Abstract
Lung development depends on accurate and precise patterning of a pulmonary anlagen, consisting of both endodermally and mesodermally-derived progenitor cells. In this process, the need to establish communication and control among individual cells is paramount. Transforming growth factor-β (TGFβ) and Wingless/int (Wnt) signaling pathways serve this need. The individual functional repertoire of the two pathways is further expanded by cross-talk and integration of signaling at multiple levels taking advantage of their hard-wired multi-component signal transduction platforms. Cross-talk creates the possibility for both specificity and versatility in signaling during development and during repair of injured tissue. Understanding the mechanics and the physiological implications of this cross-talk is necessary for therapeutic or preventive targeting of either TGFβ or Wnt signaling pathways.
Introduction
Transforming growth factor-β (TGFβ) and Wingless/int (Wnt) pathways have been around and likely interactive for the past 450 million years. Their evolutionary conservation is testimony to the key roles they play in crucial processes in development, homeostasis and response to injury. Both are necessary as early as gastrulation for establishment of Spemann’s Organizer. Recent studies have revealed considerable TGFβ~Wnt cross-talk in mammalian development and pathogenesis of disease. This brief and decidedly non-exhaustive review is focused on documented and potential cross-talk between these crucial signaling engines in the lung and their implications in physiological and pathophysiological contexts. There are a number of excellent reviews on TGFβ and Wnt signaling pathways treated separately. As the information on TGFβ~Wnt cross-talk in the lung is limited, we have incorporated findings from other organs and settings to raise potential parallels in the lung. The focus however remains on the two processes of lung development and pathogenesis of disease.
Multicomponent Nature of Signaling Pathways
Signaling pathways are multi-component, made up of receptors, receptor-associated proteins, cytoplasmic components/modifiers etcetera. In the TGFβ pathway, comprised of ligands that include TGFβ, activin, and Bmps, the binding of the ligand to a complex of cell surface receptors initiates the signaling process. For TGFβ, the receptor is a heteromeric complex of type I (TβRI) and type II transmembrane serine/threonine kinases. Downstream of the receptors, the Smad (Smad2 and Smad3 for TGFβ, Smads1, 5 and 8 for Bmp4) family of transcriptional factors are activated as intracellular effectors of TGFβ signaling. Phosphorylated Smad2 or Smad3 associate with Smad4 and translocate to the nucleus where they affect target gene transcription.
The Wnt pathway is no less complicated. Wnt ligands are cysteine-rich secreted glycoprotein homologues of Drosophila wingless. Two Wnt pathways, the canonical, mediated by β-catenin, and β-catenin-independent, “non-canonical” have been distinguished. The WNT ligands, of which there are 19 in Humans, interact with a selection of 10 seven-span transmembrane receptor molecules known as Frizzled (Fzd), and the co-receptors LRP5/6. In the absence of Wnt ligands, β-catenin (CTNNB1) is phosphorylated on Ser34, Ser37, Thr41 and Ser45 by a complex of Apc/Axin/GSK3β and degraded. Coupling of Fzd and Lrp5/6 through binding of Wnt ligands causes phosphorylation of Lrp and cytoplasmic protein, Dishevelled (Dvl), as well as membrane recruitment of the scaffold protein Axin. Stabilization of CTNNB1 is the major mechanism that regulates canonical WNT signaling. These events lead to dissociation of the Apc/Axin/GSK3β complex and inhibition of GSK3β, which results in inhibition of CTNNB1 phosphorylation. The stabilized CTNNB1 thus accumulates and translocates to the nucleus where it interacts with a complex of Lef/Tcf HMG box transcription factors to regulate target genes. The CTNNB1~Lef/Tcf transcriptional complex interacts with several factors including Smads. As will be discussed below, a number of the latter components serve as sites of interaction with the TGFβ pathway.
The multi-component hard wiring of the pathways allows for biologically meaningful interactions at multiple levels thereby creating the possibility for both specificity and versatility in signaling (Figure 1). In general and in simplistic terms the nature of the interactions could be summarized as either “interactive” or “reciprocal trans-regulation” as shown in Table 1. Much if not all of the information listed in Table 1 is based on findings in non-lung tissues and cell types, and the list is not exhaustive. The task in pulmonary research is to decipher which, if any, of the interactions occur in the lung, and to what meaningful physiologic end.
