Abstract
Prostaglandins (PGs) are multifunctional regulators of bone metabolism that stimulate both bone resorption and formation. PGs have been implicated in bone resorption associated with inflammation and metastatic bone disease and also in bone formation associated with fracture healing and heterotopic ossification. Recent studies identified roles for inducible cyclooxygenase-2 (COX-2) and PGE2 receptors in these processes. Although the effects of PGs have been most often associated with cAMP production and PKA activation, PGs can engage an extensive G-protein signaling network. Further analysis of COX-2 and PG receptors and their downstream G-protein signaling in bone could provide us with important clues to the regulation of skeletal cell growth in both health and disease.
Keywords: prostaglandin, cyclooxygenase-2, osteoblasts, osteoclasts, bone resorption, bone formation
Prostaglandins (PGs)
PGs are lipids that act in an autocrine/paracrine manner via G-protein coupled receptors (GPCRs). In the 40 years since prostaglandin E2 (PGE2) was shown to stimulate cAMP production and resorption in bone organ cultures, many studies have demonstrated that PGE2 and other prostanoids are abundantly expressed in bone and can have important roles in skeletal metabolism. PGs are produced in response to many factors that regulate bone metabolism, largely as the result of cyclooxygenase-2 (COX-2) induction.
The multiple facets of PGs and their receptors have frustrated attempts to define simple roles for PGs in bone. On the one hand, PGs may mediate bone loss associated with inflammation and osteolytic effects of metastatic cancer. On the other hand, PGs can stimulate bone formation, and studies with selective inhibitors and knockout mice have identified a role for PGs in accelerating fracture healing and led to the development of potential therapeutic agents to increase bone mass. With the recent interest in the role of PGs in enhancing tumorigenesis, it has become apparent that the local induction of COX-2/PGs is an important means by which many factors can engage the extensive G-protein signaling network that is so important for cell growth and differentiation. It seems likely that the actions of COX-2/PGs in bone, as in other tissues, will be defined by the specific COX-2 agonists inducing them and the responding cell types.
COX-2 and PG Production
All three major steps in the production of PGE2 (Box 1) are subject to regulation and each can be rate limiting. Under most conditions, the critical limiting step in the conversion of arachidonic acid to PGE2, and the step inhibited by nonsteroidal anti-inflammatory drugs (NSAIDs), is the enzyme that catalyzes both cyclooxygenase and peroxidase reactions. This enzyme, formally named prostaglandin endoperoxide H synthase or prostaglandin G/H synthase (gene name, ptgs) is now called cyclooxygenase (COX) in reference to its first function. The reason for two COX enzymes is an unanswered question. Although COX-1 and COX-2 have many similarities, including location in the endoplasmic reticulum and nuclear envelope and essentially the same catalytic mechanisms [1,2], they appear to be independently functioning biosynthetic pathways. This is due in part to differential regulation of their expression. The gene for COX-1 (ptgs1) is constantly expressed with little regulation (e.g. constitutively expressed), whereas the gene for COX-2 (ptgs2) is rapidly and transiently induced to high levels by multiple factors [3]. Now that quantitative polymerase chain reaction (PCR) is routinely used to examine gene expression, it is clear that COX-2 mRNA is constitutively expressed at levels too low under basal conditions to be easily detectible with the Northern analysis used in the past in many tissues, including bone. The ptgs2 promoter has multiple transcriptional regulatory elements typical of early response genes [2]. Cis-acting sequences shown to increase transcription of ptgs2 in osteoblastic cells include a cAMP response element (CRE), an activator protein-1 (AP-1) binding site, a C/EBP site, a Runx2 site, and a nuclear factor of activated T-cells (NFAT)/AP-1 composite binding site [4,5].
BOX 1. PG Production
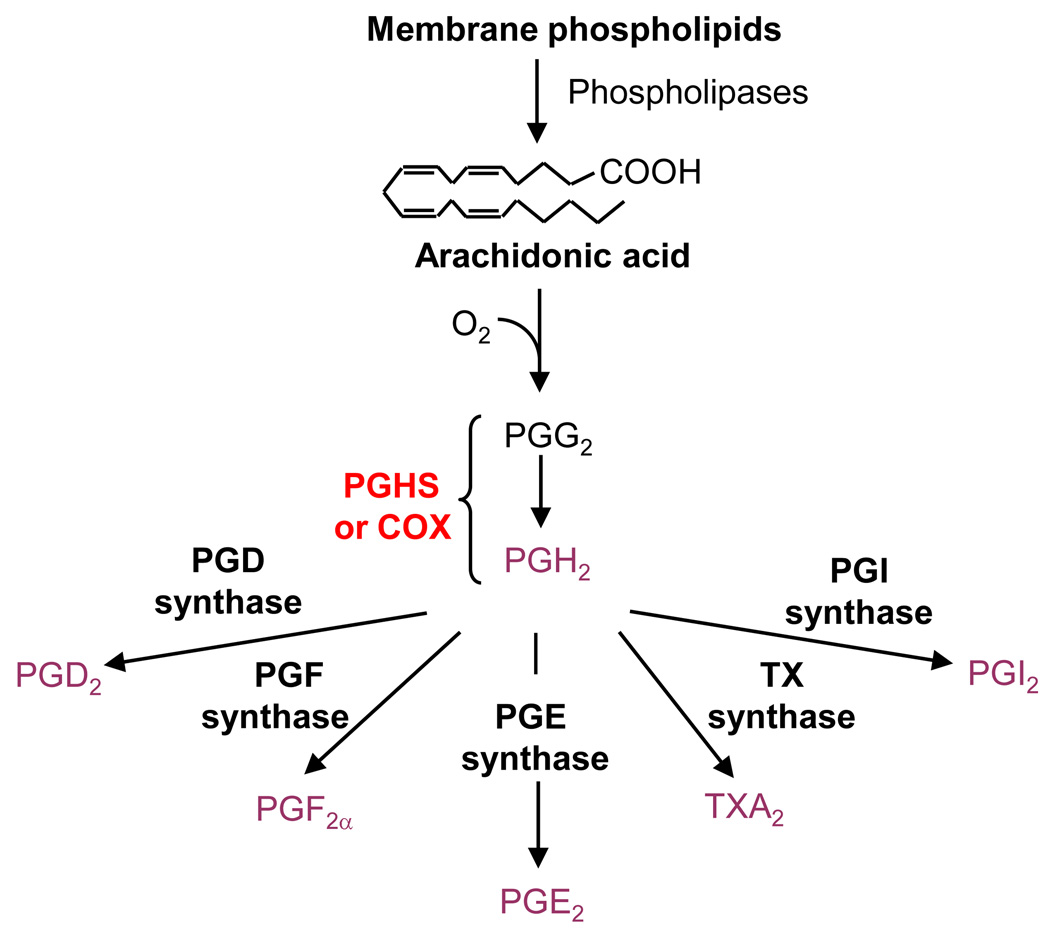
The production of prostanoids involves three major steps (Figure I): (1) mobilization of arachidonic acid (AA) from membranes; (2) conversion of AA to the unstable endoperoxide intermediates, prostaglandin G2 (PGG2) and prostaglandin H2 (PGH2); and (3) conversion of PGH2 by terminal synthases to PGE2, PGD2, PGF2α, prostacyclin (PGI2), and thromboxane (TXA2). A bifunctional enzyme converts free AA to PGG2 in a cyclooxygenase reaction and reduces PGG2 to PGH2 in a peroxidase reaction. This enzyme is popularly called cyclooxygenase (COX) in reference to its first function. Nonsteroidal anti-inflammatory drugs (NSAIDs) inhibit the activity of COX and production of PGs by competing with AA for binding to COX. There are two isoforms for COX: COX-1 and COX-2, encoded by separate genes. Slight differences in size and shape of the COX-1 and COX-2 catalytic sites have made it possible to develop NSAIDs selective for one or the other COX isoforms.
A splicing variant of COX-1, arising via the retention of intron 1, was called COX-3 and reported to be differentially sensitive to inhibition by acetaminophen. However, later studies concluded that this variant is unlikely to be a therapeutic target of acetaminophen and probably has little or no COX activity in humans [1].
