Abstract
Multiple potential uses of direct gene transfer into neurons require restricting expression to specific classes of glutamatergic neurons. Thus, it is desirable to develop vectors containing glutamatergic class-specific promoters. The three vesicular glutamate transporters (VGLUTs) are expressed in distinct populations of neurons, and VGLUT1 is the predominant VGLUT in the neocortex, hippocampus, and cerebellar cortex. We previously reported a plasmid (amplicon) Herpes Simplex Virus (HSV-1) vector that placed the Lac Z gene under the regulation of the VGLUT1 promoter (pVGLUT1lac). Using helper virus-free vector stocks, we showed this vector supported ~90 % glutamatergic neuron-specific expression in postrhinal (POR) cortex, in rats sacrificed at either 4 days or 2 months after gene transfer. We now show that pVGLUT1lac supports expression preferentially in VGLUT1-containing glutamatergic neurons. pVGLUT1lac vector stock was injected into either POR cortex, which contains primarily VGLUT1-containing glutamatergic neurons, or into the ventral medial hypothalamus (VMH), which contains predominantly VGLUT2-containing glutamatergic neurons. Rats were sacrificed at 4 days after gene transfer, and the types of cells expressing β-galactosidase were determined by immunofluorescent costaining. Cell counts showed that pVGLUT1lac supported expression in ~10-fold more cells in POR cortex than in the VMH, whereas a control vector supported expression in similar numbers of cells in these two areas. Further, in POR cortex, pVGLUT1lac supported expression predominately in VGLUT1-containing neurons, and, in the VMH, pVGLUT1lac showed an ~10-fold preference for the rare VGLUT1-containing neurons. VGLUT1-specific expression may benefit specific experiments on learning or specific gene therapy approaches, particularly in neocortex.
Keywords: herpes simplex virus vector, glutamatergic neuron-specific expression, glutamatergic neuron class, vesicular glutamate transporter1, neocortical neuron
1. Introduction
Due to the heterogeneous cellular composition of most brain areas, and particularly forebrain areas, neuron class-specific expression is advantageous for many gene transfer studies or gene therapies. Glutamatergic neurons are the predominant class of excitatory neuron in the brain, although the classes of neurons within this major class remain controversial (Nelson et al., 2006; Sugino et al., 2006). Thus, it is desirable to develop vectors that support expression in specific classes of glutamatergic neurons. One approach is to exploit promoters that are specific for specific classes of glutamatergic neurons.
Helper virus-free Herpes Simplex Virus (HSV-1) plasmid vectors (Fraefel et al., 1996) (amplicons) are attractive for gene transfer into neurons. Advantages of HSV-1 vectors include that they have a large capacity (51 kb and 149 kb HSV-1 vectors have been established (Wade-Martins et al., 2003; Wang et al., 2000)); HSV-1 vectors efficiently transduce neurons; and HSV-1 vectors that contain specific cellular promoters support long-term neuron-specific, or neuron class-specific, expression. Promoters that support cell type-specific expression from HSV-1 vectors include: The preproenkephalin (preproENK) promoter supports expression in specific enkephalinergic neuron-containing brain areas (amygdala or ventromedial hypothalamus, (Kaplitt et al., 1994). The tyrosine hydroxylase promoter (TH) supports expression in specific types of midbrain dopaminergic neurons; in particular, 40 to 60 % nigrostriatal neuron-specific expression (Jin et al., 1996; Song et al., 1997; Wang et al., 1999). The glutamic acid decarboxylase (GAD) promoter supports GABAergic neuron-specific expression in a neocortical area, postrhinal (POR) cortex (Rasmussen et al., 2007). Neuron-specific expression is supported by chimeric promoters that contain an upstream enhancer from the TH promoter fused to the neurofilament heavy gene promoter (TH-NFH promoter), or add a β-globin insulator (INS) upstream of the TH-NFH promoter (INS-TH-NFH promoter). These modified neurofilament promoters support at least 90 % neuron-specific expression in the substantia nigra pars compacta, striatum, hippocampus, and POR cortex (Cao et al., 2008; Sun et al., 2004; Zhang et al., 2000; Zhang et al., 2005). HSV-1 vectors containing each of these cell type-specific promoters support long-term expression, for two to fourteen months (see references for each promoter, above).
Glutamatergic neuron-specific expression from HSV-1 vectors has been achieved by using promoters from specific genes for either glutamate biosynthesis or transport into synaptic vesicles. The brain/kidney phosphate-activated glutaminase (PAG (Banner et al., 1988)), encoded by the GLS gene, produces most of the glutamate for release as neurotransmitter (Hertz, 2004), and PAG has been used as an immunohistochemical marker for glutamatergic neurons (Kaneko and Mizuno, 1988; Kaneko et al., 1992; Sakata et al., 2002; Van der Gucht et al., 2003). A HSV-1 vector containing the PAG promoter supports long-term (2 month) expression in PAG-containing neurons in POR cortex (Rasmussen et al., 2007). Expression in specific classes of glutamatergic neurons might be obtained by using specific vesicular glutamate transporter (VGLUT) promoters. The three VGLUTs are expressed in distinct populations of neurons (review (Fremeau et al., 2004b)). VGLUT1 is the predominant VGLUT in the neocortex, hippocampus, cerebellar cortex, and basolateral nuclei of the amygdala; VGLUT2 is found in the thalamus, deep cerebellar nuclei, hypothalamus, brainstem, and in some neocortical neurons, primarily in layer 4 but also in deeper layers; and VGLUT3 is found in neurons traditionally viewed as non-glutamatergic (Bellocchio et al., 2000; Fremeau et al., 2001; Fremeau et al., 2004b; Herzog et al., 2001; Takamori et al., 2000; Takamori et al., 2001; Varoqui et al., 2002). We previously showed that a HSV-1 vector containing the VGLUT1 promoter supports long-term (2 month) expression in PAG-containing neurons in rat POR cortex (Rasmussen et al., 2007). However, this study did not examine expression in specific classes of glutamatergic neurons.
