Abstract
The forkhead box proteins A1 and A2 (Foxa1 and Foxa2) are transcription factors with critical roles in establishing the developmental competence of the foregut endoderm and in initiating liver specification. Using conditional gene ablation during a later phase of liver development, we show here that deletion of both Foxa1 and Foxa2 (Foxa1/2) in the embryonic liver caused hyperplasia of the biliary tree. Abnormal bile duct formation in Foxa1/2-deficient liver was due, at least in part, to activation of IL-6 expression, a proliferative signal for cholangiocytes. The glucocorticoid receptor is a negative regulator of IL-6 transcription; in the absence of Foxa1/2, the glucocorticoid receptor failed to bind to the IL-6 promoter, causing enhanced IL-6 expression. Thus, after liver specification, Foxa1/2 are required for normal bile duct development through prevention of excess cholangiocyte proliferation. Our data suggest that Foxa1/2 function as terminators of bile duct expansion in the adult liver through inhibition of IL-6 expression.
The liver develops from the ventral foregut endoderm. This structure gives rise to a tissue bud that invades the septum transversum and from which originates the liver and the intrahepatic biliary tree. At the time of liver specification, liver stem cells differentiate into hepatoblasts, the precursors of hepatocytes and cholangiocytes. Hepatoblasts in contact with the mesenchyma surrounding the portal vein branches, organize into a single layered sleeve of small flat epithelial cells, called ductal plates. Ductal plates are first duplicated by a second layer of cells over variably long segments of their perimeter and then dilate to form tubular structures that are then incorporated into the nascent portal space. Once incorporated into the portal space, the immature tubules are remodelled to form individual bile ducts, while the remaining segments of the ductal plate are gradually deleted by apoptosis [1,2].
Several transcription factors (HNF1β, HNF4, HNF6) and signaling pathways (Jagged1/Notch2, Hedgehog, TGFβ, Wnt/β-catenin and FGF) that regulate the development of the intrahepatic biliary epithelium have been identified, but the signals that arrest bile duct formation at the appropriate developmental time are not known. In a recent issue of the Journal of Clinical Investigation, Dr. Li, from Klaus Kaestner's lab [3], provides convincing evidence that the Foxa1 and Foxa2 transcription factors may actually function as terminators of bile duct expansion.
Foxa1 and Foxa2 (previously known as HNF3) belong to a group of liver specific transcription factors that also includes the above mentioned HNF1α HNF1β, HNF4, HNF6, and C/EBPα,β. The Foxa family of transcription factors (from forkhead box protein A) controls embryonic development and organogenesis in a number of organs, including the liver and pancreas. If both Foxa1 and 2 are deleted, no liver is formed, indicating that, in the mouse embryo, Foxa transcription factors play a critical role in establishing the developmental competence of the foregut endoderm and in initiating liver specification [3].
Using a conditional gene ablation approach, Li and colleagues [3] showed that deletion of Foxa1/2 I after initial liver specification caused biliary hyperplasia, an increased number of dysmorphic and dilated biliary structures, along with an increased deposition of fibrous tissue. Cholangiocyte proliferation was drastically increased. These changes were not associated with changes in other pathways involved in biliary development, such as Wnt, Notch, and Hedgehog. On the other hand, persistently elevated levels of interlukin-6 (IL-6) expression were found in the biliary epithelium that also expressed increased STAT3 and p42/44 MAPK and IL-6-dependent genes. These changes were prevented by administration of inhibitory anti-IL-6 antibodies.
Foxa1/2 are “pioneer” factors that are able to open the chromatin and enhance the binding of other transcription factors to their target genes. For instance, Foxa1/2 proteins facilitate the binding of nuclear hormone receptors to their targets. One such receptor is the glucocorticoid receptor (GR). The IL-6 promoter contains closely spaced binding sites for Foxa1/2, GR and NF-κB. GR suppresses the IL-6 promoter, but NF-κB can replace GR and stimulate IL-6 transcription. Li et al. showed that Foxa1/2 are required to enable the GR to bind to the IL-6 promoter. In their absence, Nf-κB will bind to the IL-6 promoter instead of GR, resulting in stimulation of IL-6 production.
Are these findings relevant for human liver diseases? Foxa1 and Foxa2 remain expressed in normal hepatocytes and cholangiocytes in adulthood [3], suggesting that these transcription factors may act as repressors of IL-6 expression also in the adult liver. In contrast to normal biliary cells, reactive cholangiocytes are known to secrete a large amount of IL-6 [4]. Li and colleagues showed that in mice with experimental obstructive cholestasis, Foxa1 and 2 expression decreases, concomitantly with an increase in IL-6 expression [3]. This inflammatory cytokine is an important autocrine and paracrine survival factor for liver epithelial cells [5,6]. In fact, mortality following bile duct ligation is increased in mice defective for IL-6.
