Abstract
Recent language studies in aging and dementia provide two complementary lines of evidence that: (1) measures of semantic knowledge and word-finding ability show declines comparable to those of episodic memory, and greater impairment than executive function measures, during the prodromal period of Alzheimer’s disease and (2) cognitively intact older adult carriers of the apolipoprotein E (APOE) ε4 allele also demonstrate poorer object naming than their low-risk peers. Given that possible changes in the neural substrates of word retrieval (e.g., Broca’s area and fusiform gyrus) in at-risk adults may signal impending cognitive decline and serve as a prodromal marker of AD, we examined whether APOE ε4 carriers exhibit changes in brain response in regions subserving word retrieval and semantic knowledge. Eleven cognitively intact APOE ε4 older adults and 11 age, education, and family history of AD-matched APOE ε3 adults named aloud photographs of animals, tools, and vehicles during event-related fMRI. Results showed that, in the face of equivalent naming accuracy, APOE ε4 adults demonstrated more widespread brain response with greater signal change in the left fusiform gyrus, bilateral medial prefrontal cortex, and right perisylvian cortex. Findings are discussed in the context of possible compensatory mechanisms invoked to maintain performance in those at genetic risk for AD.
Keywords: apolipoprotein E, language, fMRI, word retrieval, confrontation naming
Introduction
The apolipoprotein E (APOE) ε4 allele on chromosome 19 is a major genetic risk factor for late-onset Alzheimer’s disease (AD) that may account for as much as 50% of the risk for developing the disease (Ashford & Mortimer 2002, Saunders et al 1993). Apolipoprotein E in its ε2 and ε3 isoforms plays a fundamental role in cell maintenance and repair through its function in lipid transport and cellular metabolism; however, the effectiveness of the ε4 isoform in this role is questionable. While the mechanisms underlying the role of APOE ε4 in AD are not fully understood, the APOE ε4 genotype has been linked with increases in amyloid β deposition, dysregulation of tau phosphorylation, mitochondrial damage, and disruption of cytoskeletal structure (Mahley & Huang 1999, Mahley & Huang 2006, Mahley & Huang 2009). The ε4 allele has also been associated with small vessel arteriolosclerosis and microinfarcts of the deep nuclei in autopsy-confirmed patients with AD (Mahley et al 1996, Tiraboschi et al 2004, Yip et al 2005). Non-demented APOE ε4 carriers show subtle functional and structural brain differences (Bondi et al 2005, Bookheimer et al 2000, Han et al 2007, Johnson et al 2007, Lind et al 2006, Mondadori et al 2007) and cognitive changes (Bondi et al 1999, Bretsky et al 2003, Jorm et al 2007, Lind et al 2006, Miller et al 2005, Mondadori et al 2007) that may reflect a prodromal phase of AD. In fact, these cognitive declines and functional changes in APOE ε4 carriers tend to become most evident after age 65 (Jorm et al 2007). By contrast, young ε4 carriers have been shown to demonstrate better episodic memory performance and reduced learning- and retrieval-related activity (Mondadori et al 2006), suggesting that the negative effects of the APOE ε4 allele accelerate with age.
There is strong evidence to suggest that AD results in neuropsychological and brain changes that occur several years or more prior to the frank expression of clinical symptoms that warrant a formal diagnosis of dementia (Twamley et al 2006). Although the assessment of cognitive changes in prodromal AD has largely focused on episodic memory, recent evidence suggests that AD need not begin solely as a memory disorder (Storandt et al 2006). Indeed, subtle deficits in a broad range of neuropsychological domains, including executive functions, perceptual speed, verbal ability, visuospatial skill, and attention, have been implicated during the prodromal period (Backman et al 2004, Backman et al 2005, Jacobs et al 1995, Storandt et al 2006, Twamley et al 2006). This widespread decline in cognitive abilities mirrors evidence that multiple brain regions (e.g., medial and lateral temporal lobes, frontal lobes, anterior cingulate cortex) and connectivity between these regions are impaired in prodromal AD (Albert et al 2001, Andrews-Hanna et al 2007, Small et al 2003).
Changes in language abilities during prodromal AD have been the subject of some interest. Studies have shown that these changes are characterized by word finding difficulties on tests of verbal fluency and object naming. Word finding deficits during verbal fluency and object naming tasks have also been demonstrated in cognitively intact older adults with the APOE ε4 allele compared to those without the ε4 allele (Miller et al 2005). Because word retrieval requires access to both word forms (i.e., lexical forms) and meaning (i.e., semantics), the poor performance of those at risk for AD may result from either source. Specifically, decline in the ability to strategically access, select and retrieve appropriate lexical word forms has been associated with deterioration of frontal neural substrates in the inferior frontal gyrus (e.g., Broca’s area) and medial prefrontal cortex (e.g., cingulate region) (Barch et al 2000, Carter et al 2000, Crosson et al 2005, Gabrieli et al 1998, MacDonald et al 2000, Thompson-Schill et al 1997, Wagner et al 2001), whereas a deterioration of semantic stores for representations of meaning are thought to reside, at least in part, in the inferior temporal cortex (Chao et al 1999, Ishai et al 1999, Wierenga et al 2009). Our previous studies, however, suggest that difficulty accessing lexical word forms contributes to word finding difficulties in normal aging while semantic memory remains intact (Wierenga et al 2008). In the context of equivalent word retrieval performance, healthy older adults activated frontal cortical regions in the contralateral right hemisphere (Broca’s area homologue) in addition to those both they and young adults activated, although they did not recruit additional neural substrates responsible for semantic knowledge during the word retrieval task. These results suggest that difficulty accessing lexical word forms may be a consequence of normal aging or AD, whereas a breakdown of semantic knowledge may be unique to AD and its prodrome.
A number of studies have shown that semantic memory that underlies general knowledge and language is often disturbed in patients with mild cognitive impairment (MCI; a likely AD prodrome) (Adlam et al 2006, Duong et al 2006, Hodges et al 1995, Kraut et al 2006) or early AD (Hodges et al 1995, Nebes 1989). Adults with AD exhibit a significant confrontation naming deficit (Bayles & Tomoeda 1983) characterized by semantically-based errors, thought to reflect a pronounced deterioration of semantic memory. The integrity of semantic memory in adults at genetic risk for AD by virtue of the APOE ε4 allele has not received much attention despite the finding that measures of semantic knowledge show significant declines during prodromal AD (Mickes et al 2007); see also (Cuetos et al 2007, Powell et al 2006). Mickes and colleagues (2007), for example, have shown that both semantic memory and episodic memory functions declined rapidly in a three-year period progressing to AD, whereas executive function deficits were not especially prominent. From these findings, the authors suggest that cognitive abilities thought to be subserved by the medial and lateral temporal lobes (episodic memory and semantic knowledge, respectively) may be substantially more impaired than cognitive functions subserved by the frontal lobes (executive functions). These findings are consistent with previous results of decreased semantic access in nondemented APOE ε4 older adults (Rosen et al 2005) and correspond well to the known neuropathologic encroachments of AD early in the disease process (Braak & Braak 1991). The findings are also consistent with recent reports of the ability of language tasks to predict pathologic AD six years later in non-demented individuals (Powell et al 2006).
Several lesion and functional neuroimaging studies demonstrate that the inferior temporal lobe, especially the fusiform gyrus, is a focal point for the integration of visual and semantic information. A medial-lateral distinction has reliably been found for nonliving vs. living objects, respectively, in the fusiform gyrus (Chao et al 1999, Ishai et al 1999, Mahon et al 2009, Wierenga et al 2009). In addition, we recently reported a dissociation between semantic category (living vs nonliving) and visual attribute (global vs local form) processing in the fusiform gyrus for younger and older adults performing an object naming task (Wierenga et al 2009). The ability to make such fine-grained semantic distinctions suggests that a similar object-naming paradigm could be effective in detecting subtle breakdown in semantic function that might occur in APOE ε4 carriers. This is particularly true given that a recently identified neural pathway interconnecting the mid-fusiform region with the amygdala/hippocampus is thought to be important for object recognition and memory consolidation and may be disrupted in AD (Smith et al 2009).
Only a few functional neuroimaging studies, restricted to middle-aged adults, have investigated language (e.g., verbal fluency, object naming) in APOE ε4 carriers. Results from these studies indicated that high-risk middle-aged women (e.g., positive family history of AD and an ε4 allele) had increased left parietal brain activation during covert verbal fluency and reduced inferior temporal cortex activation during verbal fluency and object-naming (Smith et al 2002). Furthermore, the reduction in inferior temporal lobe activity was correlated with eventual cognitive decline (Smith et al 2005). It is not known, however, if these results generalize to older adults given that better performance tends to correspond to decreased blood oxygen level dependent (BOLD) response in younger adults with an ε4 allele (Mondadori et al 2007) but to increased BOLD response (thought to reflect compensatory mechanisms) in older adults with an ε4 allele. These contradictory findings may reflect antagonistic pleiotropy whereby the ε4 allele could have protective effects (e.g., on survival and cognition) during young adulthood, but well-known detrimental effects during the post-reproductive years (Bloss et al 2008). The interpretation of the decreased activity in middle-aged high risk adults during object naming is further complicated by evidence that APOE ε4 and family history (at least in middle-aged adults) interact and independently influence brain function in regions thought to be involved in AD pathology (Johnson et al 2007, Johnson et al 2006, Trivedi et al 2008, Xu et al 2009).
