Abstract
Subclinical doses of Paclitaxel (PTX) given 1 day prior to a HER-2/neu (neu)-targeted, granulocyte-macrophage colony stimulating factor (GM-CSF)-secreting whole-cell vaccine enhances neu-specific T cell responses and slows neu+ tumor growth in tolerized HER-2/neu (neu-N) mice. We demonstrate that co-administration of PTX and Cyclophosphamide (CY) synergizes to slow tumor growth, and that in vitro, DC precursors exposed to PTX before LPS maturation results in greater co-stimulatory molecule expression, IL-12 production, and the ability to induce CD8+ T cells with enhanced lytic activity against neu+ tumors. PTX treatment also enhances maturation marker expression on CD11c+ DCs isolated from vaccine-draining lymph nodes. Ex vivo, these DCs activate CD8+ T cells with greater lytic capability than DC’s from vaccine alone-treated neu-N mice. Finally, PTX treatment results in enhanced antigen-specific, IFN-γ-secreting CD8+ T cells in vivo. Thus, administration of PTX with a tumor vaccine improves T cell priming through enhanced maturation of DC.
Keywords: Paclitaxel, Cyclophosphamide, Dendritic Cell, Cross-presentation, Immunotherapy, Immunomodulation, Combination Therapy
Introduction
The success of cancer vaccines as single agents has been limited in cancer bearing hosts. [1; 2] Most pre-clinical studies suggest that vaccines work best in minimal residual disease, which has led to the exploration of combining vaccines with standard cancer treatments such as chemotherapy and radiation, since these modalities can often reduce overall tumor burden or activate the immune system. [3; 4; 5; 6; 7; 8] Standard treatment doses of chemotherapy, however, are often detrimental to the effectiveness of tumor vaccines, most likely due to their cytotoxic effects on activated T cells. Therefore, subclinical doses of standard chemotherapeutic agents have been tested in both animal models and clinical trials for their ability to synergistically enhance the immune activity of vaccines. Standard agents such as Doxorubicin, 5-Fluorouracil, and Gemcitabine have been shown to enhance antigen presentation by inducing apoptosis of tumor cells. [9; 10; 11] Similarly, alkylating agents like Melphalan and Mitomycin C can up-regulate the expression of CD80 and CD86 co-stimulatory molecules on tumor cells, thereby conferring greater susceptibility as targets for CD8+ T cell lysis. [12] In addition, subclinical doses of Cyclophosphamide (CY) have been shown to increase immune activity by selectively killing T regulatory cells (Tregs) and inducing T helper 1 type T cell responses. [13; 14; 15; 16; 17] Paclitaxel (PTX) is an anticancer drug originally derived from the bark of the Pacific yew tree. It is best known clinically for its ability to stabilize microtubules, thus preventing cell division and causing cell death. [18] At sub-therapeutic doses, however, PTX has also been found to mimic the action of Toll-Like Receptor (TLR) agonists such as lipopolysaccharide (LPS), thereby inducing the maturation of mouse macrophages and major histocompatibility complex (MHC) class II expression on mouse dendritic cells (DC). [19; 20] The mechanism by which PTX results in enhanced antigen presenting cell (APC) function is thought to be due to signaling through the TLR4/MD-2 receptor complex. These data strongly suggest that PTX can enhance the activation of APCs including DC, and thus, make it an excellent candidate for combining with immunotherapeutic approaches.
We have used the neu-N model of mammary tumors to study the efficacy of granulocyte-macrophage colony stimulating factor (GM-CSF)-secreting whole cell vaccines. In the neu-N model, the rat HER-2/neu (neu) gene, under the control of the mouse mammary tumor virus (MMTV) promoter, is overexpressed in the mammary tissue of FVB/N mice. [21] The resulting neu-N mice are tolerant to neu when compared to parental FVB/N mice. [1; 2; 15] Vaccination with whole cell vaccines that express neu and secrete GM-CSF leads to delays in tumor growth, but not tumor rejection in neu-N mice. We have shown that tumor rejection can occur if a subclinical dose of Cyclophosphamide (CY) is given at the time of T cell priming, one day prior to whole cell vaccination. We also reported that administering PTX at the time of T cell priming, one day prior to vaccination with the GM-CSF-secreting whole-cell vaccine, can enhance neu-specific T cell responses and cure small tumor burdens in tolerant neu-N mice. In addition, the degree of T cell enhancement was similar to what was observed with CY. [15]
Here we show that combining both CY and PTX at the time of T cell priming, enhances the overall T cell activity of the GM-CSF-secreting vaccine, when compared with vaccine given with either chemotherapeutic agent alone. In contrast to CY, PTX does not deplete Tregs. Instead, we show that PTX enhances the maturation of early DC precursors by signaling through TLR-4. Furthermore this enhanced maturation results in enhanced priming of activated CD8+ T cells by PTX-primed CD11c+ DC in vivo. These findings have important implications for designing combinatorial vaccine approaches for the treatment of cancer.
Materials and Methods
Mice
FVB/N mice were purchased from Harlan or Taconic Laboratories. Neu-N mice [21] were purchased from Jackson Laboratories, and a breeding colony established and maintained at the Johns Hopkins University Animal Facilities. Clone 100 T cell receptor (TCR) transgenic mice, derived from FVB/N mice that express the high-avidity RNEU(420–429)–specific TCR in the majority of peripheral CD8+ T cells were generated as described, bred to a RAG background and maintained at the Johns Hopkins University Animal Facilities. [22]Experiments were performed on 8- to 12-week-old mice using AAALAC compliant protocols approved by the Animal Care and Use Committee of the Johns Hopkins University School of Medicine.
Cell Lines and Culture
The NT2.5 tumor cell line, 3T3neuGM vaccine and 3T3GM mock vaccine cell lines were cultured as previously described. [1; 2] Dendritic cells were cultured as described by Inaba. [23] Briefly, bone marrow dendritic cells were cultured at 1×106 cells/ml in 10ng/ml of GM-CSF, which was replenished on days 2 and 4 of a 6 day culture. On day 6, dendritic cells were matured with 800ng/ml LPS for 2 days prior to analysis, or as described in the figures. Where indicated, PTX was added to dendritic cells during culture and/or maturation.
Tumor Challenge Studies
Neu-N mice were challenged subcutaneously (sub-q) in the mammary fat pad with 5×104 NT2.5 cells. When applicable, on day 2, tumor challenged mice were injected intraperitoneally with CY (100mg/kg), PTX (20mg/kg), or the combination of CY+PTX. On day 3 post-tumor challenge, mice were subcutaneously injected with 3T3neuGM or 3T3GM at a dose of 1×106 cells in each of three limbs contralateral to the tumor-challenged mammary fat pad. Challenged mice were examined for tumor growth and tumor size was measured every 5 to 7 days until the tumors reached 1cm in diameter.
Dendritic Cell and T cell Isolation
Tissues were disrupted by mincing and incubation in a digestion medium containing 2mg/ml Collagenase D (Roche), 0.14 mg/ml DNAse I (Invitrogen), and RPMI (GIBCO) for 30 minutes at 37°C. Cell mixtures were pressed through a 40μM nylon mesh screen and washed twice in PBS containing 0.5% BSA (Sigma) and 2mM EDTA (GIBCO). CD11c+ cells were enriched by positive selection using magnetic beads (Miltenyi). For flow cytometry, Fcγ receptors were blocked using FcBlock anti-CD16/32 antibody (BD Pharmingen). CD8+ T cells were purified using Dynal CD8+ T cell isolation kits as per the manufacturer’s instructions.
