Abstract
Porous poly(L-lactic acid) (PLLA) scaffolds with bioactive coatings were prepared by a novel one-step method. In this process, ice-based microporogens containing bioactive molecules, such as hydroxyapatite (HA) and collagen, served as both porogens to form the porous structure and vehicles to transfer the bioactive molecules to the inside of PLLA scaffolds in a single step. Based on scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDX), X-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FTIR) analysis, the bioactive components were found to be transferred successfully from the porogens to PLLA scaffolds evenly. Osteoblast cells were used to evaluate the cellular behaviors of the composite scaffolds. After 8 days culturing, MTT assay and alkaline phosphatase (ALP) activity results suggested that HA/collagen could improve the interactions between osteoblast cells and the polymeric scaffold.
Keywords: ice-based microporogens, scaffold, collagen, hydroxyapatite, poly(L-lactic acid
1. Introduction
Scaffolding is a critical requirement in tissue engineering. The properties of scaffolds should satisfy a number of specific criteria. In terms of architecture, scaffolds should be highly porous with an interconnected pore network and appropriate pore size to facilitate cell migration and fluid diffusion, which is often dependent on the fabrication methods. A variety of techniques have been developed to produce porous scaffolds for tissue-engineering purposes, including phase separation [1], porogen leaching [2–7], gas foaming [8], rapid prototyping [9], electrospinning [10], and various combinations [11]. In porogen leaching, the polymer solution is introduced into the gaps among the microporogens to form a polymer-porogen composite. After the porogens are dissolved and removed, the remaining polymeric structure takes the form of a porous matrix as a reverse replica of the microporogen assembly. Various porogens, such as salt [2,6], gelatin [3], paraffin [4], ice [5], and sugar [7], have been utilized. For ice microspheres, the conventional porogen leaching by washing with organic solvent or water was replaced by freeze-drying, thus facilitating an efficient removal of the porogens.
A scaffold should also be biocompatible, in most cases biodegradable, exhibit adequate mechanical properties, and possess a biocompatible surface suitable for cell attachment, proliferation and differentiation. These properties are primarily dependent on the nature of scaffold material. With the approval of US Food and Drug Administrations (FDA) for specific human clinical uses, α-hydroxypolyesters, such as polylactic acid (PLA), poly(glycolic acid) (PGA), and their copolymers poly(lactide-co-glycolide) (PLGA), have drawn much interest as scaffold materials for tissue engineering. These polymers are biocompatible and offer a wide range of biomechanical and biodegradation properties to fit various applications [12–15]. They can be reliably processed into scaffolds using a number of methods [15,16]. However, their limited ability to facilitate cellular responses has been a significant concern.
Bioactive molecules such as hydroxyapatite (HA) [17–22], collagen [19–24] and other proteins [24,25] are often used as coating to modify the material surfaces of polymeric tissue engineering scaffolds to enhance the target biological responses. We have recently developed an approach to generate HA/collagen coated poly(lactic-glycolic acids) scaffolds using a porogen-leaching method [26]. The approach involves a two-step process with coating first on the paraffin microspheres and then using the coated micropheres as both the porogens and the vehicles to transfer the bioactive apatite/collagen coating. In this study, we describe the advancement of the porogen-based technology using ice-based microporogens to deposit multiple bioactive agents into the desired scaffold in a single step.
2. Materials and Methods
2.1. Materials
PLLA with an inherent viscosity of approximately 7.11 dl/g was purchased from PURAC (PURAC, Netherlands). HA [Ca10(OH)2(PO4)6], with an average grain of less than 500 nm, was obtained from The University of Hong Kong. Collagen type I was separated from fresh bovine tendon [27]. Chloroform was purchased from Acros (Belgium). Deionized water was obtained with a nanopure diamond ultrapure water systems (Barnstead, USA).
2.2. Preparation of ice-based microparticles
1% (w/v) collagen solution, 4% (w/v) HA suspension, and 1% collagen solution mixed with HA particles (HA/collagen: 80/20, w/w) were prepared and pre-cooled to 2 °C. Ice-based microparticles were prepared by spraying cold deionized water or the prepared solutions through the capillary into liquid nitrogen from a distance of 10 cm. Microparticles with diameter from 100 to 500 μm were collected. Four groups of ice-based microparticles, i.e. pure ice microparticles, ice-based microparticles formed with collagen solution, ice-based microparticles formed with HA suspension, and ice-based microparticles formed with HA/collagen solution were prepared using this approach (Fig. 1a). The whole procedure was processed in a −5 °C environment.
