Abstract
As we learn new information about the social and moral behaviors of other people, we form and update character judgments of them, and this can profoundly influence how we regard and act towards others. In the study reported here, we capitalized on two interesting neurological patient populations where this process of complex “moral updating” may go awry: patients with bilateral damage to ventromedial prefrontal cortex (vmPFC) and patients with bilateral damage to hippocampus (HC). We predicted that vmPFC patients, who have impaired emotion processing, would exhibit reduced moral updating, and we also investigated how moral updating might be affected by severe declarative memory impairment in HC patients. The vmPFC, HC, and brain-damaged comparison (BDC) participants made moral judgments about unfamiliar persons before and after exposure to social scenarios depicting the persons engaged in morally good, bad, or neutral behaviors. In line with our prediction, the vmPFC group showed the least amount of change in moral judgments, and interestingly, the HC group showed the most amount of change. These results suggest that the vmPFC and hippocampus play critical but complementary roles in updating moral character judgments about others: the vmPFC may attribute emotional salience to moral information, whereas the hippocampus may provide necessary contextual information from which to make appropriate character judgments.
Keywords: ventromedial, hippocampus, moral, social cognition, memory
INTRODUCTION
In a constantly changing environment, it is vital that people update their knowledge base as new information becomes available. For example, knowledge about the self, perceptions of others, and interpersonal motivations must continuously be updated in order to support advantageous social functioning (Lieberman, 2007). In order to appropriately update one’s social and moral knowledge base, both the emotional salience and the context of newly acquired social and moral information must be taken into account. For example, if you overhear your officemate lying to her husband, you may form a negative moral judgment of her. If you later discover that she lied to cover up a surprise birthday party for him, you may change your moral judgment, but this time in the positive direction. In this example, both the emotional salience and the context of the situation are factored into forming a moral judgment; and as a consequence, your regard for and behavior towards your officemate in the future may be very different, depending on whether or not you took all the relevant information, as well as your previous interactions with that person, into account.
When a person first meets another person, an immediate and spontaneous impression of that other person is formed based on information at hand such as verbal and nonverbal behavior and appearance. In the field of social psychology, it has been demonstrated that when people are simultaneously shown a person’s face and a behavior associated with a particular trait (such as aggression or selfishness), people form spontaneous impressions of others that are bound to that trait, and these impressions are robust and maintained in memory (e.g., Todorov & Uleman, 2002, 2004). In addition, after learning a particular person-trait pairing, when that same person is perceived again, neural substrates known for their involvement in emotion processing and social cognition, such as anterior cingulate cortex and superior temporal sulcus (STS), are recruited (Todorov, Gobbini, Evans, & Haxby, 2007).
Not surprisingly, the same neural systems involved in impression formation, or forming a social judgment of a person based on limited behavioral information, seem to be involved in moral judgment (Singer, Kiebel, Winston, Dolan, & Frith, 2004). Borrowing a definition of morality from Moll, Zahn, de Oliveira-Souza, Krueger, and Grafman (2005), moral judgment may be defined as judging the moral behavior of a person against a set of customs and values that are embraced by a cultural group to guide social conduct. Multiple lines of research, including behavioral studies with normal populations, studies with clinical populations, neuropsychological studies, and functional neuroimaging studies, have implicated brain regions involved in emotion processing and social cognition, such as STS, medial and dorsolateral prefrontal cortex (mPFC; dlPFC), ventromedial prefrontal cortex (vmPFC), and the amygdala as being important for various aspects of moral cognition (reviewed in Greene & Haidt, 2002 and Moll et al., 2005; Koenigs et al., 2007). Of these brain regions, the prefrontal cortex is consistently implicated in studies of social and moral cognition, suggesting that when people integrate new social and moral information about another person with their initial impression of them (or previous interactional history) in order to form an updated character judgment, the complex information processing that takes place relies on prefrontal cortex, and especially the vmPFC sector (Adolphs, 1999, 2003, 2006; Bar-On, Tranel, Denburg, & Bechara, 2003).
One of the putative roles of the vmPFC is to integrate moral knowledge with the emotional/affective system in order to support socially advantageous behavior, and studies of brain-damaged patients provide support for this idea. Beginning with Harlow’s description of Phineas Gage in 1868, many studies have demonstrated that circumscribed frontal lobe damage can produce severe impairments in decision-making abilities, emotion, and social behavior. Patients with damage to vmPFC exhibit behavioral deficits that are marked by a diminished ability to trigger emotional responses to socially salient stimuli, an inability to learn from social punishment, and a diminished ability to regulate their social behavior in real life (e.g., Eslinger & Damasio, 1985; Tranel 1994; Grafman et al., 1996; Anderson, Bechara, Damasio, Tranel, & Damasio, 1999; Bechara, Damasio, & Damasio, 2000; Hornak et al., 2003; Roberts et al., 2004; Anderson, Barrash, Bechara, & Tranel, 2006; Koenigs & Tranel, 2007; Koenigs et al., 2007). Various studies have also demonstrated impaired experience and recognition of complex social emotions (such as embarrassment, empathy, and regret) in patients with vmPFC damage (Beer, Heerey, Keltner, Scabini, & Knight, 2003; Shamay-Tsoory, Tomer, Berger, & Aharon-Peretz, 2003; Camille et al., 2004; Beer, John, Scabini, & Knight, 2006).
While the vmPFC plays a putative role in attributing emotional salience to newly acquired social information, the hippocampus, which supports declarative memory, may also make an important contribution. Declarative memory is required for the formation, maintenance, and updating of long-term relational representations that integrate the relevant relations among different pieces of information about a given event (e.g., an individual and their actions on a specific occasion), as well as the relationships among representations of multiple events acquired across numerous experiences, thus providing the larger contextual record of a person’s actions over time (Eichenbaum & Cohen, 2001; Cohen & Banich, 2003). Indeed, recent research has shown that patients with bilateral hippocampal damage and declarative memory deficits, who are unable to draw on the contextual information provided by these long-term relational representations, are impaired on the Iowa Gambling Task (IGT), a measure of complex decision-making (Gutbrod et al., 2006; Gupta et al., 2009). These two studies demonstrate that while amnesic patients are sensitive to punishment (i.e., they have an immediate response both behaviorally and physiologically to punishment in the IGT) and thus, demonstrate normal basic emotional learning, they are not able to form and update associations that integrate variations in reward and punishment across decks and across experiences with each deck, resulting in an impaired ability to make sustained advantageous decisions over time. It may be the case that, when forming a moral judgment of a person, declarative memory provides critical contextual information necessary for the appropriate and advantageous appraisal of the social information being evaluated (e.g., how has this person behaved towards you and others in the past; how “good” or “bad” is the current behavior in comparison to other real or imagined moral behaviors).
This background provides an intriguing foundation for investigating the neural basis of moral (“character”) judgments, which is the topic of the current study. We hypothesized that the vmPFC is critical for updating character judgments in light of new social and moral information. In addition, we wanted to investigate how moral updating would be affected by severe declarative memory impairment due to bilateral hippocampal damage. We used a novel moral “updating” task and compared the performances of patients with bilateral vmPFC damage, patients with bilateral hippocampal damage (HC), and brain-damaged comparison (BDC) patients with damage outside the vmPFC and hippocampal regions. We predicted that for valenced (morally good or bad) scenarios and relative to BDC patients, patients with vmPFC damage would show reduced moral updating (i.e., less change of moral judgments after learning new social and moral information). We also explored, in the HC patients, how moral updating might be affected in severely memory impaired individuals to investigate the potential role of declarative memory in moral updating (i.e., changing one’s moral judgment after learning new social and moral information).
METHODS
Participants
Our participant sample consisted of: a patient group with bilateral damage to vmPFC (vmPFC; n=4), a patient group with bilateral hippocampal damage (HC; n=4), and a brain-damaged comparison group (BDC; n=16). The patients were selected from the Patient Registry of the University of Iowa’s Division of Behavioral Neurology and Cognitive Neuroscience, under the auspices of which they have all been characterized neuropsychologically and neuroanatomically, according to standard protocols (Frank, Damasio, & Grabowski, 1997; Tranel, 2007). The neuropsychological, neuroanatomical, and experimental data were all collected in the chronic epoch of recovery (at least three months after lesion onset). All participants received detailed information describing the experimental procedures that were approved by the Institutional Review Board for Human Subjects Research at the University of Iowa, and gave informed consent before participating in the study in accordance with the Declaration of Helsinki.
Demographic data regarding the participants are provided in Table 1. The three groups are comparable in terms of age, education, and time since lesion onset (chronicity). There were no group differences in age (one-way ANOVA; F(2,21) = 0.89, p = 0.43); education (one-way ANOVA; F(2,21) = 2.63, p = 0.10); or chronicity (one-way ANOVA; F(2,21) = 1.33, p = 0.29). The HC group contains a higher proportion of left-handed subjects than the vmPFC and BDC groups; however, there is no a priori reason to suspect handedness should have any effect on experimental task performance in this study (and all of the target patients have bilateral lesions). We did not have equal sex ratios in the three groups, but this is a necessary limitation given the rarity of the key neurological patients (vmPFC, HC). Neuropsychological data for the three groups of patients are presented in Table 2. There were no group differences in verbal IQ (one-way ANOVA; F(2,18) = 1.93, p = 0.18); performance IQ (one-way ANOVA; F(2,18) = 2.09, p = 0.15); or full-scale IQ (one-way ANOVA; F(2,17) = 1.68, p = 0.22).
