Abstract
Organisms have developed concurrent behavioral and physiological adaptations to the strong influence of day/night cycles, as well as to unforeseen, random stress stimuli. These circadian and stress-related responses are achieved by two highly conserved and interrelated regulatory networks, the circadian CLOCK and stress systems, which respectively consist of oscillating molecular pacemakers, the Clock/Bmal1 transcription factors, and the hypothalamic-pituitary-adrenal (HPA) axis and its end-effector, the glucocorticoid receptor. These systems communicate with each other at different signaling levels, and dysregulation of either system may lead to development of pathologic conditions. In this review, we summarize the mutual physiologic interactions between the circadian CLOCK system and the HPA axis, and discuss their clinical implications.
Adjustment of internal homeostasis to major changes in circumstance
Virtually all organisms live under the strong influence of day/night cycles created by the 24-hour rotation of the planet. Organisms sense these regular external changes and synchronize their physical activities, such as behavior, food intake, energy metabolism, sleep, reproductive activity and immune function, to increase their chance for survival 1. From their early evolution, organisms have developed a highly conserved and sophisticated ubiquitous molecular “clock”, the CLOCK system, which creates internal circadian rhythmicity under the influence of light/dark information 2. This regulatory system has output connections to numerous organs and tissues, relaying information from a centrally created circadian rhythm 3.
In addition to synchronizing their activities to day/night changes, organisms continuously face unforeseen short- and long-term changes in the environment called “stressors”, which can be external (e.g. excessive heat or cold, food deprivation, trauma and invasion by pathogens) or internal (e.g. hurtful memories, splachnic injuries, neoplasias) 4. To adapt to these stressful stimuli, organisms have developed another regulatory system, the stress system, which senses environmental changes through various sensory organs, processes them in the central nervous system (CNS), and adjusts the CNS and peripheral organ activities to improve chances for survival 4. The stress system consists of the hypothalamic-pituitary-adrenal (HPA) axis and its end-effectors glucocorticoids 4, and the locus caeruleus/norepinephrine-autonomic systems and their end-effectors, norepinephrine and epinephrine. The stress system restores internal homeostasis by regulating many biological activities, including those of the CNS, intermediary metabolism, immunity and reproduction 4, 5. This system, however, may also exert an array of adverse effects on the organism when its response is not properly tailored to the stressful stimulus (Box-1). For example, acute hyper-activation of the HPA axis has been associated with development of post-traumatic stress disorder, while chronic activation of the HPA axis, and the consequently prolonged elevation of serum cortisol levels, induces visceral-type obesity and insulin resistance/dyslipidemia 4, 5.
Box-1: Pathologic conditions associated with chronic activation of the HPA axis
Prolonged activation of the HPA axis increases circulating glucocorticoid levels, thus causing pathologies such as those seen in endogenous or exogenous hypercortisolism (Cushing syndrome as described below.
Suppression of the immune function (shift from T-helper 1 to T-helper 2 response) (Immune system).
Central obesity/dyslipidemia and peripheral tissue resistance to insulin and glucose intolerance/overt diabetes mellitus type 2 (Metabolic syndrome).
Muscle wasting and osteoporosis (Musculoskeletal system).
Alteration in mood and cognition (Central nervous system).
All these pathologic changes ultimately may reduce life expectancy. From 4, 5
The CLOCK and stress systems are both fundamental for survival, and thus, communicate with each other at multiple levels to adjust numerous physiologic activities. Interestingly, dysregulation in either of these systems may lead to similar pathologic conditions. Here we review the mutual interactions of the CLOCK and stress systems in physiology and pathophysiology.
The circadian CLOCK system
The circadian CLOCK system consists of central and peripheral components. The central component is located in the suprachiasmatic nuclei (SCN) of the hypothalamus and acts as a “master” CLOCK under the strong influence of light/dark input from the eyes, whereas the peripheral components behave as “slave” CLOCKs, functioning virtually in all organs and tissues 1, 2 (Box-2). The activity of the peripheral CLOCKs is synchronized to that of the central master CLOCK through both humoral and neural connections, which have not been fully elucidated as yet 6. The “master” and “slave” CLOCKs have almost the same transcriptional regulatory machinery for generating intrinsic circadian oscillatory rhythms through coordinated activation/inactivation of a set of transcription factors 1, 3. Central among these factors are the circadian locomotor output cycle kaput (Clock) and its heterodimer partner brain-muscle-arnt-like protein 1 (Bmal1), which belong to the basic helix-loop-helix (bHLH)-PER-ARNT-SIM (PAS) superfamily of transcription factors 2, 7.
Box-2: Central CLOCK-dependent and -independent regulation of peripheral CLOCKs
The circadian CLOCK system consists of central and peripheral components.
The central CLOCK regulated by the light-dark cycle (master CLOCK) synchronizes the circadian rhythm of peripheral CLOCKs through various as yet poorly defined neural and humoral connections (Figure I of Box-2); thus, they respectively function as “master” and “slave” CLOCKs.
Destruction of central CLOCK abolishes synchronization of peripheral CLOCKs in different organs, while circadian rhythm of each peripheral CLOCK is still maintained, suggesting that peripheral CLOCKs have conditional autonomy from the central CLOCK.
Circadian rhythm of peripheral CLOCKs can also be shifted by food restriction and various other regulators, such as glucocorticoids, fibroblast growth factor, and activators of protein kinases A and C and mitogen-activated protein kinase 16.