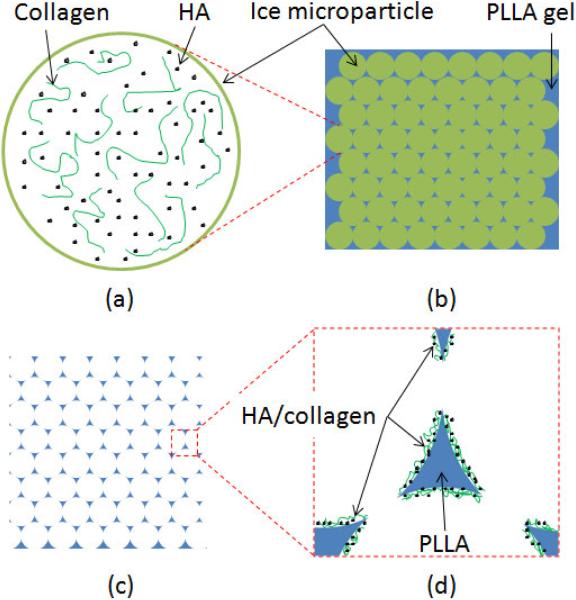
Fig. 1.
A scheme of preparation methods of PLLA scaffold with coatings. (a) ice-based microparticles with HA and collagen inside; (b) the inter space among ice-based microparticles was filled by PLLA gel; (c) porous polymeric scaffold was gotten after ice and solvent were removed; (d) HA and collagen coated on the surface of pore wall.
2.3. Fabrication of the PLLA scaffolds with bioactive coating
PLLA solution was dissolved in 1,4-dioxane (10%, w/w) and cooled to −5 °C for 12h [28,29]. Although the melting point of 1,4-dioxane is 11.8 °C, the PLLA solution became gel after it was pre-cooled. PLLA gel and the ice-based microparticles (20/80, v/v) were mixed together by a plastic spoon. A plastic syringe without a needle end was used as a mould of the PLLA scaffolds. PLLA gel and the ice-based microparticles were placed in the cylinder with caution to make sure that no air bubbles were trapped in the mold. The syringe end was placed vertically on a piece of rubber plate; and the syringe piston was pushed down gently [30]. A little extra PLLA gel was extruded from the gap between the syringe end and the rubber plate. When no more PLLA gel was observed coming out, the pressure on the piston was released. At this time, all ice-based microparticles contacted closely and tightly. The inter space among ice-based microparticles was filled completely with PLLA gel (Fig. 1b). Then the whole PLLA scaffold with ice-based microparticles was pushed out by the piston and kept in liquid nitrogen for 12h. Finally, the samples were freeze-dried at −20 °C for 7 days to completely remove the solvent and water to obtain three-dimensional porous polymeric scaffolds (Fig. 1c) with the bioactive deposition coating (Fig. 1d). The size of scaffolds used in this research is 5 mm in diameter and 5 mm in thickness.
In order to examine the inside of the samples, some scaffolds were cut in the middle after they were frozen and just before they were freeze-dried.
2.4. Characterization
The morphology and composition of the PLLA scaffolds without and with different deposition coatings were analyzed by scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDX), and X-ray diffraction (XRD). The samples were studied under a scanning electron microscope (JEOL JSM-6490, Japan) after being coated with gold. EDX was obtained without gold coating. Randomly selected areas of about 1×1 mm on the sample surface and section were examined. XRD spectra were obtained using a Philips X-ray diffractometer with a fixed incidence of 1° in the range of 10–40° using 0.06° step and 1 s/step scan speed. Cross sections of the scaffolds were examined along with pure collagen and pure HA as references. Fourier transform infrared spectroscopy (FTIR) was used to obtain information on the molecular components on the surfaces. The transmission spectra of pure HA, pure collagen, PLLA scaffolds without and with different coatings were measured with a Perkin-Elmer FTIR Spectrometer.
2.5. Cell culturing and characterization
After the scaffolds were sterilized by 70% ethanol and washed by PBS solution completely. 20 μl (about 1×105 cells) of the Saos-2 osteoblast-like cell suspensions were seeded evenly into PLLA scaffolds, PLLA/HA scaffolds, PLLA/collagen scaffolds, and PLLA/HA/collagen scaffolds with a micropipette, respectively. The seeded scaffolds were maintained in incubator for 4h for cell adhesion to the scaffolds and then 1.0 ml completed medium was added to the wells. The 24-well plate was incubated at a temperature of 37 °C in a 5% CO2 atmosphere. The medium was changed every 2 days. After incubation, any non-adherent cells on the samples were removed by aspirating the medium and washing with PBS solution.
