Abstract
The NG2 proteoglycan is a type 1-transmembrane protein expressed by a range of cell types within and outside the mammalian nervous system. NG2-expressing (NG2) cells are found in grey and white matter tracts of the developing and adult CNS and have previously been assumed to represent oligodendrocyte precursor cells: new work using transgenic mice has shown that NG2 cells generate oligodendrocytes, protoplasmic astrocytes and in some instances neurons in vivo. NG2 cells express GABAA receptors and the AMPA subtype of glutamate receptors. They make intimate contact to neurons prior to myelinating axons and also form electron-dense synaptic specialisations with axons in the cerebellum, cortex and hippocampus and with non-myelinated axons in the corpus callosum. These synaptic NG2 cells respond to neuronal release of glutamate and GABA. This neuron-glia interaction may thus regulate the differentiation and proliferation of NG2 cells. The C-terminal PDZ-binding motif of the NG2 protein binds several PDZ proteins including Mupp1, Syntenin and the Glutamate Receptor Interacting Protein (GRIP). Since GRIP can bind subunits of the AMPA receptors expressed by NG2 cells, the interaction between GRIP and NG2 may orientate the glial AMPA receptors towards sites of neuronal glutamate release. The origin, heterogeneity and function of NG2 cells as modulators of the neuronal network are important incompletely resolved questions.
Keywords: NG2, Oligodendrocyte progenitor, Neuron-glia synapse, AMPA-receptor, GABA, Cell lineage
1. Introduction
Cells expressing the proteoglycan NG2 make up 5–10% of all glia in the developing and adult CNS. They are evenly distributed in white and grey matter and some cells proliferate even in the adult, implying a continual turnover of this cell population. NG2 protein expression is down-regulated upon maturation of the cells. Expression of NG2 has in the past been used as a marker for oligodendrocyte precursor cells (OPC; see Nishiyama et al., 2009) and recent evidence discussed below has demonstrated that indeed NG2 cells give rise to oligodendrocytes, but also to subpopulations of astrocytes during normal development. The discovery at the turn of the last century of the unusual synaptic association between NG2 cells and neurones in multiple regions of the developing and adult CNS (Bergles et al., 2000; Jabs et al., 2005) has spurred widespread interest in this cell population and suggested that in addition to acting as a plastic progenitor pool for more differentiated cells, NG2 glia may form a unique glial network in continual parlance with neurones. In addition to the expression on immature myelinating glia in the CNS, NG2 is expressed in the PNS by immature Schwann cells.
In this review we will focus on the properties, progeny and origin of NG2 cells but first summarise current knowledge regarding the structure and function of the NG2 protein, the defining feature of this class of cells.
2. Structure of NG2 and its partner molecules
The NG2 protein was originally defined by antibodies directed against surface proteins on a rat cell line with glial and neuronal properties (Stallcup, 1981). Independently NG2 homologues were discovered in human and mouse (Harper et al., 1984; Niehaus et al., 1999; Pluschke et al., 1996; Schneider et al., 2001; Stegmuller et al., 2002). The mammalian protein of 330 kDa (also termed CSPG-4) is encoded by a single gene with multiple exons coding for 2327 amino acids. To date no alternatively spliced variants have been described. The amino terminus exhibits two Laminin G/Neurexin/Sex Hormone Binding Globulin (LNS) domains (Fig. 1), thus placing NG2 firmly in the large family of the neurexins; cell adhesion molecules dictating synaptic specificity in neurones and exhibiting a large degree of alternative splice forms (Missler and Sudhof, 1998). The large extracellular domain of NG2 includes sites near the single transmembrane domain which are readily cleaved by a variety of proteases in vitro and in vivo (Nishiyama et al., 2009). This can result in the deposition of the ectodomain in the extracellular matrix, thus making the identification of NG2-expressing cells by antibodies, especially in lesions rich in proteases, particularly difficult. The biological relevance of this cleavage is unclear: one possibility is that it could be a mechanism to release NG2 from a putative receptor via regulated proteolysis. The intracellular domain is rather short (76 Aa) and includes a C-terminal type I Postsynaptic density 95/Discs Large/Zonula-occludens-1 (PDZ) domain recognition motif, as well as several threonines whose phosphorylation state regulates cell behaviour such as spreading and migration (Fang et al., 1999; Lin et al., 1996; Majumdar et al., 2003; Tillet et al., 2002). In addition, a type II PDZ domain binding motif, a Src Homology type 2 (SH2) domain binding motif and a WW-domain binding motif all underline interactions of NG2 with intracellular signalling and structural proteins. The NG2 protein is a part-time proteoglycan; chondroitin sulfate gylcosaminoglycan (GAG) chains are linked to the extracellular domain (Nishiyama et al., 2009). The amount of GAG chains carried by the core protein varies with cell type and developmental stage (Schneider et al., 2001).
Figure 1. Schematic diagram of the NG2 protein.
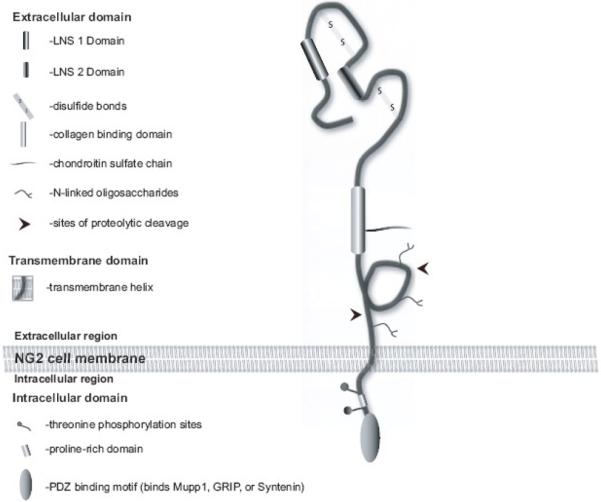
modified from (Stallcup and Huang, 2008)
Several partner molecules have been identified for the NG2 protein. These include ß1 integrins in melanoma and astrocytoma and the receptor for PDGFα: these associate in cis with NG2. PDGFAA, FGF2, Collagen V and VI, MT3MMP, Plasminogen, tPA and galectin 3 have all been described as binding directly to the large NG2 ectodomain (summarised in Nishiyama et al., 2009). Interestingly, no cell adhesion molecule ligands for the LNS domains have been described to date. In the light of the interaction of NG2 cells with axons during myelination and at synapses in development and in the adult, it is likely that neuronal receptors exist. Several binding partners for the C-terminal type I PDZ domain-binding motif have been identified. These are Multi-PDZ Domain protein 1 (MUPP1), Glutamate Receptor Interacting Protein (GRIP) and Syntenin-1(Barritt et al., 2000; Chatterjee et al., 2008; Stegmuller et al., 2003). In particular, the latter two partners may be relevant for the function of NG2 cells at synapses and in wrapping axons at early stages of myelination. GRIP binds to the GluR2/3 subunits of the AMPA receptor, glutamate receptors expressed by NG2 cells which are activated by neuronally released glutamate at the neuron-glial synapse and also influence oligodendrocyte differentiation (see articles in this issue by Steinhauser et al., (Gallo et al., 1996) and also Fig. 2). Syntenin provides connections to the cell cytoskeleton, which may be relevant for migration of NG2 cells to axons prior to myelination as well as process movement of synaptic NG2 glia. Furthermore, NG2 has been shown to recruit the small GTPase cdc42 and p130cas (crk-associated substrate) in melanoma cells (Eisenmann et al., 1999), intracellular molecules regulating diverse processes in migration and cell polarisation
Figure 2. The role of the NG2 protein at the neuron-glial synapse.
