Abstract
Objective
Early detection of glycosaminoglycan loss may provide insight into mechanisms of cartilage damage in the ACL-injured patient. We hypothesized that tibial and femoral dGEMRIC indices would be lower in the medial compartment of the ACL-injured knee than in the contralateral, uninjured knee, and that scan order (i.e. whether the injured or the uninjured knee was imaged first) would not affect the indices.
Methods
15 subjects with unilateral ACL injuries recieved a double dose of gadolinium [Gd(DTPA)2−] intravenously. After 90 minutes, both knees were sequentially imaged. The injured knee was scanned first in the odd-numbered subjects and second in the even-numbered subjects. The dGEMRIC indices of the median slice of the medial compartment were determined using the MRIMapper software. Index comparisons were made between knee status (ACL-injured versus uninjured), scan order (ACL-injured first versus uninjured first), and cartilage location (tibia versus femur) using a mixed model.
Results
There was a significant difference in the mean dGEMRIC indices of the medial compartment between injured and uninjured knees (p<0.007). On average, there was a 13% decrease in the dGEMRIC index of the injured knee compared to the uninjured knee. There were no significant effects due to test order (p=0.800) or cartilage location (p=0.439).
Conclusions
The results demonstrate lower GAG concentrations in the medial compartment of the femoral and tibial articular cartilage of the ACL-injured knee when compared to the contralateral uninjured knee. The dGEMRIC indices were not sensitive to scan order; thus, sequential imaging of both knees is possible in this patient population.
Keywords: Cartilage, ACL, Knee Injury, MRI, Imaging, Osteoarthritis, Proteoglycan
Introduction
Anterior cruciate ligament (ACL) injuries are thought to place the knee at risk for early osteoarthritis (OA), though firm conclusions regarding the prevalence of OA remain controversial, ranging from 0% to 90%1. The mechanisms of cartilage damage following ACL injury remain unknown but likely involve the initial inflammatory response, altered kinematics, abnormal contact stresses, and concomitant injuries to the menisci and subchondral bone2–10. Furthermore, animal models of ACL transection (i.e. the Pond-Nuki model) have been developed to initiate OA to study disease progression and treatments11–12. Because there are many confounding biochemical and biomechanical factors that must be considered, a biomarker that can detect early changes in cartilage metabolism within this patient population will be paramount to identify the prevalence of OA, to help establish the mechanisms and risk factors of cartilage damage, and to evaluate potential treatment strategies. Because it is estimated that more than 400,000 ACL injuries occur each year in the United States13, and that many of these patients are at increased risk for post-traumatic OA, the ability to intervene and reverse the process is critical.
One of the primary matrix molecules of cartilage is aggrecan. The large aggrecan molecules consist of many negatively-charged glycosaminoglycan (GAG) chains captured in a Type II collagen network. The GAGs are responsible for generating the swelling pressures in cartilage that enable it to support high compressive loads. GAG loss is associated with cartilage degeneration14, and follows ACL injury5, 15. Therefore, a method that monitors GAG loss in articular cartilage following ACL injury could possibly serve as an early biomarker of cartilage damage, or as an indicator as to which ACL-injured patients may be at greater risk for developing secondary OA.
Delayed Gadolinium-Enhanced MR Imaging of Cartilage (dGEMRIC) is a molecular imaging technique that has been used to study GAG loss in the articular cartilage of patients with primary OA16–17, and it can be readily used on the cruciate-injured patient18–19. With dGEMRIC, T1-maps of hyaline cartilage are created following the intravenous (IV) administration of an anionic gadolinium-based contrast agent [Gd(DTPA)2−]. Since cartilage matrix is largely composed of GAG molecules with negatively-charged carboxyl and sulfate groups, it repels the negatively charged contrast ions. As a result, the gadolinium concentrations are higher in cartilage regions with low GAG concentrations, and the cartilage T1-relaxation time (T1gd) is reduced16. The resulting dGEMRIC index (the average T1gd in a region of interest) is related to both the GAG concentration and the time between gadolinium administration and image acquisition20–22. In healthy subjects, it is optimal to acquire the MR images 90–120 minutes after IV contrast injection20. For studies of ACL-injured patients, the contralateral uninjured knee has been used as a control2, 23. However, when using dGEMRIC, serial imaging of both knees results in time differences between contrast administration and image acquisition, and thus could influence the dGEMRIC index value.
