Abstract
In this paper, we aim to characterize fibrinogen-IgG interactions, and explore how fibrinogen alters IgG-mediated phagocytosis.
Using enzyme-linked binding assays, we found that fibrinogen binding to IgG is optimized for surfaces coated with high levels of IgG. Using a similar method, we have shown that for an antigen unable to specifically bind fibrinogen, fibrinogen enhances binding of antibodies towards that antigen. For binding of IgG antibodies to cells expressing Fc receptors, we found a bimodal binding response, where low levels of fibrinogen enhance binding of antibody to Fc receptors and high levels reduce it. This corresponds to a bimodal effect on phagocytosis of IgG-coated particles, which is inhibited in the presence of excess IgG during coating of the particles with antibodies and fibrinogen.
We conclude that fibrinogen can modulate phagocytosis of IgG-coated particles in vitro by changing IgG binding behavior, and that high fibrinogen levels could negatively affect phagocytosis.
Keywords: Fibrinogen, Immunoglobulin G, Opsonization, Phagocytosis
INTRODUCTION
Human fibrinogen molecules (Fg) are plasma glycoproteins that are converted to fibrin during thrombosis, and are involved in many immune functions as well. Levels range from 1–4 mg/ml in healthy individuals, and become elevated with concentrations of 5 mg/ml and more in individuals in response to chronic inflammation. There are several variants of fibrinogen molecules of 270–420 kDa molecular weight, which is the result of degradation and splicing variants. We focused our efforts on the most common type, 340 kDa, high molecular weight fibrinogen type 1, which is the most common variant in human blood (70%) [1].
Fibrinogen γ chains interact with beta2 integrin receptors such as Mac-1 (CD11b/CD18, αMβ2) and αXβ2 (CD11c/CD18) on leukocytes [2; 3]. These interactions generally lead to cellular activation, resulting in increased phagocytosis [4] among many other enhanced functions. These effects appear to be important, as lack of fibrin(ogen) impairs bacterial clearance in defibrinated mice[5].
Human immunoglobulin G (IgG) is found in immobilized forms such as on bacteria and immune complexes, as well as in soluble forms. IgG comprises most of the antibodies in serum at variable concentrations with an average of about 10–14 mg/ml in healthy adults, with individual specific antibodies varying widely between 0–30 mg/ml, or higher in myeloma patients. These levels increase in response to infections [6; 7; 8], which reflects the primary functions of IgG antibodies: binding of antigen, neutralization, opsonization and elimination of the offending agent. In addition to binding of antigens and IgG-Fc receptors, which mediate phagocytosis of IgG-coated particles, IgG binds acute phase proteins such as complement C1q [9], C-reactive protein via C1q [10], Serum Amyloid P[11], acid alpha-1-glycoprotein[12] as well as fibronectin[13]. We recently demonstrated specific, saturable fibrinogen binding to IgG and IgA antibodies, but not IgM antibodies. Binding seems to occur predominantly on the IgG heavy chains, as specific, saturable binding demonstrable is present on Fab and Fc fragments, but strongest for Fc fragments and heavy chains [14].
Observing this effect we hypothesized that fibrinogen binding to IgG antibodies affects subsequent binding of cells to antibody coated bacteria, thus modulating phagocytosis. In this study we aim to further characterize fibrinogen-IgG binding, fibrinogen-IgG interaction specific effects on phagocytosis, effect of fibrinogen binding towards antibody-antigen binding and the effect of fibrinogen on antibody-cellular interactions. As fibrinogen bound to surface-bound IgG appears to modulate phagocytosis in distinct ways depending on the original fibrinogen concentration, we believe that these interactions might prove significant in many ways. For one, surface antigen bound IgG is commonly found on opsonized bacteria, and as fibrinogen appears to have concentration-dependent effects on phagocytosis, it might lead to decreased bacterial clearance under clinical conditions featuring high levels of plasma fibrinogen.
MATERIALS AND METHODS
Coating of bacteria, beads and wells
1 µm diameter carboxy-functionalized clear polystyrene beads (Phosphorex, Fall River, MA) were coated with GFP in bicarbonate buffer (pH 9.3), washed three times in cold PBS, and then coated with antibody and fibrinogen.
For binding assays, the protein of interest (GFP, IgG, fibrinogen-see supplemental materials) was coated onto plates at conditions described by the manufacturer’s insert for maximum absorption, or 50-fold diluted.
Beads and bacteria were suspended in PBS solutions containing 5 µg/ml anti-GFP antibody and varying concentrations of fibrinogen (0–5 mg/ml). Before adding the IgG antibody, the antibody solution was centrifuged at 15000 g for 1 minute to remove IgG aggregates. Bacteria or beads were incubated for 1 hour at room temperature, and washed 3 times with PBS. For protein binding assays, plates were blocked with 1% BSA in PBS for 1 hour, washed three times, and then treated with 10µg/ml protein for 1 hour at room temperature. THP-1 cells were coated onto adhesive plates by suspending 1×106/ ml cells in PBS pH 8.0, and allowed to adhere to the plates within one hour on ice. The plate was then washed three times with cold Gey’s balanced salt solution.
Enzyme-linked binding assays
To assess specific binding, binding assays using ELISA techniques were developed which are similar to the assays described by Makogonenko et al.[15], and have been validated by the same group who also used surface plasmon resonance with similar results.
For binding assays, protein coated plates were washed and blocked with 1% BSA. If the experiment required the receptor antibody (anti-GFP, anti-human IgG) being bound to its antigen (GFP, human IgG), the receptor antibody was allowed to bind to the antigen-coated plate at saturation levels in the presence of 1% BSA. Subsequently, specific binding of biotinylated ligand (fibrinogen, IgG) in the presence of 1% BSA was determined using a Streptavidin-Horse radish peroxidase/TMB system and excess unlabeled ligand as competitor. Absorbance readings of 450 nm were converted to bound ligand (pmol) using standards on the same plate, and specific binding calculated as the difference between total binding and unspecific binding. Data was analyzed using Graphpad Prism software, and statistical significance determined using 95% confidence intervals. We compared one binding and two binding site hypothesis using F-tests, and found no significant improvement in model fit, leading us to analyze the data with the simpler one binding site hypothesis.
To determine how much fibrinogen or IgG was immobilized on the plate during the coating process, we used similar ELISA methods and specific antibodies against the immobilized protein of choice.
If cells were used as receptor, the same method was employed except that detergent-free solutions were used, and incubation steps were performed at 4 degrees Celsius.