Differential regulation of expression cannot account for all the differences between COX-1 and COX-2. For example, ptgs1 inserted under the regulatory sequences that drive ptgs2 expression cannot substitute for all of the deficits seen with ptgs2 deletion [6]. There are clearly differences in PG production by COX-1 and COX-2, with COX-2 being more efficient at producing PGs than COX-1 in many circumstances [7]. Osteoblasts from mice with disruption of ptgs2 produce little PGE2 in culture despite the constitutive expression of COX-1, unless excess arachidonic acid is added to cultures [4]. However, there must be compensation by COX-1 in some tissues since double ptgs1/ptgs2 deficient mice die from failure of ductus arteriosus closure shortly after birth [8]. One study reported a beneficial effect of COX-1 deficiency on bone parameters in vivo [9], and another study suggested that COX-1 might compensate for COX-2 deficiency in the response of bone to mechanical loading in vivo [10]. However, the role of COX-1 in bone is still unclear.
PGs are not stored in cells and are rapidly degraded in vivo during passage through the lungs by 15-hydroxyprostaglandin dehydrogenase (PDGH) [11]. In vitro, however, PGE2 is stable and can accumulate in cell culture media. PGs can modulate the effects of agonists that induce them, and the effects of such regulation might be quite different in vitro, where PGs accumulate between media changes, and in vivo, where PGs are rapidly lost to the cells that produce them. In addition to the many cytokines, growth factors and hormones that induce COX-2, serum is also a potent stimulator of COX-2 expression and PGE2 production in cultured osteoblasts, and PGs induced by fresh serum at the time of media changes may affect the response of cells to other factors being added to the cultures [4]. In addition, PGs themselves can stimulate COX-2 expression, and this can amplify the effects of PGs and of other agonists that induce COX-2 [12]. This autoamplification effect may be particularly important in vitro where PGs are not rapidly degraded. In vivo, the autoamplifying mechanism could contribute to prolonging the effects of short periods of impact loading in skeletal tissue or the effects of cytokines in inflammatory diseases.
PGE2 Receptors and Signaling
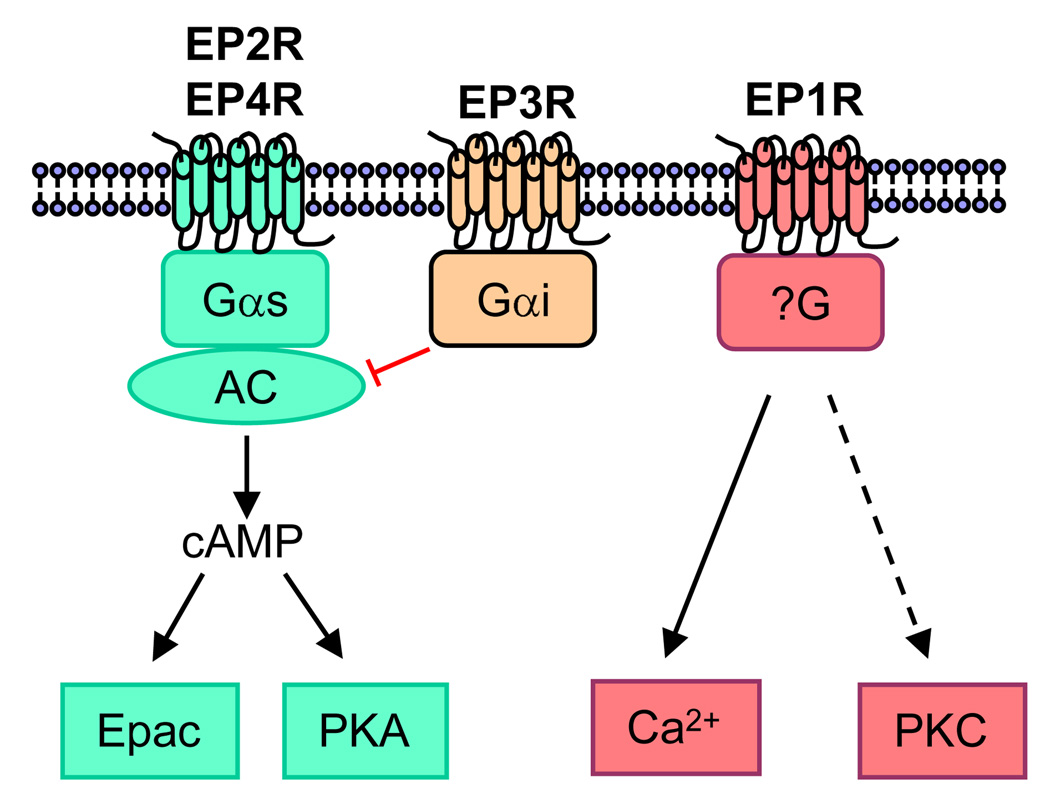
Since PGE2 was first shown to stimulate cAMP production and resorption in bone organ cultures [15], the effects of PGE2 in bone have been most often associated with cAMP production and protein kinase A (PKA) activation, indicating an important role for PGE2 receptors EP2R and EP4R, which are both coupled to Gαs (Box 2). EP2R and EP4R have been proposed to mediate the anabolic effects of PGE2 on bone [16–20], but the details of how this occurs are unknown. It is also unknown why two receptors for PGE2 are coupled to Gαs. A number of studies suggest that EP2R and EP4R have non-overlapping functions. This might be due in part to differential regulation of expression or differential desensitization of the receptors. Studies with selective EP receptor agonists suggest that selective EP2R agonists stimulate greater cAMP production and COX-2 induction than EP4R agonists [12], and hence, differential production of endogenous PGs may also contribute to differences between EP2R and EP4R.
BOX 2. PGE2 Receptors
There are at least nine G-protein linked PG receptors mediating prostanoid actions, one or more for each of the prostanoids [13]. Four classes of GPCRs are associated with the effects of PGE2: EP1, EP2, EP3, and EP4 14 (Figure I). EP1R acts largely by increasing calcium flux but perhaps also via protein kinase C (PKC). Although it may be coupled to Gαq, the absence of a phosphatidylinositide response has led to speculation that it is coupled to an as yet unidentified G protein [14]. Both EP2R and EP4R are coupled to Gαs and stimulate cyclic 3,5-adenosine monophosphate (cAMP) formation. EP3R is coupled to Gαi and acts largely by inhibiting cAMP production. However, there are multiple alternative transcripts of the EP3R receptor that may act through other signal transduction pathways. Mice deficient in each EP receptor subtype have been generated, and highly selective agonists for these receptors have been developed [14]. These agonists, which are not yet commercially available, are the basis for many reports of differential effects of EP receptors.
