Abstract
Previously we reported that prepubertally ovariectomized mice that received young, transplanted ovaries at a postreproductive age displayed a 40% increase in life expectancy. To study this increase in life expectancy in greater detail, prepubertally ovariectomized and ovary-intact CBA/J mice underwent ovarian transplantation at 11 months with 60-day-old ovaries or a sham surgery. Life span was significantly increased in transplant recipients. Body weight changes of mice in each group were measured from the time of surgery (11 months) to death. Neither ovariectomy nor ovarian transplantation influenced the amount of peak body weight attained or body weight retained at death. However, the time (days) to peak body weight was decreased by ovariectomy and ovarian transplant recipients displayed a trend toward an increase in time to peak weight. In addition, ovarian transplantation decreased the rate of weight loss to death. These results demonstrate that ovarian status, examined by means of ovariectomy and ovarian transplantation, clearly influenced the rate of weight change, but not the total amount of weight gain or loss in female mice.
Keywords: ovary, ovariectomized, ovarian transplantation, body weight, rate of gain, rate of loss, gonadal influence, life span, weight gain, weight loss, longevity
1. Introduction
The origin of accelerated accumulation of body fat at menopause, which often leads to changes in weight gain, is not well defined. Are the changes derived from reproductive alterations, the aging process per se, or are the changes a combination of factors? Reproductive hormones, which undergo drastic changes at menopause, have many actions that affect growth, body weight and adiposity, including effects on appetite, energy expenditure, gastrointestinal function, metabolism and body composition (Williams 2004). Ovariectomy in rodents is one approach to modeling human menopause and studying changes due to ovarian function. In aged rats, when irregular cycles begin to appear, estrogen levels remain relatively elevated, whereas in aged mice and humans, estrogen levels decline (Rubin et al., 2000). This physiological similarity between mice and humans has made the mouse the predominant mammalian model for studying estrogen-related aging phenomena.
Reports of the effects of ovariectomy on body weight and fat mass can be conflicting. Body fat in ovariectomized animals has been reported to be increased (Leshner and Collier, 1973; Rogers et al., 2009), decreased (Galletti and Klopper, 1964) and unchanged (Clark and Tarttelin, 1982; Holt et al., 1936; Nyda et al., 1948). Changes in the fat mass of menopausal women are reported in a similarly-conflicting manner with increases (Lovejoy et al., 2008), decreases (Aloia et al., 1991) and no changes (Aubertin-Leheudre et al., 2008; Wang et al., 1994) being reported. In mice, many studies from which the conflicting effects of ovariectomy are reported were short-term and/or utilized young mice at an age of rapid growth, making identification of any compensatory adaptations difficult.
Previously, we reported that prepubertally ovariectomized mice that received young, transplanted ovaries at a postreproductive age displayed a 40% increase in life expectancy (Cargill et al., 2003). To study this phenomenon in greater detail, prepubertally ovariectomized and ovary-intact CBA/J mice underwent ovarian transplantation with 60-day-old ovaries or a sham surgery at 11 months of age. Life span was increased in transplant recipients (Mason et al., 2009). During this study, body-weight changes of mice in each group were measured from the time of surgery (11 months) to death. Reported here are the effects of ovarian transplantation and ovariectomy on the amount and rate of weight gain and loss.
2. Materials and methods
2.1. Mice
The CBA strain (used in the current study) and the DBA strain of mice are unique in that they prematurely lose their ovarian follicles, becoming reproductively senescent by 10–11 months of age (Faddy et al., 1987; Jones and Krohn, 1961; Thung et al., 1956). Since a reduction of ovarian follicles in the human may be associated with the onset of menopause, it has been suggested that CBA and DBA mice may serve as appropriate experimental models to study age-related changes in the human reproductive system (Barnett et al., 2006; Gosden et al., 1978; Thung et al., 1956).