If cells were used as ligand, unspecific cell binding was determined by measuring cell binding to GFP and subtracting from the total number of cells bound.
Phagocytosis assay
To determine phagocytosis, ~105 cells were grown on glass cover slips in a 6 well plate and incubated with 1 ml of the treated bacteria for 60 minutes (shorter incubation times such as 15 and 30 minutes provided similar results) at 5 % CO2, 37°C and a bacteria/cell ratio of 1:100 in serum/Antibody/fibrinogen-free medium. For beads, a ratio of 1:1000 was used. In order to distinguish extracellular particles from intracellular ones, we either employed confocal fluorescence microscopy (Zeiss LSM 510 Meta NLO Confocal Microscope attached Zeiss Axioimager Z1 and Axiovert 200M) or a method described by Nuutila[16], using Trypan blue to squelch extracellular GFP fluorescence. In three randomly chosen fields of view containing a total of 100–300 cells, for each cell the phagocytic index was determined as the number of intracellular bacteria/particles per cell. Phagocytic indexes for all cells were averaged, producing the mean phagocytic index. In addition, the percentage of cells containing five or more bacteria/particles for each field of view was calculated, and averaged for each condition.
To determine statistical significance, one-way analysis of covariance with Bonferroni adjustment was performed. The significance cutoff was set at p<0.05.
Experiments were performed three times.
RESULTS
Surface density and phase type regulate fibrinogen-IgG binding
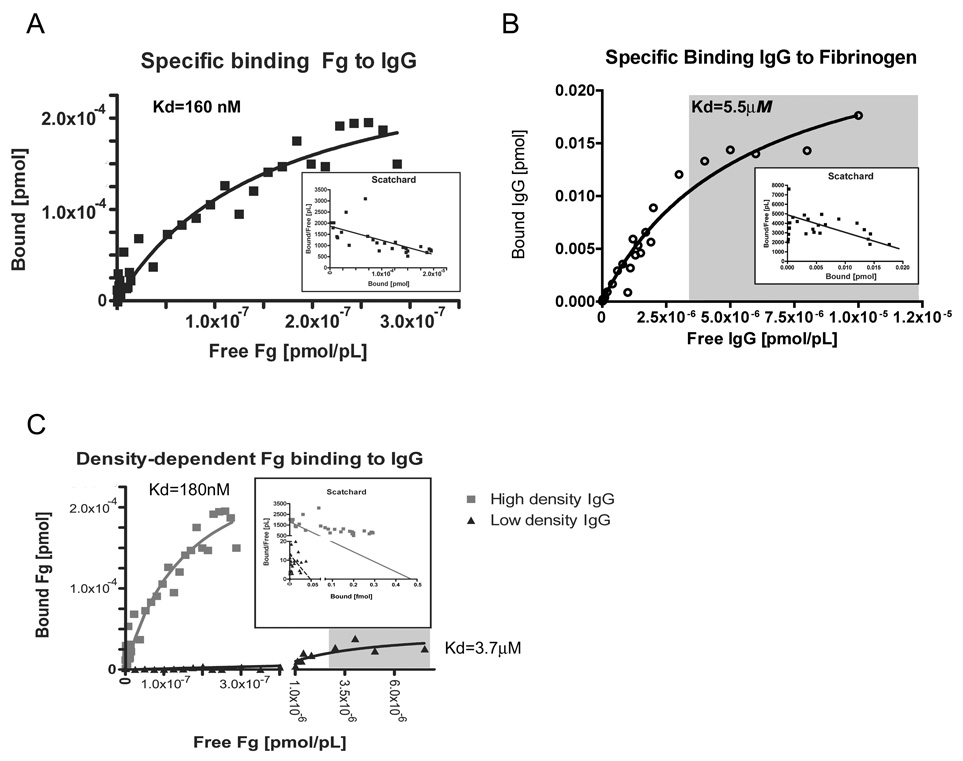
We have found recently that human IgG specifically binds to human fibrinogen type I in a saturable manner, and that binding is conserved for a variety of mammalian antibodies, including monoclonal antibodies. This binding is also specific to IgG, as it occurs much less with IgM[14] and does not occur with BSA or GFP (data not shown). Since binding occurred under physiologic concentrations of fibrinogen, we sought to determine if binding is dependent on phase and surface concentration in an effort to find optimal binding conditions and hints of physiologic function. Therefore we tested fibrinogen binding to immobilized IgG, and IgG binding to immobilized fibrinogen using the enzyme linked binding assay as described as in the materials & method section. As seen in figure 1 a, b) and Table 1, the dissociation constant (Kd) of fibrinogen binding to IgG significantly differs from the Kd for IgG binding to fibrinogen by a factor of 10. Since differences in binding could be the result of differing amounts of immobilized protein on the surface, we determined bound protein using ELISA techniques. The amount of immobilized fibrinogen at equilibrium ranged from 0.003 to 0.020 pmol [95% CI] depending on the detecting antibody, and the amount of immobilized human IgG ranged from 0.0033 to 0.0065 pmol [95% CI] using anti-human IgG mouse antibody. There is no statistically significant difference between bound amounts of IgG and fibrinogen, and we therefore assume equimolar bound amounts. Interestingly, while the total maximum amount of fibrinogen bound to human IgG (Bmax 0.030 pmol) is similar to the amount of immobilized IgG, the maximum amount of IgG bound to fibrinogen (Bmax 0.00029 pmol) is far less than the previously determined amount of immobilized fibrinogen. In order to determine if fibrinogen-IgG interactions result in a precipitin reaction similar to typical antigen-antibody interactions (such as fibrinogen-anti-fibrinogen IgG antibody), we performed a radial immunodiffusion assay, but found no evidence of precipitin reaction with human IgG and fibrinogen.
Figure 1. Fibrinogen binding to IgG is highly dependent on surface conditions.
Strong specific human fibrinogen binding to immobilized human IgG (A). When reversing phases, human IgG does not bind as strong to immobilized human fibrinogen (B). Binding strength is dependent on the amount of immobilized IgG present on the plate, with lower amounts and densities of IgG producing both weaker binding and lesser amounts of bound fibrinogen (C). Experimental Details: Fibrinogen or IgG were immobilized as indicated onto high binding polystyrene microwell plates, blocked with BSA, washed three times with PBS, and incubated with varying amounts of biotinylated ligand for 1 hour at 25°C in the presence or absence of excess unlabeled ligand. After three additional wash cycles to remove unbound ligand, bound ligand was detected using a colorimetric Streptavidin-Horseradish peroxidase system, and specifc binding calculated from the difference between bound ligand in the presence and absence of competitor. Binding data was then analyzed with GraphPad Prism using non-linear curve fitting and the one binding site model. Binding surface were coated with 1000 ng/well IgG (A), 1000 ng/well fibrinogen (B), which resulted in similar equimolar amounts of bound fibrinogen or IgG. For determining the effect of surface IgG density, binding surfaces were either coated with 1000 ng/well IgG or 20 ng/well IgG, which resulted in at least 10-fold reduction of bound IgG. During fibrinogen binding all other conditions the same (C). Gray shaded areas represent physiologic range of free ligand concentration.
