Summary
Recent advances indicate that the amygdala represents valence: a general appetitive/aversive affective characteristic that bears similarity to the neuroeconomic concept of value. Neurophysiological studies show that individual amygdala neurons respond differentially to a range of stimuli with positive or negative affective significance. Meanwhile, increasingly specific lesion/inactivation studies reveal that the amygdala is necessary for processes – e.g., fear extinction and reinforcer devaluation – that involve updating representations of value. Furthermore, recent neuroimaging studies suggest that the human amygdala mediates performance on many reward-based decision-making tasks. The encoding of affective significance by the amygdala might be best described as a representation of state value – a representation that is useful for coordinating physiological, behavioral, and cognitive responses in an affective/emotional context.
Introduction
For many years, the amygdala was thought of primarily as a center for fear in the brain. This perception was fueled by a wealth of experimental data, mainly from rodents, that emerged from the fear conditioning paradigm (summarized in [1,2]). Classic lesion and inactivation studies established that the amygdala is essential for the perception of fear, the expression of fearful behavior, and the acquisition of fear in response to stimuli that had been paired with aversive outcomes (Pavlovian fear conditioning) (e.g. [3], reviewed in [4]). Neurophysiological studies, which also were mainly limited to rodents, showed that cells in the basolateral amygdala (BLA) had firing rates that were highly sensitive to stimuli that were associated with fear-inducing events, and also with the aversive events themselves [4,5].
While new light continues to be shed on the detailed functioning of the amygdala with regard to fear learning and fearful behavior, in recent years, a surge of work in humans and non-human primates has led to an expanded conception of the amygdala’s role. The anatomical connections of the amygdala in primates hint at a reason for this: especially compared to rodents, humans and non-human primates have a hugely elaborated prefrontal cortex (PFC) [6], many parts of which – especially medial and orbital areas – have extensive bidirectional connections with the amygdala [7–10]. The amygdala receives input from a full range of higher sensory and poly-sensory areas, and projects back to them in turn, even to primary sensory targets (connections that may be unique to primates) [11–13]. Other targets of amygdala output include the hippocampus, basal ganglia, perirhinal and entorhinal cortices, the basal forebrain, and subcortical structures such as the hypothalamus [14]. In sum, the anatomical situation of the amygdala seems to imply the potential for a far more wide-ranging role than “danger alarm.”
Using a combination of new techniques and innovative extensions of old ones, neuroscientists have uncovered a role for the amygdala in a wide variety of tasks with an emotional component, whether appetitive or aversive [15]. Emotional responses frequently occur in reaction to stimuli that predict impending rewarding or aversive reinforcement, and they are often described within a framework that uses two axes to characterize emotions: arousal (from calm to excited) and valence (from extremely negative to extremely positive) [16]. As we discuss below, recent data implicates the amygdala in processing information related to both arousal and valence; we will focus mainly, but not exclusively, on processing related to valence.
The concept of positive and negative valence is related to recent work by neuroscientists seeking to understand the neural basis of economic choice. According to neuroeconomic theory, a “universal currency” of value should be encoded in the brain in order to effectively compare different economic options [17–19]; moreover, the values of stimuli, the values of actions, and “state value” – the value of the overall situation of an organism at a given moment – are essential variables in theoretical accounts of learning [20,21]. Using decision-making tasks, scientists have characterized neural signals correlated with value, where value is defined within an economic framework (reviewed in [18,19]). In contrast, most studies focused on the amygdala have not used decision-making tasks; rather, they used classical or instrumental conditioning tasks, in which subjects learn the association between conditioned stimuli (CSs) and appetitive or aversive unconditioned stimuli (USs). Many types of associations between a CS and US may be formed during conditioning [22] – such as those between the CS and the motor response elicited by the US, or the sensory properties of the US – but, as we will discuss, considerable evidence now indicates that amygdala neurons encode information about the overall affective or motivational significance of USs associated with CSs. Therefore, we would argue that information about “valence” encoded by the amygdala probably corresponds to “value” as it is studied in decision-making tasks; we will therefore use the terms “value” and “valence” interchangeably.
This review will examine recent progress in our understanding of whether and how value – in all its multifaceted senses – is encoded by the amygdala in rodents, humans, and non-human primates. We will consider the small but growing body of neurophysiological evidence concerning how the activity of individual amygdala neurons encodes the value of stimuli, and perhaps more general quantities, such as state value; and we will examine how this view of amygdala function may be supported by recent evidence from lesions and inactivation of the amygdala, and from observation of amygdala activity using functional imaging. Along the way, we will consider emerging ideas about how a representation of value might be “read out” and used by other brain areas such as the orbitofrontal cortex (OFC). Overall, recent work paints a compelling picture of the amygdala as a key brain area for the processing and propagation of signals pertaining to value, and therefore as an essential part of the neural foundation of motivated behavior and emotion.
A neurophysiological representation of value
Until recent years, neurophysiological recording in the amygdala was mostly the province of rodents, with the exception of a few pioneering experiments in non-human primates [23,24], and the majority of rodent studies were focused on fear conditioning. These studies helped to establish the viewpoint that the amygdala – particularly the lateral nucleus – is a key structure in Pavlovian conditioning, at least of the aversive valence, because it is a site of convergence for information about conditioned stimuli (CSs) and unconditioned stimuli (USs) [4], and because fear learning is closely connected with synaptic changes in the lateral nucleus (reviewed in [25]). While, at the time, neuroscientists often used the term “fear memory” to describe the representation that seems to be stored in the lateral nucleus, these results were equally consistent with the amygdala storing a representation of stimulus value. In terms of Pavlovian fear conditioning: as the previously neutral CS (often a light or tone) becomes associated with an aversive US (typically a shock), the CS acquires a negative value, which is reflected in lasting changes in the responses of individual amygdala neurons to the CS (as in [5]).