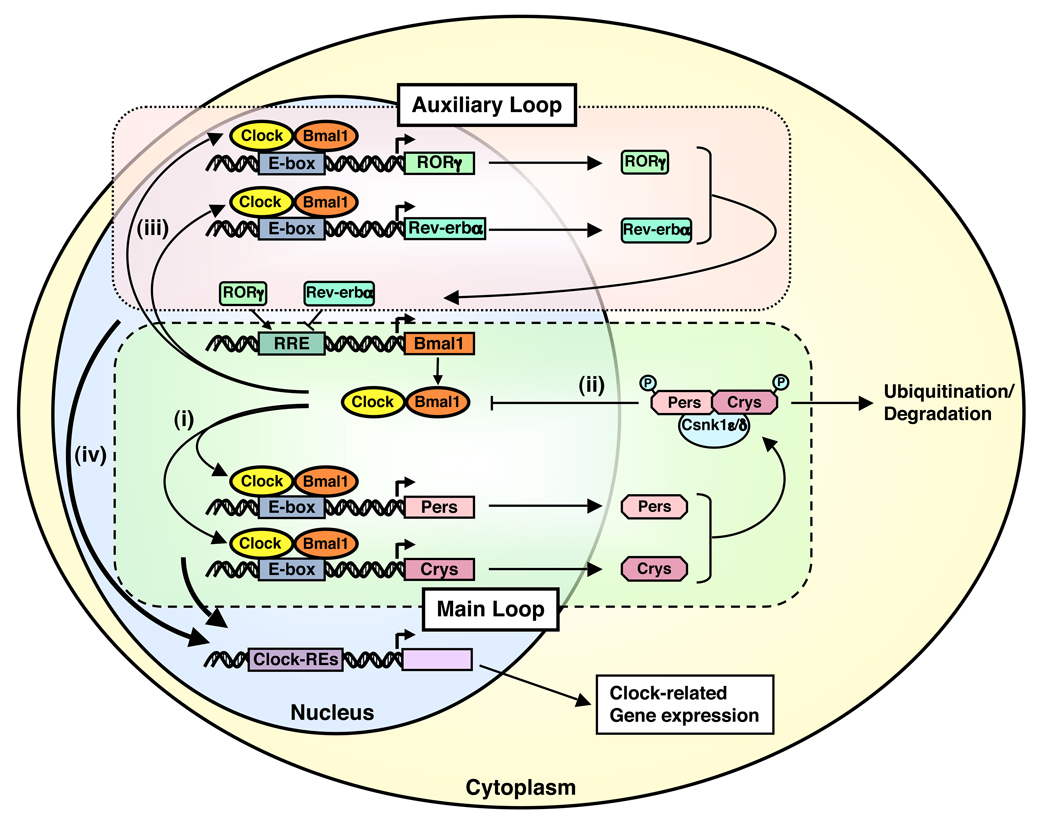
The Clock/Bmal1 heterodimer binds the E-box response elements located in the promoter region and stimulates the transcription of other essential clock genes, such as Periods (Per1, Per2 and Per3) and Cryptochromes (Cry1 and Cry2) (Figure 1). Accumulated proteins Pers and Crys then form a complex with the caseine kinase 1ε and δ, are phosphorylated, translocate into the cell nucleus and repress the transcriptional activity of the Clock/Bmal1 heterodimer by inhibiting its binding to the E-box response elements located in their own promoters, thus ultimately forming a negative feedback transcriptional loop that maintains an oscillation of their gene expression approximately every 24 hours 1, 8, 9. In addition to the regulation of this principal transcriptional loop, Clock/Bmal1 stimulates expression of other clock-related proteins, such as Rev-erbα, retinoic acid receptor-related orphan receptor α (RORα), Dec1, Dec2 and albumin gene D site-binding protein (Dbp), which form an auxiliary loop that stabilizes the main regulatory loop composed of Clock/Bmal1, Pers and Crys (Figure 1). Importantly, the transcription factors of the main and auxiliary loops control numerous “downstream” clock-responsive genes to influence a variety of biological activities 1, 2, 10.
Figure 1.
The circadian CLOCK system is regulated by a self-oscillating transcriptional loop.
The C heterodimer Clock/Bmal1 binds to E-box elements located in the promoter region and stimulates expression of essential clock transcription factors Pers and Crys (i), which in turn repress the transcriptional activity of the CLOCK/BMAL1 heterodimer by inhibiting its binding to the E-box response elements located in their own promoters through formation of a complex with and subsequent phosphorylation by the caseine kinase 1ε and δ (ii). Clock/Bmal1 also stimulates expression of other CLOCK-related proteins, such as Rev-erbα, RORα, Dec1, Dec2 and Dbp (iii), which create an auxiliary loop that helps stabilize the main regulatory loop. These clock transcription factors control numerous “downstream” CLOCK-responsive genes to influence a variety of biologic activities (iv). Bmal1: brain-muscle-arnt-like protein 1, Clock: circadian locomotor output cycle kaput, Crys: cryptochromes, Csnk1ε/δ: caseine kinase 1ε/δ, P: phosphate residue on the phsphorylated molecules, Pers: periods, RORγ: retinoic acid receptor-related orphan nuclear receptor γ.
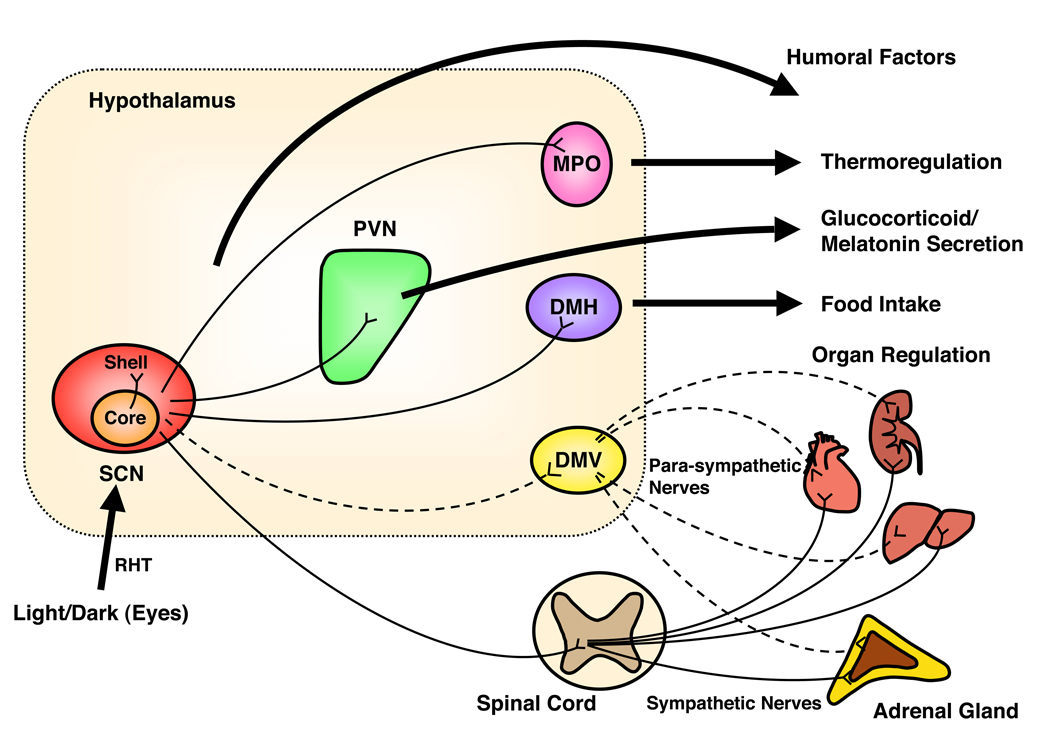
To obtain light/dark information, the central CLOCK located in SCN receives afferent neurons from retina through the retino-hypothalamic tract (RHT) 11. Neurons of the central CLOCK in the SCN send efferent projections to the other parts of the brain, such as the paraventricular nucleus (PVN), medial preoptic area (MPA) and dorsomedial nucleus (DMH) of the hypothalamus and the pineal gland, to transfer timing information and to regulate secretion of pituitary hormones and melatonin, as well as to control sleep, food intake and body temperature 1, 6, 12 (Figure I of Box 2). The central master CLOCK also employs the autonomic nervous system to regulate the peripheral CLOCKs located in various organs 1, 6. In addition to these neural connections, the central CLOCK uses several humoral mediators, such as AVP, tumor necrosis factor (TNF) α, prokineticin-2, cardiotrophin-like cytokines and neuromedin, to synchronize circadian rhythmicity of the peripheral CLOCKs 3, 6, 13, 14. Of note, peripheral CLOCKs have the ability to be influenced by zeitgebers other than the light-dark cycle independently of the central master CLOCK15 (Box-2). In agreement with this observation, food restriction in rodents shifts the circadian rhythm of peripheral CLOCKs, whereas it does not affect that of the central master CLOCK 16. A recent report revealed that the dorsomedial nucleus of the hypothalamus contained a center for this food restriction-mediated shift of circadian rhythm in peripheral CLOCKs regulating energy metabolism 17.
Figure I of Box-1.
Central CLOCK synchronizes the peripheral CLOCKs and regulates peripheral organ activities via neural and humoral interactions.