Table 1.
Fibrinogen binding is highly variable
| Receptor (immobilized) |
Ligand | Kd (95% CI) | Bmax (95% CI) |
|---|---|---|---|
| human IgG (high conc.) |
human Fibrinogen | 160 nM (67.87 … 247.8 nM) |
0.29 fmol (0.2080 … 0.3630 fmol) |
| human Fibrinogen (high conc.) |
human IgG | 5.5 µM (3.3665 … 7.6712 µM) |
27 fmol (21.47 … 33.36 fmol) |
| human IgG (high conc.) |
human Fibrinogen | 180 nM (0.0 … 426.6 nM) |
0.48 fmol (0.1545 …0.7957 fmol) |
| human IgG (low conc.) |
human Fibrinogen | 3.7 µM (0.5542 to 6.873 µM) |
50 amol (25.52 … 71.74 amol) |
Experimental Details: Fibrinogen or IgG were immobilized as indicated onto high binding polystyrene microplates, blocked with BSA, and incubated with varying amounts of biotinylated ligand for 1 hour at 25°C in the presence or absence of excess unlabeled ligand. Bound ligand was detected using a colorimetric Streptavidin-Horseradish peroxidase system, and raw binding data analyzed with GraphPad Prism using non-linear curve fitting and the one binding site model. 95% confidence intervals are given for both Kd and Bmax. Generally, we immobilized proteins with solutions containing an excess of protein (1000 ng/well) in regard to maximum binding capacity (500 ng/cm2). To determine effect of low density coating, we coated one plate with 1000 ng/well IgG and the other with 20 ng/well IgG, and determined fibrinogen binding as before under otherwise same conditions. Experiments were performed three times with similar results.
Since binding of fibrinogen seemed to preferentially occur on IgG-coated surfaces, it appeared similar to the binding characteristics of another IgG-binding protein, complement C1q, which for most efficient binding requires antibodies immobilized on a surface. Since C1q requires also a high surface density of IgG for efficient binding, we also thought that efficient fibrinogen-IgG binding might depend on IgG surface concentration. Therefore we tested if reducing IgG concentration on a surface not only decreases the maximum amount of protein bound (Bmax), but also increased the dissociation constant Kd, implying weakened binding. As seen in Figure 1.c) and Table 1, a 20-fold reduction of IgG on the surface not only lowers Bmax by a similar magnitude as expected, but also significantly increases the Kd value. We confirmed that reducing the amount of IgG in the coating solution indeed reduces the amount of immobilized IgG, as for the high density plates, IgG amounts ranged consistently between 0.06–0.07 pmol as determined through the ELISA method, and for low density plates, IgG amounts were at least 10-fold lower (0.0004–0.003 pmol).
Therefore, it appears that fibrinogen binding strength to human IgG is maximized if large amounts of human IgG are immobilized on a surface. One situation where this occurs is during opsonization of bacteria in the presence of a protective antibody response, which leads to subsequent efficient phagocytosis.
Fibrinogen has bimodal effect on IgG-mediated phagocytosis of antibody-coated particles
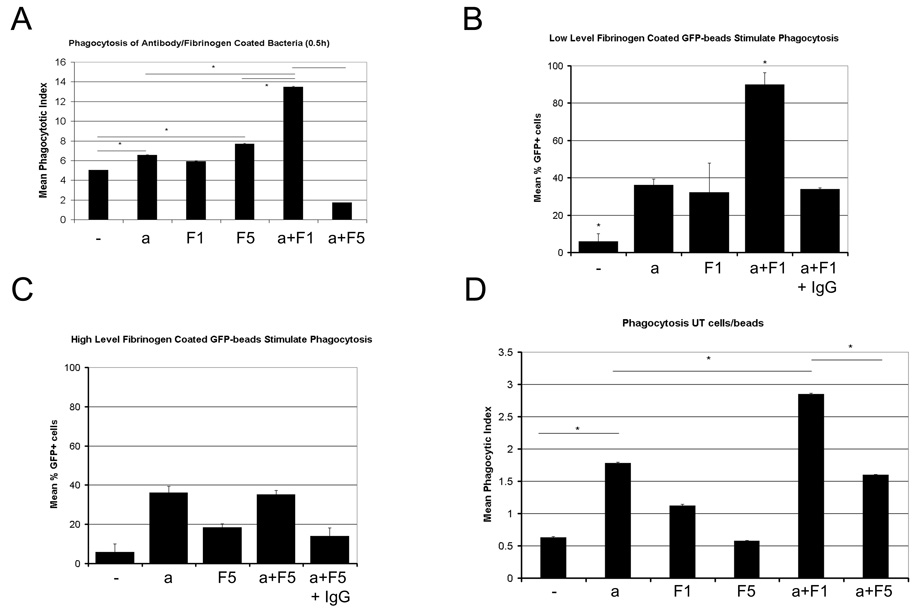
Since fibrinogen and IgG enhance phagocytosis, we expected to see a dose-dependent increase in phagocytosis if both molecules were deposited on particles such as E.coli bacteria. Using both confocal and conventional fluorescence microscopy techniques to evaluate phagocytosis, we observed that macrophages exhibit significantly enhanced phagocytosis of GFP-expressing, antibody-coated E.coli that were additionally coated with low levels (1 mg/ml) of fibrinogen (Figure 2.a). Similar effects were also seen with 2 mg/ml fibrinogen and to a lesser extent with 0.5 mg/ml fibrinogen (not shown). No significant differences in intracellular distribution (not shown) were observed. When testing the response of macrophages to bacteria coated with variety of combinations of fibrinogen (0, 0.2, 0.5, 1, 2, 5 mg/ml) and antibodies (0, 0.1, 1 µg/ml) solutions, we observed that there appears to combinations of 1–2 mg/ml fibrinogen and 1 µg/ml anti-E.coli IgG antibody maximized the phagocytic response (Table 2). We noted that this effect was also seen when creating histograms tabulating the number of cells containing x bacteria, where x is a whole number between 1 and 50. The effect of fibrinogen and antibody coating results in a shift towards higher bacterial numbers, and higher numbers of cells containing more than 1 bacterium (Supplemental Figure 2). As a result, total numbers of phagocytically active cells or percentage reflects the increase in mean phagocytic index, and correlates well (R2=0.77). As the effect of fibrinogen on phagocytosis is similar when the percentage of phagocytic cells containing more than one particle per cell is measured, this allowed us to determine effects on phagocytosis on greater numbers of cells. As seen before with the mean phagocytic index, combination treatment of 1 mg/ml fibrinogen and antibody results in significantly increased numbers of phagocytically active cells. However, as evident in all figures, high level fibrinogen during the antibody-coating process tends to diminish phagocytosis.