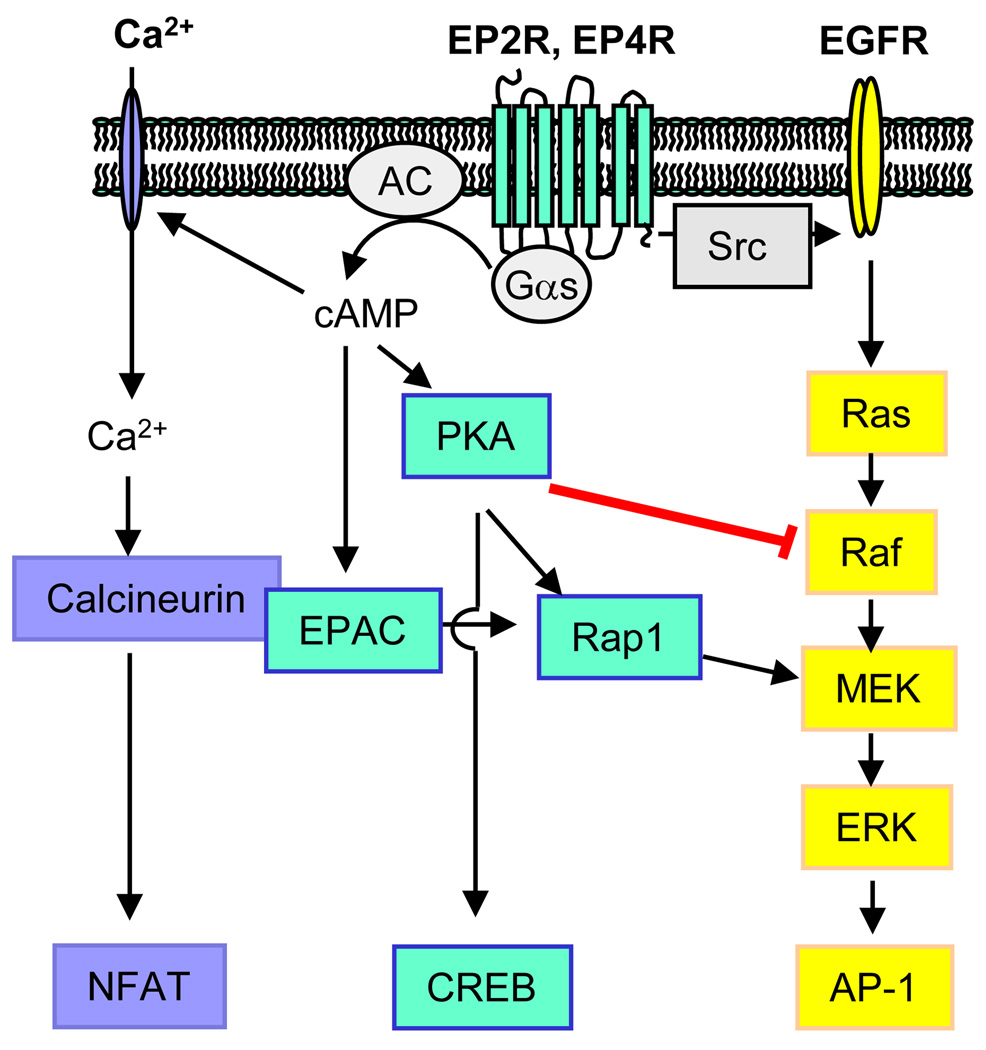
There is a growing literature on the interaction of Gαs-activated signaling with other signaling pathways to regulate mitogenic activities of cells [21]. A few of the potential pathways that may interact with or be engaged by EP2R and EP4R are illustrated in Figure 1. Studies in non-bone tissues, such as colon cancer cells, designed to elucidate the role of PGE2 in promoting tumorigenesis, suggest involvement of PI3 kinase, mitogen activated protein kinase (MAPK) and Wnt pathways in the EP2R/EP4R regulation of cell growth, migration and apoptosis [22–25]. In addition, activation of cAMP/PKA signaling in osteoblastic cells can lead to stimulation of calcium–calcineurin-NFAT signaling, and this pathway might be important for bone formation and resorption [5]. PGE2 effects might also be mediated by cAMP independent of PKA through Epac (exchange protein directly activated by cAMP), a guanine nucleotide exchange factor for the small GTPases Rap1 and Rap2 [26]. An additional mechanism for the differential effects of EP2R and EP4R activation might be differential effects on the PKA regulatory subunits, RI and RII [27]. PKA-RII can be tethered by A-kinase anchoring proteins (AKAPs) into complexes that include phosphodiesterases as well as different effectors of cAMP signaling, such as calcineurin, Epac, and protein kinase C (PKC), thereby integrating multiple signaling pathways [28]. The pathways diagrammed in Figure 1 also indicate how EP receptor signaling can lead to increased PGE2 production and autoamplification of EP receptor signaling, since ERK phosphorylation can increase phospholipase A2 activity and arachidonic acid release, and both ERK phosphorylation and the PKA-CREB pathway can increase COX-2 expression [4].
Figure 1.
Examples of pathways that may be activated by EP2R and EP4R. (i) Gαs may act via cAMP-gated channels to increase Ca2+ and Ca2+-dependent calcineurin signaling. Gαs may also act via (ii) cAMP to activate PKA or EPAC, leading to activation or inhibition of phosphorylation of extracellular signal-regulated kinase (ERK), a member of the mitogen-activated protein kinase (MAPK) family. (iii) Gαs may also act independently of cAMP to activate the MAPK pathway by transactivating epidermal growth factor receptor (EGFR) via Src. Potential target transcription factors include nuclear factor of activated T-cells (NFAT), cAMP-response element binding protein (CREB) and activator protein-1(AP-1).
Much remains to be learned about PGE2 signaling in bone cells. It is likely that several of the pathways identified in other tissues as mediating effects of PGE2 on cell growth will also be important for the anabolic effects of PGE2 in bone. In fact, a recent study suggests that some of the actions of PGE2 that stimulate formation of bone nodules in vitro are mediated by differential actions of EP2R and EP4R on MAPK signaling pathways [29]. In addition, it is proposed that the Wnt/β-catenin signaling pathway, known to be an important regulator of bone mass, is triggered by PGs in osteocytes in response to loading to transmit anabolic signals of mechanical loading to cells on the bone surface [30]. Understanding PGE2 signaling in bone cells should help us understand the actions of parathyroid hormone (PTH), which not only acts via similar GPCR pathways but is also a strong inducer of COX-2 and PG production. PTH is the major calcium-regulating hormone in the body, and when given intermittently, is a potent anabolic drug for treating osteoporosis. Because most of the data on PG signaling in bone has been obtained in rodent tissues, the relevance of these data for human cells needs to be determined. In addition, based on data from early studies in osteoblastic cells, it is generally assumed that PGE2 is the predominant PG in bone, but the roles of other eicosanoids in bone biology need to be clarified.
PGE2 and Bone Resorption
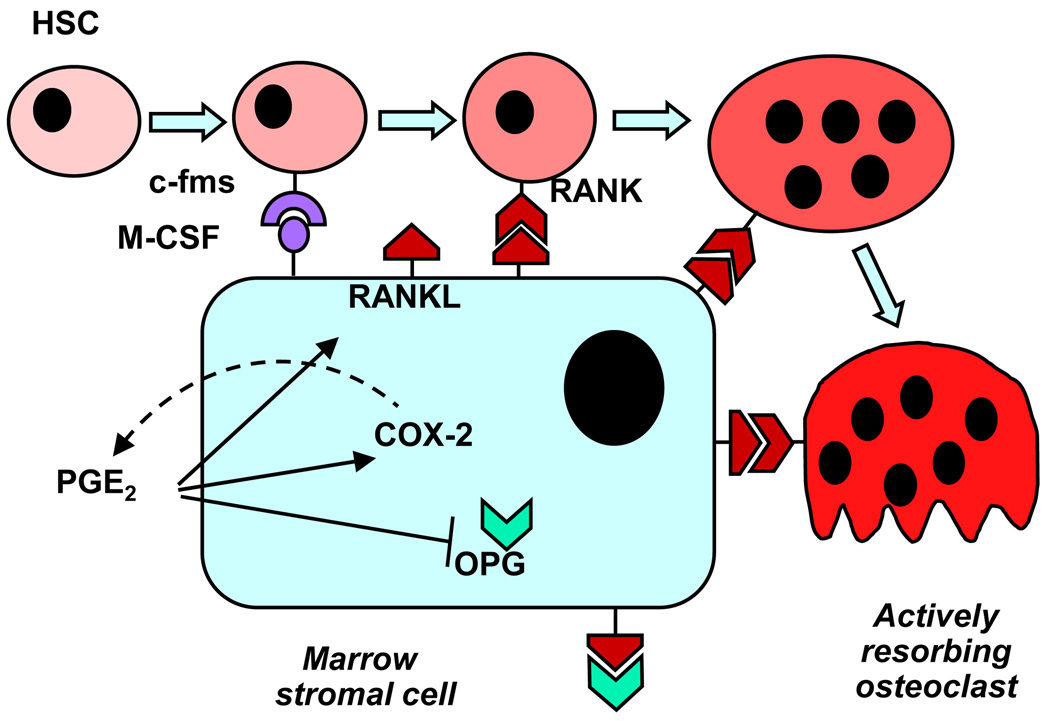
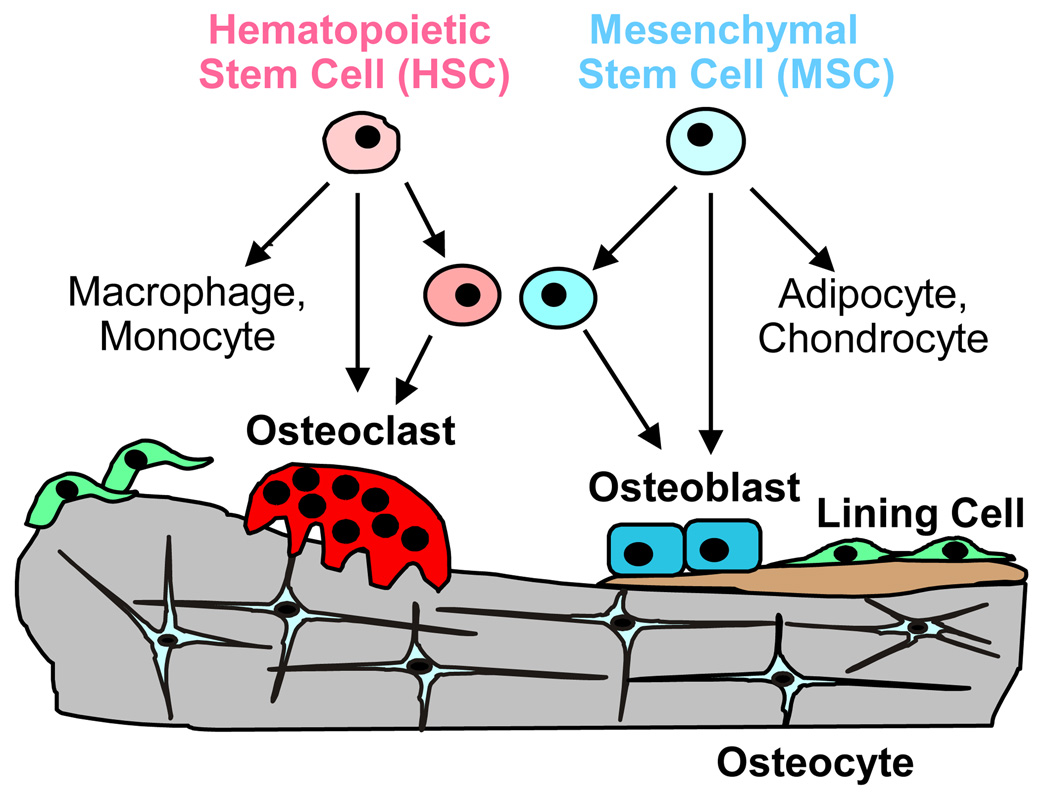
Bone turnover is the result of bone resorption and formation (Box 3), and both processes can be regulated by PGE2. Exogenous PGE2 is a strong stimulator of bone resorption in bone organ cultures and of osteoclast differentiation in marrow cultures [4]. These cultures contain precursors of both osteoblastic (mesenchymal lineage) and osteoclastic (hematopoietic lineage) cells. Studies using marrow cultures from mice with ptgs2 deleted or treated with selective COX-2 inhibitors show that endogenous PGs, produced by induction of COX-2, enhance the stimulation of osteoclast differentiation by multiple resorption agonists, including cytokines, interleukin (IL)-1, IL-6, IL-11, IL-17, and tumor necrosis factor (TNF)-α; hormones (PTH, vitamin D3); and growth factors, fibroblast growth factor (FGF)-2 and bone morphogenetic protein (BMP)-2 [4] (Box 4). The major effect of PGE2 on resorption is generally considered to occur indirectly via upregulation of receptor activator of NFκB ligand (RANKL) expression and inhibition of osteoprotegerin (OPG) expression in osteoblastic cells. Some of the roles proposed for COX-2 expression and PGE2 production in the osteoblastic support of osteoclast differentiation are diagrammed in Figure 2.
