Abstract
In the CNS, ATP is released upon injury and promotes neuroproliferation via purinergic receptors. In the olfactory epithelium, ATP promotes the synthesis and release of neurotrophic factor NPY in neonates and induces neuroproliferation in neonatal and adult mice. We tested the hypothesis that NPY is involved in ATP-induced neuroproliferation in adult mice olfactory epithelium. Intranasal instillation of ATP significantly increased protein levels and number of NPY+ cells. Pre-intranasal instillation of purinergic receptor antagonist PPADS significantly reduced ATP-induced upregulation of NPY. Intranasal instillation of NPY-Y1 receptor antagonist BIBP3226 following ATP instillation significantly inhibited the ATP-induced increase in BrdU incorporation, suggesting that NPY is released after ATP instillation and activates Y1 receptors to promote neuroproliferation. These data indicate that ATP initiates neuroproliferation via NPY upregulation, NPY release, and Y1 receptor activation, and suggests that the olfactory epithelium is good model to study neuroregenerative mechanisms in the CNS.
Keywords: Purinergic receptors, regeneration, neurotrophic factor, NPY receptors
Introduction
The olfactory epithelium (OE) is a good model to study the mechanisms of neurotrophic factor-regulated neuroregeneration as neurogenesis occurs during embryogenesis and continues throughout life under both physiological conditions and following injury (Graziadei and Monti-Graziadei, 1978). The level of neurogenesis is tightly regulated by a multitude of endogenous positive and negative chemical signals. Chemical, infectious, or traumatic damage upsets the balance of these chemical signals and increases neurogenesis. Although studies suggest that mechanisms regulating embryonic neurogenesis and adult neuroregeneration in the OE may be equivalent (Beites et al., 2005; Murdoch and Roskams, 2007; Schwob, 2002), it is not known if similar regulatory signals mediate both postnatal neurogenesis and adult maintenance. Knowledge of the endogenous factors that stimulate neurogenesis throughout development will advance neuroproliferative replacement therapies aimed at endogenous stem cell recruitment.
One possible positive regulator of neurogenesis is ATP. ATP is released following injury, and could stimulate localized proliferation and regeneration. Indeed, sustained pathologic release of ATP has been observed in spinal cord injury (Wang et al., 2004). In the olfactory system, we previously reported that ATP, via activation of P2 purinergic receptors, increases neuronal precursor cell proliferation and differentiation in neonatal and adult mouse OE (Jia et al., 2009). We found that ATP induces NPY release from neonatal mouse OE slices in vitro (Kanekar et al., 2009), suggesting that NPY may be involved in ATP-induced neuronal proliferation in neonatal mouse OE. NPY is a 36 amino acid peptide reported to induce neuronal precursor proliferation in subventricular zone of the lateral ventricle (Stanic et al., 2008), hippocampus (Ha et al., 2002; Howell et al., 2005) and retina (Milenkovic et al., 2004). In the OE, NPY is expressed in sustentacular cells (Hansel et al., 2001; Kanekar et al., 2009), and a subpopulation of microvillous cells, both of which have processes extending to the basement membrane (Kanekar et al., 2009; Montani et al., 2006), and in the olfactory ensheathing cells that reside in the lamina propria (Ubink et al., 1994). NPY Y1 receptors are expressed in the basal cell layer where the basal progenitor cells are located (Hansel et al., 2001). NPY stimulates proliferation of olfactory neuronal progenitor cells in vitro via Y1 receptor-activated extracellular signal-regulated kinase signaling cascade (Doyle et al., 2008; Hansel et al., 2001). A significant reduction in basal cell proliferation occurs in NPY deficient mice (Hansel et al., 2001) and Y1 receptor knockout mice (Doyle et al., 2008). Collectively, these data indicate that NPY is ideally situated to have a role in promoting basal neuronal progenitor cell proliferation. In the present study, we tested the hypothesis that ATP regulates neurogenesis via NPY in adult mouse OE and compared results across different developmental stages (neonatal vs. adult). We found that ATP activation of P2 purinergic receptors significantly increases NPY protein levels, upregulates NPY expression in the sustentacular and microvillous cells, and induces basal progenitor cell proliferation via activation of NPY Y1 receptors. These data suggest that ATP acts synergistically with other neurotrophic factors, such as NPY, to exert its neuroproliferative action in adult OE. Further, it implies that similar mechanisms and regulatory signals mediate postnatal neurogenesis and adult maintenance.
Materials and Methods
Animals
Adult male Swiss Webster mice (6–8 weeks) were purchased from Charles River Laboratories (Portage, MI). All mice were housed in a temperature-, humidity-, and light-controlled room (12 hr light/dark cycle, lights on from 7:00 AM to 7:00 PM). Food and water were available ad libitum. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals as approved by Michigan State University Institutional Animal Care and Use Committee.
Enzyme Immunoassay
Anesthetized mice (4% isoflurane) were intranasally instilled with 200 μmoles/kg pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonate (PPADS) or saline vehicle, followed by ATP (400 nmoles/kg) or saline vehicle 30 min later. 20 hours after ATP instillation, mice were anesthetized (4% isoflurane) and decapitated. The OE tissues were rapidly dissected and stored at −80 °C. On the day of enzyme immunoassay performance, the OE tissues were homogenized by sonication in Tris buffer [10 mM Tris-HCl (pH 7.6) containing 100 mM NaCl, 1 mM EDTA, 2 M activated Na3VO4, 50 mM NaF, and a protease inhibitor cocktail (1:1000, Sigma-Aldrich, St. Louis, MO)]. The concentration of protein in the homogenate was measured using a bicinchoninic acid protein assay kit (Pierce, Rockford, IL). Levels of NPY in the homogenate were assayed using an enzyme immunoassay kit (Phoenix Pharmaceuticals, Belmont, CA) according to manufacturer’s instructions. All samples (n = 3–5 animals per group) were run in duplicate, averaged, and reported as mean + SEM of NPY/μg protein.