The purpose of the present study was to use functional magnetic resonance imaging (fMRI) to examine changes in the neural substrates underlying word retrieval in non-demented older adults at genetic risk for AD by virtue of the APOE ε4 allele. Because semantic knowledge and word-finding ability have been shown to deteriorate as much as episodic memory, and more than executive function, during the prodromal period of AD (Mickes et al 2007, Oldfield 1971), such changes may herald the onset of cognitive decline. Furthermore, group differences in functional activity in either frontal or inferior temporal regions would help clarify the neural substrates of word retrieval changes in at-risk older adults. Therefore, we hypothesized that APOE ε4 adults would show increased brain response in frontal regions, thought to reflect compensatory recruitment of executive control mechanisms underlying the ability to retrieve lexical information, but decreased response in inferior temporal regions consistent with dysfunction in posterior brain regions affected in early AD (Braak & Braak 1991).
Materials and Methods
Participants
Twenty-four healthy older adults from a larger pool of more than 80 volunteers enrolled in a longitudinal study of healthy aging participated. Participants were recruited through newspaper advertisements and community lectures (i.e., no clinic-based or medical referral sources). All participants were considered normal based on extensive medical, neurologic, laboratory, and neuropsychologic evaluations. All participants were native English speakers and strongly right-handed (Oldfield 1971). Potential participants were excluded if they reported a history of neurological disease, dementia or Mild Cognitive Impairment, cardiovascular disease, uncontrolled hypertension, or a Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition Axis 1 diagnosis of learning disability, attention deficit disorder, or substance abuse. In addition, participants were excluded if they had metal in their body other than dental fillings, reported poor visual acuity, or if they were taking prescription psychoactive medications. No participant reported a significant level of depressive symptoms on the Geriatric Depression Scale (i.e., GDS ≥ 10). Participants were instructed to abstain from caffeine the day of the scan. Of the 24 adults scanned, two individuals were excluded from further analysis due to excessive movement (i.e., met a priori criterion of excessive motion as determined by the number of outliers in the time series exceeding 1.2 standard deviations above the group mean as determined by AFNI’s 3dToutcount program).
All participants in the larger pool were genotyped for APOE using a polymerase chain reaction-based method (Saunders et al 1993). In a yoked sample design, the first 12 eligible ε4 adults and the first 12 demographically-matched eligible ε3 adults scheduled for their annual assessment were enrolled in the study. Of the 22 adults included in the study, 11 participants were ε3/ε3 homozygotes, 2 were ε4/ε4 homozygotes, and 9 were ε3/ε4 heterozygotes. As shown in Table 1, the APOE ε3 group and the APOE ε4 group did not differ significantly in age, level of education, sex distribution, or family history of Alzheimer’s disease (defined as having at least one first degree relative with AD). Moreover, on formal neuropsychological testing, the two groups did not differ in global cognitive functioning as assessed by the Mattis Dementia Rating Scale-II (DRS), in estimated premorbid verbal IQ as measured by the American version of the National Adult Reading Test (ANART), or in confrontation naming, verbal fluency, aspects of executive function, or memory measured by the Wechsler Memory Scale-Revised (WMS-R) or the California Verbal Learning Test (CVLT). Participants did not demonstrate deficits on cognitive screening or formal neuropsychological testing, nor did they demonstrate significant affective disturbance, functional impairment, or difficulties with activities of daily living (Table 1). Furthermore, groups did not differ on structural MR indices of whole brain, gray matter, white matter or hippocampal volumes, white matter lesion burden, or cortical thickness in frontal and temporal regions of interest (see Table 1). This research was approved by the Ethics Committee and Institutional Review Board at the University of California at San Diego and VA San Diego Healthcare System. Written informed consent was obtained from all participants according to guidelines established by the Declaration of Helsinki.
Table 1.
Demographics, neuropsychological, and brain structural characteristics of the APOE ε3 and ε4 groups
| Variables |
APOE |
|||||
|---|---|---|---|---|---|---|
| ε3, n = 11 | ε4, n = 11 | t or Χ2, value | p-value (two-tailed) | |||
| Demographics | ||||||
| Age, y | 77.55 | (6.01) | 78.64 | (7.16) | −0.39 | 0.70 |
| Education, y | 15.82 | (2.48) | 16.27 | (2.15) | −0.46 | 0.65 |
| Women/men | 6 | 5 | 5 | 6 | 0.18 | 0.67 |
| + FH AD/−FH AD | 7 | 4 | 6 | 5 | 0.19 | 0.67 |
| Global cognition | ||||||
| DRS total (144 points total) | 141.00 | (2.83) | 140.27 | (4.45) | 0.46 | 0.65 |
| ANART VIQ | 118.80 | (8.01) | 121.64 | (4.15) | −1.03 | 0.32 |
| Language | ||||||
| Boston Naming Test | 58.27 | (2.57) | 56.55 | (4.08) | 1.19 | 0.25 |
| Letter Fluency (FAS Total) | 44.45 | (14.34) | 49.00 | (9.87) | −0.87 | 0.40 |
| Category Fluency (animals) | 23.55 | (8.08) | 19.82 | (3.82) | 1.38 | 0.18 |
| Learning and memory | ||||||
| WMS-R LM immediate recall | 27.18 | (8.15) | 27.00 | (8.14) | 0.05 | 0.96 |
| WMS-R LM delayed recall | 23.82 | (10.27) | 23.73 | (8.43) | 0.02 | 0.98 |
| WMS-R VR immediate recall | 33.38 | (8.12) | 32.43 | (7.98) | 0.23 | 0.82 |
| WMS-R VR delayed recall | 30.25 | (7.94) | 29.43 | (9.23) | 0.19 | 0.86 |
| CVLT List 1–5 total recall | 49.00 | (13.23) | 46.90 | (11.59) | 0.36 | 0.70 |
| CVLT long delay free recall | 9.55 | (2.88) | 9.60 | (4.45) | −0.03 | 0.97 |
| CVLT recognition hits | 14 | (1.94) | 13.5 | (2.33) | 0.50 | 0.63 |
| Executive function | ||||||
| Trails B, seconds | 87.00 | (52.29) | 83.73 | (41.92) | 0.16 | 0.88 |
| WCST-48, categories | 5.50 | (1.27) | 5.45 | (0.82) | 0.10 | 0.92 |
| WCST-48, total errors | 6.20 | (8.88) | 7.18 | (5.17) | −0.31 | 0.76 |
| Emotional function | ||||||
| GDS | 3.30 | (3.65) | 2.27 | (2.53) | 0.76 | 0.46 |
| b Brain volume (Z-scores)* | ||||||
| Total brain volume | −0.06 | (1.04) | 0.06 | (0.83) | −0.29 | 0.77 |
| White matter | 0.29 | (0.90) | −0.29 | (0.89) | 1.44 | 0.17 |
| Gray matter | −0.28 | (0.95) | 0.28 | (0.84) | −1.41 | 0.18 |
| Hypointensities | 0.23 | (0.69) | −0.23 | (1.09) | 1.13 | 0.28 |
| Left hippocampus | 0.30 | (0.96) | −0.30 | (0.81) | 1.51 | 0.15 |
| Right hippocampus | 0.24 | (0.88) | −0.24 | (0.94) | 1.19 | 0.25 |
| c Cortical thickness (Z-scores)* | ||||||
| Left fusiform gyrus | 0.02 | (0.93) | −0.03 | (1.03) | 0.12 | 0.91 |
| Right fusiform gyrus | −0.16 | (1.06) | 0.19 | (0.80) | −0.81 | 0.43 |
| Left pars orbitalis | −0.06 | (0.58) | 0.07 | (1.31) | −0.29 | 0.77 |
| Right pars orbitalis | 0.00 | (0.53) | −0.00 | (1.33) | 0.02 | 0.99 |
| Left pars triangularis | −0.01 | (0.99) | 0.01 | (0.94) | −0.05 | 0.96 |
| Right pars triangularis | −0.33 | (0.63) | 0.41 | (1.14) | −1.8 | 0.08 |
| Left pars opercularis | −0.18 | (0.90) | 0.22 | (1.00) | −0.95 | 0.36 |
| Right pars opercularis | −0.24 | (0.94) | 0.30 | (0.92) | −1.29 | 0.21 |
| Left rostral anterior cingulate | −0.16 | (0.69) | 0.19 | (1.20) | −0.82 | 0.43 |
| Right rostral anterior cingulate | −0.15 | (0.88) | 0.18 | (1.05) | −0.75 | 0.46 |
| Left caudal anterior cingulate | −0.04 | (1.00) | 0.05 | (0.93) | −0.19 | 0.85 |
| Right caudal anterior cingulate | −0.16 | (0.91) | 0.20 | (1.00) | −0.86 | 0.40 |
statistically significant pairwise comparison: ε3< ε4 adults.
ε3 n=10, ε4 n=10
ε3 n=11, ε4 n=9
standardized residual z-scores after controlling for age, gender and intracranial volume for volume comparisons and controlling for age and gender for cortical thickness comparisons.
+FH AD = positive family history of Alzheimer’s disease; DRS = Dementia Rating Scale; ANART VIQ = American National Adult Reading Test Verbal Intelligence Quotient estimate; WMS-R = Wechsler Memory Scale-Revised, LM = Logical Memory subtest; VR = Visual Reproduction subtest; CVLT = California Verbal Learning Test; WCST-48 = Wisconsin Card Sorting Test-48 card version; GDS = Geriatric Depression Scale
Experimental Design and Procedure
FMRI naming task
Participants alternated between an overt picture naming task and a passive viewing task during four functional imaging runs (similar to the task previously reported by Wierenga and colleagues (Wierenga et al 2008, Wierenga et al 2009). During the visual naming task, 20 grayscale photographs of animals, 20 grayscale photographs of tools or implements, and 20 grayscale photographs of vehicles were presented for a total of 60 naming trials during the scanning session. Photographs were chosen based on the results of a previous study (Wierenga et al 2009) that assessed the amount of high spatial frequency content needed for object identification between categories. Photographs were equated for size and resolution. A training phase that used a different set of photographs preceded the experimental phase.
