelia led to the efficient induction of cervical cancer when K14Crep53f/f mice were treated with estrogen for 6 months. HPV E6 must not completely inactivate p53 because K14E6WT (16) or K14E6WTp53f/f (this study) mice did not develop cervical cancers when only treated with estrogen for 6 months. K14Crep53f/f mice developed nonreproductive tumors in the absence of estrogen. These tumors consisted primarily of squamous cell carcinomas and mammary tumors, similar to those found in previous studies that did not include treatment with exogenous estrogen (22–24). Interestingly, mammary tumors developed with a shortened latency in K14Crep53f/f mice treated with estrogen, were of mixed cell types, and were estrogen receptor a (ERα) positive. These observations suggest that estrogen and the loss of p53 synergize to result in early-onset tumors that are dependent on estrogen and may arise from multipotent progenitor cells in the mammary gland. Furthermore, a subset of the mammary tumors arising in the K14Crep53f/f mice treated with estrogen exhibited signs indicative of an epithelial to mesenchymal transition (EMT), including loss of E-cadherin and the expression of vimentin (25, 26). Addition of HPV E6 and/or E7 onto the K14Crep53f/f background significantly shortened tumor latency and increased tumor multiplicity in both nonreproductive and reproductive sites, indicating that p53-independent effects of E6 act together with or in some tissue contexts independently of HPV E7 to increase its oncogenic potential.
Materials and Methods
Mouse lines
The previously described p53f/f (27), K14Cre (23), K14E6WT (11), K14E6Δ146-151 (K14E6Δ herein; ref. 28), and K14E7WT (8) mouse strains were maintained on the inbred FVB background. K14Cre mice were crossed to p53f/f mice to generate K14Crep53f/f mice in which p53 is deleted by Cre-mediated excision in the basal layer of stratified epithelia. K14E6WT, K14E6Δ, and K14E7WT transgenic mice were crossed to p53f/f and then intercrossed with K14Crep53f/f mice to generate K14E6p53f/f, K14E6Δp53f/f, K14E7WTp53f/f, K14E6WTE7WTp53f/f, K14E6WTCrep53f/f, K14E6ΔCrep53f/f, K14E7WTCrep53f/f, and K14E6WTE7WTCrep53f/f mice. All mice were bred and maintained in the American Association for Accreditation of Laboratory Animal Care–approved McArdle Laboratory Animal Care Facility in accordance with an institutionally approved animal protocol.
Estrogen treatment and analysis of reproductive tracts
For acute studies, 5- to 6-wk-old virgin female mice were treated with 17β-estradiol (0.05 mg, 60-d release pellets) for 6 wk to achieve a state of persistent estrus (29) to eliminate variability in the thickness of the reproductive epithelia. The mice were then either exposed to 12 Gy of ionizing radiation from a 137Cs source or untreated and sacrificed 24 h later. One hour before sacrifice, the nucleotide analogue 5-bromo-2′-deoxyuridine (BrdUrd; 12.5 mg/mL in PBS) was injected i.p. at 10 μL/g body weight to label any newly synthesized DNA.
For long-term cancer studies, 5-wk-old virgin female mice were either untreated or treated with 17h-estradiol (0.05 mg, 60-d release pellets) for a period of 6 mo, after which the reproductive tracts were harvested and fixed according to previously described methods (16) and sectioned. Every tenth, 5-Am section was stained with H&E and evaluated histopathologically, with the worst lesion scored as the final diagnosis.
Quantification of DNA synthesis and DNA damage response
To quantify the levels of DNA synthesis in the epithelium of the cervix, basal and suprabasal BrdUrd-positive cells were counted and divided by the total number of cells and multiplied by 100 to determine the percentage. To quantify the DNA damage response, the total number of BrdUrd-positive cells was counted and divided by the total number of cells in the epidermis and multiplied by 100. BrdUrd was counted in ten 40× microscopic fields per mouse, with a total of at least three or more mice per genotype group.
Statistical analysis
The MSTAT software program1 was used in determining statistical significance. A two-sided Wilcoxon rank sum test was used for determining statistical significance in the quantification of DNA synthesis, tumor multiplicity, and tumor area. A two-sided Fisher’s exact test was used in determining significance for cancer incidence, whereas the log-rank test was used for Kaplan-Meier analyses.
Immunohistochemistry and immunofluorescence
Immunohisto-chemistry and immunofluorescence were carried out as previously described (16, 17) and described further in Supplementary Methods.
Results
p53 is efficiently deleted in the epithelium of the reproductive tract in K14Crep53f/f mice
The primary goal of this study was to determine whether inactivation of p53 in the presence of exogenous estrogen is sufficient for the induction of cervical cancer. Early development of lymphomas and other tumors in germ-line p53-null mice (20) makes it impossible to use these mice in long-term experiments. Thus, we obtained p53f/f mice that are homozygous for alleles of p53 in which loxP sites were inserted into introns 1 and 10 (27) and mated to ones that express Cre driven by the human K14 promoter (K14Cre; ref. 23). Cre mediates the excision of sequences flanked by the inverted loxP sites, resulting in a frameshift within the spliced transcript arising from the p53f/f allele. This frameshift produces a short unstable gene product that is undetectable in all K14Cre-expressing tissues (23).
To determine whether p53 was efficiently deleted in the epithelium of the reproductive tract in K14Crep53f/f mice, we tested whether these mice (treated with exogenous estrogen to induce persistent estrus and thereby eliminate variability in proliferative indices due to estrus/diestrus cycling) lacked the ability to mount a DNA damage response, an activity that requires p53 (30). To induce a DNA damage response, mice were either not treated or treated with ionizing radiation and sacrificed 24 h later. One hour before sacrifice, the mice were injected with the nucleotide analogue BrdUrd to label any newly synthesized DNA. The levels of p53 and BrdUrd were assessed by performing immunohistochemistry on reproductive tissue sections. p53 was not detected in the cervical epithelium of K14Crep53f/f mice in contrast to p53f/f mice on irradiation, indicating that p53 was deleted in these cells (Supplementary Fig. S1A). Furthermore, cervical cancers obtained from K14Crep53f/f mice did not express p53 (data not shown). The number of BrdUrd-positive cells was quantified (Supplementary Fig. S1B; Fig. 1A, left). DNA synthesis was reduced upon DNA damage in p53f/f mice (1.5% unirradiated versus 0.13% irradiated; P = 0.02), whereas in K14Crep53f/f mice the levels of DNA synthesis remained similar to that in unirradiated mice (2.9% unirradiated versus 2.1% irradiated; P = 0.28). These data provide functional proof that p53 was efficiently deleted in the cervical epithelia of the K14Crep53f/f mice.
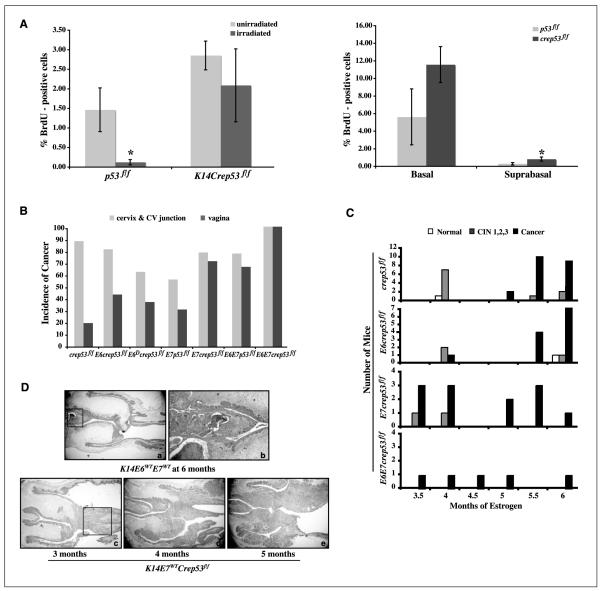
Figure 1.
