Summary
Background
Early life trauma, particularly child abuse, has been associated with aberrations in hypothalamic-pituitary-adrenal (HPA) axis functioning in adulthood. However, the relationship of early abuse and later adult neuroendocrine changes may be moderated by additional factors such as comorbid psychopathology and recent life stress. Parental exposure to child abuse may have transgenerational effects, with offspring of abuse victims showing similar neuroendocrine profiles as their mothers. The majority of previous studies in this area focus on adult offspring, and the degree to which the effects of parental child abuse can be detected earlier in the development of the offspring remains obscure.
Methods
The current study utilized a clinical sample of women with a history of MDD (N= 126), to examine the effects of maternal early life sexual and physical abuse (Childhood Trauma Questionnaire; CTQ) on both maternal and infant salivary cortisol levels during a laboratory stress paradigm at 6 months postpartum.
Results
Maternal child abuse was associated with steeper declines in cortisol in the mothers, and lower baseline cortisol in their infants. Comorbid maternal PTSD, current maternal depressive symptoms, and recent life stressors were significant moderators of maternal cortisol change. Maternal abuse history was associated with increases in cortisol levels in those mothers who experienced these additional stressors. Similarly, a history of early maternal abuse and comorbid PTSD was associated with greater increases in infant cortisol levels.
Conclusions
Maternal childhood abuse was associated with HPA axis function in both the mother and the infant during the postpartum period.
Keywords: Early Life Stress, HPA Axis, Depression, Child, Cortisol
Introduction
A history of early life stress (ELS), such as childhood trauma, is associated with a striking increase in the risk for subsequent depression (Chapman et al., 2004; Felitti et al., 1998) and anxiety disorders (McCauley et al., 1997). Several large-scale studies have shown significant associations between childhood trauma and adult depression. For example, Kendler et al. (2004) noted that the onset of major depressive disorder (MDD) in adult women is best predicted by a combination of childhood sexual abuse and current life stress. While the majority of women who develop MDD do not have a history of childhood trauma, people with adverse childhood experiences have been found to have a 4-fold increase in the risk of depression (Felitti et al., 1998).
The HPA axis is hypothesized to be an important link between ELS and the pathophysiology of later psychopathology. Heim and colleagues (2000) found that abused women showed significant cortisol increases in response to a laboratory stressor if they were currently depressed but not if they were currently euthymic. The authors hypothesize that ELS is associated with a greater sensitivity of the HPA axis to stress during adulthood. This sensitivity potentially underlies a vulnerability to the development of adult depression and suggests that current depressive symptomatology is a potentially important moderator of the ELS – HPA axis relationship.
In addition to depressive symptoms, it has been hypothesized that recent life stressors (Young & Breslau, 2004) and PTSD (de Kloet et al., 2007) might also moderate the relationship between ELS and HPA axis function. The impact of early child abuse combined with these moderating factors on perinatal HPA axis function remains unknown. Adult females with a history of sexual abuse with current PTSD have demonstrated increased urinary cortisol excretion and increased glucocorticoid feedback sensitivity (Lemieux & Coe, 1995; Stein et al., 1997). Neuroendocrine responses (such as pituitary and adrenal) have also been found to be positively correlated with the degree of the abuse and the severity of the PTSD and depression (Heim et al., 2001).
Women who have had a previous depressive episode are vulnerable to perinatal depression (Marcus, Flynn, Blow, & Barry, 2003). As depression and anxiety have been linked to hypothalamic-pituitary-adrenal (HPA) axis alterations, it is not surprising that perinatal depression and anxiety are significantly related to both maternal and infant HPA axis functioning (Field et al., 2006). Our group recently reported that maternal depression was significantly associated with increased levels of baseline and mean infant cortisol levels. Maternal depression with a comorbid anxiety disorder was related to increases in infant cortisol reactivity (Brennan et al., 2008). The relationship between maternal PTSD and infant cortisol levels differs from the maternal depression-infant cortisol findings, although such research remains limited. Yehuda and colleagues, in a sample of 38 mother-infant dyads, noted that maternal PTSD following 9/11 was associated with significantly lower infant baseline cortisol levels 9 months of age (2005). Potential moderators, such as comorbid depression and recent life stress were not assessed. Perinatal maternal depression and stress have also been shown to effect many aspects of child behavior and development. Cognitive performance, behavior, and child psychopathology have all been shown to be influenced by perinatal maternal mental illness (see Brand and Brennan, 2009 for a recent review of this topic).
As addressed above, it has been suggested that ELS can influence the HPA axis of adult women and that comorbid psychopathology, recent life stress, and current depressive symptoms may moderate this relationship. Furthermore our group and others have demonstrated links between maternal psychopathology and infant cortisol levels. The primary goal of this study is to take this line of research one step further and investigate the association between maternal history of child abuse and maternal cortisol levels in a clinical sample of postpartum women, and to explore whether depressive symptoms and stressful life events, as well as comorbid PTSD moderated this relationship. Our secondary goal is to examine potential transgenerational effects, such that infants of mothers with an abuse history would show patterns of HPA axis function similar to their mothers.
Methods
Participants
Women participating in a longitudinal study of the perinatal course of mental illness at the Emory Women’s Mental Health Program (WMHP) were screened for study participation. Inclusion criteria were: (1) lifetime history of MDD as determined by SCID, (2) completion of the Childhood Trauma Questionnaire (CTQ); and (3) participation in a laboratory visit at 6 months postpartum. Subjects were excluded for: 1) multiple gestation; 2) substance abuse or dependence during pregnancy; and 3) psychotic or bipolar disorder. The Emory University Institutional Review Board approved this study, and mothers provided written informed consent.
A total of 126 mother / infant dyads qualified for the present study. The infant sample contained 62 boys and 64 girls, with a mean age of 187±17 days at the time of the laboratory study. Most (94%) of the mother/infant dyads were Caucasian, the median maternal education level was college graduate, mean maternal age was 34 ± 4 years, and 95% of the mothers were married or cohabitating at the time of the infant follow-up.