The central master CLOCK located in the SCN (core) obtains light/dark information from the retina through the retinohypothalamic track (RHT), and adjusts its circadian rhythm, while it indirectly projects several efferent neurons to transmit timing information to other parts of the brain and distant organs for synchronizing their peripheral CLOCKs and influencing their activities, such as secretion of pituitary hormones and melatonin, food intake, sleep and body temperature. The central master CLOCK employs the autonomic nervous system and humoral mediators for organ regulation. For simplicity, detailed anatomical structures for the sympathetic and parasympathetic nervous systems, such as nuclei located in the brain stem including the solitary nucleus and the ambiguous nucleus and the sympathetic and parasympathetic ganglia, are omitted.
DMH: dorsomedial nucleus of hypothalamus, DMV: dorsal motor nucleus of vagus, MPO: medial preoptic region, PVN: paraventricular nucleus, RHT: retinohypothalamic tract, SCN: suprachiasmatic nucleus.
The stress HPA axis
The HPA axis, apart from having a circadian activity, also mediates the adaptive response to stressors; it consists of the hypothalamic PVN parvocellular corticotropin-releasing hormone (CRH)- and arginine vasopressin (AVP)-secreting neurons, the corticotrophs of pituitary gland, and the adrenal gland cortices. The PVN neurons release CRH and AVP into the hypophyseal portal system located under the median eminence of the hypothalamus in response to stimulatory signals from higher brain regulatory centers. Secreted CRH and AVP reach the pituitary gland and synergistically stimulate the secretion of the adrenocorticotropic hormone (ACTH) 4, 18. ACTH released into the systemic circulation finally stimulates both production and secretion of glucocorticoids (cortisol in humans and corticosterone in rodents) from the cortex of the adrenal glands. Secreted glucocorticoids in turn suppress higher regulatory centers, the PVN and the pituitary gland, forming a closed negative feedback loop that aims to reset the activated HPA axis and restore its homeostasis 4.
Glucocorticoids are steroid hormones that act as the end-effectors of the HPA axis 19. They influence the functions of virtually all organs and tissues in the body, and are necessary for the maintenance of many important biologic activities, such as the homeostasis of the CNS, the cardiovascular system, intermediary metabolism and the immune/inflammatory reaction, influencing mRNA expression of up to 20% of the expressed genome 5, 19. Thus, the HPA axis influences virtually all organs and tissues through its end-effectors glucocorticoids, in addition to the organization of adaptive response to stressors. Glucocorticoids exert their diverse effects through a specific intracellular receptor, the glucocorticoid receptor (GR), which belongs to the nuclear receptor superfamily and is ubiquitously expressed in almost all human tissues and organs 20, 21.
In the absence of glucocorticoids, GR is located primarily in the cytoplasm, while glucocorticoid-bound GR undergo conformational changes, homo- or heterodimerize and translocate into the nucleus where the dimer interacts with glucocorticoid response elements (GREs) located in distant or proximal regulatory regions of glucocorticoid target genes to influence their transcription 20, 21. In addition to transactivation/transrepression of the glucocorticoid-responsive genes through direct binding to GREs, GR modulates transcription of many glucocorticoid-responsive genes through mutual protein-protein interactions between the GR and several specific transcription factors, such as NF-κB and STAT5, in a GRE-independent fashion. The GR, thus, regulates the transcription of genes responsive to these transcription factors by influencing their ability to regulate their own target genes 20, 21.
Crosstalk between the circadian CLOCK system and the HPA axis
Regulation of the HPA axis by the circadian CLOCK system
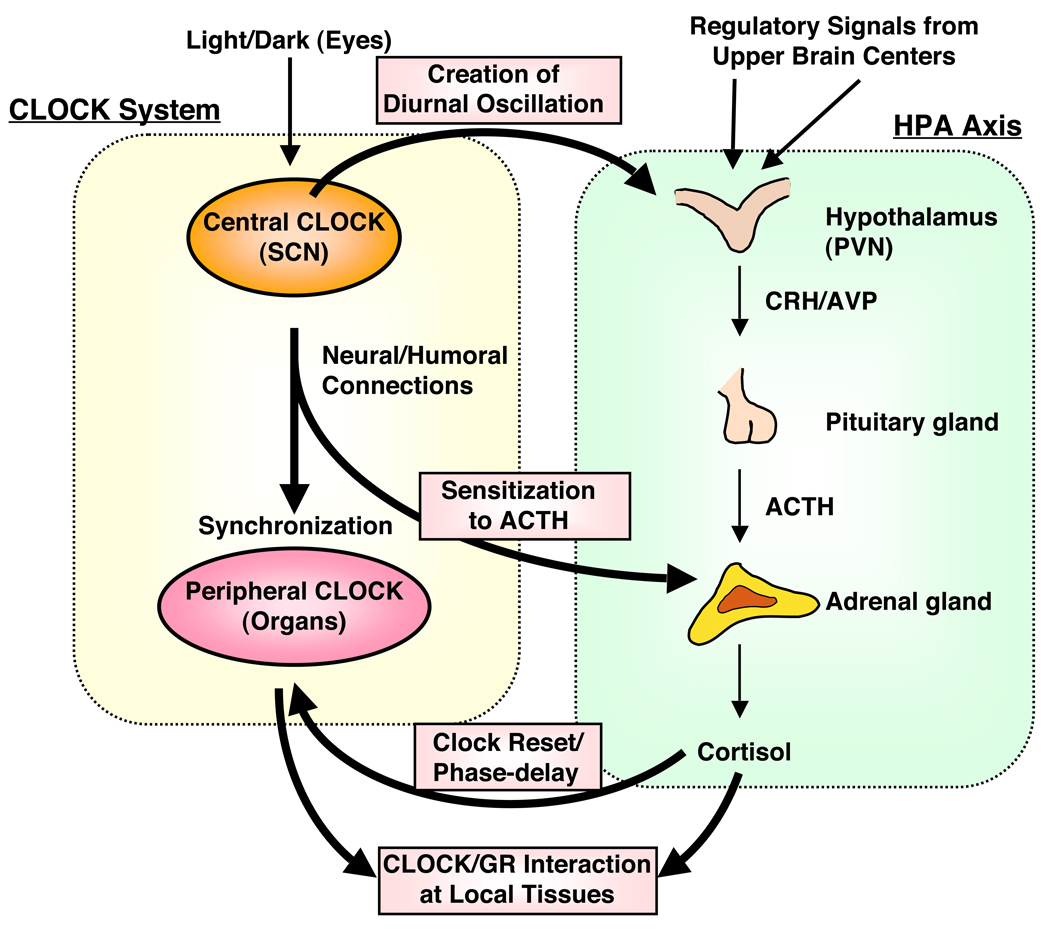
Circulating glucocorticoid levels are tightly regulated and fluctuate naturally in a circadian fashion, reaching their zenith in the early morning and their nadir in the late evening in diurnal animals, including humans 22, 23. The light-activated central master CLOCK located in the SCN orchestrates this daily rhythmic release of glucocorticoids by influencing the activity of the HPA axis through efferent connections from the SCN to the CRH/AVP-containing neurons of the PVN 6, 18, 24 (Figure 2). This central CLOCK-mediated diurnal regulation of circulating glucocorticoids is extremely important for adjusting the body’s daily activities. The central CLOCK system also modulates glucocorticoid release from the adrenal glands in a HPA axis-independent fashion by altering the sensitivity of the adrenal cortex to ACTH through SCN-mediated activation of the autonomic nervous system 6, 12, 24, 25 (Figure 2).