In this study, we established that an HSV-1 vector containing the VGLUT1 promoter supports expression predominately in VGLUT1-containing neurons in POR cortex. This vector supported only low levels of expression in the ventromedial hypothalamus (VMH), as most glutamatergic neurons in the VMH contain VGLUT2.
2. Results
2.1. An HSV-1 vector containing the VGLUT1 promoter supports expression preferentially in POR cortex glutamatergic neurons compared to VMH glutamatergic neurons
pVGLUT1lac contains the mouse VGLUT1 promoter and first intron (7 kb and 4.6 kb fragments, respectively) (Rasmussen et al., 2007). The first intron may have a role in regulating expression (Dr. Herzog, personal communication), and thus was included in pVGLUT1lac. The control vector, pINS-TH-NFHpkcΔ/INS-TH-NFHlac, contains a modified NFH promoter (Zhang et al., 2000). This vector supports expression in both glutamatergic and GABAergic neurons in the striatum, hippocampus, and POR cortex (Sun et al., 2003; Sun et al., 2004; Sun et al., 2005; Zhang et al., 2005; Zhang et al., 2009).
These two vectors were packaged into HSV-1 particles, using a helper-virus free packaging system (Fraefel et al., 1996; Sun et al., 1999). To quantify the numbers of infectious vector particles (IVP/ml), the purified vector stocks were titered on Baby Hamster Kidney (BHK) cells; at 1 day after transduction, positive cells were visualized using 5-bromo-4-chloro-3-indoyl-β-D-galactopyranoside (X-gal) staining. The titer of pVGLUT1lac was 2.0 × 106 IVP/ml, and the titer of pINS-TH-NFHpkcΔ/INS-TH-NFHlac was 3.0 × 106 IVP/ml. This titering was performed on BHK fibroblast cells as the best available assay. Of note, these fibroblast cells form a monolayer. In contrast, PC12 cells, and most neuronal cell lines, do not form a monolayer, and the titers obtained on BHK cells are higher than the titers obtained on PC12 cells (Yang et al., 2001; Zhang et al., 2000). Expression from these neuronal promoters in fibroblast cells represents ectopic expression that declined rapidly at longer times after gene transfer (not shown). We previously used a PCR assay to determine the titers of vector genomes (VG/ml) and the packaging efficiency (IVP/ml / VG/ml), which were similar for these two vectors (Rasmussen et al., 2007); the VG/ml assay was not repeated here.
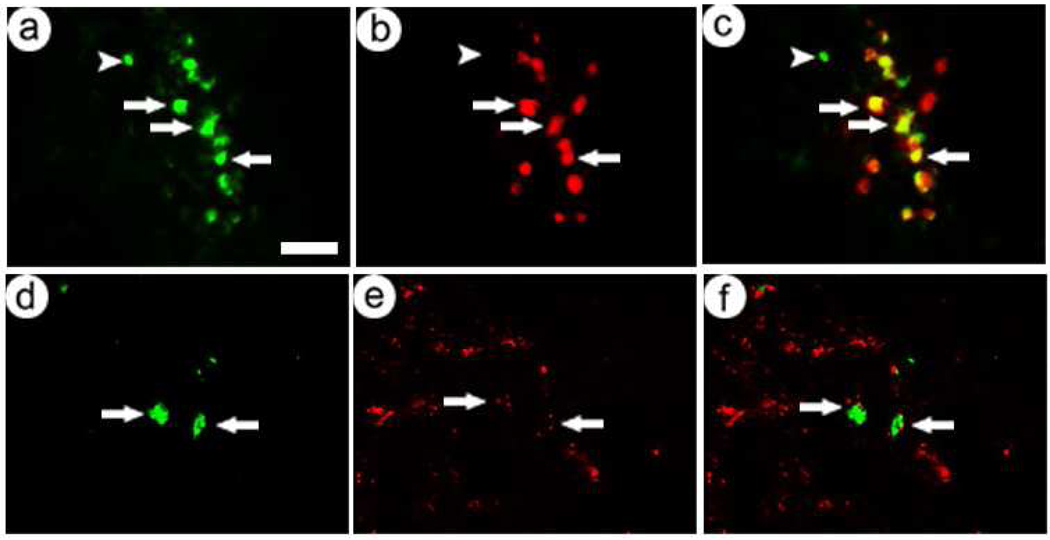
Glutamatergic neurons in POR cortex express predominately VGLUT1; in contrast, glutamatergic neurons in the VMH express predominately VGLUT2 ((Barroso-Chinea et al., 2007; Bellocchio et al., 2000; Fremeau et al., 2001; Fremeau et al., 2004b; Herzog et al., 2001; Takamori et al., 2000; Takamori et al., 2001; Varoqui et al., 2002); Allen Institute Brain Atlas, www.brainatlas.org). We injected equal titers of pVGLUT1lac or the control vector into either POR cortex or the VMH, sacrificed the rats 4 days later, and performed costaining for β-galactosidase immunoreactivity (β-gal-IR) and phosphate-activated glutaminase (PAG)-IR. PAG is a marker for glutamatergic neurons (Kaneko and Mizuno, 1988; Kaneko et al., 1992; Sakata et al., 2002; Van der Gucht et al., 2003). The specificity of the PAG antibody used here was previously established by both Western blots and preabsorption (Cangro et al., 1985; Shapiro et al., 1987). Representative photomicrographs showed that pVGLUT1lac supports expression in large numbers of glutamatergic neurons in POR cortex (Fig. 1A–C) and in fewer glutamatergic neurons in the VMH (Fig. 1D–F). In the VMH, in some proximal cells, the PAG-IR was almost adjacent; but in the merged views of β-gal-IR and PAG-IR, clear cells were visible. Omission of the primary antibodies resulted in no detectable staining (Fig. 1G–I). In contrast, the control vector, which contains a neuron-specific promoter, supported expression in similar numbers of cells in POR cortex (Fig. 2A) and the VMH (Fig 2B).