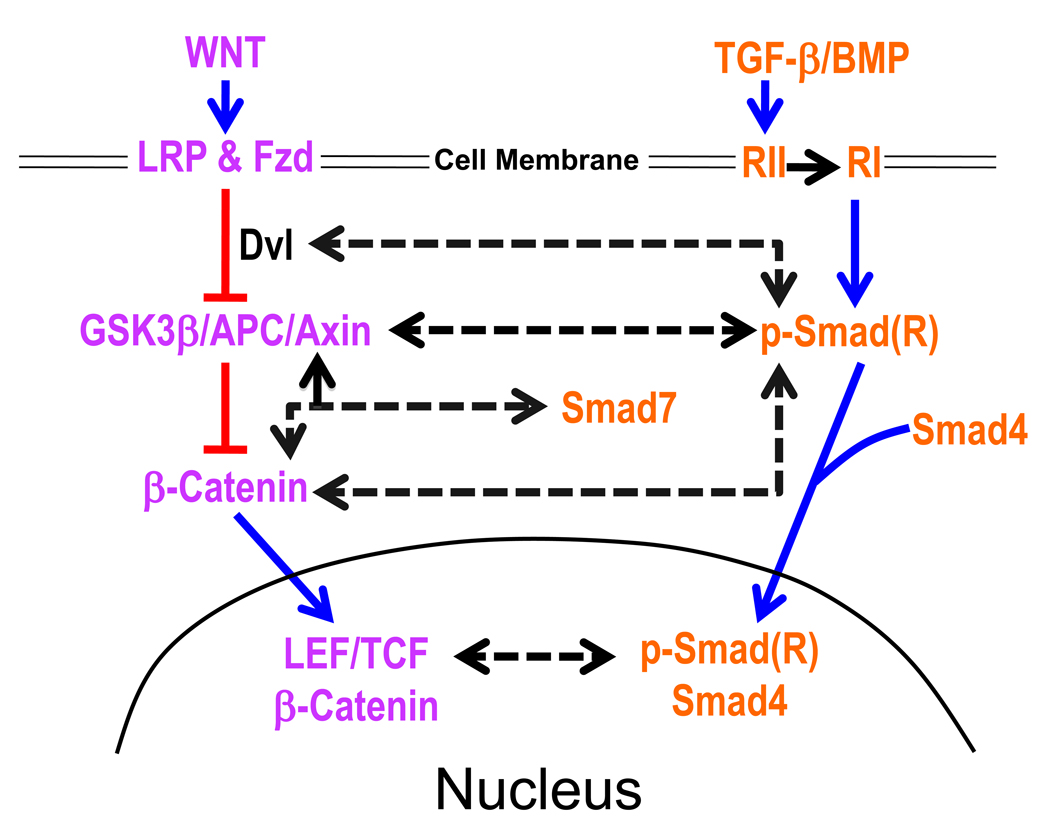
FIGURE 1.
Multi-component nature of TGFβ and Wnt Pathways. Interactions between the individual components of each pathway are shown with interrupted arrows. For references please refer to Table 1. Abbreviations used are: LRP, Low density Lipoprotein Receptor Protein, Fzd, Frizzled, RII, TGFβ type II receptor, RI, TGFβ type I receptor or Alk5, Dvl, Dishevelled, GSKβ, Glycogen Synthase Kinase 3 beta, Apc, Adenomatous Polyposis Coli, P-Smad, phosphorylated, ligand activated Smad, LEF, Lymphoid Enhancer Binding Factor, TCF, T Cell-Specific Transcription factor-1
TABLE 1.