Table 1. Demographics, chronicity, laterality, and lesion etiology of vmPFC, HC, and BDC patients.
Subject ID, subject identification number; Hand, handedness; R, right-handed; L, left-handed; M, male; F, female; Age, age at testing in years; Edu, years of formal education; Chronicity, length of time between lesion onset and current experiment in years or months; Laterality, lesion location; B, bilateral; R, right; L, left; SAH, subarachnoid hemorrhage; ACoA, anterior communicating artery aneurysm; HSE, herpes simplex encephalitis; AVM, arteriovenous malformation. Group summary statistics including mean and standard deviation (in parentheses) are presented below the solid line.
| Subject ID | Group | Hand | Sex | Age | Edu | Chronicity | Laterality | Lesion Etiology |
|---|---|---|---|---|---|---|---|---|
| 0318 | vmPFC | R | M | 66 | 14 | 26 y. | B | Meningioma resection |
| 2352 | vmPFC | R | F | 57 | 14 | 7 y. | B | SAH; ACoA aneurysm |
| 2391 | vmPFC | R | F | 60 | 13 | 2 y. | B | Meningioma resection |
| 2577 | vmPFC | R | M | 67 | 11 | 8 y. | B | SAH; ACoA aneurysm |
| 1606 | HC | R | M | 59 | 12 | 16 y. | B | Anoxia |
| 2308 | HC | L | M | 49 | 18 | 7 y. | B | HSE |
| 2363 | HC | R | M | 50 | 17 | 8 y. | B | Anoxia |
| 2563 | HC | L | M | 51 | 16 | 6 y. | B | Anoxia |
| 1404 | BDC | R | M | 33 | 16 | 18 y. | L | Temporal lobe resection |
| 1981 | BDC | R | M | 75 | 16 | 14 y. | R | Temporal lobe infarct |
| 2435 | BDC | R | M | 65 | 12 | 9 y. | L | Parietal/temporal lobe infarct |
| 2473 | BDC | R | M | 54 | 14 | 9 y. | B | R calcarine and B occipital polar infarcts |
| 2526 | BDC | R | F | 61 | 16 | 7 y. | L | Frontal meningioma resection |
| 2527 | BDC | R | F | 53 | 12 | 6 y. | L | Frontal lobe infarct |
| 2537 | BDC | R | F | 47 | 13 | 7 y. | L | Frontal lobe infarct |
| 2810 | BDC | R | F | 60 | 14 | 6 y. | L | Temporal lobe infarct |
| 2957 | BDC | R | F | 45 | 13 | 4 y. | R | Frontal cavernous angioma resection |
| 2980 | BDC | R | M | 62 | 15 | 3 y. | L | Parietal/occipital lobe infarct |
| 3052 | BDC | R | F | 46 | 12 | 3 y. | R | Parietal meningioma resection |
| 3126 | BDC | R | M | 73 | 11 | 2 y. | R | Frontal intraparenchymal hemorrhage |
| 3137 | BDC | R | M | 53 | 16 | 3 y. | L | Frontal AVM embolization |
| 3166 | BDC | R | F | 33 | 14 | 2 y. | L | Temporal lobe resection |
| 3172 | BDC | L | F | 55 | 12 | 1 y. | L | Frontal/parietal lobe infarct |
| 3427 | BDC | R | F | 75 | 12 | 7 mo. | L | Temporal/occipital lobe infarct |
| VmPFC | 4R/0L | 2M/2F | 62.5 (4.8) | 13.0 (1.4) | 10.8 (10.5) | 4B | N/A | |
| HC | 2R/2L | 4M/0F | 52.3 (4.6) | 15.8 (2.6) | 9.3 (4.6) | 4B | N/A | |
| BDC† | 15R/1L | 7M/9F | 55.6 (13.0) | 13.6 (1.8) | 5.9 (4.8) | 4R/11L/1B | N/A | |
Means and standard deviations based on data available at time of publication.
Table 2. Neuropsychological data of vmPFC, HC, and BDC patients.
Subject ID, subject identification number; WAIS-III, Wechsler Adult Intelligence Scale-III scores: (VIQ, verbal IQ; PIQ, performance IQ; FSIQ, full-scale IQ); WMS-III, Wechsler Memory Scale-III score: (GMI, general memory index); AVLT, Auditory-Verbal Learning Test at 5 and 30 minute delays, 15 maximum score; CFT, Rey-Osterrieth Complex Figure Test at 30 minute delay, 36 maximum score; TT, Token Test from the Multilingual Aphasia Examination, 44 maximum score. Defective scores are italicized and severely defective scores are bolded. Group summary statistics including mean and standard deviation (in parentheses) are presented below the solid line.
| Subject ID | Group | VIQ | PIQ | FSIQ | GMI | AVLT- 5/30 min. delay | CFT- 30 min. delay | TT |
|---|---|---|---|---|---|---|---|---|
| 0318 | vmPFC | 142 | 134 | 143 | 109 | 14/10 | 32 | 44 |
| 2352 | vmPFC | 108 | 102 | 106 | 109 | 14/11 | 16 | 44 |
| 2391 | vmPFC | 110 | 107 | 109 | 132 | 15/14 | 24 | 43 |
| 2577 | vmPFC | 89 | 80 | 84 | 96 | 7/8 | 15 | 44 |
| 1606 | HC | 94 | 89 | 91 | 66 | 6/0 | 11 | 44 |
| 2308 | HC | 95 | 78 | 87 | 45 | 5/0 | 0 | 44 |
| 2363 | HC | 112 | 83 | 98 | 73 | 9/0 | 5 | 44 |
| 2563 | HC | 98 | 105 | 102 | 75 | 7/1 | 7 | 44 |
| vmPFC | 112 (22) | 106 (22) | 111 (24) | 112 (15) | 13/11 (4/3) | 22 (8) | 44 (1) | |
| HC | 100 (8) | 89 (12) | 95 (7) | 65 (14) | 7/0 (2/1) | 6 (5) | 44 (0) | |
| BDC† | 98 (11) | 101 (9) | 99 (10) | 103 (13) | 10/8 (3/3) | 20 (8) | 41 (5) | |
Means and standard deviations based on data available at time of publication.
The participants in the vmPFC group had normal performances on measures of IQ, memory, and language at the time of testing (Table 2), and overall, the vmPFC patients are relatively intact in terms of their basic neuropsychological profiles. However, all of the vmPFC patients have defects in social and emotional functioning in the real world (Table 3). Moreover, the vmPFC patients had impaired autonomic reactivity in response to emotionally charged pictures, as well as diminished empathy, embarrassment, and guilt (reported in Koenigs et al., 2007).
Table 3. Emotional functioning, personality, and social/interpersonal functioning for individual vmPFC and HC participants.
| Group | Participant | BDI-II1 | MMPI-22 | Acquired Personality Problems3 | Social and Interpersonal Functioning4 |
|---|---|---|---|---|---|
| vmPFC | 0318 | 0 | None | Yes (3) | 3 |
| 2352 | 1 | 5(65) | Yes (3) | 2 | |
| 2391 | 8 | None | Yes (2) | 2 | |
| 2577 | 7 | None | Yes (3) | 3 | |
| HC | 1606 | 9 | 2(80)-7(75) | No | 1 |
| 2308 | 3 | None | No | 3 | |
| 2363 | 0 | 2(72) | No | 1 | |
| 2563 | 0 | 4(67)-9(65) | No | 1 |
BDI-II = Beck Depression Inventory-II. Raw scores are provided, and scores greater than 10 indicate significant depressive symptomatology.
MMPI-2 = Minnesota Multiphasic Personality Inventory-2. Clinical scales with significant elevations (i.e., T-scores above 65) are indicated, with the T-scores in parentheses (the highest three significant elevations are presented).
Acquired Personality Problems refer to whether or not the participant had acquired problems in personality functioning, as derived from data on the Iowa Rating Scales of Personality Change. The numbers in parentheses denote degree of severity, where 1 = mild, 2 = moderate, and 3 = severe.
The extent of post-lesion change or impairment in aspects of social conduct and interpersonal functioning was rated on a three-point scale, with 1 = no change or impairment, 2 = moderate change or impairment, 3 = severe change or impairment.
For the purposes of this study, the vmPFC region was defined as comprising roughly the medial one-third of the orbital frontal cortex and the ventral one-third of the medial surface of the prefrontal cortex. Lesion etiologies for this group included cerebrovascular accident (n=2) or benign tumor resection (n=2). For patient 2391, neuroanatomical analysis was based on structural magnetic resonance images (MRI) acquired with a 1.5T General Electric Signa scanner, and for patients 318, 2352, and 2577, computerized tomography (CT) images were used for analysis. In order to aid in the visualization of the overlapping lesions in the vmPFC region, each vmPFC lesion was reconstructed in three dimensions onto a normal template brain by an expert tracer using the MAP-3 technique, using the software program Brainvox (version 3.14; Frank et al., 1997). The lesion maps were then overlapped in the template volume, and a color-coded visual depiction of the sum of n lesions overlapping at any given voxel was created (Figure 1). All four vmPFC patients had lesions overlapping the ventromedial prefrontal cortices. In addition, we calculated the volume of the lesions in the vmPFC group, with the following results: 13.6, 52.5, 71.0, and 117.7 cubic cm for patients 2352, 2577, 2391, and 0318, respectively.