The NG2 protein could play a role in clustering the glial AMPA receptors towards the site of neuronal glutamate release. Glutamate acting on NG2 cells may thus regulate proliferation and differentiation and also cause a rise in intracellular calcium.
3. NG2 in evolution
NG2-like proteins are found in non-vertebrates including C.Elegans and D. Melanogaster. Interestingly, in both these species the C-terminal PDZ binding domain is conserved, furthermore in Drosophila NG2 two LNS domains are recognisable. Two recent papers have reported that Drosophila NG2 (called Kon-tiki or Perdido) associates with Drosophila GRIP demonstrating the evolutionary conservation of this interaction (Estrada et al., 2007; Schnorrer et al., 2007). Both groups reported a role of NG2 expressed by immature muscle cells in attaching to tendons. In vertebrates NG2 expression outside the nervous system includes muscle progenitor cells and pericytes (Nishiyama et al., 2009). It remains to be seen whether Drosophila glia express NG2, similar to their mammalian counterparts.
4. Functions of NG2 cells
The study of NG2 cells in situ as well as their progeny has been facilitated by the generation of new mouse lines. The DsRed protein chromophore or the Cre recombinase with the NG2 promoter has been inserted using Bacterial Artificial Chromosome technology to generate transgenic mouse lines (Nishiyama et al., 2009). Alternatively the EYFP protein has been inserted after the start AUG of the first exon of the endogeneous NG2 gene to yield a “knock-in” mouse line (Karram et al., 2008). Use of these mice to study the generation of oligodendrocytes and astrocytes from NG2 progenitors is discussed below.
Synaptic contacts formed by NG2 cells have been described with axons of neurones in the hippocampus and cerebellum (Bergles et al., 2000; Lin et al., 2005), as well as with unmyelinated axons in the corpus callosum (Kukley et al., 2007; Ziskin et al., 2007). In addition to these synapses which appear largely on the processes of NG2 cells, the cell bodies of NG2 cells are very closely apposed to neurons in many brain regions including the hippocampus, cerebellum and cortex (Fig. 3). Intimate contact between NG2 cells and neurons has been observed earlier in an electron microscopic analysis of cortex in rats, where NG2 glia were defined as beta glial cells and were considered a fourth glial cell type (Peters, 2004). In contrast to published literature where double-labelling of brain sections was carried with polyclonal antibodies to NG2 and neuronal markers (Belachew et al., 2003; Dayer et al., 2005) we have never observed expression of neuronal markers by EYFP cells in the EYFP-NG2 knockin mouse or by DsRed+ cells in the NG2-DsRed transgenic mice (Fig. 4)
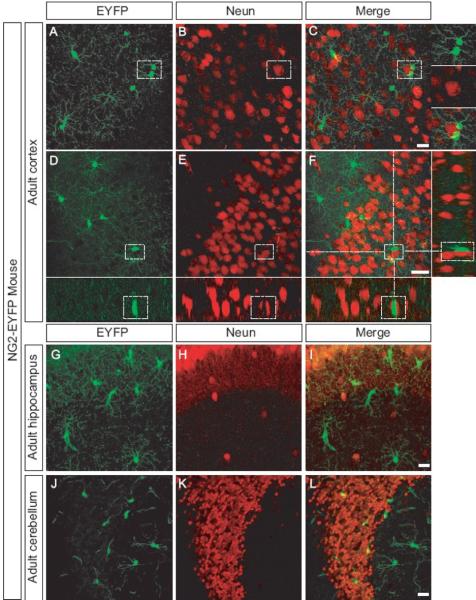
Figure 3. Intimate contact between neurons and NG2 cells in the CNS.
Confocal image scan of cortex, hippocampus, and cerebellum of adult mice expressing EYFP (A,D,G, J ) stained with an antibody that recognizes Neun (B, E, H, K). Merged images (C, F, I, L) shows no overlap, but close association between EYFP+ cell and Neun + neurons. Inserts at high magnification show EYFP+ cells close to Neun+ neurons.
Scale bars = 20 μm
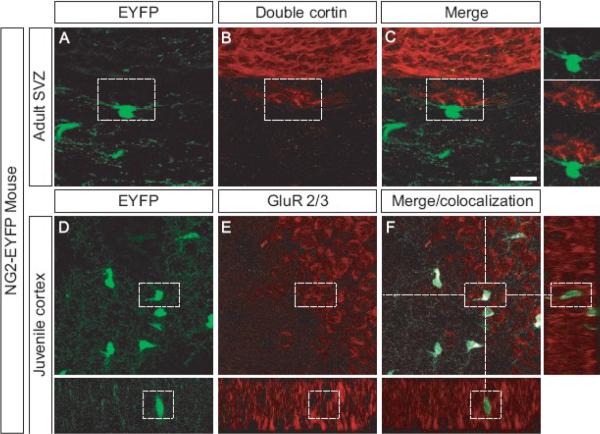
Figure 4. Intimate contact between neurons and NG2 cells in the SVZ and expression of GluR 2/3 by NG2 cells in vivo.
Confocal image scan of the SVZ of adult mice expressing EYFP (A), stained with an antibody that recognizes Doublecortin (B). Merged images (C) show no overlap but close association between EYFP+ cells and Doublecortin+ neurons. Hogh magnification inserts show EYFP+ cells very close to Doublecortin + neurons. EYFP+ cells (D) in the juvenile cortex stain with an antibody recognising GluR2/3 (E). Merge and co-localization analysis shows expression of the GluR2/3 on the processes and cell body of the EYFP+ cells.