Procedure
During the course of serial perinatal visits at the WMHP, women completed psychometric questionnaires including the CTQ. When the infants were six months old, a laboratory visit was conducted to examine HPA axis functioning in mothers and infants (Brennan et al., 2008). All laboratory visits began at 1:00 pm to control for the diurnal rhythm of cortisol. Following procedural explanation and consent, initial samples of infant and mother saliva (T0 - baseline) were obtained. Next, the mother completed a series of questionnaires, while adjacent to her, the infant was held by a research assistant. After a 20-minute period, the second saliva sample (T1 – post-separation stressor) was obtained from both the mother and infant. The infant was then placed in a car seat behind an occlusion screen, and the mother was permitted to view her infant on a TV monitor during a noise burst and an arm restraint stressor task. Saliva samples were taken from the mother and the infant immediately after the lab stressor tasks were completed (T2 - post-noise/arm stressor I), and again 20 minutes later (T3 - post-noise/arm stressor II). Our three post-stressor cortisol measures (T1, T2, T3) were taken in post-stressor time windows when cortisol levels typically increase (5 to 40 minutes) (11,12). Mothers refrained from eating, and research assistants recorded infant food (breast milk, formula) intake prior to saliva collection. Following the infant assessment, the mothers completed a clinical interview administered by a research assistant.
Measures
Maternal diagnosis
Mothers were assessed for lifetime and current psychiatric disorders using the Structured Clinical Interview for DSM-IV (SCID) (First, Spitzer, Gibbon, & Williams, 1995). A reliability analysis based on 10% of our sample yielded weighted kappas of 0.83 for MDD diagnosis and 1.0 for PTSD diagnosis.
Maternal abuse history
Mothers completed the CTQ, a validated measure assessing childhood exposure to emotional, physical, and sexual abuse (Bernstein & Fink, 1998). Child trauma was operationalized as mild or higher levels of sexual abuse or moderate or higher levels of physical abuse. We excluded emotional abuse in order to maintain consistency with the previous ELS literature.
Maternal depression
The number of women meeting SCID criteria for current MDD was too low to provide sufficient power for reliable analyses. Therefore, we used mothers’ self-reported current depressive symptoms on the Beck Depression Inventory-II (BDI) (Beck et al., 1996(Beck, Steer, & Brown, 1996) as our measure of current maternal depression. In our sample, the mean BDI score was 8.67 (SD=7.49), and the alpha for internal reliability was .88. Mothers were categorized as high vs. low on depressive symptoms, using a BDI cutoff score of ≥12 (Milgrom et. al., 2005).
Exposure to prenatal and perinatal stress
Mothers completed the psychiatric epidemiology research interview (PERI) stressful life events scale (Dohrenwend, Krasnoff, Askenasy, & Dohrenwend, 1982), focused on stressful life events occurring during pregnancy or the postpartum period.
Health history
Mothers completed an obstetrical history questionnaire detailing medical illnesses, medication exposure, exposure to toxins (e.g. nicotine, illicit drugs), method of delivery, and complications associated with the infant’s birth. Use of medications during pregnancy was determined by prospective longitudinal data collection during serial visits at the WMHP. Maternal and infant medication use, as well as eating and sleeping patterns on the day of the laboratory study were obtained. Mother’s current menstruation status and recent aerobic activity were also queried as potential confounds in relation to cortisol.
Salivary cortisol concentrations
Saliva samples were frozen at −20°C within 15 minutes of collection. Saliva was assayed for cortisol concentration using a commercially available radioimmunoassay kit (DiaSorin GammaCoat, Stillwater, Minnesota). Sensitivity for saliva cortisol is 0.05 mcg/dL, and inter- and intra-assay coefficients of variation are 6.0% and 3.5% respectively. All standards and samples were run in duplicate by a research assistant masked to maternal trauma history, maternal psychiatric diagnosis, whether sample was collected from mother or infant, and the time point of sample collection.
Mother and infant salivary cortisol measures were: 1) baseline cortisol (T0 – study entry); and 2) cortisol change calculated as the area under the curve (AUC; linear trapezoid method) for T1, T2, and T3 cortisol samples, as measured from baseline (T0).
Statistical Analysis
Descriptive statistics were calculated to provide a characterization of the study sample. To identify important covariates for hypothesis testing, preliminary univariate analyses were conducted to examine the relationship between the dependent variables and demographic (marital status, education, gender), obstetrical (prenatal maternal medication use, delivery complications, method of delivery) and other health-related (maternal and infant food and medicine intake, menstruation) predictors.
Hypothesis testing utilized analyses of covariance (ANCOVA) with maternal baseline cortisol, maternal cortisol change, infant baseline cortisol, and infant cortisol change as dependent variables. Dependent variables were log-transformed prior to ANCOVA (non-transformed values are presented in the figures for ease of interpretation). Covariates included demographic and clinical characteristics that were significantly associated with the dependent variables. All tests were two-tailed, and an alpha of 0.05 was used throughout.
Results
Descriptive / Preliminary Analyses
Thirty-eight women (30% of sample) were classified as having a history of abuse according to the CTQ (28 with sexual abuse only, 9 with physical abuse only and one with both sexual and physical abuse), and 15 (12%) had a lifetime history of PTSD. Fifty-five mothers (44%) reported a stressful life event during pregnancy or the postpartum period. At the time of laboratory assessment, 38 women (30%) had a BDI score ≥12
Two of the potential confounds were significantly associated with one or more cortisol measures and were therefore statistically controlled in subsequent analyses. Specifically, whether or not the infant was currently breast fed versus bottle fed was significantly associated with infant baseline cortisol (t=2.11, p<.05), and infant birth order (number of siblings) was significantly associated with maternal baseline cortisol (r=−.18, p<.05). Maternal marital status, maternal education, child gender, prenatal maternal medication use, delivery complications, method of delivery, infant and mother food and medicine intake and maternal menstruation cycle were unrelated to cortisol measures in this study.