Figure 1. Lesion overlap of patients with damage to vmPFC.
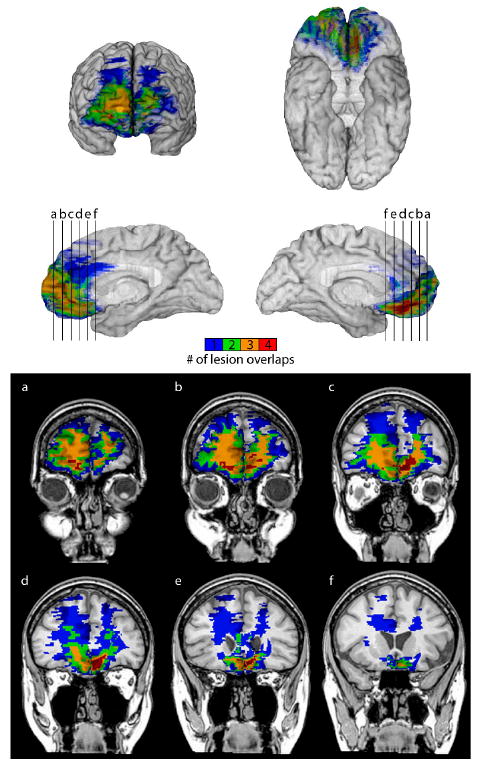
Lesions of four patients with bilateral vmPFC damage are displayed in whole brain views (anterior, ventral, and mesial) and coronal slices (with left hemisphere on the right), with the location of each coronal slice indicated by the letters a (anterior) through f (posterior) on the mesial views. The colors represent the number of overlapping lesions at each voxel, as indicated in the color bar.
All four of the HC patients had memory impairments that were sufficiently severe to interfere with daily life, including preventing them from being employed since the onset of their amnesia, and all exhibited severe memory impairment on standardized tests of memory (Table 2). Neuropsychological testing confirmed a selective and severe memory impairment disproportionate to any deficits in general cognitive (e.g., language, attention, executive functioning), or intellectual functioning. Performance of the HC patients on the Wechsler Memory Scale-III was at least 25 points lower than their performance on the Wechsler Adult Intelligence Scale-III (mean IQ-MQ difference = 29.8), with an average delay score on the memory scale (64.8) that was more than 2 standard deviations below population means. The HC patients, however, did not have major deficits in social and emotional functioning (Table 3).
Of the four patients with bilateral hippocampal damage, three sustained damage due to an anoxic/hypoxic event, and one sustained more extensive medial temporal lobe damage from herpes simplex encephalitis. Structural magnetic resonance imaging (MRI) examinations were completed on three of the four patients confirming bilateral hippocampal damage. For patients 2363 and 1606, high-resolution volumetric MRI analyses were conducted revealing significantly reduced hippocampal volumes by more than 2.5 standard deviations compared to age-matched comparison participants, but without significant extra-hippocampal damage in associated medial temporal lobe regions (for more detail, please see Allen, Tranel, Bruss, & Damasio, 2006). Patient 2563 has bilateral hippocampal damage that appears to be limited to the hippocampal region, as documented previously (Duff, Hengst, Tranel, & Cohen, 2008). Patient 2308 sustained bilateral damage to hippocampus, amygdala, and insula, with more extensive medial and lateral temporal lobe damage on the left than the right as a consequence of herpes simplex encephalitis.
The participants in the BDC group had normal performance on measures of IQ, memory, and language at the time of testing (Table 2). Lesions in the BDC group ranged from small (approximately 2 cubic cm) to large (approximately 50 cubic cm) in volume. We specifically examined whether lesion size alone affected updating of moral character judgments by analyzing all of the data comparing the vmPFC, HC, and BDC groups using the four BDC patients with the largest lesions (patients 2435, 2473, 2980, and 3427), instead of including all 16 BDC patients. The results of this analysis did not differ from that for the full group of BDC participants. Lesions in the BDC group did not involve the following neural areas that are thought to be critically involved in emotional processing or declarative memory: vmPFC, amygdala (except for patients 1404 and 3166 whose lesions include partial unilateral amygdala damage), hippocampus, anterior cingulate, STS (except for patient 2435 whose lesion includes unilateral damage to posterior STS), or insula.
Stimuli
We created 15 sets of novel stimuli each comprised of: a photograph of an actor’s face with a neutral expression, a corresponding photograph of a complex social scenario depicting the actor engaged in either morally good (n = 5), morally bad (n=5), or morally neutral (n=5) behaviors, and an auditory description of the scenario read in a neutral tone by a female actor (see Figure 2 for an example stimulus set). In order to create the images of the social scenarios, the actors were asked to perform the behavior described in the scenario and were photographed in a real world setting. Each stimulus set contained a novel actor so that each actor was only seen once. For both the morally good (n = 5) and bad (n = 5) scenarios, there were two female and three male actors for each moral type; and for the morally neutral scenarios, there were three female and two male actors, for a total of 15 actors, each corresponding to a single stimulus set. Normative ratings of the scenarios are presented below in the “Manipulation Check” section. All 15 scenarios are presented in the Appendix as text, although in the experiment, the scenarios were presented as auditory stimuli, as mentioned previously.
Figure 2. Example stimulus set used to create one “bad” scenario/trial.

2a depicts an actor’s face with a neutral expression, 2b depicts the actor engaging in a morally bad act, and 2c is the text of the verbal description heard during the presentation of 2b.
Task
The experiment was performed using Presentation® software (Version 0.70, www.neurobs.com). All stimuli were presented on a Sony Trinitron Multiscan E540, 21-inch color display. Participants were asked to make judgments of people before and after learning moral information about them. Each trial consisted of a series of screens, corresponding to a particular good, bad, or neutral moral scenario. Each trial began with a fixation cross presented for 1.5 seconds, followed by a static image of an actor’s neutral expression for five seconds (Figure 2a). Then, a second screen appeared, which asked the participants to make the following judgment: “How morally GOOD or BAD do you think this person is?” Importantly, the participants are asked to make an initial moral judgment at this point that is solely based on their first impression of the actor. Participants responded at their own pace by selecting a value on a 7-point Likert scale where 1 equals “Very Bad”; 4 equals “Somewhat [Bad/Good]”; and 7 equals “Very Good.” Participants made their selection by clicking the left and right buttons on a mouse, which would move a cursor over the seven numbers on the screen in front of them, and when the cursor highlighted the number they wanted to select, they pressed the middle mouse button to indicate their selection. The cursor always started on the far left, on the “1,” so as to discourage the participant from passively “clicking through” the experiment by selecting a “4” (neutral response, in the middle of the scale). After the participants made this baseline moral judgment, they then saw a fixation cross for 1.5 seconds, followed by a corresponding static image depicting the same actor engaging in a unique moral behavior (either morally good, bad, or neutral), and simultaneously heard an auditory description of the social scenario, which lasted for a total of ten seconds (Figure 2b,c). On the next screen, the participants viewed the same static image of the actor as before (Figure 2a) for five seconds, and then on the last screen, were asked to make an “updated” moral judgment with the same exact question as before: (“How morally GOOD or BAD do you think this person is?”). There were a total of 15 trials (five unique good, bad, and neutral scenarios), each involving a novel actor (with no reoccurring actors), as well as two practice trials. The order of the experimental trials was randomized for each participant.
Manipulation Check
The complex social scenarios were created by the first author (K.E.C.) to depict actions and events that would be readily judged as being morally “good,” “bad,” or “neutral.” In order to verify the intended moral status of the scenarios, we obtained moral ratings of the “goodness,” “badness,” or “neutrality” of the moral acts at the end of the experiment from a group of 10 normal, healthy individuals, in addition to the experimental participants (vmPFC, HC, and BDC patients). After completing all of the experimental trials, the participants saw a screen that read, “Next, you will see a picture and be asked to judge how morally good or bad the ACTION shown in the picture is.” The participants then saw each of the 15 previously viewed complex social scenes (Figure 2b), presented in a randomized order. While viewing each scene, the participants simultaneously heard a brief corresponding auditory description of the moral act (for example, “Holding someone’s head under water until they nearly drown”). Each scene was presented for a total of 4 seconds. After each of the complex social scenes, a second screen appeared, which read: “How morally GOOD or BAD do you think this ACTION is?”, and the participants responded by providing a rating as they did before, on a 7-point Likert scale.
Data Analysis
The primary comparisons of interest were the between-group differences in the updating, or changing of moral judgments. The morally good and bad scenarios were pooled together into a “valenced” group, for a total of 10 target scenarios, as we did not have any a priori reason to predict, or any hypothesis about, valence-related differences. The change scores for the five neutral scenarios served as a control measure: we did not expect changes (updates) in the moral judgments of these actors, given that the moral information provided for these was neutral (and the starting point was designed to be neutral). Data were analyzed with a one-way analysis of covariance (ANCOVA) to assess the effect of brain damage (three groups: vmPFC, HC, BDC) on updating moral judgments for both the valenced (good/bad) and neutral scenarios. We analyzed the valenced and neutral stimuli separately in order to examine pairwise comparisons between groups for both types of stimuli. The dependent measure was the absolute value of the change score, which reflects the updating of moral judgments by taking the absolute value of the difference between the baseline (pre) and updated (post) moral judgments about the actors in all 15 scenarios. Morality ratings of the moral “goodness/badness/neutrality” of the moral actions were used as a covariate to control for individual differences in basic judgments of moral actions. The statistical assumptions for the ANCOVA were checked before the analysis. All analyses were performed at a significance level of 0.05 (two-tailed) using a commercially available statistics software package (SPSS Statistics, release 17.0.0; Chicago: SPSS Inc.).