Acute and long-term carcinogenic properties of p53 insufficiency in the mouse cervix. A, conditional deletion of p53 results in loss of activity and the induction of epithelial hyperplasia in the cervix. Left, quantification of BrdUrd-positive cells in the cervical epithelium in response to radiation. p53f/f mice show a significant reduction in the amount of BrdUrd-positive cells. In contrast, K14Crep53f/f mice maintained similar levels of DNA synthesis on irradiation. The decrease in DNA synthesis on radiation in p53f/f is significant (P = 0.02), whereas no significant change is observed in K14Crep53f/f mice (P = 0.28). Right, quantification of BrdUrd-positive cells in both the basal and suprabasal layers of the cervix. There is a significant increase in the levels of DNA synthesis in the suprabasal layers of K14Crep53f/f (P = 0.03), whereas the increase in the basal layers is marginal compared with p53f/f mice (P = 0.07). All acute mice were treated with estrogen for 6 wk. For pictures of immunohistochemistry, see Supplementary Fig. S1. B, comparison of cancer incidence in both the cervix and lower reproductive tract in various genotypes of mice treated with exogenous estrogen for 6 mo. C, comparison of tumor latency in various genotypes treated with estrogen and sacrificed at the indicated time points. Large aggressive cervical cancer arises after 3.5 mo of estrogen in both K14E7Crep53f/f and K14E6WTE7WTCrep53f/f mice. K14Crep53f/f mice do not develop any cancer until after 5 mo of estrogen. There is one small MIC in K14E6Crep53f/f mice at 4 mo. D, comparison of the aggressive nature of cancers in K14E7Crep53f/f mice relative to K14E6WTE7WT. Top row, large cancer from a K14E6WTE7WT mouse filling the cervical septum after 6 mo of estrogen. The tumor in a is demarcated by the black square and is magnified in b (×40). Bottom row, cancers from K14E7Crep53f/f mice taken after 3 (c), 4 (d), or 5 (e) mo of estrogen. Note the large areas of tumor invasion by 4 mo (d) and almost the entire area in the reproductive tract is covered with tumors by 5 mo (e). All images, except b, are taken with a 1.25× objective.
Deletion of p53 in the epithelium of the cervix results in an increase in the levels of DNA synthesis
As evident from the data presented above, the overall frequency of BrdUrd-positive cervical epithelial cells in unirradiated K14Crep53f/f mice (2.9%) compared with unirradiated p53f/f mice (1.5%) was increased by ~2-fold. This difference was statistically significant (P = 0.03). To characterize further the nature of the epithelial hyperplasia in K14Crep53f/f mice, the frequency of BrdUrd-positive cells within the basal versus suprabasal compartments of the cervical epithelium of K14Crep53f/f and p53f/f mice was compared (Fig. 1A, right). K14Crep53f/f mice treated with estrogen had a marginal increase in the levels of DNA synthesis in the basal layer relative to p53f/f mice (11.6% versus 5.6%, respectively; P = 0.07). The levels of suprabasal DNA synthesis, on the contrary, were significantly increased in K14Crep53f/f relative to p53f/f mice (0.8% versus 0.3%, respectively; P = 0.03). Thus, the epithelial hyperplasia that arose in the cervix of K14Crep53f/f mice is manifested in both compartments. The levels of basal and suprabasal DNA synthesis in the cervix were also quantified in p53WT and p53–/– mice treated with estrogen and showed similar results as p53f/f and K14Crep53f/f mice, respectively (data not shown).
Inactivation of p53 in the reproductive epithelium in the presence of exogenous estrogen is sufficient for carcinogenesis
Groups of p53f/f and K14Crep53f/f mice expressing E6WT, E6Δ, or E7WT were either not treated or treated with exogenous estrogen for 6 months to determine whether deletion of p53 contributes to cervical carcinogenesis. Reproductive tracts from these mice were histopathologically analyzed to identify the worst stage of disease present by using a previously established grading system (16). Similar to previous findings, none of the untreated mice irrespective of the genotype developed cervical cancer (data not shown), even in the absence of p53. These results reiterate the observation that estrogen is a necessary cofactor in this mouse model of cervical cancer (21). As expected, none of the p53-sufficient, p53f/f mice developed high-grade lesions or cancers after 6 months of treatment with estrogen. In contrast, nearly all of the estrogen-treated K14Crep53f/f mice developed cervical cancer (88%; Fig. 1B; Table 1), which is highly significant compared with estrogen-treated p53f/f mice (P < 4× 10−5). Thus, the loss of p53 in the reproductive epithelium in combination with estrogen is sufficient for the induction of cervical cancer. Similar results were observed when we analyzed the lower reproductive tract for the incidence of vaginal intraepithelial neoplasia and cancers. Specifically, K14Crep53f/f mice had high-grade lesions and cancers (Supplementary Tables S1 and S2) in the lower reproductive tract; however, compared with the cervix, these lesions were much less frequent.
Table 1.
Cervical histopathology from mice treated with exogenous estrogen
| Genotype | NH | CIN1 | CIN2 | CIN3 | MIC | LIC | Cancer incidence (%) |
LIC incidence (%) |
Tumor multiplicity |
|---|---|---|---|---|---|---|---|---|---|
| p53f/f (n = 7) | 5 | 1 | 1 | 0 | 0 | 0.00 | |||
| K14Crep53f/f (n = 25) | 1 | 2 | 13 | 9 | 88 | 36 | 2.32 | ||
| K14E6WTp53f/f (n = 22) | 10 | 9 | 3 | 0 | 0 | 0.00 | |||
| K14E6Δp53f/f (n = 10) | 3 | 5 | 1 | 1 | 0 | 0 | 0.00 | ||
| K14E6WTCrep53f/f (n = 16) | 2 | 1 | 8 | 5 | 81.3 | 31.3 | 2.00 | ||
| K14E6ΔCrep53f/f (n = 16) | 2 | 1 | 3 | 8 | 2 | 62.5 | 12.5 | 2.25* | |
| K14E7WTp53f/f (n = 16) | 1 | 1 | 1 | 4 | 7 | 2 | 56.3 | 12.5 | 1.50 |
| K14E7WTCrep53f/f (n = 14) | 1 | 1 | 1 | 3 | 8 | 78.6 | 57.1† | 4.18‡ | |
| K14E6WTE7WTp53f/f (n = 9) | 2 | 4 | 3 | 77.7 | 33 | 3.00 | |||
| K14E6WTE7WTCrep53f/f (n = 5) | 5 | 100 | 100 | ND |
NOTE: All mice were treated with estrogen. None of the K14E6WTp53f/f mice developed any high-grade lesions or cancers, which is significantly different than K14Crep53f/f mice (P < 10−9, two-sided Fisher’s exact test). Tumor multiplicity was not determined in K14E6WTE7WTCrep53f/f mice due to difficulty in delineating individual tumors.