BOX 3. Bone Turnover
Gain or loss of bone mass is the result of the balance of bone resorption by osteoclasts and bone formation by osteoblasts (Figure I). Osteoclasts are derived from hematopoietic stem cells while osteoblasts are derived from mesenchymal stem cells. While all mature osteoclasts undergo apoptosis after completing resorption, some osteoblasts become quiescent lining cells or are incorporated into the matrix as osteocytes. Lining cells may become activated again and participate in new bone turnover. Osteocytes are thought to be strain-sensing cells, critical for bone responses to mechanical loading. In humans and larger mammals, resorption and formation occur at the same site (Haversian canals or Howship’s lacunae) and constitute a remodeling cycle in which resorption occurs first and is followed by formation. In rodents, resorption and formation may occur independently and at different sites. Different strains of mice have higher or lower basal bone turnover and may respond differently to perturbations as a result.
BOX 4. Osteoclast Differentiation
The differentiation of multinucleated bone-resorbing osteoclasts is dependent on interaction of receptor activator of NFκB ligand (RANKL) expressed by marrow stromal or osteoblastic cells with the receptor RANK expressed on osteoclastic precursors [31] (Figure 2). Many resorption agonists stimulate osteoclast differentiation primarily via inducing expression of RANKL by the osteoblastic lineage. Thus, interaction of osteoclastic and osteoblastic cells is critical at an early stage. Also produced by osteoblastic cells is macrophage colony stimulating factor (M-CSF), which is the ligand for the c-fms receptor and is necessary for early proliferation of osteoclast precursors, and osteoprotegerin (OPG), which is the decoy receptor for RANKL and inhibits osteoclast formation. In vitro, osteoclasts are defined as multinuclear cells that stain positive for tartrate resistant acid phosphase (TRAP), express receptors for calcitonin, and are capable of resorbing bone.
Figure 2.
Potential actions of COX-2 and PGE2 on osteoblastic cells to stimulate osteoclast differentiation. PGE2 increases expression of receptor activator of NFκB ligand (RANKL), which binds to its receptor RANK on osteoclast lineage cells to drive differentiation. PGE2 also inhibits the expression of osteoprotegerin (OPG), the decoy receptor for RANKL. Binding of macrophage-colony stimulating factor (M-CSF) to its receptor (c-fms) is essential to stimulate proliferation of osteoclast progenitors.
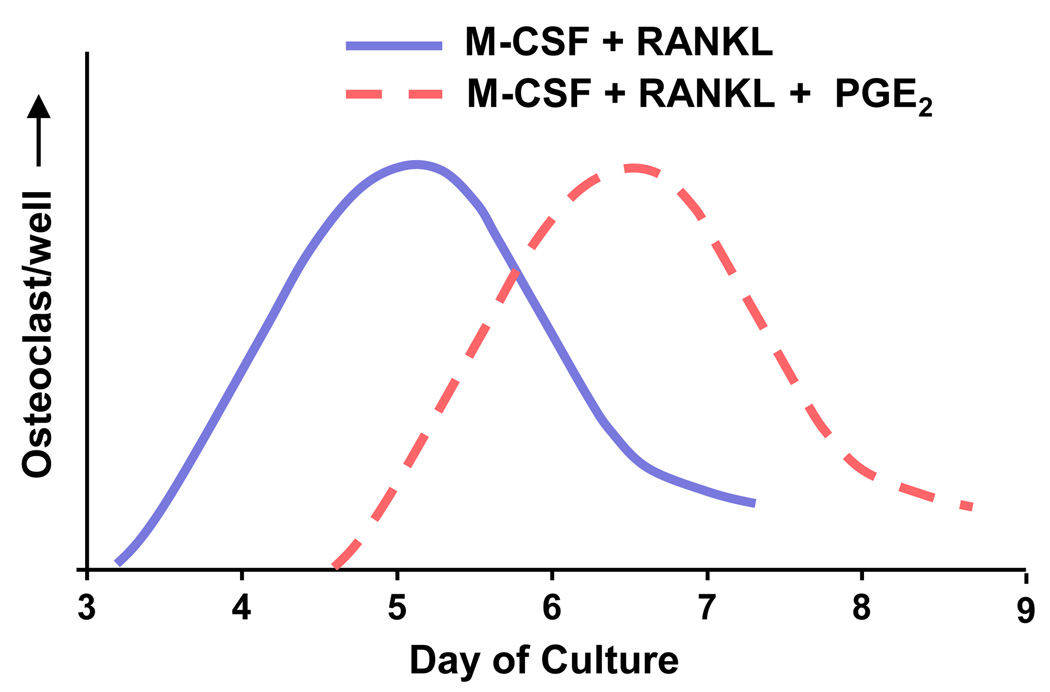
COX-2 is also expressed in cells of the hematopoietic lineage [32,33], and PGs may have direct effects on osteoclastic cells. Models used to study direct effects of factors on hematopoietic lineage cells include clonal murine RAW264.7 cells, freshly isolated spleen cells, peripheral blood mononuclear cells (PBMCs), and partially purified bone marrow macrophages (BMMs). These cultures do not produce endogenous RANKL, and either RANKL itself or cells that can be induced to produce RANKL must be added to permit osteoclast formation. PGE2 has both stimulatory and inhibitory effects on RANKL-stimulated osteoclast formation in these cultures. For example, PGE2 increases RANKL-stimulated osteoclast differentiation in RAW264.7 cells [34], but inhibits differentiation in cultured human PBMCs [35]. In spleen cells studied over an extensive time course, exogenous PGE2 had biphasic effects on RANKL-stimulated osteoclast formation, with an initial inhibitory effect and a later stimulatory effect [36]. Biphasic effects of exogenous PGE2 on RANKL-stimulated osteoclast formation have also been seen in BMM cultures [37]. An example of how conflicting conclusions among studies about the effects of PGE2 on osteoclast formation could be explained by different timing of measurements is shown in Figure 3. Finally, there are studies showing that PGs transiently inhibit the activity of isolated mature bone-resorbing osteoclasts [32,38]. The relevance of any of the effects of PGs on the hematopoietic lineage, whether stimulatory or inhibitory, to resorption and osteoclastic development in vivo is unknown.
Figure 3.
