Abstract
The vocalizing midshipman fish, Porichthys notatus, has two male morphs that exhibit alternative mating tactics. Only territorial males acoustically court females with long duration (mins- > 1h) calls, whereas sneaker males attempt to steal fertilizations. During the breeding season, morph-specific tactics are paralleled by a divergence in relative testis and vocal muscle size, plasma levels of the androgen 11-ketotestosterone (11KT) and the glucocorticoid cortisol, and mRNA expression levels in the central nervous system (CNS) of the steroid-synthesizing enzyme aromatase (estrogen synthase). Here, we tested the hypothesis that the midshipman's two male morphs would further differ in the CNS, as well as in the testis and vocal muscle, in mRNA abundance for the enzymes 11β-hydroxylase (11βH) and 11beta (β)-hydroxysteroid dehydrogenase (11βHSD) that directly regulate both 11KT and cortisol synthesis. Quantitative real-time PCR demonstrated male morph-specific profiles for both enzymes. Territorial males had higher 11βH and 11βHSD mRNA levels in testis and vocal muscle. By contrast, sneaker males had the higher CNS expression, especially for 11βHSD, in the region containing an expansive vocal pacemaker circuit that directly determines the temporal attributes of natural calls. We propose for territorial males that higher enzyme expression in testis underlies its greater plasma 11KT levels, which in vocal muscle provides both gluconeogenic and androgenic support for its long duration calling. We further propose for sneaker males that higher enzyme expression in the vocal CNS contributes to known cortisol-specific effects on its vocal physiology.
Keywords: alternative male reproductive tactics, 11-ketotestosterone, cortisol, 11βHSD, 11βH, HSD11B, CYP11B
1. Introduction
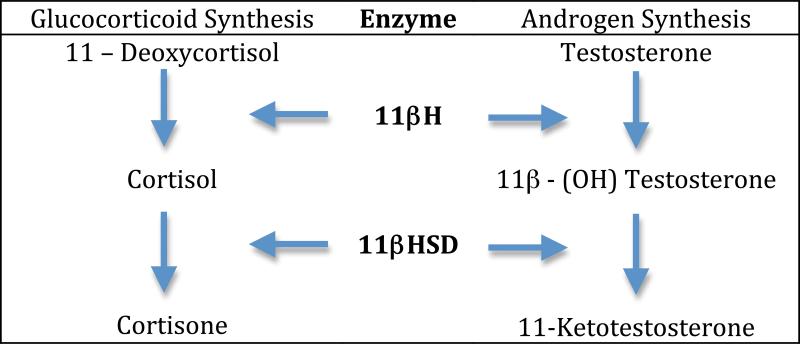
Steroid biosynthesis is a crucial mechanism among vertebrates for regulating a diverse range of physiological processes across multiple organ systems (Frieden and Lipner, 1971; Kime, 1987). Among teleost fish, elevated plasma levels of the teleost-specific androgen, 11-ketotestosterone (11KT), and the glucocorticoid cortisol occur during the breeding season and accompany changes in a large suite of neuroendocrine and behavioral traits (Bass and Grober, 2009; Oliveira, 2006). Two enzymes, 11beta (β)-hydroxysteroid dehydrogenase (11βHSD) and 11β-hydroxylase (11βH), participate in regulation of both the 11KT and cortisol signaling pathways (Fig. 1; Bury and Sturm, 2007).
Figure 1.
Overview of synthetic pathways for glucocorticoids and androgens in fish. See text and Bury and Sturm (2007) for details.
To date, studies in teleosts have identified only one 11βHSD gene that can both convert 11β-OH-testosterone to 11KT and cortisol to cortisone, with little conversion of cortisone back to cortisol (Jiang et al., 2003; Kusakabe et al., 2003; Ozaki et al., 2006) (Fig. 1). By contrast, mammals have two isoforms of 11βHSD, types 1 and 2 (Krozowski, 1999; Krozowski et al., 1999). The 11βHSD2 isoform converts either cortisol to cortisone or corticosterone to 11-dehydrocorticosterone (varies across mammalian species). In tissues with high expression of the mineralocorticoid receptor (e.g., kidney), this conversion gives aldosterone access to these receptors that have a higher affinity for cortisol and corticosterone. By contrast, 11βHSD1 converts inactive metabolites back to cortisol and corticosterone. Mammals also have two isoforms of 11βH (Bulow and Bernhardt, 2002; Lisurek and Bernhardt, 2004; Payne and Hales, 2004). It has been proposed that the 11βH gene, CYP11B, underwent a duplication event among ancestral mammals, yielding CYP11B1 and CYP11B2 that encode, respectively, 11βH and aldosterone synthase. 11β-Hydroxylase converts either 11-deoxycortisol to cortisol or 11-deoxycorticosterone to corticosterone depending on the species, while aldosterone synthase converts 11-deoxycorticosterone to aldosterone. Teleosts have a single 11βH gene, consistent with cortisol being the main glucocorticoid and no conclusive evidence for detectable levels of circulating aldosterone (Prunet et al. 2006).
Here, we investigated mRNA expression levels for the 11βHSD and 11βH genes in the midshipman fish (Porichthys notatus), a sound-producing teleost fish with two male morphs that diverge in vocal and spawning tactics (Bass, 1996). Territorial, type I males build nests in the rocky intertidal zone from where they acoustically court females with long duration (mins - >1h) advertisement calls. Sneak and satellite-spawning, type II males neither build nests nor court females but rather steal fertilizations from type I males. A large suite of somatic, neural and endocrine traits diverge between the two morphs (Bass, 1996). For example, the relative size of the testis (i.e., corrected for body size) is 9-fold greater in type II males, while relative vocal muscle size is 6-fold greater in type I males. A central vocal motor network also differs dramatically between the two males in morphology, physiology and hormonal sensitivity (Bass and Baker, 1990; Bass et al., 1994; Goodson and Bass, 2000; Remage-Healey and Bass, 2004, 2007). The androgen and cortisol-dependent sensitivity of the vocal system in midshipman fish is well-established (Bass and Remage-Healey, 2008). Both testosterone and 11KT influence vocal muscle and motoneuron size (Bass and Forlano, 2008; Brantley et al., 1993a; Lee and Bass, 2005). These androgens, along with cortisol, have male morph-specific effects on the firing pattern of an expansive pacemaker-motoneuron circuit that extends between the caudal hindbrain and rostral spinal cord and directly determines the temporal properties of natural calls (Bass and Baker, 1990; Remage-Healey and Bass, 2004, 2007; Rubow and Bass, 2009).