Abbreviation: ND, not determined.
Tumor multiplicity in K14E6ΔCrep53f/f mice is not significant relative to either K14Crep53f/f or K14E6WTCrep53f/f (P > 0.05, two-sided Wilcoxon rank sum test).
Incidence of LIC in K14E7Crep53f/f is significant relative to K14E7p53f/f (P = 0.02, two-sided Fisher’s exact test).
Tumor multiplicity in K14E7Crep53f/f mice is marginally significant relative to K14E7p53f/f (P = 0.06, two-sided Wilcoxon rank sum test).
The high penetrance of cervical cancer in K14Crep53f/f mice is in sharp contrast to K14E6WTp53f/f mice, which did not develop any cancers in the reproductive tract after treatment with estrogen for 6 months (Table 1; Supplementary Table S1). The latter result is consistent with prior studies in which K14E6WT mice on p53WT/WT background also failed to develop cervical cancers after treatment with estrogen for 6 months (16). Furthermore, the incidence of cervical cancer in K14E6WT mice was only partially penetrant (41%) when treatment with estrogen was extended to 9 months (17). The weaker oncogenic potential observed in K14E6WT mice compared with K14Crep53f/f mice suggests that HPV16 E6 is only partially inactivating p53.
HPV E6 and E7 oncogenes synergize with the deletion of p53 to shorten tumor latency
Although conditional deletion of p53 in stratified epithelia extended the life span of these mice compared with that of germ-line p53-null mice, the absence of this important tumor suppressor in other epithelial tissues resulted in the development of nonreproductive tumors that contributed to early-onset morbidity/mortality (see below and Discussion). In particular, K14E7WT and K14E6WTE7WT transgenic mice on the K14Crep53f/f background were highly susceptible to early-onset morbidity/mortality aside from expected problems of bladder distension (a phenotype induced by exogenous estrogen; refs. 9, 29) and thymic hyperplasia due to the expression of E7 (8). A subset of the K14E7WTCrep53f/f and K14E6WTE7WTCrep53f/f mice consequently died or had to be sacrificed prematurely after only 3.5 to 4 months of treatment with estrogen due to the added tumor burden in addition to the aforementioned problems. Reproductive tracts were harvested at the time of sacrifice and histologic examination revealed that 75% of K14E7WTCrep53f/f and 100% of K14E6WTE7WTCrep53f/f mice had already developed cervical cancers that were quite aggressive as evidenced by the large areas of invasion. This aggressive cancer phenotype worsened with increasing duration of treatment with estrogen (Fig. 1C and D). This led us to investigate whether cancer was also developing in K14Crep53f/f and K14E6WTCrep53f/f mice during this time frame. Therefore, mice from these two genotypes were specifically sacrificed at 4 months and histopathologically examined (Fig. 1C). None of the K14Crep53f/f mice sacrificed at 4 months had developed cancer, which is significantly different than K14E7WTCrep53f/f mice (P = 0.007). One K14E6WTCrep53f/f mouse did develop a small microinvasive cancer (MIC) in the lower reproductive tract. These results suggest that the HPV oncogene E7, and to a lesser extent E6, can shorten tumor latency in the cervix in the absence of p53.
Loss of p53 results in the development of nonreproductive tumors in the skin and mammary glands, the latter with phenotypes of EMT
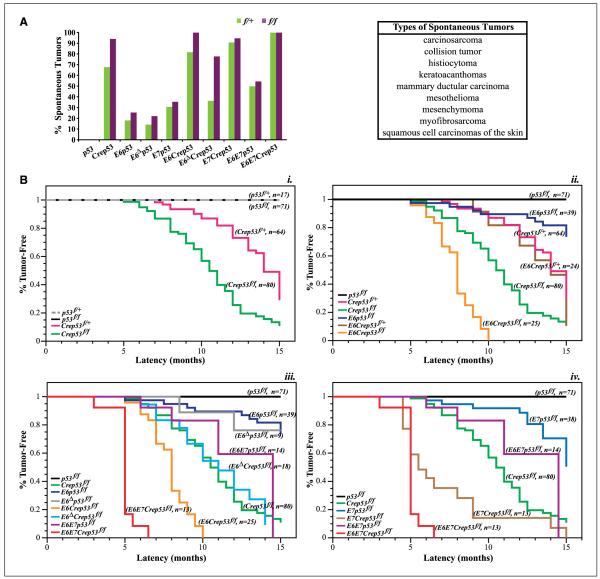
A previous study showed that conditional deletion of p53 in the basal layer of the epithelium was sufficient for the development of spontaneous tumors, such as fibrosarcomas and squamous cell carcinomas, in the skin (23). We monitored various mice over a 15-month period for the development of spontaneous tumors and observed a similar spectrum of nonreproductive tumors in our study (Fig. 2A). In addition, we observed the development of two possible cases of “collision” tumors, which are rare occurrences when two or more histologically distinct neoplasms develop adjacent to one another. To look for potential gene dosage effects on tumorigenesis and latency, we monitored mice containing one or both copies of the p53 gene disrupted. As expected, we observed an overall increase in cancer incidence (Fig. 2A) and shorter tumor latency (P =10−6; Fig. 2B, i) when both alleles of p53 were deleted. There was a highly significant reduction in median tumor latency in K14Crep53f/f mice relative to p53f/f mice (Fig. 2B, i), where the latter did not develop any tumors by the end point (median tumor latency, T50 = 10.5 versus >15 months; P < 10−24).
Figure 2.
Loss of p53 results in the development of spontaneous nonreproductive tumors. A, comparison of spontaneous tumor formation in mice of various genotypes containing either one or two floxed p53 alleles in the presence or absence of K14Cre. Cancer incidence increases when both alleles of p53 are excised. Right, list of the various types of tumors that develop in these mice. B, Kaplan-Meier curves of tumor-free survival in mice of various genotypes. i, comparison of tumor-free survival in p53f/+ (gray dashed line), p53f/f (black), K14Crep53f/+ (magenta; T50 = 14 mo), and K14Crep53f/f (green; T50 = 10.5 mo). Deletion of both alleles of p53 (K14Crep53f/f) decreases the median tumor-free survival from 14 to 10.5 mo (P =10−6). ii, comparison of genotypes from the previous graph plus K14E6WTp53f/f (blue), K14E6WTCrep53f/+ (brown; T50 = 14 mo), and K14E6WTCrep53f/f (orange; T50 = 8 mo). Again, deletion of both alleles of p53 significantly shortens tumor latency in the presence of HPV E6 (K14E6WTCrep53f/f versus K14E6WTCrep53f/+; P = 1.24 × 10−8). iii, additional comparison of K14E6Δp53f/f (gray), K14E6ΔCrep53f/f (turquoise), K14E6WTE7WTp53f/f (fuchsia), and K14E6WTE7WTCrep53f/f (red). HPV E6 synergizes with loss of p53 to shorten tumor latency in K14E6WTCrep53f/f (orange; T50 = 8 mo) relative to K14Crep53f/f (green; T50 = 10.5 mo; P = 4 × 10−8). Loss of PDZ interactions in K14E6ΔCrep53f/f does not result in a shortened latency (turquoise; T50 = 11 mo) as in K14E6WTCrep53f/f (orange; T50 = 8 mo; P = 0.0003) and is similar to K14Crep53f/f mice (P = 0.82). iv, comparison of tumor-free survival in K14E7WTp53f/f (blue), K14E7WTCrep53f/f (brown), K14E6WTE7WT (fuchsia), and K14E6WTE7WTCrep53f/f (red) mice. HPV E7 synergizes with loss of p53 to shorten tumor latency in K14E7WTCrep53f/f compared with K14E7WTp53f/f (T50 = 5.5 mo; P =5 × 10−9).