Figure 2.
The circadian CLOCK system and the HPA axis influence each other’s activity at multiple levels. The central CLOCK under the regulation of the light input controls the HPA axis and produces regular diurnal secretion of glucocorticoid hormones from the adrenal glands, while the peripheral CLOCKs, which is located in the adrenal glands and other components of the HPA axis and are regulated by the central CLOCK through the sympathetic nervous system, also contribute to the rhythmic glucocorticoid secretion from these organs. Secreted glucocorticoids in turn reset and phase-delay circadian rhythm of the peripheral CLOCKs by stimulating the expression of several CLOCK-related genes; this is especially important for temporal adjustment of body’s activity against stress. The peripheral CLOCKs also regulate glucocorticoid effect in local tissues through interaction between Clock/Bmal1 and GR, providing a local counter regulatory feedback loop to the effect of central CLOCK on the HPA axis.
ACTH: adrenocorticotropic hormone, AVP: arginine vasopressin, CRH: corticotropin-releasing hormone, PVN: paraventricular nucleus, SCN: Suprachiasmatic nucleus.
The circadian CLOCK-mediated regulation of glucocorticoid secretion from the adrenal glands is supported by recent publications, which employed mice defective in several essential clock genes (Table 1). Per1Brd mice, which express a fusion transcript containing Per1 exon 1–3 and an exogenously introduced mini gene and thus were considered to be equivalent to Per1 null mice, had markedly elevated circulating glucocorticoids without a circadian rhythm, while Per2Brd mice demonstrated elevated, but diurnally fluctuating serum glucocorticoids 26. Another group, however, reported that Per2−/− mice had the same baseline glucocorticoid concentrations as wild type mice but lost their circadian rhythm, even though their ACTH in the pituitary gland preserved its circadian rhythmicity 27. In agreement with this report, the adrenal glands from the Per2Brdm1/Cry1−/− double mutant mice had defective glucocorticoid biosynthesis 12, suggesting that the peripheral CLOCK located in the adrenal glands gates glucocorticoid production stimulated by fluctuating plasma ACTH. Taken together, these reports suggest that the central master CLOCK under the regulation of light input together with other regulators of the HPAs axis produces diurnal secretion of glucocorticoid hormones from the adrenal glands through HPA axis-dependent and -independent pathways, whereas the peripheral CLOCK located in the adrenal glands and other components of the HPA axis also contribute to rhythmic glucocorticoid secretion from this organ (Figure 2).
Table 1.
Mice Defective in Components of the Circadian CLOCK system Have a Compromised HPA axis
| Animals | CLOCK system | Effect on the HPA axis | References |
|---|---|---|---|
| Per1Brd mice (male) | Short circadian cycle | Conserved glucocorticoid circadian rhythm, but circulating levels elevated |
26 |
| Per2Brd mice (male) | Short circadian cycle with loss of persistent circadian rhythmicity |
Elevated glucocorticoid levels without circadian rhythm |
26 |
| Per2Brdm1/Cry1−/− mice (male) | Loss of circadian rhythm | Loss of ACTH and glucocorticoid circadian rhythm | 12 |
| Per2drmd1 mice (female) | Short circadian cycle with loss of persistent circadian rhythmicity |
Disturbance in the excretion of daily glucocorticoid metabolites |
60 |
| Per2tm1Brd mice | Short circadian cycle with loss of persistent circadian rhythmicity |
Absence of glucocorticoid circadian rhythm, but intact ACTH rhythm |
27 |
Influence of the HPA axis (glucocorticoids) on the circadian CLOCK system
In a reciprocal fashion, the HPA axis strongly influences the activity/circadian rhythm of the CLOCK system through glucocorticoids. These hormones affect the peripheral CLOCKs in almost all organs and tissues, while they spare the central master CLOCK in the SCN, as this brain nucleus is one of the rare areas that does not express GR 28. Thus, the central master CLOCK maintains its intrinsic circadian rhythm independently of the body’s changes caused by internal and external stressors, and acts as the “standard clock” for the peripheral slave CLOCKs 28 (Figure 2). In contrast, glucocorticoids reset their circadian rhythm by phase-shifting the expression of several clock-related genes in peripheral organs, including the liver, kidney and heart 28. Thus, the circadian rhythm of the peripheral slave CLOCKs is phase-shifted by glucocorticoids, but eventually returns to the canonical phase under the influence of the central master CLOCK. This glucocorticoid-mediated periodical resetting of the peripheral CLOCK may be particularly important during stress, as organisms need to adjust the circadian rhythm-linked activity of their bodies to properly respond to stressors. Of note, diurnally circulating glucocorticoids also contribute in part to the development of daily oscillation of peripheral CLOCKs in some tissues 29, 30.
Glucocorticoids reset the peripheral CLOCKs by influencing the expression of several clock-related genes in peripheral tissues and in some brain areas 29–32 (Table 2). For example, the Per1 gene promoter contains tandem GREs, which confer glucocorticoid-mediated Per1 mRNA expression in the liver, kidney and heart in mice 33. These tandem GREs in the mouse Per1 promoter are also present and functional in humans and rats 28, 32. Glucocorticoids stimulate Per2 mRNA expression also in a GRE-dependent fashion in mice 34; indeed, Per2 upregulation is necessary for the circadian rhythm of the central extended amygdala, a part of the limbic system that plays pivotal roles in the regulation of fear and anger, and transmits emotional information to the stress system 35. The amygdala are involved in glucocorticoid-mediated regulation of glucose metabolism by influencing the secretion of adipokines like leptin, and subsequently, the sensitivity of peripheral tissues to insulin 34. Further, glucocorticoids regulate expression of other clock genes, including the adenovirus E4 promoter-binding protein/nuclear factor regulated by IL-3 (E4BP4/NFIL3), Timeless, Rev-Erbβ, Dec2 and NPAS2, some of which contain functional GREs in their regulatory regions 34.
Table 2.