Adult (40g) CBA/J strain female mice (Jackson Laboratory, Bar Harbor, ME) were provided ad libitum access to feed (Purina Mouse Chow 5008: 23.5% protein, 6.5% fat, Purina Mills, St. Louis, MO; http://labdiet.com/pdf/5008.pdf) and water (deionized) and were housed under conditions of constant temperature (21° C ± 2° C), humidity (min. 50%), and lighting (14L: 10D, lights-on at 0700 h). Individual pups were weaned and ear-notched at 21 days (day of birth = 0 days). All female weanlings were housed individually, with added enrichment, in a 26 × 17 × 13 cm shoebox cage in a specific-pathogen-free (SPF) colony where pathology on sentinel mice was done quarterly and pathological results showed no breach in this status. Mice were maintained in an American Association for Accreditation of Laboratory Animal Care (AAALAC)-approved facility in accordance with NIH animal-use guidelines. Animal care and use protocols were developed under National Research Council guidelines found in the Guide for the Care and Use of Laboratory Animals. This project was approved by the University of California, Davis Institutional Animal Care and Use Committee.
2.2. Experimental Design
Animals were randomly assigned to Control, Sham-transplant or Ovarian-transplant groups as follows (Fig 1.):
Fig. 1.
Experimental design. IT (intact), OX (ovariectomized), S (sham), and TX (ovarian transplant).
Controls consisted of
IT: Controls included intact animals that were not subjected to any surgical procedures.
Shams consisted of
IT-S: Intact sham animals remained intact to 11 months, at which time they underwent a sham surgery where we removed and replaced both endogenous ovaries.
OX-S: Ovariectomized sham animals were ovariectomized at 21 days and subsequently, at 11 months, underwent a sham surgery where we removed the glass beads previously placed into the ovarian bursae during the 21-day ovariectomy procedure.
Ovarian Transplants consisted of
IT-TX: Intact transplant animals remained intact to 11 months, at which time their endogenous ovaries were removed and replaced with a pair of donor ovaries from a 60-day-old mouse.
OX-TX: Ovariectomized transplant animals were ovariectomized at 21 days and subsequently, at 11 months, the glass beads previously placed into the ovarian bursae during the 21-day ovariectomy procedure were removed and replaced with a pair of donor ovaries from a 60-day-old mouse.
Sham treatments served as surgery controls for transplant procedures. All animals in each experimental group remained virgin until death.
2.3. Age at Manipulation
Mice of the CBA/J strain become reproductively competent between 45 and 60 days of age. Ovariectomy at 21 days of age was chosen to avoid major up-regulation of the reproductive system at the onset of puberty and to eliminate other influences the female gonad might have in addition to direct effects of gonadal hormones. These influences may include positive or negative feedback mechanisms or system-wide “imprinting” influences the ovary may normally provide after 21 days of age. Rodents do not undergo menopause but instead have an estropause-like decrease in reproductive function. Reproductive decline in CBA/J mice usually begins with irregular cycles at 8–10 months of age. At 11 months of age, many CBA/J mice have become reproductively incompetent (Cargill et al., 2003) with a complete loss of oocytes by 12 months of age. Ovarian transplantation and sham surgeries were conducted at 11 months of age as many females in this line of mice have reached a point of reproductive failure by this time.
2.4. Surgical Procedures
Bilateral ovariectomies at 21 days and ovarian transplantation and sham surgeries at 11 months were performed as previously described (Cargill et al., 1999). Briefly, the ovaries were exposed by paralumbar incision under sodium pentobarbital anesthesia and removed by incising the ovarian bursa opposite the ovarian hilum. The ovary was gently removed from the ovarian bursa and excised by clamping the ovarian hilum to prevent bleeding. Excised ovaries were placed in cold saline prior to transfer/replacement. The ovarian bursa was closed with one to three sutures of 10-0 Ethilon monofilament (Ethicon, Inc.). The abdominal wall was sutured with 5-0 chromic gut (Ethicon, Inc.), and the skin was closed with 9 mm wound clips (MikRon Precision, Inc.). When we performed ovariectomies at 21 days of age, a sterile 1-mm diameter glass bead was inserted into each empty ovarian bursa to keep it open for future ovarian transplantation or sham surgery.
