Abstract
The aim of this study was to determine if the dietary benefits of bioflavonoids are linked to the inhibition of ATP synthase. We studied the inhibitory effect of seventeen bioflavonoid compounds on purified F1 or membrane bound F1FO E. coli ATP synthase. We found that the extent of inhibition by bioflavonoid compounds was variable. Morin, silymarin, baicalein, silibinin, rimantadin, amantidin, or, epicatechin resulted in complete inhibition. The most potent inhibitors on molar scale were morin (IC50 ~0.07mM) > silymarin (IC50 ~0.11mM) > baicalein (IC50~0.29mM) > silibinin (IC50 ~0.34mM) > rimantadine (IC50 ~2.0mM) > amantidin (IC50 ~2.5mM) > epicatechin (IC50 ~4.0mM). Inhibition by hesperidin, chrysin, kaempferol, diosmin, apigenin, genistein, or rutin was partial in the range of 40–60% and inhibition by galangin, daidzein, or luteolin was insignificant. The main skeleton, size, shape, geometry, and position of functional groups on inhibitors played important role in the effective inhibition of ATP synthase. In all cases inhibition was found fully reversible and identical in both F1Fo membrane preparations isolated purified F1. ATPase and growth assays suggested that the bioflavonoids compounds used in this study inhibited F1-ATPase as well as ATP synthesis nearly equally, which signifies a link between the beneficial effects of dietary bioflavonoids and their inhibitory action on ATP synthase.
Keywords: E. coli ATP synthase, F1Fo-ATP synthase, F1-ATPase, ATP synthesis, bioflavonoids, biological nanomotor
Introduction
Membrane bound F1Fo ATP synthase from mitochondria, chloroplast, and bacteria is responsible for ATP production through oxidative phosphorylation or photophosphorylation. This enzyme is structurally identical and highly conserved in different species. In its simplest form in the ~530 kDa Escherichia coli F1Fo ATP synthase contains eight different subunits namely α3β3γδεab2c10–15. F1 corresponds to α3β3γδε and Fo to ab2c10. ATP hydrolysis and synthesis occur on three catalytic sites in the F1 sector, whereas proton transport occurs through the membrane embedded Fo [1–2]. The γ subunit is part of the “rotor” which is composed of γ, ε, and a ring of c subunits. The “stator” is composed of b2δ. The function of the stator is to prevent co-rotation of catalytic sites as well as the a subunit with the rotor [3–4]. Proton gradient-driven clockwise rotation of γ (as viewed from the membrane) leads to ATP synthesis and anticlockwise rotation of γ results from ATP hydrolysis. The mechanism is essentially a rotary motor and in fact it is the smallest known biological nanomotor. Detailed reviews of ATP synthase structure and function may be found in references [5–11].
ATP synthase is implicated directly or indirectly in several human diseases such as Leigh syndrome, ataxia, Batten’s diseases, Alzheimer’s, angiogenesis, and increased blood pressure etc ([11] and references therein). This enzyme is not only implicated to many disease conditions but is likely to contribute to new therapies for multiple diseases such as, cancer, heart disease, mitochondrial diseases, immune deficiency, cystic fibrosis, diabetes, ulcers, and tuberculosis that affect both people and animals [12–13]. The presence of ATP synthase on the surfaces of multiple cell types, and its involvement in a number of cellular processes, makes this enzyme an attractive molecular target, in the development of treatments for numerous diseases. A wide range of natural and synthetic products are known to bind and inhibit ATP synthase [11, 13–15] and biochemical and structural studies of ATP synthase have so far revealed about ten different inhibitor binding sites. A detailed list of known inhibitors and their actions on ATP synthase in relation to human heath and disease is discussed in reference [11].
Bioflavonoids/polyphenols are a class of plant secondary metabolites. The beneficial effects of many fruits, vegetables, and tea have been attributed to the presence of bioflavonoid compounds in them. Bioflavonoids are known to exhibit antioxidants, chemopreventive, and chemotherapeutic properties [16–20]. They have been shown to have anti-allergic, anti-inflammatory [21], and anti-microbial activity [22–24]. Their mode of action is not clear, but some dietary bioflavonoids are known to block the action of enzymes and other substances that promote the growth of cancer cells by binding to the multiple molecular targets in the body including ATP synthase [11, 13, 16, 25–26]. For example one of the most common dietary polyphenol resveratrol has been shown to have multiple uses, with multiple benefits in humans, including but not limited to increased life span, anticancer/antitumor effects, and antimicrobial activities [26]. Resveratrol was also shown to induce apoptosis via mitochondrial pathways [25, 27]. Aziz et al [28] demonstrated the chemopreventive properties of resveratrol against prostate cancer. They found that treatment with resveratrol concentrations of up to 50μmol/L/day resulted in stimulation of apoptosis in androgen-responsive human prostate carcinoma cells (LNCaP). At similar concentrations resveratrol had no effect on the rate of cell death in normal human prostate cells.
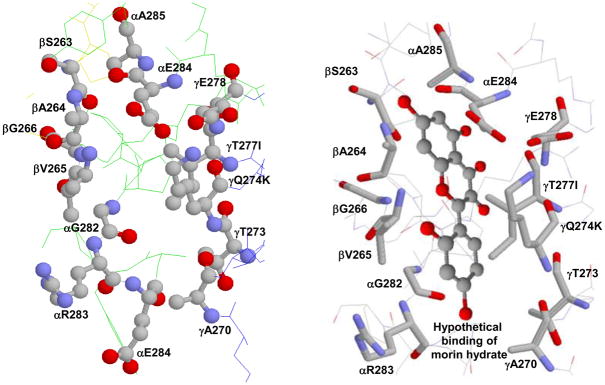
Earlier Zheng and Ramirez [15] studied the inhibitory effects of several naturally occurring polyphenolic phytochemicals on rat brain and liver mitochondrial F1Fo ATP synthase. They demonstrated that ATP synthase is molecular target for resveratrol and other aglycone isoflavones. Lately, the polyphenols resveratrol, piceatannol, quercetin, quercetrin, or quercetin-3-β-D glucoside, were shown to prevent synthetic or hydrolytic activities of E. coli and bovine mitochondrial ATP synthase [13, 16]. The proposed mode of action was binding of polyphenols at the polyphenol binding pocket of ATP synthase and blockage of clockwise or anti-clockwise rotation of the γ-subunit [16] (see Figure 1).