Immunohistochemistry
Anesthetized mice (4% isoflurane) were intranasally instilled with PPADS or saline vehicle followed by ATP or saline vehicle 30 min later as described above. 20 hrs after ATP instillation, mice were anesthetized (65mg/kg ketamine + 5mg/kg xylazine, ip), transcardially perfused with ice-cold 0.1 M phosphate-buffered saline (PBS) followed by 4% paraformaldehyde and decapitated. The lower jaw and skin was removed and tissue was postfixed overnight in 4% paraformaldehyde, rinsed in PBS, placed in RDO-Rapid Decalcifier for 4 hours (Apex Engineering Products, Aurora, IL). After decalcification, the tissues were cryoprotected with 20% sucrose and embedded in Tissue Tek OCT (Sakura Finetek, Torrance, CA). Frozen coronal sections of OE (20 μm) were collected from levels 2–6 of the mouse OE (Shipley, 2003). Tissue was always compared from equivalent levels between treatment groups (n = 3–5 animals per group).
Immunoreactivity was detected using tyramide signaling amplification kits (Invitrogen/Molecular Probes, Eugene, OR). Tissue sections were rehydrated with PBS, permeabilized with 0.02% triton X-100 and blocked with 10% blocking reagent from tyramide signaling amplification kits. The sections were incubated with rabbit anti-NPY antibody (1:50, Bachem, Torrance, CA). For double-labeling immunofluorescence, the sections were processed as described above for NPY immunoreactivity and then the sections were washed in PBS, incubated with goat anti-olfactory marker protein (OMP, 1:1000, Wako Chemical, Plano, TX), rabbit anti-calnexin (1:500, Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-notch 2 (1:200, Calbiochem, Gibbstown, NJ), rabbit anti-inositol triphosphate receptor III (IP3R3, 1:1000, Millipore/Chemicon, Billerica, MA) or rabbit anti-PLCβ2 antibody (1:50, Santa Cruz Biotechnology, Santa Cruz, CA) and followed by TRITC-conjugated donkey anti-goat or anti-rabbit immunoglobin (1:50 or 1:200, Jackson ImmunoResearch Laboratories). Immunoreactivity was visualized on an Olympus FV1000 confocal laser scanning microscope (Olympus America Inc., Center Valley, PA). Antibody specificity was examined by omitting the primary antibody, secondary antibody or neutralization of primary antibody with a 10 fold excess of NPY peptide (0.4 mg/ml). No immunoreactivity was observed in any of the controls. In some instances, the brightness and contrast of the fluorescent images were altered post-hoc. In all cases, the same changes were applied to all images collected on that given day, and it was verified that immunoreactivity was not observed in the antibody specificity controls under the new settings.
To quantify the NPY immunoreactivity (NPY-IR), the number of NPY+ cells per linear millimeter of septum, ectoturbinate 1, ectoturbinate 2, endoturbinate II, ectoturbinate 3 and endoturbinate III on three consecutive coronal sections of OE at level 4 in each animal were counted by an investigator blinded to the treatments and then normalized to the length of OE on which the NPY+ cells were scored. To assess NPY expression in rostral caudal axis of OE through the nasal cavity, we chose one animal from saline-instilled and ATP-instilled groups. The length of OE with NPY-IR was measured at three rostral caudal levels (level 3, 4 and 6) and then normalized to total length of OE at the respective levels.
In Vivo Proliferation Assay
In one experiment, anesthetized mice were intranasally instilled with saline vehicle or NPY (15μg/kg). At 42, 44 and 46 hrs after NPY instillation, mice received BrdU injections (ip, 180 mg/kg total). 48 hrs after NPY instillation, mice were perfused and the OE tissues were processed for immunohistochemistry as described above. In the other experiment, anesthetized mice were intranasally instilled with saline vehicle or ATP (400 nmoles/kg) followed by saline vehicle or NPY Y1 receptor antagonist N2-(diphenylacetyl)-N-[(4-hydroxyphenyl)methyl]-D-arginine amide (BIBP3226; 1.6 or 16 nmoles/kg) instillation at 5 min, 8, 20 and 32 hours later. At 42, 44 and 46 hrs after ATP instillation, mice received BrdU injections (ip, 60 mg/kg). This triple injection of BrdU (180 mg/kg total) gives comparable results as previously reported with lower doses (Hansel et al., 2001; Leung et al., 2007). The use of BrdU as a marker for cell proliferation was validated previously for our model system using proliferating cell nuclear antigen (PCNA) (Jia et al., 2009). 48 hrs after ATP instillation, mice were perfused and the OE tissues were processed for immunohistochemistry as described above. Tissue sections were rehydrated with PBS, permeabilized with 0.3% triton X-100 and blocked with 10% normal donkey serum (Jackson ImmunoResearch Laboratories, West Grove, PA). The sections were incubated in 2M HCl for 30 min at 65°C to denature DNA and then incubated with rat monoclonal anti-BrdU antibody (1:100 Abcam, Cambridge, MA) in 10% normal donkey serum (Jackson ImmunoResearch Laboratories) at 4°C overnight. Immunoreactivity was detected using FITC-conjugated donkey anti-rat immunoglobin (1:200, Jackson ImmunoResearch Laboratories). The number of BrdU+ cells per linear millimeter of ecto- and endo-turbinate 2 on three consecutive coronal sections of OE at level 4 in each animal were counted blindly and then normalized to the length of OE on which the BrdU+ cells were scored.
Statistical Analysis
The two-way analysis of variance (ANOVA) followed by the Bonferroni post-hoc test was performed using Prism 5 (GraphPad Software, San Diego, CA).