At 11 months of age, bilateral ovarian transplantation and sham surgeries were performed as previously described (Cargill et al., 1999). Briefly, OX-S animals were subjected to sham ovarian transplant surgery in which the glass bead placed at 21 days of age was removed, but no ovary was transplanted. Intact sham animals underwent sham ovarian transplant surgery in which their endogenous ovaries were removed, placed in cold saline and returned to the original bursae. Intact transplant animals underwent a bilateral ovariectomy and subsequent ovarian transplantation, and received a pair of 60-day-old ovaries from a donor mouse of the same strain. The OX-TX cohort underwent ovarian transplantation in which the glass beads placed at 21 days of age were removed and replaced with a pair of ovaries from a 60-day-old donor mouse of the same strain. Data on vaginal cytology were collected for at least 10 consecutive days pre- and post-surgery to ensure: 1) complete removal of the ovarian tissue, and 2) success of the ovarian transplantation procedure. Vaginal smears were taken daily beginning 10–14 days after surgery. One estrous cycle was defined as the period from the day nucleated epithelial cells first appeared (i.e., proestrus) to the day preceding the next appearance of nucleated epithelial cells in the vaginal smear, provided there was a period of leukocytic presence (i.e., diestrus) in between. Estrus was determined by the presence of large, squamous epithelial cells, with or without nuclei. After surgery, each female was housed individually in a 26 × 17 × 13 cm shoebox cage. Additionally, all serum samples submitted from mice at the time of necropsy were negative for parvovirus. Animal weights were collected at 11 months of age and at 1-month intervals thereafter. At 1-month intervals, mice were weighed daily between 6:00 AM and 9:00 AM for 3 consecutive days. Means of 3 daily weights were used to determine the monthly weight value for each mouse.
2.5. Exclusion Criteria
Presumptive ovariectomized mice that displayed signs of gonadal input prior to surgery at 11 months were excluded from analysis. Gonadal input was defined as cyclic changes within the population of vaginal cells, presumably due to cyclic influence of ovarian hormones. No gonadal input was defined as the lack of cyclic changes within the vaginal cell population. Ovarian transplant recipients that failed to display evidence of gonadal input after transplant surgery were also excluded from analysis. Gonadal input was determined by vaginal cytology analysis, as described in Surgical Procedures. Mice that displayed no cyclic activity for a 10-day period before and/or after surgery were determined to have no gonadal input for said period. Mice that displayed at least one full estrous cycle in a 10-day period before and/or after surgery were determined to have gonadal input for said period. Additional selection criteria included technical and pathological conditions that prevented accurate analysis of weight gain/loss. This additional selection included, but was not limited to, incorrectly collected data, weight gain/loss due to obvious neoplastic growth or other pathological conditions preventing collection of sufficient weight data. Mice that fit these criteria were the only mice used for analysis throughout this study.
2.6. Statistical analysis
Experimental data were analyzed preliminarily by one-way ANOVA and linear regression analysis. Significance tests for each predictor in the multiple linear regression model were implemented with the Student’s t-test, two-tailed, unequal distribution of variance assumed and Chi-square (χ2) analysis where appropriate. Test results were considered significant for P-values p < 0.05.
3. Results
3.1. Effects from birth to 11 months of age
Prior to surgery at 11 months of age, intact mice were not subjected to any surgical procedures and, as expected, no body weight changes were observed within intact groups to 11 months of age. Mice that were subjected to prepubertal ovariectomy at 21 days of age mice appeared to gain weight faster than intact mice to 11 months. While no differences were observed between any group in body weight at 11 months of age (Fig. 2A), ovariectomized mice attained a greater percentage of their ultimate peak weight by 11 months, compared with intact mice (93.4% vs. 91.0%, respectively, p = 0.012, Fig. 2B).
Fig. 2.
A) Static body weight at 11 months of age, peak weight and at death. No differences were observed between groups in regard to static body weight at 11 months, peak weight or weight at death. B) Percent of peak body weight attained by 11 months of age. By 11 months of age, ovariectomized mice had reached a higher proportion of their peak body weight, compared with intact mice.
3.2. Effects from 11 months of age to peak weight
No differences were observed between groups with respect to total peak weight attained (Fig. 2A). The effects of surgery at 11 months of age were evident in both sham and transplant groups. Compared with non-surgical control mice, intact mice that underwent a sham surgery at 11 months of age displayed a drop in body weight at 12 months of age (Fig. 3A). Ovariectomized sham mice and intact and ovariectomized transplant recipients also experienced a similar body-weight drop after surgery (Fig. 3B).
Fig. 3.
Effects of surgery at 11 months of age. A) Intact sham mice experienced a drop in body weight after surgery at 11 months of age. No drop in body weight was observed in non-surgical control mice during this same period. B) Ovariecomized sham mice and both transplant groups also experienced a drop in body weight after surgery.