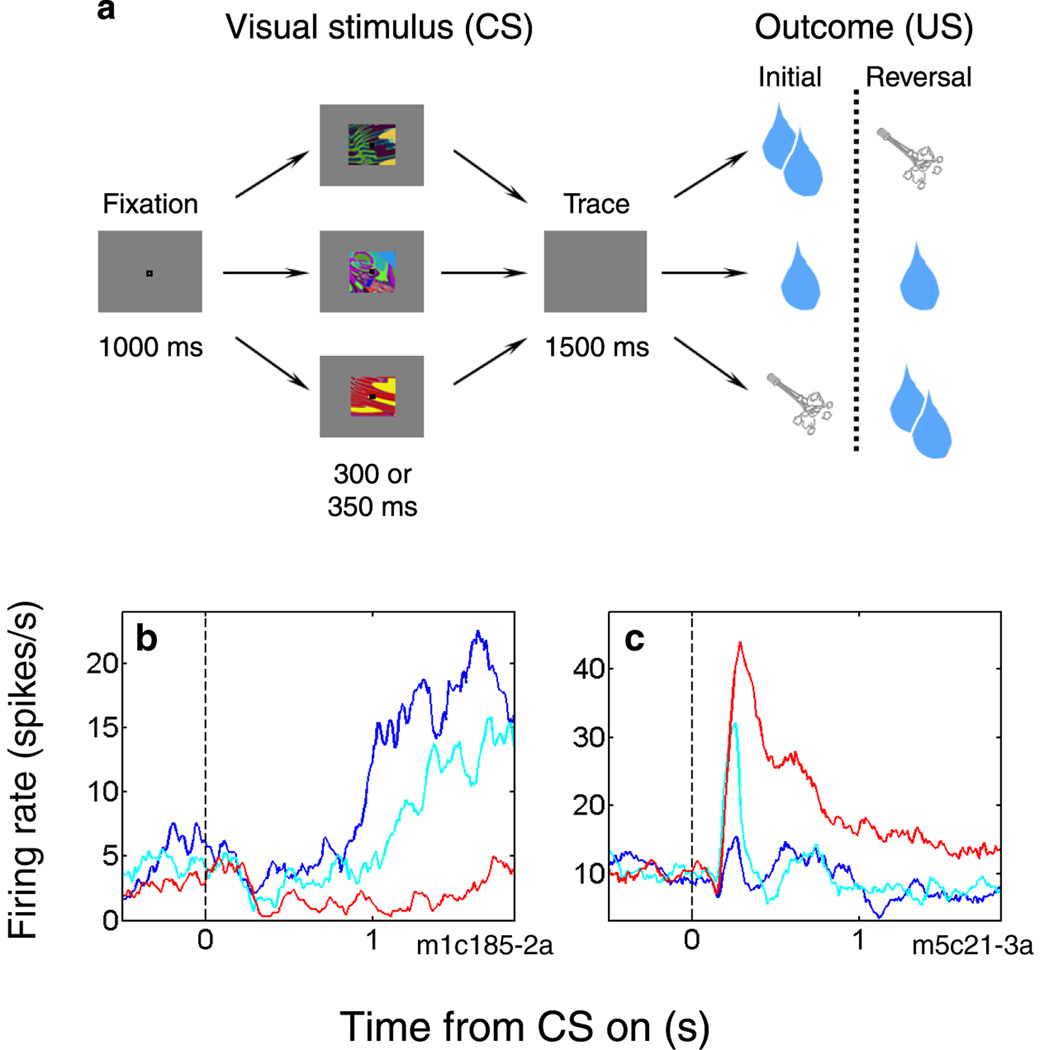
In recent years, a few groups have sought to extend the rodent work by characterizing neural signals in a non-human primate model during appetitive and aversive conditioning. In macaque monkeys, Paton and colleagues [26] found that the responses of many individual amygdala neurons reflect both the positive and negative values of visual stimuli. The authors, as is typical in the field, defined “value” operationally: positively valued stimuli are those that elicit approach behaviors, and negatively value stimuli are those that elicit defensive behavior. They used a conditioning task that combined a form of fear conditioning (pairing abstract visual stimuli with an aversive air-puff to the face) with reward learning in a single session (Fig. 1a). Contrary to the view of the amygdala as a fear center, the authors found similar numbers of neurons that respond more strongly to a CS that has been associated with reward (positive value-coding neurons) or with air-puff (negative value-coding neurons; Fig. 1b,c). More recently, Belova, Paton and colleagues added to the picture by showing that this putative value representation in the amygdala is not limited to recently learned CSs [27•]: rather, value-coding neurons respond in a consistent fashion to all events during a trial, including the rewards and air-puffs themselves (stimuli that are primary reinforcers; Fig. 2a,b) and a fixation point (a mildly positive stimulus conditioned over a long time period; Fig. 2c,d). Furthermore, as the same group also found to be the case in OFC [28], individual amygdala neurons often integrated information about impending reinforcement of both valences, and therefore did not simply represent the sensory properties of a preferred US associated with a CS [27•]. Based on these findings, the authors hypothesize that amygdala neurons may track state value – the overall value of an organism’s situation, which may be modulated as different stimuli appear in the environment.
Figure 1.
Individual neurons in primate amygdala can encode positive or negative visual stimulus value. (a) Sequence of events during an appetitive/aversive trace conditioning task; for details, see [26]. In each session, the subject learns to associate three novel, abstract visual stimuli with large reward, small reward, or an aversive air-puff to the face. After the initial reward contingencies are learned, a reversal takes place: the image associated with large reward is now followed by air-puff, and the image associated with air-puff is now followed by large reward. The reversal allows neural signals related to image value to be disentangled from neural signals related to visual characteristics of the images. (b and c) Examples of amygdala neurons that encode image value during the task shown in a. Plots are peri-stimulus time histograms aligned on the time of image presentation (black dotted line). Blue line, activity during large reward trials. Cyan line, activity during small reward trials. Red line, activity during air-puff trials. (b) A positive value-coding neuron, which fires more strongly on large reward trials than air-puff trials. (c) A negative value-coding neuron, which fires more strongly on air-puff trials than on large-reward-trials. Note that the activity on small reward trials is intermediate; for details, see [27•]. Differential activity may occur primarily during image presentation (as in c), the trace interval (as in b), or both.
Figure 2.
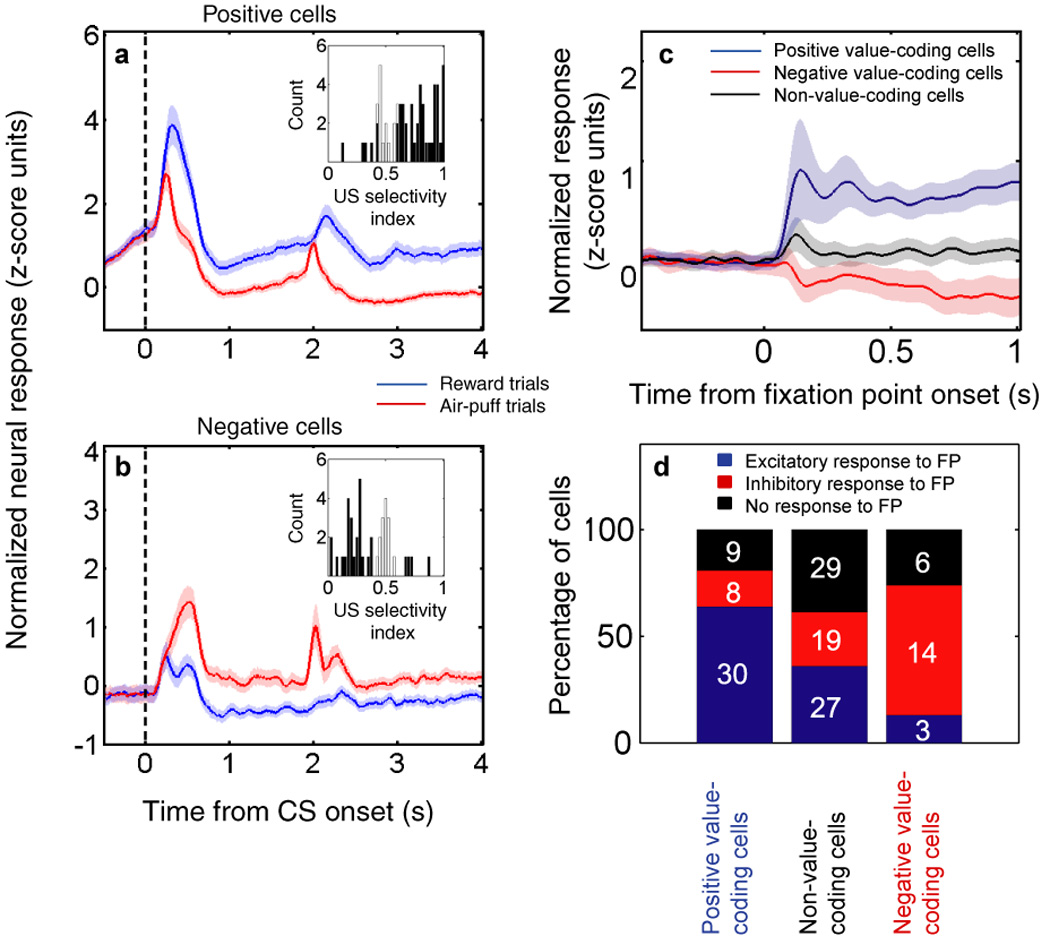
Populations of neurons in primate amygdala encode the value of conditioned stimuli, of unconditioned stimuli and of a fixation point. (a and b) Population responses to conditioned and unconditioned stimuli in a trace conditioning task in which novel, abstract images are followed by either reward or air-puff. Plots are normalized and averaged peri-stimulus time histograms (PSTHs) aligned on the time of CS presentation (vertical dashed line). Reward or air-puff occurred at either 1.8 or 1.85 s, depending on the session. Blue line, activity on rewarded trials. Red line, activity on air-puff trials. Shading indicates SEM. Inset histograms, reinforcement selectivity indices calculated for each neuron using an ROC analysis; see [29•] for details. (a) Population responses of positive value-coding cells. Note that US selectivity indices are predominantly greater than 0.5, indicating a higher firing rate for reward than for air-puff. (b) Population responses of negative value-coding cells. Note that US selectivity indices are predominantly less than 0.5, indicating a higher firing rate for air-puff than for reward. (c) Population normalized average response to fixation point (FP) presentation for positive value-coding cells (blue), negative value-coding cells (red), and non-value-coding cells (black). Shaded areas indicate SEM. Note that the FP is a mildly positive stimulus, and that positive and negative value-coding cells respond to it in a manner consistent with their responses to CSs; see [27•] for details. (d) Bar chart showing the percentage of cells with increases (blue), decreases (red), or no change (black) in activity during FP presentation. The number of cells of each type is indicated. The majority of positive value-coding cells increase firing to the FP, while the majority of negative value-coding cells decrease firing to the FP. Figure adapted with permission from [27•] and [29•].