Ductular proliferation and expansion of the cholangiocyte compartment is a compensatory response to many acute and chronic forms of liver diseases, from cholangiopathies to viral hepatitis and fulminant hepatitis. When the proliferative ability of hepatocytes is impaired, cells from the canals of Hering give rise to progenitor cells/reactive cholangiocytes capable of generating both biliary cells and hepatocytes.
In addition to IL-6, a number of autocrine and paracrine factors, including the epidermal growth factor (EGF), hepatocyte growth factor (HGF), insulin-like growth factor-1 (IGF-1), vascular endothelial growth factor (VEGF), and neurotrophins and estrogens, are able to stimulate biliary epithelia proliferation [7-9]. Furthermore, in response to liver damage, reactive cholangiocytes also secrete IL-1β, IL-8, TNFα, IFNγ, monocyte chemotactic protein-1 (MCP-1), cytokine-induced neutrophil chemo attractant (CINC), nitric oxide (NO), endothelin-1 (ET-1), platelet derived growth factor-BB (PDGF-BB), transforming growth factor-β2 (TGF-β2), connective tissue growth factor (CTGF), and VEGF-A and C [8,9]. These factors have important paracrine effects on hepatic stellate cells (HSC), portal fibroblasts, inflammatory cells, endothelial cells, and as well as on mesenchymal and endothelial precursors [8,9]. It is therefore not surprising that Foxa1/2 defective mice exhibit increased portal fibrosis, likely as a result of the paracrine effects of IL-6. In human liver disease, the extent of ductular reaction is known to correlate with the extent of portal fibrosis [10,11].
Unfortunately, the mechanisms responsible for the “activation” of reactive cholangiocytes are not well understood. It is tempting to speculate that in cholangiocytes exposed to biliary damage, the release of Foxa1/2-mediated repression enables the activation of the reactive/progenitor cells. Several of the above mentioned factors are also transitorily expressed by ductal plate cells during development. Recent observations underscore the importance of morphogenesis pathways such as Hh, Wnt, and Notch in progenitor cell-mediated liver repair [10,12,13]. Thus, liver repair mechanisms appear to recapitulate ontogenesis, and as we increase our knowledge of liver development, we foster our understanding of the pathophysiology of the diseases of adulthood. Or, in better words “So we beat on…..borne back ceaselessly into the past” [14].
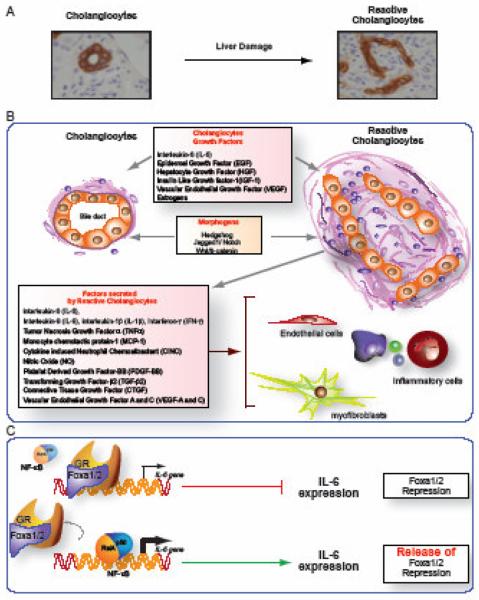
Fig. 1. Ductular proliferation and the expansion of “reactive cholangiocytes” is a common compensatory and reparative response to liver damage (panel A).
Reactive cholangiocytes, also called “liver progenitor cells” because of their ability to differentiate into hepatocytes, are thought to originate from epithelial cells lining cholangioles and ducts of Hering. Reactive cholangiocytes generate a number of growth factors, chemokines, and cytokines that generate a network of autocrine and paracrine signals that play a key role in liver repair and cross talk with the other cell types of the hepatic reparative complex (mesenchymal cells, inflammatory cells, endothelial cells) (panel B). The molecular mechanisms regulating the activation of reactive cholangiocytes are not known. The paper from Li et al. begins to shed light on these mechanisms showing that release of Foxa1/2 repression is essential to allow transcription of IL-6 in developing and reactive biliary cells. Foxa1/2 are “pioneer” factors, able to open the chromatin and enhance the binding of other transcription factors, such as the glucocorticoid receptor (GR), to their target genes. The IL-6 promoter contains closely spaced binding sites for Foxa1/2, GR and NF-κB. GR suppresses the IL-6 promoter. Li et al. showed that Foxa1/2 are required to enable the GR to bind to the interlukin-6 (IL-6) promoter. In their absence, Nf-κB, will bind to the IL-6 promoter instead of GR, resulting in stimulation of IL-6 production. It is tempting to speculate that similar models may apply to several of the other paracrine and autocrine factors generated by reactive cells (see panel C).