The surgery-associated drop in body weight in sham and transplant mice had no significant effect on the amount of peak body weight attained or body weight at death in these groups (Fig. 2A.). Differences were observed in the rate of body weight change, seen as days from 11 months to peak weight (p = 0.001), between non-surgical, control mice and the surgical groups. Surgical mice took fewer days to reach peak weight than non-surgical, control mice (469d vs. 516d, respectively, Fig. 4A).
Fig. 4.
Age when peak weight was reached. A) Surgical mice reached peak weight earlier than non-surgical control mice. B) Ovariectomized mice reached peak weight earlier than intact mice.
Since the non-surgical, control mice were the only group that did not experience a drop in body weight after 11 months, their subsequent body-weight dynamics likely occurred under different conditions than the sham and transplant mice. Consequently, non-surgical controls will be excluded from subsequent comparisons (The Intact group will include IT-S and IT-TX mice, but not IT mice).
Prepubertal ovariectomy affected the rate of weight change from 11 months to peak weight. Ovariectomized mice required less time (d) to reach peak weight than intact mice (OVX = 102d, Intact = 158d, p < 0.001, Fig. 4B), but the rate of gain (grams per day) was not different between the two groups (0.017g/d vs. 0.020g/d, respectively). Ovarian transplantation appeared to slow the rate of weight change from 11 months to peak weight, but this effect was not significant for intact or ovariectomized recipients (Fig. 5A&B).
Fig. 5.
Effect of ovarian transplantation from 11 months to peak weight.
3.3. Effects from peak weight to death
No differences were observed between groups with respect to weight retained at death (Fig. 2A). The days from peak weight to death was strongly influenced by ovariectomy and ovarian transplantation (Fig. 6A). However, mice that received ovarian transplants lived significantly longer than sham-treated mice, which heavily influenced weight-loss analysis (Mason et al., 2009).
Fig. 6.
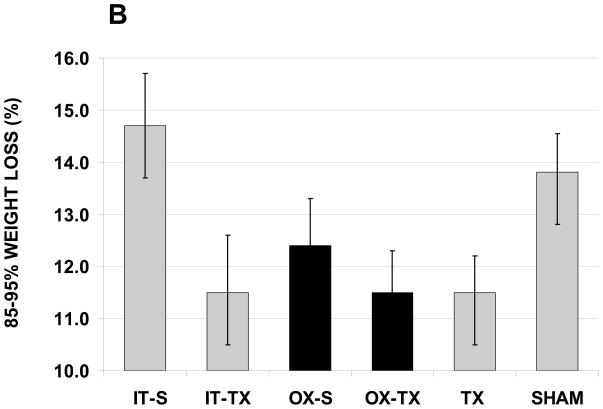
Weight loss for all groups. A) Days from peak weight to death was increased in ovariectomized mice and transplant recipients. B) Weight loss during the 85–95% survival period was significantly less in transplant recipients.
To minimize this influence, the percentage of weight loss from 85 to 95% of the life span was determined (e.g., if a mouse lived 1000 days, the percent weight loss would equal weight loss from 850 to 950 days of age, divided by weight at 11 months of age [age at peak weight was variable between groups, 11 months was not]). This period of the life span reflected the period of greatest change in survival among all groups. After this adjustment, transplant mice displayed a decrease in percentage of body weight lost during this period, compared with sham mice (11.5% vs. 13.8%, respectively, p = 0.028, Fig. 6B). To eliminate the effect of prepubertal ovariectomy on this adjustment, we compared intact transplant mice with intact sham mice and found similar results. Intact transplant mice displayed a decrease in percentage of body weight lost during this period, compared with intact sham mice (11.5% vs. 14.7%, respectively, p = 0.042).
4. Discussion
Manson and colleagues demonstrated that body weight is directly related to mortality from all causes in middle-aged women (Manson et al., 1995). Results from other studies suggest that postmenopausal weight status, body composition and body fat distribution may be related to various parameters of menstrual and reproductive history (Kirchengast et al., 1999; Troisi et al., 1995). Previous studies in rodents have shown that ovariectomy increases adiposity (Liang et al., 2002; Meli et al., 2004; Richard, 1986; Wade and Gray, 1979) and that estrogen supplementation in ovariectomized mice decreases adipose tissue mass, compared with non-supplemented ovariectomized mice (D’Eon et al., 2005).