The results of Paton, Belova and colleagues [26,27•,29•,30] demonstrate a strong connection between value signals in the amygdala and monkey behavior: notably, value signals change as fast as monkeys learn, as demonstrated by anticipatory licking and eye closure, upon reversal of reinforcement contingencies [26]; and both the value signal and licking behavior are graduated across three levels of value (strong positive, weak positive, and negative) [27•]. Recently, it has been shown that the activity of individual amygdala neurons also correlates with skin conductance responses (SCRs) in monkeys, a physiological measure connected with emotional arousal [31], whether the SCRs are evoked by viewing rewarded images or simply by the monkey’s internal state (i.e., no outside emotional stimulus is present). In another avenue of research, two groups found that individual amygdala neurons respond differentially to more complex emotional stimuli: faces or vocalizations of conspecifics with different facial expressions, ranging from appeasing to threatening [32,33]. Interestingly, both studies found more neurons that had a stronger response to threatening cues than appeasing cues, harking back to the idea of the amygdala as a fear center, and consistent with a functional imaging study that found a stronger response to threatening faces in the monkey basolateral complex (BLA) [34]. However, it is possible that the threatening faces and vocalizations simply had a stronger negative value than the appeasing cues have positive value, making it easier to activate what Paton et al. [26] called negative value-coding neurons.
In addition to encoding the valence of affectively salient stimuli, it is important to note the increasing evidence that the amygdala also carries signals related to emotional intensity, regardless of valence. Belova et al. [29•] found that the responses of many amygdala neurons are enhanced when stimuli are unexpected; some show this enhancement to rewarding stimuli only, some to aversive stimuli only, and some to both. Importantly, this latter group of neurons may contribute to valence-independent emotional processes, such as increased arousal and attention, and enhancement of memory-encoding. Similarly, Shabel and Janak [35], working in rat amygdala, have recently reported a group of neurons that respond similarly to appetitive and aversive CSs, and that have responses that are correlated with blood pressure – a physiological measure of arousal. Meanwhile, Paz et al. [36,37] have shown that activity in BLA – particularly in conjunction with signals from the medial PFC [37] – modulates transmission in the rhinal cortices, a phenomenon that could potentially contribute to the emotion-related enhancement of memory formation.
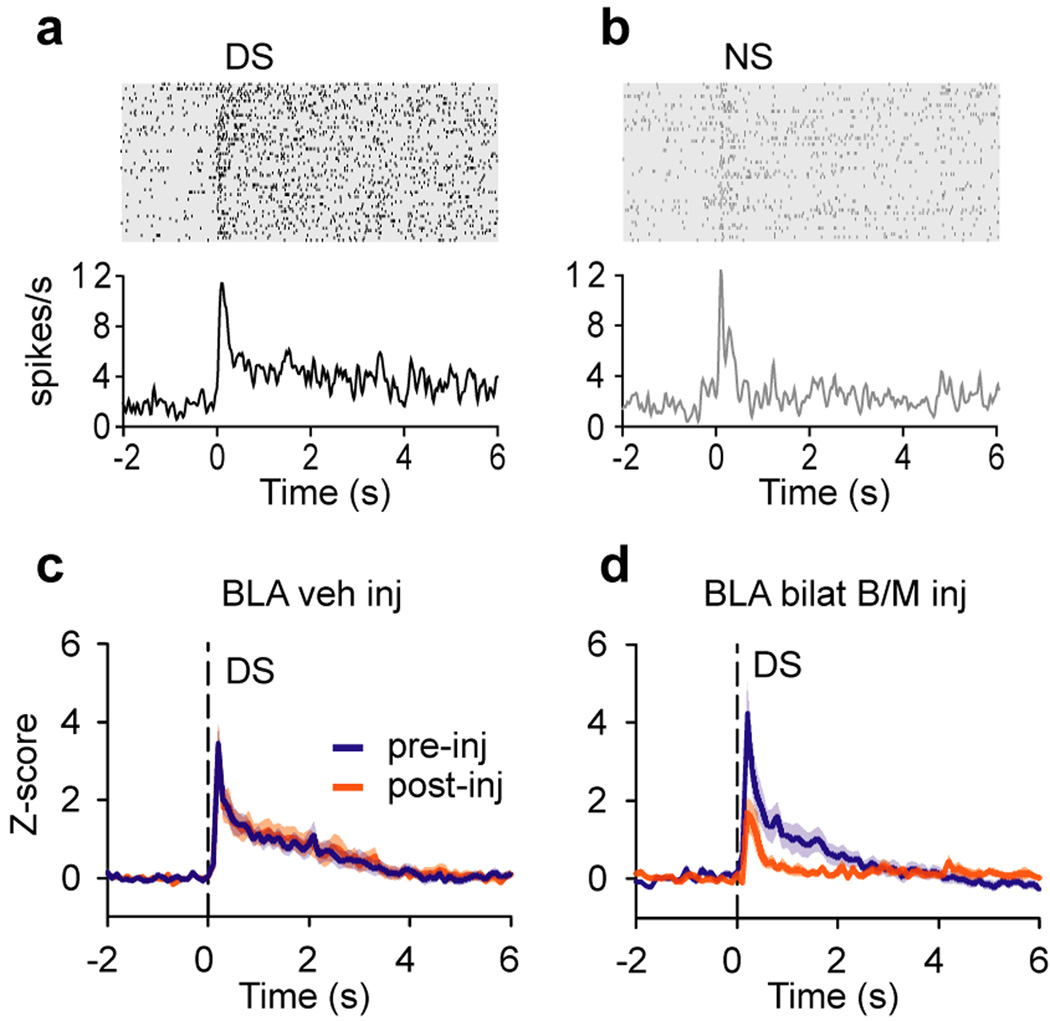
Other evidence from rodent amygdala neurophysiology, which was long focused on fear conditioning, has begun to converge with that from primate neurophysiology to support the idea of a general representation of value in the amygdala. In addition to the well-established encoding of fearful/aversive associations of stimuli, many individual neurons in rat BLA show increased responding to odor cues that are associated with a sucrose reward [38]. More recently, using the same appetitive and aversive odor cue paradigm, but now treating rats with cocaine, it was shown that cue-selective BLA neurons fail to reverse their selectivity when reinforcement contingencies are reversed – paralleling the rats’ behavioral reversal learning deficit [39]. Meanwhile, Ambroggi, Ishikawa, and colleagues showed that many BLA neurons respond preferentially to a reward-predicting auditory cue, as opposed to an unrewarded cue [40•], and that this discriminatory signal is necessary both for normal reward-seeking behavior [41] and for the development of similar signals in the nucleus accumbens ([40•]; see Fig. 3). Tye et al. [42•] use a similar task to demonstrate that successful association of a cue with sucrose reward is correlated with the number of BLA neurons that respond to the cue, and that this is based on the rapid, NMDA-dependent strengthening of thalamo-amygdala synaptic connections; in a novel extension of this line of work, Popescu et al. [43] report that coherent gamma oscillations develop between BLA and striatum in response to a tone that is associated with reward.