Like other species with alternative male reproductive tactics (Brantley et al., 1993c; Knapp and Neff, 2007; Oliveira, 2006), midshipman exhibit male morph-specific profiles in plasma levels of steroid hormones. During the pre-nesting and nesting periods of the breeding season (see Sisneros et al., 2004 for definitions), the main circulating androgen in type I males is 11KT, with plasma levels almost 10 fold that of testosterone (Brantley et al., 1993c; Knapp et al., 1999; Sisneros et al., 2004). In type II males, 11KT is essentially undetectable and testosterone is the predominant androgen (Brantley et al., 1993c). Plasma levels of cortisol, the main glucocorticoid in teleosts (Prunet et al., 2006), are 2-3 fold greater in type II males compared to type I males (Arterbery et al., 2009).
Coupled with the divergence in circulating steroids and the steroid sensitivity of the vocal system, the two male morphs also differ widely in the CNS expression of aromatase (estrogen synthase), the enzyme that converts testosterone to estrogen. During the breeding season, aromatase mRNA levels are 2-3 fold higher in type II males in the pacemaker-motoneuron circuit (Forlano and Bass, 2005; Schlinger et al., 1999). Blocking CNS aromatase action inhibits testosterone-dependent facilitation of pacemaker-motoneuron activity, thus demonstrating the physiological and behavioral relevance of locally elevated aromatase in the CNS (Remage-Healey and Bass, 2007).
Together, the above led to the current study further testing the hypothesis that expression levels of the steroidogenic enzymes 11βHSD and 11βH would also diverge between the two male midshipman morphs in different CNS regions, especially given morph-specific modulation of CNS vocal function by androgens, cortisol and aromatase (Remage-Healey and Bass, 2004, 2007). We also included the vocal muscle and testis in our investigation since they play obvious roles in establishing morph-specific vocal behaviors (Brantley and Bass, 1994) and plasma androgen levels (Brantley et al., 1993c). As shown, 11βHSD and 11βH have male morph-specific patterns of expression in both the CNS and peripheral tissues. The combined actions of these two enzymes likely play an essential role in expression of the known male morph-specific, steroid-dependent phenotypes in midshipman fish.
2. Materials and methods
2.1. Animals
Midshipman fish were hand-collected from nest sites in northern California during the breeding season (May-August), shipped to Cornell University within 72 h, and maintained in seawater tanks until sacrificed for tissue within 24 – 48 h of receipt. This study included type I males (20.6 – 25.1 g, 12 - 13.5 cm in standard length), type II males (3.94 - 5.88 g, 8 - 8.5 cm) and females (11.1 - 17.5 g, 10 - 11.5 cm). The gonad and vocal muscle of the animals were used to verify sex and male morph status (Bass, 1996). Tissue sampling was carried out following deep anesthetization (0.025% benzocaine; Sigma, St. Louis, MO). All experimental protocols were approved by the Cornell University Institutional Animal Care and Use Committee.
2.2. Cloning, sequencing, and alignment
We first cloned the 11βHSD and 11βHSD genes in midshipman fish so that we could subsequently use quantitative real time PCR to characterize their mRNA expression levels (see below). RNA was isolated and pooled from type I male whole CNS (n = 3 individuals) using Trizol (Invitrogen, Carlsbad, CA) and reverse transcribed using Superscript III Reverse Transcriptase (Invitrogen) and random hexamers following the manufacturer's protocols. The CNS samples consisted of both the brain and the rostral spinal cord that includes the caudal part of the vocal pattern generator (Bass et al., 1994). PCR on cDNA was conducted using the following degenerate primers: 11βHSD forward primer (based upon the amino acid sequence SNYRGCME): 5’ TCNAAYTAYAGRGGNTGYATGGAR 3’, Tm = 55.8°C; 11βHSD reverse primer (based upon the amino acid sequence ETKDLFQ): 5’ YTGRAANAGRTCYTTNGTYTC 3’, Tm = 50.5°C; 11βH forward primer (based upon the amino acid sequence GVFLKNG): 5’ GGNGTNTTYCTNAARAAYGGN 3’, Tm = 53.5°C; 11βH reverse primer (based upon the amino acid sequence ANITELM): 5’ CATNAGYTCNGTDATRTTNGC 3’, Tm = 51°C.
Amplification was performed with Taq polymerase (Epicentre, Madison, WI) with the following cycles: Stage 1 – 94°C for 2 min (1 cycle), Stage 2 – 94°C for 1 min, 52°C for 1 min, 72°C for 3 min (35 cycles), and Stage 3 – 72°C extension for 10 min (1 cycle). Based on other studies in teleosts (see below), expected products of 380 bp for 11βHSD and 595 bp for 11βH were verified on a 1% agarose gel. The primers contained restriction sites (not shown) on the 5’ end to increase the efficiency of ligation into the Bluescript KS (+) plasmid, and the ligations were transformed into DH5α (Invitrogen, Carlsbad, CA) competent cells and plated. Twenty individual clones were grown in liquid culture, mini-prepped (Qiagen, Germantown, MD), and sequenced at the Cornell University Life Sciences Core Laboratory Center using T3 and T7 primers. The resulting sequences were subject to a BLAST analysis (NCBI) to verify identity and aligned to other vertebrate proteins using CLustalW.
Sequences for 11βHSD alignments were obtained from the following species: Homo sapiens 11βHSD1 (Genbank accession number NM_005525.2), Mus musculus 11βHSD1 (Genbank accession number NM_001044751.1), Homo sapiens 11βHSD2 (Genbank accession number NM_000196), Mus musculus 11βHSD2 (Genbank accession number NM_008289.2), Taeniopygia guttata 11βHSD2 (Ensembl identifier ENSTGUG00000005862), Anolis carolinensis (Ensembl identifier ENSACAG00000003658), Xenopus tropicalis 11βHSD2 (Ensembl identifier ENSXETG00000004704), Danio rerio (Genbank accession number NM_212720), Oreochromis niloticus (Genbank accession number AY190043.2), Anguilla japonica (Genbank accession number AB252646), Oncorhynchys mykiss (Genbank accession number AB104415).
Sequences for 11βH alignments were obtained from the following species: Homo sapiens 11βH2 (Genbank accession number NM_000498), Mus musculus 11βH2 (Genbank accession number NM_009991.3), Homo sapiens 11βH1 (Genbank accession number NM_000497), Mus musculus 11βH1 (Genbank accession number NM_001033229.2), Anolis carolinensis (Ensembl identifier ENSACAG00000004353), Xenopus tropicalis (Ensembl identifier ENSXETG00000001446), Acanthopagrus schlegelii (Genbank accession number EF423618), Dicentrarchus labrax (Genbank accession number AF449173).