Fig. 1.
pVGLUT1lac supported expression of β-gal predominantly in glutamatergic neurons in POR cortex and the VMH, but in fewer cells in the VMH than in POR cortex. The rats were sacrificed at 4 days after gene transfer. β-gal-IR was detected using an anti-β-gal antibody, and glutamatergic neurons were identified using anti-PAG. (A–C) POR cortex contained many transduced glutamatergic neurons; (A) β-gal-IR, (B) PAG-IR, or (C) merged. Arrows, costained cells. (D–F) The VMH contained few transduced glutamatergic neurons; (D) β-gal-IR, (E) PAG-IR, or (F) merged. (G–I) Omission of the primary antibodies resulted in no detectable immunofluorescence; (G) rhodamine filter, (H) fluorescein filter, or (I) merged. Scale bar: 50 µm.
Fig. 2.
A vector containing a modified neurofilament promoter supported expression of β-gal in large numbers of cells in both POR cortex and the VMH. The rats were sacrificed at 4 days after gene transfer of pINS-TH-NFHpkcΔ/INS-TH-NFHlac, and β-gal-IR was detected. (A) POR cortex, and (B) VMH. Scale bar: 50 µm.
Using this helper virus-free system, we previously showed that following vector injection into POR cortex, the vast majority of the transduced cells are in POR cortex. Specific neocortical areas with large projections to POR cortex contain ~1 % of the number of transduced cells as POR cortex, and no transduced cells were observed in any of the subcortical areas examined (Zhang et al., 2005). Thus, in this study, we examined the transduced cells proximal to the injection site. Other studies, using other HSV-1 vector systems, have reported significant levels of retrograde transport of vectors to brain areas distant from the injection site (Martins et al., 2008). The amount of retrograde transport depends upon the strain of HSV-1, properties of the vector system, injection parameters, properties of the brain area receiving the injection, and other variables.
We performed cell counts of the numbers of expressing cells, and the numbers of expressing cells that were glutamatergic neurons, in either POR cortex or the VMH. The results showed that the control vector supported expression in an average of 1,173 cells in POR cortex, and a slightly lower number in the VMH (Table 1). This modest 15 % difference in numbers of expressing cells between POR cortex and the VMH likely reflects differences in cell density, diffusion through the extracellular space (extracellular matrix composition), cell infectivity, and other related variables. The experimental vector, pVGLUT1lac, supported expression in an average of 544 cells in POR cortex, or 46 % of the number of expressing cells as observed using the control vector. This ~2-fold decrease in number of expressing cells is likely because pVGLUT1lac supported expression predominately (>90 %) in glutamatergic neurons (Table 1 and (Rasmussen et al., 2007)), whereas the control vector supported expression in 52 % glutamatergic and 45 % GABAergic neurons in POR cortex (Zhang et al., 2005) and in 65 % glutamatergic neurons in the VMH (Table 1). Expression in both glutamatergic and GABAergic neurons likely explains the higher number of expressing cells observed using the control vector. Of note, pVGLUT1lac supported expression in an average of only 64 cells in the VMH, or only 12 % of the number of cells observed in POR cortex, and this difference was highly significant (p<0.001 t-test). Most (91 %) of the expressing cells in the VMH were glutamatergic neurons (Table 1). The low levels of expressing cells in the VMH as compared to POR cortex may be because most VMH glutamatergic neurons contain VGLUT2, but most POR cortex glutamatergic neurons contain VGLUT1; this potential explanation is explored next.
TABLE 1.
Numbers of β-gal-IR cells and % glutamatergic-specific expression in rats sacrificed at 4 days after injection of specific vectors into either POR cortex or the VMH
| Vector | Injection Site |
β-gal-IR Cells |
% PAG-IR Costaining |
|---|---|---|---|
| pVGLUT1lac | POR cortex | 544±79 | 95±2 |
| pVGLUT1lac | VMH | 64±15 | 91±3 |
| pINS-TH-NFHpkcΔ/INS-TH-NFHlac | POR cortex | 1,173±79 | NDa |
| pINS-TH-NFHpkcΔ/INS-TH-NFHlac | VMH | 911±66 | 65±5 |
Four hemispheres were analyzed for each vector and injection site; the values shown are mean±s.e.m.
ND, not done.