Figure 2. Different levels of fibrinogen present during the antibody-coating of particles produces a bimodal phagocytosis response.
Low levels of fibrinogen (1 mg/ml fibrinogen, F1) present during the coating of bacteria with antibodies (a), result in enhanced phagocytosis of these bacteria by macrophages when challenged with these bacteria in serum-free media(A). When coating sterile GFP-coated polystyrene beads with combinations of anti-GFP IgG and fibrinogen, the same effect was observed, and presence of excess non-specific IgG during the coating progress inhibited this response in macrophages (B). Similar as observed with bacteria, high levels of fibrinogen (5 mg/ml, F5) during the coating of GFP-beads with anti-GFP IgG, did not produce any subsequent enhanced phagocytosis in macrophages(C). When repeating these experiments with undifferentiated THP-1 cells that lack beta2 integrin receptors for fibrinogen, different levels of fibrinogen present during the coating process of GFP-coated beads with anti-GFP antibodies still produced different phagocytosis responses (D). Experimental Details: GFP-expressing E.coli or GFP-coated polystyrene beads were coated with antibody and/or fibrinogen at various concentrations for 1 hour at 25°C. Bacteria/beads were washed thoroughly to remove free fibrinogen and antibody, and then applied to macrophage or THP-1 cultures in serum-free media. A) Phagocytic indices after 0.5 hour in 40–70 macrophages, B, C) Mean % of cells containing more than 1 GFP bead after 0.5 hours in 130–180 macrophages. In B) particles were coated with anti-GFP antibody and/or 1 mg/ml Fibrinogen, while in C) particles were coated with anti-GFP antibody and/or 5 mg/ml Fibrinogen. D) Mean phagocytosis of anti-GFP IgG or fibrinogen coated GFP-beads in undifferentiated THP-1 cells after 1 hour. For all figures: a=antibody 1 µg/ml, F1=1 mg/ml fibrinogen, F5=5 mg/ml fibrinogen, IgG=5000-fold excess unspecific IgG added. Beads were incubated under these conditions for 1 hour and washed three times before the phagocytosis assay to remove unbound fibrinogen or antibody. All experiments were repeated three times producing consistent results.
Table 2.
Enhanced Phagocytosis in response to low level fibrinogen
| Treatment | 0.1 µg/ml Antibody | 1 µg/ml Antibody |
|---|---|---|
| 0.2 mg/ml Fibrinogen | no effect | no effect |
| 0.5 mg/ml Fibrinogen | Addition | Addition |
| 1 mg/ml Fibrinogen | N/A | Synergism |
| 2 mg/ml Fibrinogen | N/A | Addition |
| 5 mg/ml Fibrinogen | no effect | Diminished |
Type of effect seen on mean phagocytic indices (MPI) if macrophages were challenged with bacteria coated with either different concentration of fibrinogen (0–5 mg/ml), antibody (0–1 µg/ml) or both. Combination treatment resulted in: no effect (no statistical significant difference to either antibody or fibrinogen alone), additional effect (combination treatment MPI is no greater than the sum of MPIs from the ‘fibrinogen only’ and ‘antibody only’ experiments), synergistic effect (combination treatment MPI exceeds the sum of ‘fibrinogen only’ and ‘antibody only’ treatment), diminished effect (combination treatment MPI is lower than the MPIs of antibody and fibrinogen treatments). N/A: this combination was not tested.
Since E.coli is a complex organism expressing many molecules that may affect phagocytosis, we tested phagocytosis of beads coated with GFP, anti-GFP polyclonal sheep antibody and varying levels of fibrinogen to see if similar responses are observed in this simpler model. Again, a bimodal response is seen, and this effect can be inhibited with excess IgG during the coating process (Figure 2.b, c), indicating that the enhancement of phagocytosis by fibrinogen is due to fibrinogen-IgG interactions. We did notice that fibrinogen alone stimulates phagocytosis of beads, but compared to the control it was not significant. Antibodies alone did significantly stimulate phagocytosis (p<0.05), as expected.
Since macrophages also express beta2 integrins, which bind fibrinogen and might enhance binding and phagocytosis of fibrinogen/antibody-coated particles independently of Fc gamma receptors, we tested phagocytosis of GFP-beads in undifferentiated THP-1 cells, which have no detectable levels of beta 2 integrins. As predicted, a bimodal phagocytic response was still seen (Figure 2.d). When comparing additional concentrations of antibodies and fibrinogen, it becomes clear that there appears to be an optimal concentration of fibrinogen and antibodies which produces a synergistic response (Table 2). This synergistic response appears to be centered at the low end of normal physiologic fibrinogen concentrations.
Therefore, we conclude that fibrinogen bound to antibody-coated particles affects phagocytosis in a bimodal manner in these models, where low level fibrinogen (0.5, 1, 2 mg/ml) enhances phagocytosis while high levels of fibrinogen (5 mg/ml) have no added benefit.