Pictures were presented one at a time for 3400 ms each, in an event-related format, and participants named each picture aloud. An event-related design was chosen to allow for overt responding so that performance accuracy and response latency could be assessed. Between trials, participants were instructed to rest quietly, and to look at abstract patterns derived by pixelating photographs from the naming task using Adobe PhotoShop 7.0, which served to randomize the pixels while maintaining image luminance. The interstimulus interval equaled a variable intertrial interval plus 3400 ms for each picture naming trial. Intertrial intervals were pseudorandomly varied between 13600 ms (8 images), 15300 ms (9 images), 17000 ms (10 images) and 18700 ms (11 images) to mitigate effects of periodic or quasi-periodic physiological noise, and to allow the hemodynamic response to return to baseline before the participant spoke again to prevent contamination of the latter part of the hemodynamic response by movement during the subsequent response. Experimental runs began and ended with a rest interval. There were 15 trials in each of the 4 experimental runs. Each 15-trial run was 323s in length and acquired 190 functional images for each slice. Stimuli were presented using E-Prime Version 1.1 software via an LCD projector that was back-projected onto a screen at the participant’s feet. Overt verbal responses were monitored using a bidirectional dual microphone (Resonance Technology, Inc.). Microphone output was run through the penetration panel into the scanner control room and connected to a Dell Inspiron Laptop Computer with Adobe Audition 1.5 software that recorded verbal responses from each scanning run. These responses were scored for accuracy and reaction time off-line.
Image acquisition
All data were acquired on a GE Signa Excite 3-T whole body system with a body transmit coil and an 8-channel receive-only head coil. Functional images were obtained with a 1-shot gradient echo EPI scan: 24 cm FOV, 64 × 64 matrix, 3.75 mm × 3.75 mm in-plane resolution, TR=1700 msec, TE= 30 msec, flip angle=70°. Twenty-eight 5 mm thick sagittal slices covering the whole brain were acquired. Two field maps were collected to correct for distortions in EPI images due to susceptibility artifact: 24 cm FOV, 64 × 64 matrix, 3.75 mm × 3.75 mm in-plane resolution, TR=1,000 msec, TE=minimum full (1st field map) or 5.5 (2nd field map), flip angle=60°, 28 5 mm thick sagittal slices covering the whole brain. A high resolution T1-weighted 3D MP-RAGE scan was obtained to provide anatomic reference: 26 cm FOV, 256 × 256 matrix, TR=7 msec, TE=min full, flip angle=8°, inversion recovery prepared: inversion time 900 msec, bandwidth=31.25 kHz, 170 1.2 mm sagittal slices. Head motion was minimized using foam padding.
Data analysis
Behavioral data
Accuracy and response latency measures from the overt naming task during fMRI were submitted to group (APOE ε3 vs. ε4) × category (animals, tools, vehicles) ANOVAs with paired t-tests used for post-hoc pair-wise comparisons.
Neuroimaging data
fMRI data were analyzed and overlaid onto structural images with the Analysis of Functional Neuroimaging (AFNI) software package from the National Institutes of Health (Cox 1996). To minimize the effects of head motion, each individual’s functional time series were corrected for motion by alignment to that base image which necessitated the least interpolation using a three-dimensional iterated, linearized, weighted least-squares method with Fourier interpolation. Following automated motion correction, the time series was examined for uncorrected motion outliers, and time-points with more than 10 times the mean number of outliers within a run were excluded from statistical analysis (via censor file). Slice timing correction was applied to the four imaging runs for the naming task and runs were detrended of low frequency signal drifts (Birn et al 2001) and concatenated into a single time series. Percent signal change from the mean was calculated. The association between measured BOLD signal and the object naming task was calculated with multiple regression using the program 3dDeconvolve. The following predictors were included in the model: a constant, a linear trend, three parameters indicating the degree of motion correction performed in three rotational angles, and stimulus functions indicating the initiation of the 3400 ms presentation of pictures of animals, tools, and vehicles to model the hemodynamic response for each category. The 3dDeconvolve command was repeated with a stimulus function indicating the initiation of the 3400 ms presentation of all pictures to model the hemodynamic response for object naming collapsed across category for within-group comparisons. For each voxel, the observed fMRI intensity time-series was modeled as the convolution of the experimental stimulus vector (comprised of 60 picture stimuli) and the estimated best-fit 11-lag impulse response using tent functions, allowing the hemodynamic response to return to baseline. Area under the curve (AUC) of the deconvolved hemodynamic response (HDR) was the dependent variable for group analyses. AUC was calculated by adding the deconvolved image intensity at each deconvolved time point of the impulse response. The first image following stimulus presentation, during which the participant responded overtly, was excluded to eliminate stimulus-correlated signal artifact (Carter et al 2000) since the vast majority of responses occurred within the first 1.7 seconds following stimulus presentation. Functional images were spatially smoothed with a Gaussian kernel of 4 mm full-width at half-maximum. The T1-weighted anatomic images and the AUC functional activation maps were warped to the coordinates of the co-planar stereotactic atlas of Talairach & Tournoux (Talairach J. 1988) and resampled at a 4 mm3 resolution.
Region-of-interest (ROI) analysis
Based on previous studies of word retrieval-related brain response among older adults, group differences were examined in a bilateral fusiform gyrus (FG) search ROI. We manually outlined each participant’s fusiform gyrus in order to increase the specificity in this small but important brain region. Left and right fusiform gyrus ROIs were drawn on each participant’s high-resolution MP-RAGE brain image in coronal view rotated into alignment with the anterior commissure-posterior commissure (AC-PC) plane. Following the guidelines of Lee et al. (Lee et al 2002) and Behrmann et al. (Behrmann et al 2007), the outlining began one slice posterior to the appearance of the mammillary body and continued posteriorly to a slice midway between the posterior commissure and the posterior end of the occipital lobe at the AC-PC level. The collateral and occipitotemporal sulci were used to determine the medial and lateral fusiform gyrus borders, respectively. In cases in which the sulci were interrupted or duplicated, the more laterally located sulcus was used as the border. Interrater reliability was computed for the FG ROI by 2 independent raters (C.W., S.D.) who were blind to group membership. Ten cases were selected randomly for interrater reliability. An intraclass correlation coefficient used to compute interrater reliability for the 2 raters was 0.86. A voxel was classified as falling within the fusiform gyrus if it was located in the fusiform gyrus in 7 out of 11 ε4 adults and 6 out of 11 ε3 adults. These thresholds were used because the resulting region represented >50% of the participants in each group and resulted in a cluster of relatively equal volume across groups; the conjunction of these two masks was then transformed into standard atlas space and used as the final mask. The blurred and standard-space transformed AUC images for each participant were masked with the resampled 4×4×4 mm bilateral FG ROI (volume = 20,352 mm3). Significant clusters resulting from within-group t-tests comparing object naming-related activity vs. baseline in both APOE ε3 and APOE ε4 adults separately were retained. Additionally, significant clusters for the main effect of group, the main effect of category, and the group × category interaction resulting from a random effects 2 group × 3 category ANOVA were retained. Clusters in the FG ROI were considered significant if each voxel was significant at p<0.05 (t>2.2, df=10 for within group comparisons, t>4.4, df=1,20 for main effect of group, t>3.2, df=2,40 for main effect of category and group × category interaction) and had a volume of at least 448 mm3. This threshold/volume combination was determined by Monte Carlo simulation (AlphaSim program) to protect ROI-wise probability of false positives of p<0.05. Effect sizes were calculated according to the following equation: eta-squared = (t2/(t2+df)) where t = t-value and df = degrees of freedom.
Whole brain analysis
As an exploratory analysis, we examined voxel-wise task-related whole brain response using within group t-tests and a 2 group × 3 category ANOVA with subjects as a random factor and AUC of the HDR as the dependent variable. Regions were considered activated if each voxel was significant at p<0.05 (t>2.2, df=10 for within group comparisons, t>4.35, df=1,20 for main effect of group, t>3.2, df=2,40 for main effect of category and group × category interaction) and the cluster had a volume of at least 1536 mm3. This threshold/volume combination protected a whole-brain probability of false positives of p<0.05.
Given that characteristics of the BOLD signal may differ between individuals due to potential changes in cerebrovascular dynamics, we also examined the temporal characteristics of the BOLD HDRs in significant clusters of activation in ε3 and ε4 older adults during word retrieval. We generated a mask of the significant clusters that showed differences in activity between groups at previously reported threshold/volume combinations protecting a whole-brain probability of false positives of p<0.05 and derived the HDR (based on percent signal change from baseline) for each participant and averaged across voxels. The averaged HDRs for each subject and cluster were entered into a 2 (group) × 10 (image number) repeated measures ANOVA to investigate differences in the time course of the HDR in those clusters that showed a significant difference in activation between ε3 and ε4 older adults. The first image was excluded from the group × time repeated measures ANOVA to remain consistent with the 2 group × 3 category ANOVAs for AUC that eliminated the first image to reduce motion artifact from overt speaking.
Correlations with performance
To aid interpretation of observed clusters of activity during object naming, we calculated the Pearson’s correlation between mean brain response (e.g., AUC) in clusters of significant activity and naming accuracy or response time.
Anatomical analysis
T1-weighted 3D MP-RAGE image files in DICOM format were transferred to a Linux workstation for morphometric analysis. Images were reviewed for quality, and automatically corrected for spatial distortion due to gradient nonlinearity (Jovicich et al 2006) and B1 field inhomogeneity, and were then rigid body registered to a probabilistic brain atlas. Volumetric segmentation (Fischl et al 2002, Fischl et al 2004) and cortical surface reconstruction (Dale & Sereno 1993, Dale et al 1999, Fischl et al 1999, Fischl et al 2004) methods based on FreeSurfer software were used. To measure thickness, the cortical surface was reconstructed (Dale & Sereno 1993, Dale et al 1999) and parcellated into distinct regions of interest (ROIs) (Buckner et al 2004). ANCOVAs were then conducted to assess group differences in brain volume or cortical thickness. Volumetric data were corrected for individual differences by regressing the estimated total intracranial volume (eTIV), age, and gender (Wierenga et al 2009) and then group comparisons were performed on the resultant standardized residual z-scores using independent samples t-tests. For group comparisons of cortical thickness, participant age and gender were included in the model as covariates.