Scale bars = 20 μm
In contrast to observations that the NG2 protein appeared to be a repulsive molecule for cerebellar neurons in vitro (Tan et al., 2005; Tan et al., 2006), axons are clearly not repulsed by NG2 cells but in fact appear to be actively contacting them (Butt et al., 2005; Nishiyama et al., 2005; Yang et al., 2006). We have observed that axons of rat hippocampal neurones readily grow over the plasma membrane of HEK cells expressing a truncated version of NG2 lacking a large part of the extracellular domain but containing the LNS domains (Chatterjee et al., 2008); (Griemsmann and Trotter, unpublished observations). In contrast, the chondroitin sulfate side chains of NG2 are likely to be inhibitory to axonal growth (Galtrey and Fawcett, 2007). Neurones exclusively form synapses in vivo with NG2 cells but not with neighbouring astrocytes, oligodendrocytes or microglia: NG2 glial cells are thus unique in promoting presynaptic specialisation in neurones. Could the NG2 protein itself be a synapse-promoting molecule? Furthermore, it is likely that NG2 cells release synapse-modulatory substances such as Brain Derived Neurotrophic Factor (BDNF), as suggested by a recent publication (Tanaka et al., 2009)
Electrophysiological and imaging evidence has demonstrated that excitation of NG2 glia by neuronal release of GABA acting on the GABAA receptors of NG2 cells, or via glutamate acting on the AMPA receptors, invokes a calcium signal (Gallo et al., 2008; Hamilton et al., 2009; Paukert and Bergles, 2006). Recent work has linked the GABA-induced signals in OPC to activation of sodium channels prior to changes in calcium and a stimulation of migration (Tong et al., 2009). It will be important to define events subsequent to this rise in calcium, for example changes in gene expression in NG2 cells.
5. Heterogeneity of the NG2 population
Since NG2 cells can give rise to different cell types (see below), an obvious question is whether NG2 cells are a heterogeneous population. Are there regional or developmental differences in the functions and differentiation potential of NG2 cells? All NG2 cells appear to express the receptor for PDGF AA. Analysis of transcription factor expression by NG2 cells in different regions of the brain have shown that almost all NG2 cells express Olig 2 and Sox 10 (Karram et al., 2008; Kitada and Rowitch, 2006; Ligon et al., 2006a; Ligon et al., 2006b). The NG2 population is heterogeneous when examined for the expression of glutamine synthestase, at least in the hippocampus (Karram et al., 2008). Study of a transgenic mouse in which EGFP was fused to the 3' UTR of the PLP gene, demonstrated two populations of NG2 cells in the developing subventricular zone: one population expressed EGFP and another lacked EGFP expression. The authors suggested that one population generated oligodendrocytes while the other population was more immature (Mallon et al., 2002). Several groups have shown heterogeneity in the NG2 cell population based on electrophysiological measurements. NG2 cells in the white and grey matter areas of the developing mouse were shown to differ in morphology and electrophysiological properties: furthermore, a few NG2+ cells in the grey matter elicited depolarization-induced spikes similar to immature action potentials (Chittajallu et al., 2004). In contrast, two other groups (Ge et al., 2009; Karadottir et al., 2008) reported subpopulations of NG2 cells in white matter which were able to generate action potentials; a finding which has generated controversy in the field as it would force a reclassification of these NG2 cells as bona-fide neurons. Within the hippocampus, a grey matter area, the EYFP+ cells are heterogeneous at a given developmental stage based on their electrophysiological properties (Karram et al., 2008). Unfortunately, neither the immunohistochemical nor the electrophysiological studies permit a distinction of functional diversity within the lineage from lineage heterogeneity. This question can better be addressed by studies using the transgenic mouse lines described below.
6. Progeny of NG2 cells
The fate of NG2 cells has been a subject of intense debate and has been studied using a variety of approaches. Here we will review primarily the recent literature on in vivo fate mapping of endogenous NG2 cell using the Cre-loxP technology. This method utilizes transgenic mouse lines that express the site-specific recombinase Cre driven by various promoters that are active in NG2 cells. When these mouse lines are crossed to Cre reporter mouse lines, the expression of the reporter gene is activated permanently in cells that express Cre, thereby allowing identification of their progeny by persistent reporter expression.
In NG2creBAC transgenic mice, constitutively active Cre is expressed from a large BAC (bacterial artificial chromosome) transgene in the context of 200 kb of sequence including all of the regulatory sequences of the 34 kb NG2 (CSPG4) gene. Using these mice, Zhu et al. (Zhu et al., 2008a; Zhu et al., 2008b) demonstrated that NG2 cells generate oligodendrocytes throughout the gray and white matter of the brain and spinal cord. Quantification of oligodendrocytes that expressed the reporter in these mice revealed that the percentage of oligodendrocytes that expressed the reporter gene was similar to the Cre recombination efficiency, indicating that all of the oligodendrocytes are derived from cells that express NG2 at some time in their life. In addition, a subpopulation of protoplasmic astrocytes in the gray matter of ventral forebrain and spinal cord appear to be derived from NG2 cells. Surprisingly, none of the GFAP+ astrocytes in the white matter were generated from NG2 cells under normal conditions, suggesting heterogeneity of the source of astrocytes.
Other studies have used inducible Cre lines in which Cre-mediated excision is activated by tamoxifen in transgenic lines that express a fusion protein consisting of Cre and various forms of the mutated ligand-binding domain of estrogen receptor engineered to bind tamoxifen with a higher affinity than endogenous estradiol(Metzger and Chambon, 2001), 2001). Rivers et al. (2008) generated Pdgfra-creERT2BAC transgenic mice that express CreERT2 under the regulatory sequences of PDGFRA, which is expressed in all NG2 glial cells (Rivers et al., 2008). Induction of Cre in adult Pdgfra-creERT2BAC transgenic mice revealed that the vast majority of the reporter+ cells were either NG2 cells or oligodendrocytes. No astrocytes expressed the reporter when Cre was activated in adult mice. In addition to oligodendrocyte lineage cells, a small number of cells with the morphology of projection neurons were detected in the piriform cortex, and these cells expressed the neuronal antigens NeuN and MAP2 but not markers of interneurons. The observation that the number of reporter+ neurons gradually increased after Cre induction suggests that they had been generated from NG2+/PDGRα+ cells. The identity of these PDGFRα+ neuronal precursors and whether they express NG2 remain unknown.
Using Olig2-creER™ transgenic mice that were generated by inserting CreER™ (Danielian et al., 1993) into the Olig2 gene(Takebayashi et al., 2002), Dimou et al., (2008) observed that when Cre was induced in adult mice, the reporter gene was expressed almost exclusively in either NG2 cells or mature oligodendrocytes and in a few protoplasmic astrocytes in gray matter but not in neurons (Dimou et al., 2008). The number of reporter+ oligodendrocytes in the white matter increased over time to >80% of the total induced cells. By contrast, those in the gray matter reached a plateau at <20% of total reporter+ cells. These findings differ slightly from the results obtained by Cre activation at similar ages in NG2creER™BAC transgenic mice, where the number of reporter+ oligodendrocytes continued to rise in both gray and white matter for 60 days after induction, although the rate of increase was smaller in the gray matter ((Zhu et al., 2008c); manuscript submitted).