Maternal current depressive symptoms were not significantly correlated with maternal PTSD diagnosis (r= −.02 , p= .83) or recent stressful life events (r= .17, p=.06). Maternal PTSD diagnosis and recent stressful life events were significantly correlated, but the strength of the correlation was low (r=.18 , p=.04). Therefore each moderator was assessed in a separate ANCOVA; post-hoc analyses controlling for each of the other moderators did not change the results described below.
Cortisol change in response to stress was inversely correlated with baseline cortisol (r=−.66, p<.001 for mothers and r=−.62, p<.001 for infants), confirming the law of initial value (commonly noted inverse correlation between baseline and reactivity in physiological responses) (Wilder, 1958). All analyses examining cortisol change therefore included baseline cortisol as a covariate.
Maternal Abuse History and Maternal Cortisol
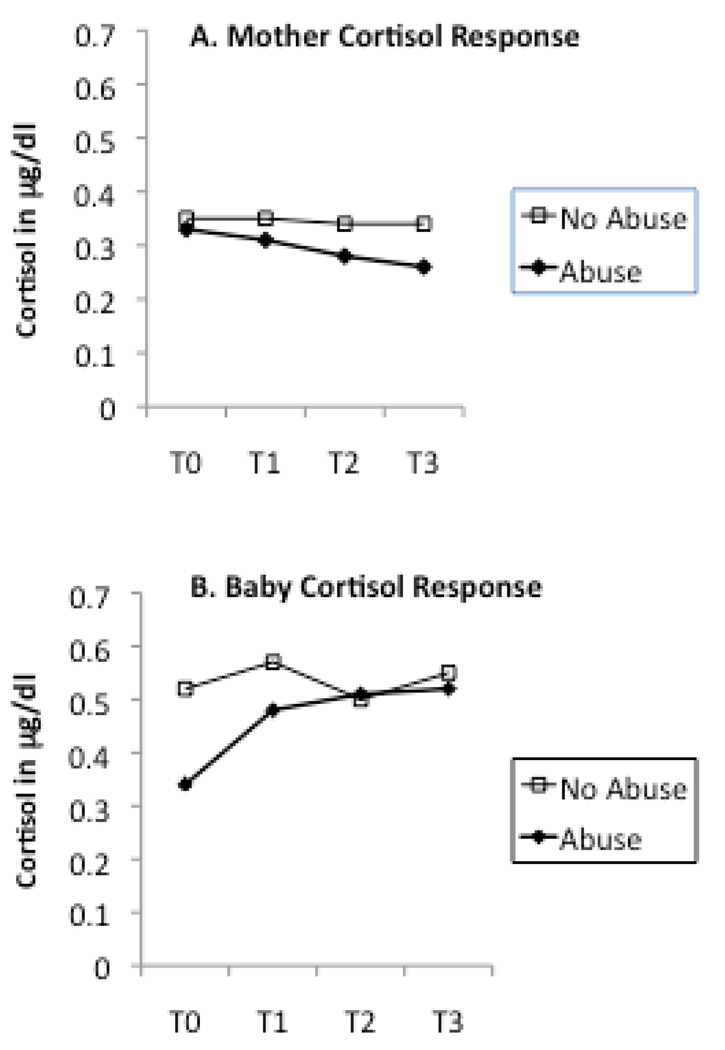
ANCOVAs revealed a statistically significant association between maternal abuse history and mother cortisol change (F(1,111)=4.82, eta squared=.04, p=.03) but not maternal cortisol at baseline (F(1,119)=1.37, eta squared=.01, p=.24). The maternal post-stressor cortisol concentrations were lower for mothers with a history of childhood abuse compared to mothers with no such history (cf. Figure 1A).
Figure 1.
Mother abuse history as determined as present or absent by the CTQ, and cortisol levels measured from baseline (T0) to post-stressor (T1,T2,T3) time points. Mother abuse history is associated with significantly less cortisol reactivity in the mother and a significantly lower cortisol baseline in the baby.
Moderators of Maternal Abuse History and Maternal Cortisol
Table 1 presents the significance levels of the interaction terms from the ANCOVAs examining potential moderators of the relationship between maternal abuse history and maternal cortisol. As can be seen, maternal depressive symptoms, exposure to stressful life events, and history of PTSD significantly moderated the relationship between maternal abuse history and maternal cortisol change in response to stress; however none of these factors moderated the relationship with maternal baseline cortisol.
Table 1.
Moderators of maternal history of abuse and maternal cortisol.