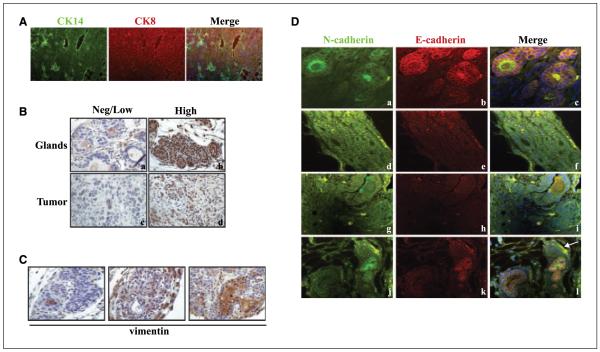
Other studies have shown that spontaneous mammary carcinomas develop when BRCA2 (23) or BRCA1 (24) is conditionally deleted in combination with p53, with a median latency of 10 and 7.5 months, respectively. We also observed the development of mammary tumors in females on the K14Crep53f/+ and K14Crep53f/f background, without concomitant deletion of BRCA. Interestingly, mammary tumors developed more quickly in mice treated with exogenous estrogen, appearing as early as 3 to 4 months. These tumors were large and aggressive as surgical removal of the tumor(s) provided a minor reprieve of ~2 weeks before a quick resurgence of growth. Furthermore, treatment with estrogen resulted in tumors that were often ERα positive (Fig. 3B), in contrast to tumors obtained in the absence of exogenous estrogen (24). In the absence of exogenous estrogen, the breeding or virgin females displayed a longer tumor latency (data not shown). Various histologic types of mammary tumors (adenocarcinomas, myoepithelial carcinomas, and spindle cell carcinomas; data not shown) were observed in estrogen-treated mice on the K14Crep53f/f background, many of which differentially expressed markers of both myoepithelial (K14) and luminal (keratin 8) cell types (Fig. 3A).
Figure 3.
Loss of p53 in the presence of estrogen results in spontaneous ERα-positive and ERα-negative mammary tumors. A, cross-sections of mammary tumors. Sections were stained by immunofluorescence for cytokeratin 8 (CK8; red) and cytokeratin 14 (CK14; green). Nuclei are shown by 4′,6-diamidino-2-phenylindole (DAPI) stain. B, cross-sections of mammary glands (a and b) attached to solid tumors (c and d) taken from mice on the K14Crep53f/f background and stained for ERα (1:100; brown nuclei). Both low and high levels of ERα were observed. C, cross-sections of mammary glands from tumors that had reduced levels of E-cadherin and evaluated for the expression of vimentin by immunohistochemistry (brown). D, cross-sections of mammary glands attached to solid tumors taken from K14Crep53f/f mice. Sections were stained by immunofluorescence for N-cadherin (green; a, d, g, and j) and E-cadherin (red; b, e, h, and k). Nuclei are shown in DAPI. Both N-cadherin and E-cadherin were observed in the majority of glands as shown in c. In a subset of tumors, expression of E-cadherin was decreased or lost (e, h, and k). The arrow in l points to a section of the gland that has lost expression of E-cadherin, whereas the connected neighboring tissue retains expression of both N-cadherin and E-cadherin.
In addition to a shortened latency, a subset of mammary tumors arising in the estrogen-treated mice displayed spindle cell type morphology or contained mixtures of both epithelial and mesenchymal cell types. We were therefore curious to determine whether there was an occurrence of EMT in these tumors. In the course of EMT, expression of E-cadherin is often reduced, and N-cadherin increased (25). Thus, the expression of both N-cadherin and E-cadherin was evaluated by immunofluorescence. The majority of these tumors expressed both N-cadherin and E-cadherin; however, a subset of these tumors irrespective of the presence of either HPV E6 or E7 displayed a loss of E-cadherin. These tumors also expressed vimentin, an intermediate filament marker up-regulated in EMT (Fig. 3C and D; ref. 26).
Cervical cancers arising in p53 conditionally deficient mice display alterations in the p16/pRb pathway
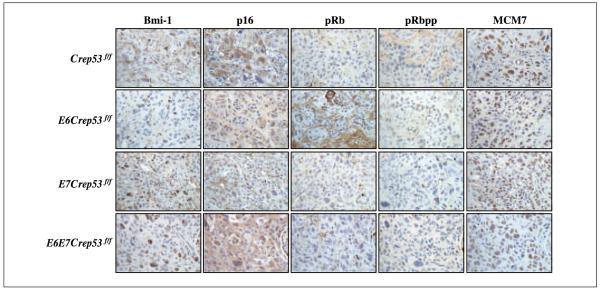
The cyclin kinase inhibitor p16 has been established as a marker for high-risk HPV-positive infection in dysplastic lesions and cancers in the reproductive tract in both humans (31) and mice (17). Moreover, HPV E7 accounts for most of the induction of p16, as tumors from K14E6WT transgenic mice express reduced levels of p16 and exhibit an inverse staining pattern compared with tumors arising in K14E7WT mice (17). The “E7” staining pattern, however, was regained when the ability of E6 to degrade p53 was hindered in K14E6I128T mice. Therefore, we hypothesized that cervical cancers arising in K14Crep53f/f mice would express low levels of p16. Immunohistochemistry for p16 was performed on tumors from mice on the K14Crep53f/f background in the presence or absence of E6 and E7. Unexpectedly, tumors from K14Crep53f/f mice predominantly overexpressed p16 (Fig. 4; Supplementary Table S3), a result opposite to that observed in tumors from K14E6WT mice (17). A small subset of K14Crep53f/f tumors, however, expressed lower amounts of p16, resembling more the pattern observed in K14E6WT mice (data not shown). Tumors from K14E6WTCrep53f/f and K14Crep53f/f mice had similar levels of p16, suggesting that deletion of p53 is dominant with regard to the induction of p16. As expected, tumors from K14E7WTCrep53f/f and K14E6WTE7WTCrep53f/f mice expressed robust levels of p16 presumably due to the presence of HPV E7.
Figure 4.
Evaluation of the p16/pRb pathway in tumors without p53. Images are cross-sections of cervical tumors taken from mice of the indicated genotypes and evaluated for levels of Bmi-1, p16, pRb, phospho-Rb (pRbpp), and MCM7 (brown nuclei) expression. Both tumors from K14Crep53f/f and K14E6WTCrep53f/f mice have low expression of Bmi-1, pRb, and phospho-Rb, whereas expression of p16 and MCM7 was higher. Both tumors from K14E7WTCrep53f/f and K14E6WTE7WTCrep53f/f mice have similar expression patterns, such that Bmi-1, p16, and MCM7 were high and expression of pRb and phospho-Rb was low (more detailed description in Supplementary Table S3).