Results
ATP increases NPY protein levels via activation of P2 purinergic receptors in adult mouse OE
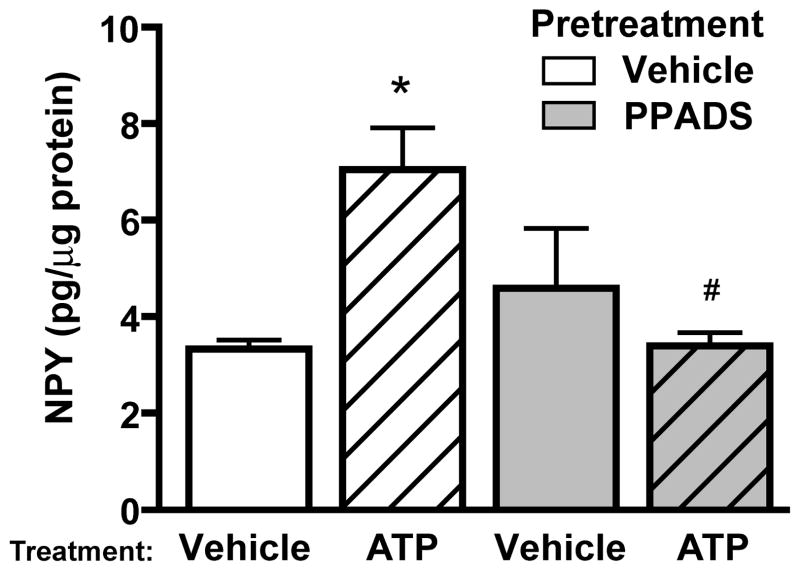
Our previous studies showed that extracellular ATP significantly induced the release but not the synthesis of NPY in OE slices of neonatal mouse in vitro (Kanekar et al., 2009). In the present in vivo study, we tested whether extracellular ATP has a role in NPY synthesis in adult mouse OE using both enzyme immunoassays and immunohistochemistry. We measured NPY protein levels by enzyme immunoassays in the presence and absence of ATP and P2 purinergic receptor antagonist PPADS. Mice were intranasally instilled with P2 purinergic receptor antagonist PPADS (200 μmoles/kg) or saline vehicle 30 min prior to instillation of ATP (400 μmoles/kg) or saline vehicle. In the vehicle pre-treatment groups, intranasal instillation of ATP significantly increased the NPY levels 20 hrs post-instillation compared to vehicle control (Figure 1; 7.1 ± 0.9 v. 3.3 ± 0.2 pg NPY/μg protein, n = 5, 4 mice, respectively; p<0.05). Pre-treatment with P2 antagonist PPADS followed by saline vehicle instillation had no effect on NPY protein levels compared to the saline vehicle control, (Figure 1; 4.6 ± 1.2 v. 3.3 ± 0.2 pg NPY/μg protein, n = 4 mice in both groups; p >0.05). However, PPADS significantly abolished the ATP-induced increase in NPY back to control levels (Figure 1; 3.4 ± 0.3 pg NPY/μg protein, n = 3 mice; p<0.05). These data indicate that ATP activation of P2 purinergic receptors upregulates NPY expression in adult mouse OE.
Figure 1. ATP upregulates NPY expression in adult mouse OE via P2 purinergic receptor activation.
Mice were instilled with vehicle or P2 purinergic receptor antagonist PPADS (200 μmoles/kg) prior to intranasal instillation of vehicle or ATP (400 nmoles/kg). 20 hrs later, OE tissue was collected and assayed for NPY levels by enzyme immunoassay. n = 5, 4, 4, 3 animals each treatment.*, p<0.05 v. vehicle/saline group. #, p<0.05 v. vehicle/ATP group (two-way ANOVA followed by Bonferroni post-hoc test).
Since there are reported regional differences in relative differentiation and cell survival in the adult OE (Vedin et al., 2009) and ATP promotes neuroproliferation throughout the whole adult OE without zonal differences (Jia et al., 2009), we also investigated whether there are zonal differences in ATP-induced NPY expression via immunohistochemistry. We examined NPY expression in seven areas of OE covering zone 1 to zone 4: septum, ectoturbinate 1, 2, and 3, endoturbinate II, and III, and olfactory bulb (Figure 2A). There were very few cells (5–10 cells/mm OE) with endogenous NPY-immunoreactivity (NPY-IR) in saline vehicle-treated animals (Figure 2B, Table 1). Intranasal instillation of ATP significantly increased the number of NPY-IR cells at all locations examined (Table 1; 42–61 cells/mm OE, p < 0.05). Compared to saline vehicle -instilled animals, ATP increased NPY expression in septum (Figure 2C, D), ectoturbinate 1 (Figure 2E), ectoturbinate 2 (Figure 2F, G), endoturbinate II (Figure 2B, J–L), ectoturbinate 3 (Figure 2H), and endoturbinate III (Figure 2I). PPADS pretreatment alone did not alter the number of NPY-IR cells but significantly blocked the ATP-induced increase in the number of NPY-IR cells back to control levels in all locations examined (Table 1, p < 0.05). Preadsorption of the antibody with NPY resulted in no immunoreactivity, confirming antibody specificity (Figure 2B″).
Figure 2. ATP upregulates NPY expression in septum and turbinates of adult mouse OE.
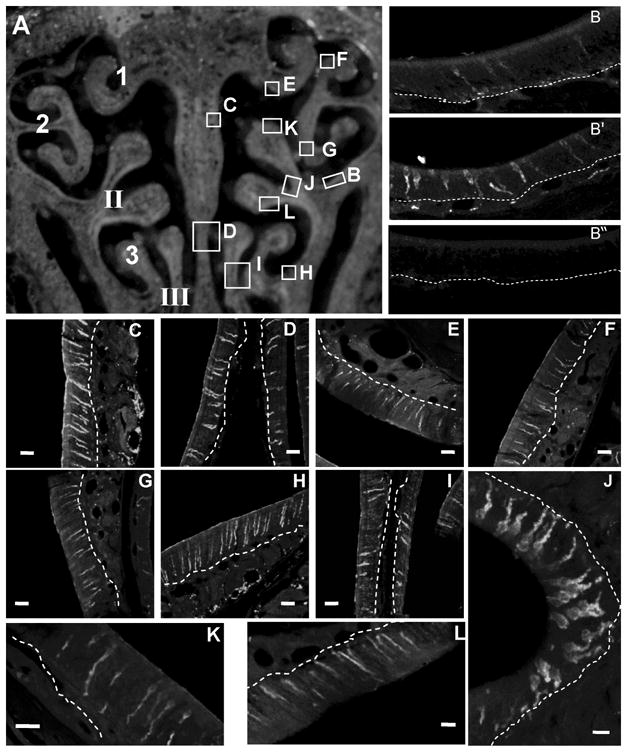
(A) Schematic image of the regions examined. Left: The endoturbinates II, III, and ectoturbinates 1–3 are numbered. Right: Lettered regions correspond to the images shown in B–L. (B, B′, B″) Representative NPY-immunoreactivity (NPY-IR) from a saline-instilled mouse (B), an ATP-instilled mouse (B′) and a peptide neutralization control (B″). (C–L) Representative images of ATP-induced NPY-IR from the lettered regions designated in A. Dashed white lines indicate basement membrane. Scale bars = 5μm.
Table 1.