The MTT assay was used to visualize the metabolically active cells. The scaffolds were removed and placed into a new 24-well dish, to which 1.0 ml of growth medium containing 0.5 mg/ml [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrasodium bromide] (MTT) was added. Cells were allowed to incubate at 37 °C for 4h, after which the medium and scaffolds were gently removed. Thereafter the formazan crystals formed were solubilized by adding DMSO. Then the optical density (OD) of formazan in the solution was read using a microplate reader at 570 nm (BIO-RAD Model 550, BIO-RAD, USA).
Alkaline phosphatase (ALP) activity was measured using an alkaline phosphatase assay kit (Zhongsheng Beikong, China). Cells were incubated in scaffolds for 8 days. After removing the culture medium, the cells were washed with PBS and then detached with trypsin/EDTA. After centrifugation, the cell pellets were washed with PBS and resuspended by vortexing them in 0.025 ml of deionized water with 0.1% Triton X-100. The cell pellets were disrupted via a cyclic freezing/thawing process. The prepared cell lysates were used to determine ALP activity according to the manufacturer's instructions.
Experiments were run in triplicate per sample. All data were expressed as mean ± standard deviation (SD) with n=3. Single factor analysis of variance (ANOVA) technique was used to assess statistical significance of results. Difference with p values < 0.05 were considered to be significant.
3. Results
3.1. SEM of PLLA/HA/collagen scaffolds
After PLLA gel and ice-based microspheres were freeze-dried, the spaces occupied by ice-based microspheres were emptied as pores. The PLLA located in the inter space among the ice-based microspheres became the pore wall of the PLLA scaffold. The contact areas between two adjacent ice-based microparticles became small holes connecting the adjacent bigger pores. The resultant PLLA scaffold was highly porous with good pore connectivity (Fig. 2). The diameter of these holes ranged from 100 to 500 μm which was consistent with the size of the ice-based microparticles. These connected holes would serve adequately for the passages of cells, nutrients, and waste products in and out of the scaffold.
Fig. 2.
SEM micrographs of PLLA scaffolds fabricated using ice-based porogen.
Under the low air pressure of freezing-drying, the ice microparticles with collagen and HA/collagen should form sponges after the ice was removed. The collagen sponge or HA/collagen sponge collapsed and fall on the pore surface and resulted in the formation of coating in humid atmosphere after the vacuum was released. HA, collagen or HA/collagen were transferred by deposition onto the inner surface of the pores within the PLLA scaffold, and could be observed by SEM (Fig. 3).
Fig. 3.
SEM micrographs of PLLA scaffolds fabricated using ice-based porogen: (a) PLLA scaffold; (b) PLLA scaffold with collagen coating; (c) PLLA scaffold with HA coating; (d) PLLA scaffold with HA/collagen hybrid coating.
3.2. EDX spectrum
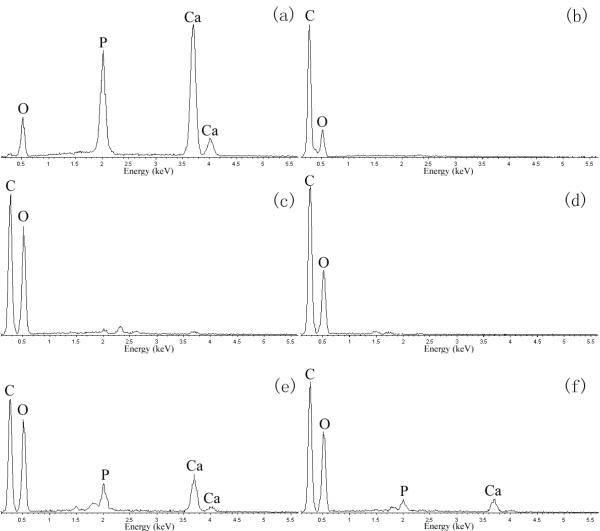
Pure HA powder, pure collagen film and the surface and middle section of scaffolds were examined by EDX. The EDX spectrum (Fig. 4) showed that the main elements are carbon and oxygen for collagen [Fig. 4(b)], PLLA scaffold [Fig. 4(c)], and PLLA scaffold with collagen coating [Fig. 4(d)]. For the PLLA scaffolds coated with HA [Fig. 4(e)] and with HA/collagen [Fig. 4(f)], calcium and phosphorus were detected which were derived from the HA particles [Fig. 4(a)]. These results suggested that HA was successfully coated on the surface of the pore wall inside PLLA scaffolds. No coating difference was detected between the surface and section areas of a same sample.
Fig. 4.