Figure 1.
X-ray crystallographic structure of polyphenol binding site of ATP synthase. (A) Empty and (B) hypothetical binding of morin hydrate at the polyphenol binding pocket. Residues from α, β, and γ subunits involved in interaction with polyphenols are identified. In bovine two variants, Q274K and T277I, occur in the γ subunit and are identified in the figure. PDB file 2jj1 [16] with RasMol [55] was used to generate this figure.
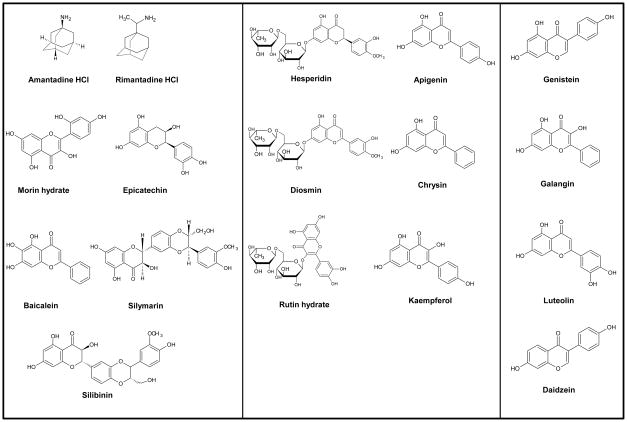
The question arises (i) whether the dietary bioflavonoids have differential inhibitory actions on E. coli ATP synthase and (ii) what kind of effect dietary bioflavonoids have on the intact E. coli cell growth which will be an indicate their effect on ATP synthesis. Thus we studied the inhibitory effect of seventeen bioflavonoid/polyphenol compounds illustrated in Figure 2 on E. coli ATP synthase using both purified F1-ATPase and membrane bound F1Fo ATP synthase preparations. This study shows that dietary bioflavonoids bind and inhibit E. coli ATP synthase in differential manner. Our results also reaffirm that the beneficial effect of dietary polyphenols as antitumor or antimicrobial agents may be at least in part are through their inhibitory action on ATP synthase.
Figure 2. Structures of bioflavonoids are shown in three groups.
(I) exerting complete inhibition of F1-ATPase activity, (II) exerting incomplete inhibition of F1-ATPase activity, and (III) exerting insignificant inhibition of F1-ATPase activity.
Materials and Methods
Source of bioflavonoids and other chemicals
Ultra pure bioflavonoid compounds were purchased from Sigma-Aldrich Chemical Company. Catalog numbers for all bioflavonoids used in this study are presented in Table 1. Silymarin used in this study was a mixture of anti-hepatotoxic flavonolignans from the fruit of Silybum marianum while silibinin, a pure compound, is the principal component of silymarin. Also, we followed the supplier’s directions in the handing of all compounds such as kaempferol was light sensitive so it was protected from light. All the compounds were resuspended in DMSO immediately before use for the desired concentration and were stored in −20 °C. In ATPase assays the final volume of DMSO was not more that 25%. Earlier we noted that up to 40% DMSO has no effect on membrane bound F1Fo of E. coli ATP synthase [13]. All other chemicals used in this study were ultra pure analytical grade, and purchased from either Sigma –Aldrich Chemical Company or Fisher Scientific Company.
Table 1.
Growth of Escherichia coli cells and residual ATPase activity in presence of bioflavonoid compounds
| Polyphenolic compounds | Sigma catalog numbers | a Growth on succinate plates | b Growth yield in limiting glucose (%) | c Residual ATPase Activity (%) | IC50 values (mM) |
|---|---|---|---|---|---|
| d Control | N/A | ++++ | 100 | N/A | N/A |
| e Null | N/A | - | 45 | N/A | N/A |
| Morin hydrate | M4008-5G | - | 46 | 0 | 0.07 |
| Silymarin | S0292-10G | - | 51 | 0 | 0.11 |
| Baicalein | 465119-100MG | - | 48 | 0 | 0.29 |
| Silibinin | S0417-1G | - | 47 | 0 | 0.34 |
| Rimantadine | 390593-1G | - | 52 | 0 | 2.0 |
| Amantadin | A1260-5G | - | 51 | 0 | 2.5 |
| (−)-Epicatechin | E1753-1G | - | 53 | 0 | 4.0 |
| Hesperidin | H5254-25G | ++ | 76 | 40 | N/A |
| Chrysin | C80105-25MG | ++ | 73 | 40 | N/A |
| Kaempferol | K0133-10MG | ++ | 74 | 45 | N/A |
| Diosmin | D3525-5G | +++ | 84 | 50 | N/A |
| Apigenin | A3145-25MG | +++ | 84 | 60 | N/A |
| Genistein | G6649-25MG | +++ | 87 | 62 | N/A |
| Rutin hydrate | R5143-50G | +++ | 87 | 60 | N/A |
| Galangin | 282200-25MG | ++++ | 93 | 100 | N/A |
| Daidzein | D7802-25MG | ++++ | 96 | 90 | N/A |
| Luteolin | L9283-10MG | ++++ | 87 | 80 | N/A |
Growth on succinate plates after 3 days was determined by visual inspection. ++++, high growth; +++, moderate growth; ++, low growth; or -, no growth.
Growth yield on limiting glucose was measured as OD595 after ~20 hours growth at 37 °C.
Residual activity is the left over ATPase activity
Control, pBWU13.4/DK8 which contains UNC+ gene encoding ATP synthase; Null, pUC118/DK8 with UNC− gene. Growth of positive and negative controls in absence of polyphenol compounds. Data are means of four to six experiments each at 37 °C. Each individual experimental point is itself the mean of duplicate assays.
