Abstract
The Abelson Murine Leukemia Virus (A-MuLV) encodes v-Abl, an oncogenic form of the ubiquitous cellular non-receptor tyrosine kinase, c-Abl. A-MuLV specifically transforms murine B cell precursors both in vivo and in vitro. Inhibition of v-Abl by addition of the small molecule inhibitor STI-571 causes these cells to arrest in the G1 phase of the cell cycle prior to undergoing apoptosis. We found that inhibition of v-Abl activity results in upregulation of transcription of the pro-apoptotic TNF-family ligand TRAIL (tumor-necrosis factor-related apoptosis-inducing ligand). Similarly to BCR-Abl-transformed human cells, activation of the transcription factor Foxo3a led to increased TRAIL transcription and induction of a G1 arrest in the absence of v-Abl inhibition, and this effect could be inhibited by the expression of a constitutively active AKT mutant. Multiple pathways act to inhibit FoxO3a activity within Abelson cells. In addition to diminishing transcription factor activity via inhibitory phosphorylation by AKT family members, we found that inhibition of IKKβ activity results in an increase in the total protein level of FoxO3a. Furthermore over-expression of the p65 subunit of NF-κB results in an increase in TRAIL transcription and in apoptosis and deletion of IKKα and β diminishes TRAIL expression and induction. We conclude that in Abelson cells, the inhibition of both NF-κB and FoxO3a activity is required for suppression of TRAIL transcription and maintenance of the transformed state.
Constitutively active forms of the ubiquitous protein tyrosine kinase c-Abl are associated with oncogenic transformation in both mouse and man. In humans, a chromosomal translocation results in fusion of the Breakage Cluster Region (BCR) and c-Abl loci resulting in the production of a BCR-Abl fusion protein that is present in nearly 95% of chronic myelogenous leukemia (CML) and about 10% of acute lymphocytic leukemia (ALL) cases (Advani and Pendergast, 2002). A specific protein tyrosine kinase inhibitor, STI-571 (Gleevec), has shown great clinical utility in the treatment of these diseases (Druker et al., 1996; Mauro and Druker, 2001). In mice, a fusion between the retroviral Gag locus in the Abelson Murine Leukemia Virus (AMuLV) with c-Abl results in the v-Abl gene, which is capable of transforming murine bone marrow, resulting in acute pro-B cell leukemia (Abelson and Rabstein, 1970; Green et al., 1989). In the absence of IL-7 signaling, normal pro-B cells undergo apoptosis both in vivo and in vitro (Marshall et al., 1998). v-Abl transformation results in cytokine-independent growth, in part due to the activation of many of the signaling pathways activated by IL-7 stimulation (Banerjee and Rothman, 1998). Inhibition of v-Abl activity by STI-571 results in the loss of this cytokine-independent growth and leads to apoptosis (Muljo and Schlissel, 2003). The addition of IL-7 to cells in which v-Abl has been inhibited is capable of delaying both the G1 arrest and apoptosis (Banerjee and Rothman, 1998). Though both BCR-Abl and v-Abl fusion proteins produce leukemias, the signaling pathways initiated by the proteins differ. For example, BCR-Abl-transformed cells have high levels of active NF-κB and little to no ERK activity, while the reverse is true in v-Abl transformed cells (Hakansson et al., 2008; Klug et al., 1994; Zou and Calame, 1999).