Fibrinogen binding to IgG does not diminish antigen binding
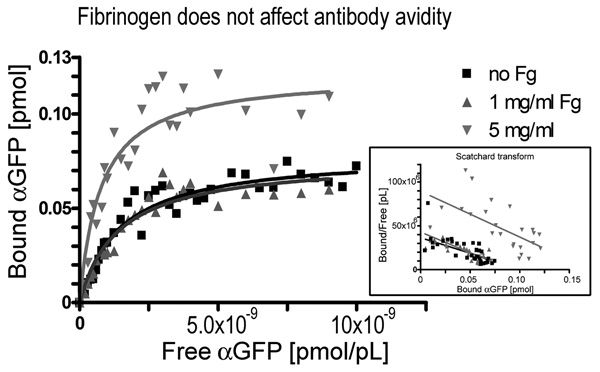
As high fibrinogen levels present during opsonization appear to interfere with phagocytosis, we hypothesized that high levels of fibrinogen might interfere with antigen binding of antibodies, resulting in less bound antibody for cell binding through Fc receptors. To test this hypothesis we chose green fluorescent protein (GFP) as a model antigen for the following reasons: First, GFP appears to lack specific fibrinogen binding. Second, polyclonal sheep anti-GFP antibody binds specifically to our recombinant GFP with typical avidity (Kd=1.0 nM). Third, fibrinogen binds to this GFP antibody, and fourth, fibrinogen binding is not significantly affected if this antibody is bound to GFP. (95% Confidence Intervals: Fg to anti-GFP: Kd=0.0–5.3 µM, Bmax=0.0–196.9 fmol; Fg to anti-GFP bound to GFP: Kd=0.66–2.2 µM, Bmax=5.07–10.04 fmol).
Using this GFP model, we tested how fibrinogen affects antigen-antibody binding. As seen in figure 3 and Table 3, fibrinogen does not interfere with antigen binding at 0–5 mg/ml concentrations. To the contrary, there is a significant increase in Bmax at high fibrinogen levels, indicating binding of additional antibody to existing antibody-antigen complexes. Different antigen/antibody pairs produce similar results and confirm the notion that fibrinogen does not diminish antigen-antibody binding at physiologic levels, but may enhance total antibody binding. It is possible that this may be due to fibrinogen avidity effects, where high levels of fibrinogen could link additional antibody to antigen-bound antibody. However, despite adding more antibody to the antigen, this does not result in enhanced phagocytosis as seen in Fig 2. One possibility is that the additional bound antibody might interfere with efficient Fc receptor binding to antigen-antibody complexes, which will be explored in the next set of experiments.
Figure 3. Fibrinogen does not diminish antigen-antibody binding.
Fibrinogen has no discernible effect on binding strength, but high levels of fibrinogen (5 mg/ml) results in increased total binding of fibrinogen to immobilized anti-GFP IgG. Experimental details: GFP was immobilized onto high binding polystyrene microplates, blocked with BSA, and incubated with varying amounts of biotinylated antibody for 1 hour at 25°C in the presence or absence of fibrinogen (0, 1 or 5 mg/ml). To assess unspecific binding, excess GFP was added as competitor. Bound ligand was detected using a colorimetric Streptavidin-Horseradish peroxidase system, and specific binding determined as the difference between bound antibody in the absence of GFP and bound antibody in the presence of GFP. Binding data was analyzed with GraphPad Prism using non-linear curve fitting and the one binding site model. Experiment was performed three times with similar results.
Table 3.
Fibrinogen affects antigen-antibody binding
| Receptor (immobilized) |
Ligand | Kd (95% CI) | Bmax (95% CI) |
|---|---|---|---|
| GFP | polyclonal anti-GFP sheep IgG & no Fg |
1.5 nM (1.049 … 1.868 nM) |
79 fmol (72.55 … 85.79 fmol) |
| GFP | polyclonal anti-GFP sheep IgG + 1mg/ml Fg |
1.4 nM (0.8772 … 1.906 nM) |
76 fmol (66.32 …85.12 fmol) |
| GFP | polyclonal anti-GFP sheep IgG + 5 mg/ml Fg |
0.77 nM (0.3981 … 1.137 nM) |
120 fmol (105.9 … 136.7 fmol) |
Experimental Details: GFP was immobilized onto high binding polystyrene microplates, blocked with BSA, and incubated with varying amounts of biotinylated antibody for 1 hour at 25°C in the presence or absence of fibrinogen. To assess unspecific binding, excess GFP was added as competitor. Bound ligand was detected using a colorimetric Streptavidin-Horseradish peroxidase system, and raw binding data analyzed with GraphPad Prism using non-linear curve fitting and the one binding site model. 95% confidence intervals are given for both Kd and Bmax. Experiments were performed three times with similar results.
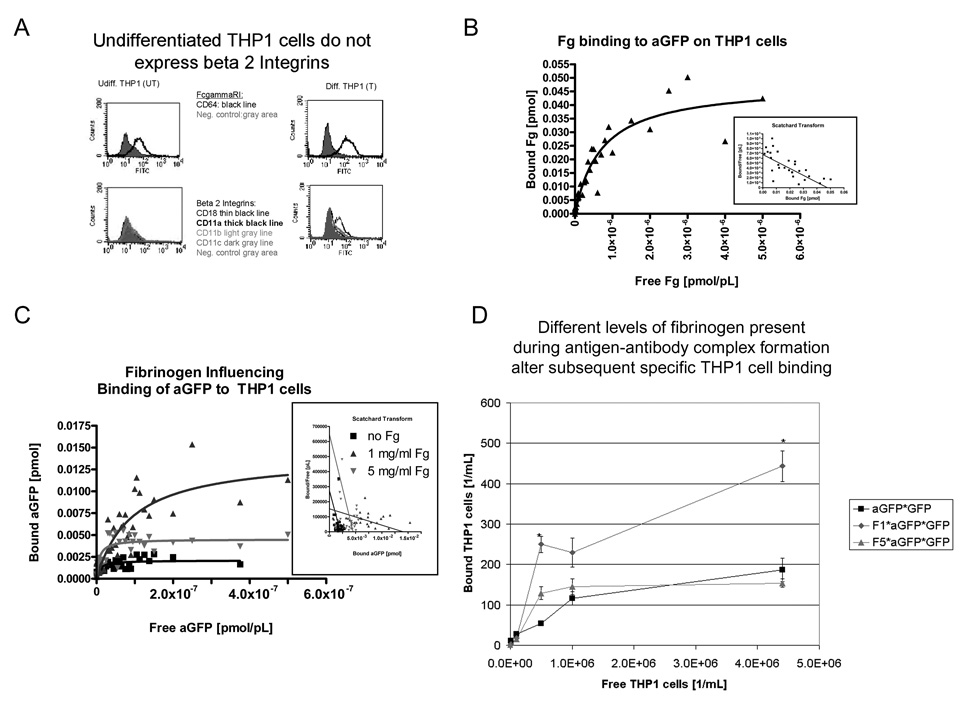
Fibrinogen binding to IgG creates bimodal IgG binding to FcR expressing cells
Since we did not observe any inhibitory effects of high fibrinogen concentrations at the antigen-antibody interface, we suspected that fibrinogen acts on the antibody-Fc receptor interface. Since the fibrinogen-IgG interaction appears highly dependent on surface density, we wanted to observe effects of fibrinogen on IgG-FcR binding on whole cells. We evaluated these effects on the surface of cultured macrophages, and on an undifferentiated THP-1 cells that do not expresses beta2-integrins (Figure 4.a). To ensure that these cells express functional FcR binding polyclonal sheep anti-GFP antibody, we confirmed specific binding of anti-GFP antibody to the immobilized THP-1 cells (95% confidence interval Kd=23–69 nM). We tested if fibrinogen binds to the same cells, and as expected from the absence of beta2 integrins found no specific, saturable binding (data not shown). As expected, if we loaded the cells with high concentration polyclonal anti-GFP, fibrinogen bound in a saturable manner to the cells with similar binding characteristics as for the anti-GFP IgG bound to GFP (Figure 4.b, 95% confidence interval Kd=392–1030 nM).