We and others have shown that the levels of p16 and pRb are inversely correlated in both tumors and tissue culture (32, 33). Thus, immunohistochemistry for pRb was also performed to determine if the inverse correlation with p16 is maintained in tumors without p53 (Fig. 4). pRb was low but detectable in tumors from K14Crep53f/f and K14E6WTCrep53f/f mice. Consistent with the ability of E7 to inactivate and degrade pRb, the levels of pRb were decreased further or undetectable in tumors from K14E7WTCrep53f/f and K14E6WTE7WTCrep53f/f mice. These results suggest that the inverse relationship between p16 and pRb is maintained in tumors without p53.
Bmi-1 has been reported to regulate p16 through targeting of the INK4a locus (34). Bmi-1 is overexpressed in many different cancers (35, 36) and can immortalize certain epithelial cells when expressed exogenously (37). We performed immunohistochemistry to assess the status of Bmi-1 in cervical tumors (Fig. 4). Tumors from K14Crep53f/f, K14E6WTCrep53f/f, K14E7WTCrep53f/f, and K14E6WTE7WTCrep53f/f mice all expressed Bmi-1. Bmi-1 was inversely correlated with the expression of p16 in K14Crep53f/f and K14E6WTCrep53f/f tumors. In the presence of E7 (K14E7WTCrep53f/f and K14E6WTE7WTCrep53f/f), the levels of Bmi-1 were increased further but primarily retained an inverse relationship with p16.
The E2F-responsive gene MCM7 has also been established as a biomarker for dysplastic lesions and cancers positive for high-risk HPV infections (38). Induction of MCM7 likely occurs in part through the ability of E7 to target pRb for degradation, resulting in an up-regulation of E2F target genes. HPV E6 can also dysregulate the pRb pathway (39), in a manner distinct from HPV E7, leading to the up-regulation of expression of E2F target genes, such as MCM7 (17). We assessed the status of MCM7 by immunohistochemistry on tumors from this study (Fig. 4; Supplementary Table S3). The majority of tumors from K14Crep53f/f mice expressed intermediate to high levels of MCM7, consistent with the pattern observed in cervical tumors from K14E6WT mice (17). We hypothesized that the ability of E6 to hyperphosphorylate pRb (39) results in the up-regulation of the E2F-responsive gene MCM7. To determine whether E6 can hyperphosphorylate pRb in the tumors that arise in our mice, immunohistochemistry was performed with an antibody recognizing phosphorylated pRb specifically at Ser807 and Ser811, two sites that when phosphorylated can disrupt the binding of E2F to pRb (40). Tumors from both K14Crep53f/f and K14E6WTCrep53f/f expressed phosphorylated pRb, suggesting that the inactivation of pRb by phosphorylation drives the up-regulation of E2F target genes, such as MCM7, in these tumors in the absence of E7. K14E6WTCrep53f/f tumors expressed a modest qualitative increase in the levels of phosphorylated pRb relative to K14Crep53f/f tumors. This increase suggests that the ability of E6 to hyperphosphorylate pRb is in part independent of its ability to inactivate p53, consistent with previous findings (39). In the presence of E7, almost all of the pRb is degraded and only occasional pRb and phosphorylated pRb-positive cells were detected (Fig. 4).
Discussion
In this study, we compared the oncogenic potential of the HPV16 E6 oncogene to the genetic disruption of the tumor suppressor p53 in the context of a mouse model for cervical carcinogenesis that relies on treatment with exogenous estrogen. Conditional deletion of p53 was sufficient for the induction of reproductive (cervical and vaginal) cancers only in the presence of estrogen and the addition of HPV E6 and E7 shortened tumor latency and produced more aggressive tumors. In the course of this study, we also discovered that estrogen and the loss of p53 synergized to induce early onset of mammary tumors. These mammary tumors are ERα positive, composed of mixed cell types, thus suggestive of a multipotent progenitor and displayed evidence of EMT. Below, we discuss the implications of our studies in the context of understanding cervical carcinogenesis associated with HPVs as well as mammary tumorigenesis.
HPV E6 does not completely inactivate p53
We have previously shown that K14E6WT transgenic mice treated with exogenous estrogen do not succumb to the development of high-grade lesions or cancers unless the estrogen treatment period was extended from 6 to 9 months (17). In contrast, K14E7WT transgenic mice efficiently developed cancer after only 6 months of treatment with estrogen, suggesting that HPV E6 has a weaker oncogenic potential in the reproductive tract (16). These results are in stark contrast to previous observations in the epidermis of the skin, where E6 was more potent relative to E7 (10). Similar findings were observed in this study. None of the K14E6WTp53f/f mice developed high-grade lesions or cancers after 6 months of treatment with estrogen. Treatment of K14Crep53f/f mice with estrogen, however, resulted in a wide spectrum of high-grade lesions, including cancers in both the cervix (Table 1) and the lower reproductive tract (Supplementary Tables S1 and S2). Furthermore, the incidence of large invasive cancer (LIC) in the cervix of K14E7WTCrep53f/f mice (57.1%) was higher than in K14E6WTE7WTp53f/f mice (33%). Whereas this increase was not highly significant (P = 0.40), the increase in LIC in the lower reproductive tract of K14E7WTCrep53f/f mice (57.1%) compared with K14E6WTE7WTp53f/f mice (11%) was statistically significant (P = 0.04). These results not only suggest that the conditional inactivation of p53 in the reproductive epithelium is sufficient to give rise to cancer in concert with exogenous estrogen but also support the hypothesis that HPV E6 is not completely inactivating p53 in the reproductive tract.
Similar observations were drawn from monitoring the development of nonreproductive tumors. K14E6WTp53f/f mice were significantly less tumor prone as evidenced by a reduced hazard ratio (0.17), cancer incidence (25.6%), and tumor multiplicity (0.3) compared with that observed in K14Crep53f/f mice (all P < 0.0001; Table 2). Thus, HPV E6 is incompletely inactivating p53 at all evaluated tissue sites. The analysis of human cervical cancers has led to the same conclusion in that some human cervical cancers still express detectable p53 protein, indicative of E6 not completely degrading it (41, 42). Thus, our mouse model for cervical cancer recapitulates a feature of human cervical cancer in that p53 is not completely inactivated by E6.
Table 2.
Comparison of spontaneous nonreproductive tumor development
| Genotype | Cancer incidence (%) |
Tumor multiplicity |
Median tumor latency (mo) |
|---|---|---|---|
| p53f/f (n = 71) | 0 | 0 | 0 |
| K14Crep53f/f (n = 80) | 94.1 | 2.41 | 10.5 |
| K14E6WTp53f/f (n = 39) | 25.6* | 0.30* | -* |
| K14E6Δp53f/f (n = 9) | 22.2* | 0.25* | - |
| K14E6WTCrep53f/f (n = 25) | 100 | 3.88† | 8† |
| K14E6ΔCrep53f/f (n = 18) | 77.8‡ | 2.57 | 11‡ |
| K14E7WTp53f/f (n = 38) | 35.5§ | 1.27§ | -§ |
| K14E7WTCrep53f/f (n = 24) | 94.7 | 2.33 | 5.5* |
| K14E6WTE7WTp53f/f (n = 14) | 54.5 | 1.40 | - |
| K14E6WTE7WTCrep53f/f (n = 13) | 100 | 4.17 | 5 |
NOTE: All statistics were two sided (further descriptions in Materials and Methods). These mice were not treated with estrogen.