Biphasic effect of PGE2 on osteoclast formation in bone marrow macrophage (BMM) cultures. BMMs stimulated to become osteoclasts by treatment with RANKL and M-CSF. The addition of PGE2 to RANKL and M-CSF delays the appearance of osteoclasts. If there is only one measurement time, the conclusion as to the effect of PGE2 on RANKL-stimulation of osteoclast differentiation will depend on the day of measurement. On day 5, PGE2 “inhibits” and on day 7, it “stimulates.”
Similar to the effects of PGE2 on bone formation, PGE2-stimulation of bone resorption in rodents is largely dependent on Gαs-activation via EP4R and EP2R. In vitro data from rodents indicate that PGE2 stimulated resorption is predominantly mediated via EP4R, with some contribution from EP2R [39]. A marked decrease in resorptive response to PGE2 was found in cultured calvariae from mice with EP4R gene deletion, and there was impaired response to PGE2 even in calvariae from mice with only one allele for EP4R disrupted [40,41]. However, aged mice with EP4R gene deletion have increased osteoclast numbers on trabecular surfaces and increased eroded endocortical surface compared to wild type mice [42], and administration of an EP4R selective agonist to rats subjected to protocols causing bone loss, such as immobilization, increases bone formation rather than resorption [16]. These data suggest that more in vivo studies comparing effects of EP receptor gene deletion on resorption are needed to confirm conclusions from in vitro data.
PGE2 and Bone Formation
Although PGE2 was initially recognized for its effects on bone resorption, it soon became evident that it also stimulates bone formation. Similar to PTH, which also has major actions via cAMP, systemic injection of PGE2 increases both bone resorption and formation; however, formation can be greater than resorption, resulting in substantial increases in bone mass in rats, dogs and humans [4]. Similar to PTH, continuous PGE2 administration in rats can lead to bone loss, whereas intermittent administration is anabolic [43]. Selective agonists for both EP2 and EP4 receptors have been developed with the goal of stimulating the anabolic effects of PGE2 without the undesirable side effects of systemic PGE2 injection, such as diarrhea and hypotension. Local application of EP2R agonists [20] and local and systemic application of EP4R agonists [16,19,44] have anabolic effects in vivo and enhance bone healing in rodent models. It is unclear if these selective agonists also have limiting side effects when given systemically.
The most consistent osteogenic effects of PGE2 are seen in cultures designed to study osteoblastic differentiation. PGE2 stimulates osteoblastic differentiation in marrow stromal cell and primary calvarial cell cultures [4], and osteoblastic differentiation is decreased in marrow stromal cell cultures from mice deficient in endogenous PGs due to deletion of ptgs2 [45,46]. Consistent with the anabolic effects of EP2R and EP4R agonists in vivo, both EP2R and EP4R have been implicated in the osteogenic effects of PGE2 in vitro [17,29,47]. Using NSAIDs or cells from mice with disruption of ptgs2, it has been shown that induction of endogenous PG production by freshly added serum [4] and some osteogenic agonists, such as strontium ranelate [46] and BMP-2 [48], can enhance the stimulation of osteoblast differentiation in vitro. Thus, the induction of COX-2 and PG production by these factors might be the means of engaging additional osteogenic Gαs-activated signaling pathways that stimulate the proliferation or differentiation of early osteoblastic precursors.
Multiple studies show that PTH is a potent inducer of COX-2 and PG production [4]. PTH and PGE2 have many similarities, in addition to similar cAMP signaling pathways. They both stimulate bone formation and bone resorption in vivo and osteoclast differentiation in vitro. One puzzling difference is that continuous treatment of osteoblastic cells in vitro with PGE2 stimulates osteoblastic differentiation, whereas continuous treatment with PTH does not [49]. By “continuous”, we mean that treatment is added at each media change. PTH has been shown to be stable in culture up to 72 h between medium changes [50]. However, continuous treatment with PTH stimulates osteoblast differentiation in marrow stromal cultures when COX-2 expression is absent or COX-2 activity is inhibited by selective NSAID [51]. Mice with disruption of ptgs2 also have an enhanced anabolic response to intermittent PTH in vivo [52]. Thus, endogenous PGs can enhance or inhibit the osteogenic or anabolic effects of factors inducing them, depending on the inducing factor.
Endogenous PGs in Pathology
Whether the catabolic or anabolic function of endogenous PGs will predominate in vivo to stimulate either bone resorption or formation has been difficult to predict. Recently a familial disorder, primary idiopathic hypertrophic osteoarthropathy, was found to be associated with mutation in the enzyme 15-hydroxyprostaglandin dehydrogenase (PDGH) that inactivates PGs. These patients have chronically elevated PGE2 levels, digital clubbing and evidence of both increased bone formation and resorption in their phalanges [11].
On the other hand, elevated PG production associated with inflammatory diseases, such as rheumatoid arthritis, can cause bone loss. Many cytokines involved in inflammation are potent inducers of COX-2 expression and PGE2 production. Although cytokines themselves are potent inducers of bone resorption independent of PG production, selective inhibition of COX-2 can reduce bone loss and cartilage destruction associated with inflammatory joints in rodent models [53] and reduce osteoclast surface and number [54]. Lipopolysaccharide (LPS)-induced bone loss in mice can be reduced by deletion of membrane bound PGE synthase-1, the terminal synthase in PGE2 production [55]. However, inflammatory bone loss may reflect not only increased bone resorption but also decreased bone formation. Cytokines can inhibit production of bone matrix proteins independently of cytokine-induced PG production, and it is possible that cytokine-induced PG production stimulates a compensatory anabolic effect. For example, in IL-1α transgenic mice, treatment in vivo with a COX-2 inhibitor reduced joint inflammation but increased osteopenia by suppressing bone formation [56].
PGs have been implicated in carcinogenesis and may also enhance bone metastases. In breast cancer models, inhibition of COX-2 activity decreased bone metastases, while tumors that expressed higher levels of COX-2 showed increased bone destruction by osteoclasts [57,58]. In a model of malignant melanoma, an EP4R antagonist suppressed osteolysis [59]. In models of prostate cancer, a soluble EP2R fragment blocked both tumor growth and osteolysis by bone metastases [60]. In these studies, it can be difficult to distinguish effects of PGs on tumor growth from effects on metastatic processes.
Endogenous PGs also impact fracture healing. Multiple studies in animal models show that bone repair is impaired or delayed by treatment with NSAIDs or by the absence of COX-2 [44,61–63]. In mouse models, PGs produced from COX-2 in the early inflammatory phase of bone repair are critically involved in bone healing [64]. On the other hand, PGs can lead to excessive bone formation that results in heterotopic ossification after bone surgery or trauma [65]. Thus, whether or not PGs have a beneficial effect in bone repair processes might depend on the context or the duration of COX-2 induction.
Endogenous PGs in Physiology
PGs may mediate some of the anabolic responses of bone to mechanical loading [66], responses that are important for skeletal adaptations throughout life. Many studies have shown that increased COX-2 expression and PG production are early responses to mechanical loading of bone cells, and some early studies showed that inhibition by NSAIDs of PG production also inhibited new bone formation in response to applied loading in vivo [4]. A role for COX-2 produced PGs in triggering the anabolic Wnt/β-catenin signaling pathway in osteocytes in response to loading has been proposed that would make PGs a key player in the anabolic response to loading [30]. Although such a critical role for PGs in mechanical loading remains to be confirmed, it seems likely that PGs are one of several factors, such as Ca2+ and nitric oxide, important for mechanotransduction.
Much of the data on physiologic role(s) of PGs in vivo comes from rodent models. Mice with global knockout of ptgs1, ptgs2 or genes for the EP receptors do not have dramatic skeletal phenotypes. Early studies of COX-1 and COX-2 deficiency were done in mice with a mixed C57Bl/6/129 background. In this background, mice with absence of both COX-1 and COX-2 die shortly after birth from failure of ductus arteriosus closure [8]. COX-1 deficient mice in this background survive normally, while COX-2 deficient mice have increased mortality and shortened lifespan secondary to renal dysplasia [67]. COX-2 deficient females in this background are infertile, with multiple failures in reproductive processes, including ovulation, fertilization, and implantation [68]. Some of the COX-2 deficient mice have marked hyperparathyroidism (HPTH) secondary to renal failure, and this HPTH can cause increased bone resorption and bone loss [69]. Wild-type C57Bl/6 or 129 mice may lack type GIIA secretory phospholipase A2, which contributes to releasing substrate for both COX-1 and COX-2, and therefore, there might be less compensation by COX-1 for COX-2 deficiency in this background [70]. When COX-2 deficient mice are bred in the outbred CD-1 background, they do not have renal dysfunction, and null females are fertile [68,71]. However, male COX-2 deficient mice in the CD-1 background still have HPTH, thus making it difficult to determine the direct effects of COX-2 deficiency on bone [71]. COX-2 deficient mice on all backgrounds studied have small deficits in bone mass relative to wild type mice [9,10,69,72], although female mice may be protected from this loss [73]. However, it is unclear if the increased loss observed in COX-2 deficient males is due to changes in bone formation or resorption. Future studies targeting the deletion of ptgs2 gene expression to bone may help elucidate the specific pathways involved in COX-2 signaling in bone.