EDX spectra of (a) HA powder; (b) collagen film; (c) PLLA scaffold without coating; (d) PLLA scaffold with collagen coating; (e) PLLA scaffold with HA coating; and (f) PLLA scaffold with HA/collagen hybrid coating.
3.3. XRD results
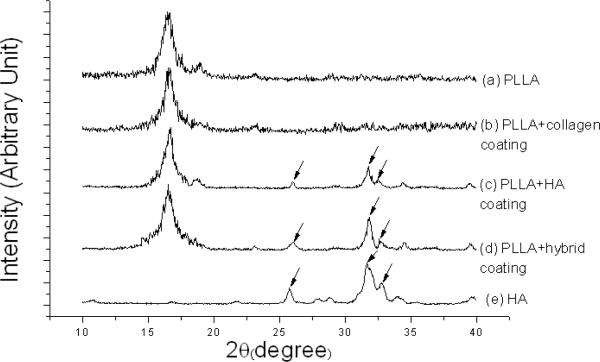
Pure HA powder and the middle section of PLLA scaffolds without or with different coatings were scanned by EDX. Three peaks (at 26, 32 and 33°) were observed in the X-ray diffraction (XRD) patterns of PLLA scaffolds with HA and HA/collagen coating (traces `c' and `d' in Fig. 5). They were the characteristic XRD peaks of pure HA (trace `e' in Fig. 5). Thus, these results also suggested that the HA was successfully transferred onto the PLLA scaffolds.
Fig. 5.
X-ray diffraction patterns of (a) PLLA scaffold without coating; (b) PLLA scaffold with collagen coating; (c) PLLA scaffold with HA coating; (d) PLLA scaffold with HA/collagen hybrid coating; and (e) pure HA.
3.4. FTIR spectra
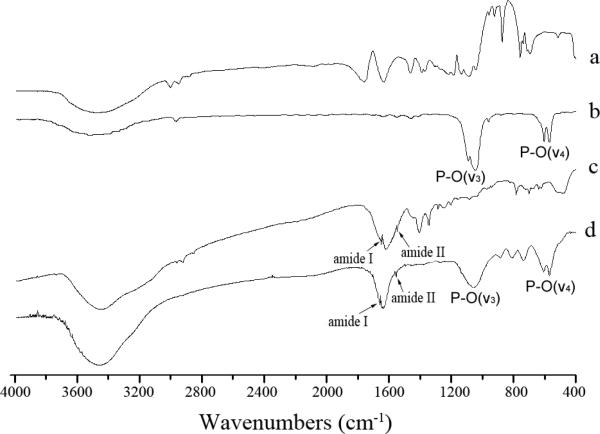
FTIR spectroscopy was used to gain additional information on the chemical structure of the substrate and the coating (Fig. 6). The FTIR spectrum of PLLA scaffold with HA coating [Fig. 6(b)] showed strong peaks at 1031 cm−1 and 602/567 cm−1, indicative of the v3 and v4 vibration of PO4−3, respectively [31]. FTIR spectrum of PLLA/collagen [Fig. 6(c)] showed two peaks at 1655 and 1550 cm−1, indicative of amide I and II. Both the PO4−3 and amide peaks were observed in the FTIR spectrum of PLLA scaffold with HA/collagen coating [Fig. 6(d)]. These analysis therefore confirmed that both HA and collagen were successfully transferred into PLLA scaffold.
Fig. 6.
FTIR spectra of (a) PLLA; (b) PLLA scaffold with HA coating; (c) PLLA scaffold with collagen coating. (d) PLLA scaffold with HA/collagen coating.
3.5. MTT assay and ALP
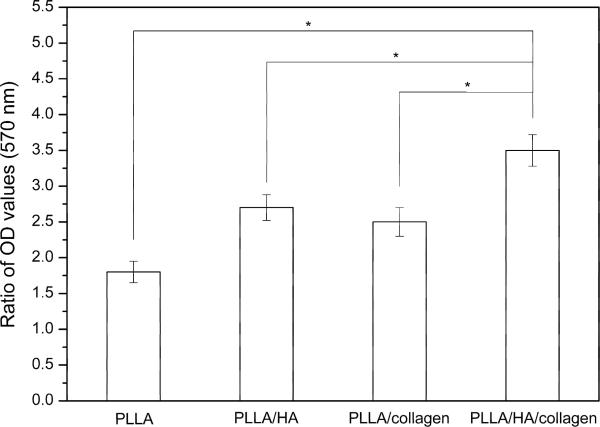
MTT assay involves a reduction reaction which reduces MTT reagent to a blue formazan product when incubated with viable cells. Thus the absorbance of formazan indirectly reflected the level of cell metabolism in culture. In order to reveal the change of cell metabolism after 8 days culture, scaffolds were also examined by MTT after cell seeding procedure as control. Fig. 7 showed the ratio of OD value of formazan produced by cells on different scaffolds before and after 8 days culture. Compared to pure PLLA scaffold, the highest absorbance was obtained on the PLLA/HA/collagen scaffold with p < 0.05.