Significant relative to K14Crep53f/f (P < 0.0001).
Significant relative to K14Crep53f/f (P = 0.04).
Significant relative to K14E6WTCrep53f/f (P = 0.02).
Significant relative to K14E7WTCrep53f/f (P < 0.001).
Differences in the oncogenic potential of E6 among epithelial tissues
Whereas E6 (K14E6WTp53f/f) did induce nonreproductive tumors (Table 2), it failed to induce any reproductive tumors (Table 1; Supplementary Table S1). This indicates that E6 has stronger oncogenic potential in nonreproductive sites. Tumor development in nonreproductive sites, in part, may require a lower threshold of inactive p53. Alternatively, p53-independent activities of E6 may be more influential for tumor development in nonreproductive sites compared with that in the reproductive tract. The latter possibility is supported by the earlier onset of tumors in nonreproductive sites in K14E6WTCrep53f/f compared with that in K14Crep53f/f mice (Fig. 2B, ii).
The E7 oncogene synergizes with the loss of p53 to induce cancer
p53 is regarded as the “guardian of the genome” (43) because it mediates the response of DNA damage and other stress response pathways. Our studies herein show that the deletion of p53 is sufficient to cause the induction of LICs of the reproductive tract in the presence of exogenous estrogen. Prior studies of HPV transgenic mice, however, determined that induction of LIC in the reproductive tract was highest when both HPV E6 and E7 were expressed (16). Thus, we investigated whether placement of HPV E7 onto the K14Crep53f/f background led to a further increase in LIC. Indeed, the incidence of LIC in the cervix (Table 1) was significantly higher in K14E7WTCrep53f/f relative to K14E7WTp53f/f (57.1% versus 12.5%, respectively; P = 0.02). The difference in the incidence of LIC in the lower reproductive tract (Supplementary Table S2) was even greater (57.1% versus 0%; P = 0.002). In addition, HPV E7 shortened tumor latency and fostered more aggressive tumor progression when placed on the p53-deficient genetic background (Fig. 1C and D). Thus, HPV E7 cooperates with the loss of p53 to increase cancer incidence, shorten tumor latency, and promote extensive tumor invasion.
p53-independent activities of HPV E6 contribute to the development of nonreproductive tumors independent of HPV E7 but only contribute to cervical carcinogenesis in the presence of HPV E7
The spectrum of histopathology observed in the K14E6WTCrep53f/f and K14Crep53f/f mice was quite similar for the cervix, indicating that E6 is not contributing any p53-independent effects. The p53-independent activities of E6, however, became apparent in the presence of E7. Whereas only 57% of the K14E7WTCrep53f/f mice developed LIC, 100% of the K14E6WTE7WTCrep53f/f mice developed LIC (Table 1). These results indicate that the p53-independent activities of E6 contribute to cervical carcinogenesis but only do so in synergy with some function(s) of HPV E7. This synergy was observed previously with mice on the p53-sufficient background (17). Interestingly, in the lower reproductive tract, the p53-independent activities of E6 by itself were weakly evident, as evidenced by the development of LIC in K14E6WTCrep53f/f, which was not observed in the K14Crep53f/f mice, and a significantly higher tumor multiplicity (P = 0.05) in K14E6WTCrep53f/f mice (Supplementary Table S2). In the presence of E7, the cooperative effects of the p53-independent activities of E6 were again manifested in the lower reproductive tract, as evidenced by the higher incidence of LIC in K14E6WTE7WTCrep53f/f mice (Supplementary Table S2). These observations reiterate the synergy between the p53-independent activities of E6 and some function(s) of E7.
Although p53-independent effects of E6 were only weakly evident in the lower reproductive tract and completely absent in the cervix, we clearly observed these effects, independent of E7, in nonreproductive sites. Because deletion of p53 resulted in a high incidence of cancers at other sites (Fig. 2A), we did not observe dramatic increases in incidence in the presence of E6 and E7. However, we did note p53-independent effects of E6 when we compared median tumor latency (T50 = 8 versus 10.5 months; P < 0.0001; Table 2; Fig. 2B, ii) and tumor multiplicity in K14E6WTCrep53f/f mice relative to that in K14Crep53f/f mice (3.9 versus 2.6, respectively; P = 0.04). Similar to the cervix, these p53-independent effects of E6 are likely to reflect interactions with PDZ partners given that K14E6ΔCrep53f/f mice do not display the same increase in tumor multiplicity or shortened tumor latency. Tumor multiplicity in these mice was 2.6 and median tumor latency was 11 months (Fig. 2B, iii), which were not significantly different from that observed in the K14Crep53f/f mice (P > 0.05 for both comparisons). These results suggest that in nonreproductive sites p53-independent effects of HPV E6 enhance the oncogenic potential of E6 even in the absence of E7, whereas in the reproductive tract p53-independent effects of E6 largely (in the lower reproductive tract) or entirely (in the cervix) require the presence of HPV E7.
As indicated above, these p53-independent activities of E6 likely include the interactions of E6 with PDZ partners. Specifically, we previously found that K14E6Δ mice, which express a form of E6 unable to bind PDZ domain partners, have similar incidences of cancer and tumor sizes as K14E6WT mice when treated with estrogen for 9 months. K14E6ΔE7WT mice, however, were reduced in both tumor multiplicity and tumor size when compared with K14E6WTE7WT mice (17). Thus, the interactions of E6 with PDZ partners synergize with some activity of HPV E7 in inducing cervical cancer. The specific activities of E7 necessary for this synergy with the p53-independent activities of E6 have yet to be determined. Human cervical cancers have altered localization and expression of Dlg and Scribble (44, 45), two PDZ partners of E6. Based on the aforementioned findings, the roles of Dlg and Scribble warrant further investigation to determine whether they are indeed modulated by E6 as part of its p53-independent effects that synergize with E7 in cervical carcinogenesis.
Modulation of the p16/pRb pathway
Analysis of various biomarkers surprisingly revealed that cervical cancers from K14Crep53f/f mice have similar expression patterns as tumors expressing HPV E7. From previous results, we expected an expression pattern similar to K14E6WT tumors, where levels of p16 were low and levels of pRb were high. Instead, we observed predominantly elevated levels of p16 and low levels of pRb. Observations from this study show that HPV E6 does not completely inactivate p53. We conclude that the complete inactivation of p53 in K14Crep53f/f mice alters the mechanism of regulating the p16/pRb pathway differently from that caused by HPV E6, which only partially inactivates p53. Tumors in the K14Crep53f/f mice also express low levels of Bmi-1 and pRb, indicating that there is a lack of cell cycle checkpoints and regulation of p16 activity, at least by Bmi-1. An inverse relationship between Bmi-1 and p16 has been observed in some (35, 36) but not all (46) human cancers. In our studies of cervical tumors on a p53-deficient background, we also saw an inverse relationship but with converse results (i.e., we observed heightened levels of p16 and modest levels of Bmi-1). Furthermore, pRb that was observed in the p53-deficient tumors included forms that were phosphorylated and correlated with heightened levels of the E2F-responsive gene MCM7. Thus, in tumors arising in p53-deficient epithelial tissues of mice treated with estrogen, the p16/pRb pathway is grossly dysregulated.