In vivo studies of the skeletal phenotype of EP4R deficient mice have been limited by the high rate of neonatal death (>95%) occurring shortly after birth as a result of failure of the ductus arteriosus to close [74]. In an attempt to improve their survival, EP4R deficient mice have been bred as mixtures of multiple genetic backgrounds. Aged EP4R deficient mice in a highly mixed background were shown to have reduced bone mass and impaired fracture healing compared to wild type mice [42]. Bone mechanical properties were reduced in both EP2R and EP4R deficient mice [75,76]. In vivo studies of receptor deletions targeted to cells in bone and studied in the same carefully controlled backgrounds are needed to dissect out the functions of these receptors in bone.
NSAIDs and Bone Loss in Humans
Most of what is known about the role of endogenous PGs in bone metabolism comes from animal studies, while the application to humans remains to be confirmed. Selective COX-2 inhibitors affect bone mineral density (BMD) differently in men and women age 65 and older [76]. In men, daily COX-2 inhibitor use was associated with a 2.4–5.3% lower hip and spine BMD compared to nonusers, but in postmenopausal women not on estrogen replacement therapy, it was associated with a 0.9–5.7% higher BMD. There was no effect of COX-2 inhibitor in women on estrogen replacement. The authors speculated that beneficial effects of mechanical loading might be reduced by COX-2 inhibition in men, while the proinflammatory state and increased bone turnover associated with estrogen withdrawal might be suppressed by COX-2 inhibition in postmenopausal women. Although studies suggest that female mice with COX-2 deficiency might be protected from the bone loss seen in male mice with COX-2 deficiency [73], a causal role for endogenous PGs in the bone loss due to estrogen deficiency has not been confirmed [4]. These interesting observations provide an impetus for further examination of the role of sex differences in skeletal responses to PGs.
Large scale clinical studies on the skeletal effects of inhibiting COX-2 in healthy humans are unlikely to be undertaken since selective COX-2 inhibitors may elevate the risk of myocardial infarction and stroke, perhaps because they inhibit PGI2, which opposes the thrombotic actions of TXA2 produced by COX-1 [78]. Inhibitors of terminal synthases, downsteam of COX-2, that do not inhibit PGI2 might diminish this risk. Observational studies of the effect of NSAIDs on bone mass may be misleading since the regular use of NSAIDs may be associated with other characteristics that can affect bone, such as decreased level of physical activity or presence of inflammatory conditions. An additional limitation of observational studies is that NSAID use by subjects in the general population is unlikely to be frequent enough or at a high enough dose to completely inhibit COX activity, which would bias studies toward a smaller effect size. There is also considerable variability at an individual level in the degree of COX-2 inhibition and selectivity attained by selective COX-2 inhibitors [79]. However, given the widespread use of NSAIDs for musculoskeletal pain, the potential importance of inhibiting the COX-2 pathway in treating or preventing cancer, and the role of COX-2 in accelerating bone repair, as well as the unexpected inhibitory effects of COX-2 activity on the anabolic response to PTH, a better understanding of the role of endogenous PGs on skeletal health is needed.
Concluding Remarks
PGs are induced by multiple factors that have diverse actions on bone. Understanding the role of PGs in skeletal metabolism has been complicated because PGs act locally and transiently, are regulated at multiple levels, have multiple receptors and can have opposing effects depending on the test system. It seems likely that a major function of PGE2 is to modulate or integrate multiple signaling pathways by engaging additional Gαs-activated signaling pathways. Whether or not the regulatory function of PGs contributes to the catabolic or anabolic actions of the factor that induces them, and ultimately to bone loss or gain, will depend both on the factor inducing PG production and on the local cellular milieu. For example, the induction of PG production by BMP-2 enhances the BMP-2 stimulation of both osteoclastic and osteoblastic differentiation, whereas induction of PG production by PTH enhances the PTH stimulation of osteoclastic differentiation but inhibits the PTH stimulation of osteoblastic differentiation. Although this complexity makes it more difficult to understand the role of PGs in bone, clarifying the mechanisms of PG action could lead to a better understanding of the physiology and pathophysiology of bone and to new therapeutic applications associated with altering PGs in skeletal disorders.
Figure 1 of Box 1.
Biosynthesis of prostaglandins (PGs). Arachidonic acid is released from membrane phospholipids by phospholipase A2 enzymes and converted by prostaglanding G/H synthase (PGHS), also called cyclooxygenase (COX), to PGH2 in a two-step reaction. Terminal synthases convert PGH2 into PGs and thromboxane (TXA2).
Figure I of Box 2.
Association of PGE2 receptors with G-protein coupled receptors. (i) EP2R and EP4R activate adenylyl cyclase (AC) and cyclic AMP (cAMP)-dependent protein kinase A (PKA) or PKA-independent EPAC (exchange protein directly activated by cAMP) signaling. (ii) EP3R inhibits the synthesis of cAMP. (iii) EP1R activates Ca2+ and possibly (hashed line) protein kinase C (PKC) signaling.
Figure I of Box 3.
Bone turnover. Old bone is resorbed by multinucleated osteoclasts and new bone is formed by osteoblasts. Some osteoblasts become incorporated into the matrix as osteocytes, while others may become lining cells.
ACKNOWLEDGMENTS
The preparation of this review has been supported by NIH grants to CP (AR47673 and DK48361), LGR (AR18063) and KAB (F30 AG034013).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Kis B, et al. Acetaminophen and the cyclooxygenase-3 puzzle: sorting out facts, fictions, and uncertainties. J. Pharmacol. Exp. Ther. 2005;315:1–7. doi: 10.1124/jpet.105.085431. [DOI] [PubMed] [Google Scholar]
- 2.Kang YJ, et al. Regulation of intracellular cyclooxygenase levels by gene transcription and protein degradation. Prog. Lipid Res. 2007;46:108–125. doi: 10.1016/j.plipres.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rouzer CA, Marnett LJ. Cyclooxygenases: structural and functional insights. J Lipid Res. 2009;50 Suppl:S29–S34. doi: 10.1194/jlr.R800042-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pilbeam CC, et al. Prostaglandins and Bone Metabolism. In: Bilezikian JP, et al., editors. Principles of Bone Biology. Third edn. Elsevier/Academic Press; 2008. pp. 1235–1271. [Google Scholar]
- 5.Huang H, et al. PTH Induces COX-2 in MC3T3-E1 Osteoblasts Via cAMP-PKA and Calcium-Calcineurin Pathways. J Bone Miner Res. 2009 (in press) [Google Scholar]
- 6.Yu Y, et al. Targeted cyclooxygenase gene (ptgs) exchange reveals discriminant isoform functionality. J. Biol. Chem. 2007;282:1498–1506. doi: 10.1074/jbc.M609930200. [DOI] [PubMed] [Google Scholar]
- 7.Kulmacz RJ, et al. Comparison of the properties of prostaglandin H synthase-1 and -2. Prog. Lipid Res. 2003;42:377–404. doi: 10.1016/s0163-7827(03)00023-7. [DOI] [PubMed] [Google Scholar]
- 8.Loftin CD, et al. Failure of ductus arteriosus closure and remodeling in neonatal mice deficient in cyclooxygenase-1 and cyclooxygenase-2. Proc. Natl. Acad. Sci. U. S. A. 2001;98:1059–1064. doi: 10.1073/pnas.031573498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Myers LK, et al. The isozyme-specific effects of cyclooxygenase-deficiency on bone in mice. Bone. 2006;39:1048–1052. doi: 10.1016/j.bone.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 10.Alam I, et al. Mechanotransduction in bone does not require a functional cyclooxygenase-2 (COX-2) gene. J Bone Miner. Res. 2005;20:438–446. doi: 10.1359/JBMR.041124. [DOI] [PubMed] [Google Scholar]