Results
Behavioral results
Performance during the FMRI task is presented in Table 2. In terms of naming accuracy, there were no significant main effects of group [F(1,20) = 0.76, p = 0.40] or category [F(2,40) = 1.64, p = 0.21], and no interaction between group and category [F(2,40) = 1.02, p = 0.37]. In addition, there was no significant main effect of group [F(1,20) = 0.42, p = 0.53] and no group by category interaction effect [F(2,40) = 0.60, p = 0.56] for response time (RT) (correct responses only). However, RT for correctly identified objects differed significantly between categories when performance was collapsed across subjects [F(2,40) = 8.73, p = .00]. Participants responded more quickly to tools than vehicles [t = −4.27, p = 0.000] or animals [t = 3.49, p = 0.002], but responded similarly to vehicles and animals [t = 0.41, p = 0.69]. Since naming accuracy did not differ for animals, tools, or vehicles collapsed across subjects, level of naming performance is unlikely to have influenced FMRI comparisons of semantic category. Although RTs collapsed across participants differed significantly between categories, the differences were very small (e.g., less than 230 ms) and unlikely to have much bearing on the FMRI category comparisons.
Table 2.
Performance on the FMRI Naming Task for ε3 and ε4 Adults.
| Total |
Animals |
Tools |
Vehicles |
||||||
|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | ||
| Accuracy (%correct) | ε3 |
91.5 | (7.3) | 92.2 | (9.1) | 91.7 | (7.1) | 90.6 | (10.1) |
| ε4 | 93.8 | (4.5) | 92.9 | (7.0) | 96.8 | (3.4) | 91.7 | (6.7) | |
| Response Time (ms) | ε3 |
1647 | (241) | 1773 | (322) | 1490 | (184) | 1679 | (321) |
| ε4 | 1736 | (345) | 1786 | (394) | 1604 | (388) | 1816 | (358) | |
FMRI results
Fusiform gyrus ROI
Within group analysis
Significant clusters of task-related brain response within the FG ROI were found in both the ε3 and ε4 adults according to within-group t-tests comparing naming objects vs. viewing pixelated images (Table 3; Fig. 1). Both the ε3 and ε4 adults activated a cluster in the right medial FG, with the ε4 adults showing slightly more posterior activity. The ε4 adults demonstrated two additional clusters of activity in the left posterior lateral and anterior medial FG during object naming. Mean brain response in these clusters of differential activation was not significantly related to naming accuracy or response time in either group.
Table 3.
Clusters of significant brain response within the fusiform gyrus search region for object naming vs. passive viewing
| Direction of response | Hemisphere | Subregion (Brodmann’s area) | Volume (mm3) | Coordinates of maximum intensity voxel | t or F-score for maximum intensity voxel | Eta-squared (Mean±SEM) |
|---|---|---|---|---|---|---|
| APOE ε3 adults | ||||||
| Name > View | Ŧ ą L | C1. Medial fusiform gyrus (37) | 768 | 22L, 57P, 4I | 8.9 | 0.56±0.04 |
| APOE ε4 adults | ||||||
| Name > View | L | C1. Lateral posterior fusiform gyrus (37) | 1024 | 34L, 63P, 12I | 4.6 | 0.45±0.03 |
| R | C2. Medial fusiform gyrus (37) | 640 | 26R, 53P, 12I | 4.1 | 0.48±0.03 | |
| ą L | C3. Medial fusiform gyrus | 512 | 26L, 45P, 12I | 4.6 | 0.49±0.04 | |
| Main Effect of Group | ||||||
| APOE ε4 > APOE ε3 | R | C1. Medial fusiform gyrus (37) | 448 | 34R, 53P, 12I | 6.3 | 0.58±0.02 |
Clusters shown survived our cluster threshold alpha-protection procedure (p < 0.05, volume > 448 mm3, whole brain p < 0.05; see text for details). Note: L = left, R = right, A = anterior, P = posterior, S = superior, C = cluster.
= brain response negatively correlated with naming accuracy performance at trend level (r’s >0.38)
= brain response positively correlated with naming response time performance at trend level (r’s >0.38)
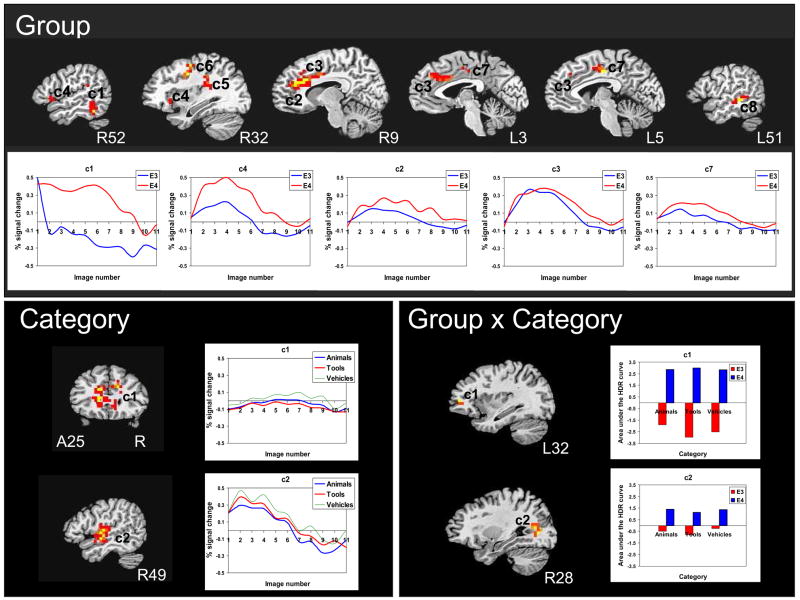
Figure 1.
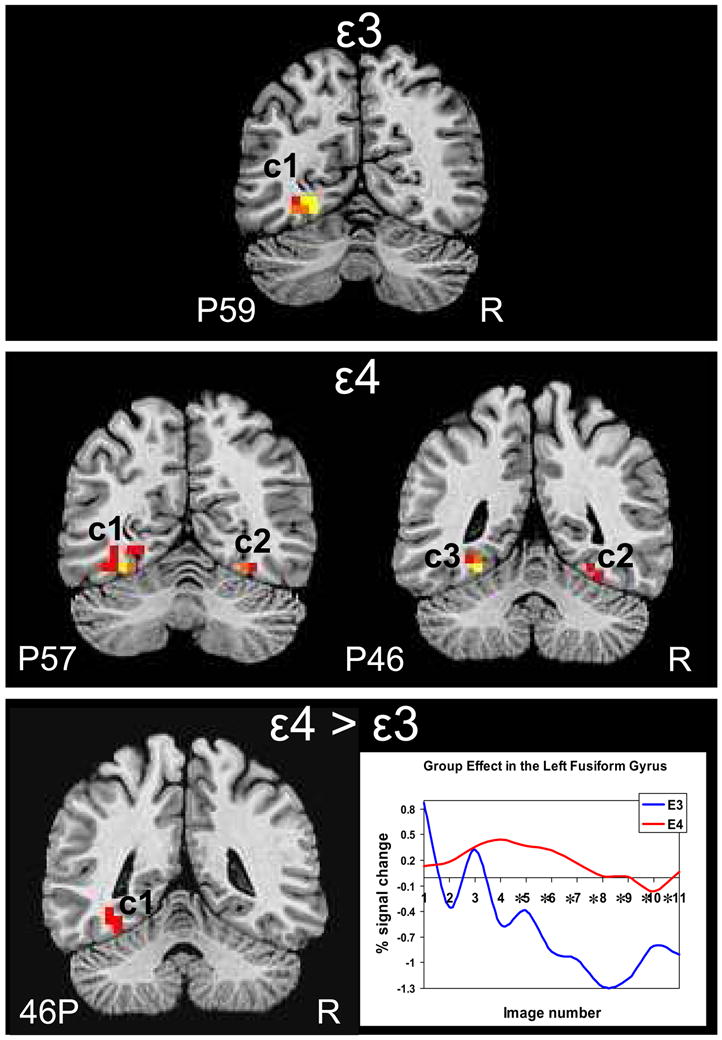
Brain response to object naming vs. viewing pixelated images in the fusiform gyrus ROI. Thresholded and clustered results (protecting an ROI-wise p≤0.05) for a single-sample t-test are presented in the top panel for the APOE ε3 participants and the middle panel for the APOE ε4 participants. Warm colors represent areas more active during object naming than passive viewing (red: p≤0.05, orange: p≤0.01, yellow: p≤0.005). The bottom panel shows the results of the group main effect of the 2 × 3 repeated measures ANOVA with the corresponding hemodynamic response function for each group. Red: p≤0.05. Asterisks indicate images numbers at which signal intensity differed significantly (p <0.05) between groups. Results are overlaid onto coronal slices of a high-resolution anatomical image (R: right, P: posterior).
Between group analysis
Results of the group × category ANOVA revealed a main effect of group in the left anterior medial FG with ε4 adults showing greater brain response than ε3 adults. Analysis of the temporal dynamics of the HDR revealed that the group × image number interaction was not significant for the left FG [F(9, 180) = 1.55, p = 0.13], although a visual examination of the response curves reveals strikingly different time courses between groups. The ε4 adults appear to exhibit a typical quadratic shape while the ε3 adults show predominantly negative signal change. No correlations were found between naming performance and brain response in the cluster that showed significant brain response according to the main effect of group. In contrast to our previous findings (Mickes et al 2007), no main effect of category or group × category interaction was found in the FG ROI.