Cellular apoptosis is executed by activation of the caspase cascade, resulting in degradation of essential cellular proteins and genomic DNA. Caspase activation can be initiated by two known pathways operating either independently or in combination (Roth and Reed, 2002; Scaffidi et al., 1998). In the cell intrinsic pathway, permeability of the outer mitochondrial membrane to necessary components of the apoptosome is controlled by the binding of pro- and anti-apoptotic Bcl2 family members (Cory et al., 2003; Xu and Shi, 2007). In the cell extrinsic pathway, stimulation of so-called death receptors by ligand binding results in the formation of the death inducing signaling complex (DISC) (Chen and Wang, 2002; Xu and Shi, 2007). One such ligand, TNF-related apoptosis-inducing ligand (Apo2L/TRAIL), can bind to several receptors, only one of which serves to activate apoptosis in mice (Sheridan et al., 1997). The remaining “decoy” receptors serve to inhibit or block TRAIL signaling, thus protecting cells from apoptosis. In initial tests, TRAIL showed specific anti-tumor activity while leaving normal cells undisturbed, in stark contrast to other members of the TNF family (Schaefer et al., 2007). Currently, several TRAIL-receptor agonists are in various stages of clinical trials as cancer therapeutic agents (Henson et al., 2008; Kruyt, 2008; Lee et al., 2007).
The regulation of TRAIL transcription in lymphocytes and cell lines has been linked to both NF-κB and FoxO3a (Baetu et al., 2001; Ghaffari et al., 2003). Though NF-κB activity is often associated with anti-apoptotic programs of gene expression, in Jurkat T cells it is essential for the activation of TRAIL transcription downstream of surface receptor stimulation (Baetu et al., 2001). FoxO3a is a member of the FoxO subgroup of forkhead transcription factors and is capable of binding directly to the TRAIL promoter and activating TRAIL transcription (Ghaffari et al., 2003). Phosphorylation of FoxO family members by various kinases such as AKT, SGK, and CDK2 results in their cytoplasmic sequestration and inactivation (Huang and Tindall, 2007). Inactivation of FoxO3a specifically appears to be important for oncogenic growth and transformation, presumably in part due to its link to TRAIL expression. In tumors that lack active AKT, FoxO3a is excluded from the nucleus due to phosphorylation by the IκB Kinase beta (IKKβ). IKKβ phosphorylation leads to a ubiquitin-dependent proteosomal degradation of FoxO3a (Hu et al., 2004). This phosphorylation site is not shared by the other FoxO family members.
TRAIL transcription is down-regulated in BCR-Abl-transformed human leukemic cells in an AKT-dependent manner (Ghaffari et al., 2003). Expression of a constitutively active (non-AKT phosphorylatable) FoxO3a mutant was capable of inducing TRAIL expression, G1 arrest, and apoptosis in multiple BCR-Abl dependent cell lines in the presence of an active BCR-Abl kinase (Ghaffari et al., 2003; Kikuchi et al., 2007). In the present study, we found that v-Abl kinase activity results in suppression of TRAIL transcription through multiple mechanisms. In addition to the suppression of FoxO3a activity due to its phosphorylation by AKT-family members, we found that inhibition of IKKβ resulted in an increase in the total protein level of FoxO3a and an increase in TRAIL transcription. Cells lacking both IKKα/β had greatly reduced basal levels of TRAIL transcription, and diminished TRAIL upregulation in response to STI-571 treatment. Additionally, activation of NF-κB results in an increase in TRAIL transcription within v-Abl-transformed cells, in contrast to what occurs within BCR-Abl transformed cells. Thus, leukemic transformation by the various forms of the Abl kinase engage distinct mechanisms to circumvent TRAIL induced apoptosis.