Measurement of growth yield in limiting glucose medium; preparation of E. coli membranes; purification of E. coli F1; assay of ATPase activity of membrane bound F1Fo or purified F1
Bothe membrane bound F1Fo and purified F1 were isolated from the E. coli strain pBWU13.4/DK8 [29]. Growth yield in limiting glucose was measured as in [30]. E. coli membrane bound F1Fo or purified F1 were prepared as in [31]. It should be noted that this procedure involves three washes of the initial membrane pellets. The first wash is performed in buffer containing 50 mM TES pH 7.0, 15% glycerol, 40 mM 6-aminohexanoic acid, and 5 mM p-aminobenzamidine. The following two washes are performed in buffer containing 5 mM TES pH 7.0, 15% glycerol, 40 mM 6-aminohexanoic acid, 5 mM p-aminobenzamidine, 0.5 mM DTT, and 0.5 mM EDTA. Prior to experiments, membranes were washed twice more by resuspension and ultracentrifugation in 50 mM TrisSO4 pH 8.0, 2.5 mM MgSO4. F1 was purified as described in Ref [32]. Prior to the experiments, F1 samples (100μl) were twice passed through 1-ml centrifuge columns (Sephadex G-50) equilibrated in 50mM TrisSO4 pH 8.0, to remove catalytic site bound-nucleotide. ATPase activity was measured in 1 ml of assay buffer containing 10 mM NaATP, 4 mM MgCl2, and 50 mM TrisSO4, at pH 8.5 and 37 °C. Reactions were started by addition of 1 ml of assay buffer to the purified F1 or membranes, and stopped by addition of SDS to a 3.3% final concentration. Pi released was assayed as in [33]. For membrane bound F1Fo (30 – 50 μg protein), reaction times were 20–30 min. For purified F1 (20μg protein), reaction times were 2–5 min. All reactions were shown to be linear with time and protein concentration. SDS-gel electrophoresis on 10% acrylamide gels was as in [34]. Immunoblotting with rabbit polyclonal anti-F1-α and anti-F1-β antibodies was as in [35].
Inhibition of ATPase activity by bioflavonoid compounds
Membrane bound F1Fo or purified F1 (0.2–1.0 mg/ml) were preincubated with varied concentrations of bioflavonoid compounds for 60 min at room temperature, in 50 mM TrisSO4 pH 8.0. The volume of bioflavonoid compounds added was in the ranged from 0–20μl in a total reaction volume of 550μl. Then 1 ml of ATPase assay buffer was added to measure the enzyme activity. Inhibitory exponential decay curves were generated using SigmaPlot 10.0. The best fit lines and IC50 values for the curves were obtained using a single 3 parameter model. The range of absolute specific activity for membrane bound F1Fo was 20–26 and for purified F1 was 28–42μmol/min/mg at 37 °C for different preparations. These absolute values were used as 100% bench mark to calculate the relative ATPase activity.
Reversal of purified F1 or membrane bound enzyme ATPase activity from the inhibition of bioflavonoid compounds
Reversibility experiments were performed by dilution of the membrane enzyme and by passing the inhibited purified F1 through centrifuge columns. For the measurement of reversibility by dilution, membranes were first reacted with inhibitory concentrations of bioflavonoids for 1 hour at room temperature. These concentrations were used based on the maximal observed inhibition of the ATP synthase (see Figure 3–5). 50 mM TrisSO4, pH 8.0 buffer was then added to bring the concentrations down to non inhibitory levels and incubation continued for 1 additional hour at room temperature before the ATPase assay. Reversibility was also tested by passing the bioflavonoid inhibited purified F1 enzyme through 1 ml centrifuge columns twice before measuring the ATPase activity. Control samples without bioflavonoids were incubated for the same time periods as the samples with bioflavonoids.
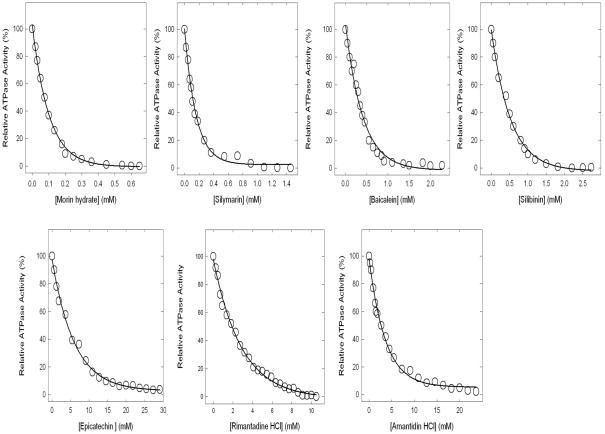
Figure 3. Complete inhibition of ATPase activity in purified F1 or membrane-bound ATP synthase.
Membranes or purified F1 were preincubated for 60 min at 23°C with varied concentration of bioflavonoids shown in the figure and then aliquots added to 1 ml of assay buffer and ATPase activity determined. Details are given in Materials and Methods. Each data point represents average of at least four experiments done in duplicate tubes, using two independent membrane or F1 preparations. Results agreed within ± 10%.
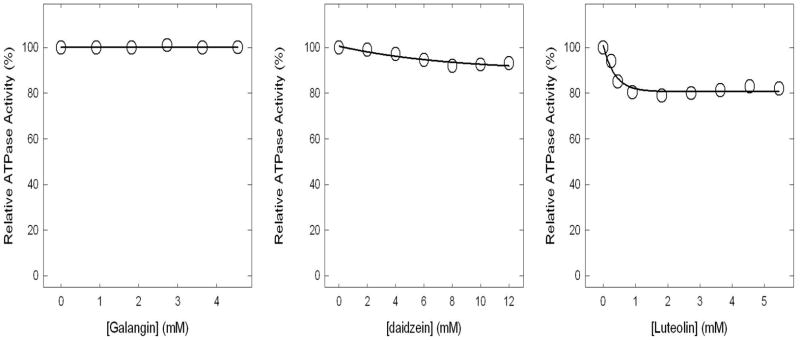
Figure 5. Insignificant or no inhibition of ATPase activity in purified F1 or membrane-bound ATP synthase.
Membranes or purified F1 were preincubated for 60 min at 23°C with varied concentration of bioflavonoids identified in the figure and then aliquots added to 1 ml of assay buffer and ATPase activity determined. Each data point represents average of at least four experiments done in duplicate tubes, using two independent membrane or F1 preparations. Results agreed within ± 10%.