A list of two potential modes of interactions between TGFβ and Wnt Pathways. “Interactive” refers to cross-communication as depicted in Figure 1. “Reciprocal Trans-regulation” defines regulation of one pathway’s components by those of the other.
| INTERACTIVE | |||
|---|---|---|---|
| SITE of INTERACTIONS |
TGFβ vs WNT | Function | References |
| Cytoplasm | Smad1~Dvl1 | Bmps antagonize Wnt signaling and retard bone marrow mesenchymal stem cell proliferation |
Liu et al., 2006 |
| Smad3~Axin | promotes Smad3 degradation | Guo et al., 2008 | |
| Smad2~CTNNB1 | promotes EMT | Kim et al., 2009 | |
| Smad7~ CTNNB1 | promotes CTNNB1degradation | Han et al., 2006 | |
| Smad7~Axin | stabilizes CTNNB1; promotes Smad7 degradation |
Tang et al., 2008; Liu et al., 2006 |
|
| Nucleus | Smad1/4~Lef1 | activates Msx2 promoter | Hussein et al., 2003 |
| Smad3~Lef1 | activates Xtwin transcription | Labbe et al., 2000 | |
| Smad1~ CTNNB1 ~Tcf4 |
controls Myc transcription | Hu and Rosenblum, 2005 | |
| RECEPROCAL TRANS-REGULATION | ||
|---|---|---|
| WNT→TGFβ mediators | activation of Bmp4 expression | Shu et al., 2005 |
| activation of Nodal expression | Rodriguez-Esteban et al., 2001 | |
| TGFβ → WNT mediators | activation of Lef1 expression | Nawshad & Hay, 2003 |
| activation of WNT-8 expression |
Hoppler and Moon, 1998 | |
| increase in CTNNB1 transcripts |
Satterwhite and Neufeld, 2004 | |
| increase in CTNNB1 protein in human fibroblast cells |
Caraci et al., 2008 | |
Primordial Specification
From the outset, both TGFβ and Wnt signaling are critical for lung primordial specification. Compound Wnt2/2b mutant mice have no lungs, but morphogenesis of other foregut endoderm-derived organs is normal. This phenotype is recapitulated by an endoderm-restricted deletion of CTNNB1 via Shhcre (Goss et al., 2009), demonstrating that Wnt2/2b signaling acts through the canonical Wnt pathway to specify lung endodermal progenitors within the foregut. Lung agenesis can also be caused by retinoic acid deficiency. Global gene expression analysis of RA-deficient foreguts revealed expression of a large number of TGFβ targets, suggesting that repression of TGFβ may be required for lung formation (Chen et al., 2007). Functional TGFβ~Wnt interactions in lung primordial specification remain unknown, but likely, as even in the earliest stages of cell fate determination interplay between TGFβ and Wnt appears to be critical. The pluripotent human embryonic stem cells are capable of undergoing indefinite self-renewal and serve to generate derivatives of all three primary germ layers. In the undifferentiated state, maintenance of pluripotency requires activation of SMAD2/3 pathway via TGFβ~Wnt interactions (James et al., 2005). By logical extension, a balance between TGFβ and Wnt signaling may be critical for loss of multipotency in endodermal cells that subsequently commit to narrow their developmental options to lung lineage.
Cell Differentiation
The final product of lung morphogenesis is a functional gas exchange organ with remarkable structural and cellular complexity. This process requires both TGFβ and Wnt signaling. Conditional inactivation of Alk3, a Bmp type I receptor caused defects in lung morphogenesis, cell proliferation, and differentiation (Sun et al., 2008). In the mutant lungs, Wnt signaling activity was increased in association with decreased expression of Wnt inhibitory factor-1 (WIF-1). This observation suggests interactions between the TGFβ family member Bmp and canonical Wnt signaling in controlling cell proliferation and differentiation during lung development. In a recent study, lung epithelial-specific accumulation of stabilized CTNNB1 activated the Wnt canonical pathway in airway progenitor cells & caused polyp formation(Li et al., 2009). Accumulated CTNNB1 also robustly activated Bmp4, and inhibited cell differentiation. Conversely, inhibition of WNT signaling either by over-expression of Wnt inhibitor Dkk1 or deletion of CTNNB1 inhibited Bmp4 expression in lung epithelial cells (Shu et al., 2005). Thus, Wnt signaling inhibits lung epithelial cell differentiation via activation of TGFβ.
There is also evidence for interactions between non-canonical Wnt5a and members of the TGFβ family during lung cellular differentiation and vasculogenesis. Mis- or, over-expression of Wnt5a impairs patterning of both distal air ways and vascular tubulogenesis. The phenotype includes severe pulmonary hypoplasia, associated with changes in gene expression, including that of Bmp4, a member of the TGFβ superfamily (Li et al., 2005).