2.2. pVGLUT1lac supports expression predominately in VGLUT1-containing glutamatergic neurons in POR cortex, and in low numbers of VGLUT1- or VGLUT2-containing neurons in the VMH
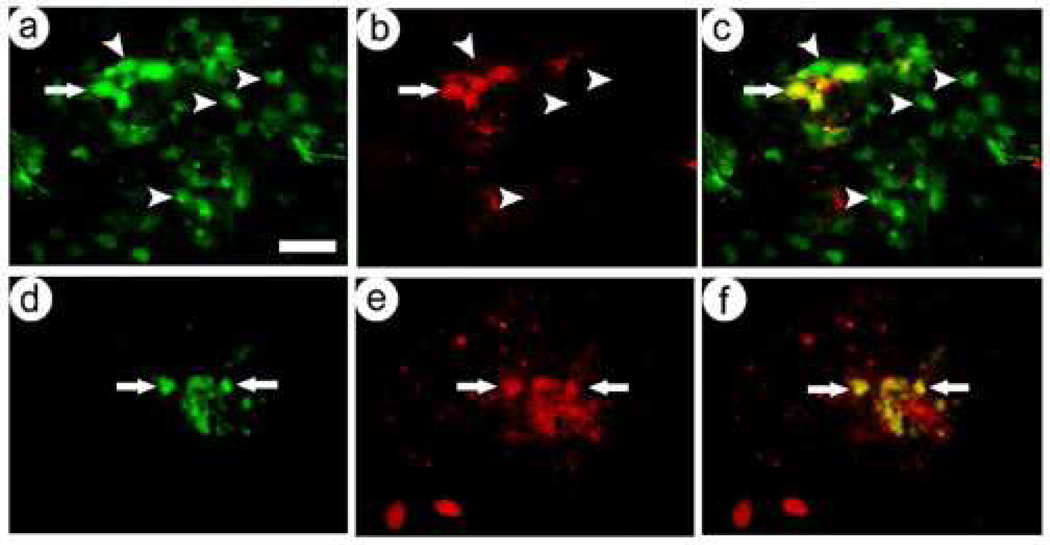
We injected equal titers of pVGLUT1lac or the control vector into either POR cortex or the VMH, sacrificed the rats 4 days later, prepared four sets of serial sections, and assayed a specific set of sections for either β-gal-IR and VGLUT1-IR or β-gal-IR and VGLUT2-IR. The specificity of the VGLUT1 and VGLUT2 antibodies used here were previously established by Western blots (Fremeau et al., 2001; Fremeau et al., 2004a). Photomicrographs showed that pVGLUT1lac supported expression in numerous VGLUT1-containing neurons in POR cortex (Fig. 3A–C), and some of the few β-gal-IR cells in the VMH contained VGLUT1-IR (Fig. 3D–F). In contrast, most of the β-gal-IR cells in POR cortex lacked VGLUT2-IR (Fig. 4A–C). However, although the VMH contained few expressing cells, a significant fraction of these β-gal-IR cells contained VGLUT2-IR (Fig. 4D–F).
Fig. 3.
pVGLUT1lac supported expression of β-gal predominantly in VGLUT1-containing neurons in POR cortex, and preferentially in the few VGLUT1-containing neurons in the VMH. The rats were sacrificed at 4 days after gene transfer. β-gal-IR was detected using anti-β-gal, and the sections were costained with anti-VGLUT1. (A–C) POR cortex contained many transduced VGLUT1-containing neurons; (A) β-gal-IR, (B) VGLUT1-IR, or (C) merged. Arrows, costained cells; arrowheads, singly stained cells. (D–F) The VMH contained transduced VGLUT1-containing neurons; (D) β-gal-IR, (E) VGLUT1-IR, or (F) merged. Scale bar: 50 µm.
Fig. 4.
pVGLUT1lac supported expression of β-gal in few VGLUT2-containing neurons in POR cortex, and the VMH contained few expressing cells, but many of these cells contained VGLUT2. The rats were sacrificed at 4 days after gene transfer. β-gal-IR was detected using anti-β-gal, and the sections were costained with anti-VGLUT2. (A–C) In POR cortex, a small fraction of the transduced neurons contained VGLUT2; (A) β-gal-IR, (B) VGLUT2-IR, or (C) merged. Arrows, costained cells; arrowheads, singly stained cells. (D–F) The VMH contained only a small number of expressing cells, but many contained VGLUT2; (D) β-gal-IR, (E) VGLUT2-IR, or (F) merged. Scale bar: 50 µm.
We performed cell counts on the numbers of expressing cells that contained either VGLUT1-IR or VGLUT2-IR, in either POR cortex or the VMH. The results showed that in POR cortex, ≥90 % of the expressing cells contained VGLUT1, but only 11 % of the expressing cells contained VGLUT2 (Table 2). The VMH contained only 10–15 % of the number of β-gal-IR cells as observed in POR cortex (Table 2), similar to the experiment that assayed PAG-IR (Table 1; the VGLUT-1 and VGLUT-2 assays each used one of the four sets of sections, and the numbers of β-gal-IR cells in POR cortex or the VMH in Table 2 are ~25 % of those in Table 1). Of the few β-gal-IR cells in the VMH, only 7 % contained VGLUT1, and 90 % contained VGLUT2 (Table 2). Thus, most of the remaining, low-level expression in the VMH was inappropriate expression in VGLUT2-containing neurons.
TABLE 2.
Numbers of β-gal-IR cells, and % VGLUT1- or VGLUT2-specific expression, in rats sacrificed at 4 days after injection of pVGLUT1lac into either POR cortex or the VMH
| VGLUT1 Neurons | VGLUT2 Neurons | |||
|---|---|---|---|---|
| Injection Site |
Average β-gal-IR cells |
% VGLUT1-IR Costaining |
Average β-gal-IR cells |
% VGLUT2-IR costaining |
| POR cortex | 132±22 | 93±1 | 147±19 | 11±1 |
| VMH | 14±1 | 7±4 | 20±4 | 90±3 |
Four hemispheres were analyzed for each injection site; every 4th section was analyzed for either β-gal-IR and VGLUT1-IR or β-gal-IR and VGLUT2-IR; the values shown are mean±s.e.m.
To confirm that the VGLUT1 promoter supported VGLUT1-spectiic expression, we further investigated the inappropriate expression in VGLUT2-containing neurons in the VMH. We used the Allen Institute Brain Atlas (www.brainatlas.org) to compare the numbers of VGLUT1-containing and VGLUT2-containing neurons in the mouse VMH. We examined seven sections that were hybridized with a VGLUT1-specific probe, and seven sections that were hybridized with a VGLUT2-specific probe; these sections were spaced at ~100 µm intervals and together spanned the VMH. Cell counts revealed 99.6 % VGLUT2-specific expression and only 0.4 % VGLUT1-specific expression (1,387 VGLUT2-containing cells, 6 VGLUT1-containing cells). In contrast, pVGLUT1lac supported expression in ~10 % VGLUT1-containing cells and ~90 % VGLUT2-containing cells in the VMH (Table 2). Thus, pVGLUT1lac supported at least a 10-fold preference for VGLUT1-containing neurons in the VMH, but most of the low level expression was inappropriate expression in VGLUT2-containing neurons.