For the analysis of the manipulation check data, the dependent variable, morality ratings of morality per se in the complex social scenarios, was analyzed with a repeated measures ANOVA with moral type (good, bad, or neutral) as the within-subjects factor, and group (vmPFC, HC, BDC) as the between-subjects factor. We expected a main effect of moral type (good > neutral > bad), but no group main effect or interaction.
RESULTS
Manipulation Check
The group of 10 normal, healthy individuals perceived the moral scenarios in the manner intended. On average, “morally bad” scenarios were rated 1.5, “morally good” scenarios were rated 6.2, and “morally neutral” scenarios were rated 4.3 on a 7-point Likert scale where 1 equals “Very Bad”; 4 equals “Somewhat [Bad/Good]”; and 7 equals “Very Good.” These results support the construct validity of the stimuli.
In general, the three groups of experimental participants (vmPFC, HC, BDC) rated the moral scenarios similarly: on average, “morally bad” scenarios were rated between 1-2, “morally good” were rated between 6-7, and “morally neutral” were rated between 4-6 (Figure 3). The two-factor repeated measures ANOVA with moral type (good, bad, or neutral) as the within-subjects factor and group (vmPFC, HC, BDC) as the between-subjects factor revealed a significant main effect of moral type (F(2,42) = 499.1, p < 0.001). Pairwise comparisons based on estimated marginal means with a Bonferroni adjustment for multiple comparisons revealed significant differences (p < 0.001) between the mean ratings of the three scenario types, where good (6.4) > neutral (4.9) > bad (1.4). The analysis also revealed a significant between-subjects effect of group (F(2,21) = 5.3, p = 0.01), as well as a significant group by moral type interaction (F(4,42) = 5.74, p = 0.001). These results are primarily driven by the HC group, which rated the good and neutral stimuli more morally good and the bad stimuli more morally bad than both the BDC and vmPFC groups.
Figure 3. Average group ratings of complex social stimuli for experimental participants.
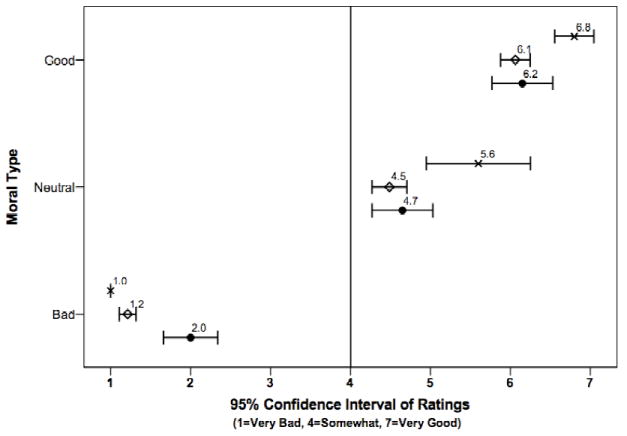
This figure shows the means and 95% confidence intervals for the three group’s ratings of the moral “goodness/badness” of the scenarios. On average, “bad” scenarios are rated between 1-2, “good” are rated between 6-7, and “neutral” are rated between 4-6. The vmPFC group is indicated by closed circles, the BDC group by open diamonds, and the HC group by crosses.
These findings indicate that in general, the three groups apprehended and judged the intrinsic moral goodness or badness of the scenarios similarly, and in the manner intended by the construction of the stimuli. However, because there were significant group differences in the ratings of the intrinsic moral “goodness” or “badness” of the complex social stimuli actions, an analysis of covariance (ANCOVA) with the morality ratings as the covariate was employed to compare group differences on the “updating” variable.
Moral Updating
In support of the main hypothesis, we found that for valenced scenarios and in comparison to the BDC group, patients with vmPFC damage showed reduced moral updating (i.e., less changing of moral judgments after learning social/moral information). Surprisingly and interestingly, we found that the HC group exhibited significantly exaggerated moral updating (i.e., more change of moral judgments after learning new social and moral information), in comparison to the BDC group, and in stark contrast to the vmPFC group. These findings are graphed in Figure 4.
Figure 4. Moral updating for valenced scenarios as a function of group.
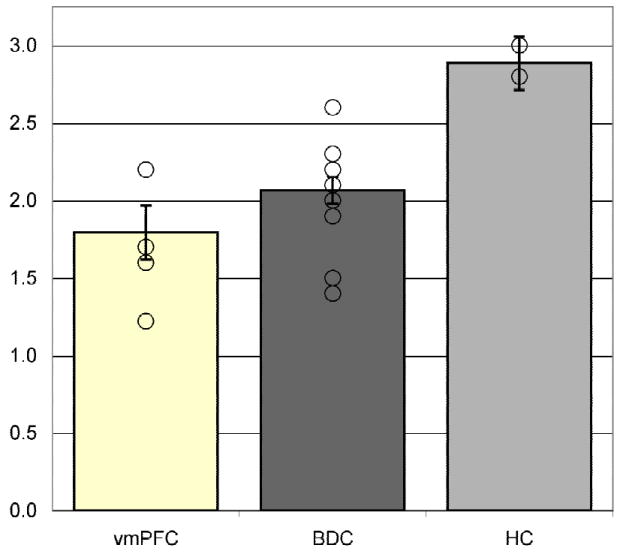
This figure shows the group changes in moral judgments (in absolute Likert scale units) for morally good and bad (valenced) scenarios. Group means represent adjusted values after taking into account the effects of the covariate. Individual raw data points are plotted as open circles. Error bars represent SEM.
Testing for group differences in moral updating for the valenced (pooled good/bad) scenarios was the principal analysis of interest. Preliminary checks did not reveal a significant effect of error variance of the dependent measure across groups (F(2,236) = 2.3, p > 0.05). As expected, the covariate (ratings of moral actions) was found to significantly predict the updating of moral judgments (F(1,235) = 107.0, p < 0.001). However, significant group differences in the updating of moral judgments remained after controlling for individual differences in ratings of moral actions (F(2,235) = 11.7, p < 0.001; Figure 4), with a moderate effect size (partial eta squared = 0.09). Pairwise multiple comparisons among estimated marginal means using the Bonferroni test revealed that the HC group updated their moral judgments of valenced stimuli significantly more than the BDC group (p < 0.001) and the vmPFC group (p < 0.001; mean change 2.89 versus 2.07 and 1.79, respectively). The vmPFC group updated their moral judgments less than the BDC group (mean of 1.79 versus 2.07 Likert units), although this difference did not reach statistical significance, which is likely due to the limited power of the test because of the small sample sizes.
For the morally neutral scenarios, preliminary checks did not reveal a significant effect of error variance of the dependent measure across groups (F(2,122) = 0.6, p > 0.05). The ANCOVA revealed that the covariate (ratings of moral actions) did not significantly predict the updating of moral judgments for morally neutral scenarios (F(1,121) = 0.41, p > 0.05). There were no significant group differences in the updating of moral judgments of neutral scenarios after controlling for individual differences in ratings of moral actions (F(2,121) = 1.0, p > 0.05; Figure 5).
Figure 5. Moral updating for neutral scenarios.
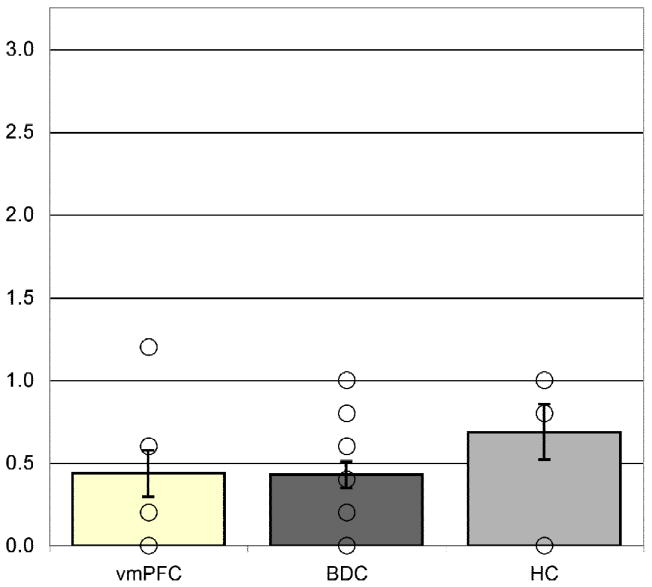
All groups changed their moral judgments on average, less than one Likert unit for the morally neutral stimuli. Group means represent adjusted values after taking into account the effects of the covariate. Individual raw data points are plotted as open circles. Error bars represent SEM.
Confidence intervals and Cohen’s d effect sizes of pairwise comparisons were calculated, and the outcomes support the overall pattern of group differences in change scores for the valenced and neutral complex social scenarios (Table 4). Specifically, for the contrasts of vmPFC versus HC, and BDC versus HC for the valenced stimuli, the effect sizes were large (3.63 and 2.51, respectively), and the 95% confidence interval of the mean differences did not include zero. The contrast of vmPFC versus BDC revealed a large effect size (0.83), although the 95% confidence interval of the mean difference included zero. The pairwise comparisons for the neutral stimuli revealed negligible (vmPFC versus BDC; 0.03) and medium effect sizes (vmPFC versus HC and BDC versus HC; 0.93 and 0.84, respectively), and all of the 95% confidence intervals included zero.