Glucocorticoids Influence CLOCK Circadian Rhythm and Expression of Clock-related Genes
| Experiment conditions | Tissues and cells examined | Effect on clock genes expression | References |
|---|---|---|---|
| Dexamethasone administration to mice with ablated SCN |
Liver | Regulation of 60% of the hepatic circadian transcriptome (e.g. Per1, Bmal1, and Cry1) |
61 |
| Corticosterone administration to intact and bilaterally adrenalectomized rats (male) |
BNSTov and CEA of the amygdala |
Control of Per2 gene expression by the daily fluctuation of corticosterone secretion from the adrenal gland |
30, 62 |
| Exposure of cultured cells to dexamethasone and/or prednisolone |
Human bronchial epithelial cells |
Induction of human and rat Per1 | 29, 32 |
| Human peripheral mononuclear cells Rat fibroblasts |
|||
| Acute physical stress in mice | Liver, heart, lung and stomach | Induction of Per1 mRNA | 33 |
| GRNesCre mice (tissue-specific GR knockout) |
BNSTov and CEA of the amygdala |
The presence of GR confers rhythmic Per2 expression |
35 |
| Dexamethasone treatment | Mouse primary mesenchymal stem cells |
Regulation of mRNA expression of Per1, Per2, E4BP4 and Timeless. Identification of functional GREs in the promoter region of the Per1, Per2 and E4BP4 genes. |
34 |
Clock/Bmal1 directly regulates GR transcriptional activity through acetylation
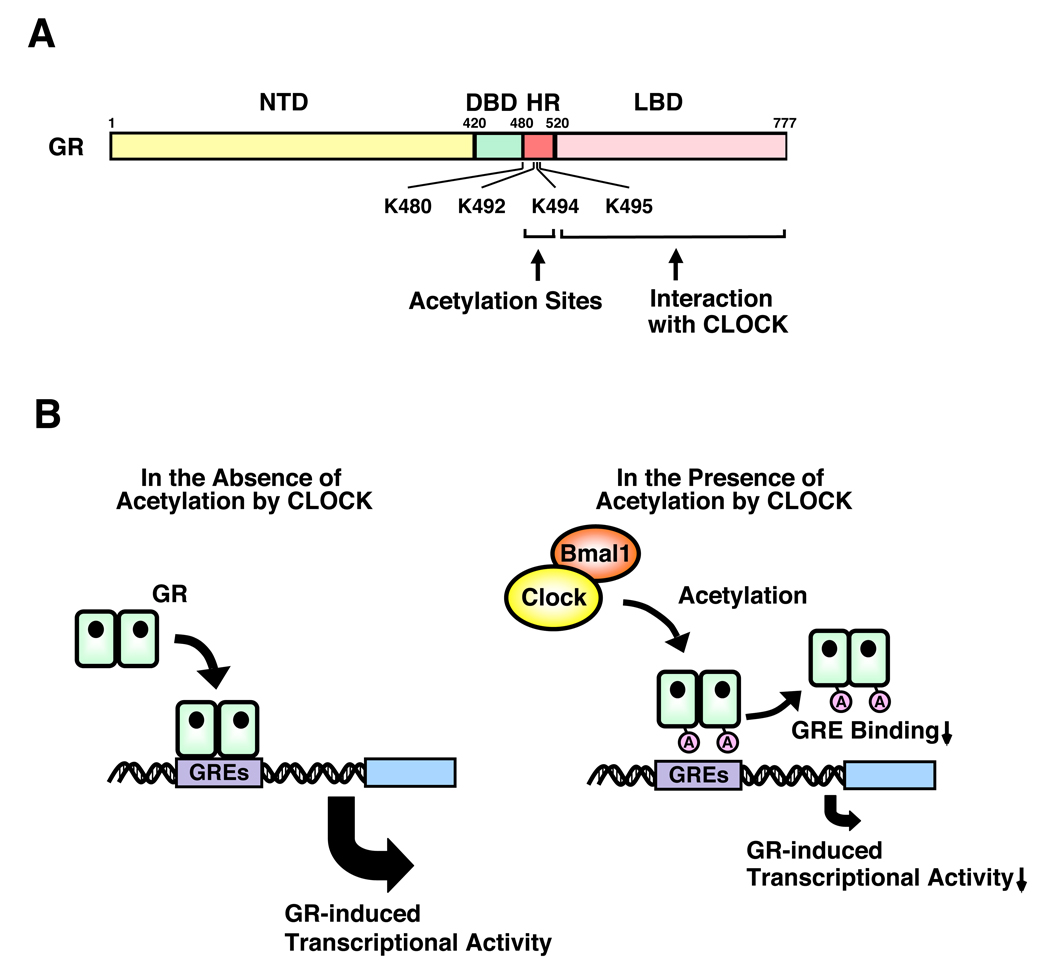
The CLOCK and stress systems crosstalk with each other at peripheral target tissues through the GR. Indeed, Clock/Bmal1 physically interact with the ligand-binding domain of the GR through a region enclosed in the C-terminal part of the Clock protein, and suppressed GR-induced transcriptional activity 36. In serum shock-synchronized cells, GR transactivational activity, assessed by mRNA expression of an endogenous glucocorticoid-responsive gene that contains functional GREs in its promoter region, fluctuated spontaneously in a circadian fashion, in reverse phase with Clock/Bmal1 mRNA expression 36. Clock shares high amino acid and structural similarity with the activator of thyroid receptor (ACTR), a member of the p160-type nuclear receptor coactivator family with inherent histone acetyltransferase (HAT) activity, and, not surprisingly, has such enzymatic function as well 37. Clock acetylated a lysine cluster located in the hinge region of the human GR, including lysines at amino acid positions 480, 492, 494 and 495, reducing GR’s affinity for its cognate DNA sequence GREs 36. These findings indicate that Clock/Bmal1 is a reverse phase negative regulator of glucocorticoid action in target tissues, antagonizing the biological actions of diurnally fluctuating circulating glucocorticoids and providing a local target tissue counter regulatory feedback loop to the central CLOCK on the HPA axis.
Implications of the CLOCK-HPA axis interaction for the development of metabolic and immune disorders
As evidenced in the sections above, the CLOCK system and HPA axis influence the activity and function of the CNS and peripheral tissues, while the master CLOCK system clearly dictates the circadian activity of the HPA axis. In the following sections, we discuss these mutual interactions and their implications in the development of pathologic conditions, with metabolic and immune disorders as illustrative examples.
Intermediary metabolism handles the turnover of carbohydrates, proteins and lipids, as well as energy production/storage, all functions essential for survival. Expression of a substantial number (~10%) of the energy-controlling genes, including those encoding nuclear hormone receptors and enzymes for glucose and lipid metabolism, are under circadian regulation, in a tissue-specific fashion 38–41. One nuclear receptor, Rev-erbα, which is also a negative regulator of the circadian CLOCK transcription circuit, suppresses gluconeogenesis and lipid metabolism in the liver, inhibits adipocyte differentiation and represses the transcriptional activity of several other nuclear receptors, including PPARγ and RORα 42, 43. Mice defective in the Clock or Bmal1 gene demonstrate disturbances in gluconeogenesis and defective diurnal variation of circulating glucose and triglycerides, and develop obesity, hyperlipidemia and diabetes mellitus 44, 45, as well as elevated expression of the plasminogen activator inhibitor-1 (PAI-1), all known risk factors for obesity, diabetes and cardiovascular disease 46–48. Another CLOCK protein, Per2, represses PAI-1 expression in a Clock/Bmal1-dependent manner, indicating that Per2 is also an interface for the development of these metabolic diseases upon dysregulation of the CLOCK system 48. In humans, rotating shift workers whose circadian system is continuously reset by night-time activity/day-time sleep show a higher risk of obesity, hypertension, high triglycerides, insulin resistance and subsequent development of ischemic heart disease 49, 50. Furthermore, specific single nucleotide polymorphisms (SNPs) within the Clock gene have been associated with development of obesity 51, 52, while mRNA expression of Bmal1, Per2 and Cry1 in visceral fat are associated with increased waist circumference, an indicator of visceral adiposity and the metabolic syndrome 53.
Persistent stimulation of the HPA axis by various stressors or administration of high doses of glucocorticoids strongly shift intermediary metabolism toward catabolism and visceral fat accumulation 19, 22. Elevated circulating glucocorticoids stimulate gluconeogenesis, glycogenolysis, lipolysis and degradation of proteins into amino acids, and over time cause central obesity and insulin resistance/overt diabetes mellitus, hyper(dys)lipidemia and muscle loss and thinning of the skin, all of which are typically observed in patients with Cushing’s syndrome 19, 22, 54.