| Mother Baseline Cortisol | ||||
| Moderator | df | F | eta2 | p |
| Depressive Symptoms | 115 | .35 | .00 | .55 |
| Recent Life Events | 119 | 1.26 | .01 | .27 |
| History of PTSD | 119 | .02 | .00 | .90 |
| Mother Cortisol Change | ||||
| Moderator | df | F | eta2 | p |
| Depressive Symptoms | 107 | 4.12 | .04 | .045 |
| Recent Life Events | 111 | 4.27 | .04 | .04 |
| History of PTSD | 111 | 6.13 | .06 | .02 |
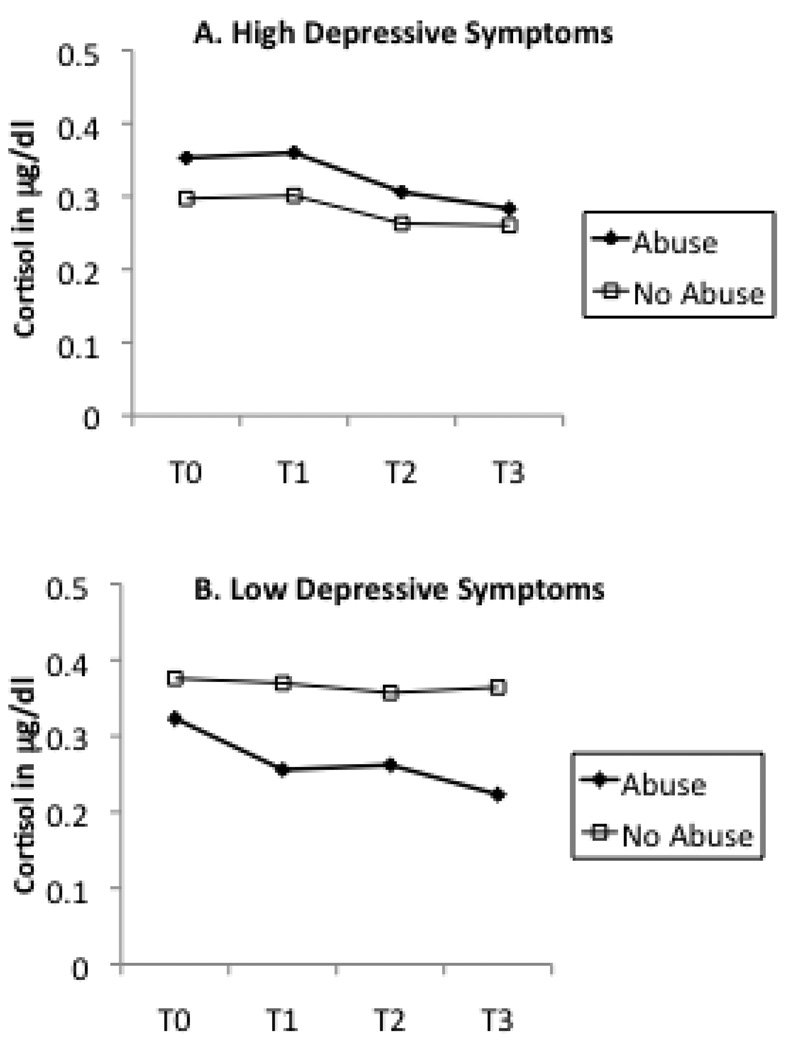
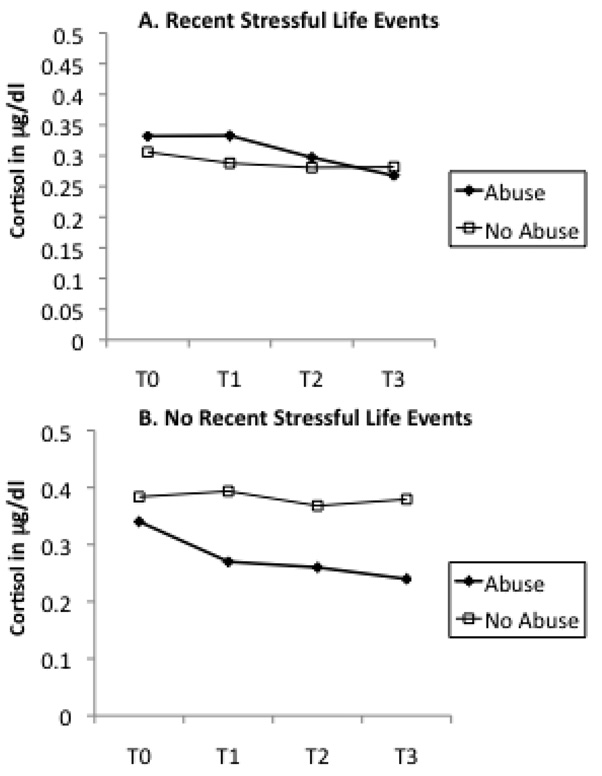
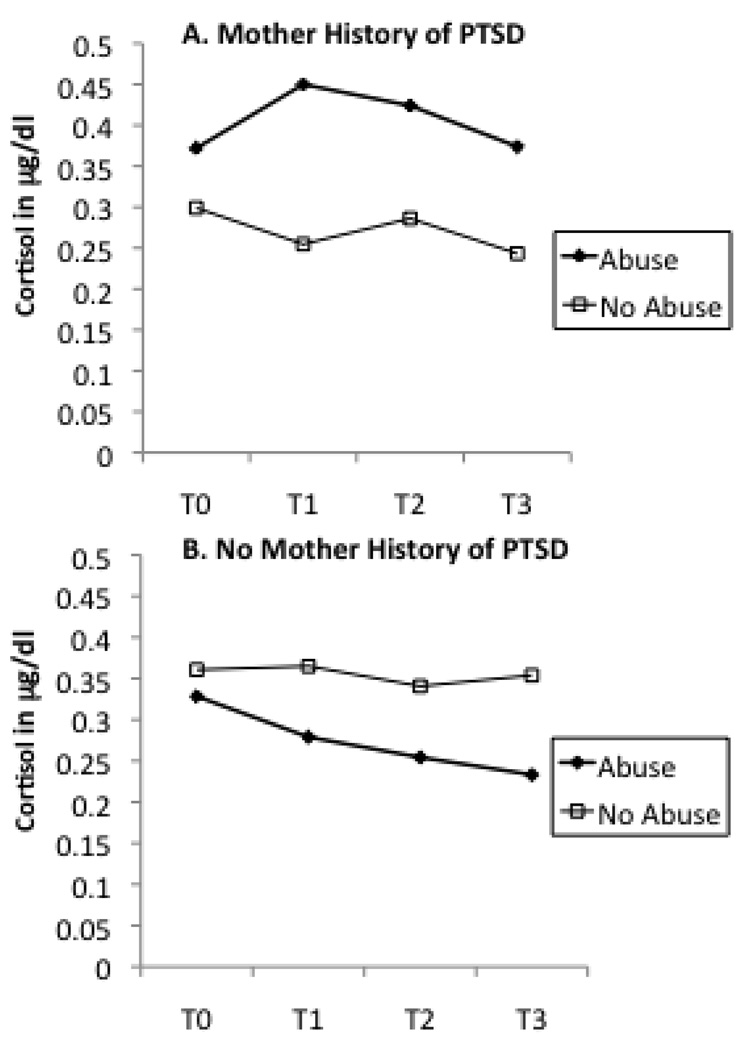
In order to interpret these interactive effects, we plotted maternal abuse history as a dichotomous variable by maternal cortisol levels across T0 to T3 in groups with or without current maternal depression (Figure 2), recent stressful life events (Figure 3), and maternal lifetime history of PTSD (Figure 4). A similar pattern was noted across Figure 2 through Figure 4. In each instance, the presence of an additional risk factor (i.e., maternal depression, stress, or PTSD) resulted in a pattern of higher cortisol levels for women with a history of abuse in comparison to women with no such history. In the absence of these additional risk factors, maternal history of abuse was associated with a decrease in cortisol, similar to that noted in the sample as a whole. This divergence in cortisol levels was most apparent in response to the initial separation from the infant (change in cortisol from T0 to T1).