Table 4. Confidence intervals of the mean difference and effect sizes of pairwise comparisons between vmPFC, HC, and BDC groups.
Confidence intervals and effect sizes are based on independent samples t-tests between groups.
| Scenario Type | Comparison Groups | 95% Confidence Interval of the Difference | Effect Size (Cohen’s d) | |
|---|---|---|---|---|
| Lower Bound | Upper Bound | |||
| Valenced | vmPFC vs. HC | -1.684 | -0.501 | 3.63 |
| Valenced | BDC vs. HC | 0.355 | 1.284 | 2.51 |
| Valenced | vmPFC vs. BDC | -0.743 | 0.197 | 0.83 |
| Neutral | vmPFC vs. HC | -0.786 | 0.286 | 0.93 |
| Neutral | BDC vs. HC | -0.204 | 0.719 | 0.84 |
| Neutral | vmPFC vs. BDC | -0.385 | 0.4 | 0.03 |
Pre Versus Post Judgments
In addition to analyzing the change score data, we also examined the raw “pre” and “post” judgments separately. The first moral judgments (“pre”) represent the moral judgments made before the participant knows anything about the actors, but they are asked to make a moral judgment of them, nonetheless. The second judgments (“post”) represent the “updated” moral judgments made after the participant has learned that the actor has engaged in either morally good, bad, or neutral behavior. The results are plotted in Figure 6 and show the “pre” data individually from the “post” data. For the “pre” data, a 3 (Group: vmPFC, BDC, HC) × 3 (Moral Type: good, bad, neutral) between-groups analysis of variance was conducted and revealed a significant main effect of Group (F(2,350) = 20.3, p < 0.001). The main effect of Moral Type and the interaction were both nonsignificant. Bonferroni post hoc tests on the overall group differences revealed that the HC group provided significantly higher “pre” judgments than the BDC group (p < 0.001) and the vmPFC group (p < 0.001; mean of 5.4 versus 4.6 and 4.7, respectively on a 7 point Likert scale) across all three moral types.
Figure 6. Pre and post moral judgments of bad/neutral/good complex social stimuli.
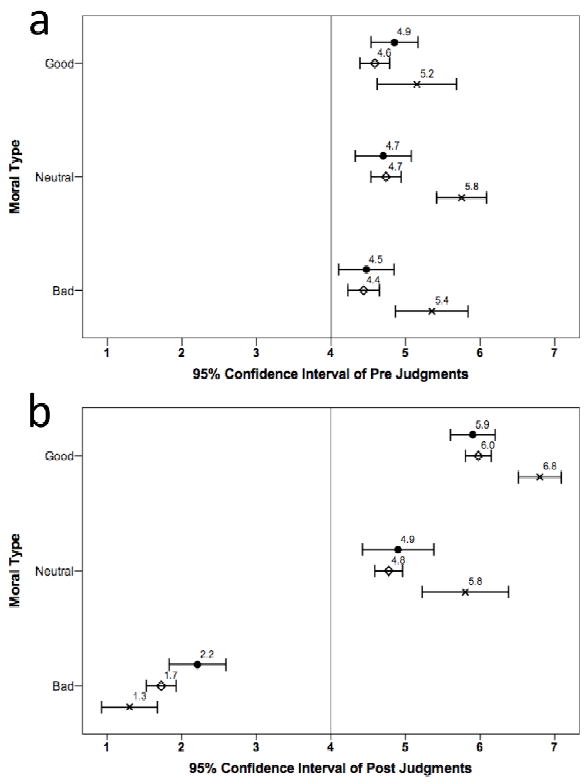
This figure shows the means and 95% confidence intervals for the three group’s raw moral judgments before (a) and after (b) learning moral information about persons. The vmPFC group is indicated by closed circles, the BDC group by open diamonds, and the HC group by crosses.
For the “post” data, a 3 (Group: vmPFC, BDC, HC) × 3 (Moral Type: good, bad, neutral) between-groups analysis of variance was conducted and revealed a significant main effect of Group (F(2,350) = 7.7, p = 0.001), a main effect of Moral Type (F(2,350) = 592.1, p < 0.001), and a Group × Moral Type interaction (F(4,350) = 8.8, p < 0.001). The significant interaction reflects the fact that for the bad scenarios, the vmPFC group provided less negative “post” judgments than the BDC group and the HC group (mean of 2.2 versus 1.7 and 1.3, respectively); for the good scenarios, the HC group provided higher “post” judgments than the vmPFC group and the BDC group (mean of 6.8 versus 5.9 and 6.0, respectively); and for the neutral scenarios, the HC group provided higher “post” judgments than the vmPFC group and the BDC group (mean of 5.8 versus 4.9 and 4.8, respectively).
Good Versus Bad Valence
Although we had no a priori prediction about moral updating being differentially influenced by good versus bad valence, it was of interest to examine this factor in a follow-up analysis. Examination of the morally good and bad change scores revealed between-group differences for both the morally good and bad scenarios (Figure 7). A 3 (Group: vmPFC, BDC, HC) × 2 (Valence: good, bad) between-groups analysis of covariance was conducted. As was done for the main analysis of group differences for the pooled valenced stimuli, the dependent measure was the absolute value of the change score, as a result of updating one’s moral judgment of others, and ratings of the moral actions were used as a covariate to control for individual differences in basic judgments of moral actions. Preliminary checks did not reveal a significant effect of error variance of the dependent measure across groups (F(5,213) = 2.0, p > 0.05). The covariate (ratings of moral actions) did not significantly predict the updating of moral judgments (F(1,212) = 1.5, p > 0.05). However, significant between-group differences in the updating of moral judgments were found, even after controlling for individual differences in ratings of moral actions (F(2,212) = 12.5, p < 0.001), with a moderate to large effect size (partial eta squared = 0.11). The main effect of moral type (good vs. bad) was also significant (F(1,212) = 17.2, p < 0.001), with a moderate effect size (partial eta squared = 0.08). Additionally, there was a significant interaction between group and moral type (F(2,212) = 4.2, p < 0.05), which reflects the different pattern of moral updating for the bad and good moral scenarios. Multiple pairwise comparisons using a Bonferroni adjustment revealed that the HC group was significantly different from the vmPFC and BDC groups (HC > vmPFC and BDC; p < 0.01 for both comparisons) and the difference between the vmPFC and BDC groups trended towards significance (vmPFC < BDC; p = 0.08). For both the good and bad scenarios, the vmPFC group had the lowest change scores. The HC group updated their moral judgments significantly more than both the BDC and vmPFC groups for the morally bad scenarios (mean change of 4.4 vs. 3.2 and 2.6, respectively), and slightly more than the BDC and vmPFC groups for the good scenarios (mean change of 1.3 vs. 1.1 and 0.7, respectively).
Figure 7. Moral updating for bad and good scenarios, as a function of group.
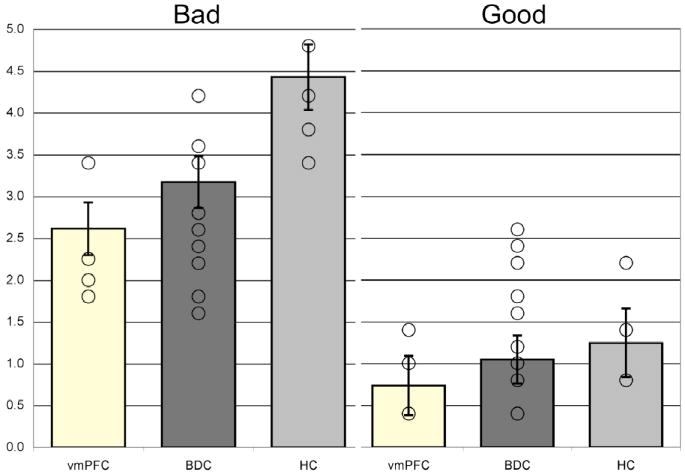
This figure shows changes in moral judgments (in absolute Likert scale units) for the bad and good (valenced) scenarios. Group means represent adjusted values after taking into account the effects of the covariate. Individual raw data points are plotted as open circles. Error bars represent SEM.
DISCUSSION
The question of how we go about formulating, refining, and updating our judgments of the moral character of other individuals is of great interest and importance, and gaining a handle on the neural correlates of this process is of similar import. The current study provides some provocative insights into these issues. Our findings come from small numbers of neurological patients, and the results need to be checked with larger samples of relevant patients. Nonetheless, the basic effects we observed are quite intriguing. To summarize, we found that patients with bilateral vmPFC damage had reduced moral updating, i.e., less change of moral judgments after learning new social and moral information about persons. By contrast, patients with bilateral hippocampal damage had exaggerated moral updating, i.e., more change of moral judgments after being exposed to new social and moral information about persons. In what follows, we consider qualifications and caveats regarding our findings, and then discuss some of the potential implications.
To begin with, the manipulation check data supported the efficacy of our stimuli –all of the morally good complex social scenarios were perceived as morally good, all the bad scenarios were perceived as bad, and all the neutral scenarios were perceived as neutral, by all three groups of participants, and by a group of healthy, normal individuals. Overall, the stimuli were effective in terms of providing the participants three types of complex social scenarios in order to judge moral behavior: morally good, bad, and neutral, and the mean ratings of each scenario type by all three groups are clearly distinguishable in the manner intended by the design of the stimuli.