2.3. Absolute-quantitative real time PCR (qPCR)
Tissue used for qPCR was not pooled from multiple individuals. Rather, the abundance of the 11βHSD and 11βHSD genes was determined for each tissue from each of 4-5 animals of each reproductive morph. RNA was isolated from CNS, vocal muscle, and testis using Trizol (Invitrogen, Carlsbad, CA), treated twice with DNaseI (Invitrogen, Carlsbad, CA) to remove genomic DNA contamination, and reverse transcribed using Superscript III Reverse Transcriptase (Invitrogen, Carlsbad, CA) following the manufacturer's protocols. As in similar studies with midshipman fish (Arterbery et al., 2009; Forlano et al., 2005; Forlano et al., 2009; Schlinger et al., 1999), the CNS was divided into three parts: a rostral, forebrain-only region that included the olfactory bulb, telencephalon and preoptic area; a middle region including the midbrain tectum and tegmentum, diencephalon, and cerebellum; a caudal region with the remaining hindbrain and rostral spinal cord that is dominated by vocal motor circuitry (Bass et al., 1994). The forebrain and hindbrain-spinal regions assayed here were specifically isolated from other regions because of their prominent role in vocal motor patterning (Bass and Remage-Healey, 2008; Goodson and Bass, 2000) and abundance of estrogen and androgen receptor mRNA, aromatase mRNA and protein, and neuropeptide-containing neurons (Forlano et al., 2005, Forlano et al., 2009; Goodson et al., 2003).
Absolute qPCR was conducted on cDNA from all tissues using gene specific primer pairs designed from the nucleotide sequence of midshipman genes that 1) amplified a product about 150 nucleotides in length, 2) isolated a single product with no dimer pairs, and 3) had a standard efficiency (R2) of no less than 98%. For the 11βHSD gene, the forward primer 5’ CCGAGCCTCCGTCATTCTGC 3’ (Tm = 60.6°C) and the reverse primer (5’ AAAGAGGTCCTTGGTCTCAGCACG 3’ (Tm = 60.2°C) were the primer pair (R2=0.982). For the 11βH gene, the forward primer 5’ CGTGCCCCCTTGTGGACA 3’ (Tm = 60.8°C) and the reverse primer 5’ GAACTCCGGTGTACTGGTGTCCTT 3’ (Tm = 60.4°C) were the primer pair (R2=0.987).
All real time reactions were run in triplicate for the above tissue samples and no template controls, with each reaction containing the following: 10 μl of 2X Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA), 2 μl of forward and reverse primer at a concentration of 100 nM, 4 μl of H2O, and 2 μl of the appropriate cDNA. Reactions were run on an Applied Biosystems 7900 HT Sequence Detection System at the Cornell University Life Sciences Core Laboratory Center under the default manufacturer's conditions (SDS 2.1 software, Applied Biosystems, Foster City, CA) using 60°C as an annealing temperature.
Gene copy number was determined using standard curve analysis for all gene primer sets, including beta actin that was used as a reference gene for normalizing gene copy number (see Arterbery et al., 2009 for primer design). The standards covered a linear range of 5×10^6 to 100 copy/μl. Briefly, the raw Ct values were converted to copy number with the standard curve produced using the SDS 2.1. Each target gene copy number was normalized using the beta actin copy number from the same tissue sample. The normalized values were determined for each individual. As discussed elsewhere (Arterbery et al., 2009), we recognize the potential limits of using beta actin as the single reference gene for qPCR.
2.4. Statistics
Normalized mRNA values were compared between morphs for each tissue sampled. Statistics were performed on all data sets using ANOVA analysis and post-hoc Tukey-HSD tests. Statistics were performed using JMP 7.0 software. In addition, ANOVA was performed for each comparison and Welch-ANOVA tests conducted on those with unequal variance to verify any significant result. While variation in expression of the reference gene (beta actin, see above) was less than 5% between morphs within a given tissue, it was much greater than 5% between the CNS and peripheral tissues. Hence, we only made statistical comparisons across either central or peripheral tissues.
3. Results
3.1. Cloning, sequencing, and alignment
The first step in our investigation was to clone the midshipman genes for 11βHSD and 11βH so that we could quantify their mRNA levels in central and peripheral tissues. We first identified conserved blocks of amino acids in vertebrates for 11βHSD and 11βH and then designed degenerate primers to those regions to amplify PCR products from cDNA of type I male CNS. The resulting PCR products of 380 bp for the 11βHSD gene (HSD11B) and 595 bp for the 11βH gene (CYP11B) were subcloned. The sequences of 20 independent clones revealed that a single gene product was amplified in each case. The translated protein sequence can be viewed on Genbank on the NCBI webserver (Genbank Accession No. EU530638 for HSD11B and EU869313 for CYP11B).
Amino acid alignments of the midshipman enzymes with other vertebrate enzymes revealed high sequence identity (alignment in Supp. Fig. 1 and sequence identity in Supp. Table 1 for 11βHSD; alignment in Supp. Fig. 2 and sequence identity in Supp. Table 2 for 11βH).