Acknowledgments
supported by NIH DK079005 grant, by the Yale University Liver Center NIH DK34989 grant and by PKD foundation. The support of Fondazione S. Martino, Bergamo is also gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lemaigre FP. Mechanisms of liver development: concepts for understanding liver disorders and design of novel therapies. Gastroenterology. 2009;137:62–79. doi: 10.1053/j.gastro.2009.03.035. Epub 2009 Mar 27. [DOI] [PubMed] [Google Scholar]
- 2.Raynaud P, Carpentier R, Antoniou A, Lemaigre FP. Biliary differentiation and bile duct morphogenesis in development and disease. Int J Biochem Cell Biol. 2009;6:6. doi: 10.1016/j.biocel.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 3.Li Z, White P, Tuteja G, Rubins N, Sackett S, Kaestner KH. Foxa1 and Foxa2 regulate bile duct development in mice. J Clin Invest. 2009;119:1537–45. doi: 10.1172/JCI38201. doi: 10.1172/JCI38201. Epub 2009 May 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demetris AJ, Fontes P, Lunz JG, 3rd, Specht S, Murase N, Marcos A. Wound healing in the biliary tree of liver allografts. Cell Transplant. 2006;15:S57–65. doi: 10.3727/000000006783982386. [DOI] [PubMed] [Google Scholar]
- 5.Ezure T, Sakamoto T, Tsuji H, Lunz JG, 3rd, Murase N, Fung JJ, et al. The development and compensation of biliary cirrhosis in interleukin-6-deficient mice. Am J Pathol. 2000;156:1627–39. doi: 10.1016/s0002-9440(10)65034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsumoto K, Fujii H, Michalopoulos G, Fung JJ, Demetris AJ. Human biliary epithelial cells secrete and respond to cytokines and hepatocyte growth factors in vitro: interleukin-6, hepatocyte growth factor and epidermal growth factor promote DNA synthesis in vitro. Hepatology. 1994;20:376–82. [PubMed] [Google Scholar]
- 7.Alvaro D, Mancino MG, Glaser S, Gaudio E, Marzioni M, Francis H, et al. Proliferating cholangiocytes: a neuroendocrine compartment in the diseased liver. Gastroenterology. 2007;132:415–31. doi: 10.1053/j.gastro.2006.07.023. Epub 2006 Jul 24. [DOI] [PubMed] [Google Scholar]
- 8.Lazaridis KN, Strazzabosco M, Larusso NF. The cholangiopathies: disorders of biliary epithelia. Gastroenterology. 2004;127:1565–77. doi: 10.1053/j.gastro.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Strazzabosco M, Fabris L, Spirli C. Pathophysiology of cholangiopathies. J Clin Gastroenterol. 2005;39:S90–S102. doi: 10.1097/01.mcg.0000155549.29643.ad. [DOI] [PubMed] [Google Scholar]
- 10.Fabris L, Cadamuro M, Guido M, Spirli C, Fiorotto R, Colledan M, et al. Analysis of liver repair mechanisms in Alagille syndrome and biliary atresia reveals a role for notch signaling. Am J Pathol. 2007;171:641–53. doi: 10.2353/ajpath.2007.070073. Epub 2007 Jun 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richardson MM, Jonsson JR, Powell EE, Brunt EM, Neuschwander-Tetri BA, Bhathal PS, et al. Progressive fibrosis in nonalcoholic steatohepatitis: association with altered regeneration and a ductular reaction. Gastroenterology. 2007;133:80–90. doi: 10.1053/j.gastro.2007.05.012. Epub 2007 May 16. [DOI] [PubMed] [Google Scholar]
- 12.Omenetti A, Yang L, Li YX, McCall SJ, Jung Y, Sicklick JK, et al. Hedgehog-mediated mesenchymal-epithelial interactions modulate hepatic response to bile duct ligation. Lab Invest. 2007;87:499–514. doi: 10.1038/labinvest.3700537. Epub 2007 Mar 5. [DOI] [PubMed] [Google Scholar]
- 13.Thompson MD, Monga SP. WNT/beta-catenin signaling in liver health and disease. Hepatology. 2007;45:1298–305. doi: 10.1002/hep.21651. [DOI] [PubMed] [Google Scholar]
- 14.Fitzgerald FS. The Great Gatsby. 1925 [Google Scholar]