EXPERIMENTAL PROCEDURES
Cell Lines and plasmids
The following Abelson transformed pro-B cell lines were used in this work: PD31 (Lewis et al., 1982), 220-8 (Alt et al., 1984), 7G-S (Amin and Schlissel, 2008). An IKKα/β dual knockout v-Abl cell line was created from the bone marrow of knockout mice (Derudder et al., 2009) using the previously described method (Amin and Schlissel, 2008). All Abelson cell lines were maintained in RPMI 1640 with L-glutamine (Invitrogen), supplemented with 5% FCS, 1x Pen/Strep (Gibco), and 50μM β-mercaptoethanol. EcoPak2 cells were maintained in DMEM (Invitrogen) supplemented with 5% FCS and 1X sodium pyruvate (Gibco) in the absence of antibiotics. All cells were incubated at 37°C with 5% CO2. Retroviral plasmids expressing FoxO3a, FoxO3a(A3)-ER, and Foxo1(A3)-ER were a gift from David Fruman (UC Irvine) (Amin and Schlissel, 2008). The Foxo3a(A3)-ER H215R mutant was created by R. Amin. MSCV IκBΔN IRES Thy1.1 was provided by William Sha (UC Berkeley). MSCV-FADD-DN-Zeo was a gift from Astar Winoto (UC Berkeley). Myr-AKT was provided by N. Rosenberg and cloned into an ER-fusion cassette by R. Amin (Schlissel lab). The Bcl-xL expression plasmid, pAW3neoBclX, was a gift of T. Behrens (U. Minnesota) (Fang et al., 1995). FoxO3a and FoxO1 shRNA expressing cells were previously described (Amin and Schlissel, 2008). The following kinase inhibitors were used in this work: STI-571 (Novartis), AKT inhibitor VIII (Calbiochem), IKKβ Inhibitor III (2.5 μM) (Calbiochem). Tamoxifen (4OHT) (Calbiochem) was used for ER-induction.
Retroviral production and infection were performed as previously described (Amin and Schlissel, 2008). Selection for retrovirally infected cells was performed by sorting for the expression of the retrovirally expressed markers, Thy1.1 ((OX-7, Pharmingen)) and human CD4 (RPA-T4, eBiosciences). Stable transfection and drug selections were performed as described previously (Sheehy and Schlissel, 1999)
RT-PCR Analysis
RNA was isolated from 1 – 10 × 106 cells using the RNeasy kit (Qiagen). 3 μg of total RNA were reverse transcribed using random hexamers and M-MLV reverse transcriptase (Invitrogen) according to the manufacturers protocol. All real time-PCR analyses were performed with JumpStart Taq (Sigma) measuring EvaGreen incorporation. Primers: HPRT 5’: CTGGTGAAAAGGACCTCTCG. HPRT 3’: TGAAGTACTCATTATAGTCAAGGGCA. TRAIL 5’: TGCGGAAAGAAAGCAAGTCTC. TRAIL 3’: TCCAGCCACAGACACTTTCG. Standard deviations shown in the paper are for the fold changes in TRAIL transcription.
PI Staining
1 × 106 cells were fixed with ethanol then stained with propidium iodide (Sigma) in the presence of RNAse A in PBS. Cells with a sub-G1 DNA content were excluded from the analyses presented here.
Apoptosis assay
1 × 106 cells were stained with Annexin V-FITC (BDBiosciences) and 7AAD (eBiosciences) in PBS containing calcium chloride and BSA prior to analysis by flow cytometry.
Western Blot Analysis
The following primary antibodies were used in this work: anti-phospho-T32 Foxo3a (Upstate), anti-Foxo3a (Upstate), anti-IKKβ (H4, Santa Cruz), anti-phosphoserine (Zymed) anti-actin (C11, Santa Cruz). For Western blot analysis, cells were lysed in 2X sample buffer (2% SDS, 0.1% bromphenol blue, 10% β-mercaptoethanol, 20% glycerol, 50 mM Tris pH 6.8 ) by boiling for 20 minutes at a concentration of 1 × 106 cells/ 10 μL. 2 million cell equivalents were electrophoresed through a 4-10% SDS-polyacrylamide gel, transferred to Immobilon-FL PVDF (Millipore), and blotted with the indicated primary antibodies. IR conjugated secondary antibodies were purchased from Rockland (IRdye800) or Molecular Probes (Alexa Fluor 680)
Immunofluoresence
All steps were carried out at room temperature. For each experiment, approximately 5 × 105 Abelson cells were cytospun onto glass slides and fixed with 4% paraformaldehyde for 10 minutes. Fixed samples were blocked and permeabilized by incubation with blocking buffer (1X PBS, 0.2% Triton X-100, 5% normal rat serum, 5% fetal calf serum) for 20 minutes, followed by incubation with the primary antibody, anti-p65 (C20, Santa Cruz), diluted in blocking buffer for 30 minutes. Following washing, secondary antibody, anti-rabbit-IgG, Cy3 conjugated (Jackson immunoresearch), were incubated for 30 minutes in blocking buffer, followed by identical washes. Cells were overlaid with anti-fade solution. Images were obtained using a fluorescence imaging microscope and SlideBook software.