3.2. Absolute qPCR
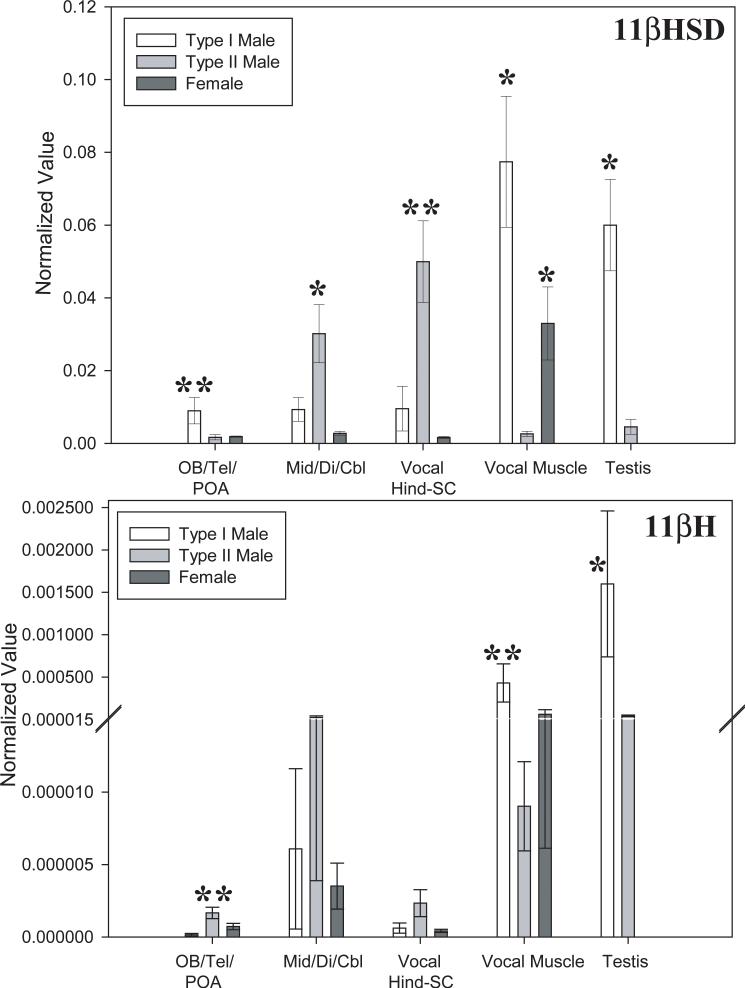
Using qPCR, we found that the male morph-specific suite of traits in midshipman fish (see Introduction) was paralleled by differential expression in both the CNS and periphery of the genes encoding 11βHSD and 11βH. In general, sneak-spawning type II males had the higher CNS levels of 11βHSD and 11βH. By contrast, the advertisement-calling type I males had higher mRNA levels for both genes in the vocal muscle and the testis. The results are illustrated in Figure 2 and presented in numerical detail in Supplemental Tables 3 (11βHSD) and 4 (11βH). Variance analysis verified both morph (Welch-ANOVA p<0.0001) and gene (Welch-ANOVA p<0.006) specific differences. These differences were found to be significant for 11βHSD with p≤0.008 and for 11βH with p≤ 0.02.
Figure 2.
Normalized mRNA values are plotted against tissue type for 11βHSD and 11βH genes for each midshipman reproductive morph (see legend, top left). Note break in y-axis of the 11βH bar graph to accomodate high values for type I male gonad (testis). Standard errors are shown. Single and double asterisks indicate significance over, respectively, one and two other morph(s). See text and Supplemental Tables 3 and 4 for numerical data. The CNS was divided into three regions: forebrain, including olfactory bulb (OB), telencephalon (Tel) and preoptic area (POA); a middle region including the midbrain tectum and tegmentum (Mid), diencephalon (Di) and cerebellum (Cbl); the remaining hindbrain (Hind) and rostral spinal cord (SC) that is predominated by an expansive vocal pattern generator circuit (Bass et al., 1994).
3.2.1. 11βHSD
For 11βHSD mRNA, male-morph specific profiles in the CNS were most prominent for the hindbrain-spinal cord (HSC) region that is predominated by the vocal pacemaker-motoneuron circuit (Bass et al., 1994). Type II males showed significantly greater, 5-fold higher levels over type I males in this region. Type II males also had 3-fold higher levels over type I males in the middle CNS region, although the difference did not reach significance. The forebrain pattern also showed male morph specificity, but in this case type I males instead showed significantly higher levels over type II males.
The vocal muscle of type I males had significantly greater, nearly 30-fold higher levels of 11βHSD mRNA than the muscle of type II males. The testis of type I males also showed significantly greater, nearly 13-fold higher 11βHSD mRNA levels than the testis of type II males.
For females, 11βHSD mRNA levels were uniformly low in all CNS regions. They did not differ significantly from type I males in the vocal HSC and middle CNS region, and from type II males in the forebrain. For the vocal muscle, female levels were significantly greater than those of type II males, but lower than those of type I males. The magnitude of the difference with type I males was, however, far less in comparison to that between the two male morphs and did not reach significance.
3.2.2. 11βH
As with 11βHSD mRNA, the CNS expression levels for 11βH mRNA diverged between the two male morphs. In this case, type II males expressed higher 11βH levels than type I males in all CNS regions, although the difference was only significant in the forebrain.
Also like 11βHSD mRNA, the vocal muscle of type I males had significantly higher 11βH mRNA levels than the muscle of type II males. Similarly, the testis of type I males had much higher 11βH mRNA levels than the testis of type II males. Elevated levels of both 11βH and 11βHSD mRNA in type I male testis is consistent with the essential role of both enzymes in the synthesis of 11KT (Fig. 1), which circulates at much higher levels in type I males (Brantley et al., 1993c).
For females, the 11βH mRNA levels in all CNS regions were similar to those of type I males, while the levels for the vocal muscle were not significantly different from those of type II males.
4. Discussion
The results of this study in midshipman fish show male morph-specific patterns in the CNS, vocal muscle and testis in the mRNA expression levels for the 11βHSD and 11βH enzymes essential to androgen and glucocorticoid synthesis (Fig. 1). We propose that these results support the general hypothesis that greater abundance of the 11βHSD and 11βH genes in any given tissue directly relates to local modulation of glucocorticoid and/or androgen synthesis and metabolism, and the performance of male morph-specific behaviors. Thus, higher expression in type I male testis underlies the high plasma 11KT levels found in type I males alone that can support 11KT's known induction of type I male vocal traits. Greater abundance in type I male vocal muscle likely provides gluconeogenic and androgenic support for the long duration calling exhibited by type I males alone. Finally, higher expression in the vocal CNS of type II males likely contributes to known cortisol-specific effects on vocal motor activity in type II males. Before a more extended discussion of each of the above points, we will briefly compare the 11βHSD and 11βH sequences in midshipman fish to those in other teleosts, and vertebrates in general.
4.1. 11βHSD and 11βH sequence comparisons
Like other teleosts so far studied (Jiang et al., 2003; Kusakabe et al., 2003; Ozaki et al., 2006), midshipman fish apparently have only one form of the 11βHSD gene. The midshipman 11βHSD sequence is very similar to those in other teleosts, all of which are most like the type 2 isoform of 11βHSD in mammals (Supplemental Figure 1) that regulates the conversion of cortisol and corticosterone to inactive metabolites (see Introduction; Bury and Sturm, 2007). Midshipman fish also have only one form of 11βH, again like other teleosts (Supplemental Figure 2). The midshipman 11βH enzyme is most similar to the mammalian 11βH type 1 isoform that converts 11-deoxycortisol to cortisol in humans and 11-deoxycorticosterone to corticosterone in rodents (Payne and Hales, 2004). The presence of only one form of 11βH in teleosts is consistent with the lack of evidence for circulating aldosterone in teleosts (Prunet et al., 2006), including midshipman (Arterbery et al., 2009), and the role of the second 11βH isoform in aldosterone synthesis among mammals (see Introduction; Bury and Sturm, 2007).