Loss of p53 synergizes with estrogen to induce a shortened latency for the development of mammary tumors, some with properties of EMT
Originally, mammary tumors were not detected in K14Crep53f/f mice without conditional deletion of BRCA2 (23). However, recent follow-up studies determined that spontaneous breast cancers do develop in K14Crep53f/f mice, with a median latency of 11 months (22), or when either a MMTV- or WAP-directed Cre was used to disrupt the floxed p53 allele (47). In our studies, we also observed mammary tumors in female K14Crep53f/f in the absence of any deletion of BRCA2 or BRCA1. Importantly, we also observed that treatment of K14Crep53f/f mice with exogenous estrogen greatly shortened the time of onset of mammary tumors. Similar findings for p53 and breast cancer have been observed in transplantation studies of p53-null mammary glands into p53 wild-type BALB/c mice where exogenous hormones, such as estrogen, significantly shortened tumor latency relative to untreated mice (48). These observations suggest that exogenous estrogen is driving proliferation and enhancing tumorigenicity in the mammary gland deleted for p53.
Approximately 70% of all human breast cancers are ERα positive (49) and many of these tumors are estrogen dependent. Estrogen dependency is not frequently observed in mouse models for human breast cancer. In our study, exogenous estrogen not only shortened tumor latency but also resulted in a shift in the nuclear hormone receptor ERα status from completely negative (23) to predominantly positive (this study; Fig. 3B). These results lead us to predict that our mouse model system produces estrogen-dependent mammary tumors. The mammary tumors displayed characteristics of both myoepithelial and luminal cell types consistent with the tumors arising from a multipotent progenitor cell. Furthermore, the loss of p53 and exogenous estrogen resulted in phenotypes suggestive of an EMT occurrence in a subset of the mammary tumors. EMT is generally associated with a more aggressive disease, as cancer cells can migrate and metastasize to other sites to establish secondary tumors. These results suggest that estrogen not only increased tumor incidence and shortened tumor latency but also altered the tumor phenotype to one more representative of human breast cancer.
Supplementary Material
Acknowledgments
Grant support: CA098428, CA022443, and CA009135.
We thank Drs. Caroline Alexander, Norman Drinkwater, and Bill Sugden for critical reading of this manuscript; members of the Lambert laboratory for insightful discussions; Dr. Caroline Alexander for assistance in the analysis of mammary tumors; Dr. Chris Bradfield for access to equipment for tumor measurements; and Dr. Anton Berns for sharing the K14Cre and p53f/f mouse strains.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
References
- 1.zur Hausen H. Papillomavirus infections—a major cause of human cancers. Biochim Biophys Acta. 1996;1288:F55–78. doi: 10.1016/0304-419x(96)00020-0. [DOI] [PubMed] [Google Scholar]
- 2.Sankaranarayanan R, Ferlay J. Worldwide burden of gynaecological cancer: the size of the problem. Best Pract Res Clin Obstet Gynaecol. 2006;20:207–25. doi: 10.1016/j.bpobgyn.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 4.Durst M, Kleinheinz A, Hotz M, Gissmann L. The physical state of human papillomavirus type 16 DNA in benign and malignant genital tumours. J Gen Virol. 1985;66:1515–22. doi: 10.1099/0022-1317-66-7-1515. [DOI] [PubMed] [Google Scholar]
- 5.Jeon S, Lambert PF. Integration of human papilloma-virus type 16 DNA into the human genome leads to increased stability of E6 and E7 mRNAs: implications for cervical carcinogenesis. Proc Natl Acad Sci U S A. 1995;92:1654–8. doi: 10.1073/pnas.92.5.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munger K, Phelps WC, Bubb V, Howley PM, Schlegel R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989;63:4417–21. doi: 10.1128/jvi.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hawley-Nelson P, Vousden KH, Hubbert NL, Lowy DR, Schiller JT. HPV16 E6 and E7 proteins cooperate to immortalize human foreskin keratinocytes. EMBO J. 1989;8:3905–10. doi: 10.1002/j.1460-2075.1989.tb08570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herber R, Liem A, Pitot H, Lambert PF. Squamous epithelial hyperplasia and carcinoma in mice transgenic for the human papillomavirus type 16 E7 oncogene. J Virol. 1996;70:1873–81. doi: 10.1128/jvi.70.3.1873-1881.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arbeit JM, Howley PM, Hanahan D. Chronic estrogen-induced cervical and vaginal squamous carcinogenesis in human papillomavirus type 16 transgenic mice. Proc Natl Acad Sci U S A. 1996;93:2930–5. doi: 10.1073/pnas.93.7.2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song S, Liem A, Miller JA, Lambert PF. Human papillomavirus types 16 E6 and E7 contribute differently to carcinogenesis. Virology. 2000;267:141–50. doi: 10.1006/viro.1999.0106. [DOI] [PubMed] [Google Scholar]
- 11.Song S, Pitot HC, Lambert PF. The human papillomavirus type 16 E6 gene alone is sufficient to induce carcinomas in transgenic animals. J Virol. 1999;73:5887–93. doi: 10.1128/jvi.73.7.5887-5893.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76–9. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- 13.Huibregtse JM, Scheffner M, Howley PM. A cellular protein mediates association of p53 with the E6 oncoprotein of human papillomavirus types 16 or 18. EMBO J. 1991;10:4129–35. doi: 10.1002/j.1460-2075.1991.tb04990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dyson N, Howley PM, Munger K, Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989;243:934–7. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- 15.Chellappan S, Kraus VB, Kroger B, et al. Adenovirus E1A, simian virus 40 tumor antigen, and human papillomavirus E7 protein share the capacity to disrupt the interaction between transcription factor E2F and the retinoblastoma gene product. Proc Natl Acad Sci U S A. 1992;89:4549–53. doi: 10.1073/pnas.89.10.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riley RR, Duensing S, Brake T, Munger K, Lambert PF, Arbeit JM. Dissection of human papillomavirus E6 and E7 function in transgenic mouse models of cervical carcinogenesis. Cancer Res. 2003;63:4862–71. [PubMed] [Google Scholar]
- 17.Shai A, Brake T, Somoza C, Lambert PF. The human papillomavirus E6 oncogene dysregulates the cell cycle and contributes to cervical carcinogenesis through two independent activities. Cancer Res. 2007;67:1626–35. doi: 10.1158/0008-5472.CAN-06-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen M, Song S, Liem A, Androphy E, Liu Y, Lambert PF. A mutant of human papillomavirus type 16 E6 deficient in binding α-helix partners displays reduced oncogenic potential in vivo. J Virol. 2002;76:13039–48. doi: 10.1128/JVI.76.24.13039-13048.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Chen JJ, Gao Q, et al. Multiple functions of human papillomavirus type 16 E6 contribute to the immortalization of mammary epithelial cells. J Virol. 1999;73:7297–307. doi: 10.1128/jvi.73.9.7297-7307.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donehower LA, Harvey M, Slagle BL, et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–21. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 21.Brake T, Lambert PF. Estrogen contributes to the onset, persistence, and malignant progression of cervical cancer in a human papillomavirus-transgenic mouse model. Proc Natl Acad Sci U S A. 2005;102:2490–5. doi: 10.1073/pnas.0409883102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Derksen PW, Liu X, Saridin F, et al. Somatic inactivation of E-cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell. 2006;10:437–49. doi: 10.1016/j.ccr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 23.Jonkers J, Meuwissen R, van der Gulden H, Peterse H, van der Valk M, Berns A. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet. 2001;29:418–25. doi: 10.1038/ng747. [DOI] [PubMed] [Google Scholar]