Recently, another NG2 cell fate-mapping study was performed using PLP-creERT transgenic mice, which express CreERT under the control of the PLP promoter (Guo et al., 2009). Previous studies using PLP-EGFP transgenic mice had revealed that PLP transcriptional activity is detected in a subpopulation of NG2 cells in adult mice as described above(Mallon et al., 2002). When Cre was activated at postnatal day 7 (P7) in PLP-creERT transgenic mice, 90 – 94% of the reporter+ cells in the forebrain were NG2+, while only 27% of the induced cells were NG2+ in the spinal cord, presumably due to activation of Cre in oligodendrocytes that were more prevalent in the caudal CNS at this age. Eight days after Cre induction, the majority of the reporter+ cells in the white matter of the forebrain were oligodendrocytes, while those in the gray matter consisted of a mixture of oligodendrocytes and NG2 cells. In addition, reporter expression was detected in some protoplasmic astrocytes in the ventral forebrain and scattered neurons throughout the forebrain. However, a prior study using plp-cre transgenic mice had shown that PLP transcription is activated not only in oligodendrocyte lineage cells but also in neuronal progenitor cells prior to glial development (Delaunay et al., 2008).
While all of these studies consistently support the oligodendrocyte fate of NG2 cells in both white and gray matter, the findings related to astrocyte and neuronal fate of NG2 cells vary. The precise reason for the differences observed in the fate of NG2 cells among these studies remains unclear, but it is likely that differences in the specificity of Cre-targeting to NG2 cells and the efficacy of Cre induction contribute to the different results obtained in these studies.
7. Origin of NG2 cells
In the spinal cord, the majority of oligodendrocyte lineage cells arise from discrete ventral domains under the influence of Sonic hedgehog (Shh) (Lu et al., 2000; Orentas et al., 1999; Richardson et al., 2006; Takebayashi et al., 2000; Zhou et al., 2000) et al., 2000). Cells that express Olig1 and Olig2 in the pMN domain and those that express Nkx2.2 in the ventrally adjacent P3 domain comprise the early committed oligodendrocyte lineage cells. NG2 becomes detectable after these cells migrate out of the ventricular zone and expand to occupy the entire spinal cord (Nishiyama et al., 1996a; Pringle and Richardson, 1993). While the majority of oligodendrocytes arise from the ventral sources, some PDGFRα+ oligodendrocyte precursors in the dorsal spinal cord arise from the dorsal structures independently of Shh in the absence of the homeodomain transcription factor Nkx6.1 (Cai et al., 2005; Vallstedt et al., 2005), and some arise from the dorsal domains defined by Dbx1 expression via radial glia (Fogarty et al., 2005).
The origin of NG2 cells in the forebrain is somewhat more complex, but as in the spinal cord, the ventral subpallial regions appear to be the major source of oligodendrocytes. The first PDGFRα+ cells appear in the anterior entopeduncular region (AEP) between the median ganglionic eminence (MGE) and anterior hypothalamus at E13.5 in the rat (Pringle et al., 1992; Tekki-Kessaris et al., 2001) and subsequently expand dorsolaterally. Their appearance is dependent on the homeodomain transcription factor Nkx2.1, which is necessary for the correct expression of Shh (Nery et al., 2001; Tekki-Kessaris et al., 2001).
Prior to E14 in the mouse or E15 in the rat, NG2 expression in the forebrain is confined to the vasculature. The first NG2+ parenchymal cells appear after E15, at least 2 days after the first appearance of PDGFRα+ cells, and all the non-vascular NG2+ cells also express PDGFRα (Nishiyama et al., 1996b). These cells are likely to be the earliest NG2 cells that appear in the forebrain. At this early stage a small number of PDGFRα+ cells without detectable NG2 is seen scattered throughout the parenchyma, along with a larger number of cells that co-express NG2 and PDGFRα. By the end of the embryonic development, there is an almost complete overlap in the expression of NG2 and PDGFRα, with the exception of the SVZ, where there are PDGFRα+NG2− cells (Nishiyama et al., 1996b). This is consistent with the finding that >99% of PDGFRα-immunopanned cells from E19 rat brains were NG2+ (Tekki-Kessaris et al., 2001).
Genetic fate mapping using three different Cre transgenic mouse lines revealed that oligodendrocytes are generated in three waves (Kessaris et al., 2006). The earliest PDGFRα+ cells are generated from Nkx2.1+ cells in the MGE and AEP beginning around E12. Using quail/chick transplantation, Olivier et al. (2001) demonstrated that cells in AEP supplies all the oligodendrocytes in the telencephalon. In the mouse, PDGFRα+ cells derived from Gsh2+ cells in the lateral ganglionic eminence (LGE) appear after E16.5 and eventually replace the earlier Nkx2.1-derived cells. A third wave of PDGFRα+ cells appears mainly postnatally from dorsal Emx1+ cells and generates oligodendrocytes in the pallium including the neocortex and the corpus callosum. The original Nkx2.1-derived cells disappear after birth. It has not been tested whether NG2 is expressed equally in the both the early PDGFRα+ cells derived from Nkx2.1+ cells and the later generated Gsh2-derived PDGFRα+ cells. Both Gsh2-derived and Emx1-derived cells are capable of generating oligodendrocytes, but it has not yet been tested whether NG2 cells derived from the different sources differ in their ability to interact with neurons or generate astrocytes.
The literature on the development of NG2 cells from ventral progenitor cells that express the DLX family of homeodomain transcription factors varies. Migration studies using DiI labeling and cross transplantation in slice cultures revealed that cells that are generated early from MGE migrate dorsally and become dispersed widely through the neocortex, while those that develop later from LGE and MGE migrate into the proliferative zones of the neocortex to form the future subventricular zone (Anderson et al., 2001. The DLX family of transcription factors that includes Dlx1, Dlx2, Dlx5, and Dlx6 is expressed in the embryonic ganglionic eminences. Short-term fate mapping of Dlx2+ cells using DLX2-tau-lacZ transgenic mice revealed that the location of the cluster of (−galactosidase+ cells representing the progeny of Dlx2+ cells shifts from LGE in late embryonic brain to the central core region of the dorsolateral SVZ in the early postnatal stage {Marshall, 2002 #463). Among the progeny of Dlx2+ cells found at P6–10 were GFAP+ astrocytes and CNPase+ oligodendrocytes in the neocortex and corpus callosum. In another study, cells from the corpus callosum and subcortical white matter from E18 mice were found to coexpress NG2 and Dlx1/2/4/5 (He et al., 2001). By contrast, a recent fate-mapping study using the enhancers URE2 and I12b from DLX1 and 2 genes revealed that DLX1/2+ cells give rise to GABAergic interneurons but very few oligodendrocytes and no astrocytes (Potter et al., 2009). It was also recently shown that Dlx1/2 negatively regulates oligodendrocyte development by repressing Olig2, and that lack of Dlx1/2 increased the number of PDGFRα+ cells in early MGE and LGE (Petryniak et al., 2007). Thus, it appears that Dlx1/2 is upregulated as the progenitor cells become committed to a neuronal fate and is lost in cells that become committed to an oligodendrocyte lineage. The function of Dlx1/2 is in turn repressed by Mash1 (Petryniak et al., 2007) which has been shown to be required for the development of NG2 cells in some regions (Parras et al., 2004).