4.2. Male morph divergence in 11βHSD and 11βH mRNA abundance
We discuss the male morph-specific patterns in 11βHSD and 11βH mRNA expression in midshipman fish in the context of both glucocorticoid and androgen signaling pathways (Fig. 1). We provide both parsimonious and testable hypotheses to explain the results based, in part, on numerous earlier investigations of the midshipman's two male morphs at multiple levels of analysis, ranging from molecular to neurophysiological and behavioral. The current study can now direct future ones using enzymatic assays to investigate the tissue-specific activity levels of 11βHSD and 11βH, and anatomical methods for the cellular localization of 11βHSD and 11βH mRNAs as we have done for aromatase and steroid receptors (Forlano et al., 2001; Forlano et al., 2005; Forlano et al., 2009).
4.2.1. Testis
To our knowledge, this is the first report that provides quantitative results supporting the hypothesis that elevated 11βHSD and 11βH in the testis are directly related to male morph-specific androgen production in species with alternative male reproductive tactics. The relative levels of 11βHSD and 11βH mRNAs are 13-40 fold higher in the testis of territorial type I males than the testis of the sneaker type II males. The results strongly support the proposal (e.g., see Knapp, 2003) that 11βHSD, the one enzyme essential for the final conversion of testosterone to 11KT (Fig. 1), is predominantly expressed in the testis of the territorial/ displaying male morph (type I male midshipman in this case) in teleosts with alternative male reproductive tactics. This is consistent with elevated plasma 11KT in only type I male midshipman and other territorial/ displaying male morphs (Brantley et al., 1993c; Oliveira, 2006), and the reported absence of C21-hydroxylase in fish testis that leads to glucocorticoid synthesis (Li et al., 2003; also see Ozaki et al., 2006). The elevated 11βH gene levels in type I males compared to type II males is also consistent with the detection in some type I males of 11β-OH-testosterone (Brantley et al., 1993c), an intermediate in the conversion of testosterone to 11KT (Fig. 1).
11Beta-hydroxysteroid dehydrogenase's capacity to convert cortisol to cortisone could figure prominently in protecting sperm development from the potentially detrimental effects of locally high cortisol levels (see Ozaki et al., 2006). However, we would have expected 11βHSD levels to be either comparable or higher in the testis of type II males given their 2-3 fold higher plasma levels of cortisol in comparison to type I males (Arterbery et al., 2009).
In sum, the available evidence best supports the hypothesis that the higher expression levels of the 11βHSD and 11βH genes in type I male testis relates to their high circulating levels of 11KT that, in turn, are linked to the induction of type I male vocal and reproductive traits (see Introduction and below).
4.2.2. Vocal muscle
The 11βHSD and 11βH mRNA levels are widely divergent between the two male morphs in the vocal muscle, in this case 30-35 fold higher in type I males. A role for both enzymes in 11KT synthesis at the level of the vocal muscle may seem doubtful given 11KT's high plasma levels in type I males (Brantley et al., 1993c). However, testosterone and/or 11β-OH-testosterone available from the circulation (Brantley et al., 1993c) could support local synthesis and provide added androgenic support for 11KT's likely role in the induction of increased muscle mass at the onset of the breeding season (Sisneros et al., 2008). This hypothesis is consistent with 11KT's potent induction of type I male muscle traits in juvenile males (Brantley et al., 1993a, c).
An alternative, but not mutually exclusive, hypothesis is that both enzymes play a prominent role in glucocorticoid signaling pathways. Elevated 11βH in the vocal muscle of type I males could lead to locally elevated cortisol levels (Fig. 1). This would parallel the close to 3-fold higher levels of glucocorticoid receptor mRNA in the vocal muscle of type I males compared to type II males (Arterbery et al., 2009). The co-elevation of local cortisol and glucocorticoid receptor levels could then aid in the gluconeogenic demand of the type I male vocal muscle during the repetitive production of advertisement calls (“hums”), each perhaps lasting more than 1h, on successive nights during the breeding season (Brantley and Bass, 1994; Bass et al., 1999; Ibara et al., 1983). Higher 11βHSD expression in the type I male vocal muscle could also convert cortisol to cortisone, thereby providing a local mechanism for moderating the potential catabolic effects of cortisol on muscle (e.g., see Peeters et al., 2008).
Together, the available data support the combined hypothesis that co-elevated 11βH and 11βHSD in the vocal muscle of type I males functions in both androgen (i.e., 11KT synthesis) and glucocorticoid mechanisms. Future studies should both determine local steroid levels in the vocal muscle of both male morphs and possible interactions between androgen and glucocorticoid receptors (e.g., see Chen et al., 1997) that might balance the known anabolic/ catabolic effects of these two classes of steroids on vertebrate striated muscle (Peeters et al., 2008).
4.2.3. Central nervous system (CNS): vocal hindbrain-spinal cord
We expected a morph-specific pattern for the CNS expression of 11βHSD and 11βH like that observed for the periphery since 11KT is the predominant circulating androgen in type I males (Brantley et al., 1993c), and 11KT modulates the vocal pattern generator of type I males alone (Remage-Healey and Bass, 2004, 2007). Surprisingly, expression levels for 11βHSD are, in general, several fold higher in much of the CNS of type II males. Compared to 11βHSD, 11βH mRNA levels are 2-3 orders of magnitude lower than the lowest 11βHSD levels in any CNS region of any adult morph (Fig. 2; note break in y-axis for 11βH; also see Suppl. Tables 3, 4). While we cannot discount a role for 11βH in local steroid synthesis in the CNS until local steroid levels are determined, the evidence mainly supports an important CNS function for 11βHSD in both male morphs. Given this, the remaining discussion focuses on 11βHSD.