NPY-IR cells in the olfactory epithelium (Mean ± SEM / mm OE)
| Vehicle-Saline | Vehicle-ATP | PPADS-Saline | PPADS-ATP | |
|---|---|---|---|---|
| Septum | 8.3 ± 1.6 | 49.1 ± 7.7*** | 9.7 ± 2.0 | 11.5 ± 5.1## |
| Ectoturbinate 1 | 4.8 ± 1.8 | 50.5 ± 6. 7*** | 5.2 ± 1.3 | 9.9 ± 2.9## |
| Ectoturbinate 2 | 10.2 ± 4.0 | 61.4 ± 5.1*** | 5.1 ± 2.2 | 7.1 ± 2.4### |
| Endoturbinate II | 9.2 ± 0.8 | 57.9 ± 6.3*** | 7.0 ± 2.0 | 9.6 ± 1.0### |
| Ectoturbinate 3 | 9.2 ± 2.7 | 59.3 ± 15.5* | 5.4 ± 0.3 | 9.4 ± 3.1# |
| Endoturbinate III | 6.5 ± 0.5 | 42.1 ± 7.8** | 5.2 ± 1.6 | 6.3 ± 1.1# |
p < 0.001;
p < 0.01;
p < 0.05; Vehicle-Vehicle vs. Vehicle-ATP.
p < 0.001;
p < 0.01;
p < 0.05; Vehicle-ATP vs. PPAD-ATP.
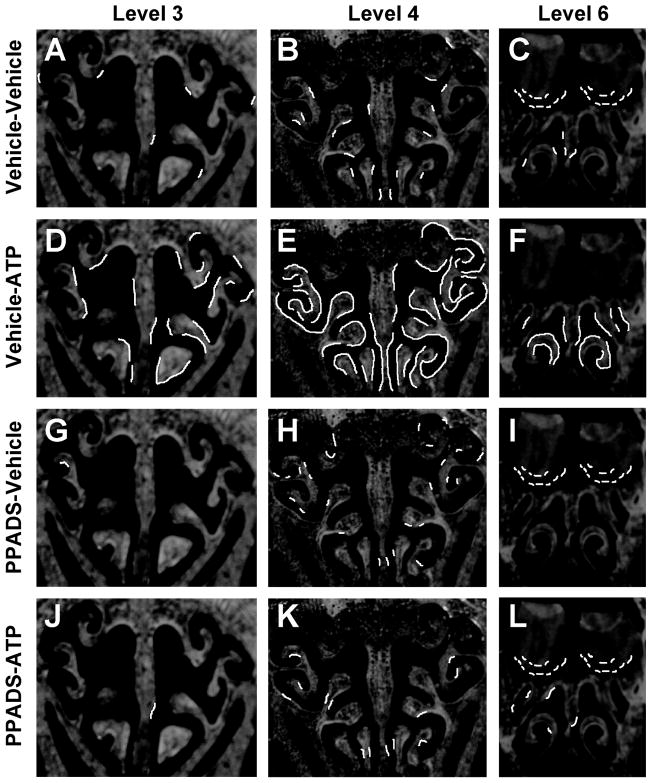
In order to assess whether there were differences in ATP-induced NPY expression in the rostral-caudal axis of OE through the nasal cavity, we measured the length of OE containing NPY-IR at three distinct rostral-caudal levels (levels 3, 4 and 6) in one animal from each group. In saline vehicle-instilled animals, 3.5 % of OE in level 3, 7.3% of OE in level 4 and 5.0% of OE in level 6 contained NPY-IR (Figure 3A–C). In ATP-instilled animals, the percentage of OE containing NPY-IR was increased to 27.3% at level 3, 64.1% at level 4 and 32.2% at level 6 of the OE (Figure 3D–F). These data indicate that ATP induces NPY expression through the whole rostral caudal axis of OE in adult mouse OE. However, ATP-induced NPY-IR was more robustly increased (approximately 7.8 times) in level 4 of the OE.
Figure 3. ATP activation of P2 purinergic receptors induces NPY expression in the OE through the whole nasal cavity.
(A–L) NPY-IR expression patterns are overlaid as white lines on representative cryostat sections at levels 3, 4 and 6 of the OE. Very little endogenous NPY-IR is observed in the vehicle/saline group (A–C). ATP induces NPY-IR expression in the vehicle/ATP group (D–F). NPY-IR expression patterns in PPADS/saline group is not different from vehicle/saline group (G–I) however, PPADS pre-treatment blocks ATP-induced increases in NPY expression (J–L).
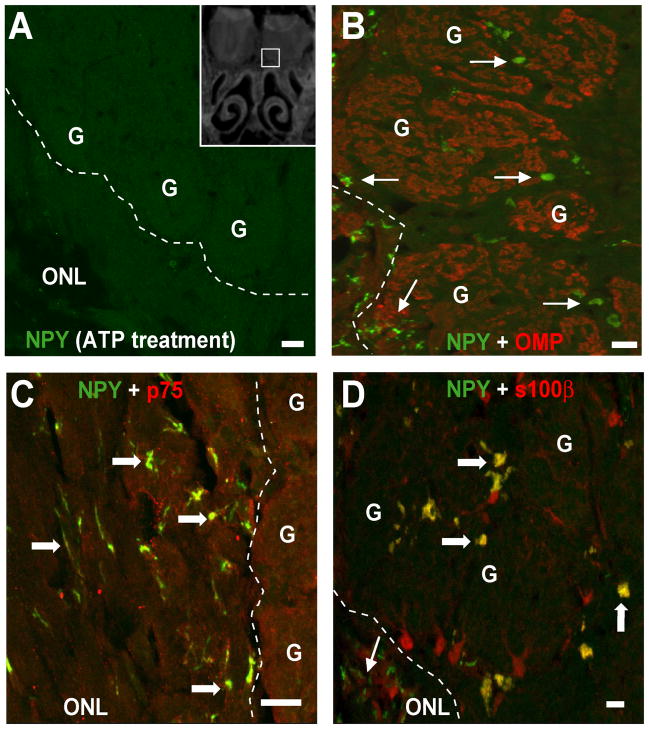
Interestingly, in level 6 we observed that vehicle-ATP instillation reduces NPY-IR in the outer nerve layer and glomerular layer of the olfactory bulbs (Figure 3F, 4A) compared to the vehicle/vehicle-instilled group (Figure 3C, 4B–D) and both groups treated with PPADS (Figure 3I, L). Previous studies show that NPY is expressed in the ensheathing cells in the nerve bundles in the lamina propria and in the bulb (Ubink et al., 1994). In the olfactory bulb (level 6), the NPY-IR was not co-localized with olfactory marker protein (OMP), which is a specific marker of olfactory sensory neurons (Figure 4B), suggesting that NPY is not released from axon terminals. The NPY+ cells were co-localized with olfactory ensheathing cell marker p75 in the outer nerve layer (Figure 4C) and with olfactory ensheathing cell marker S100β in the glomerular layer (Figure 4D), suggesting that the NPY+ cells in the olfactory bulb regions are olfactory ensheathing cells.