Figure 3.
Neurons in the rat BLA develop preferential responses to a rewarded cue. (a and b) Responses of a BLA neuron in the discriminative stimulus (DS) task, in which rats learned to press a lever in response to an auditory cue (the DS) to receive sucrose. Another interleaved auditory cue, the nonrewarded stimulus (NS), was not reinforced. (a) Response of an example BLA neuron to the DS. (b) Response of the same neuron to the NS. Rasters and peri-stimulus time histograms are aligned on cue presentation. (c and d) Population average normalized firing rate of nucleus accumbens core (NAc) neurons to the DS before and after injection of a vehicle (c) or baclofen and muscimol (B/M) (d) into the bilateral BLA. Shading indicates SEM. Inactivation of BLA dramatically reduced the discrimination of the DS and NS by NAc neurons; for further details, see [40•]. Figure adapted with permission from [40•].
Notably, even coming from the sub-specialty of rodent fear conditioning, there is increasing evidence that individual amygdala neurons, even when they are task-responsive, are not uniformly excited by aversive stimuli. Herry et al. [44•] recently showed that two distinct subpopulations of BLA neurons are involved in fear conditioning, extinction of fear, and subsequent fear renewal: neurons that are preferentially excited by cues that are currently associated with shock (whether during initial conditioning or renewal), and neurons that are excited by cues that were previously associated with shock, but are now extinguished (i.e., are associated with safety). Interestingly, these two populations of neurons even show distinct patterns of connectivity to the hippocampus and PFC. Future experiments are needed to clarify whether these neuronal subpopulations can be identified with the positive and negative value-coding neurons found in primates [26], and whether these subpopulations also participate in distinct neural circuits.
Taking the amygdala’s representation of value off-line
Non-human primate studies
Lesions of the amygdala, which produce an extraordinarily wide range of behavioral and emotional effects, have historically provided a rich source of data about possible functions of the amygdala [45,46] (reviewed in [47]). In recent years, neuroscientists working in rodents and non-human primates have used newer techniques – such as temporary pharmacological inactivation – to silence the amygdala, and those who work with lesions have increasingly used anatomically precise techniques that spare fibers of passage, such as excitotoxic chemical injections. These studies provide invaluable clues as to how signals provided by the amygdala – which, as we have seen, carry information about stimulus value, and perhaps even state value – might be utilized in neural circuits that underlie a wide range of motivated behavior.
Recent fiber-sparing lesion studies in monkeys have confirmed many of the findings that emerged from rodent studies, and from less-precise lesions in primates. For example, Antoniadis et al. have shown that the primate amygdala is indeed necessary for fear learning resulting from Pavlovian conditioning [48,49]. In these studies, complete amygdala lesions made using ibotenic acid injections prevented acquisition of conditioned fear, as measured by the fear-potentiated startle reflex; however, they did not prevent the memory or expression of conditioned fear that was acquired before the lesions. This latter finding contrasts with a number of earlier findings in rats (most recently [50]), but is consistent with findings in human patients with amygdala lesions (e.g., [51]), and therefore seems to reflect a genuine species difference. Expanding upon the ideas of Antoniadis et al., we would speculate that the orbitofrontal cortex (OFC) or other prefrontal areas – structures that are highly elaborated in primates as compared with rodents, and that are interconnected with the amygdala – might acquire information during or after learning sufficient to support expression of previously learned fear.
Similarly, new fiber-sparing lesion studies confirm the importance of the amygdala in behavioral and physiological responding to naturally fear-inducing stimuli. In a pair of recent studies, Machado et al. found that monkeys with amygdala lesions show decreased defensive behaviors towards a rubber snake or an unfamiliar human intruder, both of which are inherently aversive stimuli for monkeys [52,53]. Notably, amygdala lesions did not disrupt defensive behavior towards objects with learned connotations of fear (including both objects and social signals) [53], which seems consistent with the amygdala being necessary for learning, but not retention or expression, of conditioned fear.
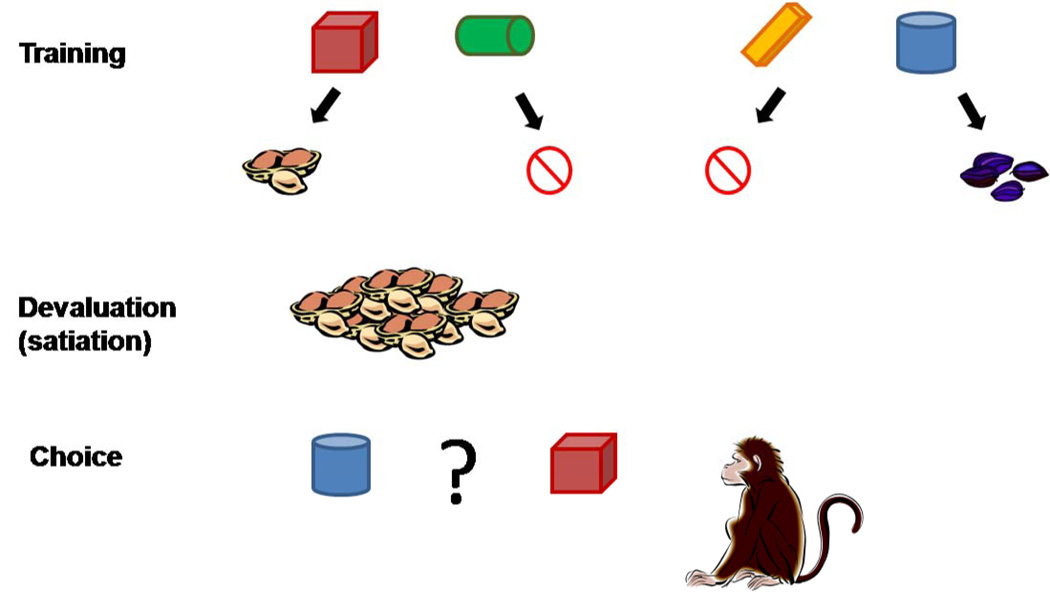
Moving beyond the fear domain, new lesion studies in non-human primates continue to support an essential role for the amygdala in reward assessment [54,55], and particularly in updating the value of a reward during a reinforcer devaluation procedure ([55–57•]; see Fig. 4). In this experimental paradigm, monkeys are selectively satiated on a particular reward (e.g., peanuts); following satiation, normal subjects are immediately less likely to choose objects that were previously associated with the now-devalued reward. In contrast, monkeys with excitotoxic amygdala lesions [56,57•] or temporary inactivation of the amygdala during the satiation procedure [58] are unable to refrain from choosing objects associated with the devalued reward. Interestingly, amygdala inactivation after satiation – during the object choice task – does not impair monkeys’ choices [58]. Thus, it seems that updating the value signal in the amygdala is required for reinforcer devaluation, but an on-line value representation in the amygdala is not necessary for expression of the updated preferences, which might be guided by expected outcome signals in the OFC or elsewhere in the absence of amygdala activity.
Figure 4.
Schematic diagram of a reinforcer devaluation task. In the training phase, the subject learns to associate pairs of cues with one of two food reward (e.g., peanuts and raisins) or non-reinforcement. Typically a large set of cue pairs is used, although only two are shown here. After training, and before testing, the subject is offered free access to one of the rewards (e.g., peanuts) until satiation (the subject voluntarily stops consumption of the reward). The monkey’s reward preferences are assessed both before initial training, and after satiation. In the test phase, the subject is offered a choice between cues that are associated with the devalued food (in this case, peanuts) or the non-devalued food (raisins). Normal monkeys will preferentially choose the cue associated with the non-devalued food. Monkeys lacking amygdala function continue to choose the cue associated with the devalued food; see [56] for more details.