Interestingly, most of the metabolic phenotypes associated with dysregulation of the CLOCK system and the HPA axis overlap with each other (Table 3). Whether disruption of the former system leads to metabolic problems through modulation of the HPA axis, or these distinct systems influence the same metabolic pathways independently is not known. We hypothesize that both mechanisms are functional in the development of the metabolic syndrome upon dysregulation of the CLOCK system. Indeed, several molecules, such as PPARγ, PAI-I and PGC1α, which play key roles in intermediary metabolism, respond to both the CLOCK system and glucocorticoids; furthermore, mice defective in the clock genes, such as Per1−/− and Per2−/− mice, have defective glucose metabolism and obesity with an impaired HPA axis and altered diurnal glucocorticoid rhythm 26, 27. Furthermore, Clock/Bmal1-mediated modulation of GR-induced transcriptional activity may also participate in the development of metabolic abnormalities, as these transcription factors suppress glucocorticoid-induced mRNA expression of glucose-6 phosphatase, a rate-limiting enzyme in the process of glycogenolysis 36.
Table 3.
Metabolic Disturbances Associated with Loss of Circadian Rhythm or Glucocorticoid Excess.
| Signs and symptoms | Loss of circadian rhythm | Glucocorticoid excess |
|---|---|---|
| Glucose metabolism | ||
| Elevation of plasma glucose | ++ | ++ |
| Peripheral insulin resistance | ++ | ++ |
| Fat metabolism | ||
| Elevation of circulating triglycerides | ++ | + |
| Elevation of circulating cholesterol | + | + |
| Fatty liver | + | + |
| Central obesity | ++ | ++ |
| Protein turnover | Biosynthesis ↓? | Catabolism ↑ |
| Appetite | ↑ | ↑ |
| Hypertension | + | + |
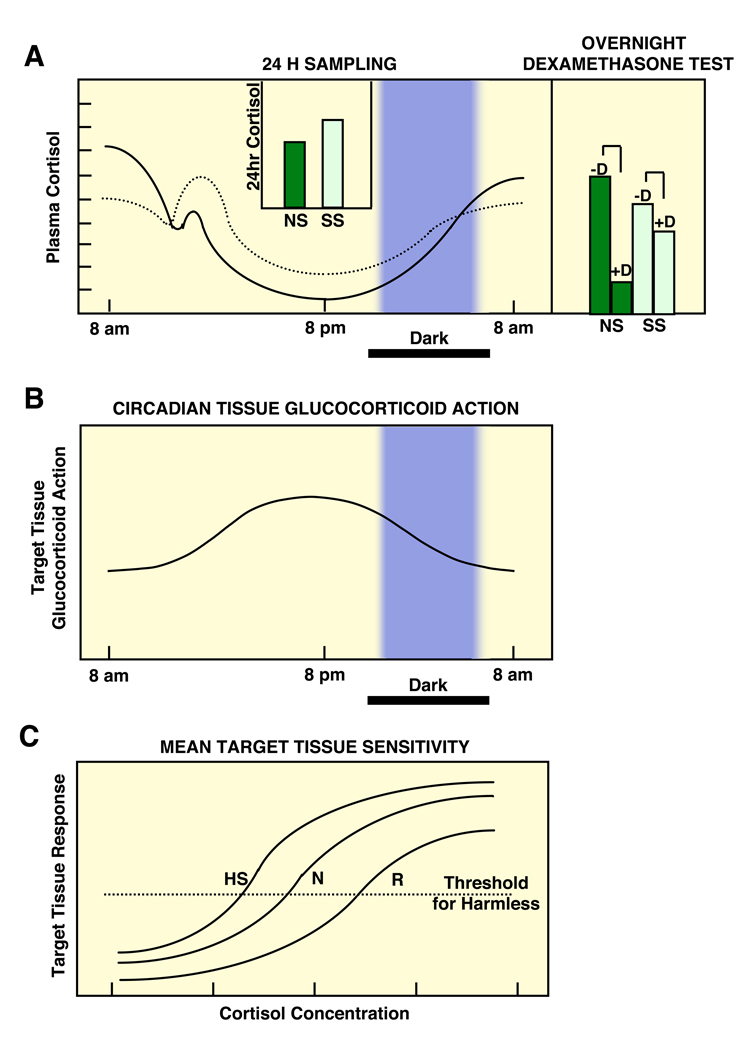
The circadian activity in the sensitivity of glucocorticoid target tissues to cortisol may explain the development of central obesity and the metabolic syndrome in chronically stressed individuals, whose HPA axis circadian rhythm is characterized by blunting of the evening decreases of circulating glucocorticoids, as a result of enhanced input of higher centers upon the hypothalamic PVN secretion of CRH and AVP (Figure 4). Similarly disrupted coupling of the secretion of cortisol and target tissue sensitivity to glucocorticoids may explain the increased cardiometabolic risk of persons who are exposed to frequent jet lag because of traveling across time zones 55, 56.
Figure 4.
A heuristic scheme of the circadian secretion of cortisol in non-stressed and chronically stressed humans (top left panel) and their responses to midnight dexamethasone administration (top right panel), the corresponding circadian changes of target tissue sensitivity to glucocorticoids (middle panel) and the mean target tissue sensitivity to glucocorticoids in the human population (bottom panel).
It is obvious that even mild evening cortisol elevations, as those seen in chronically stressed individuals, will exert disproportionately increased glucocorticoid effects because of the natural circadian target tissue sensitivity increase at this time of the day. CS: Chronically stressed individuals, D: midnight dexamethasone administration, HS: hypersensitivity, N: normal sensitivity, NS: non-stressed individuals, R: resistance Modified from 65.
Similar interactions between the CLOCK system and the HPA axis are also observed in the regulation of immune function. In both humans and rodents, the CLOCK system produces a circadian fluctuation of several cytokines, including interferon (IFN) γ, interleukin-1 (IL-1)β, IL-6, and TNFα in T- and B-lymphocytes and natural killer cells 57, 58. Indeed, knockout mice defective in components of the CLOCK system demonstrate various immune dysfunctions, such as a blunted and/or absent circadian rhythm in circulating leukocytes and cytokine secretion, an impaired response to lipopolysaccharide (LPS)-induced endotoxic shock and defective B-lymphocyte development 57, 58. Activation of the HPA axis and subsequent release of glucocorticoids, in turn, strongly influence immune activity and the inflammatory reaction 4, 19, 22. Physiologic concentrations of glucocorticoids are crucial for proper function of the immune system, whereas pharmacologic doses and/or high concentrations observed under stress strongly suppress its activity primarily by repressing the activity of transcription factors/other molecules crucial for the regulation of immune function/inflammation, such as activator protein-1 (AP-1), NF-κB and STAT5, through protein-protein interaction with GR 20, 21, 59. Since many immune cells are in the circulation, with decreased access to the CNS, their peripheral CLOCK system seems to be mainly regulated through humoral factors, including glucocorticoids, that are under the control of the central CLOCK 58. The CLOCK system may also regulate immune function and lead to development of immunologic defects in part through modulation of endogenously secreted glucocorticoid actions on numerous components of the immune system via mutual interaction between transcription factors Clock/Bmal1 and GR.