In the lung, smooth muscle cell (SMC) differentiation is also controlled by both Wnt and TGFβ signaling. TGFβ mediates myofibroblast differentiation and activates α-smooth muscle actin expression. Inhibition of Wnt signaling by deletion of Wnt7b or deletion of CTNNB1 disrupts smooth muscle development (Shu et al., 2002). Alternatively, activation of Wnt signaling in lung mesenchymal cells activates the expression of both PDGF receptors, Pdgfr-α and Pdgfr-β and increases proliferation of SMC precursors (Cohen et al., 2009). Whether TGFβ and Wnt signaling pathways interact to regulate SMC differentiation remains to be determined. However, it is known that TGFβ-stimulated α-Smooth Muscle Actin expression is inhibited by induction of Egr1 (Early Growth response-1), a zinc-finger transcriptional regulator whose expression is inhibited by the Wnt pathway. Thus, Egr1 serves as a potential link in the cross-talk between TGFβ and Wnt pathways during muscle cell differentiation in the lung.
Progenitor/Stem Cells & Repair of Injury
The individual roles of TGFβ and Wnt signaling in stem cell biology is well recognized. In the lung, little to no published information is currently available on the role of TGFβ in resident progenitor/stem cells. Unpublished data from our group suggest that TGFβ/Alk5 is a key regulator of Clara cell ontogeny from the pool of airway epithelial progenitors. Whether maintenance or recruitment of such progenitor/stem cells in the process of injury/repair requires WNT/TGFβ input remains to be examined. However the role of Wnt in lung progenitor/stem cells has been recognized. For example, stabilization of CTNNB1 in CC10 expressing cells expands the stem cell pools in the adult lung (Reynolds et al., 2008). Conversely, deletion of CTNNB1 exons 2–6 via Ccspcre in airway epithelial cells had no impact on expression of Clara cell differentiation or their sensitivity to naphthalene. Repair of the naphthalene-injured airway was also normal. Thus, CTNNB1 may not be necessary for maintenance or efficient repair of the bronchiolar epithelium (Zemke at al., 2009). Pten a phosphatase tumor suppressor which links TGFβ and Wnt signaling, is a key regulator of stem cell pools in many organs. Pten is regulated by TGFβ and activates Wnt via phosphorylation of GSK3. In the lung, epithelial-specific Pten deletion via Nkx2-1cre expands all putative stem cell pools concomitant with activation of CTNNB1 and Wnt signaling (Tiozzo et al., 2009) This increase also protects the airways from naphthalene injury.
In lung disorders such as Idiopathic/Interstitial Pulmonary Fibrosis, IPF, there is clear involvement of both TGFβ and Wnt pathways. Epithelial Mesenchymal Transition or EMT, a process involved in many biological settings and currently believed to play a role in IPF, provides the most evidence-based example of TGFβ~Wnt interactions in lung injury. One of the major events in EMT is inhibition of E-cadherin. Through a non-Smad pathway, TGFβ activates a cascade of downstream molecules including MEK and ERK and eventually transcription factors such as Elk1, c-Jun and c-Fos. The sum of such reactions leads to formation of activator protein-1 or AP1. AP1 is a recognized inducer of Snail, a zinc-finger transcriptional factor that has been identified in cancer-associated EMT. Snail inhibits E-cadherin gene expression, thereby promoting EMT. Furthermore, TGFβ-stimulated phospho-Smad2/Smad4 complex can activate transcription of Lef-1. Lef1 is an HMG transcription and a target of Wnt signaling, thus serving as another interface between TGFβ and Wnt pathways. In response to TGFβ, phospho-Smad2/Smad4 complex can activate transcription of Lef-1, which in turn forms a transcriptional complex with Smad2 and Smad4 to inhibit E-Cadherin and hence EMT (Nawshad et al., 2007).
A recent study found the laminin receptor, α3β1integrin to serve as a novel interface between TGFβ and Wnt signaling in EMT. Mice with epithelial-specific deletion of α3β1 exhibit relative resistance to bleomycin-induced EMT and IPF. In the presence of active TGFβ, α3β1 facilitates tyrosine, rather than inhibiting serine phosphorylation of CTNNB1. Stabilized tyrosine-phosphorylated– CTNNB1 forms a transcriptional complex with phosphorylated Smad2 thus promoting EMT (Kim et al., 2009).