Flow Cytometry
T cells and dendritic cells were washed once in FACS buffer (HBSS, 2 % FBS, .05% NaAzide), resuspended in FACS buffer containing specific antibodies diluted to the manufacturer’s recommendations, and incubated at 4 degrees Celcius for 30mins. Cells were washed twice in FACS buffer and analyzed on a Facscaliber (BD). Data was analyzed using FlowJo (Treestar, San Diego, CA). Anti-CD11c-APC (HL3), anti-CD40-PE (3/23), anti-CD86-PE (GL1), anti-MHC class II PE (M5/114.15.2) anti-IL12-PE (C15.6) were purchased from BD Pharmingen.
Chemotherapeutic Agents
Paclitaxel (PTX (Bristol-Myers Squibb, Princeton, NJ) and Cyclophosphamide (CY, Mead Johnson) were diluted in PBS prior to injection. CY was given intraperitoneally (i.p.) at a dose of 100mg/kg. PTX was injected i.p. at 20mg/kg. In vitro, PTX was used at ranges of 5nM to 100uM as described in the figures.
Peptides
RNEU(420–429) (PDSLRDLSVF) and NP(118–126) (RPQASGVYM) peptides were synthesized to greater than 95% purity at the Johns Hopkins Biosynthesis and Sequence Facility. RNEU(420–429) is the immunodominant epitope of rat HER-2/neu in FVB/N mice, and has been described previously[24]. The NP(118–126) peptide is from the lymphocytic choriomeningitis virus nucleoprotein. [25]
In vitro T cell generation
T cells were isolated from the spleens of C100/RAG mice by nylon wool purification and CD8+ T cell isolation kit (Invitrogen/Dynal) and cultured with in vitro generated dendritic cells. Dendritic cells were cultured as described with or without PTX, matured with LPS, and pulsed with either NP control or RNEU(420–429) peptide. T cells and DCs were cultured at a 1:1 ratio for 5 days prior to the chromium release assay.
Chromium Release Assay
Lysis assays were performed in triplicate in 96-well V-bottom plates as previously described [1]. Briefly, target cells were resuspended in 100 ul CTL medium and labeled at a concentration of 0.2 mCi of Cr-51 per 2 × 106 target cells. Target cells were incubated at 37°C and 5% CO2 for 1 h. Cells were washed in CTL medium and resuspended at 6 × 104 cells/ml in RPMI 1640. To pulse peptide onto targets, 100 ul of peptide in RPMI 1640 was added to 50 ul targets for 1 h at room temperature in each well. After the removal of 100 ul of supernatant, 150 ul of T cells in CTL medium was added for the indicated E:T ratio. After a 4-h incubation, 100 ul of supernatant was assayed for Cr-51 release and percent specific lysis was determined by the formula: ((51Cr release sample - spontaneous 51Cr release target alone)/(maximum 51Cr release target alone - spontaneous 51Cr release target alone)) × 100.
TLR Blocking Studies
Dendritic cells were isolated as described above, and cultured in Complete medium (RPMI, FBS 10% (Invitrogen), L-glutamine .5% (Invitrogen), Penicillan/Streptomycin 1% (Invitrogen)) for 6 days prior to maturation with 800ng LPS. Blocking was achieved by adding 2 or 10 ug of the anti-TLR4 antibody MTS510 (eBiosciences) at the beginning of culture up to the LPS maturation.
Statistical Methods
Data were analyzed using ANOVA, Kruskal-Wallace (assuming no Gaussian distribution), and Unpaired Student’s T-test as appropriate as described in the figure legends.
Results
PTX and CY demonstrate enhanced vaccine induced antitumor activity in vivo when compared with vaccine given with either agent alone
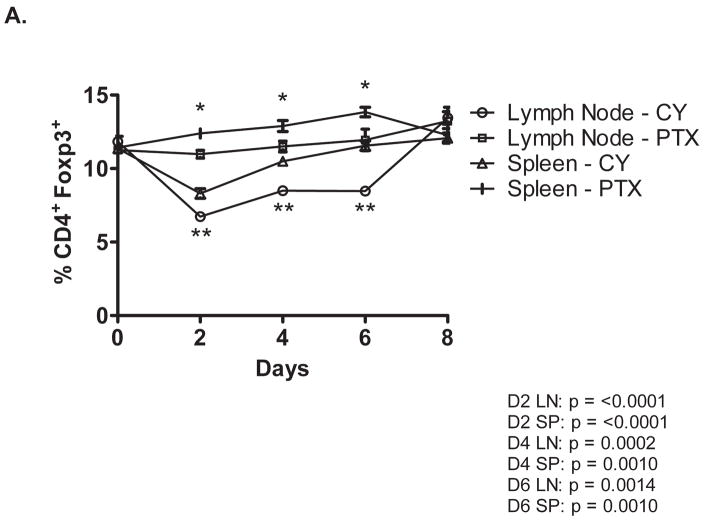
We previously demonstrated that sub-clinical doses of PTX and CY given one day prior to vaccination with a GM-CSF secreting, neu-targeted whole cell vaccine can enhance neu-specific T cell responses and cure small burdens of tumor in neu-N mice. [15] In addition, we reported that CY enhances the vaccine efficacy by eliminating cycling CD4+CD25+FOXP3+ T regulatory cells (T regs) and recruiting high avidity T cells to the immune response. [13] PTX is cytotoxic to immune cells when given at high doses. However, the mechanism by which it enhances vaccine induced antitumor immunity when given at low doses has not been completely studied. We hypothesized that if PTX and CY work through similar mechanisms, we would not expect to see a synergistic anti-tumor response when PTX and CY are combined with the GM-CSF secreting tumor vaccine compared to the response seen when either agent is given alone with the vaccine. To confirm that PTX works by a different mechanism than CY, tumor challenged neu-N transgenic mice were given the same doses of PTX, CY, or CY+PTX, and lymphocytes isolated from the spleen and tumor draining lymph nodes of mice were analyzed daily for up to 8 days post injection for CD4+FoxP3-expressing Tregs. As expected, PTX did not alter the number of Tregs from baseline. In addition, the combination of PTX+CY did not deplete Tregs to a greater extent than CY alone, indicating that the two drugs work by different mechanisms (Figure 1A).
Figure 1. CY and PTX have synergistic antitumor effects when combined with a HER-neu specific whole cell vaccine.
A. Tumor-bearing neu-N mice received 20mg/kg PTX, 100mg/kg CY, or both via intraperitoneal injection followed by vaccination with 3T3neuGM or 3T3GM mock vaccine cells. Spleens and vaccine-draining lymph nodes were collected at the indicated time points post vaccination. CD4 and Foxp3 expression was measured by flow cytometry and the percent of CD4+ cells positive for Foxp3 was determined. Data were analyzed each day with P values < .05 for both ANOVA and Kruskal Wallace. Individual groups were then compared by Unpaired Student’s T-test with * P < 0.001 for CY vs PTX treated splenocytes; ** P < 0.001 for CY vs PTX treated lymph node cells. B. The combination of CY and PTX delays the growth of NT2.5 tumors compared to CY or PTX alone. Mice were tumor challenged and treated as described in the Methods. Mice were monitored every 5 to 7 days. Weekly time points are shown to simplify the graph. Groups from day 29 to 48 were analyzed by Kruskal-Wallace and ANOVA p <.002 and .0001 respectively. Individual time points were analyzed by Unpaired Students t-Test. * P < 0.01 for Cy + PTX + Vac versus CY + Vac and PTX + Vac; ** P < 0.001 for CY + PTX + Vac combination versus CY + Vac, PTX + Vac, and combination + mock vaccine. Vac = vaccine. Data not shown: Mock: overlays Vac, CY + Mock: Overlays PTX + Mock, and CY + PTX + Mock: overlays CY + Vac.