Figure 4. NPY expression in ensheathing cells in the olfactory bulb is inhibited by intranasal instillation of ATP.
Z-stack images of NPY-IR in the olfactory nerve layer and bulbs. (A) Intranasal instillation of ATP inhibits the NPY-IR in the olfactory ensheathing cells. Inset: representative cryostat section depicting the region from which images A–D were taken. Scale bars = 20μm. Dashed line distinguishes the outer nerve layer (ONL) from the glomeruli (G). (B) NPY-IR (green) was not co-localized with olfactory sensory neuron marker olfactory marker protein (OMP)-IR (red). Thin arrows, single localization of NPY. (C) The NPY-IR (green) between glomeruli was co-localized with p75 (red). Thick arrows, co-localization of NPY. (D) NPY-IR in the outer nerve layer was co-localized with s100-β (red).
ATP induces NPY expression in non-neuronal sustentacular and microvillous cells
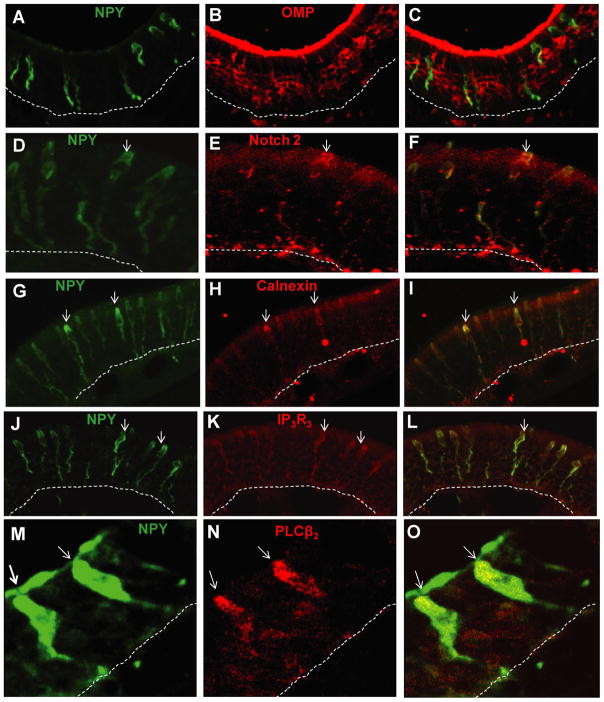
NPY is expressed in sustentacular and microvillar cells in neonatal and adult mouse OE (Hansel et al., 2001; Kanekar et al., 2009; Montani et al., 2006). In this study, we observed that ATP induced NPY expression in cell somas located prominently in the apical layer of OE. The cells were morphologically similar to both sustentacular cells, with cell somas in the upper third of OE and a thin cytoplasmic extension and endfoot process that terminates at the basement membrane, and microvillous cells, with flask-shaped cell somas, a short thick apical dendrite, and a long process. To confirm the cell types in which ATP-induced expression of NPY occurs, we performed double-labeling of NPY with sustentacular cell markers, calnexin (Czesnik et al., 2006) and notch 2 (Carson et al., 2006), microvillous cell markers, phospholipase C β2 (PLCβ2) and inositol triphosphate receptor III (IP3R3) (Elsaesser et al., 2005) or the OSN marker, OMP. ATP-induced NPY expression was not observed in OMP+ olfactory sensory neurons (Figure 5A–C). However, NPY did co-localize with notch 2+ (Figure 5D–F) and calnexin+ (Figure 5G–I) sustentacular cells, and IP3R3+ (Figure 5J–L) and PLC β2+ (Figure 5M-O) microvillar cells. These data indicate that ATP induces NPY expression in the non-neuronal sustentacular and microvillous cells in adult mouse OE.
Figure 5. ATP induces NPY expression in sustentacular and microvillar cells but not olfactory sensory neurons.
NPY-IR (green) was not co-localized with olfactory sensory neuron marker olfactory marker protein (OMP)-IR (red) (A, B and C), but was co-localized with sustentacular cell markers notch2-IR (red; D, E and F) and calnexin-IR (red; G, H and I), and with microvillar cell marker phospholipase C β2 (PLCβ2) -IR (red; J, K and L) and inositol triphosphate receptor III (IP3R3)-IR (red; M, N and O). Dashed lines indicate basement membrane.