In the postnatal CNS throughout adulthood, the SVZ appears to play an important role in the generation of NG2 cells. Retroviral marking of perinatal SVZ showed that NG2 cells in the corpus callosum and neocortex are generated from neonatal SVZ (Levison et al., 1999). In adult mice, retroviral marking of GFAP+ SVZ type B cells resulted in generation of NG2 cells in the corpus callosum in normal and demyelinated states (Gonzalez-Perez et al., 2009; Menn et al., 2006). Although a small number of NG2 cells are found in the SVZ, and it has been reported that NG2 cells represent transit-amplifying progenitor cells in the SVZ (Aguirre et al., 2004), recent studies suggest that the majority of these progenitor cells in the SVZ do not express NG2, but NG2 is highly expressed in the parenchyma surrounding the SVZ (Cesetti et al., 2009; Diers-Fenger et al., 2001; Komitova et al., 2009; Platel et al., 2009). Besides the SVZ, local proliferation of NG2 cells also contributes to the maintenance of the NG2 cell population in the mature CNS.
In addition to the ventral sources of tangentially migrating cells in embryonic stages and the neocortical SVZ in postnatal rodents, radial glia have also been implicated as a source for NG2 cells. By using human GFAP-cre fate mapping, the predominant fate of radial glia in the striatum was found to be NG2 cells and oligodendrocytes, while neurons comprised the majority of the progeny of radial glial in the neocortex(Malatesta et al., 2000; Malatesta et al., 2003). In another study, when neonatal dorsal radial glia were marked with adenovirus expressing Cre, Cre reporter was detected in NG2 cells in the neocortex and subcortical white matter, indicating that at least some NG2 cells are derived from dorsal radial glia (Ventura and Goldman, 2007).
These observations from recent studies suggest that the general pattern of NG2 cell development in the forebrain is fundamentally similar to those in the spinal cord in that cells arising from the early ventral source become intermingled with cells that arise later from dorsal regions. Further studies are needed to determine whether the NG2 cells that originate from different regions are functionally equivalent in various aspects of NG2 cell function described above.
8. Conclusion
Recent insights discussed above address the function and lineage of NG2-expressing cells in the CNS. However, we are still far from a complete understanding of the functional roles they play in the CNS. Multiple questions remain to be answered concerning their heterogeneity, response to CNS damage and most importantly, mechanism of integration into and modulation of the neuronal network. NG2-expressing cells may vary between different CNS regions with regard to expression of proteins and mRNAs. During the next few years, we expect to unravel the mysteries surrounding these exciting NG2-expressing cells.
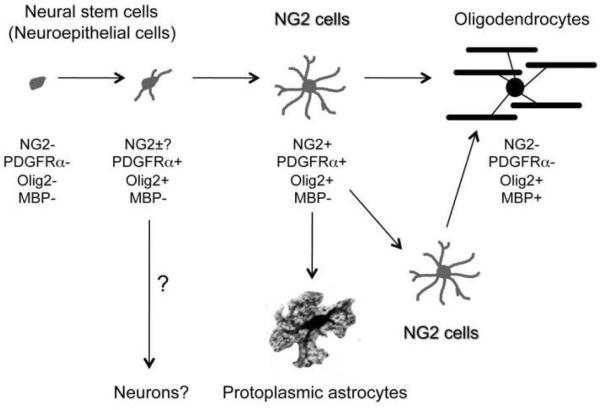
Figure 5. A schematic diagram showing the NG2 cell lineage.
NG2 cells originate from NG2−/PDGFRα− cells in the germinal zone and acquire NG2 expression as they migrate to their destination. They have the ability to self-renew and generate oligodendrocytes at all ages. NG2 cells in the immature brain also generate a subpopulation of protoplasmic astrocytes. The neuronal fate of NG2 cells is still debated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguirre AA, Chittajallu R, Belachew S, Gallo V. NG2-expressing cells in the subventricular zone are type C-like cells and contribute to interneuron generation in the postnatal hippocampus. J Cell Biol. 2004;165:575–89. doi: 10.1083/jcb.200311141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SA, Marin O, Horn C, Jennings K, Rubenstein JL. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001;128:353–63. doi: 10.1242/dev.128.3.353. [DOI] [PubMed] [Google Scholar]
- Barritt DS, Pearn MT, Zisch AH, Lee SS, Javier RT, Pasquale EB, Stallcup WB. The multi-PDZ domain protein MUPP1 is a cytoplasmic ligand for the membrane-spanning proteoglycan NG2. J Cell Biochem. 2000;79:213–24. doi: 10.1002/1097-4644(20001101)79:2<213::aid-jcb50>3.0.co;2-g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belachew S, Chittajallu R, Aguirre AA, Yuan X, Kirby M, Anderson S, Gallo V. Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol. 2003;161:169–86. doi: 10.1083/jcb.200210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DE, Roberts JD, Somogyi P, Jahr CE. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187–91. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- Butt AM, Hamilton N, Hubbard P, Pugh M, Ibrahim M. Synantocytes: the fifth element. J Anat. 2005;207:695–706. doi: 10.1111/j.1469-7580.2005.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Qi Y, Hu X, Tan M, Liu Z, Zhang J, Li Q, Sander M, Qiu M. Generation of oligodendrocyte precursor cells from mouse dorsal spinal cord independent of Nkx6 regulation and Shh signaling. Neuron. 2005;45:41–53. doi: 10.1016/j.neuron.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Cesetti T, Obernier K, Bengtson CP, Fila T, Mandl C, Holzl-Wenig G, Worner K, Eckstein V, Ciccolini F. Analysis of stem cell lineage progression in the neonatal subventricular zone identifies EGFR+/NG2− cells as transit-amplifying precursors. Stem Cells. 2009;27:1443–54. doi: 10.1002/stem.74. [DOI] [PubMed] [Google Scholar]
- Chatterjee N, Stegmuller J, Schatzle P, Karram K, Koroll M, Werner HB, Nave KA, Trotter J. Interaction of syntenin-1 and the NG2 proteoglycan in migratory oligodendrocyte precursor cells. J Biol Chem. 2008;283:8310–7. doi: 10.1074/jbc.M706074200. [DOI] [PubMed] [Google Scholar]
- Chittajallu R, Aguirre A, Gallo V. NG2-positive cells in the mouse white and grey matter display distinct physiological properties. J Physiol. 2004;561:109–22. doi: 10.1113/jphysiol.2004.074252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielian PS, White R, Hoare SA, Fawell SE, Parker MG. Identification of residues in the estrogen receptor that confer differential sensitivity to estrogen and hydroxytamoxifen. Mol Endocrinol. 1993;7:232–40. doi: 10.1210/mend.7.2.8469236. [DOI] [PubMed] [Google Scholar]