Finally, a recent fiber-sparing lesion study [57•], along with re-analysis of previous data [59], has caused neuroscientists to reconsider the role of the amygdala in reversal learning – a frequently used paradigm in which stimulus-reinforcement contingencies are switched. The primate neurophysiology studies cited above [26,29] confirm that the responses of individual amygdala neurons change upon reversal, and that the timing of the changes coincides with behavioral indicators of learning. In contrast, Izquierdo and Murray [57•] found that monkeys with excitotoxic amygdala lesions learned reversals of object-reward associations just as quickly as controls, even though the same monkeys were impaired at reinforcer devaluation. There are several possible reasons for this apparent contradiction: one is that the amygdala may normally participate in reversal learning, but in the absence of value signals coming from the amygdala, the OFC and/or other areas may be able to support this simple kind of learning as well. Another explanation, favored by Izquierdo and Murray [56,57•] and not incompatible with the first, is that the kind of object-reward reversal task that they used can also be treated as a visual association task, and may therefore not require the manipulation of objects’ associations with reward values. Third, the task used by Paton et al. [26], in contrast to that of Izquierdo and Murray, required the learning of not only a reward/no-reward switch, but a reward/punishment reversal. As the amygdala seems to be unequivocally required for acquisition of conditioned fear, it is possible that the aversive element of the task may preferentially engage the amygdala.
Rodent studies
In parallel with the rise of sophisticated techniques for silencing the amygdala in non-human primates, recent studies in rodents have increasingly used fiber-sparing lesions and pharmacological inactivation of the amygdala. Many of these experiments have confirmed and extended the results of amygdala lesion studies in non-human primates; for example, two studies [60,61•] have recently confirmed that excitotoxic lesions of the rat BLA cause deficits in reinforcer devaluation, just as they do in monkeys. This result holds true across types of conditioning (Pavlovian or instrumental) and types of devaluation (satiation vs. food-illness pairing), although the BLA apparently assumes less importance when only a single outcome is available and devalued [61•]. Interestingly, the BLA (and specifically opioids therein) also seems to be necessary for re-associating actions with increased reward values, a procedure known as reward inflation: Wassum et al. [62•] found that injection of naloxone into the BLA blocks a food deprivation-related increase in sucrose-seeking activity, even though the increase in palatability of sucrose was unaffected.
Based on reinforcement revaluation experiments such as those described above, it appears that the amygdala plays a key role in representing the value of a reinforcer (US), and reconnecting the changing value of a US to its associated cue (CS) or action. This is consistent with the neurophysiological findings that individual amygdala neurons encode the values of USs in a graduated manner [27•,29•,30]. However, a pair of recent studies by Rabinak et al. [63,64] complicate this picture by demonstrating that the amygdala does not seem to be required for aversive reinforcer inflation. Pharmacological inactivation of either the BLA [63,64] or the central nucleus (CEA) [64] during administration of intensified aversive USs did not affect increased freezing behavior in response to either a context or a tone cue associated with the US. In contrast, the same group recently confirmed that inactivation of the BLA [64] and/or CEA [65] can block the initial acquisition of conditioned fear in a variety of conditions. The apparent contradiction between the findings of Rabinak et al. [63,64] and those regarding revaluation of positive reinforcements might be explained in a number of ways; perhaps the most convincing is that the paradigm of Rabinak et al. involved only a single outcome, which, as previously suggested [61•], might simplify the reinforcer revaluation problem enough that it can be solved by other brain areas without input from the amygdala.
Working with rodents, neuroscientists have taken advantage of a large toolbox of established techniques from experimental psychology, many of which are well adapted to explore the idea of motivational value. One such tool is reward discounting, which can be based on the amount of risk, effort, or time that must be endured before receiving a reward. Two recent studies have established that pharmacological inactivation of the rat BLA disrupts optimal choice behavior – indeed, sometimes prevents rats from making any choice at all – when either high effort [66,67] or high risk [67] is required to obtain the larger of two available rewards. This finding is consistent with recent studies in human patients, which have shown that amygdala lesions impair decision-making under conditions of risk [68,69], specifically resulting from difficulty in weighing potential gains (but not losses) [69]. These impairments are all understandable if we posit that a loss of online calculation of value by the amygdala, whether of cues or expected outcomes, prevents effective choice assessment by cortical areas such as OFC.
Another key tool from experimental psychology is extinction learning: learning to inhibit a previously acquired fear response when the feared CS is repeatedly presented without an aversive US. In rodents, BLA activity (specifically, NMDA receptor activation) is required for the acquisition and retention of extinction [70–72], although not relearning of extinction after fear conditioning has been reinstated [71,72]. Current thinking about extinction (summarized in [73]) holds that the original CS-US association is preserved in the brain, but amygdala-dependent inhibitory mechanisms suppress the fear response in the presence of the extinguished cue. As noted by Laurent and Westbrook [72], extinction is context-dependent; thus, the amygdala’s role in extinction may be to interact with the PFC to adjust the value of the extinguished CS when it occurs in the right context.
The value signal in BOLD
Consistent with the historically prevalent view of the amygdala as a fear center, functional imaging studies in humans have often focused on the amygdala in the context of aversive events. Therefore, there is a large body of literature – including many ongoing research efforts – that connects increases in the blood-oxygenation level-dependent (BOLD) signal in the amygdala to stimuli that induce fear or apprehension. Many studies have shown that activation of the amygdala is correlated with fear learning in Pavlovian conditioning paradigms; recently, for example, amygdala activity was connected to the association of specific faces with shock [74,75], an effect which seems to be abolished (both behaviorally and neurally) by the pro-social effects of oxytocin [75]. Moreover, Schiller et al. [76] recently showed that amygdala activation discriminates between a CS (an angry face) that is currently predictive of shock and a CS that is no longer predictive of shock in an aversive reversal-learning paradigm. In addition to direct fear conditioning, BOLD signal in the amygdala correlates with fear learning that takes place via observation of aversive events happening to others [77]. These results are consistent with the idea that, just as in monkeys and rodents, human fear learning is likely to involve the representation of the negative value of learned and inherently aversive stimuli in the amygdala.
In recent years, neuroscientists have increasingly used fMRI to study the amygdala in the context of reward-related tasks and conditions that combine elements of reward and punishment (many of which are reviewed in [78]). Indeed, a recent meta-analysis [79] reveals that, across many functional imaging studies, a core region of the human amygdala – tentatively identified with the basal and lateral nuclei – reliably responds to both appetitive and aversive stimuli. This is consistent with neurophysiological findings in non-human primates [26,27•], as is the demonstration that human amygdala activation increases with the selection of larger rewards [80]. Meanwhile, there is increasing evidence from fMRI that the human amygdala – specifically, a circuit involving amygdala and PFC – participates in the extinction of fear learning [81], in addition to its initial acquisition, and also in learning to avoid aversive outcomes [82]. Moreover, Hampton et al. [83•] use functional imaging to demonstrate that lesions of the amygdala disrupt reward expectation signals in ventromedial PFC, implying that a representation of stimulus value in PFC may be “downstream” from the value signal in the amygdala.
Notably, Talmi et al. [84] have recently demonstrated a correlation between BOLD signal in the human amygdala and magnitude of pavlovian-instrumental transfer (PIT): the automatic increase in vigor of a reward-associated instrumental response (in this case, squeezing a hand-grip) in the presence of a CS that has previously predicted reward. This is consistent with the idea that the amygdala encodes the motivational value of CSs, whether they are acting as a cue to expect reward, or as secondary reinforcers – stimuli that have acquired the ability to act as rewards for instrumental behaviors – as suggested by Tye et al. [85].
Finally, just as neurophysiologists have begun to look at value in an economic context, practitioners of fMRI have started to look for brain areas that are activated by economic tasks in the most literal sense – tasks that involve gaining and losing money. In an interesting twist, functional imaging during a gambling task [86] shows that amygdala activation correlates with the strength of the “framing effect” – the economic phenomenon of risk-aversion when a gamble is presented in terms of gains, and risk-seeking when the same gamble is presented in terms of losses. De Martino et al. [86] show that, given a certain amount of money, subjects made more decisions to “play it safe” if allowed to keep 40% of the money; but they made more decisions to “risk it all” if the other option is to lose 60% of the money. Decisions in keeping with the framing effect, in both directions, are correlated with BOLD signal in the amygdala; and in fact, the framing effect on both behavior and neural activity is enhanced in subjects who are homozygous for the short variant of the serotonin transporter gene [87•], a condition that enhances amygdala reactivity in a variety of situations [88]. The framing effect might result from presenting the same choice in two different emotional contexts, necessitating a recalculation of the value of each option in the current context, which is reflected in amygdala activity.