- 11.Uppal S, et al. Mutations in 15-hydroxyprostaglandin dehydrogenase cause primary hypertrophic osteoarthropathy. Nat. Genet. 2008;40:789–793. doi: 10.1038/ng.153. [DOI] [PubMed] [Google Scholar]
- 12.Sakuma Y, et al. Stimulation of cAMP production and cyclooxygenase-2 by prostaglandin E(2) and selective prostaglandin receptor agonists in murine osteoblastic cells. Bone. 2004;34:827–834. doi: 10.1016/j.bone.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi T, Narumiya S. Function of prostanoid receptors: studies on knockout mice. Prostaglandins Other Lipid Mediat. 2002;68–69:557–573. 557–573. doi: 10.1016/s0090-6980(02)00055-2. [DOI] [PubMed] [Google Scholar]
- 14.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J. Biol. Chem. 2007;282:11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 15.Klein DC, Raisz LG. Prostaglandins: stimulation of bone resorption in tissue culture. Endocrinology. 1970;86:1436–1440. doi: 10.1210/endo-86-6-1436. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida K, et al. Stimulation of bone formation and prevention of bone loss by prostaglandin E EP4 receptor activation. Proc. Natl. Acad. Sci. U. S. A. 2002;99:4580–4585. doi: 10.1073/pnas.062053399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raisz LG, Woodiel FN. Effects of selective prostaglandin EP2 and EP4 receptor agonists on bone resorption and formation in fetal rat organ cultures. Prostaglandins Other Lipid Mediat. 2003;71:287–292. doi: 10.1016/s1098-8823(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 18.Paralkar VM, et al. An EP2 receptor-selective prostaglandin E2 agonist induces bone healing. Proc. Natl. Acad. Sci. U. S. A. 2003;100:6736–6740. doi: 10.1073/pnas.1037343100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ke HZ, et al. A nonprostanoid EP4 receptor selective prostaglandin E2 agonist restores bone mass and strength in aged, ovariectomized rats. J Bone Miner. Res. 2006;21:565–575. doi: 10.1359/jbmr.051110. [DOI] [PubMed] [Google Scholar]
- 20.Li M, et al. Prostaglandin E(2) receptors in bone formation. Int. Orthop. 2007;31:767–772. doi: 10.1007/s00264-007-0406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rozengurt E. Mitogenic signaling pathways induced by G protein-coupled receptors. J Cell Physiol. 2007;213:589–602. doi: 10.1002/jcp.21246. [DOI] [PubMed] [Google Scholar]
- 22.Castellone MD, et al. Cyclooxygenase-2 and colorectal cancer chemoprevention: the beta-catenin connection. Cancer Res. 2006;66:11085–11088. doi: 10.1158/0008-5472.CAN-06-2233. [DOI] [PubMed] [Google Scholar]
- 23.Fujino H, Regan JW. EP(4) prostanoid receptor coupling to a pertussis toxin-sensitive inhibitory G protein. Mol. Pharmacol. 2006;69:5–10. doi: 10.1124/mol.105.017749. [DOI] [PubMed] [Google Scholar]
- 24.Cha YI, DuBois RN. NSAIDs and cancer prevention: targets downstream of COX-2. Annu. Rev. Med. 2007;58:239–252. 239–252. doi: 10.1146/annurev.med.57.121304.131253. [DOI] [PubMed] [Google Scholar]
- 25.Rao R, et al. Prostaglandin E2-EP4 receptor promotes endothelial cell migration via ERK activation and angiogenesis in vivo. J. Biol. Chem. 2007;282:16959–16968. doi: 10.1074/jbc.M701214200. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, et al. The RAP1 guanine nucleotide exchange factor Epac2 couples cyclic AMP and Ras signals at the plasma membrane. J. Biol. Chem. 2006;281:2506–2514. doi: 10.1074/jbc.M508165200. [DOI] [PubMed] [Google Scholar]
- 27.Kleiveland CR, et al. Human mesenchymal stem cell proliferation is regulated by PGE2 through differential activation of cAMP-dependent protein kinase isoforms. Exp. Cell Res. 2008;314:1831–1838. doi: 10.1016/j.yexcr.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Dodge-Kafka KL, Kapiloff MS. The mAKAP signaling complex: integration of cAMP, calcium, and MAP kinase signaling pathways. Eur. J. Cell Biol. 2006;85:593–602. doi: 10.1016/j.ejcb.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Minamizaki T, et al. EP2 and EP4 receptors differentially mediate MAPK pathways underlying anabolic actions of prostaglandin E2 on bone formation in rat calvaria cell cultures. Bone. 2009;44:1177–1185. doi: 10.1016/j.bone.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42:606–615. doi: 10.1016/j.bone.2007.12.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008;473:139–146. doi: 10.1016/j.abb.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hackett JA, et al. Prostaglandin production by human osteoclasts in culture. J. Rheumatol. 2006;33:1320–1328. [PubMed] [Google Scholar]
- 33.North TE, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–1011. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kobayashi Y, et al. Prostaglandin E2 enhances osteoclastic differentiation of precursor cells through protein kinase A-dependent phosphorylation of TAK1. J Biol. Chem. 2005;280:11395–11403. doi: 10.1074/jbc.M411189200. [DOI] [PubMed] [Google Scholar]
- 35.Take I, et al. Prostaglandin E2 strongly inhibits human osteoclast formation. Endocrinology. 2005;146:5204–5214. doi: 10.1210/en.2005-0451. [DOI] [PubMed] [Google Scholar]
- 36.Ono K, et al. Biphasic effect of prostaglandin E2 on osteoclast formation in spleen cell cultures: role of the EP2 receptor. J Bone Miner Res. 2005;20:23–29. doi: 10.1080/14041040510033842. [DOI] [PubMed] [Google Scholar]
- 37.Blackwell KA, et al. Bone morphogenetic protein 2 enhances PGE2 stimulated osteoclast formation in murine bone marrow cultures. Prostaglandins Other Lipid Mediat. 2009;90:76–80. doi: 10.1016/j.prostaglandins.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okamoto F, et al. Prostaglandin E2 activates outwardly rectifying Cl(−) channels via a cAMP-dependent pathway and reduces cell motility in rat osteoclasts. Am. J Physiol Cell Physiol. 2004;287:C114–C124. doi: 10.1152/ajpcell.00551.2003. [DOI] [PubMed] [Google Scholar]
- 39.Suzawa T, et al. The role of prostaglandin E receptor subtypes (EP1, EP2, EP3, and EP4) in bone resorption: an analysis using specific agonists for the respective EPs. Endocrinology. 2000;141:1554–1559. doi: 10.1210/endo.141.4.7405. [DOI] [PubMed] [Google Scholar]
- 40.Miyaura C, et al. Impaired bone resorption to prostaglandin E2 in prostaglandin E receptor EP4-knockout mice. J Biol. Chem. 2000;275:19819–19823. doi: 10.1074/jbc.M002079200. [DOI] [PubMed] [Google Scholar]
- 41.Zhan P, et al. Effect of deletion of the prostaglandin EP4 receptor on stimulation of calcium release from cultured mouse calvariae: Impaired responsiveness in heterozygotes. Prostaglandins Other Lipid Mediat. 2005;78:19–26. doi: 10.1016/j.prostaglandins.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 42.Li M, et al. Osteopenia and impaired fracture healing in aged EP4 receptor knockout mice. Bone. 2005;37:46–54. doi: 10.1016/j.bone.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 43.Tian XY, et al. Continuous PGE2 leads to net bone loss while intermittent PGE2 leads to net bone gain in lumbar vertebral bodies of adult female rats. Bone. 2008;42:914–920. doi: 10.1016/j.bone.2007.12.228. [DOI] [PubMed] [Google Scholar]
- 44.Tanaka M, et al. Prostaglandin E2 receptor (EP4) selective agonist (ONO-4819.CD) accelerates bone repair of femoral cortex after drill-hole injury associated with local upregulation of bone turnover in mature rats. Bone. 2004;34:940–948. doi: 10.1016/j.bone.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 45.Zhang X, et al. Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair. J Clin. Invest. 2002;109:1405–1415. doi: 10.1172/JCI15681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choudhary S, et al. Strontium ranelate promotes osteoblastic differentiation and mineralization of murine bone marrow stromal cells: involvement of prostaglandins. J. Bone Miner. Res. 2007;22:1002–1010. doi: 10.1359/jbmr.070321. [DOI] [PubMed] [Google Scholar]