Whole brain analysis
Within-group analysis
Whole brain voxel-wise within-group t-test analysis revealed several areas of task-related brain response (Table 4). Specifically, ε3 adults showed task-related activation when naming objects vs. viewing pixelated images in a large region that included the lateral and medial frontal cortex bilaterally, and in several smaller posterior regions that included the left perisylvian region, the lingual gyrus and occipital cortex bilaterally, the left precuneus and superior parietal lobe, and the left and right fusiform gyrus. In addition, they showed greater activity for viewing pixelated images (i.e., the baseline condition) than for object naming in regions of the right cingulate gyrus and right superior temporal gyrus. The ε4 adults showed a widespread task-related brain response that encompassed large regions of the frontal and temporal cortices bilaterally, and smaller clusters of activity localized to the right perisylvian cortex, bilateral cuneus, bilateral medial superior parietal cortex, right fusiform gyrus, right middle frontal gyrus, right culmen and left middle occipital gyrus. Greater activity for viewing pixelated images than for object naming was observed in the anterior aspect of the right superior temporal gyrus. In the ε3 group, task-related brain response in the right precentral gyrus was significantly negatively correlated with naming accuracy (r=−0.69, p=0.04), task-related brain response in the left precuneus/superior parietal lobe was significantly negatively correlated with response time (r=−0.77, p=0.02), and baseline-related brain response in the right cingulate gyrus was significantly positively correlated with naming accuracy (r=0.74, p=.02). In the ε4 group, task-related brain response in the right medial superior parietal cortex was significantly negatively correlated with naming accuracy (r=−0.64, p=0.03).
Table 4.
Clusters of significant brain response across the whole brain for within subject contrast of picture naming vs passive viewing and 2 group × 3 category ANOVA.
| Direction of response | Hemisphere | Subregion (Brodmann’s area) | Volume (mm3) | Coordinates of maximum intensity voxel | t or F-score for maximum intensity voxel | Eta-squared (Mean±SEM) |
|---|---|---|---|---|---|---|
| APOE ε3 adults | ||||||
| Name > View | ŧ L & R | L lateral frontal cortex, L & R medial frontal cortex | 76,864 | 38L, 15A, 28S | 7.3 | 0.49±0.00 |
| L & R | L & R lingual gyrus, occipital cortex | 4352 | 2R, 81P, 12I | 5.1 | 0.44±0.01 | |
| ąą R | R precentral gyrus | 2880 | 54R, 5P, 20S | 5.3 | 0.47±0.02 | |
| ŧŧĄ L | L precuneus, superior parietal lobe | 1856 | 30L, 61P, 32S | 3.6 | 0.39±0.01 | |
| L | L perisylvian cortex, superior temporal gyrus | 1792 | 58, 25, 4 | 4.2 | 0.41±0.02 | |
| R | R fusiform gyrus, lingual gyrus | 1536 | 18R, 65P, 8I | 4.8 | 0.44±0.02 | |
| L | L fusiform gyrus, lingual gyrus | 1536 | 22L, 57P, 4I | 8.9 | 0.51±0.03 | |
| View > Name | ||||||
| R | R cingulate gyrus | 2944 | 22R, 25P, 40S | −4.2 | 0.44±0.01 | |
| R | R superior temporal gyrus, caudate tail | 2880 | 22R, 37P, 12S | −4.2 | 0.42±0.01 | |
| ĄĄ R | R cingulate gyrus | 1728 | 10R, 13P, 28S | −5.9 | 0.50±0.02 | |
| APOE ε4 adults | ||||||
| Name > View | R & L | Left lateral frontal cortex, L superior parietal lobe, L & R medial frontal cortex, L & R inferior temporal cortex, L & R thalamus, R inferior frontal gyrus | 183,424 | 42L, 5P, 20S | 9.1 | 0.49±0.00 |
| R | R perisylvian cortex (superior temporal gyrus, precentral gyrus) | 7808 | 58R, 5P, 4S | 5.4 | 0.44±0.01 | |
| L & R | L & R cuneus | 3456 | 6R, 77P, 12S | 3.9 | 0.42±0.01 | |
| ąą R | R medial superior parietal cortex | 2688 | 10R, 37P, 64S | 5.9 | 0.45±0.01 | |
| Ŧ L | L medial superior parietal cortex | 2624 | 14L, 41P, 60S | 4.8 | 0.48±0.01 | |
| R | R fusiform gyrus | 2304 | 42R, 61P, 16I | 4.6 | 0.44±0.02 | |
| Ŧ R | R middle frontal gyrus | 1984 | 26R, 11A, 36S | 5.5 | 0.40±0.01 | |
| ą R | R culmen | 1920 | 34R, 45P, 36I | 3.6 | 0.41±0.01 | |
| L | L middle occipital gyrus | 1728 | 34L, 77P, 4S | 6.7 | 0.44±0.02 | |
| View > Name | ||||||
| R | R superior temporal gyrus, anterior aspect | 5312 | 26R, 15A, 24I | −25.7 | 0.29±0.00 | |
| Main Effect of Group | ||||||
| APOE ε4 > APOE ε3 | ||||||
| R | C1. Fusiform gyrus, inferior temporal gyrus, middle temporal gyrus, superior temporal gyrus (37) | 3008 | 50R, 45P, 16I | 12.27 | 0.62±0.02 | |
| R | C2. Anterior cingulate, rostral cingulate zone, pre-SMA (24, 6) | 2304 | 10R, 23A, 28S | 13.27 | 0.69±0.02 | |
| R & L | C3. Anterior cingulate, rostral cingulate zone, pre-SMA (24, 6) | 1984 | 2R, 35A, 32S | 9.08 | 0.63±0.02 | |
| R | C4. Inferior frontal gyrus, insula (47, 13) | 1856 | 38R, 11A, 4S | 27.29 | 0.66±0.02 | |
| R | C5. Insula, superior temporal gyrus, supramarginal gyrus, inferior parietal lobe (13, 41) | 1728 | 38R, 33P, 20S | 12.33 | 0.68±0.02 | |
| R | C6. Middle frontal gyrus, medial frontal gyrus, precentral gyrus (6) | 1664 | 18R, 13P, 60S | 17.92 | 0.73±0.02 | |
| L | C7. Cingulate gyrus, supplementary motor area (24, 6) | 1664 | 14L, 25P, 52S | 12.14 | 0.69±0.02 | |
| L | C8. Middle temporal gyrus, superior temporal gyrus, insula (22, 21, 13) | 1536 | 50L, 33P, 0 | 12.17 | 0.66±0.02 | |
| Main Effect of Category | ||||||
| R & L | C1. Anterior cingulate, posterior cingulate (24, 31, 32) | 71488 | 6L, 13P, 24S | 59.61 | 0.65±0.01 | |
| R | C2. Superior temporal gyrus, insula (21, 22, 41, 42, 13) | 6080 | 46R, 5P, 4I | 9.90 | 0.52±0.01 | |
| Interaction of Group X Category | ||||||
| L | C1. Superior frontal gyrus, middle frontal gyrus, anterior cingulate (10, 24) | 2176 | 22L, 51A, 12S | 7.53 | 0.49±0.02 | |
| R | C2. Posterior cingulate, middle occipital gyrus (31) | 1664 | 30R, 69P, 4S | 6.81 | 0.50±0.02 | |
Clusters shown survived our cluster threshold alpha-protection procedure (p < 0.05, volume > 1536 mm3, whole brain p < 0.05; see text for details). Note: L = left, R = right, A = anterior, P = posterior, S = superior, C = cluster.
= brain response positively correlated with naming accuracy performance (p<0.05)
= brain response positively correlated with naming accuracy performance at trend level (r’s >0.38)
= brain response negatively correlated with naming accuracy performance (p<0.05)
= brain response negatively correlated with naming accuracy performance at trend level (r’s >0.38)
= brain response positively correlated with naming response time performance at trend level (r’s >0.38)
= brain response negatively correlated with naming response time performance (p<0.05)
= brain response negatively correlated with naming response time performance at trend level (r’s >0.38)
Between group analysis
Results of the whole-brain voxel-wise group × category ANOVA revealed a main effect of group in 1) the right fusiform gyrus extending to the inferior, middle and superior temporal gyri, 2) regions of the right and left anterior cingulate extending to the rostral cingulate zone, pre-SMA, and SMA, 3) the right inferior frontal gyrus (BA 47), 4) the right insula extending to the superior temporal gyrus and supramarginal gyrus, 5) the right middle frontal gyrus, and 6) the left middle temporal gyrus extending to the superior temporal gyrus and insula (Table 4, Fig. 2). In all of these regions, the ε4 adults showed greater activity than the ε3 adults. A main effect of category was found in a large cluster extending the length of the cingulate gyrus (vehicles greater than animals and tools, though small signal change) and in the right superior temporal gyrus (vehicles greater than animals greater than tools). A group × category interaction was found in the left superior frontal gyrus extending to the middle frontal gyrus and anterior cingulate (BAs 10, 24) whereby the ε4 group showed greater brain response for tools compared to animals and vehicles and the ε3 group showed a negative brain response with greater response to animals than vehicles and tools. A group × category interaction effect was also found in the posterior cingulate (BA 31), whereby ε4 adults showed greater brain response to animals and vehicles compared to tools and ε3 adults showed a negative brain response with activity greater for vehicles compared to animals and tools. No correlations were found between naming performance and brain response in clusters that showed significant activation in main effects of group or category.
Figure 2.