Figure 2.
Mother abuse history as determined as present or absent by the CTQ and cortisol levels measured from baseline (T0) to post-stressor (T1,T2,T3) time points divided by current depressive symptoms. The presence of current depression resulted in a different pattern of cortisol response to the stressor.
Figure 3.
Mother abuse history as determined as present or absent by the CTQ and cortisol levels measured from baseline (T0) to post-stressor (T1,T2,T3) time points divided by recent stressful life events. The presence of recent stressful life events resulted in a different pattern of cortisol response to the stressor.
Figure 4.
Mother abuse history as determined as present or absent by the CTQ and cortisol levels measured from baseline (T0) to post-stressor (T1,T2,T3) time points divided by maternal history of PTSD. The presence of a history of PTSD resulted in a different pattern of cortisol response to the stressor.
Maternal Abuse History and Infant Cortisol
ANCOVA revealed a statistically significant association between maternal abuse history and infant baseline cortisol (F(1,119)=5.15, eta squared=.04, p=.03). As can be seen Figure 1B, infants of mothers with a history of childhood abuse had lower baseline cortisol levels than controls. In contrast, infant cortisol change in response to the stressors was not associated with maternal abuse history, once differences in baseline were controlled (F(1,105)=.34, eta squared=.00, p=.56).
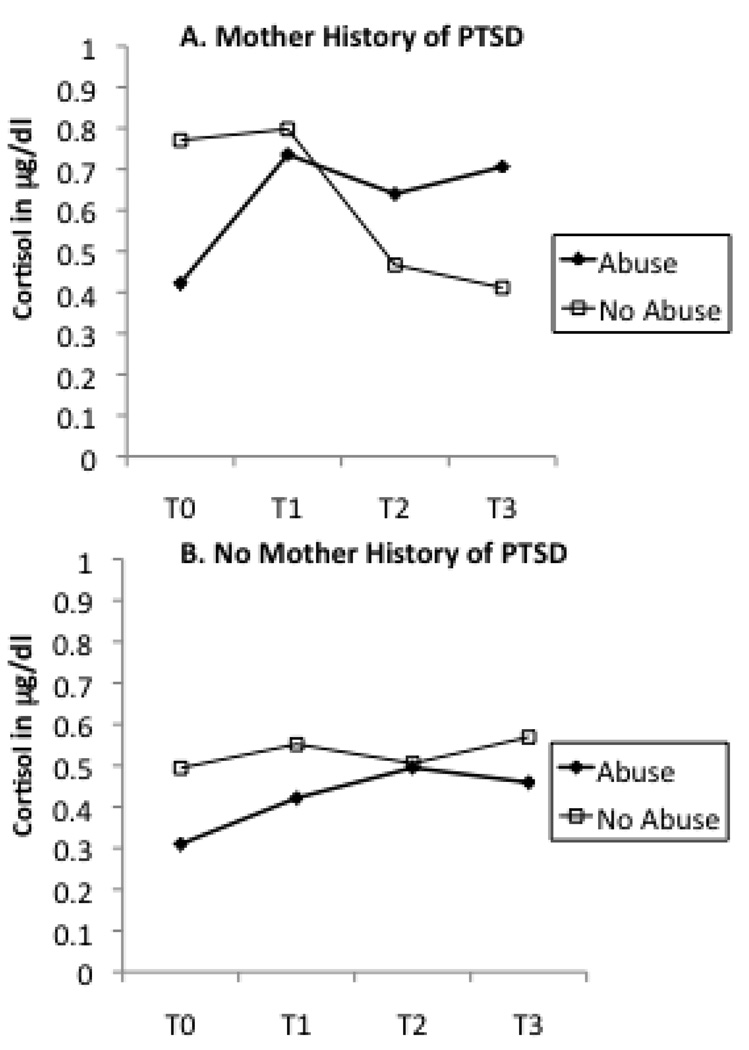
Table 2 presents the results of ANCOVAs testing whether current maternal depression, recent stressful life events, and maternal lifetime history of PTSD acted as moderators of the relationship between maternal abuse history and infant cortisol. Only one moderator term was statistically significant. Maternal lifetime history of PTSD interacted with maternal abuse history to predict infant change in cortisol. Figure 5 presents the cortisol levels of infants from T0 to T3 plotted according to maternal abuse history and PTSD. Infants whose mothers have a history of both child abuse and PTSD demonstrated the greatest increase in cortisol relative to baseline.
Table 2.
Moderators of maternal history of abuse and infant cortisol.
| Infant Baseline Cortisol | ||||
| Moderator | df | F | eta2 | p |
| Depressive Symptoms | 115 | 1.04 | .01 | .31 |
| Recent Life Events | 119 | 2.11 | .02 | .15 |
| History of PTSD | 119 | 1.68 | .02 | .20 |
| Infant Cortisol Change | ||||
| Moderator | df | F | eta2 | p |
| Depressive Symptoms | 102 | .78 | .01 | .38 |
| Recent Life Events | 105 | .12 | .00 | .73 |
| History of PTSD | 105 | 4.24 | .04 | .04 |
Figure 5.
Mother abuse history as determined as present or absent by the CTQ and infant cortisol levels measured from baseline (T0) to post-stressor (T1,T2,T3) time points divided by maternal history of PTSD. Infants of a mother with a history of both child abuse and PTSD demonstrate the greatest increase in cortisol relative to baseline.