Fig. 7.
MTT assay. The ratio of OD value of formazan produced by cells on different scaffolds after cell seeding and 8 days culture (n=3). * Significant difference with p<0.05 between the samples highlighted by the lines.
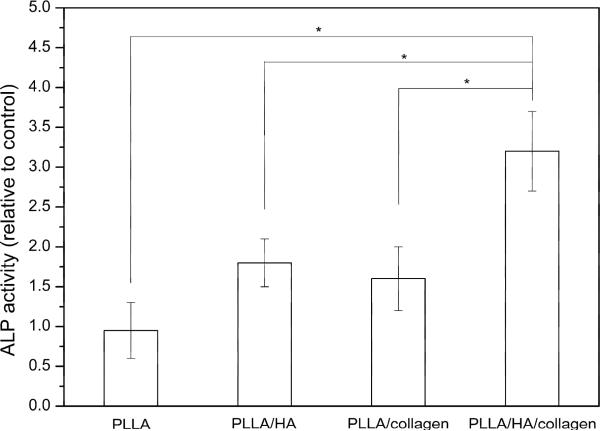
Alkaline phosphatase activity was measured to assess the differentiated osteogenic activity of the cell constructs. Fig. 8 showed the relative ALP activity of osteoblast cells cultured for 8 days on PLLA scaffolds with different coatings when pure PLLA scaffolds were used as control. The ALP activity of Saos-2 osteoblast-like cell cultured on PLLA/HA/collagen scaffold was significantly higher than that on the PLLA, PLLA/HA and PLLA/collagen scaffolds (p < 0.05).
Fig. 8.
The relative ALP activity of osteoblast cells cultured for 8 days on PLLA scaffolds with different coatings when pure PLLA scaffolds were used as control (n=3). * Significant difference with p<0.05 between the samples highlighted by the lines.
4. Discussion
In previous reports, we have developed a two-step approach to generate apatite, collagen, and HA/collagen coated poly(lactic-glycolic acids) scaffolds using paraffin-based porogens [27,30,32]. Because ice can be easily removed by freeze-drying, without the use of additional organic solvents for porogen leaching, it has been used for the fabrication of scaffolds in a porogen leaching procedure [5]. In this paper, ice was applied as both the microporogen and the vehicle to transfer bioactive coatings. Our results showed that HA, collagen, and hybrid HA/collagen have all been successfully transferred onto the pore wall of PLLA scaffolds using ice-based microporogen in one step.
As primary constituents of bone matrix, apatite and collagen have been coated on various biomaterials to mimic the natural microenvironment and to promote cell adhesion for bone tissue engineering [20–22,33,34]. The process for collagen coating or apatite coating usually involves immersing scaffolds in collagen solution or simulated body fluid (SBF) for a few days or even weeks. However, this approach is hampered by the fact that biodegradable polymeric materials, such as poly(α-hydroxy acids), degrade in aqueous as well as humid environment via hydrolysis. We have thus accelerated this incubation process to no more than 24 hours to reduce this risk [18–22]. In this study, the method of ice-based microporogens totally circumvents this risk of scaffold degradation during the coating process.
The spatial distribution of coating was also a concern in the immersion method. Usually less coating was found on the internal surfaces than on the peripheral ones because of more ready exchange between the peripheral surfaces and the surrounding fluid [35,36]. This non-uniform distribution of coating could happen especially in large size scaffolds with small pores. In the one-step method, the bioactive components introduced by the ice-based microporogens are distributed evenly onto the pore surface within the scaffolds, even for relatively thick scaffolds with small pores.
Furthermore, in the immersion method the amount of coating is influenced by several factors, such as the ionic concentrations of the immersion solution, the incubation period, application of hydrostatic pressure, the pore size and pore connectivity of the scaffolds, etc. In one-step method, the coating process is incorporated into the scaffold fabrication process. In principle, the amount of coating can be controlled by varying the concentration of agents in the microporogens. In the two-step microporogen method, the process of porogens dissolution by organic solvent or water may increase the chance of the coating being partially washed away with the solvent fluxes. In the one-step method, agents embedded in ice-based microporogens should have a better chance to be transferred to and remain on the polymeric surface because the process of solvent washing is replaced by freeze-drying.