In the current study, prepubertal ovariectomy at 21 days of age produced no differences in the amount of body weight at 11 months, peak body weight attained or body weight retained at death. Other authors have reported increases in body weight after ovariectomy (Davidge et al., 2001; Shimomura et al., 2002; Wade and Gray, 1979), but many of these studies were focused on the period immediately after ovariectomy, whereas our study was focused on long-term effects and followed the mice from 11 months of age to death. Couse et al. (1999) reported that α-estrogen receptor knockout (αERKO) mice display an increase in body weight at 8 months of age compared with wild-type controls. These authors also reported that by 12 months of age the wild-type controls had significantly increased their body weights and had diminished the bodyweight gap between the two genotypes, suggesting that the lack of the estrogen-receptor affected the rate of gain, but not the total amount of weight gained, similar to the findings in our mice.
In our mice, prepubertal ovariectomy had no effect on the total amount of weight gained, but did effect the time to peak weight. Ovariectomized mice reached peak body weight 56 days sooner than intact mice. Even when mice received new ovaries, ovariectomized recipient mice still reached peak weight 50 days sooner than non-ovariectomized recipients. Ovariectomized transplant mice had a similar rate-of-gain as ovariectomized sham mice, from 11 months of age to attainment of peak weight, which represents the period of influence of the new ovaries. Neuroendocrine feedback mechanisms continue to evolve between weaning and adulthood (Goldman et al.,1973). This observation suggests ‘plasticity’ of the neuroendocrine system during this period in development, which may provide an opportunity for epigenetic reprogramming of metabolism or an ‘imprinting’ event to occur. Lack of gonadal input, due to prepubertal ovariectomy at 21 days of age, may have left an ‘imprint’ on the neuroendocrine system of these mice that could not be overcome, even by the transplantation of new ovaries.
Clark and Tarttelin (1982) reported that estrogen treatment of ovariectomized animals reduced both the rate of body-weight gain and the ultimate body weight. The current results show that the transplantation of new ovaries at 11 months of age affected the rate of body weight gain, similar to that seen with estrogen supplementation. However, in contrast to the report of Clark and Tarttelin (1982), the total amount of weight gained was not influenced by the new ovaries. The ultimate body weight appears to be independent of ovarian status. Additionally, Shaw et al. (1983) reported that ovariectomy appears to have no effect on rate of weight gain. In the current study, ovariectomy at 21 days increased the rate of body weight gain from birth to peak weight. The CBA/J strain of mice used in the current study is a special strain that looses its ovarian follicles by 1 year of age (Parkening et al., 1984) making direct comparisons with mice of other strains or rats difficult. Differences in results may be related to experimental design, as Shaw et al. (1983) utilized congenitally obese Zucker rats. Clark and Tarttelin (1982) utilized Sprague-Dawley rats of the Simonsen strain and final data collection extended to only 15 weeks of age.
The current results demonstrate that ovarian status, examined by means of ovariectomy and ovarian transplantation, clearly influenced the rate of weight change, but had no significant influence on the total amount, or set-point, for weight attained at 11 months of age (the normal time of reproductive senescence in female CBA/J mice), total peak weight attained or weight retained at death. Attributes that can influence rate-of-change without altering set-point values may be suggestive of metabolic influences. Resting energy expenditure in humans appears to decrease more during the menopausal transition than what could normally be attributed to the aging process per se (Ravussin et al., 1988). Ovariectomy in mice decreases energy expenditure without altering energy intake (Rogers et al., 2009), suggesting an energy surplus, which may be stored as increased adipose tissue mass and could be influential in the rate of body-weight gain.
Hormone replacement therapy is known to correct many menopausal symptoms and to attenuate gains in fat mass and losses of fat-free mass (Aloia et al., 1991; Svendsen et al., 1994). While fat mass and fat-free mass were shown to be similar between postmenopausal women using hormone replacement therapy and those not using therapy, resting energy expenditure was greater in the women using hormone therapy (Aubertin-Leheudre et al., 2008). Hormone-treated subjects with a greater resting energy expenditure and similar energy intake would be expected to have a decrease in surplus energy and have less energy to store in adipose tissue. This phenomenon may be reflective of the conditions in our ovarian transplant recipients with their presumptive increased exposure to ovarian hormones and decreased rate of weight gain.