Since CY and PTX work through different mechanisms, we hypothesized that the combination of PTX + CY given one day prior to the GM-CSF-secreting vaccine would lead to better survival than either drug given alone. To test this hypothesis, we vaccinated NT tumor-bearing neu-N mice with 3T3 cells transfected with the neu and GM-CSF genes (3T3neuGM vaccine) or 3T3GM mock vaccine in combination with ip injections of the active doses of PTX (20mg/kg), CY (100mg/kg), or PTX + CY given one day prior to vaccination. [13; 15] Mice were monitored twice a week for tumor development and changes in tumor size. The combination of PTX+CY administered one day prior to 3T3neuGM vaccination reduced the rate of tumor growth compared to groups that received vaccine with either PTX or CY alone. In addition, between 50–70% of mice receiving the combination of PTX+CY remained tumor free up to 31 days post tumor implantation before progression was observed (Figure 1B). These data demonstrate that the combination of CY+PTX+3T3neuGM vaccine is more potent at treating tumor-bearing neu-N mice than either chemotherapeutic agent alone, thereby supporting our hypothesis that CY and PTX work by different mechanisms.
PTX treatment of DC precursors prior to LPS maturation enhances maturation marker expression on DC
We previously reported that PTX must be given at immune modulating doses 1 day prior to whole-cell GM-CSF-secreting vaccination to adequately enhance the induction of T cell-mediated anti-tumor immunity. [15] These in vivo findings and the data presented in Figure 2 suggest that PTX affects DC differentiation at an early time point rather than at the final maturation stage that likely occurs about 5–6 days after DC exposure to GM-CSF. To examine this possibility, bone marrow derived cells were cultured with 10nM PTX and GM-CSF for 6 days prior to maturation with LPS. DCs cultured with the combination of PTX+GM-CSF from day 0 of culture and not matured showed slight increases in MHCII and IL-12 expression. (Figure 2, 1st Column Set) Upon maturation with LPS on day 6, DCs pre-cultured with PTX+GM-CSF showed a significant increase in expression of CD86, CD40, MHCII, and IL-12 compared to DCs cultured with GM-CSF alone and matured with LPS (Figure 2, 2nd Column Set). DCs cultured in PTX followed by maturation with various concentrations of PTX lead to an increase in MHC II and CD86 expression, but not to the same degree as observed when DCs cultured in PTX are then matured with LPS (Figure 2, Column Set 3 through 5). In follow up experiments, bone marrow derived DCs were also exposed to PTX beginning at different time points (day 1, day 3 and day 5) following the initiation of an in vitro culture to determine whether PTX can enhance DC differentiation at different stages of DC differentiation. Enhanced maturation was not observed when DCs were exposed to PTX at these later time points (data not shown). PTX was used as a maturation signal instead of LPS, but did not act as a maturation signal using increased MHC II, CD40, and CD86 expression as readouts (data not shown), which concurs with our earlier in vivo finding that PTX must be given early in the vaccination cycle. Together, these findings suggest that PTX affects DCs at an early developmental stage and may have a synergistic effect with GM-CSF on DC progenitor cells since PTX alone does not induce the degree of differentiation seen with GM-CSF alone.
Figure 2. PTX affects DC phenotype at an early differentiation stage.
Maturation marker expression levels were analyzed by flow cytometry in DCs cultured for 6 days in either GM-CSF (dark columns) or 10 nM PTX + GM-CSF (white columns) w/o maturation with LPS (None), DCs cultured with PTX + GM-CSF and matured with LPS (LPS), and DCs cultured with PTX + GM-CSF and matured with various concentrations of PTX (column sets 3–5) At least 10,000 DCs were analyzed for expression of CD86, CD40, MHC Class II, and IL-12. Data are shown as the mean plus standard deviation and are representative of 3 individual experiments. P values from Student’s t-Test compare DCs cultured with GM and matured with LPS and DCs cultured in GM + PTX and matured with LPS.
PTX-treated DC induce tumor antigen-specific T cells with enhanced lytic ability
The experiments described above demonstrate that PTX can enhance the early maturation of DCs in vitro. In addition, we have previously demonstrated that 30% of neu-N mice treated with a sub-clinical dose of CY and 3T3neuGM vaccine reject neu-expressing (NT) tumors. [15] In CY+vaccine treated mice, rejection of pre-established tumors is associated with the induction of a high avidity T cell response to the immunodominant RNEU(420–429) peptide [13]. In contrast, high avidity T cells are not found in neu-N mice with progressing tumors. [24] Therefore, we assessed whether this enhanced maturation of DCs in the presence of PTX would also enhance DC activation of naïve high avidity RNEU(420–429)-specific T cells. Bone marrow derived DCs from FVB/N mice cultured in PTX+GMCSF or GM-CSF alone and matured with LPS, were pulsed with RNEU(420–429) peptide and cultured with naïve high avidity RNEU(420–429)-specific CD8+ transgenic T cells (C100 T cells). C100 T cells stimulated for 5 days were then tested for their ability to lyse neu-expressing tumors in a 4-hr 51chromium release assay. As shown in figure 3A, C100 T cells, stimulated with in vitro PTX-cultured DCs, demonstrated a 2-fold enhanced ability to kill neu-expressing tumors when compared with DCs cultured in GM-CSF alone, suggesting that the enhanced expression of activation markers by PTX-cultured DCs results in enhanced activation of CD8+ T cell responses. As a more physiologic approach, we also evaluated whether in vivo-generated DCs naturally activated by either the GM-CSF vaccine alone or in combination with ip. PTX can also activate naïve T cells isolated from C100 transgenic mice. To address this, CD11c+ DCs were purified from vaccine draining lymph nodes of mice vaccinated six days earlier with the 3T3neuGM alone or the 3T3neuGM vaccine given in sequence with PTX. DCs were then incubated for 5 days with naïve C100 T cells. Our data demonstrate that in vivo PTX+vaccine-generated DC can generate RNEU(420–429)-specific T cells with enhanced lytic compared to DC from mice treated with vaccine alone (Figure 3B). In addition, this enhanced ability of DCs to prime CD8+ T cell responses suggest that PTX stimulation through TLR4 may be enhancing the ability of DC to cross present antigen.
Figure 3. PTX matures DCs with enhanced ability to induce high avidity CD8+ T cells with higher lytic activity.