Group performance on the 10 valenced experimental trials demonstrates that after viewing scenarios depicting an actor engaged in a morally good or bad deed, the HC group changed their moral judgments 28 percent more than the BDC group, and 38 percent more than the vmPFC group (Figure 4). For the HC group, the differences for the valenced stimuli were driven by the morally bad scenarios, for which the HC group changed their moral judgments in the negative direction 28 percent more than the BDC group and 41 percent more than the vmPFC group (Figure 7). For the vmPFC group, we found that they had the least change of moral judgments (for both good and bad scenarios), relative to the other two groups, although the difference between the vmPFC and BDC groups did not reach statistical significance (likely due to small n’s). As expected, there were no between-group differences for changes in moral judgments of morally neutral scenarios (Figure 5).
The raw “pre” and “post” data support the pattern of reduced moral updating by the vmPFC group, and exaggerated moral updating by the HC group, relative to a group of comparison participants that was found in the change score data. As shown in Figure 6a, for all three moral scenario types, the HC group’s initial (“pre”) judgment was higher than both the BDC and vmPFC groups (whose initial judgments were almost exactly alike), suggesting that the HC patients tended to have a slight “positivity bias” in their initial appraisals of the stimulus persons. The “post” data provide further insight into group differences in their moral judgments. For the valenced stimuli (good/bad scenarios), the vmPFC group’s mean “post” judgment is almost one Likert unit different from the HC group’s mean (0.9 more for the bad scenarios and 0.9 less for the good scenarios; Figure 6b), and also reflected less change in comparison to the BDC group (a difference of 0.4 for the bad scenarios and 0.1 for the good scenarios).
The main effect of group in the valenced stimuli was driven by the morally bad scenarios. Lesser moral “updating” for the morally good scenarios was observed across groups of participants (Figure 7). There are multiple reasons why the morally bad scenarios are carrying this effect. The presence of an inherent negativity bias, or an asymmetry in the “potency” of good versus bad valence (with bad entities significantly more potent and of higher salience than good) is a common observation in all stimulus domains, including judgments of character and is well established in the literature (Reeder, 1993; Reader & Brewer 1979; Ito, Larsen, Smith, & Cacioppo, 1998; reviewed in Rozin & Royzman, 2001). In addition, all groups provided slightly positive (i.e., between 4-6 on a 7 point Likert scale) initial judgments of the actors the first time they observed them across all three moral types (Figure 6a). Thus, when presented with morally good scenarios for these actors, the participants had less “room” to change in the positive direction in terms of their moral judgments, at least insofar as our Likert scale was concerned, and this may have contributed to this negative bias as well.
“Moral Updating”
Due to the specific nature of the task used in this study, some clarification of the term “moral updating” is warranted. For the purposes of the current study, we have used the term “moral updating” to simply mean the process of forming a moral judgment based on an initial impression of a person, then learning a moral (good/bad/neutral) behavior that the person took part in, and then updating the judgment to reflect the learning of moral information. Here, the participant does not have to employ reversal learning to make the appropriate updated moral judgment, as there are not multiple and conflicting facts to take into consideration. Instead, the participant first forms a spontaneous initial impression based on the actor’s appearance alone, then provides an “updated” moral judgment, based on what they learned about the actor.
One possible account to explain our observations is that in order to form an appropriately-gauged moral judgment of a person, one must process and integrate both the emotional salience of the moral information (how emotionally potent the moral act is), and the context in which it is presented (how bad is a bad moral act when compared against one’s compendium of all previously learned moral acts). In the current study, these processes are dissociated in two patient groups who are known to have impairments in either emotional processing (the vmPFC group) or flexibly representing associated information with previous experiences (the HC group). Alternative explanations for the reduced “moral updating” seen in the vmPFC patients and the exaggerated “moral updating” seen in the HC patients are offered below. We acknowledge that “moral updating” in the current experiment is limited in that it does not incorporate multiple pieces of learned moral information over time, but we use the term nonetheless because we feel that it accurately describes the cognitive process assessed by the task used in the current study.
Role of vmPFC in Moral Updating
The idea that emotion plays a critical role in decision-making is well supported in the literature; however, the neural mechanisms underlying how emotion influences or modulates decision-making are still not entirely clear. One framework to help conceptualize how one forms a social/moral judgment in the brain is the somatic marker hypothesis (Damasio, 1996). This hypothesis purports that when one is forced with making a complex decision, the vmPFC utilizes previously experienced emotional information to perform, in a sense, a cost/benefit analysis of the various options available. Once the different affective values of potential outcomes have been “calculated,” other neural substrates (dlPFC and mPFC, and perhaps subcortical circuits) make use of this information to construct and implement a plan of action (Wallis, 2007). Damage to vmPFC disrupts this mechanism and as a consequence, patients with damage to vmPFC fail to base their choices on the emotional value with which a particular choice would normally be associated, even in the context of normal declarative memory for all of the available options (Damasio, Tranel, & Damasio, 1990; Damasio, 1994; Bechara et al., 2000; Bechara, 2004). This formulation can explain well the findings from the current study, in that the vmPFC patients, who have intact declarative memory but impaired emotional processing, had the lowest updating of moral character judgments. In other words, the vmPFC patients appeared to give less “weight” to the new, valenced moral information, and thus changed their ratings less.
An alternative explanation may be that the observed impairment is not specific to moral learning and instead, may be attributed to impoverished affect or a general learning impairment. The fact that the vmPFC patients provided mostly normal ratings for the moral acts themselves suggests that they apprehended the stimuli more or less the same as the BDC participants (although their ratings were slightly less “bad” for the bad scenarios; see Figure 3), and that impoverished affect alone could not account for the results. Presumably, the vmPFC patients would be impaired on other emotional learning tasks, perhaps manifesting as perseveration or poor reversal learning, but this would not extend to a general learning impairment. It could also be the case that the more complex the representation that needs to be updated, the more impaired vmPFC patients are in updating it, and therefore, moral/social representations of other people that typically require blending and weighting of many different pieces of value-laden information, are precisely the kinds of representations that they have the most difficulty updating. This study provides a foundation from which to further investigate many of these outstanding questions.
Role of Hippocampus in Moral Updating
While the vmPFC plays a putative role in attributing emotional salience to newly acquired social information, other neural structures such as the hippocampus are also likely involved in forming and updating character judgments. In our relationships with others, our judgment of, and behavior towards, other people would seem to rely extensively on declarative memory: accessing multiple lines of associated information often remote in time and space, and flexibly integrating that information with previous experiences (e.g., how people have behaved towards us in the past, what we know about how they conduct their affairs, and the like). While the role of declarative memory in character judgments may seem intuitive, remarkably, there are actually very few published studies investigating a potential role of the hippocampus in social and moral judgments. A few studies have shown that patients with hippocampal amnesia can attribute affective valence (i.e., good or bad) to new social information (i.e., faces of unfamiliar people) in the absence of declarative memory for the social stimuli (Johnson, Kim, & Risse, 1985; Tranel & Damasio, 1993; Todorov & Olson, 2008).
The current study demonstrates that patients with hippocampal damage and declarative amnesia have exaggerated moral updating. The declarative memory system provides the relational database from which individuals draw the specific situational or contextual information of a given moral behavior from which to formulate a moral judgment, and supports the ability to generate and maintain on-line events (real or imagined) for comparison (e.g., how bad a given scenario is in comparison to the full range of possible bad acts). These abilities depend on declarative memory and because the HC patients have severe declarative memory impairments, we believe these patients overvalue the emotional signal at hand, leading them to make more polarized judgments. In other words, hearing that someone has performed a morally objectionable act such as stealing money from the church could very well, in the absence of any additional information and without the capacity to recall anything learned before about the person, lead to the appraisal that the person who did that act is very, very bad. If one has normal declarative memory, one would remember that the person who committed the objectionable act had been rated previously as “neutral” (or even slightly positively), and thus there would be an “averaging” (or some combinatorial) process that would lead to a lesser degree of change. We are aware that the accuracy of this explanation depends on further investigation, where amnesic patients are explicitly tested for their declarative memory of the individuals they are rating, but our account provides a plausible starting point for interpreting the behavior of the amnesic participants.
Patient 2308: The Potential Role of Amygdala in Moral Updating
Moral judgments require the contribution and orchestration of multiple neural substrates and cognitive systems. Based on previous research and the results of the current study, two separate but related systems appear to contribute to moral judgments: a system that assigns an emotional value to a given representation (supported by vmPFC and probably also the amygdala) and a system for the flexible formation and maintenance of relational representations over time (supported by hippocampus). Of the four amnesic patients included in this study, patient 2308 is notable in that this patient sustained extensive medial temporal lobe damage, including bilateral amygdala damage. Given that the amygdala is thought to have a role in moral cognition, we examined 2308’s performance to check whether it differed from the other HC patients (who did not have amygdala damage). In fact, no significant differences between 2308 and the other three HC patients were found. We also examined whether participant 2308 contributed disproportionately to the group results. When we removed this patient’s data from the HC group data, the results remained the same: the HC group still exhibited significantly exaggerated change scores for valenced moral scenarios, in comparison to the other two groups.