Discussion
This study examined the relationship between early life trauma and HPA axis function in response to a laboratory stressor paradigm in a clinical sample of mother/infant dyads during the postpartum period. Infants whose mothers had a history of trauma showed significantly lower levels of baseline cortisol. No differences in baseline cortisol were found for mothers with a history of child abuse; however, these women demonstrated greater decreases in cortisol in the context of the infant stressor paradigm. Our finding that maternal cortisol decreases in response to stress is consistent with those seen in healthy women with a history of childhood maltreatment (Carpenter et al., 2007). The HPA axis hyporeactivity findings from the current investigation and the Carpenter study parallel those noted in primate and rodent studies of chronic social stress and subordination (Pohorecky et al., 2004; Saltzman et al., 2006). However, they do stand in contrast to the findings from the clinical samples linking ELS to HPA axis hyperreactivity (e.g., Heim et al., 2000). Further research is needed to see whether the contrasting results from community versus clinical samples can be accounted for by a higher level of current symptomology (e.g., depression, current life stress) in clinical samples, which we found to be moderating factors for HPA axis reactivity in the mothers in our study.
An examination of the moderating effect of depressive state, stressful life events, and comorbid PTSD on the relationship between early trauma and cortisol levels yielded statistically significant results for infant and maternal cortisol. The general pattern of results suggests that in mothers reporting childhood abuse and an additional risk factor, maternal cortisol levels were higher. These findings were also similar to those of Schechter and colleagues (Schechter et al., 2004) who found that mothers with higher levels of PTSD symptoms had the highest cortisol response to separation from their child. The majority of previous studies in clinical samples have found increased HPA axis reactivity in association with early life trauma or abuse, and that these effects are more prevalent in individuals with depressive disorders or symptoms (Heim, Mletzko, Purselle, Musselman, & Nemeroff, 2008; Heim et al., 2000; Young & Breslau, 2004). Our study suggests that current life stress and comorbid PTSD may also be important moderators of the ELS-HPA axis relationship.
MDD and PTSD are both associated with dysregulation of the HPA axis and more specifically down regulation of pituitary CRH receptors (Shea et al., 2004). Depressed patients have consistently exhibited increased basal cortisol levels in urine, cerebral spinal fluid, and plasma, and show an exaggerated cortisol response to ACTH. The biological findings in PTSD are less consistent with some investigators showing decreased cortisol levels in urine and plasma (Yehuda et al., 1995) and others have showing increased cortisol levels as compared to controls (Maes et al., 1998). As Shea and colleagues point out, these differential findings could be due to a number of factors such as type of trauma, the amount of time elapsed since the trauma, and the presence of comorbid MDD. Furthermore, the presence or absence of ELS influences the relationship between psychopathology and HPA axis dysregulation.
Our findings support the hypotheses of Heim and colleagues (2001) that ELS may alter the set point of the stress response system. Specifically, they propose that ELS may lead to sensitization of the anterior pituitary to CRF, perhaps reflecting a biological vulnerability to the effects of stress (2001). This vulnerability in turn increases the risk for eventual depression or anxiety in adulthood. While this study did not specifically examine CRF concentrations, our findings are consistent with this existing literature, and additionally extend the previous findings of the moderating effects of MDD to current life stress and PTSD.
ELS was not found to effect maternal baseline cortisol levels. One potential reason for the lack of expected findings is that our measure was not a “true” baseline. Before coming into the laboratory the mother had been informed about the nature of the study, and therefore could have already been anticipating the stress of the study when the first samples were taken. In the infants, no anticipation stress would occur, and therefore the baseline measure may have been more reflective of a “true” baseline. In the future, this study should be replicated using measures at home to better capture both maternal and infant baseline levels of cortisol.
The secondary aim of this study was to investigate possible transgenerational effects of ELS. While transgenerational effects were found, the infants did not show similar neuroendocrine profiles as their mothers. We have already noted the possibility that baseline measures taken from the mother and the infant may not have been comparable, due to the fact that the mother may have been anticipating the study in advance of her arrival to the laboratory. There are many other potential explanations for non parallel findings between the mothers and the infants in terms of their HPA axis responses. The first explanation is developmental. The HPA axis continues to develop throughout childhood and adolescence (Walker et al., 2001), and it is possible that after these infants go through adolescence, their neuroendocrine profiles will more closely resemble their mothers. The infants may also be in a developmental period of hyporesponsivity to stress, which would dampen findings related to the reactivity measure in particular. Alternatively, it may be the mother’s HPA axis that has changed over time. Lower baseline cortisol may have initially followed the childhood trauma (King et al., 2001), but may not have been sustained into adulthood, particularly if the mother eventually developed major depression, as all of the women in our sample eventually did. It is also possible that the laboratory paradigm used in the current study did not confer the same level of stress to both mother and baby. The stress paradigm utilized in this study was directed toward the infant rather than the mother; the impact of the “stressors” on the mother was arguably limited. The fact that such a minimal stressor produced significant group differences in cortisol responsivity suggests that our finding may be a conservative estimate of the relationship between childhood trauma and cortisol reactivity in depressed women.
Maternal comorbid PTSD also significantly moderated the relationship between ELS and infant cortisol levels, a finding consistent with Yehuda and colleagues (e.g. Yehuda et al., 2005, Yehuda et al., 2007). Our study found that maternal PTSD was a moderator for infant cortisol reactivity rather than baseline. In our sample, maternal childhood trauma was associated with lower infant baseline cortisol, regardless of maternal PTSD status. We noted a hyperactive cortisol response to stress in infants, however, only if their mother reported both early childhood trauma and PTSD. Our study also differed from Yehuda et al. (2005) in that we focused on the effects of maternal ELS on infant cortisol levels, whereas Yehuda focused on the effects of trauma and maternal PTSD experienced during pregnancy. More research is needed to understand how the timing of the stressor interacts with maternal PTSD to predict infant HPA axis abnormalities.