Y1 receptors mediate ATP-induced basal progenitor cell proliferation in adult mouse OE
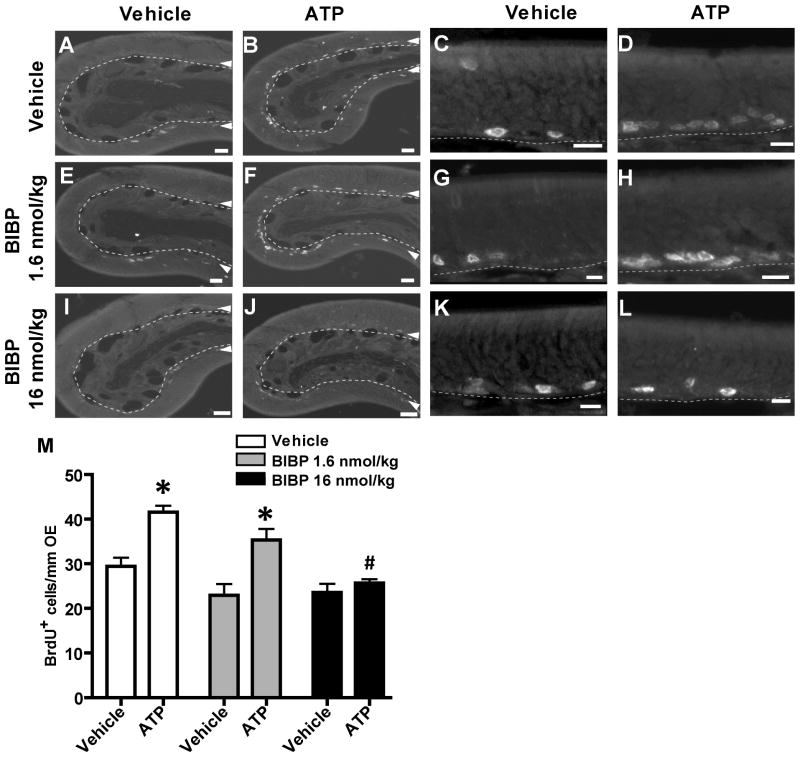
The premise of our hypothesis that NPY is involved in ATP-induced neuroproliferation requires that ATP induces the release of NPY with subsequent activation of Y1 receptors to promote basal cell proliferation. We previously demonstrated that ATP induces the release of NPY in neonatal OE slices (Kanekar et al., 2009), however, it is hard to make viable OE slices from adults as the bones supporting the OE are calcified. Therefore, we indirectly examined NPY release by measuring ATP-induced proliferation in the presence and absence of Y1 receptor antagonist BIBP3226. If ATP induces the release of NPY with subsequent activation of Y1 receptors on the basal progenitor cells and promotion of proliferation, then incubation of the Y1 antagonist BIBP3226 will significantly reduce ATP-stimulated proliferation in the OE. Mice were intranasally instilled with ATP or saline vehicle followed by instillation of saline vehicle or NPY Y1 receptor antagonist BIBP3226 (1.6 or 16 nmoles/kg) at 5 min, 8, 20, and 32 hours. In vehicle-treated animals, ATP instillation significantly increased BrdU-labeled proliferating cells by 41% above the vehicle control from 29.5 ± 1.9 to 41.6 ± 1.5 BrdU+ cells/mm OE (Figure 6A–D, M; p<0.01, n=3). Intranasal treatment with 1.6 or 16 nmoles/kg BIBP3226 alone did not significantly alter the number of BrdU-labeled cells compared to the vehicle-treated groups, (Figure 6E, G, I, K, M; 23.0 ± 2.5 and 23.6 ± 1.9, respectively; p>0.05). ATP instillation significantly increased BrdU-immunoreactive cells by 54% above the vehicle control in the 1.6 nmoles/kg BIBP3226-treated group (Figure 6F, H, M; 35.3 ± 2.4, p<0.01, n=3). However, 16 nmoles/kg BIBP3226 treatment completely blocked the ATP-induced increase in BrdU-labeled cells back to control levels (Figure 6J, L, M; 25.7 ± 0.9, p<0.001, n=3). These data indicate that ATP evokes NPY release and NPY promotes neuroregeneration via activation of Y1 receptors in adult mouse OE.
Figure 6. NPY Y1 receptor antagonist BIBP3226 significantly blocks ATP-induced neuroproliferation in adult mouse OE.
Mice were intranasal instilled with saline vehicle (A, C, E, G, I, K) or ATP (400 nmoles/kg; B, D, F, H, J, L) followed by saline vehicle (A–D) or BIBP3226 (1.6 or 16 nmoles/kg; E–H, and I–L, respectively) 5 min, 8, 20 and 32 hours later and tissue was collected at 48 hours post-ATP instillation. Right two columns are representative higher magnification images. Dashed lines indicate basement membrane; Δ, basal cell layer where BrdU incorporation can be seen; Scale bar = 20μm, A, B, E, F, I, J or 10 μm, C, D, G, H, K, L. (M) Data represents mean BrdU+ cells/mm OE ± SEM. *, p<0.01 v. respective saline vehicle group; #, p<0.001 v. vehicle/ATP group.
Discussion
We investigated whether NPY mediates ATP-induced neuroproliferation in adult mouse OE in vivo. NPY Y1 receptor antagonist BIBP3226 significantly blocked ATP-induced increases in BrdU-incorporation in basal cells in adult mouse OE. Moreover, the proliferation rates in antagonist-instilled animals, in the absence of exogenous ATP, were lower but not significantly lower compared to the vehicle-instilled animals. This result suggests that NPY may have a role in normal maintenance of neurons in the OE, but that other factors may also contribute. In support of this observation, chronic NPY deficiency (Hansel et al., 2001) or blockade of NPY effects by knockout of Y1 receptors (Doyle et al., 2008) produces a significant reduction in olfactory neuronal precursor proliferation. Taken together, these data imply that NPY and Y1 receptors have a role in normal OE maintenance in the adult. In neonates, ATP induces proliferation via purinergic receptor activation (Jia et al., 2009), and induces the release of NPY, suggesting that similar mechanisms and chemical signals mediate both post-natal and adult neurogenesis under normal conditions.
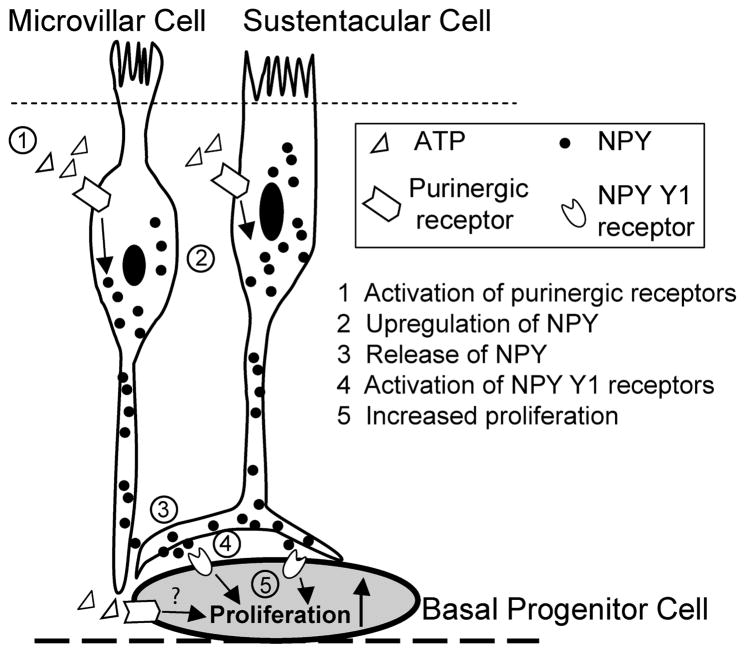
The present study provides a potential mechanism for the initiation of neuroregeneration following injury, whereby injured cells release ATP. This initiates a purinergic receptor mediated signaling cascade that evokes the release of NPY, and NPY Y1 receptors are activated, resulting in proliferation of neural precursor cells (Figure 7). In support of this mechanism, ATP upregulates NPY expression in the adult mouse OE. The protein levels of NPY and NPY+ cells in the OE were significantly increased 20 hrs after ATP instillation. ATP-induced NPY expression was observed in non-neuronal sustentacular and microvillous cells but not olfactory sensory neurons which corroborates the localization reported in previous studies (Hansel et al., 2001; Kanekar et al., 2009; Montani et al., 2006). This provides evidence that sustentacular cells and microvillous cells may have a role in the regulation of neuronal regeneration by stimulus-induced release of NPY. Due to the phenomenon of zonal expression of antigenically distinct progenitor cells in the OE (Murdoch and Roskams, 2008), we examined the ATP-induced NPY expression in the septum and turbinates through rostracaudal axis of OE which cover zone 1 to zone 4 (Ressler et al., 1993; Vassar et al., 1993). We found that ATP-induced NPY expression was observed in the septum and all turbinates through zone 1 to zone 4, with little difference in distribution across zones. These data suggest that if the upregulation of NPY is involved in ATP-induced neuroproliferation, there should no zonal or regional difference in ATP-induced proliferation in the OE. Indeed, our previous study (Jia et al., 2009) found no significant difference in ATP-induced increases in BrdU-incorporation in basal cells in septum vs. turbinates, suggesting that ATP does not preferentially induce proliferation in specific locations within the OE, but rather induces proliferation throughout the entire epithelium. Taken together, these data suggest that ATP and NPY may have a universal, broad effect in the OE and the upregulation of NPY may mediate ATP-induced neuroregeneration in the OE.