The objectives of this study were to evaluate the dGEMRIC indices of the tibial and femoral articular cartilage of the medial compartment of the knee following ACL injury, and to compare them to those of the contralateral, uninjured “control” knee in one imaging session. Additionally, we intended to establish whether scan order (i.e. whether the injured or uninjured knee was imaged first) would influence the indices. We hypothesized that the tibial and femoral dGEMRIC indices would be lower in the ACL-injured knees when compared to the uninjured knees, and that the scan order would not affect the dGEMRIC indices. Since the time between injury and MR imaging was variable between patients, our secondary hypothesis was that the dGEMRIC indices of the ACL-injured knee were not dependent on the time from injury.
Materials and Methods
SUBJECTS
A total of 67 consecutive subjects were invited to participate in the study. 51 (76%) declined participation, most commonly citing lack of time required within our local scheduling constraints or unwillingness to receive the IV infusion of contrast agent. Sixteen subjects were included in the study, but in one case the time to follow up was more than 10 years, and this subject was thus excluded from the analysis leaving 15 subjects (7 male and 8 female), who were candidates for unilateral arthroscopic ACL reconstruction and met the inclusion criteria for this study. All subjects had consulted with an orthopaedic surgeon for the treatment of a traumatic ACL injury to correct problems associated with joint instability (Table 1), and were included if they were between the ages of 18 and 50 years, had no prior history of injury to either knee, and no predisposing conditions for arthritis (i.e. rheumatoid arthritis, diabetes). Subjects were excluded if they had concomitant ligament injuries, significant mensical damage (greater than 1/3 involvement), and/or if they had any chondral lesions as determined by clinical examination and verified by diagnostic MRI. The diagnostic MRIs were also used to evaluate the presence of traumatic bone marrow lesions (BMLs) and subchondral fractures in the tibial plateau and femoral condyles. The median age was 26 years (range 19–48) and the median time from injury to MR imaging was 82 days (range 21–424). Study approval was granted by the local Institutional Review Board of Rhode Island Hospital prior to initiating subject recruitment. All subjects gave their informed consent prior to participating.
Table 1.
Characteristics medial compartment dGEMRIC values for the study subjects.
| Femur T1gd (ms) | Tibia T1gd (ms) | |||||||
|---|---|---|---|---|---|---|---|---|
| Sub# | Age | Sex | Injury | Days* | Injured | Control | Injured | Control |
| 1 | 20 | M | Skiing | 128 | 410 | 486 | 405 | 505 |
| 2 | 26 | F | Basketball | 78 | 384 | 379 | 361 | 439 |
| 3 | 48 | F | Work (fall) | 170 | 438 | 490 | 441 | 456 |
| 4 | 20 | M | Skateboarding | 46 | 421 | 517 | 369 | 491 |
| 5 | 20 | M | Soccer | 21 | 467 | 501 | 440 | 480 |
| 6 | 31 | M | Work (twist) | 94 | 375 | 453 | 378 | 451 |
| 7 | 48 | M | Work (fall) | 424 | 422 | 401 | 425 | 435 |
| 8 | 37 | F | Skiing | 90 | 432 | 487 | 433 | 451 |
| 9 | 39 | F | Volleyball | 61 | 456 | 525 | 434 | 484 |
| 10 | 44 | F | Skiing | 82 | 361 | 424 | 411 | 372 |
| 11 | 44 | F | Skiing | 56 | 389 | 404 | 336 | 394 |
| 12 | 23 | F | Ultimate Frisbee | 45 | 381 | 381 | 385 | 499 |
| 13 | 25 | M | Work (fall) | 115 | 375 | 417 | 378 | 400 |
| 14 | 19 | M | Basketball | 119 | 342 | 689 | 359 | 615 |
| 15 | 19 | F | Soccer | 68 | 424 | 422 | 436 | 448 |
Days = “Time from Injury”
MR IMAGING
The MR images were performed on a 1.5T magnet (Symphony; Siemens, Erlangen, Germany) using a quadrature knee coil (Siemens, Erlangen, Germany). Since we intended to perform the dGEMRIC analyses on the femoral and tibial articular cartilage from a single, 3 mm-thick slice through the median plane of the medial femoral condyle (Fig. 1), a localizer sequence [axial T1-weighted gradient echo] was first obtained. Five turbo spin-echo inversion recovery sequences [inversion times of 1650ms, 650ms, 350ms, 150ms, and 28ms; TR=1800ms; TE=19ms; Bandwidth = 326Hz; FOV=160mm; Matrix=384 × 384; voxel size=0.4 × 0.4 × 3mm; NEX = 1] were then acquired for subsequent T1gd mapping to compute the dGEMRIC indices of the medial femoral and tibial articular cartilage.
Figure 1.