Differential gene expression between midshipman male morphs in single tissue types such as the testis and vocal muscle leads to obvious hypotheses given their well-known roles in, respectively, steroid biosynthesis and vocalization. By contrast, studies of entire CNS regions that are involved in myriad functions inherently constrain functional interpretations. Recognizing this limitation, we restrict the discussion of the CNS to the hindbrain-spinal cord region (HSC) that contains the expansive vocal pacemaker-motoneuron circuit (Bass et al., 1994). Previous studies of the vocal-HSC have led to cogent, testable hypotheses regarding the functional significance of divergent patterns of mRNA and protein abundance in this CNS region. A striking example of this noted earlier was the initial demonstration of elevated aromatase activity levels in the same vocal-HSC region studied here that predicted localization of aromatase mRNA and protein to the vocal motor system (Schlinger et al., 1999; Forlano et al., 2001). Subsequent neuroanatomical studies supported this hypothesis (Forlano and Bass, 2005; Bass and Forlano, 2008), while neurophysiology showed that blocking aromatase action could interfere with testosterone-dependent modulation of vocal motor patterning (Remage-Healey and Bass, 2007). Neurophysiological studies also demonstrated estrogen and androgen modulation of the vocal system (Remage-Healey and Bass, 2004; Remage-Healey and Bass, 2007), supported by in situ hybridization showing estrogen and androgen receptor mRNA expression in the vocal-HSC (Forlano et al., 2005, 2009). Similarly, we predict the anatomical localization of 11βHSD mRNA and protein to the vocal-HSC and its greater abundance in type II males compared to type I males, as observed here using qPCR.
Given the 2-3 fold higher plasma cortisol levels in type II males compared to type I males (Arterbery et al., 2009) and the predominant role of 11βHSD in the conversion of cortisol to cortisone in teleosts (Fig. 1), elevated 11βHSD levels in the vocal-HSC of type II males could lead to the rapid conversion of locally high cortisol levels to cortisone that could serve two purposes related to vocal function. First, it likely contributes to the fast (within 5 mins), morph-specific actions of cortisol on the activity of the vocal pattern generator; cortisol suppresses vocal motor activity in type II males, but facilitates it in type I males (Remage-Healey and Bass, 2004, 2007). Secondly, cortisol conversion to an inactive metabolite could protect the vocal pattern generator from cortisol-related neurotoxicity (Joels et al., 2007). Cortisone may also be a neuroactive compound in the vocal motor system and potentially underlie the divergence in vocal patterning between the two male morphs. Neurophysiological and biochemical studies can begin to test these hypotheses.
5. Concluding comments
This new study provides an initial assessment of localized expression patterns in discrete central nervous and peripheral somatic tissues of genes encoding enzymes that regulate both androgen and glucocorticoid synthesis in a teleost fish with alternative male reproductive morphs. The higher expression of 11βHSD and 11βH in the testis and vocal muscle of type I males is consistent with prior studies showing that 11KT circulates at much higher levels in type I males, and that both androgens and glucocorticoids could support type I male vocal muscle function related to extended periods of advertisement calling during the breeding season. Male morph-specific patterns of expression are also observed in the CNS, especially in the vocal hindbrain-spinal cord region. The higher expression levels for both enzymes in this region in type II males is likely to be fundamental to the known morph-specific effects of cortisol on the CNS vocal pattern generator.
Defining the relationship between dynamic changes in steroid and 11βHSD/11βH levels in local CNS regions such as the vocal-HSC will help delineate the role of steroidogenic enzymes in modulating the neurophysiological mechanisms of a specific behavior, in this case vocalization (see Remage-Healey et al., 2008). Future studies should also be aimed at other steroidogenic enzymes known to have roles in glucocorticoid and androgen synthesis such as C21α hydroxylase, C17α hydroxylase, and 3βHSD and 17βHSD (see Bury and Sturm, 2007) to increase the resolution of morph-specific comparisons in steroid synthesis in localized CNS regions such as the vocal-HSC and peripheral tissues such as the vocal muscle and testis.
Given the widespread occurrence of alternative mating tactics among teleosts (Mank and Avise, 2006; Oliveira et al., 2008), and vertebrates in general (Rhen and Crews, 2002), the male-morph specific mRNA expression profiles reported here predict similarly divergent patterns of gene expression in these other species. More broadly, stress and reproductive regulation via, respectively, glucocorticoid- and androgen-related proteins are important factors influencing behavioral and physiological variation in teleost fish and other vertebrates including humans (Korte et al., 2005; Pasquali et al., 2008; Sapolsky et al., 2000). Comparative approaches to the interaction of multiple, steroid signaling axes can provide a more basic, molecular and evolutionarily-based foundation showing how systemic hormonal properties contribute to both adaptive and maladaptive mechanisms of behavioral modulation via steroid signaling mechanisms (also see Korte et al., 2005; Sapolsky et al., 2000).
Supplementary Material
Supplemental Figure Legends
Supplemental Figure 1: An alignment of the deduced amino acid sequence of midshipman (P. notatus) and other vertebrate 11βHSD enzymes reveals high sequence identity between mammals and teleost fishes. Black and gray shading indicate identical and similar amino acids, respectively. Both the forward and reverse primers used in qPCR for the midshipman 11βHSD are underlined.
Supplemental Figure 2: An alignment of deduced amino acid sequence of midshipman (P. notatus) and other vertebrate 11βH enzymes reveals high sequence identity between mammals and teleost fishes. Black and gray shading indicate identical and similar amino acids, respectively. Both the forward and reverse primers used in qPCR for the midshipman 11βH are underlined.