Results
Complete inhibition of ATPase activity of purified F1 or F1Fo ATP synthase in membranes by morin, silymarin, baicalein, silibinin, rimantadin, amantidin, or, epicatechin
Polyphenol bound X-ray structure has shown the bioflavonoid/polyphenols bound in the polyphenol binding pocket of ATP synthase. This binding pocket located at the interface of α, β, and γ-subunits [16] (Figure 1). 1 The bound bioflavonoids can form hydrophobic interactions with γGln274 (γLys-260), γThr-277 (γIle-263), βAla-264 (βAla-278), or βVal-265 (βVal-279), and additional non polar interactions with residues γAla-270 (γAla-256), γThr-273 (γThr-259), γGlu-278 (γGlu-264), αGly-282 (αGly-290), or αGlu-284 (αGlu-292) which are within 4Å of the bound compounds (Figure 1). E. coli residue numbers are used throughout. Bovine mitochondrial residue numbers are shown in parentheses. Polyphenol binding pocket residues of E. coli ATP synthase are identical to the bovine polyphenol binding pocket residues except for two changes, namely γQ274K and γT277I, where Gln is replaced by Lys and Thr is replaced by Ile in bovine. The seventeen bioflavonoids used in this study (Fig. 2, Table 1) are dived into three groups: (I) potent inhibitors (~0% residual activity), (II) partial inhibitors (~40–60% residual activity), and (III) weak inhibitors (~80–100% residual activity).
Figure 3 shows the inhibition of ATPase activity of purified F1 or membrane bound enzyme in presence of varied concentrations of morin, silymarin, baicalein, silibinin, rimantadin, amantidin, or, (−)- epicatechin.. All seven bioflavonoids caused in complete (~100%) inhibition. On molar scale morin hydrate was the most potent inhibitor. The relative potency was morin (IC50 ~0.07mM) > silymarin (IC50 ~0.11mM) > baicalein (IC50 ~0.29mM) > silibinin (IC50 ~0.34mM) > rimantadine (IC50 ~2.0mM) > amantidin (IC50 ~2.5mM) > (−)-epicatechin (IC50 ~4.0mM). We consistently found that the F1 data and the membrane data were the same for these inhibitors. This is in agreement with our previously established interpretation that inhibition of ATPase activity can be assayed using either membrane bound F1Fo preparations or purified F1 with equivalent results [13, 36–40].
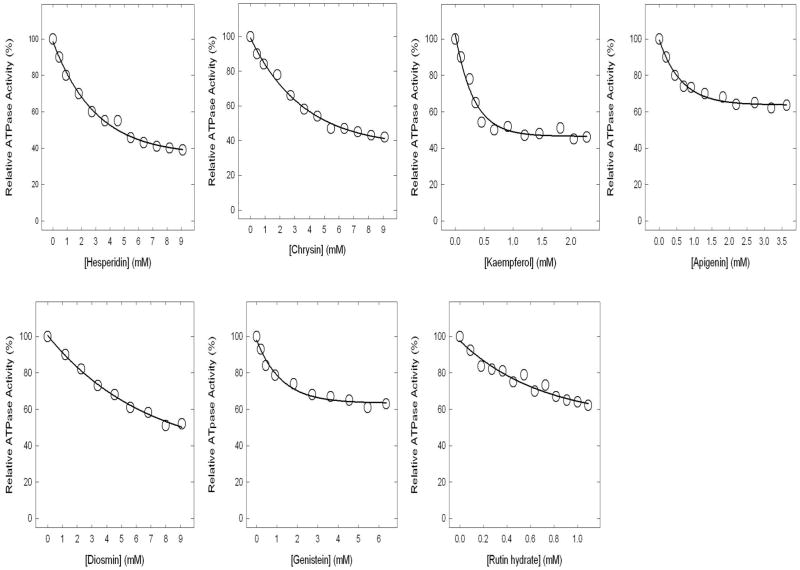
Partial inhibition of ATPase activity of purified F1 or F1Fo ATP synthase in membranes by hesperidin, chrysin, kaempferol, diosmin, apigenin, genistein, or rutin
Figure 4 shows the inhibitory effect of hesperidin, chrysin, kaempferol, diosmin, apigenin, genistein, or rutin. These seven bioflavonoids exert partial inhibition of about 40–60%. As before the F1 data and the membrane bound F1Fo data were the same for all inhibitors.
Figure 4. Incomplete inhibition of ATPase activity in purified F1 or membrane-bound ATP synthase.
Membranes or purified F1 were preincubated for 60 min at 23°C with varied concentration of bioflavonoids identified in the figure and then aliquots added to 1 ml of assay buffer and ATPase activity determined. Details can be found Materials and Methods section. Each data point represents average of at least four experiments done in duplicate tubes, using two independent membrane or F1 preparations. Results agreed within ± 10%.
Insignificant or no inhibition by galangin, daidzein, or luteolin of ATPase activity of purified F1 and F1Fo ATP synthase in membranes
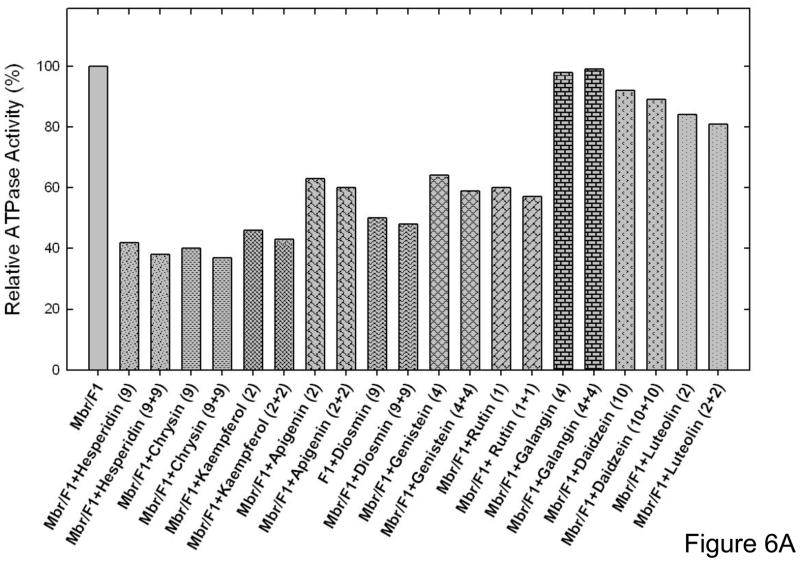
The maximal inhibition in the presence of luteolin was ~20%, daidzein was ~10%, and galangin showed no inhibition at all (Fig 5). Partial or slight inhibition of ATP synthase is not uncommon. In previous studies [7, 13, 36–43], we have noted several instances where mutant or wild-type ATP synthase were incompletely inhibited by inhibitors like fluoroaluminate, fluoroscandium, sodium azide, NBD-Cl, polyphenols, or amphibian peptides. To ensure that the maximal inhibition with bioflavonoids hesperidin, chrysin, kaempferol, diosmin, apigenin, genistein, rutin, galangin, daidzein, or luteolin had been reached, we incubated each membrane bound F1Fo preparation or purified F1 with hesperidin (9mM), chrysin (9mM), kaempferol (1 mM), diosmin (9mM), apigenin (1.5mM), genistein (2mM), rutin (1 mM), galangin (4mM), daidzein (10mM), or luteolin (2mM) by the maximal inhibitory concentrations, for 1 h as in Figure 3 and 4. This was followed by supplementary pulses of the same inhibitory bioflavonoid concentrations and incubation was continued for an additional hour before ATPase assay. As shown in Figure 6A very little or no additional inhibition occurred, which was consistent with Figure 4 and 5 data. This shows that the inhibition by the above bioflavonoids was maximal, and fully inhibited F1 or membrane bound enzyme retained residual activity. Although, we used a 1 hour incubation time, it was observed that the maximal inhibition of purified F1 or membrane bound enzyme was achieved within 15 minutes. Earlier resveratrol was shown to inhibit mitochondrial F1Fo ATP synthase within 1–2 minutes[15].