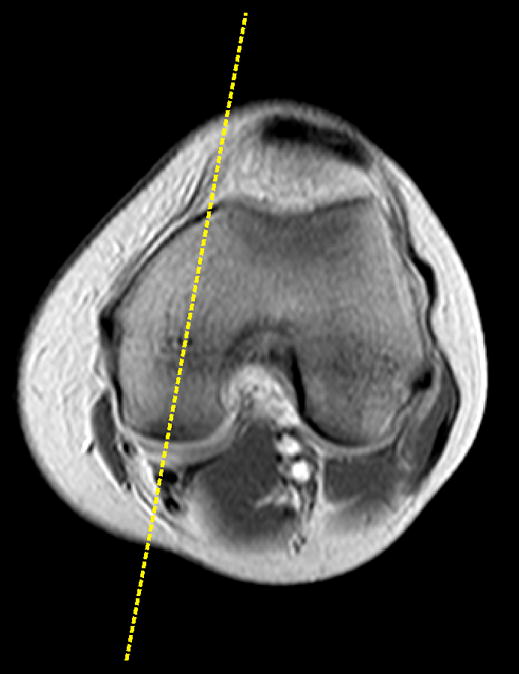
The median slice through the medial femoral condyle was selected for dGEMRIC analysis. Both the tibial and femoral cartilage segments were then evaluated within this slice.
dGEMRIC PROTOCOL
Each subject received gadolinium diethylene triamine penta-acetic acid (Magnevist; Bayer Healthcare Pharmaceuticals, Montville, NJ), 0.2 mmol/Kg, administered by slow IV infusion through a catheter placed in the antecubital vein. The contrast agent injection time was less than 5 minutes. The subject then walked vigorously for 10 minutes to promote delivery of the contrast agent to the joint. The walk was followed by 80 minutes of rest to enable the contrast agent to diffuse into the articular cartilage. The first dGEMRIC protocol was initiated 90 minutes after contrast administration.
The dGEMRIC scans were performed of the ACL-injured and contralateral uninjured knees sequentially. For the odd-numbered subjects, the ACL-injured knee was scanned first. For the even-numbered subjects, the uninjured knee was scanned first. The first knee was imaged 90 minutes post-contrast administration; the total imaging time was 11:50 minutes. The second knee was imaged approximately 105 minutes after the injection. This approach allowed us to evaluate the effects of scan order on the dGEMRIC indices.
IMAGE PROCESSING
The MRIMapper software package (2006a R2.1: Beth Israel Deaconess Medical Center, Boston, MA) was used to create T1gd maps of the femoral and tibial cartilage segments in the medial compartment (Fig. 2). First, the slice was viewed on a PC workstation. The femoral and tibial cartilage segments were segmented manually from the slice by an experienced examiner (Fig. 2). T1 maps were constructed and the average dGERMIC indices (T1gd) were calculated. Analyses were performed on both the femoral and tibial articular cartilage.
Figure 2.
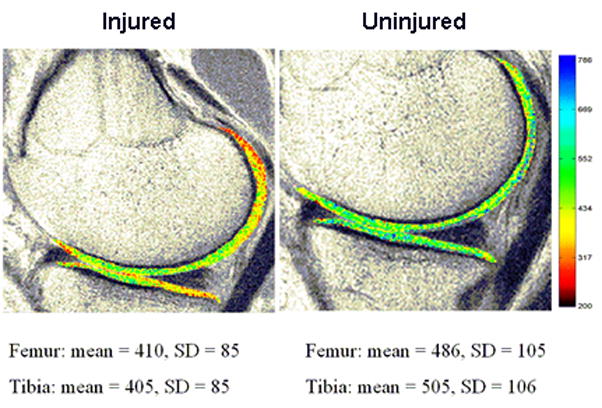
T1 maps were generated of the medial compartment of the tibia and the femur using the MRIMapper software package, and the mean dGEMRIC indices were calculated. The blue and red regions denote high and low GAG concentrations, respectively.
STATISTICAL ANALYSES
Injured and uninjured dGEMRIC indices of the medial compartment were compared using a three-way mixed linear model. The three factors were cartilage location (tibia versus femur), knee status (injured versus uninjured), and scan order (injured first versus uninjured first). The mixed linear model allowed us to consider cartilage location and knee status as within-subject factors and scan order as an across-subject factor. All main effects, two-way interactions, and the three-way interaction were included in the model. Cartilage location was included as a random effect within subject using an unstructured variance-covariance structure, grouped by knee after it was established that there was no significant covariance between cartilage location across knees within individuals. The three-way and the two-way interactions including scan order were tested for order effects. The main effects of knee status and scan order tested the primary hypotheses. The study was powered (post-hoc) to detect a mean difference in the tibial and femoral dGEMRIC indices of 52ms and 65ms, respectively.