RESULTS
TRAIL is activated during STI-571-induced apoptosis in v-Abl-transformed cells
As noted above, STI-571 is a highly specific Abl-family tyrosine kinase inhibitor used in the treatment of BCR-Abl+ CML and ALL in humans. Treatment of these cells with STI-571 increases TRAIL transcription, leading to apoptosis (Ghaffari et al., 2003; Kikuchi et al., 2007). Given that the treatment of v-abl transformed cell lines with STI-571 results in their apoptosis, we examined whether a similar TRAIL-induction was involved (Muljo and Schlissel, 2003). Upon STI-571 treatment, we observed a 5- to 20-fold upregulation of TRAIL mRNA levels in each of several v-Abl-transformed pro-B (Abelson) cell lines tested (Fig. 1a and supplemental Fig. 1). Next, we tested whether interfering with the TRAIL signaling pathway would have an effect on STI-571-induced apoptosis. The binding of TRAIL to its receptors initiates apoptosis via the FADD-dependent cell extrinsic apoptotic pathway (Scaffidi et al., 1998; Zhang and Fang, 2005). We used a retroviral expression vector to transduce an Abelson pro-B cell leukemia line with a gene encoding a dominant negative (DN) mutant of FADD (FADD-DN) (Newton et al., 1998). Our FADD-DN lacks the death effecter domain that is required for the activation of the caspases downstream of the DISC complex (Newton et al., 1998; Zhang and Winoto, 1996). Compared to the parental line, the cells expressing FADD-DN showed a 3-fold decrease in the percent of AnnexinV positive apoptotic cells after 36 hours in the presence of STI-571 (Fig. 1b). We also stably transfected cells with a cDNA expression vector encoding the anti-apoptotis protein Bcl-xL whose expression has been shown to protect Abelson cells from death after v-Abl inhibition (Banerjee and Rothman, 1998; Chen et al., 1994; Cory et al., 2003). Unlike the partial rescue of the FADD-DN construct, cells expressing Bcl-xL showed a near-complete protection from apoptosis in the time course of the assay (Fig. 1b). Expression of both FADD-DN and Bcl-xL did not result in cumulative protection. TRAIL-induced apoptosis has been reported to follow a type 2 apoptotic mechanism in numerous cell lines (Deng et al., 2002; Roth and Reed, 2002; Zhang and Fang, 2005). Type 2 apoptosis is sensitive to levels of anti-apoptotic Bcl-2 family members due to the relatively low recruitment of the DISC complex and a decreased activation of Caspase 8. Our data support such a conclusion for STI-571-induced apoptosis in Abelson cells.
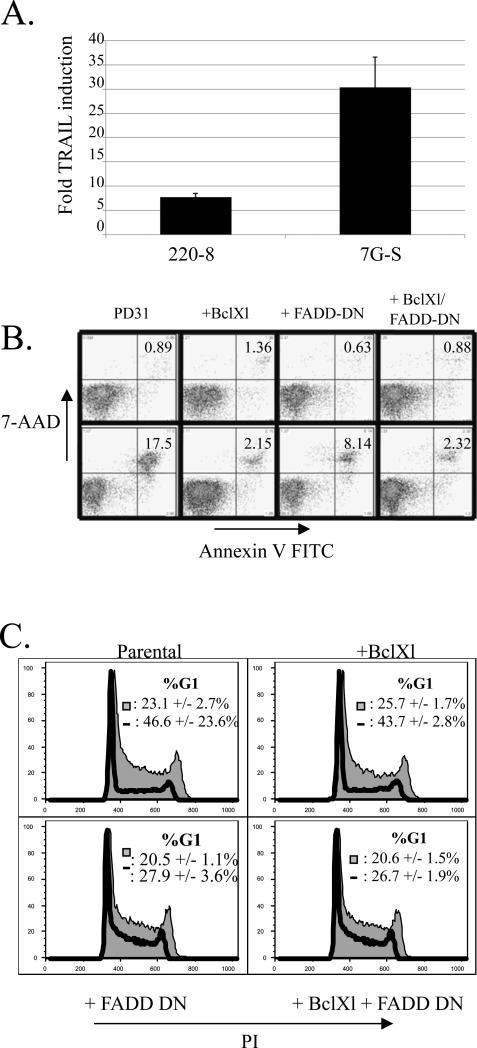
Figure 1. TRAIL upregulation in response to STI treatment plays a role in G1 arrest and cell death.