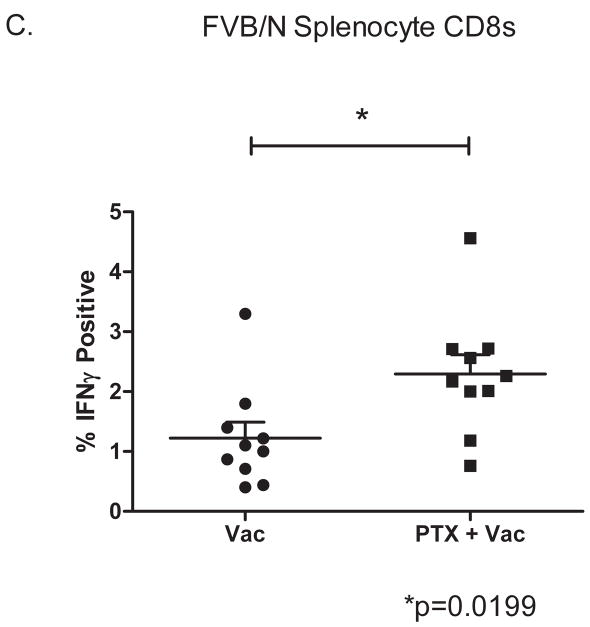
A. FVB/N-derived bone marrow DCs were cultured in 20ng/ml GM-CSF with or without 10nM PTX and matured with LPS as described in the Methods. At maturation, DCs were pulsed with 20ug of RNEU(420–429) and cultured with Clone100 transgenic T cells for five days. C100 T cells were then used in a 4hr chromium release assay of NT2.5 tumor cells. Data shown is representative of 3 total experiments, and standard deviations for each point are less than 10% of the mean. P values from a Student’s t-Test are less than .02 for the points indicated. B. DCs generated in vivo with PTX enhance CD8+ T cell lysis. Tumor challenged neu-N mice were vaccinated with or without PTX as described in the Methods. Seven days post-vaccination, DCs were isolated from vaccine draining nodes, pulsed with 10ug of RNEU(420–429) (Upper panels) or used directly (Lower panels) to stimulate naïve C100 T cells for 5 days prior to Cr-51 release assay. Targets were peptide-pulsed T-2Dq (Left panels) or NT2.5 tumor cells (Right panels). T-2Dq cells pulsed with NP(118–126) were used as a specificity control. In experiments using T-2Dq as target cells, groups were tested by ANOVA and Kruskal-Wallace p < .02. In the experiment using freshly isolated DCs and T-2Dq targets, individual points from PTX + vaccine versus vaccine alone were compared by Student’s t-Test p<.002. For experiments using NT2.5 tumor cells as target, individual dilution points were compared by Student’s t-Test. The p values are shown on the graph. Each experiment was repeated 3 times with similar results. C. PTX treatment increases the frequency of antigen-specific CD8+ T cells in vivo. CD8+ T cells were isolated from FVB/N mice 9 days after vaccination with 3T3neuGM or PTX+3T3GM and tested for RNEU(420–429) reactivity by ICS as described in the Methods. Percent IFN-γ cells are shown. Data is representative of 2 experiments.
The above studies demonstrate that in vitro and in vivo matured DC when exposed to PTX can enhance the activation of a population of clonal T cells. However, a more biologically relevant question is whether the in vivo matured DC can also enhance naturally occurring endogenous T cell responses. To address this question, endogenous CD8+ T cells isolated from FVB/N mice treated with the 3T3neuGM vaccine alone or PTX+vaccine were tested for functional reactivity to RNEU(420–429) peptide nine days after vaccination. Isolated and purified CD8+ T cells taken from the spleen of each mouse were incubated for 5 hours with T-2Dq cells pulsed with RNEU(420–429) peptide and assessed for the number of RNEU(420–429)-specific CD8+ T cells expressing IFN-γ. As expected, a greater percentage of IFN-γ-secreting RNEU-specific CD8+ T cells were indeed isolated from mice treated with the combination of PTX+vaccine when compared with mice treated with vaccine alone (Figure 3C). These data clearly demonstrate that PTX treatment of DCs enhances the in vivo induction of endogenous RNEU(420–429)- specific CD8+ T cell responses.
TLR4 blockade prior to PTX exposure abrogates DC maturation in vitro
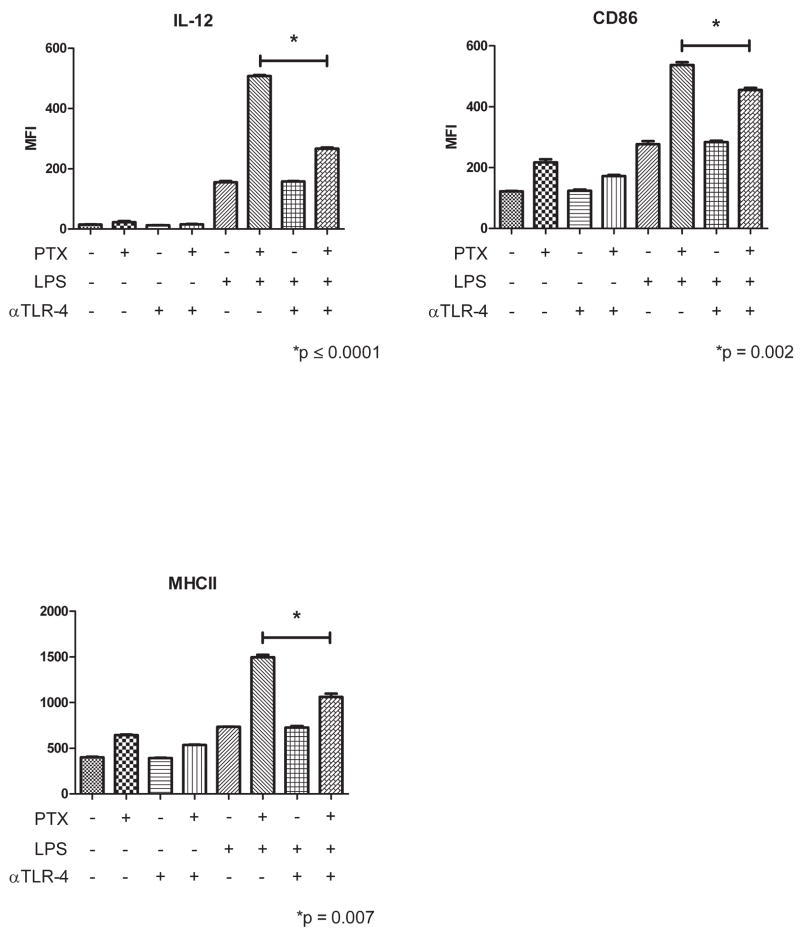
PTX has been shown to mimic LPS in Natural Killer (NK) cells and macrophages by signaling through TLR4. [19; 20] It is therefore probable that PTX acts through TLR4 in DC albeit at an earlier developmental stage as suggested by the data above. To investigate this possibility, bone marrow derived DCs were treated with either GM-CSF alone, GM-CSF+PTX, or GM-CSF+PTX and the anti-TLR4 blocking antibody, MTS510. The TLR4 blocking antibody was added to the culture for the first 6 days of in vitro exposure to PTX to insure that the effects observed were on the initial PTX binding to DC. DCs were LPS matured on day 6 and tested for the expression of DC activation markers. As shown in Figure 4, the enhanced maturation by PTX was decreased by 50% when DCs were exposed to TLR4 blockade. In addition, the surface phenotype of DCs cultured in the presence of the anti-TLR4 blocking antibody mimicked the phenotype of DCs cultured in the absence of PTX. These data further support our findings demonstrating that PTX acts on DC maturation at an early time point and by signaling through the TLR-4 receptor.