Adult female rats and mice, as well as women, eat less during the periovulatory phase of the ovarian cycle than during other phases, an effect under the control of cyclic changes in estradiol secretion (Geary, 2004). The observation that exogenous estrogen inhibited eating only in postpubertal rats suggests that maturation of the hypothalamic-pituitary-gonadal axis may be required for the estrogenic inhibition of eating (Asarian and Geary, 2006; Wade, 1974; Wade and Zucker 1970). In our mice, maturation of the hypothalamic-pituitary-gonadal axis was likely disturbed by prepubertal ovariectomy at 21 days of age. This observation may, in part explain the rate decrease in our transplant recipients and the rate increase in our ovariectomized mice.
The decrease in rate of weight loss in transplant recipients may be reflective of a difference in body composition or a decreased storage of adipose tissue in transplant recipients. Fat mass is lost more rapidly than fat-free mass (Aloia et al., 1991) and would be expected to directly affect the rate of weight loss. The minimal significance of the observed difference in rate of loss (p = 0.047) may be due to the relatively short period for adaptation to new ovaries in these mice. As mentioned previously, transplant recipients possessed new ovaries for only a short period before attainment of peak weight, allowing only a short time for the new ovaries to influence body composition at peak-weight.
The vulnerability to eating disorders and the increased frequency of morbid obesity in menopausal women (Flegal et al., 2002; Freedman et al., 2002) suggest that the effects of ovarian hormones on human energy balance may hold pathophysiological significance. Our data indicate that ovarian influence affects the rate of body-weight gain and loss, but not the set-point or total amount of weight gained or lost in female mice. Further research into the mechanisms of these phenomena may lead to ovarian influence-based treatments for suboptimal weight gain and loss in menopausal women.
Acknowledgments
We gratefully acknowledge Dr. Thomas Famula, Alice Moyer and Sandra Weisker for their intellectual support and technical assistance. Research was funded, in part, by NIA/NIH grants PO1 AG022500-01 and PO1 AG08761-10 and the Center for the Economics and Demography of Aging, UC Berkeley. JBM was supported, in part, by an American Physiological Society Porter Physiology Fellowship and a University of California, Davis Lyons Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aloia JF, McGowan DM, Vaswani AN, Ross P, Cohn SH. Relationship of menopause to skeletal and muscle mass. Am J Clin Nutr. 1991;53:1378–1383. doi: 10.1093/ajcn/53.6.1378. [DOI] [PubMed] [Google Scholar]
- Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philos T Roy Soc B. 2006;361:1251–1263. doi: 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubertin-Leheudre M, Goulet EDB, Dionne IJ. Enhanced rate of resting energy expenditure in women using hormone-replacement therapy: Preliminary results. J Aging Phys Activ. 2008;16:53–60. doi: 10.1123/japa.16.1.53. [DOI] [PubMed] [Google Scholar]
- Barnett KR, Schilling C, Greenfeld CR, Tomic D, Flaws JA. Ovarian follicle development and transgenic mouse models. Hum Reprod Update. 2006;12:537–555. doi: 10.1093/humupd/dml022. [DOI] [PubMed] [Google Scholar]
- Cargill SL, Medrano JF, Anderson GB. Age of ovary determines remaining life expectancy in old ovariectomized mice. Aging Cell. 2003;2:185–190. doi: 10.1046/j.1474-9728.2003.00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cargill SL, Medrano JF, Anderson GB. Infertility in a line of mice with the high growth mutation is due to luteal insufficiency resulting from disruption at the hypothalamic-pituitary axis. Biol Reprod. 1999;61:283–287. doi: 10.1095/biolreprod61.1.283. [DOI] [PubMed] [Google Scholar]