Given that patients also varied in terms of their time from injury, a repeated measures mixed linear model was used to probe the secondary hypothesis; the dGEMRIC indices did not vary as a function of time from injury. The model included terms for time from injury (days), cartilage location (tibia versus femur), and knee status (injured versus uninjured), as well as all the two- and three-way interactions.
Comparisons between the two groups regarding the demographic parameters were performed using Fisher’s exact test (gender distribution; bone marrow lesion distribution) or t-tests (all other demographic parameters).
Results
The demographics of Groups 1 and 2 are presented in Table 2. The median time between knee injury and MR imaging was 117 and 68 days for Groups 1 and 2, respectively. Fourteen subjects were imaged between 21 and 170 days after knee injury and one was imaged approximately one year after knee injury (424 days). Results of the study did not change when repeat analyses were performed after excluding the one subject imaged one year after injury (Table 1; Subject 7).
Table 2.
The demographics of Groups 1 and 2 were relatively comparable.
| Group 1 | Group 2 | p-value | |
|---|---|---|---|
| Age | 31 (13) | 31 (9) | 0.998 |
| Gender | 3F/5M | 5F/2M | 0.314 |
| Time from Injury (days)* | 117 | 68 | 0.181 |
| Height (cm) | 175 (44.9) | 165 (8.1) | 0.138 |
| Weight (Kg) | 88 (21.0) | 71 (9.6) | 0.063 |
| BMI | 28.0 (4.9) | 24.8 (2.3) | 0.167 |
| BML (injured) | 8/8 | 7/7 | 1.000 |
| BML (uninjured) | 1/8 | 0/7 | 1.000 |
Note that Group 1 consisted of the ACL-injured subjects in which the injured knee was imaged first, while Group 2 consisted of those subjects in which the uninjured knee was imaged first. The values provided are the means (standard deviation) except where noted.
indicates the median value. BMI = body mass index. BML = presence of traumatic bone marrow lesions.
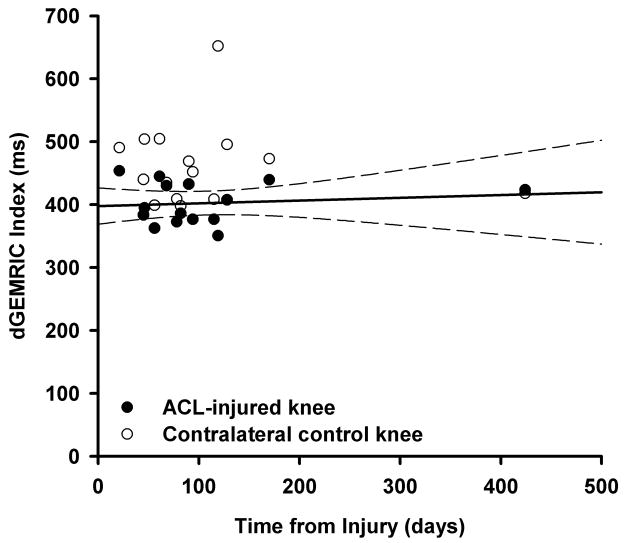
The medial compartment dGEMRIC indices of the ACL-injured knees were significantly less than those of the contralateral uninjured knees (p=0.007). The dGEMRIC indices of the tibial and femoral cartilage were not statistically different (p=0.800). There was no significant effect due to test order (p=0.439), and no significant interactions (p>0.373). On average, there was a 13% decrease in the dGEMRIC indices for the femoral cartilage (60±86.9 ms decrease) and the tibial cartilage (62±69.5 ms decrease) of the injured knee when compared to that of the contralateral uninjured knee (Fig 3). In addition, there was no significant effect due to days from injury (p=0.240; Fig. 4) and no significant interactions between days from injury with knee status or cartilage location (p>0.207). Time from injury did not influence dGEMRIC indices for either the medial femur or medial tibia (Fig. 4).
Figure 3.
Mean dGEMRIC indices from the mid-sagittal slice of the medial compartment of the tibia and femur. The dGEMRIC indices of the ACL-injured knees were significantly less than those of the contralateral uninjured knees (p=0.007). The indices between the tibia and femur were not significantly different (p=0.800). There was no significant difference due to imaging order (p=0.439). The dotted line represents the upper threshold from patients with radiographic evidence of OA25.
Figure 4.
The medial compartment dGEMRIC indices for the ACL-injured (closed circles) and the contralateral control knee (open circles) are shown for all subjects who were imaged within one year of injury. For each pair, the dGEMRIC index for the injured knee is less than that of the uninjured knee. The regression line and the associated 95% confidence intervals for the injured knees are also shown. The lower dGEMRIC index remains relatively constant over time.