Figure 4. TLR4 blockade abrogates the effect of PTX on DC development.
DCs were cultured as described in the Methods with or without the addition of 10μg/ml anti-TLR4 until maturation with LPS on day 6. The p values shown compare PTX-treated, LPS matured DC’s vs PTX-treated, LPS matured DC’s incubated in the presence of TLR4 blocking antibody by paired Students t-Test. Data was pre-analyzed by ANOVA and Kruskal-Wallace. All eight columns and the last four columns were compared. For IL-12, CD86, and MHCII, overall ANOVA and Kruskal-Wallace scores were p<.0001 and <.002, respectively. For the last four columns the scores were ANOVA p<.0001 and Kruskal-Wallace p<.02. The experiment shown is representative of 3 repeated experiments.
Discussion
To our knowledge, this is the first report demonstrating that PTX can enhance antigen-specific CD8+ T cell responses through enhanced maturation of DC precursors that have been recruited to a vaccine site. The data reported here reveal two findings that elucidate an important mechanism by which PTX can enhance DC maturation and function. First, we show that PTX, acting through the TLR4 signaling pathway on DC, can induce more rapid and effective differentiation and function of DC. Unlike LPS, which provides a late and final maturation signal to DC through the TLR-4 signaling pathway, PTX acts on the earliest DC precursors. Second, PTX can enhance DC surface expression of co-stimulatory molecules and the secretion of the T cell activating cytokine, IL-12.
Chemotherapeutic agents, like PTX, have long been a first line, cytotoxic, treatment for cancer. [26] However, increasing evidence suggests that these agents can have both positive and negative consequences on the immune system. Agents like 5′-Aza-2′-deoxycytidine can induce antigen expression in tumor cells through an epigenetic demethylation process, thereby increasing the immune response to that antigen. [27] CY, an alkylating agent, is cytotoxic to all T cells at standard doses of antitumor therapy. However, at sub-clinical doses, it can selectively eliminate Tregs, allowing tumor-specific T cells to become activated. [13; 28] Similarly, clinical doses of PTX given in conjunction with immunotherapeutic vaccines are cytotoxic to the T cells that are supposed to be activated by the vaccine. Yet, a few studies have shown that PTX can also have immune modulating effects when given at sub-clinical doses with vaccination. [15; 29; 30] Thus, characterizing the action of subclinical doses of chemotherapeutic agents on the immune system will provide new insights into how to integrate chemotherapies with immunotherapies, thereby enhancing the effects of both treatment modalities.
Our previously reported data has shown that PTX, at sub-clinical doses, enhances the potency of GM-CSF-based whole cell vaccines. Specifically, PTX given one day prior to vaccination, at the time of initial T cell priming, induced greater numbers of IFN gamma-secreting T cells that were associated with tumor growth retardation. Furthermore, altering the sequence of PTX and vaccine failed to improve and often worsened the antitumor immune effects of the combination, suggesting that enhancing CD8+ T cell activity through the improved activation of DC was one possible mechanism that PTX was improving tumor therapy. [15] In our previous work and the work presented here, PTX given with mock vaccine (3T3GM) results in a slight delay in tumor growth suggesting that PTX may have a small direct cytotoxic effect on the tumor. However, we have focused this work on PTX’s role as a DC function enhancer because it results in a significant increase in CD8+ T cell activity and improve antitumor activity in vivo. PTX has also been reported to enhance the function of APCs including macrophages and DC in multiple ways. PTX can increase the rate of tumor apoptosis, which is thought to lead to DC activation through release of heat shock protein associated complexes and the Damage Associated Membrane Protein (DAMP) pathway. [31; 32; 33; 34; 35; 36] Release of ATP from tumor cells can also activate DCs through the purigenic P2RX7 receptors. [37] Alternatively, PTX has been shown to activate macrophages and DCs through the TLR-4 pathway in a similar way to what has been observed for LPS. Specfically, PTX induced maturation and secretion of TNFα, IP-10 and IL-1β in murine macrophages. [38; 39] Recently, doses of PTX that cause minimal tumor death have been shown to increase the activity of GTPases in DCs in vitro. In addition, low-dose PTX, has been shown to increase lymphocyte IFN-γ secretion in tumor draining lymph nodes in conjunction with intratumoral DC vaccination. [40; 41] One other study has shown that PTX can enhance DC activation in vitro (antigen presentation to CD8+ T cells, IL-12 secretion, and costimulatory marker upregulation). [42] Finally, PTX, like LPS, has been reported to provide a late maturing signal to differentiated APCs, resulting in the final upregulation of surface co-stimulatory molecules required for effective T cell activation. [43]
We have extended these previously reported findings to show that PTX given with a GM-CSF-secreting whole cell vaccine can enhance DC antigen presentation, IL-12 secretion, and CD8+ T cell activation in vivo, demonstrating the utility of PTX as adjuvant for immunotherapy. Our data also demonstrate that PTX is acting through the TLR-4 pathway. However, in contrast to what has been previously reported for macrophages, we have found that PTX does not have the ability to provide the final maturation signal to DCs in the same way as LPS.[44] Instead, PTX appears to act at the earliest stages of DC differentiation. Blocking TLR-4 does inhibit this PTX function by 50% when DCs are treated in the presence of both a TLR-4 blocking antibody and PTX. It is not surprising that TLR-4 blocking does not completely inhibit DC maturation since PTX still requires the presence of GM-CSF, the most important DC growth and differentiation factor, to achieve enhanced maturation. Our finding that PTX is acting through TLR-4 on DC is in agreement with previously reported data demonstrating that PTX can act through MyD88 and the TLR-4 pathway on mouse monocytes. [45] Our in vivo experiments further suggest that PTX is enhancing the immune response through a synergistic interaction with our GM-CSF vaccine because low-dose PTX given alone cannot slow tumor growth. Therefore, it is unlikely that immune modulating doses of PTX enhance CD8+ T cell responses through enhanced tumor destruction by itself, even if apoptotic tumor cells are taken up more efficiently by DC. However, PTX given in conjunction with our mock vaccine did slightly retard tumor growth, indicating that some DC activation may have occurred through a small amount of tumor destruction when GM-CSF is also present to attract DC, however, the immune response induced by the mock vaccine is not long-lasting and does not result in long-term tumor suppression.
We also previously reported that PTX enhanced the induction of IFN-γ-secreting CD4+ T cells in response to neu-expressing mammary tumors in vivo. [15] Findings from these studies can now explain this observation. Our studies demonstrate that PTX given in sequence with a GM-CSF secreting tumor vaccine induces activated DC to secrete IL-12 in the vaccine draining lymph nodes. PTX has been shown to increase IL-12 secretion by DCs in the presence of dying tumor cells, which in turn act as adjuvants for DCs. [36] In addition, other studies have shown that low dose PTX given systemically prior to intratumoral DC vaccinations leads to a generalized increase in IFN-γ secretion and T cell infiltration. [41] It is also likely that the PTX induced increased expression of MHC class II and co-stimulatory molecules by DCs enhances the activation of tumor-specific IFN-γ-expressing CD4+ T cells. Furthermore, increased CD4+ T cell activation has been shown to lead to reciprocal “super activation” of DCs and increased CD8+ T cell activation through the process of licensing [46; 47; 48] Finally, increased IL-12 production leads to increased IFN-γ production and a Th1 type immune response, which is advantageous in anti-tumor responses. Thus, our GM-CSF-secreting whole cell tumor vaccine likely provides multiple avenues for PTX enhanced DC activation. Specifically, our whole cell vaccine is irradiated prior to subcutaneous administration, which enhances the degradation of the vaccine cells allowing them to act as an adjuvant. At the same time, GM-CSF secretion leads to DC maturation. With TLR-4 activation as a consequence of systemic low-dose PTX, and the neu antigen being presented in the draining lymph nodes, DCs are “super-activated” at the site of vaccination allowing for superior priming of CD4+ and CD8+ T cells.