- Clark RG, Tarttelin MF. Some effects of ovariectomy and estrogen replacement on body composition in the rat. Physiol Behav. 1982;28:963–969. doi: 10.1016/0031-9384(82)90161-5. [DOI] [PubMed] [Google Scholar]
- Couse JF, Korach KS. Estrogen receptor null mice: What have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- D’Eon TM, Souza SC, Aronovitz M, Obin MS, Fried SK, Greenberg AS. Estrogen regulation of adiposity and fuel partitioning - Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J Biol Chem. 2005;280:35983–35991. doi: 10.1074/jbc.M507339200. [DOI] [PubMed] [Google Scholar]
- Davidge ST, Zhang YL, Stewart KG. A comparison of ovariectomy models for estrogen studies. Am J Physiol-I. 2001;280:R904–R907. doi: 10.1152/ajpregu.2001.280.3.R904. [DOI] [PubMed] [Google Scholar]
- Faddy MJ, Telfer E, Gosden RG. The kinetics of pre-antral follicle development in ovaries of CBA/Ca mice during the first 14 weeks of life. Cell Tissue Kinet. 1987;20:551–560. doi: 10.1111/j.1365-2184.1987.tb01364.x. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults. 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- Freedman DS, Khan LK, Serdula MK, Galuska DA, Dietz WH. Trends and correlates of class 3 obesity in the United States from 1990 through 2000. JAMA. 2002;288:1758–1761. doi: 10.1001/jama.288.14.1758. [DOI] [PubMed] [Google Scholar]
- Galletti F, Klopper A. The effect of progesterone on the quantity and distribution of body fat in the female rat. Acta Endocrinol-Cop. 1964;46:379–86. doi: 10.1530/acta.0.0460379. [DOI] [PubMed] [Google Scholar]
- Geary N. The estrogenic inhibition of eating. In: Stricker EM, Woods SC, editors. Handbook of Behavioral Neurobiology, Vol 14, Neurobiology of food and fluid intake. 2. Kluwer Academic/Plenum; New York: 2004. pp. 307–345. [Google Scholar]
- Goldman L, Winget C, Hollingshead GW, Levine S. Postweaning development of negative feedback in the pituitary-adrenal system of the rat. Neuroendocrinology. 1973;12:199–211. doi: 10.1159/000122169. [DOI] [PubMed] [Google Scholar]
- Gosden RG, Jones EC, Jacks F. Pituitary-ovarian relationships during post-reproductive phase of inbred mice. Exp Gerontol. 1978;13:159–166. doi: 10.1016/0531-5565(78)90008-6. [DOI] [PubMed] [Google Scholar]
- Holt H, Keeton RW, Vennesland B. The effect of gonadectomy on body structure and body weight in rats. Am J Physiol-- Legacy Content. 1936;114:515–525. [Google Scholar]
- Jones EC, Krohn PL. Relationship between age, numbers of oocytes and fertility in virgin and multiparous mice. J Endocrinol. 1961;21:469–495. doi: 10.1677/joe.0.0210469. [DOI] [PubMed] [Google Scholar]
- Kirchengast S, Gruber D, Sator M, Huber J. Postmenopausal weight status, body composition and body fat distribution in relation to parameters of menstrual and reproductive history. Maturitas. 1999;33:117–126. doi: 10.1016/s0378-5122(99)00042-0. [DOI] [PubMed] [Google Scholar]
- Leshner AI, Collier G. The effects of gonadectomy on the sex differences in dietary self-selection patterns and carcass compositions of rats. Physiol Behav. 1973;11:671–676. doi: 10.1016/0031-9384(73)90253-9. [DOI] [PubMed] [Google Scholar]
- Liang YQ, Akishita M, Kim S, Ako J, Hashimoto M, Iijima K, Ohike Y, Watanabe T, Sudoh N, Toba K, Yoshizumi M, Ouchi Y. Estrogen receptor beta is involved in the anorectic action of estrogen. Int J Obesity. 2002;26:1103–1109. doi: 10.1038/sj.ijo.0802054. [DOI] [PubMed] [Google Scholar]
- Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obesity. 2008;32:949–958. doi: 10.1038/ijo.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson JE, Willett WC, Stampfer MJ, Colditz GA, Hunter DJ, Hankinson SE, Hennekens CH, Speizer FE. Body-weight and mortality among women. New Engl J Med. 1995;333:677–685. doi: 10.1056/NEJM199509143331101. [DOI] [PubMed] [Google Scholar]