Discussion
In this study we hypothesized that the dGEMRIC indices of the tibial and femoral cartilage of the medial compartment would be lower in the ACL-injured knees when compared to the contralateral uninjured (control) knees, and that imaging order would not affect these indices. The results support both hypotheses. We found that the average dGEMRIC indices of the cartilage in the medial femoral condyle and medial tibial plateau of the ACL-injured knees were 13% less than those of the uninjured knees. It should be noted that the demographics, including age, weight, height, BMI and the presence of BMLs, which might influence the scan order results, were similar between the two groups of subjects. Because scan order did not influence the dGEMRIC indices, the contralateral uninjured knee can be used as a matched control for investigating cartilage health in ACL-injured patients.
The cross-sectional sample of the present study also allowed us to explore the changes in dGEMRIC indices of the medial compartment with respect to time from injury. The analyses revealed that the indices were not dependent on the time from injury, and that the dGEMRIC indices of the injured knees remain lower than those of the contralateral uninjured knees (p<0.0001). This data should be interpreted cautiously, however, because the time from injury for all but one subject was less than 6 months (Table 1).
The dGEMRIC technique has been used to evaluate changes in articular cartilage metabolism following cruciate ligament injury18–19. Tiderius et al. evaluated 24 patients with acute ACL injuries and compared dGEMRIC indices to an activity level-matched, uninjured control group18. The average time from injury to imaging was 21 days (range 11–38 days). They reported that the average dGEMRIC indices of the medial femoral condyles were 376 ms and 428 ms in the ACL-injured and matched controls, respectively. These values were less than those measured in the current study, which were 406 ms and 462 ms, respectively. The differences in the absolute dGEMRIC indices may be due to the differences in the time between contrast injection and MR imaging of the two studies (120 minutes versus 105 and 90 minutes), differences in time between injury and imaging (3 vs 15 weeks), and differences in the gadolinium concentrations (triple vs double dose). Nonetheless, the relative differences between knees from the two studies were nearly identical (52 ms versus 56 ms). The data in the present study support those reported previously by Tiderius et al. but over a wider range of time from injury18. Because we found no difference in dGEMRIC indices due to scan time (90 vs 105 minutes), our data suggests that identification of a separate control group may not be necessary in future studies.
For studies of primary OA, it has been shown that the dGEMRIC indices are lower (<400 ms) in patients with radiographic evidence of joint space narrowing (JSN) compared to those without25. The dGEMRIC indices for many of the ACL-injured knees reported in the current study are within the range of those reported for arthritic patients with radiographic evidence of JSN25. Eight out of fifteen subjects had dGEMRIC values less than 400 ms in either the tibia or femur of the injured knee as compared to four out of fifteen in the tibia or the femur of the uninjured knees (Table 1). Most of the studies to date relating dGEMRIC indices to the degree of OA have been cross-sectional studies. Recently, Owman et al. determined that dGEMRIC indices were lower at baseline in patients with knee pain and arthroscopically visualized cartilage damage but no radiographic evidence of OA who then presented with radiographic evidence of OA 6 years later26. This finding demonstrates the prognostic potential of the dGEMRIC index.
Given that the dGEMRIC index is a promising marker of cartilage health, and that the lower dGEMRIC index reflects the loss of proteoglycans in the articular cartilage of the knee 16–17, 26–27, dGEMRIC may prove valuable for understanding why certain ACL-injured patients go on to develop early OA. In this study, the dGEMRIC indices of the ACL-injured knees were typically lower than those of the contralateral uninjured knees. However, in 5 out of 15 of the patients, either the tibial or femoral cartilage indices were greater than those of the contralateral knee. Therefore a range of dGEMRIC indices occurs following ACL-injury across patients. Thus, future prospective cohort studies could be designed to determine if ACL-injured knees with a lower dGEMRIC index in the early stages of injury predict those patients who will present with OA later, and to possibly relate the initial dGEMRIC indices to other factors that may place the ACL-injured patient at risk for OA (i.e. meniscal injury and subchondral bone lesions). Randomized controlled trials could then be planned to determine whether current interventions (i.e. conservative rehabilitation and/or ACL reconstruction) can restore the dGEMRIC index, and therefore the GAG concentration, to that of the contralateral uninjured knee.