The enhanced CD8+ T cell activity observed when PTX is given with a GM-CSF-secreting tumor vaccine may occur for several reasons. First, PTX induces increased surface expression of co-stimulatory molecules on DCs, which in turn may lead to enhanced co-stimulation of both CD4+ and CD8+ T cell populations during priming. It is also possible that PTX enhances DC expression of peptide:MHC complexes on its surface leading to enhanced CD4+ and CD8+ T cell activation. Finally, it is likely that PTX activation of DCs early during their maturation process that is occurring in the presence of tumor antigens, enhances antigen processing and presentation through cross-priming, ultimately leading to enhanced CD8+ T cell responses. We have demonstrated that PTX does enhance co-stimulatory molecule expression by DCs. Others have shown that PTX upregulates co-stimulatory molecules as well as the antigen processing machinery of DCs including the immunoproteosome subunits LMP 7 and 10. In one study, 5nM PTX increased LMP 7 and 10 expression by at least 30% which could affect the activation of some T cell populations. [42] This correlates well with our data using PTX ranges of 1–10nM. Given that the transgenic T cells used in our studies are high avidity, it is more likely that the signaling threshold through the TCR was at a maximum and that enhanced co-stimulation led to enhanced T cell activation. [49] However, an increase in both DC antigen presentation and co-stimulatory molecule expression is likely required to explain the enhanced expansion of CD8+ T cells also observed with PTX treatment.
Our data provide additional support for combining PTX with vaccines for testing in patients with cancer. In addition, understanding the mechanism by which PTX synergizes with the vaccine opens up new avenues for combining chemotherapeutic agents with multiple mechanisms of immune enhancing activity to be given together with vaccines. As an example, our data also demonstrate that the combination of CY and PTX can synergize to enhance the efficacy of a GM-CSF-secreting whole cell vaccine approach compared to each agent given alone with the vaccine. We previously demonstrated that CY can preferentially deplete Tregs and in doing so, enhance the activation and expansion of high avidity neu-specific CD8+ T cells. [13] With the addition of PTX to CY and the vaccine, we now demonstrate that tumor growth is retarded and that overall survival is increased when compared to vaccine given with either agent alone. Thus, it should be possible to target different mechanisms of immune suppression by combining low doses of approved chemotherapeutic agents with a vaccine thereby enhancing the antitumor responses of different arms of the immune system at the same time.
PTX has been tested clinically in multiple fashions including combinations with tumor-specific monoclonal antibodies, radiotherapy, DC adoptive transfer, and as a single agent. In each case the combination and has been proven to enhance the antitumor effects of the tested form of immunotherapy or demonstrated increased immune activation. These studies have focused mostly on standard cytotoxic doses of PTX and on its direct tumoricidal killing effects in combination with tumor-specific monoclonal antibodies. [50; 51; 52; 53] Tumor-specific antibodies have been shown to inhibit tumor growth through direct tumor cytotoxicity as a result of targeting an important growth factor signaling pathway. [54] However, increasing data suggests that monoclonal antibodies also induce antitumor effects through a number of immune based mechanisms including ADCC and enhanced DC cross-priming by FC-receptor activation of DCs. [55; 56; 57; 58; 59]} Our study provides new evidence for incorporating low doses of PTX for the direct enhancement of DC priming. However, additional studies are warranted to determine whether immune modulating doses of PTX can synergize with other types of immunotherapies even those that do not target DC.
Acknowledgments
The authors wish to thank Dr. Elizabeth Sugar for her help with the statistical analysis.
This work was funded by a National Cancer Institute Spore grant in gastrointestinal cancers (P50CA62924), Spore grant in breast cancer (), the National Cancer Institute NCDDG (U19CA113341), and an National Cancer Institute RO1 (CA122081). We are grateful for the support given by the Skip Viragh Pancreatic Center for Clinical Research and Patient Care, the Sol Goldman Pancreatic Cancer Center, and the Dana and Albert Broccoli Foundation.
Footnotes
Conflict of Interest Statement. Under a licensing agreement between Cell Genesys and the Johns Hopkins University, Johns Hopkins University is entitled to milestone payments and royalties on sales of the human vaccine product that is similar to the mouse vaccine described in this report. The terms of these arrangements are being managed by the Johns Hopkins University in accordance with its conflict of interest policies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reilly RT, Gottlieb MB, Ercolini AM, Machiels JP, Kane CE, Okoye FI, Muller WJ, Dixon KH, Jaffee EM. HER-2/neu is a tumor rejection target in tolerized HER-2/neu transgenic mice. Cancer Res. 2000;60:3569–76. [PubMed] [Google Scholar]
- 2.Reilly RT, Machiels JP, Emens LA, Ercolini AM, Okoye FI, Lei RY, Weintraub D, Jaffee EM. The collaboration of both humoral and cellular HER-2/neu-targeted immune responses is required for the complete eradication of HER-2/neu-expressing tumors. Cancer Res. 2001;61:880–3. [PubMed] [Google Scholar]
- 3.Laheru D, Lutz E, Burke J, Biedrzycki B, Solt S, Onners B, Tartakovsky I, Nemunaitis J, Le D, Sugar E, Hege K, Jaffee E. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res. 2008;14:1455–63. doi: 10.1158/1078-0432.CCR-07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emens LA, Asquith JM, Leatherman JM, Kobrin BJ, Petrik S, Laiko M, Levi J, Daphtary MM, Biedrzycki B, Wolff AC, Stearns V, Disis ML, Ye X, Piantadosi S, Fetting JH, Davidson NE, Jaffee EM. Timed sequential treatment with cyclophosphamide, doxorubicin, and an allogeneic granulocyte-macrophage colony-stimulating factor-secreting breast tumor vaccine: a chemotherapy dose-ranging factorial study of safety and immune activation. J Clin Oncol. 2009;27:5911–8. doi: 10.1200/JCO.2009.23.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrara TA, Hodge JW, Gulley JL. Combining radiation and immunotherapy for synergistic antitumor therapy. Curr Opin Mol Ther. 2009;11:37–42. [PMC free article] [PubMed] [Google Scholar]