Whole brain response to object naming vs. passive viewing overlaid onto sagittal slices of a high-resolution anatomical image. Thresholded and clustered results (protecting an ROI-wise p≤0.05; red: p ≤0.05, orange: p ≤0.01, yellow: p≤0.005) for the 2 group × 3 category repeated measures ANOVA are presented in the top panel for the main effect of group (with corresponding hemodynamic response functions for each group), the lower left panel for the main effect of category (with corresponding hemodynamic response functions for each category), and the lower right panel for the interaction of group × category with a graphical representation of the AUC for each category per group (R: right, L: left).
Anatomical results
Anatomical image data from two participants (one APOE ε4 and one APOE ε3 adult) did not pass quality control analysis for inclusion in the group analysis of brain volume. In addition, anatomical image data from two different participants (both APOE ε4) did not pass quality control analysis for inclusion in the group comparison of cortical thickness. These participants were excluded from further anatomical analysis. After controlling for total intracranial volume, age, and gender, no group differences were found for brain volume in any ROIs. The ε4 and ε3 groups did not differ in total brain volume, cerebral gray matter, cerebral white matter, white matter lesions, right hippocampal volume, or left hippocampal volume (Table 1). Furthermore, after controlling for age and gender, groups did not differ on measures of cortical thickness in regions of interest that included the left and right fusiform gyrus, left and right pars orbitalis, left and right pars opercularis, left and right pars triangularis, left and right caudal anterior cingulate, and left and right rostral anterior cingulate (Table 1).
Discussion
In light of recent findings that decline in aspects of language (e.g., vocabulary, naming, category fluency) can be as severe as decline in episodic memory in the years prior to a dementia diagnosis (Mickes et al 2007), the present study sought to elucidate differences in brain response to word retrieval and semantic memory in cognitively intact older adults with (APOE ε4 allele) or without (no ε4 allele) a genetic risk for AD. An object naming task that involved a semantic manipulation (e.g., required naming living and nonliving items from 3 categories controlled for visual attributes) was utilized in order to determine whether word retrieval problems in at-risk adults stem from changes in the neural substrates subserving the ability to access and retrieve lexical information or from changes in brain regions supporting the semantic stores themselves. A major finding of the current study is that APOE ε4 adults exhibited greater object naming-related brain response than APOE ε3 adults in a fusiform gyrus search region of interest, and in the left inferior temporal lobe (including fusiform gyrus), right perisylvian region (including the insula, inferior frontal gyrus, superior temporal gyrus and inferior parietal lobe), and medial prefrontal cortex bilaterally (including anterior cingulate, rostral cingulate zone, pre-SMA and SMA). This greater brain response in ε4 than in ε3 adults occurred in the context of equivalent naming accuracy and response time during the fMRI task, which suggests that differences in brain response are not due to differences in naming performance. Furthermore, the ε4 and ε3 groups were equated in age, level of education, family history of AD, sex, and performance on a comprehensive neuropsychological assessment battery, thereby reducing the likelihood that these factors contributed to group differences in brain response. The groups also did not differ in brain volume or cortical thickness in any of the regions that were assessed, suggesting that functional changes were not directly related to morphological differences between the groups. Finally, within group analyses that compared task (object naming) to baseline (viewing pixilated images) provided essential confirmation that activity associated with naming occurred in expected regions (including frontal and inferior temporal cortices).
While the present findings support our hypothesis that older individuals at risk for AD (i.e., with an ε4 allele) would show a compensatory increase (relative to ε3 adults) in activation in frontal regions during object naming, they do not support our hypothesis of a decrease in activation in inferior temporal lobe regions due to early AD changes. On the contrary, both ε3 and ε4 adults showed right medial fusiform gyrus activation during object naming compared to when viewing pixelated images (with ε4 adults activating both anterior and posterior aspects), but the ε4 adults showed additional activation in regions of the left medial and lateral fusiform that were not activated in the ε3 group. Activation in the fusiform gyrus was not significantly correlated with naming accuracy or response time for either group.
The discrepancy between the current results and previous results that showed neural specialization for living and nonliving stimuli in the fusiform gyrus (Chao et al 1999, Ishai et al 1999, Mahon et al 2009) may be due to inclusion of only older adults in the present study, or to reduced power to detect signal differences due to fewer stimuli or a smaller sample size. Post-hoc within group whole brain voxel-wise paired t-tests comparing animals and tools showed a region of the right medial fusiform gyrus and parahippocampal gyrus, extending to the middle occipital gyrus, that was more responsive to animals than tools in the ε3 group, but no difference between animals and tools was found for the ε4 group. The lack of the expected category distinction in the fusiform gyrus for the ε4 adults might reflect changes in neural specialization for semantic information, but the inconsistent results for the ε3 group weakens this argument and indicates that further research is needed to understand the neural substrates of semantic memory in those at risk for AD.
The expectation of diminished brain response in the fusiform gyrus of the ε4 group rested in part on the assumption of greater underlying AD pathology in this region. However, recent work has shown that regions susceptible to early AD pathology are in a particularly dynamic state of change across the spectrum of declining cognition (Dickerson et al 2005). For example, older adults in the early stages of MCI showed increased fMRI brain response in the medial temporal lobes during episodic encoding, whereas those in the later stages of MCI (i.e., nearing progression to AD) showed decreased brain response in these same brain regions. These findings suggest that brain regions particularly susceptible to AD pathology may be those most likely to engage dynamic compensatory mechanisms, and the increased BOLD response observed in the fusiform regions in the ε4 carriers in the present study is consistent with this possibility. An interaction of group by category was seen in a left frontal region (including superior and middle frontal gyri and anterior cingulate) and in the right posterior cingulate, regions that have previously been implicated in early AD (Reiman et al 1996, Reiman et al 2004). Notably, no interaction of group by category was found in previous comparisons of young versus older adults (Wierenga et al 2008). If viewed as a continuum, this suggests that more widespread group × category interactions may be seen in early AD.
The greater signal for object naming collapsed across category exhibited by the ε4 adults in the left cingulate gyrus and right anterior cingulate (BA 32) is consistent with the increased frontal activity observed with normal aging. This suggests that ε4 adults may require more resources than ε3 adults to perform internally generated tasks that place demands on executive functions involved in accessing and manipulating verbal information. Furthermore, greater right perisylvian activation that extended to the IFG (BA 47) in ε4 adults is consistent with Cabeza’s (Cabeza 2002) hemispheric asymmetry reduction in old adults (HAROLD) model. Such activity in contralateral areas in the right hemisphere has traditionally been thought to reflect additional neurocognitive effort to maintain an equivalent level of performance. We previously found that “high” performing older adults showed a positive correlation between accuracy and BOLD response in both the left and right inferior frontal gyrus (BA 47, 45), suggesting that the ability to recruit contralateral regions of the inferior frontal gyrus (in the right hemisphere), including Broca’s area homologue and pars orbitalis, assists in object naming performance in older adults. Increased activity in BA 47 may reflect the need for more effortful semantic retrieval in ε4 adults(Hernandez 2009). This finding provides further support for frontally-mediated compensatory mechanisms in those at risk for AD and generally concurs with the notion that executive functions may be better preserved than episodic or semantic memory functions during the prodromal period of AD (Mickes et al 2007).
Given that the majority of fMRI studies of cognition in non-demented APOE ε4 older adults have investigated episodic memory performance (Bondi et al 2005, Bookheimer et al 2000, Han et al 2007, Stoub et al 2006), a unique contribution of the current study is the examination of another cognitive domain—namely language and semantic memory—affected early in the disease process. Overall, findings of the current study are consistent with previous reports of increased BOLD response in APOE ε4 older adults. However, there are some general limitations of the current study. Although we refer to ε4 adults as at-risk for AD, it is unclear whether their differential brain response (compared to ε3 adults) indicates prodromal AD or is simply a genetic phenotype. Unfortunately, this limitation plagues all studies of genetic susceptibility that do not follow their subjects longitudinally to determine who among the sample ultimately progresses to AD. It could also be argued that our sample, given its older age, may not represent all ε4 carriers or even those ε4 carriers at risk for AD, but rather those who managed to avoid cognitive decline (perhaps for other genetic or lifestyle reasons) due to incident AD. However, this premise does not bear out unequivocally, as the finding of an effect of APOE ε4 on age at onset of AD has not been universally replicated—Dal Forno et al. (Dal Forno et al 1996) and Bennett et al. (Bennett et al 1995) fail to confirm this notion. We argue that, against the backdrop of completely cognitively normal older adults, the APOE ε4 effect is isolated even more.
It must also be acknowledged that the ability to discriminate changes in activity that represent differences in cognitive function from those that represent physiological change due to possession of the APOE ε4 allele is a challenge for fMRI research, especially since neurovascular coupling processes may change with age or disease risk (Buckner et al 2000, D’Esposito et al 2003). We attempted to decrease the possibility of confounding compromised vascular responses with changes in cognitive processing by excluding individuals with cerebrovascular disease. Furthermore, groups did not differ on quantitative measures of cerebrovascular integrity (e.g., white matter hyperintensities) or the temporal characteristics of the hemodynamic response curve in the activated clusters that were examined. We also measured resting cerebral blood flow in the majority of the participants studied (data presented elsewhere), and groups did not differ in average whole brain gray matter cerebral blood flow (CBF) [t(18) = −1.33, p = 0.20], although there was a trend for the APOE ε4 adults to show slightly greater CBF (ε4 mean CBF = 57.4 ml/100g/min; ε3 mean CBF = 46.7 ml/100g/min). In healthy brain function the BOLD signal is negatively correlated with CBF (i.e., increased CBF corresponds to decreased BOLD) (Cohen et al 2004) and we have previously suggested that low cerebral perfusion may lead to exaggerated BOLD responses in brain regions affected by prodromal AD processes (Bangen et al 2009, Restom et al 2007). If found, this relationship would weaken the argument for neural compensatory processes since it suggests that BOLD and cerebral perfusion would be confounded. In our sample, however, the finding that ε4 adults showed both increased CBF (though nonsignificant) and increased BOLD response supports the neural compensation hypothesis and suggests that group differences in vascular response likely did not contribute to the BOLD effects. It should be noted, however, that inclusion of direct measures of vascular responsiveness (e.g., hypercapnia) is needed to adequately assess changes in the physiological basis of group differences in the BOLD response.