Both TGFβ and WNT pathways figure importantly in pathogenesis of neonatal chronic lung disease, Bronchopulmonary Dysplasia or BPD. TGFβ is increased in the lungs of neonates at risk for BPD (Lecart et al., 2000). Recently, both TGFβ and WNT pathways were found to be stimulated in a mouse phenocopy model of BPD. Blocking the activation of TGFβ and WNT pathways, represented by levels of p-smad3 and Lef-1, respectively, protected against BPD (Dasgupta et al., 2009). The mediators of their interactions remain unknown.
What’s Missing?
As shown in Figure 1, TGFβ and Wnt can interact via multiple components that make up each of their respective signal transduction pathways. In the lung, evidence for some of the interactions is currently unavailable. For example, an important intercept of TGFβ~Wnt cross-talk occurs at the level of Axins. Axin1 and Axin2 are functionally equivalent and are known to inhibit Wnt/CTNNB1 and stimulate TGFβ signaling. In chondrocytes, maturation is regulated by precise integration of signals between the Wnt and TGFβ,–pathways via Axins. Little information is currently available on the role of Axins in lung development. Axins have been studied in lung cancer cells however their role in the TGFβ-response of these cells remains entirely un-explored.
The cytoplasmic proteins, Dishevelled (Dvl) act as mediators of Wnt signaling. Available preliminary data on their role and interactions with TGFβ is confounded by their multiplicity and redundancy. We found that conventional deletion of individual Dvl1 and Dvl2 has no discernible impact on lung morphogenesis (Li, Wynshaw-Boris & Minoo, Unpublished data). Wnt/ CTNNB1 and Bmp signaling pathways interact at the level of Dvl1/Smad1 to regulate differentiation of bone marrow mesenchymal stem cells to osteoblasts. Bmp2, a TGFβ family member antagonizes Wnt signaling in osteoblast progenitors by promoting an interaction between Smad1 and Dvl1 that restricts CTNNB1 activation (Liu et al., 2006).
There is also evidence for regulation of Wnt signaling by TGFβ via Apc. In non-transformed epithelial cells, TGFβ rapidly reduces Apc protein levels by a post-transcriptional mechanism, and increases nuclear localized CTNNB1. This finding demonstrates that TGFβ mimics the effect of Wnt signaling. No information on the role of Apc in lung development currently exists. Epithelial-specific deletion of Apc causes significant abnormalities in the lung associated with increased Bmp4 signaling (Our Unpublished data).
Practical and Functional Implications
What are the practical implications of cross-talk between the TGFβ and Wnt pathways? The evolution of the vertebrate has benefited from integration of conserved positive and negative regulatory mechanisms during development. Such The hard wiring of such interactive system from promotes precise and finely tuned regulation of key genes, allowing for selectivity and specificity that must inevitably have contributed to emergence of novel functions and organs during evolution. Phylogenetic history of TGFβ and Wnt engines suggests that they evolved together to provide such balance/fine tuning in gene regulation and hence development. Thus, while one pathway may promote cell division, the other may inhibit it. This is well demonstrated in the chondrogenesis example given above; TGFβ slows the rate of chondrocyte maturation, while Wnt promotes it. Another important consideration is that future therapies directed at manipulating either of the two pathways or their components must take into account their interactions and the intercepts to avoid untoward side-effects. In the chondrogenesis example, treatment with TGFβ suppresses Axin 1 and 2, but curtails TGFβ signaling and instead enhances Wnt/CTNNB1 signaling. In the lung, TGFβ~Wnt cross-talk has proven critical for normal development and repair after injury as well as in cancer. As more elegant genetic tools and models come online, the significance and functional implications of this molecular chattering should increase in clarity.