Finally, it should be pointed out that the method described here also allows for “multicompartment” loading of the micropores. Specifically, sequential introduction of ice porogens, which could be different in size as regulated by controlled atomization, loaded with different bioactive agents, could be carried out. In this manner, it should be possible to produce bioactive scaffolds that consist of multiple compartments of difference pore size that contain different bioactive agents.
It has been reported that fibroblast growth factors could be immobilized on PLLA surface to enhance cell growth [24]. The one-step method should be applicable for the introduction of growth factors and other proteinaceous signaling molecules with retention of biological activity, since these molecules are embedded in ice and not exposed to harsh organic solvents.
In this study, two cell affinity materials, collagen and HA, were coated on the pore wall surface of PLLA scaffolds by the one step method. According to MTT assay of pure PLLA scaffolds, more viable cells were detected after 8 days culture than 0 day culture. But the highest increase of OD value were found on PLLA scaffold with HA and collagen. It meant that HA and collagen provided a suitable surface for cell attachment and proliferation. The ALP results confirmed the similar trend. In our lab, biomimetic apatite and collagen were coated on PLLA scaffold via a combined phase-separation technique and an accelerated biomimetic coating process to enhance osteoblast-like cells attachment and activity [20]. The investigation also demonstrated that cell-compatibility of PLLA scaffolds was greatly enhanced with combined apatite/collagen coating. The attachment and total activity of osteoblast-like cells on the apatite/collagen coating were found increasing more than on apatite coating.
Although there are still some disadvantages, such as: the relative weak coating strength between the bioactive agents and the pore wall of the scaffold, the one-step method opens a new way to prepare and modify bioactive scaffolds for tissue engineering.
5. Conclusions
A novel one-step method of preparing porous three-dimensional biodegradable scaffolds with deposition of bioactive hydroxyapatite and collagen for tissue engineering was developed using pre-prepared ice-based microparticles as porogens. EDX, XRD and FTIR results revealed the even distribution of bioactive agents which may be used to enhance subsequent cell seeding, proliferation and differentiation for tissue engineering applications. MTT and ALP results suggested that HA/collagen coating could improve the interactions between osteoblast cells and the polymeric scaffold. The one-step method should be particularly invaluable to transfer bioactive agents inside of large scaffolds with relatively small pores.
Acknowledgements
This study was supported by a PolyU grant (G-U408). RST is supported by the Intramural Research Program of NIAMS, NIH (Z01 AR41131).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Lo H, Ponticiello MS, Leong KW. Fabrication of controlled release biodegradable foams by phase separation. Tissue Eng. 1995;1:15–28. doi: 10.1089/ten.1995.1.15. [DOI] [PubMed] [Google Scholar]
- [2].Mikos AG, Sarakinos G, Leite SM, Vacanti JP, Langer R. Laminated three-dimensional biodegradable foams for use in tissue engineering. Biomaterials. 1993;14:323–330. doi: 10.1016/0142-9612(93)90049-8. [DOI] [PubMed] [Google Scholar]
- [3].Gong Y, Zhou Q, Gao C, Shen J. In vitro and in vivo degradability and cytocompatibility of poly(l-lactic acid) scaffold fabricated by a gelatin particle leaching method. Acta Biomater. 2007;3:531–540. doi: 10.1016/j.actbio.2006.12.008. [DOI] [PubMed] [Google Scholar]
- [4].Ma PX, Choi JW. Biodegradable polymer scaffolds with well-defined interconnected spherical pore network. Tissue Eng. 2001;7:23–33. doi: 10.1089/107632701300003269. [DOI] [PubMed] [Google Scholar]