- Dayer AG, Cleaver KM, Abouantoun T, Cameron HA. New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. J Cell Biol. 2005;168:415–27. doi: 10.1083/jcb.200407053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaunay D, Heydon K, Cumano A, Schwab MH, Thomas JL, Suter U, Nave KA, Zalc B, Spassky N. Early neuronal and glial fate restriction of embryonic neural stem cells. J Neurosci. 2008;28:2551–62. doi: 10.1523/JNEUROSCI.5497-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diers-Fenger M, Kirchhoff F, Kettenmann H, Levine JM, Trotter J. AN2/NG2 protein-expressing glial progenitor cells in the murine CNS: isolation, differentiation, and association with radial glia. Glia. 2001;34:213–28. doi: 10.1002/glia.1055. [DOI] [PubMed] [Google Scholar]
- Dimou L, Simon C, Kirchhoff F, Takebayashi H, Gotz M. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J Neurosci. 2008;28:10434–42. doi: 10.1523/JNEUROSCI.2831-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenmann KM, McCarthy JB, Simpson MA, Keely PJ, Guan JL, Tachibana K, Lim L, Manser E, Furcht LT, Iida J. Melanoma chondroitin sulphate proteoglycan regulates cell spreading through Cdc42, Ack-1 and p130cas. Nat Cell Biol. 1999;1:507–13. doi: 10.1038/70302. [DOI] [PubMed] [Google Scholar]
- Estrada B, Gisselbrecht SS, Michelson AM. The transmembrane protein Perdido interacts with Grip and integrins to mediate myotube projection and attachment in the Drosophila embryo. Development. 2007;134:4469–78. doi: 10.1242/dev.014027. [DOI] [PubMed] [Google Scholar]
- Fang X, Burg MA, Barritt D, Dahlin-Huppe K, Nishiyama A, Stallcup WB. Cytoskeletal reorganization induced by engagement of the NG2 proteoglycan leads to cell spreading and migration. Mol Biol Cell. 1999;10:3373–87. doi: 10.1091/mbc.10.10.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty M, Richardson WD, Kessaris N. A subset of oligodendrocytes generated from radial glia in the dorsal spinal cord. Development. 2005;132:1951–9. doi: 10.1242/dev.01777. [DOI] [PubMed] [Google Scholar]
- Gallo V, Zhou JM, McBain CJ, Wright P, Knutson PL, Armstrong RC. Oligodendrocyte progenitor cell proliferation and lineage progression are regulated by glutamate receptor-mediated K+ channel block. J Neurosci. 1996;16:2659–70. doi: 10.1523/JNEUROSCI.16-08-02659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V, Mangin JM, Kukley M, Dietrich D. Synapses on NG2-expressing progenitors in the brain: multiple functions? J Physiol. 2008 doi: 10.1113/jphysiol.2008.158436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galtrey CM, Fawcett JW. The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res Rev. 2007;54:1–18. doi: 10.1016/j.brainresrev.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Ge WP, Zhou W, Luo Q, Jan LY, Jan YN. Dividing glial cells maintain differentiated properties including complex morphology and functional synapses. Proc Natl Acad Sci U S A. 2009;106:328–33. doi: 10.1073/pnas.0811353106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Perez O, Romero-Rodriguez R, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Epidermal Growth Factor Induces the Progeny of Subventricular Zone Type B Cells to Migrate and Differentiate into Oligodendrocytes. Stem Cells. 2009;27:2032–2043. doi: 10.1002/stem.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Ma J, McCauley E, Bannerman P, Pleasure D. Early postnatal proteolipid promoter-expressing progenitors produce multilineage cells in vivo. J Neurosci. 2009;29:7256–70. doi: 10.1523/JNEUROSCI.5653-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton N, Vayro S, Wigley R, Butt AM. Axons and astrocytes release ATP and glutamate to evoke calcium signals in NG2-glia. Glia. 2009 doi: 10.1002/glia.20902. [DOI] [PubMed] [Google Scholar]
- Harper JR, Bumol TF, Reisfeld RA. Characterization of monoclonal antibody 155.8 and partial characterization of its proteoglycan antigen on human melanoma cells. J Immunol. 1984;132:2096–104. [PubMed] [Google Scholar]
- He W, Ingraham C, Rising L, Goderie S, Temple S. Multipotent stem cells from the mouse basal forebrain contribute GABAergic neurons and oligodendrocytes to the cerebral cortex during embryogenesis. J Neurosci. 2001;21:8854–62. doi: 10.1523/JNEUROSCI.21-22-08854.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabs R, Pivneva T, Huttmann K, Wyczynski A, Nolte C, Kettenmann H, Steinhauser C. Synaptic transmission onto hippocampal glial cells with hGFAP promoter activity. J Cell Sci. 2005;118:3791–803. doi: 10.1242/jcs.02515. [DOI] [PubMed] [Google Scholar]
- Karadottir R, Hamilton NB, Bakiri Y, Attwell D. Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat Neurosci. 2008;11:450–6. doi: 10.1038/nn2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karram K, Goebbels S, Schwab M, Jennissen K, Seifert G, Steinhauser C, Nave KA, Trotter J. NG2-expressing cells in the nervous system revealed by the NG2-EYFP-knockin mouse. Genesis. 2008;46:743–57. doi: 10.1002/dvg.20440. [DOI] [PubMed] [Google Scholar]
- Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci. 2006;9:173–9. doi: 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada M, Rowitch DH. Transcription factor co-expression patterns indicate heterogeneity of oligodendroglial subpopulations in adult spinal cord. Glia. 2006;54:35–46. doi: 10.1002/glia.20354. [DOI] [PubMed] [Google Scholar]
- Komitova M, Zhu X, Serwanski DR, Nishiyama A. NG2 cells are distinct from neurogenic cells in the postnatal mouse subventricular zone. J Comp Neurol. 2009;512:702–16. doi: 10.1002/cne.21917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukley M, Capetillo-Zarate E, Dietrich D. Vesicular glutamate release from axons in white matter. Nat Neurosci. 2007;10:311–20. doi: 10.1038/nn1850. [DOI] [PubMed] [Google Scholar]