Concluding remarks
The circadian CLOCK and stress systems regulate each other’s activity through multi-level interactions to ultimately coordinate homeostasis against the day/night change and various unforeseen random internal and external stressors. As the day/night changes are more general and continuously happen, the circadian CLOCK system controls the stress system, while the latter adjusts the circadian rhythmicity of the non-master peripheral components of the former in response to various stressors. Thus, dysfunction in either system may alter internal homeostasis and cause pathologic changes virtually in all organs and tissues, including those responsible for intermediary metabolism and immunity. Only recently, the mutual interactions of the CLOCK circadian system and the stress HPA axis have been investigated, thus most of their physiologic as well as pathologic inter-communications remain to be examined. Specifically, nutritional availability, an environmental factor essential for survival of organisms and a major regulator of intermediary metabolism, strongly influences both of these systems, whereas little has been reported on their mutual regulations regarding this biologic activity. Indeed, traveling across time zone is now quite frequent and approximately 8.6 million Americans perform night-shift work, while prevention of overfeeding-associated metabolic abnormalities and subsequent development of cardiovascular diseases are crucial for public health 56. Thus, further studies are required to understand the exact molecular interactions between these systems and their interplay in the development of human pathology. Such research will eventually help develop rational therapies for various disorders, including behavioral, metabolic and immune diseases.
Figure 3.
Clock/Bmal1 suppresses GR-induced transcriptional activity through acetylation. (a) Clock physically interacts with the ligand-binding domain of the GR through the domain enclosed in it s C-terminal part and suppresses GR-induced transcriptional activity by acetylating via its intrinsic HAT activity a lysine cluster located in the hinge region of the GR, through which (b) Clock reduces affinity of GR to its cognate DNA sequences GREs.
A: acetylation, Bmal1: brain-muscle-arnt-like protein 1, DBD: DNA-binding domain, GR: glucocorticoid receptor, GRE: glucocorticoid response element, HR: hinge region, K: lysine residue, LBD: ligand-binding domain, NTD: N-terminal domain C: A heuristic model of the physiologic implications of this study. Modified from 36.
Acknowledgments
Literary work of this article was funded partly by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD.
References
- 1.Takahashi JS, et al. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15(Spec No 2):R271–R277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- 3.Hastings M, et al. Circadian clocks: regulators of endocrine and metabolic rhythms. J Endocrinol. 2007;195:187–198. doi: 10.1677/JOE-07-0378. [DOI] [PubMed] [Google Scholar]
- 4.Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- 5.Chrousos GP, Kino T. Glucocorticoid action networks and complex psychiatric and/or somatic disorders. Stress. 2007;10:213–219. doi: 10.1080/10253890701292119. [DOI] [PubMed] [Google Scholar]
- 6.Kalsbeek A, et al. SCN outputs and the hypothalamic balance of life. J Biol Rhythms. 2006;21:458–469. doi: 10.1177/0748730406293854. [DOI] [PubMed] [Google Scholar]
- 7.Cermakian N, Sassone-Corsi P. Multilevel regulation of the circadian clock. Nat Rev Mol Cell Biol. 2000;1:59–67. doi: 10.1038/35036078. [DOI] [PubMed] [Google Scholar]
- 8.Kondratov RV, et al. Post-translational regulation of circadian transcriptional CLOCK(NPAS2)/BMAL1 complex by CRYPTOCHROMES. Cell Cycle. 2006;5:890–895. doi: 10.4161/cc.5.8.2684. [DOI] [PubMed] [Google Scholar]
- 9.Kiyohara YB, et al. The BMAL1 C terminus regulates the circadian transcription feedback loop. Proc Natl Acad Sci U S A. 2006;103:10074–10079. doi: 10.1073/pnas.0601416103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ripperger JA, Schibler U. Rhythmic CLOCK-BMAL1 binding to multiple E-box motifs drives circadian Dbp transcription and chromatin transitions. Nat Genet. 2006;38:369–374. doi: 10.1038/ng1738. [DOI] [PubMed] [Google Scholar]
- 11.Meijer JH, Schwartz WJ. In search of the pathways for light-induced pacemaker resetting in the suprachiasmatic nucleus. J Biol Rhythms. 2003;18:235–249. doi: 10.1177/0748730403018003006. [DOI] [PubMed] [Google Scholar]
- 12.Oster H, et al. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab. 2006;4:163–173. doi: 10.1016/j.cmet.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Kraves S, Weitz CJ. A role for cardiotrophin-like cytokine in the circadian control of mammalian locomotor activity. Nat Neurosci. 2006;9:212–219. doi: 10.1038/nn1633. [DOI] [PubMed] [Google Scholar]
- 14.Mori K, et al. Neuromedin S: discovery and functions. Results Probl Cell Differ. 2008;46:201–212. doi: 10.1007/400_2007_054. [DOI] [PubMed] [Google Scholar]
- 15.Yoo SH, et al. PERIOD2∷LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Damiola F, et al. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuller PM, et al. Differential rescue of light- and food-entrainable circadian rhythms. Science. 2008;320:1074–1077. doi: 10.1126/science.1153277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bao AM, et al. The stress system in depression and neurodegeneration: focus on the human hypothalamus. Brain Res Rev. 2008;57:531–553. doi: 10.1016/j.brainresrev.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Kino T, Chrousos GP. Glucocorticoid effect on gene expression. In: Steckler T, et al., editors. Handbook on Stress and the Brain. Elsevier BV; 2005. pp. 295–312. [Google Scholar]
- 20.Chrousos GP, Kino T. Intracellular glucocorticoid signaling: a formerly simple system turns stochastic. Sci STKE. 2005;2005:48. doi: 10.1126/stke.3042005pe48. [DOI] [PubMed] [Google Scholar]
- 21.Kino T, Chrousos GP. Glucocorticoid and mineralocorticoid receptors and associated diseases. Essays Biochem. 2004;40:137–155. doi: 10.1042/bse0400137. [DOI] [PubMed] [Google Scholar]
- 22.Chrousos GP. Glucocorticoid therapy. In: Felig P, Frohman LA, editors. Endocrinology & Metabolism. 4th edn. McGraw-Hill; 2001. pp. 609–632. [Google Scholar]
- 23.Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–1362. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 24.Ishida A, et al. Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell Metab. 2005;2:297–307. doi: 10.1016/j.cmet.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Ulrich-Lai YM, et al. Adrenal splanchnic innervation contributes to the diurnal rhythm of plasma corticosterone in rats by modulating adrenal sensitivity to ACTH. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1128–R1135. doi: 10.1152/ajpregu.00042.2003. [DOI] [PubMed] [Google Scholar]
- 26.Dallmann R, et al. Impaired daily glucocorticoid rhythm in Per1 (Brd) mice. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006;192:769–775. doi: 10.1007/s00359-006-0114-9. [DOI] [PubMed] [Google Scholar]
- 27.Yang S, et al. The role of mPer2 clock gene in glucocorticoid and feeding rhythms. Endocrinology. 2009;150:2153–2160. doi: 10.1210/en.2008-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balsalobre A, et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 29.Fukuoka Y, et al. Glucocorticoid administration increases hPer1 mRNA levels in human peripheral blood mononuclear cells in vitro or in vivo. J Biol Rhythms. 2005;20:550–553. doi: 10.1177/0748730405279866. [DOI] [PubMed] [Google Scholar]