Lastly, the current study examined the BOLD response as it related to individual performance variables, but few regions showed a correlation between behavioral performance and brain response. This lack of correlation may be due to a restricted range of scores for the performance measures that occurred because cognitively impaired individuals were excluded and task demands were relatively simple. Despite these limitations, there are only a few studies to date that have investigated language processing in dementia risk and to our knowledge this is the first study to examine changes in the neural substrates of object naming in older adults with the APOE ε4 allele. This is an area of study that has been overlooked despite research that shows that anomia and semantic impairments are some of the earliest signs of AD. (Chertkow & Bub 1990, Jacobs et al 1995).
In summary, the more widespread--predominantly frontal--increased brain response during object naming in ε4 older adults than in ε3 older adults appears more similar to the brain response seen in aging than in AD. However, more widespread activation in posterior regions in ε4 than in ε3 adults, as well as group differences in response to categories in regions involved in AD (e.g., anterior and posterior cingulate), may be indicative of early semantic involvement that could reflect prodromal AD. Further research is needed to elucidate the specific posteriorly-mediated semantic processes involved in word retrieval difficulties in at-risk older adults.
Acknowledgments
This work was supported by grant IIRG 07-59343 from the Alzheimer’s Association (M.W.B.), National Institute on Aging grants R01 AG012674 (M.W.B.), K24 AG026431 (M.W.B.) and P50 AG05131 (D.P.S.), National Institutes of Health Ruth L. Kirschstein National Research Service Award MH18399-20 (C.E.W.) and NINDS F31 NS059193 (K.J.B.). The authors thank Sheena Dev for her assistance with reliability analysis of the fusiform ROI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adlam AL, Bozeat S, Arnold R, Watson P, Hodges JR. Semantic knowledge in mild cognitive impairment and mild Alzheimer’s disease. Cortex. 2006;42:675–84. doi: 10.1016/s0010-9452(08)70404-0. [DOI] [PubMed] [Google Scholar]
- Albert MS, Moss MB, Tanzi R, Jones K. Preclinical prediction of AD using neuropsychological tests. J Int Neuropsychol Soc. 2001;7:631–9. doi: 10.1017/s1355617701755105. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, et al. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–35. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashford JW, Mortimer JA. Non-familial Alzheimer’s disease is mainly due to genetic factors. J Alzheimers Dis. 2002;4:169–77. doi: 10.3233/jad-2002-4307. [DOI] [PubMed] [Google Scholar]
- Backman L, Jones S, Berger AK, Laukka EJ, Small BJ. Multiple cognitive deficits during the transition to Alzheimer’s disease. J Intern Med. 2004;256:195–204. doi: 10.1111/j.1365-2796.2004.01386.x. [DOI] [PubMed] [Google Scholar]
- Backman L, Jones S, Berger AK, Laukka EJ, Small BJ. Cognitive impairment in preclinical Alzheimer’s disease: a meta-analysis. Neuropsychology. 2005;19:520–31. doi: 10.1037/0894-4105.19.4.520. [DOI] [PubMed] [Google Scholar]
- Bangen KJ, Restom K, Liu TT, Jak AJ, Wierenga CE, et al. Differential age effects on cerebral blood flow and BOLD response to encoding: associations with cognition and stroke risk. Neurobiol Aging. 2009;30:1276–87. doi: 10.1016/j.neurobiolaging.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Sabb FW, Noll DC. Anterior cingulate and the monitoriing of response conflict: evidence from an fMRI study of overt verb generation. J Cogn Neurosci. 2000;12:298–309. doi: 10.1162/089892900562110. [DOI] [PubMed] [Google Scholar]
- Bayles KA, Tomoeda CK. Confrontation naming impairment in dementia. Brain Lang. 1983;19:98–114. doi: 10.1016/0093-934x(83)90057-3. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Avidan G, Gao F, Black S. Structural imaging reveals anatomical alterations in inferotemporal cortex in congenital prosopagnosia. Cereb Cortex. 2007;17:2354–63. doi: 10.1093/cercor/bhl144. [DOI] [PubMed] [Google Scholar]
- Bennett C, Crawford F, Osborne A, Diaz P, Hoyne J, et al. Evidence that the APOE locus influences rate of disease progression in late onset familial Alzheimer’s Disease but is not causative. Am J Med Genet. 1995;60:1–6. doi: 10.1002/ajmg.1320600102. [DOI] [PubMed] [Google Scholar]
- Birn RM, Saad ZS, Bandettini PA. Spatial heterogeneity of the nonlinear dynamics in the FMRI BOLD response. Neuroimage. 2001;14:817–26. doi: 10.1006/nimg.2001.0873. [DOI] [PubMed] [Google Scholar]
- Bloss CS, Delis DC, Salmon DP, Bondi MW. Decreased cognition in children with risk factors for Alzheimer’s disease. Biol Psychiatry. 2008;64:904–6. doi: 10.1016/j.biopsych.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi MW, Houston WS, Eyler LT, Brown GG. fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology. 2005;64:501–8. doi: 10.1212/01.WNL.0000150885.00929.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi MW, Salmon DP, Galasko D, Thomas RG, Thal LJ. Neuropsychological function and apolipoprotein E genotype in the preclinical detection of Alzheimer’s disease. Psychol Aging. 1999;14:295–303. doi: 10.1037//0882-7974.14.2.295. [DOI] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, et al. Patterns of brain activation in people at risk for Alzheimer’s disease. N Engl J Med. 2000;343:450–6. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Bretsky P, Guralnik JM, Launer L, Albert M, Seeman TE. The role of APOE-epsilon4 in longitudinal cognitive decline: MacArthur Studies of Successful Aging. Neurology. 2003;60:1077–81. doi: 10.1212/01.wnl.0000055875.26908.24. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–38. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Sanders AL, Raichle ME, Morris JC. Functional brain imaging of young, nondemented, and demented older adults. J Cogn Neurosci. 2000;12(Suppl 2):24–34. doi: 10.1162/089892900564046. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, et al. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci U S A. 2000;97:1944–8. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LL, Haxby JV, Martin A. Attribute-based neural substrates in temporal cortex for perceiving and knowing about objects. Nat Neurosci. 1999;2:913–9. doi: 10.1038/13217. [DOI] [PubMed] [Google Scholar]
- Chertkow H, Bub D. Semantic memory loss in dementia of Alzheimer’s type. What do various measures measure? Brain. 1990;113 (Pt 2):397–417. doi: 10.1093/brain/113.2.397. [DOI] [PubMed] [Google Scholar]
- Cohen ER, Rostrup E, Sidaros K, Lund TE, Paulson OB, et al. Hypercapnic normalization of BOLD fMRI: comparison across field strengths and pulse sequences. Neuroimage. 2004;23:613–24. doi: 10.1016/j.neuroimage.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crosson B, Moore AB, Gopinath K, White KD, Wierenga CE, et al. Role of the right and left hemispheres in recovery of function during treatment of intention in aphasia. J Cogn Neurosci. 2005;17:392–406. doi: 10.1162/0898929053279487. [DOI] [PubMed] [Google Scholar]
- Cuetos F, Arango-Lasprilla JC, Uribe C, Valencia C, Lopera F. Linguistic changes in verbal expression: a preclinical marker of Alzheimer’s disease. J Int Neuropsychol Soc. 2007;13:433–9. doi: 10.1017/S1355617707070609. [DOI] [PubMed] [Google Scholar]
- Dal Forno G, Rasmusson DX, Brandt J, Carson KA, Brookmeyer R, et al. Apolipoprotein E genotype and rate of decline in probable Alzheimer’s disease. Arch Neurol. 1996;53:345–50. doi: 10.1001/archneur.1996.00550040085017. [DOI] [PubMed] [Google Scholar]
- Dale A, Sereno MI. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction--A linear-approach. Journal of Cognitive Neuroscience. 1993;5:162–76. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat Rev Neurosci. 2003;4:863–72. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65:404–11. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong A, Whitehead V, Hanratty K, Chertkow H. The nature of lexico-semantic processing deficits in mild cognitive impairment. Neuropsychologia. 2006;44:1928–35. doi: 10.1016/j.neuropsychologia.2006.01.034. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD, Poldrack RA, Desmond JE. The role of left prefrontal cortex in language and memory. Proc Natl Acad Sci U S A. 1998;95:906–13. doi: 10.1073/pnas.95.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SD, Houston WS, Jak AJ, Eyler LT, Nagel BJ, et al. Verbal paired-associate learning by APOE genotype in non-demented older adults: fMRI evidence of a right hemispheric compensatory response. Neurobiol Aging. 2007;28:238–47. doi: 10.1016/j.neurobiolaging.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez AE. Language switching in the bilingual brain: what’s next? Brain Lang. 2009;109:133–40. doi: 10.1016/j.bandl.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Graham N, Patterson K. Charting the progression in semantic dementia: implications for the organisation of semantic memory. Memory. 1995;3:463–95. doi: 10.1080/09658219508253161. [DOI] [PubMed] [Google Scholar]