- Levison SW, Young GM, Goldman JE. Cycling cells in the adult rat neocortex preferentially generate oligodendroglia. J Neurosci Res. 1999;57:435–46. [PubMed] [Google Scholar]
- Ligon KL, Fancy SP, Franklin RJ, Rowitch DH. Olig gene function in CNS development and disease. Glia. 2006a;54:1–10. doi: 10.1002/glia.20273. [DOI] [PubMed] [Google Scholar]
- Ligon KL, Kesari S, Kitada M, Sun T, Arnett HA, Alberta JA, Anderson DJ, Stiles CD, Rowitch DH. Development of NG2 neural progenitor cells requires Olig gene function. Proc Natl Acad Sci U S A. 2006b;103:7853–8. doi: 10.1073/pnas.0511001103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SC, Huck JH, Roberts JD, Macklin WB, Somogyi P, Bergles DE. Climbing fiber innervation of NG2-expressing glia in the mammalian cerebellum. Neuron. 2005;46:773–85. doi: 10.1016/j.neuron.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Lin XH, Grako KA, Burg MA, Stallcup WB. NG2 proteoglycan and the actin-binding protein fascin define separate populations of actin-containing filopodia and lamellipodia during cell spreading and migration. Mol Biol Cell. 1996;7:1977–93. doi: 10.1091/mbc.7.12.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu QR, Yuk D, Alberta JA, Zhu Z, Pawlitzky I, Chan J, McMahon AP, Stiles CD, Rowitch DH. Sonic hedgehog--regulated oligodendrocyte lineage genes encoding bHLH proteins in the mammalian central nervous system. Neuron. 2000;25:317–29. doi: 10.1016/s0896-6273(00)80897-1. [DOI] [PubMed] [Google Scholar]
- Majumdar M, Vuori K, Stallcup WB. Engagement of the NG2 proteoglycan triggers cell spreading via rac and p130cas. Cell Signal. 2003;15:79–84. doi: 10.1016/s0898-6568(02)00045-1. [DOI] [PubMed] [Google Scholar]
- Malatesta P, Hartfuss E, Gotz M. Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development. 2000;127:5253–63. doi: 10.1242/dev.127.24.5253. [DOI] [PubMed] [Google Scholar]
- Malatesta P, Hack MA, Hartfuss E, Kettenmann H, Klinkert W, Kirchhoff F, Gotz M. Neuronal or glial progeny: regional differences in radial glia fate. Neuron. 2003;37:751–64. doi: 10.1016/s0896-6273(03)00116-8. [DOI] [PubMed] [Google Scholar]
- Mallon BS, Shick HE, Kidd GJ, Macklin WB. Proteolipid promoter activity distinguishes two populations of NG2-positive cells throughout neonatal cortical development. J Neurosci. 2002;22:876–85. doi: 10.1523/JNEUROSCI.22-03-00876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26:7907–18. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger D, Chambon P. Site- and time-specific gene targeting in the mouse. Methods. 2001;24:71–80. doi: 10.1006/meth.2001.1159. [DOI] [PubMed] [Google Scholar]
- Missler M, Sudhof TC. Neurexins: three genes and 1001 products. Trends Genet. 1998;14:20–6. doi: 10.1016/S0168-9525(97)01324-3. [DOI] [PubMed] [Google Scholar]
- Nery S, Wichterle H, Fishell G. Sonic hedgehog contributes to oligodendrocyte specification in the mammalian forebrain. Development. 2001;128:527–40. doi: 10.1242/dev.128.4.527. [DOI] [PubMed] [Google Scholar]
- Niehaus A, Stegmuller J, Diers-Fenger M, Trotter J. Cell-surface glycoprotein of oligodendrocyte progenitors involved in migration. J Neurosci. 1999;19:4948–61. doi: 10.1523/JNEUROSCI.19-12-04948.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB. Interaction between NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells is required for optimal response to PDGF. J Neurosci Res. 1996a;43:315–30. doi: 10.1002/(SICI)1097-4547(19960201)43:3<315::AID-JNR6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB. Co-localization of NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells in the developing rat brain. J Neurosci Res. 1996b;43:299–314. doi: 10.1002/(SICI)1097-4547(19960201)43:3<299::AID-JNR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Yang Z, Butt A. Astrocytes and NG2-glia: what's in a name? J Anat. 2005;207:687–93. doi: 10.1111/j.1469-7580.2005.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- Orentas DM, Hayes JE, Dyer KL, Miller RH. Sonic hedgehog signaling is required during the appearance of spinal cord oligodendrocyte precursors. Development. 1999;126:2419–29. doi: 10.1242/dev.126.11.2419. [DOI] [PubMed] [Google Scholar]
- Parras CM, Galli R, Britz O, Soares S, Galichet C, Battiste J, Johnson JE, Nakafuku M, Vescovi A, Guillemot F. Mash1 specifies neurons and oligodendrocytes in the postnatal brain. Embo J. 2004;23:4495–505. doi: 10.1038/sj.emboj.7600447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paukert M, Bergles DE. Synaptic communication between neurons and NG2+ cells. Curr Opin Neurobiol. 2006;16:515–21. doi: 10.1016/j.conb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Peters A. A fourth type of neuroglial cell in the adult central nervous system. J Neurocytol. 2004;33:345–57. doi: 10.1023/B:NEUR.0000044195.64009.27. [DOI] [PubMed] [Google Scholar]
- Petryniak MA, Potter GB, Rowitch DH, Rubenstein JL. Dlx1 and Dlx2 control neuronal versus oligodendroglial cell fate acquisition in the developing forebrain. Neuron. 2007;55:417–33. doi: 10.1016/j.neuron.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platel JC, Gordon V, Heintz T, Bordey A. GFAP-GFP neural progenitors are antigenically homogeneous and anchored in their enclosed mosaic niche. Glia. 2009;57:66–78. doi: 10.1002/glia.20735. [DOI] [PubMed] [Google Scholar]
- Pluschke G, Vanek M, Evans A, Dittmar T, Schmid P, Itin P, Filardo EJ, Reisfeld RA. Molecular cloning of a human melanoma-associated chondroitin sulfate proteoglycan. Proc Natl Acad Sci U S A. 1996;93:9710–5. doi: 10.1073/pnas.93.18.9710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter GB, Petryniak MA, Shevchenko E, McKinsey GL, Ekker M, Rubenstein JL. Generation of Cre-transgenic mice using Dlx1/Dlx2 enhancers and their characterization in GABAergic interneurons. Mol Cell Neurosci. 2009;40:167–86. doi: 10.1016/j.mcn.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle NP, Mudhar HS, Collarini EJ, Richardson WD. PDGF receptors in the rat CNS: during late neurogenesis, PDGF alpha-receptor expression appears to be restricted to glial cells of the oligodendrocyte lineage. Development. 1992;115:535–551. doi: 10.1242/dev.115.2.535. [DOI] [PubMed] [Google Scholar]