The presence of traumatic bone marrow lesions (BMLs) and subchondral bone fractures have been proposed as risk factors for early OA in the ACL-injured knee10. Approximately 60% of ACL-injured knees display BMLs one year after injury10. It has also been suggested that BMLs have a median healing time of 42 weeks24. It was not surprising, therefore, that every ACL-injured knee within our study had signs of BMLs on MRI. Additionally, one subject (Subject 12) had a BML in the uninjured knee (Table 2). Although the presence of BMLs has been associated with cortical depression fractures10, no such fractures were detected with MR imaging in our subjects. It is important to note that the presence of BMLs in the injured knees was not significantly different between groups. Thus, this factor should not affect our conclusion regarding the impact of scan order on dGEMRIC in the medial compartment. It should be noted that in the ACL-injured knee, BMLs are most common in the lateral compartment10, while the dGEMRIC measurements were performed in the medial compartment in this study. Future work is required to establish if BMLs and cortical depression fractures are risk factors of OA in this patient population.
We have previously shown that the GAG concentration in synovial fluid of the knee is significantly elevated following an acute ACL injury when compared to the uninjured contralateral knee, and that it remains elevated for up to one year5. Considered in the light of the present study, these findings suggest that GAGs are released from the articular cartilage into synovial fluid soon after knee injury. The dGEMRIC index has been used to study GAG loss following joint trauma18–19, 28, and the results of the current study suggest that it could be a suitable marker of cartilage damage in this patient population.
The dGEMRIC index was performed on a median slice in the medial compartment of the tibiofemoral joint. This slice was selected by concensus of the investigator and the MR technologist using anatomical landmarks. The regions of interest in the tibial and femoral articular cartilage were manually segmented. For this study, the intra-observer variations due to repeated segmentation on the dGERMIC indices were 0.8% and 1.2% for the femur and tibia, respectively.
Several study limitations should be considered. First, the time between knee injury and MR imaging ranged from 21 days to 424 days. However, this cross-sectional experimental design was adequate for evaluating our primary hypotheses comparing the dGEMRIC indices as a function of knee injury status and scan order. Additional long-term studies that evaluate the change of the dGEMRIC index over time after injury are necessary. Second, it is possible that the decrease in the mean dGEMRIC index in the ACL-injured knee is due to either a decrease in GAG concentration, an increase in water content, or combination thereof. However, Tiderius et al used a pre-contrast MRI to estimate the tissue hydration by comparing the T1 relaxation time of the cartilage in the acutely injured knees to intact control knees, and they found that they were similar in the medial compartment18. Thus, they argued that hydration was not the primary factor influencing the dGEMRIC index. Third, the dGEMRIC analyses were performed in the medial compartment only in an effort to limit the total MR imaging time. The medial compartment was selected for two reasons: 1) because cartilage degradation appears to be greatest in the medial compartment following ACL injury29–31, and 2) because it has been shown that the dGEMRIC indices are similar in both compartments following acute ACL injury18. Recent modifications to the dGEMRIC sequences may reduce scan acquisition time, and allow cartilage imaging in three dimensions32–33. Finally, there may be a selection bias associated with this study because 76% of the eligible subjects either denied participation or could not be scheduled. Improvements in recruitment may be achieved by using a dedicated research magnet with a more flexible schedule.
In conclusion, the dGEMRIC indices of the medial compartment of ACL-injured knees were significantly lower than those of the contralateral, uninjured knees. The dGEMRIC indices were not sensitive to imaging order. Sequential imaging of both knees for dGEMRIC analyses is therefore possible in this patient population. Future work should aim to determine whether a lower dGEMRIC index in the acute stages after ACL injury predicts the development of early post-traumatic osteoarthritis.