Figure 7. NPY and NPY Y1 receptor-activation mediates ATP-induced neuroproliferation in adult mouse olfactory epithelium.
(1) Exogenous ATP or injury-released ATP activates P2 purinergic receptors on the sustentacular and microvillar cells. (2) P2 receptor activation leads to increased expression of NPY and (3) subsequent NPY release. (4) Released NPY binds with NPY Y1 receptors on the basal progenitor cells, and (5) promotes neuroproliferation in the olfactory epithelium.
Additionally, we have also observed ATP-induced calcium increases in sustentacular cells (Hegg et al., 2003; Hegg et al., 2009) and microvillar cells (Hegg, unpublished data). Thus, in the cell types in which NPY upregulation was observed, ATP induces intracellular calcium increases which may evoke calcium-dependent exocytosis of NPY. There is direct evidence that ATP induces the release of NPY from neonatal OE slices (Kanekar et al., 2009), and here, we provide indirect evidence of ATP-induced NPY release in the adult in vivo. The Y1 receptor antagonist BIBP3226 significantly inhibited ATP-induced increases in BrdU incorporation, indicating that ATP evokes NPY release and NPY activation of Y1 receptors stimulates neuroproliferation in adult mouse OE.
Previous studies show that NPY is expressed in the ensheathing cells in the nerve bundles below the OE and in the bulb (Ubink et al., 1994). In the present study, we observed that NPY is expressed in two subsets of olfactory ensheathing cells located in the outer nerve layer and glomerular layer in the mature mouse. Intranasal instillation of ATP reduces or removes the NPY-IR in the olfactory ensheathing cells. During embryonic development of the olfactory system, NPY is expressed in the ensheathing cells before both the maturation of olfactory sensory neurons and the formation of glomerular layers in the olfactory bulb, suggesting that NPY might be involved in the guidance and growth of olfactory sensory axons toward their target glomeruli in the bulb (Ubink et al., 1994; Ubink and Hokfelt, 2000). It is tempting to speculate that ATP-induced NPY release from olfactory ensheathing cells may also play a role in axon guidance of newly formed neurons. However, we observed a decrease in NPY expression 48 hrs post-ATP treatment. If ATP were a signal of cellular damage, and if NPY had a role in axon guidance, then NPY release would be expected to occur when immature neurons begin to extend axons towards the bulb. However, at 2 days post-ATP treatment, when NPY depletion is observed, the ATP-induced proliferating cells express MASH1, a marker for neuronal progenitor cells, but not GAP43, a marker for immature neurons (Jia et al., 2009), suggesting that NPY depletion does not correlate to an axon guidance function. Another speculation could be that ATP-induced NPY release provides trophic support to the neurons following injury. Further experimentation will be necessary to determine the physiological role of ATP-induced NPY release from the olfactory ensheathing cells.
Collectively, these data suggest that neuroproliferation induced via purinergic receptor and NPY Y1 receptor activation may occur in both postnatal neuronal expansion and adult maintenance of neurons in the OE, as well as in response to cell injury. These data clearly have implications for the pharmacological modulation of neural progenitor cell proliferation in the olfactory epithelium. Currently there are few neuroprotective or restorative treatments available. To be successful, neural replacement therapies may need to include multiple factors jointly administered to promote regeneration. Antagonists and agents that modify the effect of NPY and purinergics and their receptors are readily available and could therapeutically modulate neuron proliferation and differentiation.