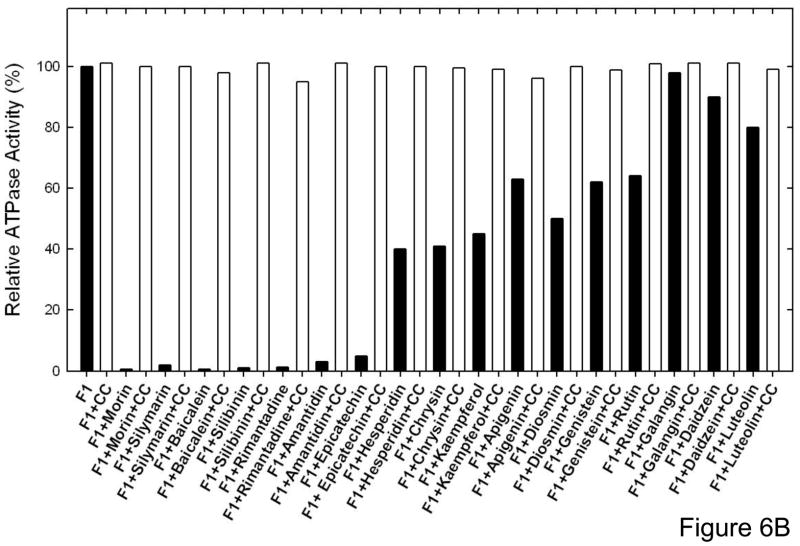
Figure 6. Results of Extra pulses of bioflavonoid compounds and reversal of inhibition by passing through centrifuge columns.
(A), Membrane bound ATP synthase (Mbr) or purified F1 (F1) was inhibited with inhibitory concentrations of the bioflavonoid compounds shown in the figure for 60 min under conditions as described in Fig 3–4. Then a further pulse of identical inhibitory concentrations was added and incubation continued for 1 h before assay. The last digits represent the compound concentrations in [μM]. (B) Purified F1 was incubated with inhibitory concentrations of bioflavonoid compounds for 60 min under conditions as described in Fig 2–4. Then the inhibited samples were passed twice through 1 ml centrifuge columns and ATPase activity was measured. The first bar is for purified F1 with no compound (F1), followed by bars in presence of compounds.
Reversal of ATPase activity of purified F1 or membrane bound enzyme from the bioflavonoid inhibition
Here we examined whether the bioflavonoid induced inhibition of ATPase is reversible or not. Reversibility data is shown in Figure 6B. This experiment was carried out in two ways. (i) the purified F1 or membrane bound enzyme was inhibited with the maximum inhibitory concentrations of bioflavonoids for 1 hr at RT as in Figures 3–5. Samples were then diluted to a non-inhibitory concentration and ATPase activity was measured. (ii) 20 μg of purified F1 samples were incubated with maximum inhibitory concentrations of bioflavonoids for 1 hr at RT. As before the inhibitory concentrations were determined based on data from figures 3 and 4. Inhibited samples were then passed twice through 1 ml sephadex G50 centrifuge columns and ATPase activity measured. Inhibition by all bioflavonoids was found to be fully reversible.
Inhibition of growth on LB, limiting glucose, and succinate medium in presence of bioflavonoid compounds
Inhibitory effects on ATP synthesis were studied by growing the wild-type E. coli strain pBWU13.4/DK8 on succinate plates (a non-fermentable carbon source), or limiting glucose, in the presence or absence of bioflavonoid compounds. The abrogation of growth was in proportion to the inhibition ATPase activity (see Table 1).
Discussion
There is increasing interest in the effects of natural dietary compounds as antimicrobial and antitumor agents. For example, polyphenols like resveratrol, piceatannol, quercetin, quercetrin, or quercetin-3-β-glucoside were shown to bind and inhibit ATP synthase suggesting that the dietary benefits of these polyphenols are in part linked to the inhibition of ATP synthesis in tumor cells, thereby leading to apoptosis [11, 13, 15–16, 44]. Thus, the ultimate goal of this study was to examine if the antimicrobial or anticancer properties of dietary bioflavonoids are possibly associated with the inhibition of ATP synthase. Moreover, results from E. coli ATP synthase have added advantage in understanding the antimicrobial effects of dietary bioflavonoid compounds.
Bioflavonoid compounds used in this study resulted in complete, partial, slight, or no inhibition of ATP synthase. This trend can be attributed to the interaction between the bioflavonoid compounds and the polyphenol binding pocket residues. Compound skeleton, size, shape, geometry, and functional group presence or position played important role in binding and inhibition (Figure 1 and 2). For example presence of sugar moieties in hesperidin, diosmin, or rutin might cause sterical hindrance resulting in partial inhibition. Fewer –OH group or the extended positioning of –OH group also resulted in partial or insignificant inhibition. On the other hand multiple –OH groups in morin, baichalein, epicatechin, silymarin, or silibinin and presence of NH2 groups in rimantadine or amantadin resulted in complete inhibition of ATPase activity.