a) Two representative Abelson virus transformed pro-B cell lines were cultured for 16 hours in the presence or absence of 5 μM STI-571. Graph shows fold-induction of TRAIL transcript levels normalized to HPRT as measured by real time RT-PCR. Error bars are standard deviation of triplicate samples. The data is representative of at least three similar experiments.
b) PD31 cells were retrovirally transduced with a vector expressing FADD-DN or stably transfected with a Bcl-xL expression vector or with both and selected for drug resistance. Cells were cultured in the presence (upper panels) or absence (lower panels) of 2.5 μM STI-571 for 36 hours, then stained with Annexin V-FITC and 7-AAD. The numbers indicate percentage of total cells in the gate. Results represent one of 3 similar experiments
b) Cells in (b) were cultured in the presence or absence of 2.5 μM STI for 17 hours, ethanol fixed, and then stained with PI in the presence of RNase. Cells were analyzed for cell cycle status by flow cytometry, excluding cells with a sub-G1 DNA content. Numbers indicate the percentage of cells in G1 as determined with the Watson (pragmatic) protocol +/- the standard deviation of 3 replicates. Shaded histogram = untreated; solid line = STI treated.
When Abelson cells are cultured in the presence of STI-571, they undergo a G1 cell cycle arrest prior to apoptosis (Muljo and Schlissel, 2003). In addition to its well-documented role in the apoptotic machinery, FADD also has been shown to promote the cell cycle in primary T cells. FADD-deficient T cells show impaired proliferation after activation, suggesting that FADD is a necessary component of the cell cycle machinery in T cells (Kabra et al., 2001; Zhang et al., 2001). However, FADD is generally thought to be uncoupled from cycling in transformed cells and cell lines (Hueber et al., 2000; Newton et al., 1998; Park et al., 2005; Zhang et al., 1998). We determined the cell cycle profile of the parental and FADD-DN-expressing cells by staining permeabilizied cells with propidium iodide. We found that expression of FADD-DN delayed the onset of G1 arrest (Fig. 1c). This was not simply due to blocking the apoptotic program since cells expressing Bcl-xL underwent a normal G1 arrest despite being significantly protected from the onset of apoptosis (Fig. 1b, c). Our data indicate that TRAIL signaling plays an important role in STI-induced G1 arrest and apoptosis in Abelson cells, and that FADD may be playing a different role in promotion of the cell cycle in Abelson cells than in thymocytes.
FoxO3a activates TRAIL transcription and G1 arrest in Abelson cells
We went on to ask which transcription factors were involved in the upregulation of TRAIL upon STI-571 treatment. FoxO3a is capable of binding to the TRAIL promoter and has been linked to TRAIL transcription in BCR-Abl-transformed cell lines (Ghaffari et al., 2003; Modur et al., 2002). FoxO3a, like other members of the FoxO family, is inhibited by phosphorylation of three residues (T32, S253, and S315 for FoxO3a) by kinases such as AKT and SGK, resulting in its cytoplasmic retention (Biggs et al., 1999; Huang and Tindall, 2007).