Acknowledgments
Supported by NHLBI, NIH and The Hastings Foundation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Caraci F, Gili E, Calafiore M, Failla M, La Rosa C, Crimi N, et al. TGF-beta1 targets the GSK-3beta/beta-catenin pathway via ERK activation in the transition of human lung fibroblasts into myofibroblasts. Pharmacol Res. 2008;57:274–282. doi: 10.1016/j.phrs.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Chen F, Desai TJ, Qian J, Niederreither K, Lü J, Cardoso WV. Inhibition of Tgf beta signaling by endogenous retinoic acid is essential for primary lung bud induction. Development. 2007;134:2969–2979. doi: 10.1242/dev.006221. [DOI] [PubMed] [Google Scholar]
- Cohen ED, Ihida-Stansbury K, Lu MM, Panettieri RA, Jones PL, Morrisey EE. Wnt signaling regulates smooth muscle precursor development in the mouse lung via a tenascin C/PDGFR pathway. J Clin Invest. 2009;119:2538–2549. doi: 10.1172/JCI38079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta C, Sakurai R, Wang Y, Guo P, Ambalavanan N, Torday JS, et al. Hyperoxia-induced neonatal rat lung injury involves activation of TGF-{beta} and Wnt signaling and is protected by rosiglitazone. Am J Physiol Lung Cell Mol Physiol. 2009;296:L1031–L1041. doi: 10.1152/ajplung.90392.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss AM, Tian Y, Tsukiyama T, Cohen ED, Zhou D, Lu MM, et al. Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev Cell. 2009;17:290–298. doi: 10.1016/j.devcel.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Ramirez A, Waddell DS, Li Z, Liu X, Wang XF. Axin and GSK3- control Smad3 protein stability and modulate TGF- signaling. Genes Dev. 2008;22:106–120. doi: 10.1101/gad.1590908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han G, Li AG, Liang YY, Owens P, He W, Lu S, et al. Smad7-induced beta-catenin degradation alters epidermal appendage development. Dev Cell. 2006;11:301–312. doi: 10.1016/j.devcel.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Hoppler S, Moon RT. BMP-2/-4 and Wnt-8 cooperatively pattern the Xenopus mesoderm. Mech Dev. 1998;71:119–129. doi: 10.1016/s0925-4773(98)00004-5. [DOI] [PubMed] [Google Scholar]
- Hu MC, Rosenblum ND. Smad1, beta-catenin and Tcf4 associate in a molecular complex with the Myc promoter in dysplastic renal tissue and cooperate to control Myc transcription. Development. 2005;132:215–225. doi: 10.1242/dev.01573. [DOI] [PubMed] [Google Scholar]
- Hussein SM, Duff EK, Sirard C. Smad4 and beta-catenin co-activators functionally interact with lymphoid-enhancing factor to regulate graded expression of Msx2. J Biol Chem. 2003;278:48805–48814. doi: 10.1074/jbc.M305472200. [DOI] [PubMed] [Google Scholar]
- James D, Levine AJ, Besser D, Hemmati-Brivanlou A. TGFbeta/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development. 2005;132:1273–1282. doi: 10.1242/dev.01706. [DOI] [PubMed] [Google Scholar]
- Kim KK, Wei Y, Szekeres C, Kugler MC, Wolters PJ, Hill ML, et al. Epithelial cell alpha3beta1 integrin links beta-catenin and Smad signaling to promote myofibroblast formation and pulmonary fibrosis. J Clin Invest. 2009;119:213–224. doi: 10.1172/JCI36940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Crooks H, Dracheva T, Nishanian TG, Singh B, Jen J, et al. Oncogenic beta-catenin is required for bone morphogenetic protein 4 expression in human cancer cells. Cancer Res. 2002;62:2744–2748. [PubMed] [Google Scholar]
- Labbé E, Letamendia A, Attisano L. Association of Smads with lymphoid enhancer binding factor 1/T cell-specific factor mediates cooperative signaling by the transforming growth factor-beta and wnt pathways. Proc Natl Acad Sci U S A. 2000;97:8358–8363. doi: 10.1073/pnas.150152697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecart C, Cayabyab R, Buckley S, Morrison J, Kwong KY, Warburton D, et al. Bioactive transforming growth factor-beta in the lungs of extremely low birthweight neonates predicts the need for home oxygen supplementation. Biol Neonate. 2000;77:217–223. doi: 10.1159/000014219. [DOI] [PubMed] [Google Scholar]