Importance of position and number of –OH groups is also apparent from the differential inhibitory effects of quercetin and apigenin. Both are reversible inhibitors of E. coli ATP synthase and bind non-covalently at the polyphenol binding site. Quercetin was shown to induce ~80% inhibition with IC50~33μM [13] while apigenin resulted in partial inhibition ~38% (see Figure 4). Higher affinity and potent inhibition by quercetin (IC50 ~55–65μM) in comparison to apigenin (IC50 ~100μM) was also observed in the inhibition of mitochondrial F1Fo ATP synthase [15]. This enhanced inhibitory effect of quercetin can be attributed to the two additional hydroxyl groups on its flavone skeleton, facilitating its inhibitory and binding activity to ATP synthase [13, 15, 45].
Earlier [15] comparative inhibitory effects of several naturally occurring polyphenolic phytochemicals on rat brain and liver mitochondrial F1Fo ATP synthase also indicated the importance of hydroxyl groups in a particular position. It was found that genistein, biochanin A, and daidzein all abrogated the ATPase activity with IC50 values between ~55 – 127 but genistin a 7-glucose derivative of genistein did not inhibit ATPase activity up to 140 μM. Interestingly enough the inhibitory effects dietary bioflavonoids on mitochondrial F1Fo did not yield big differences in the degree of inhibition while similar set of bioflavonoids in this study inhibited in very differential manner. We found very different extents of maximal inhibition (from zero to near 100%) as well as very widely different IC50 values (from 0.07 mM for morin to 4 mM (−)-epicatechin), representing several logs difference for only subtle modification of the chemical scaffold. Inhibitory effects of the similar set of polyphenolic compounds on mitochondrial enzyme resulted in IC50 values between ~50–125μM [15]. This difference between E. coli and mitochondrial enzyme results may be due to the fact that Zheng and Ramirez [15] employed a coupled assay to determine ATPase effects (and a direct Pi quantitation for only a few), whereas we measured Pi directly without intervening coupling enzymes. It should be noted that our methods would not be subject to this potentially indirect inhibition- i.e. if the coupling enzymes were inhibited, then this would be masked as a lower activity or false inhibition. Alternatively, it is possible that subtle differences in the E. coli enzyme are responsible- allowing a broader range of effects. Also, the use of E. coli enzyme seems more appropriate for correlation with antibacterial activities.
Based on the resveratrol, piceatannol, or quercetin bound x-ray structure of the polyphenol binding pocket residues γQ274, γT277, βV265, and βA264 seem to play a critical role in forming hydrophobic interactions with the polyphenol compounds. Other proximal residues which could form non-polar interactions with the bioflavonoid compounds are γA270, γT273, γE278, αE284, αG282, and αE284. The polyphenol binding pocket residues are highly conserved among different species including human, bovine, rat, and E. coli [46–47]. However, the E. coli enzyme residues γGln-274 and γThr-277 in the polyphenol binding pocket are replaced by γLys-274 and γIle-277 in mitochondrial enzyme. Distance measurements using Deep View Swiss-Pdb Viewer, Version 4.01 (http://spdbv.vital-it.ch/) suggests that the –OH group of γThr-277 generates an additional H-bond with the –OH group of γSer-281 [13] and may form additional H-bonds with the oxygen or –OH groups of the bioflavonoid compounds.
One important aspect of the inhibitory mechanism of bioflavonoids is their effect on rotary mechanism of the enzyme. 2 Mutagenic analysis of polyphenol binding pocket residues demonstrate that the hydrophobic binding pocket between the γ-subunit c-terminal tip and the hydrophobic inside provided by the α- or β-subunit residues is essential for the binding of polyphenol/bioflavonoid compounds.
Addition of extra pulse of compounds to the partially inhibited purified F1 or membrane bound F1Fo did not change the level of inhibition significantly (figure 5A). This suggests that the purified F1 or membrane bound enzyme was fully inhibited by the compounds and the observed extent of inhibition was accurate. Also the partial inhibition is not a result of uninhibited enzyme or degradation of the compounds with time. The process of inhibition was also found to be completely reversible. A fully reacted F1 regained activity after being passed through the centrifuge columns to remove the compounds. Similarly, purified F1 or membrane bound enzyme regained activity following exposure to higher concentrations, once returned to lower concentrations of the inhibitory compound, by dilution with buffer.
The growth pattern of E. coli in the presence of seventeen bioflavonoid compounds is presented in Table 1. Remarkably, the loss of growth and ATPase inhibition in the presence of all the compounds used in this study was nearly equivalent. The pUC118/DK8 (null strain) usually grows from 40–50% of the pBWU13.4/DK8 (wild-type, Table 1). This is because the null strain uses only glycolysis to generate ATP, whereas the wild-type uses both glyolysis and oxidative phosphorylation. Up to 40–50% retention of growth in the presence of each of bioflavonoid compounds is the result of intact substrate level phosphorylation, suggesting that loss of growth (50–60%) is caused by abrogation of oxidative phosphorylation resulting from inhibition of ATP synthesis. These results support the initial hypothesis that some of the beneficial effects of dietary bioflavonoids may be attributable to their inhibitory effects on ATP synthase. However, in earlier studies the degree of inhibition of F1-ATPase and ATP synthesis in presence of some polyphenols, peptides, or antibiotic was also found to be different [13–14, 48].
The uncontrolled growth of tumor cells requires additional energy in the form of ATP generated by ATP synthase. Thus the inhibition of ATP synthase will deprive tumor cells of required energy and lead to cell death or apoptosis [13, 16]. For example, resveratrol was shown to induce apoptosis via a mitochondrial pathway [25, 27]. Oligomycin, another specific ATP synthase inhibitor induced an apoptotic suicide response in cultured human lymphoblastoid and other mammalian cells within 12–18 hrs, but not in ρ° cells that are depleted of a functional mitochondrial respiratory chain [49]. Thus inhibition of the components of mitochondrial pathways may lead to marking of some cells, via CD14, for cell death, while allowing commitment to differentiation to occur in the surviving population [50]. Cell death can also be prompted through alteration of cellar bioenergetics. 1, 4-benzodiazepine (Bz-423) was shown to bind and inhibit the oligomycin sensitivity – conferring protein subunit of F1Fo ATP synthase. This results in significant decrease in ATP synthesis and increase in the production of free radicals which in turn activates redox regulated apoptosis [51].