Figure 4. Fibrinogen modulates IgG binding to undifferentiated THP-1 cells in a biphasic manner independent of beta2 Integrins.
A) Flowcytometry did not detect beta2 integrins in undifferentiated THP-1 cells, but using same conditions detected beta2 integrin in differentiated THP-1 cells. B) Using the previously described enzyme linked binding assay, we detect fibinogen binding to THP-1 cells that are loaded with anti-GFP IgG. C) When evaluating polyclonal anti-GFP sheep antibody binding to undifferentiated THP-1 cells in presence or absence of fibrinogen, a bimodal response is seen. Differences are significant for Bmax, as the 95% confidence intervals do not overlap. D) A similar bimodal response is also seen when THP-1 cells bind to anti-GFP IgG antibody/GFP complexes that have been created in the presence of 0, 1, 5 mg/ml fibrinogen. Experimental Details: Unspecific binding was determined by counting bound cells to GFP and substracted from total bound cells in the other conditions. The number of cells bound at 1 mg/ml and 5 mg/ml fibrinogen is significantly different as determined by one-way ANOVA. All binding was determined after 1 hour incubation time at 4 degrees Celsius. All experiments have been performed in triplicates.
Having established that fibrinogen can bind to IgG bound to THP-1 cells in the absence of beta2-integrins, we tested first how free fibrinogen at 0–5 mg/ml affects binding of free anti-GFP antibody to undifferentiated THP-1 cells. As seen in figure 4.c) and Table 4, 1 mg/ml fibrinogen enhances anti-GFP binding, whereas 5 mg/ml fibrinogen does not. Since THP-1 cells in this study lack beta2 integrins as fibrinogen receptors, the effect is likely the result of fibrinogen acting at the IgG-FcR level. Interestingly enough, primary macrophages expressing the full set of Fc-receptors and integrins showed a bimodal Bmax response as well (data not shown).
Table 4.
Fibinogen affects IgG binding to THP-1 cells
| Receptor (immobilized) |
Ligand | Kd (95% CI) | Bmax (95% CI) |
|---|---|---|---|
| THP1 (undiff) | polyclonal anti-GFP sheep IgG & no Fg |
56 nM (0.0 to 116.7 nM) |
3.2 fmol (1.856 … 4.607 fmol) |
| THP1 (undiff) | polyclonal anti-GFP sheep IgG + 1mg/ml Fg |
92 nM (32.16 … 152.2 nM) |
14 fmol (9.987 … 18.12 fmol) |
| THP1 (undiff) | polyclonal anti-GFP sheep IgG + 5 mg/ml Fg |
75 nM (0.0 … 155.3 nM) |
7.2 fmol (4.107 … 10.20 fmol) |
Experimental Details: THP-1 cells were immobilized onto high binding polystyrene microplates, blocked with BSA, and incubated with varying amounts of biotinylated antibody for 1 hour at 4°C in the presence or absence of fibrinogen. To assess unspecific binding, excess IgG was added as competitor. Bound ligand was detected using a colorimetric Streptavidin-Horseradish peroxidase system, and raw binding data analyzed with GraphPad Prism using non-linear curve fitting and the one binding site model. 95% confidence intervals are given for both Kd and Bmax. Experiments were performed three times with similar results.
We then tested if a similar effect is seen with immobilized anti-GFP/GFP complexes that were treated with 0–5 mg/ml fibrinogen. Again, we observed a bimodal response, with significantly more THP-1 cells binding anti-GFP/GFP/low level fibrinogen complexes than the other types of complexes (no or high level fibrinogen) (Figure 4.d).
DISCUSSION
In this paper, we have shown fibrinogen binding to antigen-bound IgG and that fibrinogen modifies IgG binding behavior. At low concentrations of fibrinogen, antibodies bind to surface antigens with unchanged strength or numbers compared to antibody binding in the absence of fibrinogen, although antibody will bind fibrinogen at these concentrations to near saturation. When phagocytic cells bind to antigen-antibody complexes created in the presence of low fibrinogen levels, binding is stronger even in the absence of fibrinogen receptors, indicating that fibrinogen might have a stabilizing role in the IgG-Fc receptor interaction, possibly resulting in more efficient phagocytosis.
At high levels of fibrinogen, antibodies bind to surface antigens with unchanged strength, but nearly two-fold greater numbers, which could be the result of fibrinogen aggregates linking antigen-bound IgG and fibrinogen-bound IgG. However, phagocytic cell binding to these complexes is decreased, possibly because the additional IgG and fibrinogen might interfere with efficient binding of Fc receptors, resulting in decreased phagocytosis.
In order to determine binding characteristics of diverse fibrinogen and IgG interactions, we utilized an assay derived from enzyme-linked immunosorbent assays. We considered alternative techniques to measure binding such as surface plasmon resonance and measuring concentrations at equilibrium across dialysis membranes, but each technique presented insurmountable technical difficulties. These included inability to measure fibrinogen/IgG binding to receptors on whole cells, and the lack of dialysis membranes with a suitable molecular weight cut off to separate efficiently fibrinogen-IgG complexes from single fibrinogen and IgG molecules.
When measuring the amount of bound fibrinogen or IgG, we noted that the bound amounts appeared significantly less than the maximum amount of protein (500 ng/cm2 ~ 0.030 pmol/mm2 IgG) that can be bound to the microwell plate as claimed by the manufacturer. However, we only determined bound IgG/fibrinogen, and not total bound protein, which would include a significant amount of our blocking agent, bovine serum albumin. In addition, since amount of bound protein was determined using an ELISA method, some initially bound IgG/fibrinogen was lost during subsequent washing steps. We considered other methods to determine plate-bound proteins, but other methods such as total internal reflection fluorescence appear not useful for polystyrene surfaces within a microwell plate. Although not statistically significant, fibrinogen tended to bind in greater amounts during the initial plate coating process, possibly related to its larger molecular size, allowing for greater contact area with the plate surface.