- Li C, Li A, Li M, Xing Y, Chen H, Hu L, et al. Stabilized beta-catenin in lung epithelial cells changes cell fate and leads to tracheal and bronchial polyposis. Dev Biol. 2009;334:97–108. doi: 10.1016/j.ydbio.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Hu L, Xiao J, Chen H, Li JT, Bellusci S, et al. Wnt5a regulates Shh and Fgf10 signaling during lung development. Dev Biol. 2005;287:86–97. doi: 10.1016/j.ydbio.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Liu Z, Tang Y, Qiu T, Cao X, Clemens TL. A dishevelled-1/Smad1 interaction couples WNT and bone morphogenetic protein signaling pathways in uncommitted bone marrow stromal cells. J Biol Chem. 2006;281:17156–17163. doi: 10.1074/jbc.M513812200. [DOI] [PubMed] [Google Scholar]
- Liu W, Rui H, Wang J, Lin S, He Y, Chen M, et al. Axin is a scaffold protein in TGF-beta signaling that promotes degradation of Smad7 by Arkadia. EMBO J. 2006;25:1646–1658. doi: 10.1038/sj.emboj.7601057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawshad A, Hay ED. TGFbeta3 signaling activates transcription of the LEF1 gene to induce epithelial mesenchymal transformation during mouse palate development. J Cell Biol. 2003;163:1291–1301. doi: 10.1083/jcb.200306024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawshad A, Medici D, Liu CC, Hay ED. TGFbeta3 inhibits E-cadherin gene expression in palate medial-edge epithelial cells through a Smad2-Smad4-LEF1 transcription complex. J Cell Sci. 2007;120:1646–1653. doi: 10.1242/jcs.003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SD, Zemke AC, Giangreco A, Brockway BL, Teisanu RM, Drake JA, et al. Conditional stabilization of beta-catenin expands the pool of lung stem cells. Stem Cells. 2008;26:1337–1346. doi: 10.1634/stemcells.2008-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Esteban C, Capdevila J, Kawakami Y, Izpisúa Belmonte JC. Wnt signaling and PKA control Nodal expression and left-right determination in the chick embryo. Development. 2001;128:3189–3195. doi: 10.1242/dev.128.16.3189. [DOI] [PubMed] [Google Scholar]
- Satterwhite DJ, Neufeld KL. TGF-beta targets the Wnt pathway components, APC and beta-catenin, as Mv1Lu cells undergo cell cycle arrest. Cell Cycle. 2004;3:1069–1073. [PubMed] [Google Scholar]
- Shu W, Guttentag S, Wang Z, Andl T, Ballard P, Lu MM, et al. Wnt/beta-catenin signaling acts upstream of N-myc, BMP4, and FGF signaling to regulate proximal-distal patterning in the lung. Dev Biol. 2005;283:226–239. doi: 10.1016/j.ydbio.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Shu W, Jiang YQ, Lu MM, Morrisey EE. Wnt7b regulates mesenchymal proliferation and vascular development in the lung. Development. 2002;129:4831–4842. doi: 10.1242/dev.129.20.4831. [DOI] [PubMed] [Google Scholar]
- Sun J, Chen H, Chen C, Whitsett JA, Mishina Y, Bringas P, Jr, et al. Prenatal lung epithelial cell-specific abrogation of Alk3-bone morphogenetic protein signaling causes neonatal respiratory distress by disrupting distal airway formation. Am J Pathol. 2008;172:571–582. doi: 10.2353/ajpath.2008.070286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Liu Z, Zhao L, Clemens TL, Cao X. Smad7 stabilizes beta-catenin binding to E-cadherin complex and promotes cell-cell adhesion. J Biol Chem. 2008;283:23956–23963. doi: 10.1074/jbc.M800351200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiozzo C, De Langhe S, Yu M, Londhe VA, Carraro G, Li M, et al. Deletion of Pten expands lung epithelial progenitor pools and confers resistance to airway injury. Am J Respir Crit Care Med. 2009;180:701–712. doi: 10.1164/rccm.200901-0100OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemke AC, Teisanu RM, Giangreco A, Drake JA, Brockway BL, Reynolds SD, et al. beta-Catenin is not necessary for maintenance or repair of the bronchiolar epithelium. Am J Respir Cell Mol Biol. 2009;41:535–543. doi: 10.1165/rcmb.2008-0407OC. [DOI] [PMC free article] [PubMed] [Google Scholar]