Currently, the field of biological psychology is not at a place where we can make accurate predictions about the impact of low cortisol levels (as seen in the infants of mothers with significant early life stress) on future behavioral or neuroendocrine outcomes. However previous research has shown associations between low basal cortisol levels and disruptive behavior in children (McBurnett et al., 2000). Similarly, the clinical implications of the moderator findings cannot yet be applied at an individual level of prediction. But there are studies that indicate that increased cortisol reactivity to laboratory stressors may be related to internalizing problems later in development (Ashman et al., 2002). In addition, the knowledge that a mother suffers both from ELS and PTSD or recent depressive symptoms might be a useful indicator a potential vulnerability of infant’s stress response system.
The current study has other notable limitations. The study group is demographically homogeneous, all women had a history of MDD and participated in a longitudinal study spanning over 12 months therefore potentially limiting the ability to generalize the findings to other maternal groups. Our study examined physical and sexual abuse histories, combined into one trauma indicator. Post-hoc analyses suggested that sexual abuse history largely accounted for our findings. Studies with larger samples should examine specific types of abuse histories in relation to HPA axis function.
Despite the limitations of the current study, these novel data raise questions about the impact of moderators of HPA axis function in postpartum women with a history of early abuse and provide evidence for early detection of transgenerational effects of childhood trauma. The findings from this study add to the existing knowledge of the effects of maternal trauma on biological responses by showing that they may only be evident under circumstances, such as when occurring in combination with additional life stress. Furthermore, this study demonstrates that significant early life stress can have lasting impacts on both women and their infants.
Acknowledgements
The authors thank research staff and Eric Vanman for their assistance with data collection and coding.
Role of Funding Source:
Funding for this study was provided by a NARSAD Young Investigator Grant awarded to Brennan, the Emory University Silvio O. Conte Center for the Neurobiology of Mental Disease (MH58922), and a Specialized Center of Research (SCOR) on Sex and Gender Effects (MH68036). The funding agencies had no further role in study design; in the collection, analysis and interpretation of the data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
Dr. Newport has received research support from Eli Lilly, GlaxoSmithKline (GSK), Janssen, and Wyeth as well as NARSAD and NIH, and speaker’s honoraria from Astra-Zeneca, Eli Lilly, GSK, and Pfizer. Dr. Stowe has received research support from GSK, NIH, and Wyeth, served on advisory boards for Wyeth, Bristol Myers Squibb (BMS), and GSK, and received speaker’s honoraria from Eli Lilly, GSK, Pfizer, and Wyeth. Dr. Smith receives research support from the American Society for Suicide Prevention and Schering Plough Pharmaceuticals. All of the remaining authors have no past or present financial ties to for-profit enterprises.
Contributors:
Drs. Newport, Stowe, and Brennan were involved in all aspects of study design, data collection and manuscript preparation. Drs. Weiss and Smith were involved in manuscript preparation, and Ms. Brand contributed to the statistical analyses and wrote the first draft and final draft of the manuscript. All authors contributed to and have approved the final manuscript.
References
- Ashman SB, Dawson G, Panagiotides H, Yamada E, Wilkinson CW. Stress hormone levels of children of depressed mothers. Dev. Psychopathol. 2002;14:333–349. doi: 10.1017/s0954579402002080. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. The Beck Depression Inventory. 2nd ed. San Antonio: The Psychological Corporation; 1996. [Google Scholar]
- Berstein D, Fink L. Childhood Trauma Questionnaire: A Retrospective Self-Report. San Antonio: The Psychological Corporation; 1998. [Google Scholar]
- Brennan PA, Pargas R, Walker EF, Green P, Newport DJ, Stowe Z. Maternal depression and infant cortisol: Influences of timing, comorbidity, and treatment. J. Child. Psychol. Psychiatry. 2008;49:1099–1107. doi: 10.1111/j.1469-7610.2008.01914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello AF, et al. Decreased adrenocorticotropic hormone and cortisol response to stress in healthy adults reporting significant childhood maltreatment. Biol. Psychiatry. 2007;62:1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, Anda RF. Adverse childhood experiences and the risk of depressive disorders in adulthood. J. Affect. Disord. 2004;82:217–225. doi: 10.1016/j.jad.2003.12.013. [DOI] [PubMed] [Google Scholar]
- de Kloet CS, Vermetten E, Heijen CJ, Geuze E, Lentjes EG, Westernberg HG. Enhanced cortisol suppression in response to dexamethasone administration in traumatized veterans with and without posttraumatic stress disorder. Psychoneuroendocrinology. 2007;32:215–226. doi: 10.1016/j.psyneuen.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Dohrenwend BS, Krasnoff L, Askensay AR, Dohrenwend BP. The psychiatric epidemiology research interview life events scale. In: Golberger L, Breznitz S, editors. Handbook of Stress: Theoretical and Clinical Aspects. New York: Free Press; 1982. [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The adverse childhood experiences study. Am. J. Prev. Med. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Field T, Diego M, Hernandez-Reif M. Prenatal depression effects on the fetus and newborn: a review. Infant. Behav. Dev. 2006;29:445–455. doi: 10.1016/j.infbeh.2006.03.003. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structural Clinical Interview for the DSM-IV Axis I Disorders. Washington DC: American Psychiatric Press; 1995. [Google Scholar]