Acknowledgments
This work was supported by NIH DC006897 (CCH), by Michigan State University Institutional Funds (CCH), and by the APS Shih-Chun Wang Young Investigator Award (CCH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beites CL, Kawauchi S, Crocker CE, Calof AL. Identification and molecular regulation of neural stem cells in the olfactory epithelium. Exp Cell Res. 2005;306:309–316. doi: 10.1016/j.yexcr.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Carson C, Murdoch B, Roskams AJ. Notch 2 and Notch 1/3 segregate to neuronal and glial lineages of the developing olfactory epithelium. Dev Dyn. 2006;235:1678–1688. doi: 10.1002/dvdy.20733. [DOI] [PubMed] [Google Scholar]
- Czesnik D, Kuduz J, Schild D, Manzini I. ATP activates both receptor and sustentacular supporting cells in the olfactory epithelium of Xenopus laevis tadpoles. Eur J Neurosci. 2006;23:119–128. doi: 10.1111/j.1460-9568.2005.04533.x. [DOI] [PubMed] [Google Scholar]
- Doyle KL, Karl T, Hort Y, Duffy L, Shine J, Herzog H. Y1 receptors are critical for the proliferation of adult mouse precursor cells in the olfactory neuroepithelium. J Neurochem. 2008;105:641–652. doi: 10.1111/j.1471-4159.2007.05188.x. [DOI] [PubMed] [Google Scholar]
- Elsaesser R, Montani G, Tirindelli R, Paysan J. Phosphatidyl-inositide signalling proteins in a novel class of sensory cells in the mammalian olfactory epithelium. Eur J Neurosci. 2005;21:2692–2700. doi: 10.1111/j.1460-9568.2005.04108.x. [DOI] [PubMed] [Google Scholar]
- Graziadei PPC, Monti-Graziadei GA. Continuous nerve cell renewal in the olfactory system. In: Jacobson M, editor. Handbook of Sensory Physiology. Springer; New York: 1978. pp. 55–83. [Google Scholar]
- Ha Y, Choi JU, Yoon DH, Cho YE, Kim TS. Nestin and small heat shock protein expression on reactive astrocytes and endothelial cells in cerebral abscess. Neurosci Res. 2002;44:207–212. doi: 10.1016/s0168-0102(02)00126-8. [DOI] [PubMed] [Google Scholar]
- Hansel DE, Eipper BA, Ronnett GV. Neuropeptide Y functions as a neuroproliferative factor. Nature. 2001;410:940–944. doi: 10.1038/35073601. [DOI] [PubMed] [Google Scholar]
- Hegg CC, Greenwood D, Huang W, Han P, Lucero MT. Activation of purinergic receptor subtypes modulates odor sensitivity. J Neurosci. 2003;23:8291–8301. doi: 10.1523/JNEUROSCI.23-23-08291.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegg CC, Irwin M, Lucero MT. Calcium store-mediated signaling in sustentacular cells of the mouse olfactory epithelium. Glia. 2009;57:634–644. doi: 10.1002/glia.20792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell OW, Doyle K, Goodman JH, Scharfman HE, Herzog H, Pringle A, Beck-Sickinger AG, Gray WP. Neuropeptide Y stimulates neuronal precursor proliferation in the post-natal and adult dentate gyrus. J Neurochem. 2005;93:560–570. doi: 10.1111/j.1471-4159.2005.03057.x. [DOI] [PubMed] [Google Scholar]
- Jia C, Doherty JD, Crudgington S, Hegg CC. Activation of purinergic receptors induces proliferation and neuronal differentiation in Swiss Webster mouse olfactory epithelium. Neuroscience. 2009;163:120–128. doi: 10.1016/j.neuroscience.2009.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanekar S, Jia C, Hegg CC. Purinergic receptor activation evokes neurotrophic factor neuropeptide Y release from neonatal mouse olfactory epithelial slices. J Neurosci Res. 2009;87:1424–1434. doi: 10.1002/jnr.21954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung CT, Coulombe PA, Reed RR. Contribution of olfactory neural stem cells to tissue maintenance and regeneration. Nat Neurosci. 2007;10:720–726. doi: 10.1038/nn1882. [DOI] [PubMed] [Google Scholar]
- Milenkovic I, Weick M, Wiedemann P, Reichenbach A, Bringmann A. Neuropeptide Y-evoked proliferation of retinal glial (Muller) cells. Graefes Arch Clin Exp Ophthalmol. 2004;242:944–950. doi: 10.1007/s00417-004-0954-3. [DOI] [PubMed] [Google Scholar]
- Montani G, Tonelli S, Elsaesser R, Paysan J, Tirindelli R. Neuropeptide Y in the olfactory microvillar cells. Eur J Neurosci. 2006;24:20–24. doi: 10.1111/j.1460-9568.2006.04878.x. [DOI] [PubMed] [Google Scholar]
- Murdoch B, Roskams AJ. Olfactory epithelium progenitors: insights from transgenic mice and in vitro biology. J Mol Histol. 2007;38:581–599. doi: 10.1007/s10735-007-9141-2. [DOI] [PubMed] [Google Scholar]
- Murdoch B, Roskams AJ. A novel embryonic nestin-expressing radial glia-like progenitor gives rise to zonally restricted olfactory and vomeronasal neurons. J Neurosci. 2008;28:4271–4282. doi: 10.1523/JNEUROSCI.5566-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Sullivan SL, Buck LB. A zonal organization of odorant receptor gene expression in the olfactory epithelium. Cell. 1993;73:597–609. doi: 10.1016/0092-8674(93)90145-g. [DOI] [PubMed] [Google Scholar]
- Schwob JE. Neural regeneration and the peripheral olfactory system. Anat Rec. 2002;269:33–49. doi: 10.1002/ar.10047. [DOI] [PubMed] [Google Scholar]
- Shipley MT, Puche AC, Ennis M. The olfactory system. In: Paxinos G, editor. The Rat Nervous System. Academic press; NY: 2003. [Google Scholar]
- Stanic D, Paratcha G, Ledda F, Herzog H, Kopin AS, Hokfelt T. Peptidergic influences on proliferation, migration, and placement of neural progenitors in the adult mouse forebrain. Proc Natl Acad Sci USA. 2008;105:3610–3615. doi: 10.1073/pnas.0712303105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubink R, Halasz N, Zhang X, Dagerlind A, Hokfelt T. Neuropeptide tyrosine is expressed in ensheathing cells around the olfactory nerves in the rat olfactory bulb. Neuroscience. 1994;60:709–726. doi: 10.1016/0306-4522(94)90499-5. [DOI] [PubMed] [Google Scholar]
- Ubink R, Hokfelt T. Expression of neuropeptide Y in olfactory ensheathing cells during prenatal development. J Comp Neurol. 2000;423:13–25. doi: 10.1002/1096-9861(20000717)423:1<13::aid-cne2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Vassar R, Ngai J, Axel R. Spatial segregation of odorant receptor expression in the mammalian olfactory epithelium. Cell. 1993;74:309–318. doi: 10.1016/0092-8674(93)90422-m. [DOI] [PubMed] [Google Scholar]
- Vedin V, Molander M, Bohm S, Berghard A. Regional differences in olfactory epithelial homeostasis in the adult mouse. J Comp Neurol. 2009;513:375–384. doi: 10.1002/cne.21973. [DOI] [PubMed] [Google Scholar]
- Wang X, Arcuino G, Takano T, Lin J, Peng WG, Wan P, Li P, Xu Q, Liu QS, Goldman SA, Nedergaard M. P2X7 receptor inhibition improves recovery after spinal cord injury. Nat Med. 2004;10:821–827. doi: 10.1038/nm1082. [DOI] [PubMed] [Google Scholar]