Although the molar amounts of plate-bound fibrinogen and IgG are similar, it is interesting to note that the amounts of bound ligand differ significantly. While the Bmax of IgG bound to immobilized fibrinogen approaches the amount of the plate-bound fibrinogen, the Bmax of fibrinogen bound to immobilized IgG is about 10-fold less than the amount of plate-bound IgG. One possible explanation could be that fibrinogen only binds to a subset of IgGs, and a possible candidate might be human IgG3, which makes up about 1/10 of total human IgG and involved in phagocytosis. During the binding assay, if only 1/10 of human IgG binds fibrinogen, it would limit the amount of fibrinogen binding to immobilized IgG, since only 1/10 of available receptors would bind fibrinogen. For the reverse binding scenario, the amount of IgG binding to immobilized fibrinogen would not be affected by the presence of a subset of IgG, since the amount of available fibrinogen binding IgG in solution far exceeds the number of immobilized fibrinogen molecules (<0.030 pmol), even if only 1/10 of all IgG bind (total IgG in solution near saturation conditions: ~200 pmol). Unfortunately, we did not have access to sufficient quantities of normal human IgG subsets to test this possibility. It is also possible that the Bmax differences are an artifact created by the immobilization of IgG. Since IgG is randomly deposited onto the plate surface during the coating process, fibrinogen binding sites might be obscured depending on the orientation of the antibody. This effect was seen with anti-GFP IgG antibody, where fibrinogen binding constants became much less variable once the antibody was bound on immobilized GFP.
We found the dissociation constant for fibrinogen binding to immobilized IgG is significantly lower than for the reverse binding reaction, which implies that fibrinogen binding to immobilized IgG is much stronger than IgG binding to immobilized fibrinogen. Therefore, it appears that binding kinetics are dependent on which ligand is immobilized. For physiologic concentrations of IgG and fibrinogen, this would result in fibrinogen bound to all surface-bound IgG while limited amounts of IgG might be bound to surface-bound fibrinogen. In addition, unlike typical antigen-antibody interactions, fibrinogen-IgG interactions in solution do not produce a precipitin reaction while a similar concentration of anti-fibrinogen IgG and fibrinogen produce such reaction. We therefore suspect that fibrinogen-IgG interactions in solution during physiologic concentrations of IgG and fibrinogen are much weaker than the fibrinogen interaction with immobilized IgG. We therefore speculate that the main condition where fibrinogen binding occurs is on the surface of IgG-opsonized bacteria.
We also found that binding strength of fibrinogen to immobilized IgG is significantly higher when using a surface coated with molar amounts of IgG. It is possible that this might be due to avidity effects, as a high surface density of IgG might allow for binding of one fibrinogen molecule to several IgG molecules, thus leading to increased binding strength. We have previously demonstrated the potential for multiple binding sites (Fab, Fc portions)[14], which would make fibrinogen binding to IgG analogous to IgG antibody binding to antigens. Physiologically, it appears that fibrinogen binding to IgG preferentially occurs on surfaces coated with a high density of IgG, which occurs on successfully opsonized bacteria. One has to be careful to immediately draw conclusions in vivo, as the binding experiments were performed in the presence of only fibrinogen, BSA and IgG during binding. This was done so that other proteins such as fibronectin, which are present in vivo and also bind to either fibrinogen or IgG, do not confound binding measurements.
We used confocal fluorescence microscopy for phagocytosis measurements to count bacteria within cells, and noticing a good correlation of mean phagocytic index (bacteria/cell) with the percentage of phagocytically active cells, we employed fluorescence microscopy in order to determine phagocytosis more efficiently for larger numbers of cells. Flow cytometry was not useful, as the fluorescence intensity of GFP-coated beats or GFP-expressing bacteria was not strong enough to produce reliable event counts.
We tested phagocytosis of particles that have been coated with IgG and fibrinogen in an otherwise serum-free culture medium, as we found that serum-containing medium causes fibrinogen/IgG-coated particles and macrophages to form clumps of cells that are difficult to count or measure phagocytic indices. We speculate that in vivo fibrinogen/IgG-opsonization of bacteria leads to entrapment of these bacteria and rapid clearance from circulation.
When examining the effect of fibrinogen-IgG interactions on phagocytosis, we found that presence of high fibrinogen concentrations during the coating of bacteria with anti-body resulted in less phagocytosis than the negative control, and the replicate experiments tended to show similar results. This effect diminished with the use of GFP-beads, where high levels of fibrinogen during the coating process resulted in no significant change compared to antibodies alone. One explanation might be that with E.coli as a substrate, excessive amounts of fibrinogen and accumulate, and form a protein coat that prevents efficient phagocytosis. This would be similar to fibrinogen accumulating on strains of S. pyogenes, which express fibrinogen-binding M proteins on their surface to resist phagocytosis[17].
In order to avoid possible interactions with unknown bacterial surface proteins, we elected to choose the simpler GFP-bead model, which limits interactions to GFP, anti-GFP IgG, fibrinogen, the blocking agent BSA and cellular fibrinogen and IgG receptors. In this simplified model, we still observed a bimodal response to fibrinogen levels during the coating process, with high levels of fibrinogen diminishing phagocytosis. It is possible that at high, pathologic concentrations the decrease of IgG-FcR binding might be due to macromolecular crowding. However, the protein concentrations used in this study are likely not high enough for these effects to dominate [18].
Alhough GFP alone does not appear to bind fibrinogen during the binding experiments, we still see an increase of phagocytosis with fibrinogen as sole coating agent of GFP-beads. It is possible that in this experiment fibrinogen nonspecifically adheres to the beads, resulting in a tendency for increased phagocytosis through activation of fibrinogen-binding beta2 integrins. We therefore needed to test if the dramatic increase in phagocytosis for the IgG-fibrinogen coated beads was due to non-specific binding. We tested this by using excess, non-physiologic concentrations of soluble IgG during the coating process, which competes with anti-GFP antibodies for fibrinogen binding, and therefore should eliminate fibrinogen specifically bound to antibody-GFP complexes on polystyrene beads. As a result, phagocytosis of these beads should be diminished, since the enhancement of phagocytosis specific to the IgG-fibrinogen interaction is eliminated. During our phagocytosis experiments, we did observe this result as phagocytosis of beads treated with excess IgG as fibrinogen competitor was diminished to the level of fibrinogen or anti-GFP antibody treatment alone. Moreover, if we used the THP1 cell model that lacked beta2 integrins as fibrinogen receptors, fibrinogen only enhanced phagocytosis if beads were opsonized with anti-GFP antibodies. Therefore, we conclude that bimodal enhancement of phagocytosis is due to specific fibrinogen-IgG interactions affecting antibody binding to both antigens and Fc-receptors. It remains to be seen if this bimodal fibrinogen effect can be verified in vivo, as an animal model that allows manipulation of fibrinogen levels across a wide range is not available to our knowledge. Fibrinogen knockout mice generally do not survive until adulthood, and there is no transgenic animal available that constitutively expresses fibrinogen levels greater than 4 mg/ml. This leaves partial defibrinogenation with defibrinating agents such as ancrod, or infusion of external fibrinogen as alternatives, and particle phagocytosis in these models have not been verified to our knowledge.