- 6.Palucka AK, Ueno H, Fay J, Banchereau J. Dendritic cells: a critical player in cancer therapy? J Immunother. 2008;31:793–805. doi: 10.1097/CJI.0b013e31818403bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang T, Herlyn D. Combination of active specific immunotherapy or adoptive antibody or lymphocyte immunotherapy with chemotherapy in the treatment of cancer. Cancer Immunol Immunother. 2009;58:475–92. doi: 10.1007/s00262-008-0598-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baxevanis CN, Perez SA, Papamichail M. Combinatorial treatments including vaccines, chemotherapy and monoclonal antibodies for cancer therapy. Cancer Immunol Immunother. 2009;58:317–24. doi: 10.1007/s00262-008-0576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buttiglieri S, Galetto A, Forno S, De Andrea M, Matera L. Influence of drug-induced apoptotic death on processing and presentation of tumor antigens by dendritic cells. Int J Cancer. 2003;106:516–20. doi: 10.1002/ijc.11243. [DOI] [PubMed] [Google Scholar]
- 10.Galetto A, Buttiglieri S, Forno S, Moro F, Mussa A, Matera L. Drug- and cell-mediated antitumor cytotoxicities modulate cross-presentation of tumor antigens by myeloid dendritic cells. Anticancer Drugs. 2003;14:833–43. doi: 10.1097/00001813-200311000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Correale P, Cusi MG, Del Vecchio MT, Aquino A, Prete SP, Tsang KY, Micheli L, Nencini C, La Placa M, Montagnani F, Terrosi C, Caraglia M, Formica V, Giorgi G, Bonmassar E, Francini G. Dendritic cell-mediated cross-presentation of antigens derived from colon carcinoma cells exposed to a highly cytotoxic multidrug regimen with gemcitabine, oxaliplatin, 5-fluorouracil, and leucovorin, elicits a powerful human antigen-specific CTL response with antitumor activity in vitro. J Immunol. 2005;175:820–8. doi: 10.4049/jimmunol.175.2.820. [DOI] [PubMed] [Google Scholar]
- 12.Sojka DK, Donepudi M, Bluestone JA, Mokyr MB. Melphalan and other anticancer modalities up-regulate B7-1 gene expression in tumor cells. J Immunol. 2000;164:6230–6. doi: 10.4049/jimmunol.164.12.6230. [DOI] [PubMed] [Google Scholar]
- 13.Ercolini AM, Ladle BH, Manning EA, Pfannenstiel LW, Armstrong TD, Machiels JP, Bieler JG, Emens LA, Reilly RT, Jaffee EM. Recruitment of latent pools of high-avidity CD8(+) T cells to the antitumor immune response. J Exp Med. 2005;201:1591–602. doi: 10.1084/jem.20042167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorelik L, Prokhorova A, Mokyr MB. Low-dose melphalan-induced shift in the production of a Th2-type cytokine to a Th1-type cytokine in mice bearing a large MOPC-315 tumor. Cancer Immunol Immunother. 1994;39:117–26. [PubMed] [Google Scholar]
- 15.Machiels JP, Reilly RT, Emens LA, Ercolini AM, Lei RY, Weintraub D, Okoye FI, Jaffee EM. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res. 2001;61:3689–97. [PubMed] [Google Scholar]
- 16.Brode S, Raine T, Zaccone P, Cooke A. Cyclophosphamide-induced type-1 diabetes in the NOD mouse is associated with a reduction of CD4+CD25+Foxp3+ regulatory T cells. J Immunol. 2006;177:6603–12. doi: 10.4049/jimmunol.177.10.6603. [DOI] [PubMed] [Google Scholar]
- 17.Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–8. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 18.Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971;93:2325–7. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- 19.Byrd-Leifer CA, Block EF, Takeda K, Akira S, Ding A. The role of MyD88 and TLR4 in the LPS-mimetic activity of Taxol. Eur J Immunol. 2001;31:2448–57. doi: 10.1002/1521-4141(200108)31:8<2448::aid-immu2448>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 20.Chan OT, Yang LX. The immunological effects of taxanes. Cancer Immunol Immunother. 2000;49:181–5. doi: 10.1007/s002620000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci U S A. 1992;89:10578–82. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manning EA, Ullman JG, Leatherman JM, Asquith JM, Hansen TR, Armstrong TD, Hicklin DJ, Jaffee EM, Emens LA. A vascular endothelial growth factor receptor-2 inhibitor enhances antitumor immunity through an immune-based mechanism. Clin Cancer Res. 2007;13:3951–9. doi: 10.1158/1078-0432.CCR-07-0374. [DOI] [PubMed] [Google Scholar]
- 23.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ercolini AM, Machiels JP, Chen YC, Slansky JE, Giedlen M, Reilly RT, Jaffee EM. Identification and characterization of the immunodominant rat HER-2/neu MHC class I epitope presented by spontaneous mammary tumors from HER-2/neu-transgenic mice. J Immunol. 2003;170:4273–80. doi: 10.4049/jimmunol.170.8.4273. [DOI] [PubMed] [Google Scholar]
- 25.Schulz M, Aichele P, Schneider R, Hansen TH, Zinkernagel RM, Hengartner H. Major histocompatibility complex binding and T cell recognition of a viral nonapeptide containing a minimal tetrapeptide. Eur J Immunol. 1991;21:1181–5. doi: 10.1002/eji.1830210513. [DOI] [PubMed] [Google Scholar]
- 26.Emens LA, Machiels JP, Reilly RT, Jaffee EM. Chemotherapy: friend or foe to cancer vaccines? Curr Opin Mol Ther. 2001;3:77–84. [PubMed] [Google Scholar]
- 27.Coral S, Sigalotti L, Altomonte M, Engelsberg A, Colizzi F, Cattarossi I, Maraskovsky E, Jager E, Seliger B, Maio M. 5-aza-2′-deoxycytidine-induced expression of functional cancer testis antigens in human renal cell carcinoma: immunotherapeutic implications. Clin Cancer Res. 2002;8:2690–5. [PubMed] [Google Scholar]
- 28.Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, Chauffert B, Solary E, Bonnotte B, Martin F. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–44. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 29.Choi GS, Lee MH, Kim SK, Kim CS, Lee HS, Im MW, Kil HY, Seong DH, Lee JR, Kim WC, Lee MG, Song SU. Combined treatment of an intratumoral injection of dendritic cells and systemic chemotherapy (Paclitaxel) for murine fibrosarcoma. Yonsei Med J. 2005;46:835–42. doi: 10.3349/ymj.2005.46.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eralp Y, Wang X, Wang JP, Maughan MF, Polo JM, Lachman LB. Doxorubicin and paclitaxel enhance the antitumor efficacy of vaccines directed against HER 2/neu in a murine mammary carcinoma model. Breast Cancer Res. 2004;6:R275–83. doi: 10.1186/bcr787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green DR, Ferguson T, Zitvogel L, Kroemer G. Immunogenic and tolerogenic cell death. Nat Rev Immunol. 2009;9:353–63. doi: 10.1038/nri2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haynes NM, van der Most RG, Lake RA, Smyth MJ. Immunogenic anti-cancer chemotherapy as an emerging concept. Curr Opin Immunol. 2008;20:545–57. doi: 10.1016/j.coi.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 33.Lake RA, Robinson BW. Immunotherapy and chemotherapy--a practical partnership. Nat Rev Cancer. 2005;5:397–405. doi: 10.1038/nrc1613. [DOI] [PubMed] [Google Scholar]