- Goldberg S, Levitan R, Leung E, Masellis M, Basile VS, Nemeroff CB, Atkinson L. Cortisol concentrations in 12- to 18-month-old infants: Stability over time, location and stressor. Biol. Psychiatry. 2003;54:719–726. doi: 10.1016/s0006-3223(03)00010-6. [DOI] [PubMed] [Google Scholar]
- Heim C, Mletzko T, Purselle D, Musselman DL, Nemeroff CB. The dexamethasone/corticotropin-releasing factor test in men with major depression: Role of childhood trauma. Biol. Psychiatry. 2008;63:398–405. doi: 10.1016/j.biopsych.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am. J. Psychiatry. 2001;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. J.A.M.A. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kuhn JW, Prescott CA. Childhood sexual abuse, stressful life events and risk for major depression in women. Psychol. Med. 2004;34:1475–1482. doi: 10.1017/s003329170400265x. [DOI] [PubMed] [Google Scholar]
- King JA, Mandansky D, King S, Fletcher KE, Brewer J. Early sexual abuse and low cortisol. Psychiatry and Clinical Neurosciences. 2001;55:71–74. doi: 10.1046/j.1440-1819.2001.00787.x. [DOI] [PubMed] [Google Scholar]
- Lemieux AM, Coe CL. Abuse-related posttraumatic stress disorder: evidence for chronic neuroendocrine activation in women. Psychosom. Med. 1995;57:105–115. doi: 10.1097/00006842-199503000-00002. [DOI] [PubMed] [Google Scholar]
- Maes M, Lin A, Bonaccoroso S, van Hunsel F, Van Gastel A, Delmeire L, et al. Increased 24-hour urinary cortisol excretion in patients with posttraumatic stress disorder and patients with major depression, but not in patients with fibromyalgia. Acta Psychiatr. Scand. 1998;98:328–335. doi: 10.1111/j.1600-0447.1998.tb10092.x. [DOI] [PubMed] [Google Scholar]
- Marcus SM, Flynn HA, Blow FC, Barry KL. Depressive symptoms among pregnant women screened in obstetrics settings. J. Women. Health. 2003;12:273–280. doi: 10.1089/154099903765448880. [DOI] [PubMed] [Google Scholar]
- McBurnett K, Lahey BB, Rathouz PJ, Loeber R. Low salivary cortisol and persistent aggression in boys referred for disruptive behavior. Arch. Gen. Psychiatry. 2000;57:38–43. doi: 10.1001/archpsyc.57.1.38. [DOI] [PubMed] [Google Scholar]
- McCauley J, Kern DE, Koloder K, Dill L, Schroeder AF, DeChant HK, et al. Clinical characteristics of women with a history of childhood abuse: unhealed wounds. J.A.M.A. 1997;277:1362–1368. [PubMed] [Google Scholar]
- Milgrom J, Ericksen J, Negri L, Gemmill AW. Screening for postnatal depression in routine primary care: Properties of the Edinburgh Postnatal Depression Scale in an Australian sample. Aust. N. Z. J. Psychiatry. 2005;39:833–839. doi: 10.1080/j.1440-1614.2005.01660.x. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA, Baumann MH, Benjamin D. Effects of chronic social stress on neuroendocrine responsiveness to challenge with ethanol, dexamethasone and corticotropin-releasing hormone. Neuroendocrinology. 2004;80:332–342. doi: 10.1159/000083682. [DOI] [PubMed] [Google Scholar]
- Ramsay D, Lewis M. Reactivity and regulation in cortisol and behavioral responses to stress. Child. Dev. 2003;74:456–464. doi: 10.1111/1467-8624.7402009. [DOI] [PubMed] [Google Scholar]
- Saltzman W, Hogan BK, Abbott DH. Diminished cortisol levels in subordinate female marmosets are associated with altered central drive to the hypothalamic-pituitary-adrenal axis. Biol Psychiatry. 2006;60:843–849. doi: 10.1016/j.biopsych.2005.12.006. 55. [DOI] [PubMed] [Google Scholar]
- Schechter DS, Zeanah CH, Myers MM, Brunelli SA, Liebowitz MR, Marshall RD, et al. Psychobiological dysregulation in violence-exposed mothers: salivary cortisol of mothers with very young children pre- and post-separation stress. Bull. Menninger. Clin. 2004;68:319–336. doi: 10.1521/bumc.68.4.319.56642. [DOI] [PubMed] [Google Scholar]
- Shea A, Walsh C, MacMillan H, Steiner M. Child maltreatment and HPA axis dysregulation: relationship to major depressive disorder and posttraumatic stress disorder in females. Psychoneuroendocrinology. 2004;30:162–178. doi: 10.1016/j.psyneuen.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Stein MB, Yehuda R, Koverola C, Hanna C. Enhanced dexamethasone suppression of plasma cortisol in adult women traumatized by childhood sexual abuse. Biol. Psychiatry. 1997;42:680–686. doi: 10.1016/s0006-3223(96)00489-1. [DOI] [PubMed] [Google Scholar]
- Walker EF, Walder DJ, Reynolds F. Developemntal changes in cortisol secretion in normal and at-risk youth. Dev. Psychopathol. 2001;13:721–732. doi: 10.1017/s0954579401003169. [DOI] [PubMed] [Google Scholar]
- Wilder J. Modern psychophysiology and the Law of Initial Value. Am. J. Psychotherapy. 1958;12:199–221. doi: 10.1176/appi.psychotherapy.1958.12.2.199. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Engel SM, Brand SR, Seckl J, Marcus SM, Berkowitz GS. Transgenerational effects of posttraumatic stress disorder in babies of mothers exposed to the World Trade Center attacks during pregnancy. J. Clin. Endocrinol. Metab. 2005;90:4115–4118. doi: 10.1210/jc.2005-0550. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Kahana B, Binder-Brynes K, Southwick SM, Mason JW, Giller EL. Low urinary cortisol excretion in holocaust survivors with posttraumatic stress disorder. Am. J. Psychiatry. 1995;152:982–986. doi: 10.1176/ajp.152.7.982. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Teicher MH, Seckl JR, Grossman RA, Morris A, Bierer LM. Parental posttraumatic stress disorder as a vulnerability factor for low cortisol trait in offspring of holocaust survivors. Arch. Gen. Psychiatry. 2007;64:1040–1048. doi: 10.1001/archpsyc.64.9.1040. [DOI] [PubMed] [Google Scholar]
- Young EA, Breslau N. Saliva cortisol in posttraumatic stress disorder: a community epidemiologic study. Biol. Psychiatry. 2004;56:205–209. doi: 10.1016/j.biopsych.2004.05.011. [DOI] [PubMed] [Google Scholar]