References
- 1.Oiestad BE, Engebretsen L, Storheim K, Risberg MA. Knee osteoarthritis after anterior cruciate ligament injury: a systematic review. Am J Sports Med. 2009;37:1434–1443. doi: 10.1177/0363546509338827. [DOI] [PubMed] [Google Scholar]
- 2.Li G, Moses JM, Papannagari R, Pathare NP, DeFrate LE, Gill TJ. Anterior cruciate ligament deficiency alters the in vivo motion of the tibiofemoral cartilage contact points in both the anteroposterior and mediolateral directions. J Bone Joint Surg Am. 2006;88:1826–1834. doi: 10.2106/JBJS.E.00539. [DOI] [PubMed] [Google Scholar]
- 3.Andriacchi TP, Briant PL, Bevill SL, Koo S. Rotational Changes at the Knee after ACL Injury Cause Cartilage Thinning. Clin Orthop Relat Res. 2006;442:39–44. doi: 10.1097/01.blo.0000197079.26600.09. [DOI] [PubMed] [Google Scholar]
- 4.Andriacchi TP, Mundermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Engin. 2004;33:447–457. doi: 10.1023/b:abme.0000017541.82498.37. [DOI] [PubMed] [Google Scholar]
- 5.Elsaid KA, Fleming BC, Oksendahl HL, Machan JT, Fadale PD, Hulstyn MJ, et al. Decreased lubricin concentrations and markers of joint inflammation in synovial fluids from patients with anterior cruciate ligament injury. Arthritis Rheum. 2008;58:1707–1715. doi: 10.1002/art.23495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleming BC, Hulstyn MJ, Oksendahl HL, Fadale PD. Ligament injury, reconstruction, and osteoarthritis. Curr Opin Orthop. 2005;16:354–362. doi: 10.1097/01.bco.0000176423.07865.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bray RC, Dandy DJ. Meniscal lesions and chronic anterior cruciate ligament deficiency. J Bone Joint Surg Br. 1989;71:128. doi: 10.1302/0301-620X.71B1.2914982. [DOI] [PubMed] [Google Scholar]
- 8.Levy AS, Meier SW. Approach to cartilage injury in the anterior cruciate ligament-deficient knee. Orthop Clin North Am. 2003;34:149–167. doi: 10.1016/s0030-5898(02)00065-2. [DOI] [PubMed] [Google Scholar]
- 9.Kaeding CC, Pedroza AD, Parker RD, Spindler KP, McCarty EC, Andrish JT. Intra-articular findings in the reconstructed multiligament-injured knee. Arthroscopy. 2005;21:424–430. doi: 10.1016/j.arthro.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Frobell RB, Roos HP, Roos EM, Hellio Le Graverand MP, Buck R, Tamez-Pena J, et al. The acutely ACL injured knee assessed by MRI: are large volume traumatic bone marrow lesions a sign of severe compression injury? Osteoarthritis Cartilage. 2008;16:829–836. doi: 10.1016/j.joca.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Pond MJ, Nuki G. Experimentally induced osteoarthritis in the dog. Annals Rheumatic Disease. 1973;32:387–388. doi: 10.1136/ard.32.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Libicher M, Ivancic M, Hoffmann V, Wenz W. Early changes in experimental osteoarthritis using the Pond-Nuki dog model: technical procedure and initial results of in vivo MR imaging. European Radiology. 2005;15:390–394. doi: 10.1007/s00330-004-2486-y. [DOI] [PubMed] [Google Scholar]
- 13.Junkin DM, Johnson DL, Fu FH, Miller MD, Willenborg M, Fanelli GC, et al. Knee Ligament Injuries. Orthopaedic Knowledge Update 4: Sports Medicine. In: Kibler WB, editor. Rosemont: American Academy of Orthopaedic Surgeons. 2009. pp. 135–153. [Google Scholar]
- 14.Lohmander LS, Dahlberg L, Ryd L, Heinegard D. Increased levels of proteoglycan fragments in knee joint fluid after injury. Arthritis Rheum. 1989;32:1434–1442. doi: 10.1002/anr.1780321113. [DOI] [PubMed] [Google Scholar]
- 15.Lohmander LS, Hoerrner LA, Dahlberg L, Roos H, Bjornsson S, Lark MW. Stromelysin, tissue inhibitor of metalloproteinases and proteoglycan fragments in human knee joint fluid after injury. J Rheumatol. 1993;20:1362–1368. [PubMed] [Google Scholar]
- 16.Burstein D, Gray M. New MRI techniques for imaging cartilage. J Bone Joint Surg Am. 2003;85:70–77. doi: 10.2106/00004623-200300002-00009. [DOI] [PubMed] [Google Scholar]
- 17.Gray ML, Burstein D, Kim YJ, Maroudas A. 2007 Elizabeth Winston Lanier Award Winner. Magnetic resonance imaging of cartilage glycosaminoglycan: basic principles, imaging technique, and clinical applications. J Orthop Res. 2008;26:281–291. doi: 10.1002/jor.20482. [DOI] [PubMed] [Google Scholar]