We used a retroviral vector to transduce a GFP-tagged version of the wild type FoxO3a into PD31 cells and observed a 5-fold increase in TRAIL transcription in the GFP-positive population (Fig. 2a). The cells had a growth disadvantage and were lost from a bulk population over time (data not shown). To get around the apparent toxicity of FoxO3a, we obtained versions of both FoxoO3a and FoxO1 cDNAs that had been mutated at the three AKT-target sites and fused to a modified version of the estrogen receptor hormone-binding domain (FoxO3a(A3)-ER and FoxO1(A3)-ER) (Mattioni et al., 1994). In the absence of hormone stimulation, these constructs are retained in the cytoplasm; addition of estrogen allows for the release and translocation of the proteins to the nucleus. We found that upon addition of tamoxifen (an estrogen mimic), the FoxO3a(A3)-ER-expressing cells induced TRAIL transcription to a similar magnitude as when those cells were treated with STI-571 (Fig. 2b). Tamoxifen had no affect on the uninfected parental cells (Fig. 2b). Cells expressing the FoxO1(A3)-ER did not induce TRAIL upon tamoxifen treatment, though they did induce the transcription of other known FoxO1-target genes, such as Rag1 (Fig. 2c and (Amin and Schlissel, 2008)).
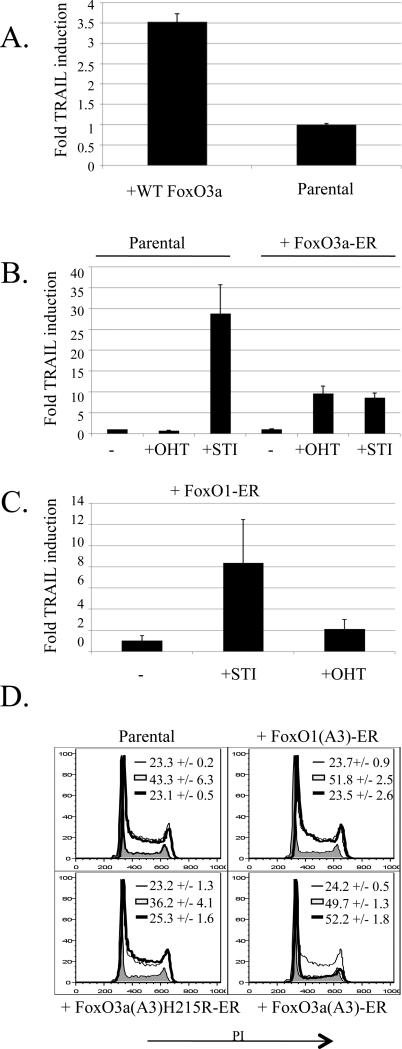
Figure 2. Expression of a constitutively active FoxO3a mutant increases TRAIL transcription and induces G1 arrest of Abelson-transformed cells.
a) PD31 cells were infected with a FoxO3a-GFP expressing retrovirus. Cells were sorted for GFP, grown for 3 days in culture, and then analyzed by real-time PCR for TRAIL and HPRT transcript levels. Graph represents fold TRAIL induction normalized for HPRT expression. Error bars are the standard deviation of triplicate samples. Results are representative of at least 3 similar experiments.
b) 7G-S cells were infected with a retroviral FoxO3a(A3)-ER construct, sorted for a marker of infection, and cultured in the presence of 4 μM tamoxifen (OHT) or 10 μM STI for 17 hours and compared to uninfected parental cells treated the same way. TRAIL and HPRT transcript levels were determined by real-time PCR. Graph represents fold TRAIL induction normalized for HPRT expression. Error bars are the standard deviation of triplicate samples. Results are representative of 3 similar experiments.
c) 7G-S cells were infected with a FoxO1(A3)-ER construct, and analyzed as in b.
d) 7G-S cells were infected with FoxO3a(A3)-ER, FoxO1(A3)-ER, or FoxO3a(A3)H215R-ER expressing retroviruses. Cells were cultured in the absence (thin line), or presence of 10 μM STI (filled region), or 4 μM OHT (thick line) for 20 hours before being analyzed for DNA content as in 1b. Numbers indicate the percentage of cells in G1 +/- the standard deviation of 3 replicates.