Polyphenol induced inhibition of proton-translocating F1-ATPase activity of S. mutans resulting in inhibition of biofilm formation and acid production [22, 52], and inhibition of ATPase activity as well as intact E. coli cell growth indicated that ATP synthase is a potential molecular target for dietary bioflavonoids. Two c-subunit mutations, D32V and A63P, of mycobacterium synthase confer resistance against the anti-tuberculosis drug diarylquinoline [53–54]. This also supports earlier conclusions that potent ATP synthase inhibitors may stimulate the development of specific antibacterial drugs, and drugs that target tumor cells without affecting normal cells [13, 16].
Acknowledgments
This work was supported by the National Institutes of Health [Grant GM085771] and East Tennessee State University Small RDC [Grant 211457] to ZA.
Abbreviations used
- NBD-Cl
7-chloro-4-nitrobenzo-2-oxa-1, 3-diazole
- Mbr
membrane containing ATP synthase
- IC50
corresponds to the concentration of inhibitor where 50% of maximal inhibition was observed.
Footnotes
E. coli residue numbers used throughout.
E. coli enzyme mutants namely αR283A/Q, αE284A/Q, βS263A/Q, γT273A/Q, γQ274A/K, γT277A/Q, and γE278A/Q resulted in the loss of inhibition by resveratrol, piceatannol and quercetin. (Dadi, P.K., and Ahmad, Z. unpublished results)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Senior AE, Nadanaciva S, Weber J. Biochim Biophys Acta. 2002;1553:188–211. doi: 10.1016/s0005-2728(02)00185-8. [DOI] [PubMed] [Google Scholar]
- 2.Abrahams JP, Leslie AGW, Lutter R, Walker JE. Nature. 1994;370:621–628. doi: 10.1038/370621a0. [DOI] [PubMed] [Google Scholar]
- 3.Diez M, Zimmermann B, Borsch M, Konig M, Schweinberger E, Steigmiller S, Reuter R, Felekyan S, Kudryavtsev V, Seidel CAM, Graber P. Nat Struct Mol Biol. 2004;11:135–141. doi: 10.1038/nsmb718. [DOI] [PubMed] [Google Scholar]
- 4.Itoh H, Takahashi A, Adachi K, Noji H, Yasuda R, Yoshida M, Kinosita K. Nature. 2004;427:465–468. doi: 10.1038/nature02212. [DOI] [PubMed] [Google Scholar]
- 5.Senior AE. Cell. 2007;130:220–221. doi: 10.1016/j.cell.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Weber J, Senior AE. FEBS Lett. 2003;545:61–70. doi: 10.1016/s0014-5793(03)00394-6. [DOI] [PubMed] [Google Scholar]
- 7.Ahmad Z, Senior AE. J Bioenerg Biomembr. 2005;37:437–440. doi: 10.1007/s10863-005-9486-8. [DOI] [PubMed] [Google Scholar]
- 8.Frasch WD. Biochim Biophys Acta. 2000;1458:310–325. doi: 10.1016/s0005-2728(00)00083-9. [DOI] [PubMed] [Google Scholar]
- 9.Ren H, Allison WS. Biochim Biophys Acta. 2000;1458:221–233. doi: 10.1016/s0005-2728(00)00075-x. [DOI] [PubMed] [Google Scholar]
- 10.Noji H, Yoshida M. J Biol Chem. 2001;276:1665–1668. doi: 10.1074/jbc.R000021200. [DOI] [PubMed] [Google Scholar]
- 11.Hong S, Pedersen PL. Microbiol Mol Biol Rev. 2008;72:590–641. doi: 10.1128/MMBR.00016-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pedersen PL. J Bioenerg Biomembr. 2007;39:1–12. doi: 10.1007/s10863-007-9070-5. [DOI] [PubMed] [Google Scholar]
- 13.Dadi PK, Ahmad M, Ahmad Z. Int J Biol Macromol. 2009;45:72–79. doi: 10.1016/j.ijbiomac.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Thorsen F, Enger PO, Wang J, Bjerkvig R, Pedersen PH. J Neurooncol. 2007;82:1–10. doi: 10.1007/s11060-006-9240-z. [DOI] [PubMed] [Google Scholar]
- 15.Zheng J, Ramirez VD. Br J Pharmacol. 2000;130:1115–1123. doi: 10.1038/sj.bjp.0703397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gledhill JR, Montgomery MG, Leslie AG, Walker JE. Proc Natl Acad Sci U S A. 2007;104:13632–13637. doi: 10.1073/pnas.0706290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barta I, Smerak P, Polivkova Z, Sestakova H, Langova M, Turek B, Bartova J. Neoplasma. 2006;53:19–25. [PubMed] [Google Scholar]
- 18.Nishino H, Murakoshi M, Mou XY, Wada S, Masuda M, Ohsaka Y, Satomi Y, Jinno K. Oncology. 2005;69:38–40. doi: 10.1159/000086631. [DOI] [PubMed] [Google Scholar]
- 19.Athar M, Back JH, Kopelovich L, Bickers DR, Kim AL. Arch Biochem Biophys. 2009;486:95–102. doi: 10.1016/j.abb.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Athar M, Back JH, Tang X, Kim KH, Kopelovich L, Bickers DR, Kim AL. Toxicol Appl Pharmacol. 2007;224:274–283. doi: 10.1016/j.taap.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto Y, Gaynor RB. J Clin Invest. 2001;107:135–142. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Percival RS, Devine DA, Duggal MS, Chartron S, Marsh PD. Eur J Oral Sci. 2006;114:343–348. doi: 10.1111/j.1600-0722.2006.00386.x. [DOI] [PubMed] [Google Scholar]