- 47.Alander CB, Raisz LG. Effects of selective prostaglandins E2 receptor agonists on cultured calvarial murine osteoblastic cells. Prostaglandins Other Lipid Mediat. 2006;81:178–183. doi: 10.1016/j.prostaglandins.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chikazu D, et al. Bone morphogenetic protein 2 induces cyclo-oxygenase 2 in osteoblasts via a Cbfa1 binding site: role in effects of bone morphogenetic protein 2 in vitro and in vivo 2002. J. Bone Miner. Res. 2005;20:1888–1898. doi: 10.1359/jbmr.2005.20.10.1887. [DOI] [PubMed] [Google Scholar]
- 49.Wang YH, et al. Comparison of the action of transient and continuous PTH on primary osteoblast cultures expressing differentiation stage-specific GFP. J Bone Miner Res. 2005;20:5–14. doi: 10.1359/JBMR.041016. [DOI] [PubMed] [Google Scholar]
- 50.Rickard DJ, et al. Intermittent treatment with parathyroid hormone (PTH) as well as a non-peptide small molecule agonist of the PTH1 receptor inhibits adipocyte differentiation in human bone marrow stromal cells. Bone. 2006;39:1361–1372. doi: 10.1016/j.bone.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 51.Choudhary S, et al. Anabolic effects of PTH in cyclooxygenase-2 knockout osteoblasts in vitro. Biochem. Biophys. Res. Commun. 2008;372:536–541. doi: 10.1016/j.bbrc.2008.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu M, et al. Intermittent PTH has increased anabolic effects in cyclooxygenase-2 knockout mice. J. Bone Miner. Res. 2007;22(S1):S66. (abstract) [Google Scholar]
- 53.Anderson GD, et al. Combination therapies that inhibit cyclooxygenase-2 and leukotriene synthesis prevent disease in murine collagen induced arthritis. Inflamm. Res. 2009;58:109–117. doi: 10.1007/s00011-009-8149-3. [DOI] [PubMed] [Google Scholar]
- 54.Taketa T, et al. Selective cyclooxygenase-2 inhibitor prevents reduction of trabecular bone mass in collagen-induced arthritic mice in association with suppression of RANKL/OPG ratio and IL-6 mRNA expression in synovial tissues but not in bone marrow cells. J Bone Miner Metab. 2008;26:143–151. doi: 10.1007/s00774-007-0808-2. [DOI] [PubMed] [Google Scholar]
- 55.Inada M, et al. Membrane-bound prostaglandin E synthase-1-mediated prostaglandin E2 production by osteoblast plays a critical role in lipopolysaccharide-induced bone loss associated with inflammation. J Immunol. 2006;177:1879–1885. doi: 10.4049/jimmunol.177.3.1879. [DOI] [PubMed] [Google Scholar]
- 56.Niki Y, et al. Administration of cyclooxygenase-2 inhibitor reduces joint inflammation but exacerbates osteopenia in IL-1 alpha transgenic mice due to GM-CSF overproduction. J. Immunol. 2007;179:639–646. doi: 10.4049/jimmunol.179.1.639. [DOI] [PubMed] [Google Scholar]
- 57.Singh B, et al. COX-2 involvement in breast cancer metastasis to bone. Oncogene. 2007;26:3789–3796. doi: 10.1038/sj.onc.1210154. [DOI] [PubMed] [Google Scholar]
- 58.Li Z, et al. Increased COX2 expression enhances tumor-induced osteoclastic lesions in breast cancer bone metastasis. Clin. Exp. Metastasis. 2008;25:389–400. doi: 10.1007/s10585-007-9117-3. [DOI] [PubMed] [Google Scholar]
- 59.Takita M, et al. Prostaglandin E receptor EP4 antagonist suppresses osteolysis due to bone metastasis of mouse malignant melanoma cells. FEBS Lett. 2007;581:565–571. doi: 10.1016/j.febslet.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 60.Takahashi T, et al. Soluble EP2 neutralizes prostaglandin E2-induced cell signaling and inhibits osteolytic tumor growth. Mol. Cancer Ther. 2008;7:2807–2816. doi: 10.1158/1535-7163.MCT-08-0153. [DOI] [PubMed] [Google Scholar]
- 61.O'Keefe RJ, et al. COX-2 has a critical role during incorporation of structural bone allografts. Ann. N. Y. Acad. Sci. 2006;1068:532–542. 532–542. doi: 10.1196/annals.1346.012. [DOI] [PubMed] [Google Scholar]
- 62.Gerstenfeld LC, et al. Selective and nonselective cyclooxygenase-2 inhibitors and experimental fracture-healing. Reversibility of effects after short-term treatment. J Bone Joint Surg. Am. 2007;89:114–125. doi: 10.2106/JBJS.F.00495. [DOI] [PubMed] [Google Scholar]
- 63.Simon AM, O'Connor JP. Dose and time-dependent effects of cyclooxygenase-2 inhibition on fracture-healing. J Bone Joint Surg. Am. 2007;89:500–511. doi: 10.2106/JBJS.F.00127. [DOI] [PubMed] [Google Scholar]
- 64.Xie C, et al. COX-2 from the injury milieu is critical for the initiation of periosteal progenitor cell mediated bone healing. Bone. 2008;43:1075–1083. doi: 10.1016/j.bone.2008.08.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rapuano BE, et al. The effects of COX-1 and COX-2 inhibitors on prostaglandin synthesis and the formation of heterotopic bone in a rat model. Arch. Orthop. Trauma Surg. 2008;128:333–344. doi: 10.1007/s00402-007-0436-2. [DOI] [PubMed] [Google Scholar]
- 66.Li L, et al. Regulation of bone biology by prostaglandin endoperoxide H synthases (PGHS): a rose by any other name. Cytokine Growth Factor Rev. 2006;17:203–216. doi: 10.1016/j.cytogfr.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 67.Norwood VF, et al. Postnatal development and progression of renal dysplasia in cyclooxygenase-2 null mice. Kidney Int. 2000;58:2291–2300. doi: 10.1046/j.1523-1755.2000.00413.x. [DOI] [PubMed] [Google Scholar]
- 68.Wang H, et al. Rescue of female infertility from the loss of cyclooxygenase-2 by compensatory up-regulation of cyclooxygenase-1 is a function of genetic makeup. J. Biol. Chem. 2004;279:10649–10658. doi: 10.1074/jbc.M312203200. [DOI] [PubMed] [Google Scholar]
- 69.Xu M, et al. Do cyclooxygenase knockout mice have primary hyperparathyroidism? Endocrinology. 2005;146:1843–1853. doi: 10.1210/en.2004-0734. [DOI] [PubMed] [Google Scholar]
- 70.Kennedy BP, et al. A natural disruption of the secretory group II phospholipase A2 gene in inbred mouse strains. J. Biol. Chem. 1995;270:22378–22385. doi: 10.1074/jbc.270.38.22378. [DOI] [PubMed] [Google Scholar]
- 71.Xu M, et al. Effects of genetic background on phenotype of cyclooxygenase-2 knockout mice. J. Bone Miner. Res. 2005;20(S1):S182. (abstract) [Google Scholar]
- 72.Chen Q, et al. Congenital lack of COX-2 affects mechanical and geometric properties of bone in mice. Calcif. Tissue Int. 2003;73:387–392. doi: 10.1007/s00223-002-0009-x. [DOI] [PubMed] [Google Scholar]
- 73.Robertson G, et al. Alteration of femoral bone morphology and density in COX-2−/− mice. Bone. 2006;39:767–772. doi: 10.1016/j.bone.2006.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Segi E, et al. Patent ductus arteriosus and neonatal death in prostaglandin receptor EP4-deficient mice. Biochem. Biophys. Res Commun. 1998;246:7–12. doi: 10.1006/bbrc.1998.8461. [DOI] [PubMed] [Google Scholar]
- 75.Akhter MP, et al. Bone biomechanical properties in prostaglandin EP1 and EP2 knockout mice. Bone. 2001;29:121–125. doi: 10.1016/s8756-3282(01)00486-0. [DOI] [PubMed] [Google Scholar]
- 76.Akhter MP, et al. Bone biomechanical properties in EP4 knockout mice. Calcif. Tissue Int. 2006;78:357–362. doi: 10.1007/s00223-005-0186-5. [DOI] [PubMed] [Google Scholar]
- 77.Richards JB, et al. The effect of cyclooxygenase-2 inhibitors on bone mineral density: results from the Canadian Multicentre Osteoporosis Study. Osteoporos. Int. 2006;17:1410–1419. doi: 10.1007/s00198-006-0142-x. [DOI] [PubMed] [Google Scholar]
- 78.Grosser T, et al. Biological basis for the cardiovascular consequences of COX-2 inhibition: therapeutic challenges and opportunities. J. Clin. Invest. 2006;116:4–15. doi: 10.1172/JCI27291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fries S, et al. Marked interindividual variability in the response to selective inhibitors of cyclooxygenase-2. Gastroenterology. 2006;130:55–64. doi: 10.1053/j.gastro.2005.10.002. [DOI] [PubMed] [Google Scholar]