- Pringle NP, Richardson WD. A singularity of PDGF alpha-receptor expression in the dorsoventral axis of the neural tube may define the origin of the oligodendrocyte lineage. Development. 1993;117:525–533. doi: 10.1242/dev.117.2.525. [DOI] [PubMed] [Google Scholar]
- Richardson WD, Kessaris N, Pringle N. Oligodendrocyte wars. Nat Rev Neurosci. 2006;7:11–8. doi: 10.1038/nrn1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;11:1392–401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S, Bosse F, D'Urso D, Muller H, Sereda MW, Nave K, Niehaus A, Kempf T, Schnolzer M, Trotter J. The AN2 protein is a novel marker for the Schwann cell lineage expressed by immature and nonmyelinating Schwann cells. J Neurosci. 2001;21:920–33. doi: 10.1523/JNEUROSCI.21-03-00920.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnorrer F, Kalchhauser I, Dickson BJ. The transmembrane protein Kon-tiki couples to Dgrip to mediate myotube targeting in Drosophila. Dev Cell. 2007;12:751–66. doi: 10.1016/j.devcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Stallcup WB. The NG2 antigen, a putative lineage marker: immunofluorescent localization in primary cultures of rat brain. Dev Biol. 1981;83:154–65. doi: 10.1016/s0012-1606(81)80018-8. [DOI] [PubMed] [Google Scholar]
- Stallcup WB, Huang FJ. A role for the NG2 proteoglycan in glioma progression. Cell Adh Migr. 2008;2:192–201. doi: 10.4161/cam.2.3.6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmuller J, Schneider S, Hellwig A, Garwood J, Trotter J. AN2, the mouse homologue of NG2, is a surface antigen on glial precursor cells implicated in control of cell migration. J Neurocytol. 2002;31:497–505. doi: 10.1023/a:1025743731306. [DOI] [PubMed] [Google Scholar]
- Stegmuller J, Werner H, Nave KA, Trotter J. The proteoglycan NG2 is complexed with alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors by the PDZ glutamate receptor interaction protein (GRIP) in glial progenitor cells. Implications for glial-neuronal signaling. J Biol Chem. 2003;278:3590–8. doi: 10.1074/jbc.M210010200. [DOI] [PubMed] [Google Scholar]
- Takebayashi H, Yoshida S, Sugimori M, Kosako H, Kominami R, Nakafuku M, Nabeshima Y. Dynamic expression of basic helix-loop-helix Olig family members: implication of Olig2 in neuron and oligodendrocyte differentiation and identification of a new member, Olig3. Mech Dev. 2000;99:143–8. doi: 10.1016/s0925-4773(00)00466-4. [DOI] [PubMed] [Google Scholar]
- Takebayashi H, Nabeshima Y, Yoshida S, Chisaka O, Ikenaka K. The basic helix-loop-helix factor olig2 is essential for the development of motoneuron and oligodendrocyte lineages. Curr Biol. 2002;12:1157–63. doi: 10.1016/s0960-9822(02)00926-0. [DOI] [PubMed] [Google Scholar]
- Tan AM, Zhang W, Levine JM. NG2: a component of the glial scar that inhibits axon growth. J Anat. 2005;207:717–25. doi: 10.1111/j.1469-7580.2005.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AM, Colletti M, Rorai AT, Skene JH, Levine JM. Antibodies against the NG2 proteoglycan promote the regeneration of sensory axons within the dorsal columns of the spinal cord. J Neurosci. 2006;26:4729–39. doi: 10.1523/JNEUROSCI.3900-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Tozuka Y, Takata T, Shimazu N, Matsumura N, Ohta A, Hisatsune T. Excitatory GABAergic Activation of Cortical Dividing Glial Cells. Cereb Cortex. 2009 doi: 10.1093/cercor/bhn238. [DOI] [PubMed] [Google Scholar]
- Tekki-Kessaris N, Woodruff R, Hall AC, Gaffield W, Kimura S, Stiles CD, Rowitch DH, Richardson WD. Hedgehog-dependent oligodendrocyte lineage specification in the telencephalon. Development. 2001;128:2545–54. doi: 10.1242/dev.128.13.2545. [DOI] [PubMed] [Google Scholar]
- Tillet E, Gential B, Garrone R, Stallcup WB. NG2 proteoglycan mediates beta1 integrin-independent cell adhesion and spreading on collagen VI. J Cell Biochem. 2002;86:726–36. doi: 10.1002/jcb.10268. [DOI] [PubMed] [Google Scholar]
- Tong XP, Li XY, Zhou B, Shen W, Zhang ZJ, Xu TL, Duan S. Ca(2+) signaling evoked by activation of Na(+) channels and Na(+)/Ca(2+) exchangers is required for GABA-induced NG2 cell migration. J Cell Biol. 2009;186:113–28. doi: 10.1083/jcb.200811071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallstedt A, Klos JM, Ericson J. Multiple dorsoventral origins of oligodendrocyte generation in the spinal cord and hindbrain. Neuron. 2005;45:55–67. doi: 10.1016/j.neuron.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Ventura RE, Goldman JE. Dorsal radial glia generate olfactory bulb interneurons in the postnatal murine brain. J Neurosci. 2007;27:4297–302. doi: 10.1523/JNEUROSCI.0399-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Suzuki R, Daniels SB, Brunquell CB, Sala CJ, Nishiyama A. NG2 glial cells provide a favorable substrate for growing axons. J Neurosci. 2006;26:3829–39. doi: 10.1523/JNEUROSCI.4247-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Wang S, Anderson DJ. Identification of a novel family of oligodendrocyte lineage-specific basic helix-loop-helix transcription factors. Neuron. 2000;25:331–43. doi: 10.1016/s0896-6273(00)80898-3. [DOI] [PubMed] [Google Scholar]
- Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008a;135:145–57. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]
- Zhu X, Hill RA, Nishiyama A. NG2 cells generate oligodendrocytes and gray matter astrocytes in the spinal cord. Neuron Glia Biol. 2008b;4:19–26. doi: 10.1017/S1740925X09000015. [DOI] [PubMed] [Google Scholar]
- Zhu X, Szuki R, Nishiyama A. The timing of NG2 cell differentiation in the brain. Neuroscience Meeting Planner. Program No. 124.9.2008. 2008c [Google Scholar]
- Ziskin JL, Nishiyama A, Rubio M, Fukaya M, Bergles DE. Vesicular release of glutamate from unmyelinated axons in white matter. Nat Neurosci. 2007;10:321–30. doi: 10.1038/nn1854. [DOI] [PMC free article] [PubMed] [Google Scholar]