- 18.Tiderius CJ, Olsson LE, Nyquist F, Dahlberg L. Cartilage glycosaminoglycan loss in the acute phase after an anterior cruciate ligament injury: delayed gadolinium-enhanced magnetic resonance imaging of cartilage and synovial fluid analysis. Arthritis Rheum. 2005;52:120–127. doi: 10.1002/art.20795. [DOI] [PubMed] [Google Scholar]
- 19.Young AA, Stanwell P, Williams A, Rohrsheim JA, Parker DA, Giuffre B, et al. Glycosaminoglycan content of knee cartilage following posterior cruciate ligament rupture demonstrated by delayed gadolinium-enhanced magnetic resonance imaging of cartilage (dGEMRIC). A case report. J Bone Joint Surg Am. 2005;87A:2763–2767. doi: 10.2106/JBJS.D.02923. [DOI] [PubMed] [Google Scholar]
- 20.Williams A, Gillis A, McKenzie C, Po B, Sharma L, Micheli L, et al. Glycosaminoglycan distribution in cartilage as determined by delayed gadolinium-enhanced MRI of cartilage (dGEMRIC): potential clinical applications. Am J Roentgenol. 2004;182:167–172. doi: 10.2214/ajr.182.1.1820167. [DOI] [PubMed] [Google Scholar]
- 21.Burstein D, Velyvis J, Scott KT, Stock KW, Kim YJ, Jaramillo D, et al. Protocol issues for delayed Gd(DTPA)(2-)-enhanced MRI (dGEMRIC) for clinical evaluation of articular cartilage. Magn Reson Med. 2001;45:36–41. doi: 10.1002/1522-2594(200101)45:1<36::aid-mrm1006>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 22.Tiderius CJ, Olsson LE, Leander P, Ekberg O, Dahlberg L. Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) in early knee osteoarthritis. Magn Reson Med. 2003;49:488–492. doi: 10.1002/mrm.10389. [DOI] [PubMed] [Google Scholar]
- 23.Andreisek G, White LM, Sussman MS, Kunz M, Hurtig M, Weller I, et al. Quantitative MR imaging evaluation of the cartilage thickness and subchondral bone area in patients with ACL-reconstructions 7 years after surgery. Osteoarthritis Cartilage. 2009;17:857–864. doi: 10.1016/j.joca.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 24.Boks SS, Vroegindeweij D, Koes BW, Bernsen RM, Hunink MG, Bierma-Zeinstra SM. MRI follow-up of posttraumatic bone bruises of the knee in general practice. AJR Am J Roentgenol. 2007;189:556–562. doi: 10.2214/AJR.07.2276. [DOI] [PubMed] [Google Scholar]
- 25.Williams A, Sharma L, McKenzie CA, Prasad PV, Burstein D. Delayed gadolinium-enhanced magnetic resonance imaging of cartilage in knee osteoarthritis: findings at different radiographic stages of disease and relationship to malalignment. Arthritis Rheum. 2005;52:3528–3535. doi: 10.1002/art.21388. [DOI] [PubMed] [Google Scholar]
- 26.Owman H, Tiderius CJ, Neuman P, Nyquist F, Dahlberg LE. Association between findings on delayed gadolinium-enhanced magnetic resonance imaging of cartilage and future knee osteoarthritis. Arthritis Rheum. 2008;58:1727–1730. doi: 10.1002/art.23459. [DOI] [PubMed] [Google Scholar]
- 27.Burstein D. Tracking longitudinal changes in knee degeneration and repair. J Bone Joint Surg Am. 2009;91 (Suppl 1):51–53. doi: 10.2106/JBJS.H.01412. [DOI] [PubMed] [Google Scholar]
- 28.Ericsson YB, Tjornstrand J, Tiderius CJ, Dahlberg LE. Relationship between cartilage glycosaminoglycan content (assessed with dGEMRIC) and OA risk factors in meniscectomized patients. Osteoarthritis Cartilage. 2009;17:565–570. doi: 10.1016/j.joca.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 29.Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR. Fate of the ACL-injured patient: A prospective outcome study. Am J Sports Med. 1994;22:632–644. doi: 10.1177/036354659402200511. [DOI] [PubMed] [Google Scholar]
- 30.Hill CL, Seo GS, Gale D, Totterman S, Gale ME, Felson DT. Cruciate ligament integrity in osteoarthritis of the knee. Arthritis Rheum. 2005;52:794–799. doi: 10.1002/art.20943. [DOI] [PubMed] [Google Scholar]
- 31.Buckland-Wright JC, Lynch JA, Dave B. Early radiographic features in patients with anterior cruciate ligament rupture. Ann Rheum Dis. 2000;59:641–646. doi: 10.1136/ard.59.8.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mamisch TC, Dudda M, Hughes T, Burstein D, Kim YJ. Comparison of delayed gadolinium enhanced MRI of cartilage (dGEMRIC) using inversion recovery and fast T1 mapping sequences. Magn Reson Med. 2008;60:768–773. doi: 10.1002/mrm.21726. [DOI] [PubMed] [Google Scholar]
- 33.Li W, Scheidegger R, Wu Y, Vu A, Prasad PV. Accuracy of T1 measurement with 3-D Look-Locker technique for dGEMRIC. J Magn Reson Imaging. 2008;27:678–682. doi: 10.1002/jmri.21244. [DOI] [PubMed] [Google Scholar]