Although we did not measure the levels of expression per cell, expression levels were sufficient to support both X-gal (Rasmussen et al., 2007) and immunofluoresence assays (this report and (Rasmussen et al., 2007)). We did not perform experiments in cultured cells to compare VGLUT1- and VGLUT2-specific expression because a suitable cell culture system is not available.
3. Discussion
In this study we found that pVGLUT1lac supports expression preferentially in VGLUT1-containing forebrain neurons. The control vector, which contains a neuron-specific promoter, supported expression in similar numbers of cells in POR cortex and the VMH. In contrast, pVGLUT1lac supported expression in approximately ten-fold more cells in POR cortex than in the VMH, and over 90 % of these cells were glutamatergic neurons. In POR cortex, over 90 % of the expressing cells were VGLUT1-containing neurons. In the VMH, of the few expressing cells, approximately 10 % contained VGLUT1 and 90 % contained VGLUT2, and this represents at least a ten-fold preference for VGLUT1-containing neurons, as 99.6 % of the glutamatergic neurons in VHM contain VGLUT2.
The VGLUT1-specific expression observed here is due to cell type-specific expression from the VGLUT1 promoter and not preferential transduction of VGLUT1-containing neurons. We previously showed that a HSV-1 vector containing the GAD promoter supports expression predominately in GABAergic neurons in POR cortex (Rasmussen et al., 2007), and a HSV-1 vector containing the modified neurofilament promoter (INS-TH-NFH promoter) supported expression in similar numbers of glutamatergic or GABAergic neurons in POR cortex, 52 % or 45 % respectively (Zhang et al., 2005).
The results reported here used rats sacrificed at 4 days after gene transfer. We previously reported (Rasmussen et al., 2007) that pVGLUT1lac supports long-term expression in POR cortex; rats sacrificed at 2 months after gene transfer contained 21 % of the number of cells observed in rats sacrificed at 4 days. This level of long-term expression is comparable to that found with other promoters; including the INS-TH-NFH, TH, preproENK, PAG, and GAD promoters; in POR cortex and other brain areas (Jin et al., 1996; Kaplitt et al., 1994; Rasmussen et al., 2007; Song et al., 1997; Sun et al., 2003; Sun et al., 2004; Sun et al., 2005; Wang et al., 1999; Zhang et al., 2000). Using pVGLUT1lac, at 2 months most of the expression was in glutamatergic neurons; similarly, the other promoters just listed also supported the appropriate cell type-specific expression at time points between 1 and 14 months after gene transfer. Some of the inappropriate expression from pVGLUT1lac in VGLUT2-containing neurons could be because at 4 days after gene transfer the chromatin structure on the vector has not reached steady state levels. However, the other promoters listed above support similar levels of cell type-specific expression at 4 days compared to the long time points.
The cause of the inappropriate expression from pVGLUT1lac is not known and may be due to multiple sources. Of note, the INS-TH-NFH, TH, preproENK, PAG, and GAD promoters each support a low level of expression in inappropriate cell types (see references cited above). Thus, some properties of the vector system likely contribute to the inappropriate expression. Potential causes of inappropriate expression include side effects from the vector system, specific missing VGLUT1 promoter elements, missing developmental gene regulatory events because gene transfer is into young adult rats, or other sources. Side effects of the vector system, including any cytotoxicity or immune response, can cause inappropriate expression. For example, using a helper virus-containing system, a vector containing a TH promoter supported 40 % catecholaminergic-specific expression (Song et al., 1997), but in the helper virus-free system, the same vector supported a higher level of catecholaminergic-specific expression, ~61 % (Wang et al., 1999). Correlatively, the helper virus-free system causes significantly less cytotoxicity and immune response (Fraefel et al., 1996; Olschowka et al., 2003). Alternatively, although pVGLUT1lac contains a large 11 kb VGLUT1 fragment (7 kb promoter and 4.6 kb first intron fragments, respectively (Rasmussen et al., 2007)), essential promoter elements may be missing. In transgenic mice, large BACs, which can be 100 kb or more, often support a higher level of cell type-specific expression than smaller fragments containing the same promoter. Another possibility is that the observed inappropriate expression could be due to genetic regulatory events that occur during glutamatergic neuron differentiation, and which are not reproduced when HSV-1 vectors transduce mature glutamatergic neurons in young adult rats. For example, at a specific time in differentiation, a specific histone on the VGLUT1 promoter may be modified (methylation, acetylation, etc.) as part of the mechanism that initiates transcription from this promoter, and this event may not occur following HSV-1 transduction of mature glutamatergic neurons.
HSV-1 vectors might be used to identify the critical elements in the VGLUT1 promoter that support VGLUT1 neuron-specific expression, or expression in specific classes of VGLUT1-containing neurons. Of note, initial results (Zhang and Geller, unpublished data) suggest that the VGLUT1 first intron in pVGLUT1lac is required for expression in specific classes of glutamatergic neurons in POR cortex. Thus, a detailed deletion analysis of the VGLUT1 promoter might yield a number of specific elements that support expression in specific classes of glutamatergic neurons. Analogously, a deletion analysis of the 6.0 kb TH promoter fragment in the TH-NFH promoter yielded two ~100 bp fragments with enhancer activity within an ~320 bp fragment (Gao et al., 2007). The capability to target expression to VGLUT1-containing neurons, or specific subclasses of VGLUT1-containing neurons, might benefit specific studies in basic neuroscience or on gene therapy, particularly in neocortex.