Conclusions
Here we have reviewed the latest progress towards understanding how value may be represented in the amygdala. In neuroscience, the terminology of “value” is used in a variety of ways – motivational value, incentive value, economic value, stimulus value, and action value, among others; however, it might be said that the idea of “state value” subsumes and encompasses all of these concepts. The value of a “state” takes into account not only external stimuli (including all the CSs and USs we have described), but internal variables such as hunger or satiation, and contexts that may be either external (e.g., location) or intangible (e.g., a rule that is in effect). Even in the context of economic choice, deciding between available options may entail comparing representations of potential future states corresponding to each choice. Moreover, the quantity of state value plays a key role in many theoretical accounts of reinforcement learning [20,21], which is a process that explicitly or implicitly underlies most of the experiments we have described above.
We would posit (as we do in [27•]) that neurons in the amygdala, as a population, encode a general representation of value that seems to incorporate learned and unlearned stimuli, contexts (such as initial or reversed reinforcement contingencies), and reinforcement history, and thus could be interpreted as state value. As we have reviewed, the great majority of recent studies that concern the amygdala – whether in rodents, monkeys, or humans – can be well understood within a framework in which the amygdala is a key area for calculating value – and recalculating it under changing circumstances, such as reinforcer devaluation or extinction. Whether value is initially calculated in the amygdala or not, it appears that signals from the amygdala are essential for value-related calculations in other brain areas such as OFC and the nucleus accumbens. Future experiments are needed to show unequivocally whether state value is initially calculated in the amygdala or passed to the amygdala from elsewhere, how inputs from many brain areas – such as the dopaminergic midbrain – might contribute to the value signal in the amygdala, and how intra-amygdalar processes might contribute to calculating state value.
Acknowledgements
This work was supported by grants from NIMH (R01 MH082017 and RC1 MH088458), NIDA (R01 DA020656), NEI (R24 EY015634) and the James S. McDonnell and Gatsby foundations. S.E.M. received support from a National Science Foundation graduate fellowship and from an individual NIMH National Research Service Award (F31 MH081620). We wish to thank J. Paton, M. Belova, and members of the Salzman lab for helpful comments and discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pare D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. Journal of Neurophysiology. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- 3.Wilensky AE, Schafe GE, LeDoux JE. Functional inactivation of the amygdala before but not after auditory fear conditioning prevents memory formation. J Neurosci. 1999;19:RC48. doi: 10.1523/JNEUROSCI.19-24-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 5.Quirk GJ, Repa C, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- 6.Wise SP. Forward frontal fields: phylogeny and fundamental function. Trends Neurosci. 2008;31:599–608. doi: 10.1016/j.tins.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghashghaei H, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- 8.Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stefanacci L, Amaral DG. Topographic organization of cortical inputs to the lateral nucleus of the macaque monkey amygdala: a retrograde tracing study. Journal of Comparative Neurology. 2000;421:52–79. doi: 10.1002/(sici)1096-9861(20000522)421:1<52::aid-cne4>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 10.Stefanacci L, Amaral DG. Some observations on cortical inputs to the macaque monkey amygdala: an anterograde tracing study. Journal of Comparative Neurology. 2002;451:301–323. doi: 10.1002/cne.10339. [DOI] [PubMed] [Google Scholar]
- 11.Amaral DG, Behniea H, Kelly JL. Topographic organization of projections from the amygdala to the visual cortex in the macaque monkey. Neuroscience. 2003;118:1099–1120. doi: 10.1016/s0306-4522(02)01001-1. [DOI] [PubMed] [Google Scholar]
- 12.Freese JL, Amaral DG. The organization of projections from the amygdala to visual cortical areas TE and V1 in the macque monkey. J Comp Neurol. 2005;486:295–317. doi: 10.1002/cne.20520. [DOI] [PubMed] [Google Scholar]
- 13.McDonald AJ. Cortical pathways to the mammalian amygdala. Progress in Neurobiology. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 14.Davis M. The role of the amygdala in conditioned and unconditioned fear and anxiety. In: Aggleton J, editor. The Amygdala: A Functional Analysis. Oxford University Press; 2000. pp. 213–287. [Google Scholar]
- 15.Murray EA. The amygdala, reward and emotion. Trends Cogn Sci. 2007;11:489–497. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Russell JA. A circumplex model of affect. Journal of Personality and Social Psychology. 1980;39:1161–1178. [Google Scholar]
- 17.Montague PR, Berns GS. Neural economics and the biological substrates of valuation. Neuron. 2002;36:265–284. doi: 10.1016/s0896-6273(02)00974-1. [DOI] [PubMed] [Google Scholar]
- 18.Sugrue LP, Corrado GS, Newsome WT. Choosing the greater of two goods: neural currencies for valuation and decision making. Nat Rev Neurosci. 2005;6:363–375. doi: 10.1038/nrn1666. [DOI] [PubMed] [Google Scholar]
- 19.Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nat Rev Neurosci. 2008;9:545–556. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sutton R, Barto A. Reinforcement Learning. Cambridge, Massachusetts: MIT Press; 1998. [Google Scholar]
- 21.Dayan P, Abbott LF. Theoretical Neuroscience. Cambridge, Massachusetts: MIT Press; 2001. [Google Scholar]
- 22.Delamater AR, Oakeshott S. Learning about multiple attributes of reward in Pavlovian conditioning. Ann N Y Acad Sci. 2007;1104:1–20. doi: 10.1196/annals.1390.008. [DOI] [PubMed] [Google Scholar]
- 23.Nishijo H, Ono T, Nishino H. Single neuron responses in amygdala of alert monkey during complex sensory stimulation with affective significance. Journal of Neuroscience. 1988;8:3570–3583. doi: 10.1523/JNEUROSCI.08-10-03570.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanghera MK, Rolls ET, Roper-Hall A. Visual responses of neurons in the dorsolateral amygdala of the alert monkey. Experimental Neurology. 1979;63:610–626. doi: 10.1016/0014-4886(79)90175-4. [DOI] [PubMed] [Google Scholar]
- 25.Maren S. Synaptic mechanisms of associative memory in the amygdala. Neuron. 2005;47:783–786. doi: 10.1016/j.neuron.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Paton J, Belova M, Morrison S, Salzman C. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439:865–870. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Belova MA, Paton JJ, Salzman CD. Moment-to-moment tracking of state value in the amygdala. J Neurosci. 2008;28:10023–10030. doi: 10.1523/JNEUROSCI.1400-08.2008. The authors elaborate upon the results in [26], which showed that individual amygdala neurons can encode the positive or negative value of images during a mixed appetitive/aversive reinforcement learning task, to demonstrate that monkey amygdala neurons also encode the values of unconditioned stimuli (rewards and punishments) and familiar stimuli long associated with reinforcement (a fixation point). This leads to the hypothesis that the amygdala tracks state value.
- 28.Morrison SE, Salzman CD. The convergence of information about rewarding and aversive stimuli in single neurons. J Neurosci. 2009;29:11471–11483. doi: 10.1523/JNEUROSCI.1815-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Belova MA, Paton JJ, Morrison SE, Salzman CD. Expectation modulates neural responses to pleasant and aversive stimuli in primate amygdala. Neuron. 2007;55:970–984. doi: 10.1016/j.neuron.2007.08.004. This study demonstrates that neural responses to reinforcement in the amygdala are generally enhanced when reward or punishment is unexpected, with some neurons showing this enhancement for reward, some for punishment, and some for both. Thus, the amygdala could participate in processing both valence (or value) and intensity of emotionally significant stimuli.