- 30.Segall LA, et al. Glucocorticoid rhythms control the rhythm of expression of the clock protein, Period2, in oval nucleus of the bed nucleus of the stria terminalis and central nucleus of the amygdala in rats. Neuroscience. 2006;140:753–757. doi: 10.1016/j.neuroscience.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 31.Oishi K, et al. Genome-wide expression analysis reveals 100 adrenal gland-dependent circadian genes in the mouse liver. DNA Res. 2005;12:191–202. doi: 10.1093/dnares/dsi003. [DOI] [PubMed] [Google Scholar]
- 32.Burioka N, et al. Circadian rhythms in the CNS and peripheral clock disorders: function of clock genes: influence of medication for bronchial asthma on circadian gene. J Pharmacol Sci. 2007;103:144–149. doi: 10.1254/jphs.fmj06003x4. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto T, et al. Acute physical stress elevates mouse period1 mRNA expression in mouse peripheral tissues via a glucocorticoid-responsive element. J Biol Chem. 2005;280:42036–42043. doi: 10.1074/jbc.M509600200. [DOI] [PubMed] [Google Scholar]
- 34.So AY, et al. Glucocorticoid regulation of the circadian clock modulates glucose homeostasis. Proc Natl Acad Sci U S A. 2009;106:17582–17587. doi: 10.1073/pnas.0909733106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Segall LA, et al. Brain glucocorticoid receptors are necessary for the rhythmic expression of the clock protein, PERIOD2, in the central extended amygdala in mice. Neurosci Lett. 2009;457:58–60. doi: 10.1016/j.neulet.2009.03.083. [DOI] [PubMed] [Google Scholar]
- 36.Nader N, et al. Circadian rhythm transcription factor CLOCK regulates the transcriptional activity of the glucocorticoid receptor by acetylating its hinge region lysine cluster: potential physiological implications. FASEB J. 2009;23:1572–1583. doi: 10.1096/fj.08-117697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doi M, et al. Circadian regulator CLOCK is a histone acetyltransferase. Cell. 2006;125:497–508. doi: 10.1016/j.cell.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 38.Yang X, et al. Nuclear receptor expression links the circadian clock to metabolism. Cell. 2006;126:801–810. doi: 10.1016/j.cell.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 39.Staels B. When the Clock stops ticking, metabolic syndrome explodes. Nat Med. 2006;12:54–55. doi: 10.1038/nm0106-54. [DOI] [PubMed] [Google Scholar]
- 40.Panda S. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 41.Storch KF, et al. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417:78–83. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- 42.Duez H, Staels B. Rev-erbα gives a time cue to metabolism. FEBS Lett. 2008;582:19–25. doi: 10.1016/j.febslet.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 43.Yin L, et al. Rev-erbα, a heme sensor that coordinates metabolic and circadian pathways. Science. 2007;318:1786–1789. doi: 10.1126/science.1150179. [DOI] [PubMed] [Google Scholar]
- 44.Rudic RD, et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2:e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turek FW, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oishi K. Plasminogen activator inhibitor-1 and the circadian clock in metabolic disorders. Clin Exp Hypertens. 2009;31:208–219. doi: 10.1080/10641960902822468. [DOI] [PubMed] [Google Scholar]
- 47.Oishi K, et al. CLOCK is involved in obesity-induced disordered fibrinolysis in ob/ob mice by regulating PAI-1 gene expression. J Thromb Haemost. 2006;4:1774–1780. doi: 10.1111/j.1538-7836.2006.02032.x. [DOI] [PubMed] [Google Scholar]
- 48.Oishi K, et al. PERIOD2 is a circadian negative regulator of PAI-1 gene expression in mice. J Mol Cell Cardiol. 2009;46:545–552. doi: 10.1016/j.yjmcc.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 49.Fujino Y, et al. A prospective cohort study of shift work and risk of ischemic heart disease in Japanese male workers. Am J Epidemiol. 2006;164:128–135. doi: 10.1093/aje/kwj185. [DOI] [PubMed] [Google Scholar]
- 50.Sookoian S, et al. Effects of rotating shift work on biomarkers of metabolic syndrome and inflammation. J Intern Med. 2007;261:285–292. doi: 10.1111/j.1365-2796.2007.01766.x. [DOI] [PubMed] [Google Scholar]
- 51.Sookoian S, et al. Genetic variants of Clock transcription factor are associated with individual susceptibility to obesity. Am J Clin Nutr. 2008;87:1606–1615. doi: 10.1093/ajcn/87.6.1606. [DOI] [PubMed] [Google Scholar]
- 52.Scott EM, et al. Association between polymorphisms in the Clock gene, obesity and the metabolic syndrome in man. Int J Obes (Lond) 2008;32:658–662. doi: 10.1038/sj.ijo.0803778. [DOI] [PubMed] [Google Scholar]
- 53.Gomez-Abellan P, et al. Clock genes are implicated in the human metabolic syndrome. Int J Obes (Lond) 2008;32:121–128. doi: 10.1038/sj.ijo.0803689. [DOI] [PubMed] [Google Scholar]
- 54.Friedman TC, et al. Carbohydrate and lipid metabolism in endogenous hypercortisolism: shared features with metabolic syndrome X and NIDDM. Endocr J. 1996;43:645–655. doi: 10.1507/endocrj.43.645. [DOI] [PubMed] [Google Scholar]
- 55.Ekstrand K, et al. Cardiovascular risk factors in commercial flight aircrew officers compared with those in the general population. Angiology. 1996;47:1089–1094. doi: 10.1177/000331979604701109. [DOI] [PubMed] [Google Scholar]
- 56.Scheer FA, et al. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu J, et al. The circadian clock Period 2 gene regulates γ interferon production of NK cells in host response to lipopolysaccharide-induced endotoxic shock. Infect Immun. 2006;74:4750–4756. doi: 10.1128/IAI.00287-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arjona A, Sarkar DK. Are circadian rhythms the code of hypothalamic-immune communication? Insights from natural killer cells. Neurochem Res. 2008;33:708–718. doi: 10.1007/s11064-007-9501-z. [DOI] [PubMed] [Google Scholar]
- 59.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 60.Pilorz V, et al. Age and oestrus cycle-related changes in glucocorticoid excretion and wheel-running activity in female mice carrying mutations in the circadian clock genes Per1 and Per2. Physiol Behav. 2009;96:57–63. doi: 10.1016/j.physbeh.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 61.Reddy AB, et al. Glucocorticoid signaling synchronizes the liver circadian transcriptome. Hepatology. 2007;45:1478–1488. doi: 10.1002/hep.21571. [DOI] [PubMed] [Google Scholar]
- 62.Segall LA, et al. Timed restricted feeding restores the rhythms of expression of the clock protein, Period2, in the oval nucleus of the bed nucleus of the stria terminalis and central nucleus of the amygdala in adrenalectomized rats. Neuroscience. 2008;157:52–56. doi: 10.1016/j.neuroscience.2008.08.055. [DOI] [PubMed] [Google Scholar]
- 63.Scott EM, et al. Association between polymorphisms in the Clock gene, obesity and the metabolic syndrome in man. Int J Obes (Lond) 2008;32:658–662. doi: 10.1038/sj.ijo.0803778. [DOI] [PubMed] [Google Scholar]
- 64.Woon PY, et al. Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc Natl Acad Sci U S A. 2007;104:14412–14417. doi: 10.1073/pnas.0703247104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chrousos GP. The role of stress and the hypothalamic-pituitary-adrenal axis in the pathogenesis of the metabolic syndrome: neuro-endocrine and target tissue-related causes. Int J Obes Relat Metab Disord. 2000;24:S50–S55. doi: 10.1038/sj.ijo.0801278. [DOI] [PubMed] [Google Scholar]