4. Materials and Methods
4.1. Materials
OptiMEM, penicillin/streptomycin, Dulbecco’s modified minimal essential medium (DMEM), and fetal bovine serum (FBS) were obtained from Invitrogen. G418 was obtained from RPI. X-gal was obtained from Sigma. Rabbit anti-E. coli β-gal was obtained from Chemicon. Mouse anti-E. coli β-gal, fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit immunoglobulin G (IgG), and tetramethylrhodamine isothiocyanate (TRITC)-conjugated goat anti-mouse IgG were obtained from Sigma. Rabbit anti-PAG was a generous gift from Dr. N. Curthoys, and mouse anti-VGLUT1 and mouse anti-VGLUT2 were generous gifts from Dr. R. Edwards.
4.2. Cells, vectors, and vector packaging
BHK21 and 2-2 cells were maintained in DMEM supplemented with 10 % FBS, 4 mM glutamine and penicillin/streptomycin. They were grown in an incubator at 37 °C, 5 % CO2 and 100 % humidity. G418 (0.5 mg/ml) was present during the growth of 2-2 cells, but was removed before plating cells for vector packaging.
pVGLUT1lac contains the mouse VGLUT1 promoter and first intron (7 kb and 4.6 kb fragments, respectively) (Rasmussen et al., 2007). The control vector, pINS-TH-NFHpkcΔ/INS-TH-NFHlac, contains a neuron-specific promoter, which is composed of the chicken β-globin INS (1.2 kb), an enhancer from the rat TH promoter (−0.5 kb to −6.8 kb), and a mouse NFH promoter (0.6 kb) (Zhang et al., 2000).
Vectors were packaged into HSV-1 particles using a modified form of the helper-virus free packaging protocol described previously (Fraefel et al., 1996; Sun et al., 1999). Purified vectors were titered on BHK cells, by counting X-gal positive cells at 24 hrs post-transduction. Wild-type HSV-1 was not detected (<10 plaque forming units/ml) in any of the vector stocks.
4.3. Stereotactic injections of vectors into rat POR cortex or the VMH
The VA Boston Healthcare System IACUC approved all the animal procedures. Adult male Sprague-Dawley rats (250–300 g) were anesthetized by ip injection of a Ketamine (20 mg/ml) Xylazine (2 mg/ml) mixture with a final dose of 60 mg/kg and 6 mg/kg, respectively. Additional anesthesia was administered as needed. Each rat received 2 injections of a specific vector into either POR cortex or the VMH, one in each hemisphere: The injection coordinates for POR cortex were anterior-posterior (AP) −8.0, medial-lateral (ML) ±6.0, dorsal-ventral (DV) −5.2) (Paxinos and Watson, 1986); the injection coordinates for the VMH were AP −2.6, ML ±1.4, DV −7.2. AP is measured relative to bregma, ML is relative to the sagittal suture and DV is relative to the bregma-lambda plane. A micropump (model 100, KD Scientific) was used for the injections; 3 µl of vector stock was injected at each site over 5 minutes, and after 5 additional minutes, the needle was slowly retracted.
4.4. Immunohistochemistry
Brains were perfused as described (Zhang et al., 2000), and 25 µm coronal sections containing, or proximal to, the injection site were prepared using a freezing microtome. Rabbit anti-PAG antibody was purified using a prepacked diethylaminoethyl/Affi-Gel blue column, following the manufacturer’s instructions. Immunohistochemistry was performed on free-floating sections as described (Zhang et al., 2000). β-gal-IR was detected using either rabbit or mouse anti-β-gal antibody (1:1,000 dilution or 1:200 dilution, respectively). Cell types were identified using rabbit anti-PAG (1:400 dilution), mouse anti-VGLUT1 (1:150 dilution), or mouse anti-VGLUT2 (1:150 dilution). Primary antibodies were visualized with either TRITC-conjugated goat anti-mouse IgG or FITC-conjugated goat anti-rabbit IgG.
4.5. Cell counts
Neuron class-specific expression was quantified by cell counts. Digital images were taken at 20× or 60× magnification, merged, and counts were performed on the merged images. In the fields that were examined, all the β-gal-IR cells were scored for containing, or lacking, a specific cell marker. All counts were done at least two separate times, and results differed by <10 %.