- 30.Salzman CD, Paton JJ, Belova MA, Morrison SE. Flexible neural representations of value in the primate brain. Ann N Y Acad Sci. 2007 doi: 10.1196/annals.1401.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laine CM, Spitler KM, Mosher CP, Gothard KM. Behavioral triggers of skin conductance responses and their neural correlates in the primate amygdala. J Neurophysiol. 2009;101:1749–1754. doi: 10.1152/jn.91110.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gothard KM, Battaglia FP, Erickson CA, Spitler KM, Amaral DG. Neural responses to facial expression and face identity in the monkey amygdala. J Neurophysiol. 2007;97:1671–1683. doi: 10.1152/jn.00714.2006. [DOI] [PubMed] [Google Scholar]
- 33.Kuraoka K, Nakamura K. Responses of single neurons in monkey amygdala to facial and vocal emotions. J Neurophysiol. 2007;97:1379–1387. doi: 10.1152/jn.00464.2006. [DOI] [PubMed] [Google Scholar]
- 34.Hoffman KL, Gothard KM, Schmid MC, Logothetis NK. Facial-expression and gaze-selective responses in the monkey amygdala. Curr Biol. 2007;17:766–772. doi: 10.1016/j.cub.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 35.Shabel SJ, Janak PH. Substantial similarity in amygdala neuronal activity during conditioned appetitive and aversive emotional arousal. Proc Natl Acad Sci U S A. 2009;106:15031–15036. doi: 10.1073/pnas.0905580106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paz R, Pelletier JG, Bauer EP, Pare D. Emotional enhancement of memory via amygdala-driven facilitation of rhinal interactions. Nature Neuroscience. 2006;9:1321–1329. doi: 10.1038/nn1771. [DOI] [PubMed] [Google Scholar]
- 37.Paz R, Bauer EP, Pare D. Measuring correlations and interactions among four simultaneously recorded brain regions during learning. J Neurophysiol. 2009;101:2507–2515. doi: 10.1152/jn.91259.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schoenbaum G, Chiba A, Gallagher M. Orbitalfrontal cortex and basolateral amygdala encode expected outcomes during learning. Nature Neuroscience. 1998;1:155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- 39.Stalnaker TA, Roesch MR, Franz TM, Calu DJ, Singh T, Schoenbaum G. Cocaine-induced decision-making deficits are mediated by miscoding in basolateral amygdala. Nat Neurosci. 2007;10:949–951. doi: 10.1038/nn1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ambroggi F, Ishikawa A, Fields HL, Nicola SM. Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron. 2008;59:648–661. doi: 10.1016/j.neuron.2008.07.004. Following up on previous work showing that the rodent BLA is necessary for normal reward-seeking behavior in response to an incentive cue [41], this study shows that BLA input, in conjunction with dopaminergic input, is necessary for normal encoding of a rewarded cue in the nucleus accumbens core.
- 41.Ishikawa A, Ambroggi F, Nicola SM, Fields HL. Contributions of the amygdala and medial prefrontal cortex to incentive cue responding. Neuroscience. 2008;155:573–584. doi: 10.1016/j.neuroscience.2008.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tye KM, Stuber GD, de Ridder B, Bonci A, Janak PH. Rapid strengthening of thalamo-amygdala synapses mediates cue-reward learning. Nature. 2008;453:1253–1257. doi: 10.1038/nature06963. Using an instrumental reward conditioning task in rats, the authors show that successful reward learning is correlated with the number of lateral amygdala (LA) neurons that show enhanced responses to the rewarded cue. Moreover, reward learning led to strengthening of glutamatergic thalamo-amygdalar synapses in the LA, and both learning and synaptic enhancement were impaired by NMDA receptor blockade.
- 43.Popescu AT, Popa D, Pare D. Coherent gamma oscillations couple the amygdala and striatum during learning. Nat Neurosci. 2009;12:801–807. doi: 10.1038/nn.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008 doi: 10.1038/nature07166. The authors identify a population of neurons in the rodent basal amygdala that is excited by cues that were previously associated with shock, but have been extinguished, along with a population that is preferentially excited by cues that are actively associated with shock. These two populations are differently connected with the hippocampus and mPFC, and thus may form part of two distinct circuits that are activated in the fearful and extinguished contexts, respectively.
- 45.Kluver H, Bucy P. Preliminary analysis of functions of the temporal lobes in monkeys. Arch Neurol Psychiatry. 1939;42:979–1000. doi: 10.1176/jnp.9.4.606. [DOI] [PubMed] [Google Scholar]
- 46.Aggleton JP, Passingham RE. Syndrome produced by lesions of the amygdala in monkeys (Macaca mulatta) Journal of Comparative and Physiological Psychology. 1981;95:961–977. doi: 10.1037/h0077848. [DOI] [PubMed] [Google Scholar]
- 47.Baxter MG, Murray EA. The amygdala and reward. Nature Reviews Neuroscience. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- 48.Antoniadis EA, Winslow JT, Davis M, Amaral DG. Role of the primate amygdala in fear-potentiated startle: effects of chronic lesions in the rhesus monkey. J Neurosci. 2007;27:7386–7396. doi: 10.1523/JNEUROSCI.5643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Antoniadis EA, Winslow JT, Davis M, Amaral DG. The nonhuman primate amygdala is necessary for the acquisition but not the retention of fear-potentiated startle. Biol Psychiatry. 2009;65:241–248. doi: 10.1016/j.biopsych.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walker DL, Paschall GY, Davis M. Glutamate receptor antagonist infusions into the basolateral and medial amygdala reveal differential contributions to olfactory vs. context fear conditioning and expression. Learn Mem. 2005;12:120–129. doi: 10.1101/lm.87105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- 52.Machado CJ, Bachevalier J. Behavioral and hormonal reactivity to threat: effects of selective amygdala, hippocampal or orbital frontal lesions in monkeys. Psychoneuroendocrinology. 2008;33:926–941. doi: 10.1016/j.psyneuen.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Machado CJ, Kazama AM, Bachevalier J. Impact of amygdala, orbital frontal, or hippocampal lesions on threat avoidance and emotional reactivity in nonhuman primates. Emotion. 2009;9:147–163. doi: 10.1037/a0014539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Machado CJ, Bachevalier J. Measuring reward assessment in a semi-naturalistic context: the effects of selective amygdala, orbital frontal or hippocampal lesions. Neuroscience. 2007;148:599–611. doi: 10.1016/j.neuroscience.2007.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Machado CJ, Bachevalier J. The effects of selective amygdala, orbital frontal cortex or hippocampal formation lesions on reward assessment in nonhuman primates. Eur J Neurosci. 2007;25:2885–2904. doi: 10.1111/j.1460-9568.2007.05525.x. [DOI] [PubMed] [Google Scholar]
- 56.Murray EA, Izquierdo A. Orbitofrontal cortex and amygdala contributions to affect and action in primates. Ann N Y Acad Sci. 2007;1121:273–296. doi: 10.1196/annals.1401.021. [DOI] [PubMed] [Google Scholar]
- 57. Izquierdo A, Murray EA. Selective bilateral amygdala lesions in rhesus monkeys fail to disrupt object reversal learning. J Neurosci. 2007;27:1054–1062. doi: 10.1523/JNEUROSCI.3616-06.2007. The authors use selective, excitotoxic lesions of the nonhuman primate amygdala to reevaluate the role of the amygdala in forming stimulus-reward associations. They find that the amygdala is indeed necessary for successful reinforcer devaluation, but, contrary to previous findings, that it is not essential for successful reversal learning in a rewarded object choice task.