In conclusion, different levels of fibrinogen bound to antigen-antibody complexes significantly affect avidity of cell binding and maximum numbers of bound cells. It appears that the bimodal phagocytic response to fibrinogen/antibody/antigen complexes is a function of the bimodal effect of fibrinogen on cell binding to antigen-antibody complexes.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Mira Edgerton for the use of Graphpad Prism software, and Dr. Moon Il Cho for providing access to fluorescence microscopy. We extend our gratitude to Robert Dunford for statistical advice. We also thank the University of Buffalo Confocal Imaging Facility for assistance with confocal microscopy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.de Maat MP, Verschuur M. Fibrinogen heterogeneity: inherited and noninherited. Curr Opin Hematol. 2005;12:377–383. doi: 10.1097/01.moh.0000169287.51594.3b. [DOI] [PubMed] [Google Scholar]
- 2.Lishko VK, Podolnikova NP, Yakubenko VP, Yakovlev S, Medved L, Yadav SP, Ugarova TP. Multiple binding sites in fibrinogen for integrin alphaMbeta2 (Mac-1) J Biol Chem. 2004;279:44897–44906. doi: 10.1074/jbc.M408012200. [DOI] [PubMed] [Google Scholar]
- 3.Ugarova TP, Yakubenko VP. Recognition of fibrinogen by leukocyte integrins. Ann N Y Acad Sci. 2001;936:368–385. doi: 10.1111/j.1749-6632.2001.tb03523.x. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez GC, Ilarregui JM, Rubel CJ, Toscano MA, Gomez SA, Beigier Bompadre M, Isturiz MA, Rabinovich GA, Palermo MS. Galectin-3 and soluble fibrinogen act in concert to modulate neutrophil activation and survival: involvement of alternative MAPK pathways. Glycobiology. 2005;15:519–527. doi: 10.1093/glycob/cwi026. [DOI] [PubMed] [Google Scholar]
- 5.Koch T, Annuss C, Schiefer HG, van Ackern K, Neuhof H. Impaired bacterial clearance after activation of the complement and coagulation systems. Shock. 1997;7:42–48. doi: 10.1097/00024382-199701000-00005. [DOI] [PubMed] [Google Scholar]
- 6.El-Ashmawy S, Taha A, Fatt-hi A, Basyouni A, Zaher S. Serum immunoglobulins in patients with chronic tonsillitis. J Laryngol Otol. 1980;94:1037–1045. doi: 10.1017/s0022215100089829. [DOI] [PubMed] [Google Scholar]
- 7.Lal H, Sachdeva OP, Mehta HR. Serum immunoglobulins in patients with chronic tonsillitis. J Laryngol Otol. 1984;98:1213–1216. doi: 10.1017/s0022215100148297. [DOI] [PubMed] [Google Scholar]
- 8.Takeuchi Y, Aramaki M, Nagasawa T, Umeda M, Oda S, Ishikawa I. Immunoglobulin G subclass antibody profiles in Porphyromonas gingivalis-associated aggressive and chronic periodontitis patients. Oral Microbiol Immunol. 2006;21:314–318. doi: 10.1111/j.1399-302X.2006.00296.x. [DOI] [PubMed] [Google Scholar]
- 9.Duncan AR, Winter G. The binding site for C1q on IgG. Nature. 1988;332:738–740. doi: 10.1038/332738a0. [DOI] [PubMed] [Google Scholar]
- 10.Kottgen E, Hell B, Kage A, Tauber R. Lectin specificity and binding characteristics of human C-reactive protein. J Immunol. 1992;149:445–453. [PubMed] [Google Scholar]
- 11.Bristow CL, Boackle RJ. Evidence for the binding of human serum amyloid P component to Clq and Fab gamma. Mol Immunol. 1986;23:1045–1052. doi: 10.1016/0161-5890(86)90003-9. [DOI] [PubMed] [Google Scholar]
- 12.Wroblewski Z. Interaction of acid alpha 1-glycoprotein with immunoglobulins. Acta Biochim Pol. 1981;28:21–30. [PubMed] [Google Scholar]
- 13.Cosio FG, Bakaletz AP. Binding of human fibronectin to antigen-antibody complexes. J Lab Clin Med. 1986;107:453–458. [PubMed] [Google Scholar]
- 14.Boehm TK, DeNardin E. Fibrinogen binds IgG antibody and enhances IgG-mediated phagocytosis. Hum Antibodies. 2008;17:45–56. [PubMed] [Google Scholar]
- 15.Makogonenko E, Tsurupa G, Ingham K, Medved L. Interaction of Fibrin(ogen) with Fibronectin: Further Characterization and Localization of the Fibronectin-Binding Site. Biochemistry. 2002;41:7907–7913. doi: 10.1021/bi025770x. [DOI] [PubMed] [Google Scholar]
- 16.Nuutila J, Lilius EM. Flow cytometric quantitative determination of ingestion by phagocytes needs the distinguishing of overlapping populations of binding and ingesting cells. Cytometry A. 2005;65:93–102. doi: 10.1002/cyto.a.20139. [DOI] [PubMed] [Google Scholar]
- 17.Podbielski A, Schnitzler N, Beyhs P, Boyle MD. M-related protein (Mrp) contributes to group A streptococcal resistance to phagocytosis by human granulocytes. Mol Microbiol. 1996;19:429–441. doi: 10.1046/j.1365-2958.1996.377910.x. [DOI] [PubMed] [Google Scholar]
- 18.Minton AP. How can biochemical reactions within cells differ from those in test tubes? J Cell Sci. 2006;119:2863–2869. doi: 10.1242/jcs.03063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.