We suspect that circumscribed bilateral amygdala damage would impair moral judgments and “moral updating,” in a manner similar to that observed in the vmPFC patients (i.e., reduced change scores), because like what we have proposed for the vmPFC patients, bilateral amygdala damage would leave declarative memory intact but cause an impaired emotional signal. The finding that patient 2308, who has both amygdala and hippocampal damage, exhibits exaggerated change scores like the rest of the HC patients suggests that the contribution of declarative memory may occur earlier in the time course of making moral judgments. From these data and other data from our laboratory (Gupta et al., 2009), it appears that bilateral hippocampal damage alone is sufficient to impair one’s ability to appropriately form and update relational representations over time in service of advantageous decision-making.
Role of vmPFC and Hippocampus in Real-World Moral Decision-Making
The relation between deficits on our moral updating task and classic vmPFC impairments that have been reported in the literature is relevant. Many classic studies of the consequences of damage to the vmPFC have noted impaired social behavior in the real world, as well as impaired social and moral reasoning (e.g., Anderson et al., 1999), and impaired judgment of moral dilemmas (e.g., Koenigs et al., 2007). Yet in all these cases, no study has directly investigated the question of whether these deficits arise from an inability to learn moral information, or merely to make judgments based upon it. Our study shows that an inability to learn appropriately-valenced new moral information about people contributes to the constellation of impaired social functioning seen following vmPFC damage.
While the impairments in real-world decision-making and social behavior in the vmPFC patients are well documented, less is known about these abilities in patients with hippocampal amnesia. Our finding that patients with hippocampal amnesia have exaggerated change scores, rating individuals as significantly more good or more bad than the BDC participants, raises interesting questions about the real-world social behavior and everyday interactions of those with profound declarative memory impairments. Previously, we reported on a densely amnesic woman who, despite being unable to provide any declarative knowledge for her interactions with a particular coworker, reported strong feelings of distrust towards this co-worker (Duff, Wszalek, Tranel, & Cohen, 2008). Interestingly, this amnesic patient indicated that she was a “good judge of character,” having to rely on gut impressions of people more since the onset of her memory impairment. While this example is consistent with our notion that in the absence of intact declarative memory, patients rely on information (e.g., an emotional signal) that is immediately at hand, additional empirical work the on real-world decision-making in patients with declarative memory impairments, and social and emotional functioning more broadly, is warranted.
Limitations and Future Directions
Taken together, we believe that in order to make successful moral judgments, not only is an intact emotional signal critical, but the contextual information of a person’s actions over time is also needed. Examination of complex behavior in multiple groups of lesion patients allows us to begin to understand the unique contributions of putative nodes in the neural networks of social and moral cognition. One challenge for future studies is to develop methods that implicitly test patients’ moral judgments in an ecologically valid setting, in an effort to capture judgments that more closely mimic real-life moral updating. In a related point, it is possible that some participants utilized verbally-mediated reasoning skills to deduce socially desirable “correct” moral ratings in our experiment. In this case, the findings in vmPFC patients in particular might be even stronger (i.e., more robust evidence of reduced moral updating) if the patients’ judgments were measured in a more implicit manner (for example, asking them to make a decision or choice based on learned moral information about various people, rather than just providing ratings). Other evidence has shown that vmPFC patients can “talk a good game” in at least some moral and social paradigms (e.g., Saver & Damasio, 1991), which would tend to obfuscate deficits that might be apparent in their real-world behavior.
An additional limitation of the current study is the small sample size. Given the rarity of circumscribed bilateral vmPFC and hippocampal lesions, a larger sample meeting all the inclusion/exclusion criteria is challenging to come by. However, examining the moral updating process in more detail and at several time points would provide more data and thus, lend more power to the analyses. Additionally, future studies replicating our findings in vmPFC and HC patients, as well as fMRI studies in healthy individuals will be essential to corroborate the initial intriguing findings from the present study. An interesting follow-up to the current study would be to examine the formation and updating of moral judgments as participants learn and integrate multiple pieces of information about a single individual, that vary in valence and time scale over which they are presented (which is more reminiscent of the challenges we face in the real world). Additionally, as alluded to earlier, it will be important to know what declarative knowledge the HC patients have at various time points, and this will require testing their memory at various stages throughout the moral updating process.
Our findings of exaggerated change scores in the HC group may appear at odds with findings from Johnson et al. (1985), who reported that a group of patients with Korsakoff’s syndrome updated their impression ratings of a “good guy” and a “bad guy” less than healthy comparison participants. One possible explanation for this apparent discrepancy is the time period between learning the social information and having to make a judgment: in the current study, this was less than a minute, whereas in the Johnson et al. study it was two hours up to one year. Differences in the patient populations and task demands (making good guy/bad guy impressions vs. moral judgments) may also contribute to the different findings. In any event, the rate of decay of the emotional signals should be examined in future studies.
CONCLUSIONS
In summary, our results suggest that both the hippocampus and vmPFC are involved in updating one’s judgment about the moral “character” of others after learning new social and moral information about those persons. The process of updating moral judgments is complex – not only must one formulate judgments in the first place, but one then has to fine tune, reconfigure, and even change one’s judgments, taking into account the emotional salience and context of new and old information. Patients with vmPFC damage may be impaired at attributing affective value to various aspects of complex moral situations, thereby contributing to impaired moral judgments and real-world social dysfunction. Patients with HC damage, lacking intact declarative knowledge of contextual information, may be left with only an emotional signal of “good” or “bad” to help guide their moral judgments. Ongoing studies in our laboratory are investigating these different components of moral learning.
Acknowledgments
We acknowledge Thomas Grabowski for reviewing the characterization of the lesions in the target and BDC groups, and the accuracy of the lesion overlap map depicted in Figure 1, and Hanna Damasio for her expert tracing of the lesions of the patients. The authors would like to acknowledge Tony Buchanan, David Rudrauf, and Joel Bruss for their assistance with experimental design, data analysis, and lesion analysis, respectively. We thank Antonio Damasio for inspiring the title.
Funding This work was supported by the National Alliance for Research on Schizophrenia and Depression; the National Institute of Neurological Disorders and Stroke [P50 NS19632]; and the National Institute on Drug Abuse [R01 DA022549].
Appendix
Morally good, bad, and neutral complex social scenarios
Morally “Good” Scenarios
Emily was at the mall and found a wallet with 65 dollars in it in the women’s bathroom. She left all the money in the wallet and turned it in to security.
Michael’s coworker stopped him in the hallway and begged him to borrow some money. Although he didn’t know her very well, she looked upset, so he gave her 20 dollars.
Chris volunteered once a month building houses for the homeless in his local community. By the end of one year, Chris had helped build 14 homes.
Jackie noticed that her friend was having a bad day at work. So on her lunch break, she bought her friend a funny card hoping that it would make her friend feel better.
Steven knew he had a rare blood type, so he often donated blood at the hospital so they could provide blood transfusions to other people with his same rare blood type.
Morally “Bad” Scenarios
Jennifer wanted a candy bar so she went into a store and when no one was looking, she took a candy bar, put it in her purse, and then quickly left the store.
Gary was in charge of the church’s finances and every once in awhile, so as not to get caught, he took some money from the church’s account and put it into his own checking account.
Jeremy’s girlfriend broke up with him, but he didn’t want to stop seeing her, so he waited for her to come home from work and then watched her through her bedroom window.
Rebecca really wanted a promotion at work, so when her coworker got the promotion instead, she went to the parking lot and keyed her coworker’s car.
After Matt found out his wife had been cheating on him, he went into the bathroom while she was taking a bath and held her head under water until she almost drowned.
Morally “Neutral” Scenarios
Mark brought a book with him to read while waiting for his doctor’s appointment. He was able to read his book for 20 minutes before the nurse called his name.
Sarah worked as a customer service representative for a clothing store. She checked her email when she got to work to see if any she had any new emails.
Andrew was running late to his dermatology appointment and couldn’t find his way to the clinic. He asked a woman in the hallway if she could help him and give him directions.
Cindy went to the file cabinet and after finding the right drawer, she placed a paper into the file and then closed the file cabinet drawer.
Sharon needed to photocopy a document to hand out to all the employees in her office, so she went to the copy room and made 200 copies.