- [5].Chen G, Ushida T, Tateishi T. Preparation of poly(L-lactic acid) and poly(DL-lactic-co-glycolic acid) foams by use of ice microparticulates. Biomaterials. 2001;22:2563–2567. doi: 10.1016/s0142-9612(00)00447-6. [DOI] [PubMed] [Google Scholar]
- [6].Laschke MW, Strohe A, Scheuer C, Eglin D, Verrier S, Alini M, Pohlemann T, Menger MD. In vivo biocompatibility and vascularization of biodegradable porous polyurethane scaffolds for tissue engineering. Acta Biomater. 2009;5:1991–2001. doi: 10.1016/j.actbio.2009.02.006. [DOI] [PubMed] [Google Scholar]
- [7].Vaquette C, Frochot C, Rahouadj R, Wang X. An innovative method to obtain porous PLLA scaffolds with highly spherical and interconnected pores. J Biomed Mater Res B. 2008;86:9–17. doi: 10.1002/jbm.b.30982. [DOI] [PubMed] [Google Scholar]
- [8].Mooney DJ, Baldwin DF, Suh NP, Vacanti JP, Langer R. Novel approach to fabricate porous sponges of poly(D, L-lactic-coglycolic acid) without the use of organic solvents. Biomaterials. 1996;17:1417–1422. doi: 10.1016/0142-9612(96)87284-x. [DOI] [PubMed] [Google Scholar]
- [9].Park A, Wu B, Griffith LG. Integration of surface modification and 3D fabrication techniques to prepare patterned poly(L-lactide) substrates allowing regionally selective cell adhesion. J Biomater Sci Polym Ed. 1998;9:89–110. doi: 10.1163/156856298x00451. [DOI] [PubMed] [Google Scholar]
- [10].Li J, Li Y, Li L, Mak AFT, Ko F, Qin L. Preparation and biodegradation of electrospun PLLA/keratin nonwoven fibrous membrane. Polym Degrad Stabil. 2009 in press. [Google Scholar]
- [11].Yang Y, Zhao J, Zhao Y, Wen L, Yuan X, Fan Y. Formation of porous PLGA scaffolds by a combining method of thermally induced phase separation and porogen leaching. J Appl Poly Sci. 2008;109:1232–1241. [Google Scholar]
- [12].Krebs MD, Sutter KA, Lin ASP, Guldberg RE, Alsberg E. Injectable poly(lactic-co-glycolic) acid scaffolds with in situ pore formation for tissue engineering. Acta Biomater. 2009 doi: 10.1016/j.actbio.2009.04.035. in press. [DOI] [PubMed] [Google Scholar]
- [13].Karp JM, Shoichet MS, Davies JE. Bone formation on two-dimensional poly(DL-lactide-coglycolide) (PLGA) films and three-dimensional PLGA tissue engineering scaffolds in vitro. J Biomed Mater Res A. 2003;64:388–396. doi: 10.1002/jbm.a.10420. [DOI] [PubMed] [Google Scholar]
- [14].Chen CC, Chueh JY, Tseng H, Huang HM, Lee SY. Preparation and characterization of biodegradable PLA polymeric blends. Biomaterials. 2003;24:1167–1173. doi: 10.1016/s0142-9612(02)00466-0. [DOI] [PubMed] [Google Scholar]
- [15].Yang F, Murugan R, Ramakrishna S, Wang X, Ma YX, Wang S. Fabrication of nano-structured porous PLLA scaffold intended for nerve tissue engineering. Biomaterials. 2004;25:1891–1900. doi: 10.1016/j.biomaterials.2003.08.062. [DOI] [PubMed] [Google Scholar]
- [16].Nam YS, Park TG. Porous biodegradable polymeric scaffolds prepared by thermally induced phase separation. J Biomed Mater Res. 1999;47:8–17. doi: 10.1002/(sici)1097-4636(199910)47:1<8::aid-jbm2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- [17].Yuan XY, Mak AFT, Li JL. Formation of bone-like apatite on poly(L-lactic acid) fibers by a biomimetic process. J Biomed Mater Res A. 2001;57:140–150. doi: 10.1002/1097-4636(200110)57:1<140::aid-jbm1153>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- [18].Chen Y, Mak AFT, Li J, Wang M, Shum A. Formation of apatite on poly(α-hydroxy acid) in an accelerated biomimetic process. J Biomed Mater Res B. 2005;73:68–73. doi: 10.1002/jbm.b.30178. [DOI] [PubMed] [Google Scholar]
- [19].Chen Y, Mak AFT, Wang M, Li J. Composite coating of bone-like apatite particles and collagen fibers on poly L-lactic acid formed through an accelerated biomimetic coprecipitation process. J Biomed Mater Res B. 2006;77:315–322. doi: 10.1002/jbm.b.30356. [DOI] [PubMed] [Google Scholar]
- [20].Chen Y, Mak AFT, Wang M, Li J, Wong MS. PLLA scaffolds with biomimetic apatite coating and biomimetic apatite/collagen composite coating to enhance osteoblast-like cells attachment and activity. Surf Coat Tech. 2006;201:575–580. [Google Scholar]