- Mason JB, Cargill SL, Anderson GB, Carey JR. Transplantation of Young Ovaries to Old Mice Increased Life Span in Transplant Recipients. J of Gerontol A-Biol. 2009;64:1207–1211. doi: 10.1093/gerona/glp134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meli R, Pacilio M, Raso GM, Esposito E, Coppola A, Nasti A, Di Carlo C, Nappi C, Di Carlo R. Estrogen and raloxifene modulate leptin and its receptor in hypothalamus and adipose tissue from ovariectomized rats. Endocrinology. 2004;145:3115–3121. doi: 10.1210/en.2004-0129. [DOI] [PubMed] [Google Scholar]
- Nyda MJ, Demajo SF, Lewis RA. The effect of ovariectomy and physiologic doses of estradiol upon body weight, linear growth and fat content of female albino rat. B Johns Hopkins Hosp. 1948;83:279–287. [PubMed] [Google Scholar]
- Parkening TA, Fabricant JD, Heussner JC, Collins TJ, Smith ER. Orthotopic ovarian transplantations in young and aged CBA mice. Exp Gerontol. 1984;19:53–61. doi: 10.1016/0531-5565(84)90031-7. [DOI] [PubMed] [Google Scholar]
- Ravussin E, Lillioja S, Knowler WC, Christin L, Freymond D, Abbott WGH, Boyce V, Howard BV, Bogardus C. Reduced rate of energy-expenditure as a risk factor for body-weight gain. New Engl J Med. 1988;318:467–472. doi: 10.1056/NEJM198802253180802. [DOI] [PubMed] [Google Scholar]
- Richard D. Effects of ovarian hormones on energy balance and brown adipose tissue thermogenesis. Am J Physiol. 1986;250:R245–R249. doi: 10.1152/ajpregu.1986.250.2.R245. [DOI] [PubMed] [Google Scholar]
- Rogers NH, Perfield JW, Strissel KJ, Obin MS, Greenberg AS. Reduced Energy Expenditure and Increased Inflammation Are Early Events in the Development of Ovariectomy-Induced Obesity. Endocrinology. 2009;150:2161–2168. doi: 10.1210/en.2008-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin GL, Zhao Y, Kalus AM, Simpson ER. Peroxisome proliferator-activated receptor gamma ligands inhibit estrogen biosynthesis in human breast adipose tissue: Possible implications for breast cancer therapy. Cancer Res. 2000;60:1604–1608. [PubMed] [Google Scholar]
- Shaw MA, Whitaker EM, Hervey E, Hervey GR. The effects of ovarian hormones on regulation of energy balance in Zucker rats. J Endocrinol. 1983;98:165–171. doi: 10.1677/joe.0.0980165. [DOI] [PubMed] [Google Scholar]
- Shimomura K, Shimizu H, Tsuchiya T, Abe Y, Uehara Y, Mori M. Is leptin a key factor which develops obesity by ovariectomy? Endocr J. 2002;49:417–423. doi: 10.1507/endocrj.49.417. [DOI] [PubMed] [Google Scholar]
- Svendsen OL, Hassager C, Christiansen C. Six months’ follow up on exercise added to a short-term diet in overweight postmenopausal women: Effects on body composition, resting metabolic rate, cardiovascular risk factors and bone. Int J Obesity. 1994;18:692–698. [PubMed] [Google Scholar]
- Troisi RJ, Wolf AM, Manson JE, Klingler KM, Colditz GA. Relation of body-fat distribution to reproductive factors in premenopausal and postmenopausal women. Obes Res. 1995;3:143–151. [PubMed] [Google Scholar]
- Thung PJ, Boot LM, Muhlbock O. Senile changes in the oestrous cycle and in ovarian structure in some inbred strains of mice. Acta Endocrinol. 1956;23:8–32. doi: 10.1530/acta.0.0230008. [DOI] [PubMed] [Google Scholar]
- Wade GN, Gray JM. Gonadal effects on food intake and adiposity: a metabolic hypothesis. Physiol Behav. 1979;22:583–593. doi: 10.1016/0031-9384(79)90028-3. [DOI] [PubMed] [Google Scholar]
- Wade GN. Interaction between estradiol-17beta and growth hormone in control of food intake in weanling rats. J Comp Physiol Psych. 1974;86:359–362. doi: 10.1037/h0035945. [DOI] [PubMed] [Google Scholar]
- Wade GN, Zucker I. Development of hormonal control over food intake and body weight in female rats. J Comp Physiol Psych. 1970;70:213–220. doi: 10.1037/h0028713. [DOI] [PubMed] [Google Scholar]
- Wang QL, Hassager C, Ravn P, Wang SL, Christiansen C. Total and regional body-composition changes in early postmenopausal women: Age-related or menopause-related? Am J Clin Nutr. 1994;60:843–848. doi: 10.1093/ajcn/60.6.843. [DOI] [PubMed] [Google Scholar]
- Williams CM. Lipid metabolism in women. P Nutr Soc. 2004;63:153–160. doi: 10.1079/PNS2003314. [DOI] [PubMed] [Google Scholar]