- 24.Liu X, Holstege H, van der Gulden H, et al. Somatic loss of BRCA1 and p53 in mice induces mammary tumors with features of human BRCA1-mutated basal-like breast cancer. Proc Natl Acad Sci U S A. 2007;104:12111–6. doi: 10.1073/pnas.0702969104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hazan RB, Qiao R, Keren R, Badano I, Suyama K. Cadherin switch in tumor progression. Ann N Y Acad Sci. 2004;1014:155–63. doi: 10.1196/annals.1294.016. [DOI] [PubMed] [Google Scholar]
- 26.Hugo H, Ackland ML, Blick T, et al. Epithelial-mesenchymal and mesenchymal-epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213:374–83. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- 27.Marino S, Vooijs M, van Der Gulden H, Jonkers J, Berns A. Induction of medulloblastomas in p53-null mutant mice by somatic inactivation of Rb in the external granular layer cells of the cerebellum. Genes Dev. 2000;14:994–1004. [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen ML, Nguyen MM, Lee D, Griep AE, Lambert PF. The PDZ ligand domain of the human papillomavirus type 16 E6 protein is required for E6’s induction of epithelial hyperplasia in vivo. J Virol. 2003;77:6957–64. doi: 10.1128/JVI.77.12.6957-6964.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elson DA, Riley RR, Lacey A, Thordarson G, Talamantes FJ, Arbeit JM. Sensitivity of the cervical transformation zone to estrogen-induced squamous carcinogenesis. Cancer Res. 2000;60:1267–75. [PubMed] [Google Scholar]
- 30.Kastan MB, Onyekwere O, Sidransky D, Vogelstein B, Craig RW. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991;51:6304–11. [PubMed] [Google Scholar]
- 31.Klaes R, Friedrich T, Spitkovsky D, et al. Over-expression of p16(INK4A) as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int J Cancer. 2001;92:276–84. doi: 10.1002/ijc.1174. [DOI] [PubMed] [Google Scholar]
- 32.Reznikoff CA, Yeager TR, Belair CD, Savelieva E, Puthenveettil JA, Stadler WM. Elevated p16 at senescence and loss of p16 at immortalization in human papillomavirus 16 E6, but not E7, transformed human uroepithelial cells. Cancer Res. 1996;56:2886–90. [PubMed] [Google Scholar]
- 33.Tsutsui T, Kumakura S, Yamamoto A, et al. Association of p16(INK4a) and pRb inactivation with immortalization of human cells. Carcinogenesis. 2002;23:2111–7. doi: 10.1093/carcin/23.12.2111. [DOI] [PubMed] [Google Scholar]
- 34.Jacobs JJ, Kieboom K, Marino S, DePinho RA, van Lohuizen M. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature. 1999;397:164–8. doi: 10.1038/16476. [DOI] [PubMed] [Google Scholar]
- 35.Vonlanthen S, Heighway J, Altermatt HJ, et al. The bmi-1 oncoprotein is differentially expressed in non-small cell lung cancer and correlates with INK4A-ARF locus expression. Br J Cancer. 2001;84:1372–6. doi: 10.1054/bjoc.2001.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song LB, Zeng MS, Liao WT, et al. Bmi-1 is a novel molecular marker of nasopharyngeal carcinoma progression and immortalizes primary human nasopharyngeal epithelial cells. Cancer Res. 2006;66:6225–32. doi: 10.1158/0008-5472.CAN-06-0094. [DOI] [PubMed] [Google Scholar]
- 37.Kim RH, Kang MK, Shin KH, et al. Bmi-1 cooperates with human papillomavirus type 16 E6 to immortalize normal human oral keratinocytes. Exp Cell Res. 2007;313:462–72. doi: 10.1016/j.yexcr.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 38.Brake T, Connor JP, Petereit DG, Lambert PF. Comparative analysis of cervical cancer in women and in a human papillomavirus-transgenic mouse model: identification of minichromosome maintenance protein 7 as an informative biomarker for human cervical cancer. Cancer Res. 2003;63:8173–80. [PubMed] [Google Scholar]
- 39.Malanchi I, Accardi R, Diehl F, et al. Human papillomavirus type 16 E6 promotes retinoblastoma protein phosphorylation and cell cycle progression. J Virol. 2004;78:13769–78. doi: 10.1128/JVI.78.24.13769-13778.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knudsen ES, Wang JY. Dual mechanisms for the inhibition of E2F binding to RB by cyclin-dependent kinase-mediated RB phosphorylation. Mol Cell Biol. 1997;17:5771–83. doi: 10.1128/mcb.17.10.5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nair P, Nair KM, Jayaprakash PG, Pillai MR. Decreased programmed cell death in the uterine cervix associated with high risk human papillomavirus infection. Pathol Oncol Res. 1999;5:95–103. doi: 10.1053/paor.1999.0161. [DOI] [PubMed] [Google Scholar]
- 42.Huang LW, Chou YY, Chao SL, Chen TJ, Lee TT. p53 and p21 expression in precancerous lesions and carcinomas of the uterine cervix: overexpression of p53 predicts poor disease outcome. Gynecol Oncol. 2001;83:348–54. doi: 10.1006/gyno.2001.6397. [DOI] [PubMed] [Google Scholar]
- 43.Lane DP. Cancer. p53, guardian of the genome. Nature. 1992;358:15–6. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 44.Lin HT, Steller MA, Aish L, Hanada T, Chishti AH. Differential expression of human Dlg in cervical intra-epithelial neoplasias. Gynecol Oncol. 2004;93:422–8. doi: 10.1016/j.ygyno.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 45.Nakagawa S, Yano T, Nakagawa K, et al. Analysis of the expression and localisation of a LAP protein, human scribble, in the normal and neoplastic epithelium of uterine cervix. Br J Cancer. 2004;90:194–9. doi: 10.1038/sj.bjc.6601465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kang MK, Kim RH, Kim SJ, et al. Elevated Bmi-1 expression is associated with dysplastic cell transformation during oral carcinogenesis and is required for cancer cell replication and survival. Br J Cancer. 2007;96:126–33. doi: 10.1038/sj.bjc.6603529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin SC, Lee KF, Nikitin AY, et al. Somatic mutation of p53 leads to estrogen receptor α-positive and -negative mouse mammary tumors with high frequency of metastasis. Cancer Res. 2004;64:3525–32. doi: 10.1158/0008-5472.CAN-03-3524. [DOI] [PubMed] [Google Scholar]
- 48.Jerry DJ, Kittrell FS, Kuperwasser C, et al. A mammary-specific model demonstrates the role of the p53 tumor suppressor gene in tumor development. Oncogene. 2000;19:1052–8. doi: 10.1038/sj.onc.1203270. [DOI] [PubMed] [Google Scholar]
- 49.Masood S. Estrogen and progesterone receptors in cytology: a comprehensive review. Diagn Cytopathol. 1992;8:475–91. doi: 10.1002/dc.2840080508. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.