Acknowledgments
We gratefully thank Drs. N. Brose and C. Rosenmund (Max Planck-Institute, Gottingen Germany) for the VGLUT1 promoter, Dr. K. O’Malley (Univ. Wash., St. Louis MO) for the TH promoter, Dr. W. Schlaepfer (Univ. PA, Philadelphia PA) for the NFH promoter, Dr. A. Davison (Institute of Virology, Glasgow UK) for HSV-1 cosmid set C, Dr. R. Sandri-Goldin (Univ. CA, Irvine CA) for 2-2 cells, Dr. N. Curthoys (CO State Univ., Fort Collins) for the anti-PAG antibody, and Dr. R. Edwards (Univ. CA, San Francisco) for the anti-VGLUT1 and anti-VGLUT2 antibodies. This work was supported by NIH Grants AG025894 (G.Z.), NS045855 and NS057558 (A.I.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Refererences
- Banner C, Hwang JJ, Shapiro RA, Wenthold RJ, Nakatani Y, Lampel KA, Thomas JW, Huie D, Curthoys NP. Isolation of a cDNA for rat brain glutaminase. Brain Res. 1988;427:247–254. doi: 10.1016/0169-328x(88)90047-2. [DOI] [PubMed] [Google Scholar]
- Barroso-Chinea P, Castle M, Aymerich MS, Perez-Manso M, Erro E, Tunon T, Lanciego JL. Expression of the mRNAs encoding for the vesicular glutamate transporters 1 and 2 in the rat thalamus. J Comp Neurol. 2007;501:703–715. doi: 10.1002/cne.21265. [DOI] [PubMed] [Google Scholar]
- Bellocchio EE, Reimer RJ, Fremeau RT, Jr., Edwards RH. Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science. 2000;289:957–960. doi: 10.1126/science.289.5481.957. [DOI] [PubMed] [Google Scholar]
- Cangro CB, Sweetnam PM, Wrathall JR, Haser WB, Curthoys NP, Neale JH. Localization of elevated glutaminase immunoreactivity in small DRG neurons. Brain Res. 1985;336:158–161. doi: 10.1016/0006-8993(85)90428-7. [DOI] [PubMed] [Google Scholar]
- Cao H, Zhang GR, Wang X, Kong L, Geller AI. Enhanced nigrostriatal neuron-specific, long-term expression by using neural-specific promoters in combination with targeted gene transfer by modified helper virus-free HSV-1 vector particles. BMC Neurosci. 2008;9:37. doi: 10.1186/1471-2202-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraefel C, Song S, Lim F, Lang P, Yu L, Wang Y, Wild P, Geller AI. Helper virus-free transfer of herpes simplex virus type 1 plasmid vectors into neural cells. J. Virol. 1996;70:7190–7197. doi: 10.1128/jvi.70.10.7190-7197.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT, Jr., Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31:247–260. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr., Kam K, Qureshi T, Johnson J, Copenhagen DR, Storm-Mathisen J, Chaudhry FA, Nicoll RA, Edwards RH. Vesicular glutamate transporters 1 and 2 target to functionally distinct synaptic release sites. Science. 2004a;304:1815–1819. doi: 10.1126/science.1097468. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr., Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004b;27:98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Gao Q, Sun M, Wang X, Geller AI. Isolation of an enhancer from the rat tyrosine hydroxylase promoter that supports long-term, neuronal-specific expression from a neurofilament promoter, in a helper virus-free HSV-1 vector system. Brain Res. 2007;1130:1–16. doi: 10.1016/j.brainres.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L. Intercellular metabolic compartmentation in the brain: past, present and future. Neurochem Int. 2004;45:285–296. doi: 10.1016/j.neuint.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Herzog E, Bellenchi GC, Gras C, Bernard V, Ravassard P, Bedet C, Gasnier B, Giros B, El Mestikawy S. The existence of a second vesicular glutamate transporter specifies subpopulations of glutamatergic neurons. J Neurosci. 2001;21:RC181. doi: 10.1523/JNEUROSCI.21-22-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin BK, Belloni M, Conti B, Federoff HJ, Starr R, Son JH, Baker H, Joh TH. Prolonged in vivo gene expression driven by a tyrosine hydroxylase promoter in a defective herpes simplex virus amplicon vector. Hum. Gene Ther. 1996;7:2015–2024. doi: 10.1089/hum.1996.7.16-2015. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Mizuno N. Immunohistochemical study of glutaminase-containing neurons in the cerebral cortex and thalamus of the rat. J. Comp. Neurol. 1988;267:590–602. doi: 10.1002/cne.902670411. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Nakaya Y, Mizuno N. Paucity of glutaminase-immunoreactive nonpyramidal neurons in the rat cerebral cortex. J Comp Neurol. 1992;322:181–190. doi: 10.1002/cne.903220204. [DOI] [PubMed] [Google Scholar]
- Kaplitt MG, Kwong AD, Kleopoulos SP, Mobbs CV, Rabkin SD, Pfaff DW. Preproenkephalin promoter yields region-specific and long-term expression in adult brain after direct in vivo gene transfer via a defective herpes simplex viral vector. Proc. Natl. Acad. Sci. U.S.A. 1994;91:8979–8983. doi: 10.1073/pnas.91.19.8979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins I, Pinto M, Wilson SP, Lima D, Tavares I. Dynamic of migration of HSV-1 from a medullary pronociceptive centre: antinociception by overexpression of the preproenkephalin transgene. Eur J Neurosci. 2008;28:2075–2083. doi: 10.1111/j.1460-9568.2008.06492.x. [DOI] [PubMed] [Google Scholar]
- Nelson SB, Sugino K, Hempel CM. The problem of neuronal cell types: a physiological genomics approach. Trends Neurosci. 2006;29:339–345. doi: 10.1016/j.tins.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Olschowka JA, Bowers WJ, Hurley SD, Mastrangelo MA, Federoff HJ. Helper-free HSV-1 amplicons elicit a markedly less robust innate immune response in the CNS. Mol. Ther. 2003;7:218–227. doi: 10.1016/s1525-0016(02)00036-9. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Vol. Sidney: Academic Press; 1986. [Google Scholar]