- Ishai A, Ungerleider LG, Martin A, Schouten JL, Haxby JV. Distributed representation of objects in the human ventral visual pathway. Proc Natl Acad Sci U S A. 1999;96:9379–84. doi: 10.1073/pnas.96.16.9379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs DM, Sano M, Dooneief G, Marder K, Bell KL, Stern Y. Neuropsychological detection and characterization of preclinical Alzheimer’s disease. Neurology. 1995;45:957–62. doi: 10.1212/wnl.45.5.957. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Ries ML, Hess TM, Carlsson CM, Gleason CE, et al. Effect of Alzheimer disease risk on brain function during self-appraisal in healthy middle-aged adults. Arch Gen Psychiatry. 2007;64:1163–71. doi: 10.1001/archpsyc.64.10.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Schmitz TW, Moritz CH, Meyerand ME, Rowley HA, et al. Activation of brain regions vulnerable to Alzheimer’s disease: the effect of mild cognitive impairment. Neurobiol Aging. 2006;27:1604–12. doi: 10.1016/j.neurobiolaging.2005.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorm AF, Mather KA, Butterworth P, Anstey KJ, Christensen H, Easteal S. APOE genotype and cognitive functioning in a large age-stratified population sample. Neuropsychology. 2007;21:1–8. doi: 10.1037/0894-4105.21.1.1. [DOI] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, et al. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage. 2006;30:436–43. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Kraut MA, Cherry B, Pitcock JA, Vestal L, Henderson VW, Hart J., Jr The Semantic Object Retrieval Test (SORT) in normal aging and Alzheimer disease. Cogn Behav Neurol. 2006;19:177–84. doi: 10.1097/01.wnn.0000213922.41008.22. [DOI] [PubMed] [Google Scholar]
- Lee CU, Shenton ME, Salisbury DF, Kasai K, Onitsuka T, et al. Fusiform gyrus volume reduction in first-episode schizophrenia: a magnetic resonance imaging study. Arch Gen Psychiatry. 2002;59:775–81. doi: 10.1001/archpsyc.59.9.775. [DOI] [PubMed] [Google Scholar]
- Lind J, Ingvar M, Persson J, Sleegers K, Van Broeckhoven C, et al. Parietal cortex activation predicts memory decline in apolipoprotein E-epsilon4 carriers. Neuroreport. 2006;17:1683–6. doi: 10.1097/01.wnr.0000239954.60695.c6. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–8. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Huang Y. Apolipoprotein E: from atherosclerosis to Alzheimer’s disease and beyond. Curr Opin Lipidol. 1999;10:207–17. doi: 10.1097/00041433-199906000-00003. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Huang Y. Apolipoprotein (apo) E4 and Alzheimer’s disease: unique conformational and biophysical properties of apoE4 can modulate neuropathology. Acta Neurol Scand Suppl. 2006;185:8–14. doi: 10.1111/j.1600-0404.2006.00679.x. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Huang Y. Alzheimer disease: multiple causes, multiple effects of apolipoprotein E4, and multiple therapeutic approaches. Ann Neurol. 2009;65:623–5. doi: 10.1002/ana.21736. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Nathan BP, Pitas RE. Apolipoprotein E. Structure, function, and possible roles in Alzheimer’s disease. Ann N Y Acad Sci. 1996;777:139–45. doi: 10.1111/j.1749-6632.1996.tb34412.x. [DOI] [PubMed] [Google Scholar]
- Mahon BZ, Anzellotti S, Schwarzbach J, Zampini M, Caramazza A. Category-specific organization in the human brain does not require visual experience. Neuron. 2009;63:397–405. doi: 10.1016/j.neuron.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickes L, Wixted JT, Fennema-Notestine C, Galasko D, Bondi MW, et al. Progressive impairment on neuropsychological tasks in a longitudinal study of preclinical Alzheimer’s disease. Neuropsychology. 2007;21:696–705. doi: 10.1037/0894-4105.21.6.696. [DOI] [PubMed] [Google Scholar]
- Miller KJ, Rogers SA, Siddarth P, Small GW. Object naming and semantic fluency among individuals with genetic risk for Alzheimer’s disease. Int J Geriatr Psychiatry. 2005;20:128–36. doi: 10.1002/gps.1262. [DOI] [PubMed] [Google Scholar]
- Mondadori CR, de Quervain DJ, Buchmann A, Mustovic H, Wollmer MA, et al. Better Memory and Neural Efficiency in Young Apolipoprotein E {varepsilon}4 Carriers. Cereb Cortex. 2006 doi: 10.1093/cercor/bhl103. [DOI] [PubMed] [Google Scholar]
- Mondadori CR, de Quervain DJ, Buchmann A, Mustovic H, Wollmer MA, et al. Better memory and neural efficiency in young apolipoprotein E epsilon4 carriers. Cereb Cortex. 2007;17:1934–47. doi: 10.1093/cercor/bhl103. [DOI] [PubMed] [Google Scholar]
- Nebes RD. Semantic memory in Alzheimer’s disease. Psychol Bull. 1989;106:377–94. doi: 10.1037/0033-2909.106.3.377. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Powell MR, Smith GE, Knopman DS, Parisi JE, Boeve BF, et al. Cognitive measures predict pathologic Alzheimer disease. Arch Neurol. 2006;63:865–8. doi: 10.1001/archneur.63.6.865. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, et al. Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334:752–8. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci U S A. 2004;101:284–9. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restom K, Bangen KJ, Bondi MW, Perthen JE, Liu TT. Cerebral blood flow and BOLD responses to a memory encoding task: a comparison between healthy young and elderly adults. Neuroimage. 2007;37:430–9. doi: 10.1016/j.neuroimage.2007.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen VM, Sunderland T, Levy J, Harwell A, McGee L, et al. Apolipoprotein E and category fluency: evidence for reduced semantic access in healthy normal controls at risk for developing Alzheimer’s disease. Neuropsychologia. 2005;43:647–58. doi: 10.1016/j.neuropsychologia.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43:1467–72. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- Small BJ, Mobly JL, Laukka EJ, Jones S, Backman L. Cognitive deficits in preclinical Alzheimer’s disease. Acta Neurol Scand Suppl. 2003;179:29–33. doi: 10.1034/j.1600-0404.107.s179.6.x. [DOI] [PubMed] [Google Scholar]
- Smith CD, Andersen AH, Kryscio RJ, Schmitt FA, Kindy MS, et al. Women at risk for AD show increased parietal activation during a fluency task. Neurology. 2002;58:1197–202. doi: 10.1212/wnl.58.8.1197. [DOI] [PubMed] [Google Scholar]
- Smith CD, Kryscio RJ, Schmitt FA, Lovell MA, Blonder LX, et al. Longitudinal functional alterations in asymptomatic women at risk for Alzheimer’s disease. J Neuroimaging. 2005;15:271–7. doi: 10.1177/1051228405277340. [DOI] [PubMed] [Google Scholar]
- Smith CD, Lori NF, Akbudak E, Sorar E, Gultepe E, et al. MRI diffusion tensor tracking of a new amygdalo-fusiform and hippocampo-fusiform pathway system in humans. J Magn Reson Imaging. 2009;29:1248–61. doi: 10.1002/jmri.21692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storandt M, Grant EA, Miller JP, Morris JC. Longitudinal course and neuropathologic outcomes in original vs revised MCI and in pre-MCI. Neurology. 2006;67:467–73. doi: 10.1212/01.wnl.0000228231.26111.6e. [DOI] [PubMed] [Google Scholar]
- Stoub TR, deToledo-Morrell L, Stebbins GT, Leurgans S, Bennett DA, Shah RC. Hippocampal disconnection contributes to memory dysfunction in individuals at risk for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2006;103:10041–5. doi: 10.1073/pnas.0603414103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach JTP. Co-Planar stereotaxic atlas of the human brain. New York: Thiem Medical Publishers; 1988. [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc Natl Acad Sci U S A. 1997;94:14792–7. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiraboschi P, Hansen LA, Masliah E, Alford M, Thal LJ, Corey-Bloom J. Impact of APOE genotype on neuropathologic and neurochemical markers of Alzheimer disease. Neurology. 2004;62:1977–83. doi: 10.1212/01.wnl.0000128091.92139.0f. [DOI] [PubMed] [Google Scholar]
- Trivedi MA, Schmitz TW, Ries ML, Hess TM, Fitzgerald ME, et al. fMRI activation during episodic encoding and metacognitive appraisal across the lifespan: risk factors for Alzheimer’s disease. Neuropsychologia. 2008;46:1667–78. doi: 10.1016/j.neuropsychologia.2007.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twamley EW, Ropacki SA, Bondi MW. Neuropsychological and neuroimaging changes in preclinical Alzheimer’s disease. J Int Neuropsychol Soc. 2006;12:707–35. doi: 10.1017/S1355617706060863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Pare-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31:329–38. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- Wierenga CE, Benjamin M, Gopinath K, Perlstein WM, Leonard CM, et al. Age-related changes in word retrieval: role of bilateral frontal and subcortical networks. Neurobiol Aging. 2008;29:436–51. doi: 10.1016/j.neurobiolaging.2006.10.024. [DOI] [PubMed] [Google Scholar]
- Wierenga CE, Perlstein WM, Benjamin M, Leonard CM, Rothi LG, et al. Neural substrates of object identification: Functional magnetic resonance imaging evidence that category and visual attribute contribute to semantic knowledge. J Int Neuropsychol Soc. 2009;15:169–81. doi: 10.1017/S1355617709090468. [DOI] [PubMed] [Google Scholar]
- Xu G, McLaren DG, Ries ML, Fitzgerald ME, Bendlin BB, et al. The influence of parental history of Alzheimer’s disease and apolipoprotein E epsilon4 on the BOLD signal during recognition memory. Brain. 2009;132:383–91. doi: 10.1093/brain/awn254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip AG, McKee AC, Green RC, Wells J, Young H, et al. APOE, vascular pathology, and the AD brain. Neurology. 2005;65:259–65. doi: 10.1212/01.wnl.0000168863.49053.4d. [DOI] [PubMed] [Google Scholar]