- 23.Cushnie TP, Lamb AJ. Int J Antimicrob Agents. 2005;26:343–356. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duarte AR, Gordillo MD, Cardoso MM, Simplicio AL, Duarte CM. Int J Pharm. 2006;311:50–54. doi: 10.1016/j.ijpharm.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Pervaiz S. FASEB J. 2003;17:1975–1985. doi: 10.1096/fj.03-0168rev. [DOI] [PubMed] [Google Scholar]
- 26.Pirola L, Frojdo S. IUBMB Life. 2008;60:323–332. doi: 10.1002/iub.47. [DOI] [PubMed] [Google Scholar]
- 27.Clement MV, Hirpara JL, Chawdhury SH, Pervaiz S. Blood. 1998;92:996–1002. [PubMed] [Google Scholar]
- 28.Aziz MH, Nihal M, Fu VX, Jarrard DF, Ahmad N. Mol Cancer Ther. 2006;5:1335–1341. doi: 10.1158/1535-7163.MCT-05-0526. [DOI] [PubMed] [Google Scholar]
- 29.Ketchum CJ, Al-Shawi MK, Nakamoto RK. Biochem J. 1998;330:707–712. doi: 10.1042/bj3300707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Senior AE, Latchney LR, Ferguson AM, Wise JG. Arch Biochem Biophys. 1984;228:49–53. doi: 10.1016/0003-9861(84)90045-6. [DOI] [PubMed] [Google Scholar]
- 31.Senior AE, Langman L, Cox GB, Gibson F. Biochem J. 1983;210:395–403. doi: 10.1042/bj2100395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weber J, Lee RS, Grell E, Wise JG, Senior AE. J Biol Chem. 1992;267:1712–1718. [PubMed] [Google Scholar]
- 33.Taussky HH, Shorr E. J Biol Chem. 1953;202:675–685. [PubMed] [Google Scholar]
- 34.Laemmli UK. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 35.Rao R, Perlin DS, Senior AE. Arch Biochem Biophys. 1987;255:309–315. doi: 10.1016/0003-9861(87)90398-5. [DOI] [PubMed] [Google Scholar]
- 36.Ahmad Z, Senior AE. J Biol Chem. 2004;279:31505–31513. doi: 10.1074/jbc.M404621200. [DOI] [PubMed] [Google Scholar]
- 37.Ahmad Z, Senior AE. J Biol Chem. 2005;280:27981–27989. doi: 10.1074/jbc.M503955200. [DOI] [PubMed] [Google Scholar]
- 38.Brudecki LE, Grindstaff JJ, Ahmad Z. Arch Biochem Biophys. 2008;471:168–175. doi: 10.1016/j.abb.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 39.Li W, Brudecki LE, Senior AE, Ahmad Z. J Biol Chem. 2009;284:10747–10754. doi: 10.1074/jbc.M809209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laughlin TF, Ahmad Z. Int J Biol Macromol. 2010;46:367–374. doi: 10.1016/j.ijbiomac.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahmad Z, Senior AE. J Biol Chem. 2004;279:46057–46064. doi: 10.1074/jbc.M407608200. [DOI] [PubMed] [Google Scholar]
- 42.Ahmad Z, Senior AE. FEBS Lett. 2005;579:523–528. doi: 10.1016/j.febslet.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 43.Ahmad Z, Senior AE. FEBS Lett. 2006;580:517–520. doi: 10.1016/j.febslet.2005.12.057. [DOI] [PubMed] [Google Scholar]
- 44.Zheng J, Ramirez VD. Biochem Biophys Res Commun. 1999;261:499–503. doi: 10.1006/bbrc.1999.1063. [DOI] [PubMed] [Google Scholar]
- 45.Wu D, Kong Y, Han C, Chen J, Hu L, Jiang H, Shen X. Int J Antimicrob Agents. 2008;32:421–426. doi: 10.1016/j.ijantimicag.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 46.Walker EH, Pacold ME, Perisic O, Stephens L, Hawkins PT, Wymann MP, Williams RL. Mol Cell. 2000;6:909–919. doi: 10.1016/s1097-2765(05)00089-4. [DOI] [PubMed] [Google Scholar]
- 47.Sicheri F, Moarefi I, Kuriyan J. Nature. 1997;385:602–609. doi: 10.1038/385602a0. [DOI] [PubMed] [Google Scholar]
- 48.Perlin DS, Latchney LR, Senior AE. Biochim Biophys Acta. 1985;807:238–244. doi: 10.1016/0005-2728(85)90254-3. [DOI] [PubMed] [Google Scholar]
- 49.Wolvetang EJ, Johnson KL, Krauer K, Ralph SJ, Linnane AW. FEBS Lett. 1994;339:40–44. doi: 10.1016/0014-5793(94)80380-3. [DOI] [PubMed] [Google Scholar]
- 50.Mills KI, Woodgate LJ, Gilkes AF, Walsh V, Sweeney MC, Brown G, Burnett AK. Biochem Biophys Res Commun. 1999;263:294–300. doi: 10.1006/bbrc.1999.1356. [DOI] [PubMed] [Google Scholar]
- 51.Johnson KM, Cleary J, Fierke CA, Opipari AW, Jr, Glick GD. ACS Chem Biol. 2006;1:304–308. doi: 10.1021/cb600143j. [DOI] [PubMed] [Google Scholar]
- 52.Duarte S, Gregoire S, Singh AP, Vorsa N, Schaich K, Bowen WH, Koo H. FEMS Microbiol Lett. 2006;257:50–56. doi: 10.1111/j.1574-6968.2006.00147.x. [DOI] [PubMed] [Google Scholar]
- 53.Andries K, Verhasselt P, Guillemont J, Gohlmann HWH, Neefs JM, Winkler H, Van Gestel J, Timmerman P, Zhu M, Lee E, Williams P, de Chaffoy D, Huitric E, Hoffner S, Cambau E, Truffot-Pernot C, Lounis N, Jarlier V. Science. 2005;307:223–227. doi: 10.1126/science.1106753. [DOI] [PubMed] [Google Scholar]
- 54.Cole ST, Alzari PM. Science. 2005;307:214–215. doi: 10.1126/science.1108379. [DOI] [PubMed] [Google Scholar]
- 55.Sayle RA, Milner-White EJ. Trends Biochem Sci. 1995;20:374. doi: 10.1016/s0968-0004(00)89080-5. [DOI] [PubMed] [Google Scholar]