Acknowledgments
We would like to thank the Cornell Statistical Consulting Unit, Cornell Life Sciences Core Laboratories Center for statistical assistance and Paul Forlano and two anonymous reviewers for helpful comments on the manuscript. Support from Cornell University Minority and Provost Diversity Fellowships and NIH Institutional predoctoral training grant GM007469 (ASA), and NSF research grant IOB 0516748 (AHB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arterbery AS, Deitcher DL, Bass AH. Corticosteroid receptor expression in a teleost fish that displays alternative male reproductive tactics. Gen. Comp. Endocrinol. 2009 doi: 10.1016/j.ygcen.2009.06.004. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass AH. Shaping brain sexuality. Am. Sci. 1996;84:352–363. [Google Scholar]
- Bass AH, Bodnar DA, Marchaterre MA. Complementary explanations for existing phenotypes in an acoustic communication system. In: Hauser M, Konishi M, editors. Neural Mechanisms of Communication. MIT Press; Cambridge, MA: 1999. pp. 493–514. [Google Scholar]
- Bass AH, Forlano PH. Neuroendocrine mechanisms of alternative reproductive tactics: The chemical language of social plasticity. In: Oliveira RF, Taborsky M, Brockmann HJ, editors. Alternative Reproductive Tactics - an Integrative Approach. Cambridge Univ. Press; 2008. pp. 109–131. [Google Scholar]
- Bass AH, Grober MS. Reproductive Plasticity in fish: Evolutionary liability in the patterning of neuroendocrine and behavioral traits underlying divergent sexual phenotypes. In: Pfaff D, Arnold A, Etgen A, Fahrbach S, Moss R, Rubin R, editors. Hormones, Brain, and Behavior. Academic Press; 2009. [Google Scholar]
- Bass AH, Horvath BJ, Brothers EB. Nonsequential developmental trajectories lead to dimorphic vocal circuitry for males with alternative reproductive tactics. J. Neurobiol. 1996;30:493–504. doi: 10.1002/(SICI)1097-4695(199608)30:4<493::AID-NEU5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Bass AH, Marchaterre MA, Baker R. Vocal-acoustic pathways in a teleost fish. J. Neurosci. 1994;14:4025–4039. doi: 10.1523/JNEUROSCI.14-07-04025.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass AH, Remage-Healey L. Central pattern generators for social vocalization: androgen-dependent neurophysiological mechanisms. Horm. Behav. 2008;53:659–672. doi: 10.1016/j.yhbeh.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantley RK, Bass AH. Alternative male spawning tactics and acoustic signals in the plainfin midshipman fish Porichthys notatus Girard (Teleostei, Batrachoididae). Ethology. 1994;96:213–232. [Google Scholar]
- Brantley RK, Marchaterre MA, Bass AH. Androgen effects on vocal muscle structure in a teleost fish with inter- and intra-sexual dimorphism. J. Morphol. 1993a;216:305–318. doi: 10.1002/jmor.1052160306. [DOI] [PubMed] [Google Scholar]
- Brantley RK, Tseng J, Bass AH. The ontogeny of inter- and intrasexual vocal muscle dimorphisms in a sound-producing fish. Brain, Behav. Evol. 1993b;42:336–349. doi: 10.1159/000114170. [DOI] [PubMed] [Google Scholar]
- Brantley RK, Wingfield JC, Bass AH. Sex steroid levels in Porichthys notatus, a fish with alternative reproductive tactics, and a review of the hormonal bases for male dimorphism among teleost fishes. Horm. Behav. 1993c;27:332–347. doi: 10.1006/hbeh.1993.1025. [DOI] [PubMed] [Google Scholar]
- Bulow HE, Bernhardt R. Analyses of the CYP11B gene family in the guinea pig suggest the existence of a primordial CYP11B gene with aldosterone synthase activity. Eur. J. Biochem. 2002;269:3838–3846. doi: 10.1046/j.1432-1033.2002.03076.x. [DOI] [PubMed] [Google Scholar]
- Bury NR, Sturm A. Evolution of the corticosteroid receptor signalling pathway in fish. Gen. Comp. Endocrinol. 2007;153:47–56. doi: 10.1016/j.ygcen.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Chen S, Wang J, Yu G, Liu W, Pearce D. Androgen and glucocorticoid receptor heterodimer formation. J. Biol. Chem. 1997;272:14087–14092. doi: 10.1074/jbc.272.22.14087. [DOI] [PubMed] [Google Scholar]
- Forlano PM, Bass AH. Seasonal plasticity of brain aromatase mRNA expression in glia: divergence across reproductive and vocal phenotypes. J. Neurobiol. 2005;65:37–49. doi: 10.1002/neu.20179. [DOI] [PubMed] [Google Scholar]
- Forlano PM, Deitcher DL, Myers DA, Bass AH. Neuroanatomical basis for high aromatase levels in teleost fish: aromatase enzyme and mRNA expression identify glia as source. J. Neurosci. 2001;21:8943–8955. doi: 10.1523/JNEUROSCI.21-22-08943.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlano PM, Deitcher DL, Bass AH. Distribution of estrogen receptor alpha mRNA in the brain and inner ear of a vocal fish with comparisons to sites of aromatase expression. J. Comp. Neurol. 2005;483:91–113. doi: 10.1002/cne.20397. [DOI] [PubMed] [Google Scholar]
- Forlano PM, Marchaterre MA, Deitcher DL, Bass AH. Distribution of androgen receptor mRNA expression in vocal, auditory and neuroendocrine circuits in a teleost fish. J. Comp. Neurol. 2009 doi: 10.1002/cne.22233. Available online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieden E, Lipner H. Biochemical Endocrinology of the Vertebrates. Prentice-Hall, Inc.; Englewood Cliffs, NJ: 1971. [Google Scholar]
- Goodson JL, Bass AH. Forebrain peptides modulate sexually polymorphic vocal circuitry. Nature. 2000;403:769–772. doi: 10.1038/35001581. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Bass AH. Putative isotocin distributions in sonic fish: relation to vasotocin and vocal-acoustic circuitry. J. Comp. Neurol. 2003;462:1–14. doi: 10.1002/cne.10679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibara RM, Penny LT, Ebeling AW, van Dykhuizen G, Calliet G. The mating call of the plainfin midshipman fish, Porichthys notatus. In: Noakes DGL, Lindquis DG, Helfmann GS, Ward JA, editors. Predators and prey in fishes. Junk Press; The Hague, Netherlands: 1983. pp. 205–212. [Google Scholar]
- Jiang J, DS W, Senthilkumaran B, Kobayashi T, Kobayashi H, Yamaguchi A, Ge W, Young G, Nagahama Y. Isolation, characterization and expression of 11 beta-hydroxysteroid dehydrogenase type 2 cDNAs from the testes of Japanese eel (Anguilla japonica) and Nile tilapia (Oreochromis niloticus). J. Mol. Endocrinol. 2003;31:305–315. doi: 10.1677/jme.0.0310305. [DOI] [PubMed] [Google Scholar]
- Joels M, Karst H, Krugers HJ, Lucassen PJ. Chronic stress: implications for neuronal morphology, function and neurogenesis. Front. Neuroendocrinol. 2007;28:72–96. doi: 10.1016/j.yfrne.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Kime DE. Fundamentals of Comparative Vertebrate Endocrinology. Plenum Press; NY, NY: 1987. [Google Scholar]