- 34.Nowak AK, Lake RA, Robinson BW. Combined chemoimmunotherapy of solid tumours: improving vaccines? Adv Drug Deliv Rev. 2006;58:975–90. doi: 10.1016/j.addr.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Tesniere A, Apetoh L, Ghiringhelli F, Joza N, Panaretakis T, Kepp O, Schlemmer F, Zitvogel L, Kroemer G. Immunogenic cancer cell death: a key-lock paradigm. Curr Opin Immunol. 2008;20:504–11. doi: 10.1016/j.coi.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 36.Tsuda N, Chang DZ, Mine T, Efferson C, Garcia-Sastre A, Wang X, Ferrone S, Ioannides CG. Taxol increases the amount and T cell activating ability of self-immune stimulatory multimolecular complexes found in ovarian cancer cells. Cancer Res. 2007;67:8378–87. doi: 10.1158/0008-5472.CAN-07-0327. [DOI] [PubMed] [Google Scholar]
- 37.Aymeric L, Apetoh L, Ghiringhelli F, Tesniere A, Martins I, Kroemer G, Smyth MJ, Zitvogel L. Tumor cell death and ATP release prime dendritic cells and efficient anticancer immunity. Cancer Res. 70:855–8. doi: 10.1158/0008-5472.CAN-09-3566. [DOI] [PubMed] [Google Scholar]
- 38.Ding AH, Porteu F, Sanchez E, Nathan CF. Shared actions of endotoxin and taxol on TNF receptors and TNF release. Science. 1990;248:370–2. doi: 10.1126/science.1970196. [DOI] [PubMed] [Google Scholar]
- 39.Manthey CL, Brandes ME, Perera PY, Vogel SN. Taxol increases steady-state levels of lipopolysaccharide-inducible genes and protein-tyrosine phosphorylation in murine macrophages. J Immunol. 1992;149:2459–65. [PubMed] [Google Scholar]
- 40.Shurin GV, Tourkova IL, Shurin MR. Low-dose chemotherapeutic agents regulate small Rho GTPase activity in dendritic cells. J Immunother. 2008;31:491–9. doi: 10.1097/CJI.0b013e318176fae4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhong H, Han B, Tourkova IL, Lokshin A, Rosenbloom A, Shurin MR, Shurin GV. Low-dose paclitaxel prior to intratumoral dendritic cell vaccine modulates intratumoral cytokine network and lung cancer growth. Clin Cancer Res. 2007;13:5455–62. doi: 10.1158/1078-0432.CCR-07-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shurin GV, Tourkova IL, Kaneno R, Shurin MR. Chemotherapeutic agents in noncytotoxic concentrations increase antigen presentation by dendritic cells via an IL-12-dependent mechanism. J Immunol. 2009;183:137–44. doi: 10.4049/jimmunol.0900734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaneno R, Shurin GV, Tourkova IL, Shurin MR. Chemomodulation of human dendritic cell function by antineoplastic agents in low noncytotoxic concentrations. J Transl Med. 2009;7:58. doi: 10.1186/1479-5876-7-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hertz CJ, Kiertscher SM, Godowski PJ, Bouis DA, Norgard MV, Roth MD, Modlin RL. Microbial lipopeptides stimulate dendritic cell maturation via Toll-like receptor 2. J Immunol. 2001;166:2444–50. doi: 10.4049/jimmunol.166.4.2444. [DOI] [PubMed] [Google Scholar]
- 45.Kawasaki K, Akashi S, Shimazu R, Yoshida T, Miyake K, Nishijima M. Mouse toll-like receptor 4.MD-2 complex mediates lipopolysaccharide-mimetic signal transduction by Taxol. J Biol Chem. 2000;275:2251–4. doi: 10.1074/jbc.275.4.2251. [DOI] [PubMed] [Google Scholar]
- 46.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–80. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 47.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–8. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 48.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–3. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 49.Zang X, Allison JP. The B7 family and cancer therapy: costimulation and coinhibition. Clin Cancer Res. 2007;13:5271–9. doi: 10.1158/1078-0432.CCR-07-1030. [DOI] [PubMed] [Google Scholar]
- 50.Braly P, Nicodemus CF, Chu C, Collins Y, Edwards R, Gordon A, McGuire W, Schoonmaker C, Whiteside T, Smith LM, Method M. The Immune adjuvant properties of front-line carboplatin-paclitaxel: a randomized phase 2 study of alternative schedules of intravenous oregovomab chemoimmunotherapy in advanced ovarian cancer. J Immunother. 2009;32:54–65. doi: 10.1097/CJI.0b013e31818b3dad. [DOI] [PubMed] [Google Scholar]
- 51.Christodoulou C, Klouvas G, Pateli A, Mellou S, Sgouros J, Skarlos DV. Prolonged administration of weekly paclitaxel and trastuzumab in patients with advanced breast cancer. Anticancer Res. 2003;23:737–44. [PubMed] [Google Scholar]
- 52.Demaria S, Volm MD, Shapiro RL, Yee HT, Oratz R, Formenti SC, Muggia F, Symmans WF. Development of tumor-infiltrating lymphocytes in breast cancer after neoadjuvant paclitaxel chemotherapy. Clin Cancer Res. 2001;7:3025–30. [PubMed] [Google Scholar]
- 53.Kimura H, Iizasa T, Ishikawa A, Shingyouji M, Yoshino M, Kimura M, Inada Y, Matsubayashi K. Prospective phase II study of post-surgical adjuvant chemo-immunotherapy using autologous dendritic cells and activated killer cells from tissue culture of tumor-draining lymph nodes in primary lung cancer patients. Anticancer Res. 2008;28:1229–38. [PubMed] [Google Scholar]
- 54.Baselga J, Albanell J. Mechanism of action of anti-HER2 monoclonal antibodies. Ann Oncol. 2001;12(Suppl 1):S35–41. doi: 10.1093/annonc/12.suppl_1.s35. [DOI] [PubMed] [Google Scholar]
- 55.Schneider-Merck T, Lammerts van Bueren JJ, Berger S, Rossen K, van Berkel PH, Derer S, Beyer T, Lohse S, Bleeker WK, Peipp M, Parren PW, van de Winkel JG, Valerius T, Dechant M. Human IgG2 antibodies against epidermal growth factor receptor effectively trigger antibody-dependent cellular cytotoxicity but, in contrast to IgG1, only by cells of myeloid lineage. J Immunol. 184:512–20. doi: 10.4049/jimmunol.0900847. [DOI] [PubMed] [Google Scholar]
- 56.Taylor RP, Lindorfer MA. Immunotherapeutic mechanisms of anti-CD20 monoclonal antibodies. Curr Opin Immunol. 2008;20:444–9. doi: 10.1016/j.coi.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim PS, Armstrong TD, Song H, Wolpoe ME, Weiss V, Manning EA, Huang LQ, Murata S, Sgouros G, Emens LA, Reilly RT, Jaffee EM. Antibody association with HER-2/neu-targeted vaccine enhances CD8 T cell responses in mice through Fc-mediated activation of DCs. J Clin Invest. 2008;118:1700–11. doi: 10.1172/JCI34333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wolpoe ME, Lutz ER, Ercolini AM, Murata S, Ivie SE, Garrett ES, Emens LA, Jaffee EM, Reilly RT. HER-2/neu-specific monoclonal antibodies collaborate with HER-2/neu-targeted granulocyte macrophage colony-stimulating factor secreting whole cell vaccination to augment CD8+ T cell effector function and tumor-free survival in Her-2/neu-transgenic mice. J Immunol. 2003;171:2161–9. doi: 10.4049/jimmunol.171.4.2161. [DOI] [PubMed] [Google Scholar]
- 59.Dalle S, Thieblemont C, Thomas L, Dumontet C. Monoclonal antibodies in clinical oncology. Anticancer Agents Med Chem. 2008;8:523–32. doi: 10.2174/187152008784533071. [DOI] [PubMed] [Google Scholar]