Footnotes
Conflict of Interest None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R. Social cognition and the human brain. Trends In Cognitive Sciences. 1999;3(12):469–479. doi: 10.1016/s1364-6613(99)01399-6. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Cognitive neuroscience of human social behaviour. Nature Reviews Neuroscience. 2003;4(3):165–178. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- Adolphs R. How do we know the minds of others? Domain-specificity, simulation, and enactive social cognition. Brain Research. 2006;1079(1):25–35. doi: 10.1016/j.brainres.2005.12.127. [DOI] [PubMed] [Google Scholar]
- Allen J, Tranel D, Bruss J, Damasio H. Correlations between regional brain volumes and memory performance in anoxia. Journal of Clinical and Experimental Neuropsychology. 2006;28(4):457–476. doi: 10.1080/13803390590949287. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Barrash J, Bechara A, Tranel D. Impairments of emotion and real-world complex behavior following childhood- or adult-onset damage to ventromedial prefrontal cortex. Journal of the International Neuropsychological Society. 2006;12(2):224–235. doi: 10.1017/S1355617706060346. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nature Neuroscience. 1999;2(11):1032–1037. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- Bar-On R, Tranel D, Denburg NL, Bechara A. Exploring the neurological substrate of emotional and social intelligence. Brain. 2003;126(Pt 8):1790–1800. doi: 10.1093/brain/awg177. [DOI] [PubMed] [Google Scholar]
- Bechara A. Disturbances of emotion regulation after focal brain lesions. International Review of Neurobiology. 2004;62:159–193. doi: 10.1016/S0074-7742(04)62006-X. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex. 2000;10(3):295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Beer JS, Heerey EA, Keltner D, Scabini D, Knight RT. The regulatory function of self-conscious emotion: Insights from patients with orbitofrontal damage. Journal of Personality and Social Psychology. 2003;85(4):594–604. doi: 10.1037/0022-3514.85.4.594. [DOI] [PubMed] [Google Scholar]
- Beer JS, John OP, Scabini D, Knight RT. Orbitofrontal cortex and social behavior: Integrating self-monitoring and emotion-cognition interactions. Journal of Cognitive Neuroscience. 2006;18(6):871–879. doi: 10.1162/jocn.2006.18.6.871. [DOI] [PubMed] [Google Scholar]
- Camille N, Coricelli G, Sallet J, Pradat-Diehl P, Duhamel JR, Sirigu A. The involvement of the orbitofrontal cortex in the experience of regret. Science. 2004;304(5674):1167–1170. doi: 10.1126/science.1094550. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Banich MT. Memory. In: Banich MT, editor. Neuropsychology:The neural bases of mental function. 2. Boston: Houghton-Mifflin; 2003. pp. 322–364. [Google Scholar]
- Damasio AR. Descartes’ Error. New York, NY: HarperCollins; 1994. [Google Scholar]
- Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 1996;351(1346):1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, Damasio H. Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behavioural Brain Research. 1990;41(2):81–94. doi: 10.1016/0166-4328(90)90144-4. [DOI] [PubMed] [Google Scholar]
- Duff MC, Hengst JA, Tranel D, Cohen N. Collaborative discourse facilitates efficient communication and new learning in amnesia. Brain and Language. 2008;106(1):41–54. doi: 10.1016/j.bandl.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff MC, Wszalek T, Tranel D, Cohen N. Successful life outcome and management of real-world memory demands despite profound anterograde amnesia. Journal of Clinical and Experimental Neuropsychology. 2008;30(8):931–945. doi: 10.1080/13803390801894681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From conditioning to conscious recollection: Memory systems of the brain. New York, NY: Oxford University Press; 2001. [Google Scholar]
- Eslinger PJ, Damasio AR. Severe disturbance of higher cognition after bilateral frontal lobe ablation: Patient EVR. Neurology. 1985;35(12):1731–1741. doi: 10.1212/wnl.35.12.1731. [DOI] [PubMed] [Google Scholar]
- Frank RJ, Damasio H, Grabowski TJ. Brainvox: An interactive, multimodal visualization and analysis system for neuroanatomical imaging. NeuroImage. 1997;5(1):13–30. doi: 10.1006/nimg.1996.0250. [DOI] [PubMed] [Google Scholar]
- Grafman J, Schwab K, Warden D, Pridgen A, Brown HR, Salazar AM. Frontal lobe injuries, violence, and aggression: A report of the Vietnam head injury study. Neurology. 1996;46(5):1231–1238. doi: 10.1212/wnl.46.5.1231. [DOI] [PubMed] [Google Scholar]
- Greene J, Haidt J. How (and where) does moral judgment work? Trends In Cognitive Science. 2002;6(12):517–523. doi: 10.1016/s1364-6613(02)02011-9. [DOI] [PubMed] [Google Scholar]
- Gupta R, Duff MC, Denburg NL, Cohen NJ, Bechara A, Tranel D. Declarative memory is critical for sustained advantageous complex decision-making. Neuropsychologia. 2009;47(7):1686–1693. doi: 10.1016/j.neuropsychologia.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutbrod K, Krouzel C, Hofer H, Müri R, Perrig W, Ptak R. Decision-making in amnesia: do advantageous decisions require conscious knowledge of previous behavioural choices? Neuropsychologia. 2006;44(8):1315–1324. doi: 10.1016/j.neuropsychologia.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Harlow JM. Recovery from the passage of an iron bar through the head. Publications of the Massachusetts Medical Society. 1868;2:327–347. [Google Scholar]
- Hornak J, Bramham J, Rolls ET, Morris RG, O’Doherty J, Bullock PR, et al. Changes in emotion after circumscribed surgical lesions of the orbitofrontal and cingulate cortices. Brain. 2003;126(7):1691–1712. doi: 10.1093/brain/awg168. [DOI] [PubMed] [Google Scholar]
- Ito TA, Larsen JT, Smith NK, Cacioppo JT. Negative information weighs more heavily on the brain: The negativity bias in evaluative categorizations. Journal of Personality and Social Psychology. 1998;75(4):887–900. doi: 10.1037//0022-3514.75.4.887. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Kim JK, Risse G. Do alcoholic Korsakoff’s syndrome patients acquire affective reactions? Journal of Experimental Psychology: Learning, Memory, and Cognition. 1985;11(1):22–36. doi: 10.1037//0278-7393.11.1.22. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Young L, Adolphs R, Tranel D, Cushman F, Hauser M, et al. Damage to the prefrontal cortex increases utilitarian moral judgements. Nature. 2007;446(7138):908–911. doi: 10.1038/nature05631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Tranel D. Irrational economic decision-making after ventromedial prefrontal damage: Evidence from the Ultimatum Game. Journal of Neuroscience. 2007;27(4):951–956. doi: 10.1523/JNEUROSCI.4606-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD. Social cognitive neuroscience: A review of core processes. Annual Review of Psychology. 2007;58:259–289. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- Moll J, Zahn R, de Oliveira-Souza R, Krueger F, Grafman J. Opinion: The neural basis of human moral cognition. Nature Reviews Neuroscience. 2005;10:799–809. doi: 10.1038/nrn1768. [DOI] [PubMed] [Google Scholar]
- Reeder GD. Trait–behavior relations in dispositional inference. Personality and Social Psychology Bulletin. 1993;19:586–593. [Google Scholar]
- Reeder GD, Brewer MB. A schematic model of dispositional attribution in interpersonal perception. Psychological Review. 1979;86:61–79. [Google Scholar]
- Roberts NA, Beer JS, Werner KH, Scabini D, Levens SM, Knight RT, et al. The impact of orbital prefrontal cortex damage on emotional activation to unanticipated and anticipated acoustic startle stimuli. Cognitive, Affective, & Behavioral Neuroscience. 2004;4(3):307–316. doi: 10.3758/cabn.4.3.307. [DOI] [PubMed] [Google Scholar]
- Rozin P, Royzman EB. Negativity bias, negativity dominance, and contagion. Personality and Social Psychology Review. 2001;5:296–320. [Google Scholar]
- Saver JL, Damasio AR. Preserved access and processing of social knowledge in a patient with acquired sociopathy due to ventromedial frontal damage. Neuropsychologia. 1991;29(12):1241–1249. doi: 10.1016/0028-3932(91)90037-9. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Tomer R, Berger BD, Aharon-Peretz J. Characterization of empathy deficits following prefrontal brain damage: The role of the right ventromedial prefrontal cortex. Journal of Cognitive Neuroscience. 2003;15(3):324–337. doi: 10.1162/089892903321593063. [DOI] [PubMed] [Google Scholar]
- Singer T, Kiebel SJ, Winston JS, Dolan RJ, Frith CD. Brain responses to the acquired moral status of faces. Neuron. 2004;41(4):653–662. doi: 10.1016/s0896-6273(04)00014-5. [DOI] [PubMed] [Google Scholar]
- Todorov A, Gobbini MI, Evans KK, Haxby JV. Spontaneous retrieval of affective person knowledge in face perception. Neuropsychologia. 2007;45(1):163–173. doi: 10.1016/j.neuropsychologia.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Todorov A, Olson IR. Robust learning of affective trait associations with faces when the hippocampus is damaged, but not when the amygdala and temporal pole are damaged. Social Cognitive and Affective Neuroscience. 2008;3(3):195–203. doi: 10.1093/scan/nsn013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov A, Uleman JS. Spontaneous trait inferences are bound to actors’ faces: Evidence from a false recognition paradigm. Journal of Personality and Social Psychology. 2002;83(5):1051–1065. [PubMed] [Google Scholar]
- Todorov A, Uleman JS. The person reference process in spontaneous trait inferences. Journal of Personality and Social Psychology. 2004;87(4):482–493. doi: 10.1037/0022-3514.87.4.482. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio AR. The covert learning of affective valence does not require structures in hippocampal system or amygdala. Journal of Cognitive Neuroscience. 1993;5(1):79–88. doi: 10.1162/jocn.1993.5.1.79. [DOI] [PubMed] [Google Scholar]
- Tranel D. “Acquired sociopathy”: the development of sociopathic behavior following focal brain damage. In: Fowles DC, Sutker P, Goodman SH, editors. Progress in Experimental Personality and Psychopathology Research. New York, NY: Springer; 1994. pp. 285–311. [PubMed] [Google Scholar]
- Tranel D. Theories of clinical neuropsychology and brain-behavior relationships: Luria and beyond. In: Morgan JE, Ricker JH, editors. Textbook of clinical neuropsychology. New York: Taylor and Francis; 2007. pp. 27–39. [Google Scholar]
- Wallis JD. Orbitofrontal cortex and its contribution to decision-making. Annual Review of Neuroscience. 2007;30:31–56. doi: 10.1146/annurev.neuro.30.051606.094334. [DOI] [PubMed] [Google Scholar]