- Knapp R. Endocrine mediation of vertebrate male alternative reproductive tactics: the next generation of studies. Integr. Comp. Biol. 2003;43:658–668. doi: 10.1093/icb/43.5.658. [DOI] [PubMed] [Google Scholar]
- Knapp R, Neff BD. Steroid hormones in bluegill, a species with male alternative reproductive tactics including female mimicry. Biol. Lett. 2007;3:628–631. doi: 10.1098/rsbl.2007.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp R, Wingfield JC, Bass AH. Steroid hormones and paternal care in the plainfin midshipman fish (Porichthys notatus). Horm. Behav. 1999;35:81–89. doi: 10.1006/hbeh.1998.1499. [DOI] [PubMed] [Google Scholar]
- Korte SM, Koolhaas JM, Wingfield JC, McEwen BS. The Darwinian concept of stress: benefits of allostasis and costs of allostatic load and the tradeoffs in health and disease. Neurosci. Biobehav. Rev. 2005;29:3–38. doi: 10.1016/j.neubiorev.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Krozowski Z. The 11beta-hydroxysteroid dehydrogenases: functions and physiological effects. Mol. Cell. Endocrinol. 1999;151:121–127. doi: 10.1016/s0303-7207(98)00256-1. [DOI] [PubMed] [Google Scholar]
- Krozowski Z, Li KX, Koyama K, Smith RE, Obeyesekere VR, Stein-Oakley A, Sasano H, Coulter C, Cole T, Sheppard KE. The type I and type II 11beta-hydroxysteroid dehydrogenase enzymes. J. Steroid Biochem. Mol. Biol. 1999;69:391–401. doi: 10.1016/s0960-0760(99)00074-6. [DOI] [PubMed] [Google Scholar]
- Kusakabe M, Nakamura I, Young G. 11Beta-hydroxysteroid dehydrogenase complementary deoxyribonucleic acid in rainbow trout: cloning, sites of expression, and seasonal changes in gonads. Endocrinol. 2003;144:2534–2545. doi: 10.1210/en.2002-220446. [DOI] [PubMed] [Google Scholar]
- Lee JS, Bass AH. Differential effects of 11-ketotestosterone on dimorphic traits in a teleost with alternative male reproductive morphs. Horm. Behav. 2005;47:523–531. doi: 10.1016/j.yhbeh.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Li YY, Inoue K, Takei Y. Interrenal steroid 21-hydroxylase in eels: primary structure, progesterone-specific activity and enhanced expression by ACTH. J Mol. Endocrinol. 2003;31:327–40. doi: 10.1677/jme.0.0310327. [DOI] [PubMed] [Google Scholar]
- Lisurek M, Bernhardt R. Modulation of aldosterone and cortisol synthesis on the molecular level. Mol. Cell. Endocrinol. 2004;215:149–159. doi: 10.1016/j.mce.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Mank J, E, Avise JC. Comparative phylogenetic analysis of male alternative reproductive tactics in ray-finned fishes. Evolution. 2006;60:1311–1316. [PubMed] [Google Scholar]
- Oliveira RF. Neuroendocrine mechanisms of alternative reproductive tactics in fish. In: Sloman KA, Wilson RW, Balshine S, editors. Behavior and Physiology of fish. Academic Press-Elsevier; 2006. pp. 297–357. [Google Scholar]
- Oliveira RF, Taborsky M, Brockmann HJ. Alternative Reproductive Tactics - an Integrative Approach. Cambridge Univ. Press; 2008. [Google Scholar]
- Ozaki Y, Higuchi M, Miura C, Yamaguchi S, Tozawa Y, Miura T. Roles of 11beta-hydroxysteroid dehydrogenase in fish spermatogenesis. Endocrinol. 2006;147:5139–46. doi: 10.1210/en.2006-0391. [DOI] [PubMed] [Google Scholar]
- Pasquali R, Vicennati V, Gambineri A, Pagotto U. Sex-dependent role of glucocorticoids and androgens in the pathophysiology of human obesity. Int. J. Obes. (London) 2008;32:1764–1779. doi: 10.1038/ijo.2008.129. [DOI] [PubMed] [Google Scholar]
- Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 2004;25:947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- Peeters GMEE, van Schoor NM, van Rossum EFC, Visser M, Lips P. The relationship between cortisol, muscle mass, muscle strength in older persons and the role of genetic variaitons in the glucocortiocid receptor. Clin. Endocrinol. 2008;69:673–682. doi: 10.1111/j.1365-2265.2008.03212.x. [DOI] [PubMed] [Google Scholar]
- Prunet P, Sturm A, Milla S. Multiple corticosteroid receptors in fish: from old ideas to new concepts. Gen. Comp. Endocrinol. 2006;147:17–23. doi: 10.1016/j.ygcen.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Bass AH. Rapid, hierarchical modulation of vocal patterning by steroid hormones. J. Neurosci. 2004;24:5892–5900. doi: 10.1523/JNEUROSCI.1220-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Bass AH. Plasticity in brain sexuality is revealed by the rapid actions of steroid hormones. J. Neurosci. 2007;27:1114–1122. doi: 10.1523/JNEUROSCI.4282-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nat. Neurosci. 2008;11:1327–1324. doi: 10.1038/nn.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhen T, Crews D. Variation in reproductive behaviour within a sex: neural systems and endocrine activation. J. Neuroendocrinol. 2002;14:517–531. doi: 10.1046/j.1365-2826.2002.00820.x. [DOI] [PubMed] [Google Scholar]
- Rubow TK, Bass AH. Reproductive and diurnal rhythms regulate vocal motor plasticity in a teleost fish. J. Exp. Biol. 2009;212:3252–3262. doi: 10.1242/jeb.032748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schlinger BA, Greco AC, Bass AH. Aromatase activity in the hindbrain vocal region of a teleost fish: divergence among males with alterantive reproductive tactics. Proc. R. Soc. Lond., Ser. B: Biol. Sci. 1999;266:131–136. [Google Scholar]
- Sisneros JA, Alderks PW, Leon K, Sniffen B. Morphometric changes associated with the reproductive cycle and behaviour of the intertidal-nesting, male plainfin midshipman Porichthys notatus. J. Fish Biol. 2008 doi: 10.1111/j.1095-8649.2008.02104.x. early view. [DOI] [PubMed] [Google Scholar]
- Sisneros JA, Forlano PM, Knapp R, Bass AH. Seasonal variation of steroid hormone levels in an intertidal-nesting fish, the vocal plainfin midshipman. Gen. Comp. Endocrinol. 2004;136:101–116. doi: 10.1016/j.ygcen.2003.12.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure Legends
Supplemental Figure 1: An alignment of the deduced amino acid sequence of midshipman (P. notatus) and other vertebrate 11βHSD enzymes reveals high sequence identity between mammals and teleost fishes. Black and gray shading indicate identical and similar amino acids, respectively. Both the forward and reverse primers used in qPCR for the midshipman 11βHSD are underlined.
Supplemental Figure 2: An alignment of deduced amino acid sequence of midshipman (P. notatus) and other vertebrate 11βH enzymes reveals high sequence identity between mammals and teleost fishes. Black and gray shading indicate identical and similar amino acids, respectively. Both the forward and reverse primers used in qPCR for the midshipman 11βH are underlined.