- [21].Chen Y, Mak AFT, Wang M, Li JS, Wong MS. In vitro behavior of osteoblast-like cells on PLLA films with a biomimetic apatite or apatite/collagen composite coating. J Mater Sci-Mater M. 2008;19:2261–2268. doi: 10.1007/s10856-007-3335-8. [DOI] [PubMed] [Google Scholar]
- [22].Chen Y, Cho MR, Mak AFT, Li J, Wang M, Sun S. Morphology and adhesion of mesenchymal stem cells on PLLA, apatite and apatite/collagen surfaces. J Mater Sci-Mater M. 2008;19:2563–2567. doi: 10.1007/s10856-007-3195-2. [DOI] [PubMed] [Google Scholar]
- [23].Suh H, Hwang YS, Lee JE, Han CD, Park JC. Behavior of osteoblasts on a type I atelocollagen grafted ozone oxidized poly L-lactic acid membrane. Biomaterials. 2001;22:219–230. doi: 10.1016/s0142-9612(00)00177-0. [DOI] [PubMed] [Google Scholar]
- [24].Ma Z, Gao C, Gong Y, Shen J. Cartilage tissue engineering PLLA scaffold with surface immobilized collagen and basic fibroblast growth factor. Biomaterials. 2005;26:1253–1259. doi: 10.1016/j.biomaterials.2004.04.031. [DOI] [PubMed] [Google Scholar]
- [25].Kokubo S, Fujimotob R, Yokotac S, Fukushimaa S, Nozakia K, Takahashia K, Miyataa K. Bone regeneration by recombinant human bone morphogenetic protein-2 and a novel biodegradable carrier in a rabbit ulnar defect model. Biomaterials. 2003;24:1643–1651. doi: 10.1016/s0142-9612(02)00551-3. [DOI] [PubMed] [Google Scholar]
- [26].Li J, Yuan XY, He F, Mak AFT. Hybrid coating of hydroxyapatite and collagen within poly(DL-lactic-co-glycolic acid) scaffold. J Biomed Mater Res B. 2008;86:381–388. doi: 10.1002/jbm.b.31031. [DOI] [PubMed] [Google Scholar]
- [27].Yang HY, Zhang QQ. Preparation and characterization of collagen-GAGS bioactive matrices for tissue engineering. J Mater Sci Technol. 2001;17:495–500. [Google Scholar]
- [28].Chen VJ, Ma P. Nano-fibrous poly(L-lactic acid) scaffolds with interconnected spherical macropores. Biomaterials. 2004;25:2065–2073. doi: 10.1016/j.biomaterials.2003.08.058. [DOI] [PubMed] [Google Scholar]
- [29].Ho MH, Kuo PY, Hsieh HJ, Hsien TY, Hou LT, Lai JY, Wang DM. Preparation of porous scaffolds by using freeze-extraction and freeze-gelation methods. Biomaterials. 2004;25:129–138. doi: 10.1016/s0142-9612(03)00483-6. [DOI] [PubMed] [Google Scholar]
- [30].Li J, Beaussart A, Chen Y, Mak AFT. Transfer of apatite coating from porogens to scaffolds: Uniform apatite coating within porous poly(DL-lactic-co-glycolic acid) scaffold in vitro. J Biomed Mater Res A. 2007;80:226–233. doi: 10.1002/jbm.a.31096. [DOI] [PubMed] [Google Scholar]
- [31].Rehman I, Bonfield W. Characterization of hydroxyapatite and carbonated apatite by photo acoustic FTIR spectroscopy. J Mater Sci-Mater M. 1997;8:1–4. doi: 10.1023/a:1018570213546. [DOI] [PubMed] [Google Scholar]
- [32].Li J, Mak AFT. Transfer of collagen coating from porogen to scaffold: homogeneous coating of collagen within poly(DL-lactic-co-glycolic acid) scaffold. Compos Part B-Eng. 2007;38:317–323. [Google Scholar]
- [33].Chen G, Ushida T, Tateishi T. A biodegradable hybrid sponge nested with collagen microsponges. J Biomed Mat Res. 2000;51:273–279. doi: 10.1002/(sici)1097-4636(200008)51:2<273::aid-jbm16>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- [34].Chen G, Ushida T, Tateishi T. Poly(DL-lactic-co-glycolic acid) sponge hybridized with collagen microsponges and deposited apatite particulates. J Biomed Mater Res. 2001;57:8–14. doi: 10.1002/1097-4636(200110)57:1<8::aid-jbm1135>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- [35].Zhang R, Ma PX. Biomimetic polymer/apatite composite scaffolds for mineralized tissue engineering. Macromol Biosci. 2004;4:100–111. doi: 10.1002/mabi.200300017. [DOI] [PubMed] [Google Scholar]
- [36].Chen Y, Mak AFT, Wang M. Formation of apatite within biodegradable scaffolds by an accelerated biomimetic process in the shaking condition and flow condition. Key Engin Mater. 2007;(334–335):1213–1216. [Google Scholar]