- Rasmussen M, Kong L, Zhang G, Liu M, Wang X, Szabo G, Curthoys NP, Geller AI. Glutamatergic or GABAergic neuron-specific, long-term expression in neocortical neurons from helper virus-free HSV-1 vectors containing the phosphate-activated glutaminase, vesicular glutamate transporter-1, or glutamic acid decarboxylase promoter. Brain Res. 2007;1144:19–32. doi: 10.1016/j.brainres.2007.01.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata S, Kitsukawa T, Kaneko T, Yamamori T, Sakurai Y. Task-dependent and cell-type-specific Fos enhancement in rat sensory cortices during audio-visual discrimination. Eur J Neurosci. 2002;15:735–743. doi: 10.1046/j.1460-9568.2002.01905.x. [DOI] [PubMed] [Google Scholar]
- Shapiro RA, Haser WG, Curthoys NP. Immunoblot analysis of glutaminase peptides in intact and solubilized mitochondria isolated from various rat tissues. Biochem J. 1987;242:743–747. doi: 10.1042/bj2420743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Wang Y, Bak SY, Lang P, Ullrey D, Neve RL, O'Malley KL, Geller AI. An HSV-1 vector containing the rat tyrosine hydroxylase promoter enhances both long-term and cell type-specific expression in the midbrain. J. Neurochem. 1997;68:1792–1803. doi: 10.1046/j.1471-4159.1997.68051792.x. [DOI] [PubMed] [Google Scholar]
- Sugino K, Hempel CM, Miller MN, Hattox AM, Shapiro P, Wu C, Huang ZJ, Nelson SB. Molecular taxonomy of major neuronal classes in the adult mouse forebrain. Nat Neurosci. 2006;9:99–107. doi: 10.1038/nn1618. [DOI] [PubMed] [Google Scholar]
- Sun M, Zhang GR, Yang T, Yu L, Geller AI. Improved titers for helper virus-free herpes simplex virus type 1 plasmid vectors by optimization of the packaging protocol and addition of noninfectious herpes simplex virus-related particles (previral DNA replication enveloped particles) to the packaging procedure. Hum. Gene Ther. 1999;10:2005–2011. doi: 10.1089/10430349950017365. [DOI] [PubMed] [Google Scholar]
- Sun M, Zhang G, Kong L, Holmes C, Wang X, Zhang W, Goldstein DS, Geller AI. Correction of a rat model of Parkinson’s disease by coexpression of tyrosine hydroxylase and aromatic amino acid decarboxylase from a helper virus-free herpes simplex virus type 1 vector. Hum. Gene Ther. 2003;14:415–424. doi: 10.1089/104303403321467180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Kong L, Wang X, Holmes C, Gao Q, Zhang W, Pfeilschifter J, Goldstein DS, Geller AI. Coexpression of Tyrosine Hydroxylase, GTP Cyclohydrolase I, Aromatic Amino Acid Decarboxylase, and Vesicular Monoamine Transporter-2 from a Helper Virus-Free HSV-1 Vector Supports High-Level, Long-Term Biochemical and Behavioral Correction of a Rat Model of Parkinson’s Disease. Hum. Gene Ther. 2004;15:1177–1196. doi: 10.1089/hum.2004.15.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Kong L, Wang X, Lu X, Gao Q, Geller AI. Comparison of protection of nigrostriatal neurons by expression of GDNF, BDNF, or both neurotrophic factors. Brain Res. 2005;1052:119–129. doi: 10.1016/j.brainres.2005.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature. 2000;407:189–194. doi: 10.1038/35025070. [DOI] [PubMed] [Google Scholar]
- Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of differentiation-associated brain-specific phosphate transporter as a second vesicular glutamate transporter (VGLUT2) J Neurosci. 2001;21:RC182. doi: 10.1523/JNEUROSCI.21-22-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Gucht E, Jacobs S, Kaneko T, Vandesande F, Arckens L. Distribution and morphological characterization of phosphate-activated glutaminase-immunoreactive neurons in cat visual cortex. Brain Res. 2003;988:29–42. doi: 10.1016/s0006-8993(03)03332-8. [DOI] [PubMed] [Google Scholar]
- Varoqui H, Schafer MK, Zhu H, Weihe E, Erickson JD. Identification of the differentiation-associated Na+/PI transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. J Neurosci. 2002;22:142–155. doi: 10.1523/JNEUROSCI.22-01-00142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade-Martins R, Saeki Y, Chiocca EA. Infectious delivery of a 135-kb LDLR genomic locus leads to regulated complementation of low-density lipoprotein receptor deficiency in human cells. Mol. Ther. 2003;7:604–612. doi: 10.1016/s1525-0016(03)00060-1. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang G, Yang T, Zhang W, Geller AI. Fifty-one kilobase HSV-1 plasmid vector can be packaged using a helper virus-free system and supports expression in the rat brain. BioTechniques. 2000;27:102–106. doi: 10.2144/00281st05. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yu L, Geller AI. Diverse stabilities of expression in the rat brain from different cellular promoters in a helper virus-free herpes simplex virus type 1 vector system. Hum. Gene Ther. 1999;10:1763–1771. doi: 10.1089/10430349950017446. [DOI] [PubMed] [Google Scholar]
- Yang T, Zhang G, Zhang W, Sun M, Wang X, Geller AI. Enhanced reporter gene expression in the rat brain from helper virus-free HSV-1 vectors packaged in the presence of specific mutated HSV-1 proteins that affect the virion. Molec. Brain. Res. 2001;90:1–16. doi: 10.1016/s0169-328x(01)00059-6. [DOI] [PubMed] [Google Scholar]
- Zhang G, Wang X, Yang T, Sun M, Zhang W, Wang Y, Geller AI. A tyrosine hydroxylase--neurofilament chimeric promoter enhances long-term expression in rat forebrain neurons from helper virus-free HSV-1 vectors. Molec. Brain. Res. 2000;84:17–31. doi: 10.1016/s0169-328x(00)00197-2. [DOI] [PubMed] [Google Scholar]
- Zhang G, Wang X, Kong L, Lu X, Lee B, Liu M, Sun M, Franklin C, Cook RG, Geller AI. Genetic enhancement of visual learning by activation of protein kinase C pathways in small groups of rat cortical neurons. J Neurosci. 2005;25:8468–8481. doi: 10.1523/JNEUROSCI.2271-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Liu M, Cao H, Kong L, Wang X, Cook RG, Geller AI. Improved spatial learning in aged rats by genetic activation of protein kinase C in small groups of rat hippocampal neurons. Hippocampus. 2009;19:413–423. doi: 10.1002/hipo.20506. [DOI] [PMC free article] [PubMed] [Google Scholar]