- 58.Wellman LL, Gale K, Malkova L. GABAA-mediated inhibition of basolateral amygdala blocks reward devaluation in macaques. Journal of Neuroscience. 2005;25:4577–4586. doi: 10.1523/JNEUROSCI.2257-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rudebeck PH, Murray EA. Amygdala and orbitofrontal cortex lesions differentially influence choices during object reversal learning. J Neurosci. 2008;28:8338–8343. doi: 10.1523/JNEUROSCI.2272-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ostlund SB, Balleine BW. Differential involvement of the basolateral amygdala and mediodorsal thalamus in instrumental action selection. J Neurosci. 2008;28:4398–4405. doi: 10.1523/JNEUROSCI.5472-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Johnson AW, Gallagher M, Holland PC. The basolateral amygdala is critical to the expression of pavlovian and instrumental outcome-specific reinforcer devaluation effects. J Neurosci. 2009;29:696–704. doi: 10.1523/JNEUROSCI.3758-08.2009. Along with [60], this study confirms that specific, excitotoxic BLA lesions in rats interfere with reinforcer devaluation under both Pavlovian and instrumental conditioning, and using either satiation or conditioned taste aversion for devaluation. However, the BLA does not seem necessary for devaluation when only a single outcome is available.
- 62. Wassum KM, Ostlund SB, Maidment NT, Balleine BW. Distinct opioid circuits determine the palatability and the desirability of rewarding events. Proc Natl Acad Sci U S A. 2009;106:12512–12517. doi: 10.1073/pnas.0905874106. In contrast to classic reward devaluation studies, the authors use food deprivation in rats to induce inflation of a sucrose reward. Blockade of opioid receptors in BLA blocks reward inflation-related changes in sucrose-seeking behavior while leaving intact the deprivation-related increase in sucrose palatability.
- 63.Rabinak CA, Maren S. Associative structure of fear memory after basolateral amygdala lesions in rats. Behav Neurosci. 2008;122:1284–1294. doi: 10.1037/a0012903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rabinak CA, Orsini CA, Zimmerman JM, Maren S. The amygdala is not necessary for unconditioned stimulus inflation after Pavlovian fear conditioning in rats. Learn Mem. 2009;16:645–654. doi: 10.1101/lm.1531309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zimmerman JM, Rabinak CA, McLachlan IG, Maren S. The central nucleus of the amygdala is essential for acquiring and expressing conditional fear after overtraining. Learn Mem. 2007;14:634–644. doi: 10.1101/lm.607207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Floresco SB, Ghods-Sharifi S. Amygdala-prefrontal cortical circuitry regulates effort-based decision making. Cereb Cortex. 2007;17:251–260. doi: 10.1093/cercor/bhj143. [DOI] [PubMed] [Google Scholar]
- 67.Ghods-Sharifi S, St Onge JR, Floresco SB. Fundamental contribution by the basolateral amygdala to different forms of decision making. J Neurosci. 2009;29:5251–5259. doi: 10.1523/JNEUROSCI.0315-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brand M, Grabenhorst F, Starcke K, Vandekerckhove MM, Markowitsch HJ. Role of the amygdala in decisions under ambiguity and decisions under risk: evidence from patients with Urbach-Wiethe disease. Neuropsychologia. 2007;45:1305–1317. doi: 10.1016/j.neuropsychologia.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 69.Weller JA, Levin IP, Shiv B, Bechara A. Neural correlates of adaptive decision making for risky gains and losses. Psychol Sci. 2007;18:958–964. doi: 10.1111/j.1467-9280.2007.02009.x. [DOI] [PubMed] [Google Scholar]
- 70.Sotres-Bayon F, Bush DE, LeDoux JE. Acquisition of fear extinction requires activation of NR2B-containing NMDA receptors in the lateral amygdala. Neuropsychopharmacology. 2007;32:1929–1940. doi: 10.1038/sj.npp.1301316. [DOI] [PubMed] [Google Scholar]
- 71.Laurent V, Marchand AR, Westbrook RF. The basolateral amygdala is necessary for learning but not relearning extinction of context conditioned fear. Learn Mem. 2008;15:304–314. doi: 10.1101/lm.928208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Laurent V, Westbrook RF. Distinct contributions of the basolateral amygdala and the medial prefrontal cortex to learning and relearning extinction of context conditioned fear. Learn Mem. 2008;15:657–666. doi: 10.1101/lm.1080108. [DOI] [PubMed] [Google Scholar]
- 73.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Petrovic P, Kalisch R, Pessiglione M, Singer T, Dolan RJ. Learning affective values for faces is expressed in amygdala and fusiform gyrus. Soc Cogn Affect Neurosci. 2008;3:109–118. doi: 10.1093/scan/nsn002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Petrovic P, Kalisch R, Singer T, Dolan RJ. Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. J Neurosci. 2008;28:6607–6615. doi: 10.1523/JNEUROSCI.4572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schiller D, Levy I, Niv Y, LeDoux JE, Phelps EA. From fear to safety and back: reversal of fear in the human brain. J Neurosci. 2008;28:11517–11525. doi: 10.1523/JNEUROSCI.2265-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Olsson A, Nearing KI, Phelps EA. Learning fears by observing others: the neural systems of social fear transmission. Soc Cogn Affect Neurosci. 2007;2:3–11. doi: 10.1093/scan/nsm005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dolan RJ. The human amygdala and orbital prefrontal cortex in behavioural regulation. Philos Trans R Soc Lond B Biol Sci. 2007;362:787–799. doi: 10.1098/rstb.2007.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ball T, Derix J, Wentlandt J, Wieckhorst B, Speck O, Schulze-Bonhage A, Mutschler I. Anatomical specificity of functional amygdala imaging of responses to stimuli with positive and negative emotional valence. J Neurosci Methods. 2009;180:57–70. doi: 10.1016/j.jneumeth.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 80.Smith BW, Mitchell DG, Hardin MG, Jazbec S, Fridberg D, Blair RJ, Ernst M. Neural substrates of reward magnitude, probability, and risk during a wheel of fortune decision-making task. Neuroimage. 2009;44:600–609. doi: 10.1016/j.neuroimage.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Delgado MR, Nearing KI, Ledoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59:829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Delgado MR, Jou RL, Ledoux JE, Phelps EA. Avoiding negative outcomes: tracking the mechanisms of avoidance learning in humans during fear conditioning. Front Behav Neurosci. 2009;3:33. doi: 10.3389/neuro.08.033.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hampton AN, Adolphs R, Tyszka MJ, O'Doherty JP. Contributions of the amygdala to reward expectancy and choice signals in human prefrontal cortex. Neuron. 2007;55:545–555. doi: 10.1016/j.neuron.2007.07.022. The authors use fMRI to examine two rare patients with specific, bilateral amygdala lesions. In a reward conditioning task with reversal, the patients exhibited major differences from normal controls in the patterns of ventromedial PFC activation, implying that the amygdala contributes to normal reward expectation and behavioral choice-related signals in PFC.
- 84.Talmi D, Seymour B, Dayan P, Dolan RJ. Human pavlovian-instrumental transfer. J Neurosci. 2008;28:360–368. doi: 10.1523/JNEUROSCI.4028-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tye KM, Janak PH. Amygdala neurons differentially encode motivation and reinforcement. J Neurosci. 2007;27:3937–3945. doi: 10.1523/JNEUROSCI.5281-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.De Martino B, Kumaran D, Seymour B, Dolan RJ. Frames, biases, and rational decision-making in the human brain. Science. 2006;313:684–687. doi: 10.1126/science.1128356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Roiser JP, de Martino B, Tan GC, Kumaran D, Seymour B, Wood NW, Dolan RJ. A genetically mediated bias in decision making driven by failure of amygdala control. J Neurosci. 2009;29:5985–5991. doi: 10.1523/JNEUROSCI.0407-09.2009. This study takes advantage of the framing effect – the phenomenon in which subjects are more likely to make the same gamble when it is presented in terms of losses than in terms of gains. Building on previous findings [86] which showed that amygdala activity is correlated with decisions in keeping with the framing effect, the authors demonstrate that this neural and behavioral phenomenon is enhanced in subjects that are homozygous for the “short” serotonin transporter allele.
- 88.Munafo MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biol Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]