In addition to inducing TRAIL transcription, activation of FoxO3a(A3)-ER with tamoxifen resulted in a pronounced G1 arrest (Fig. 2d). This activity was dependent upon the DNA binding capabilities of FoxO3a as a non-DNA binding point mutant (H215R; (Ramaswamy et al., 2002)) did not affect the cell cycle (Fig. 2d). FoxO1(A3)-ER activation was unable to halt the cell cycle (Fig. 2d). These results show that active FoxO3a is capable of increasing TRAIL transcription and causing a G1 arrest in Abelson cells, even in the presence of an active v-Abl kinase, and that this activity is specific to FoxO3a.
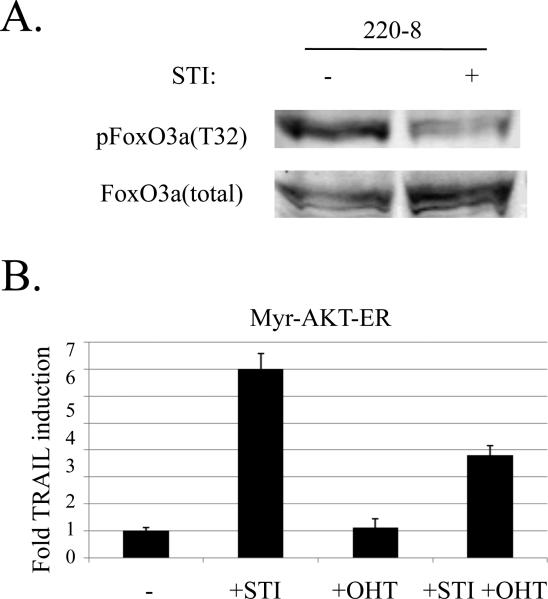
The activity of FoxO3a, like all FoxO family members, is regulated by its phosphorylation state. To address whether STI-571 treatment of Abelson transformed cells results in the dephosphorylation and activation of FoxO3a, we examined the phosphorylation status of FoxO3a in STI-571 treated and untreated cells. We found that STI-571 treatment greatly reduced the fraction of T32-phosphorylated FoxO3a in v-Abl cells (Fig. 3a). To test whether this dephosphorylation played a role in the induction of TRAIL transcription, we prevented dephosphorylation by expressing an inducible, constitutively active version of AKT (myr-AKT-ER) within these cells. AKT has been shown to directly phosphorylate and inactivate FoxO3a in numerous cell lines (Biggs et al., 1999). Indeed, in the setting of constitutive AKT activity, we observed an approximately 50% reduction in the magnitude of the TRAIL induction upon STI-571 treatment (Fig. 3b). Surprisingly, the expression of a constitutively active AKT construct was toxic to Abelson cells (data not shown). We showed previously that Abelson cell lines have a low level of basal AKT activity (Amin and Schlissel, 2008). This indicates that another kinase is likely responsible for the phosphorylation of FoxO3a observed within Abelson cells. The Serum and Glucocorticoid-inducible Kinase (SGK) has been shown to have a greater preference for phosphorylating FoxO3a T32 in vivo when compared to AKT (Brunet et al., 2001). Taken together, these results suggest that FoxO3a might be inactivated within Abelson cells in part via SGK-mediated FoxO3A phosphorylation, and the dephosphorylation and activation of FoxO3a is a part of the mechanism of STI-571 and TRAIL-induced v-Abl-transformed cell death.
Figure 3. FoxO3a is dephosphorylated upon v-Abl inhibition and TRAIL transcription is diminished in the presence of a constitutively active AKT.
a) Lysates from 220-8 Abelson cells cultured for 16 hours in the presence (+) or absence (-) of 10 μM STI were analyzed by Western blot for T32-phosphorylated or total FoxO3a as indicated.
b) 7G-S cells were infected with a retrovirus expressing ER-myr-Akt, and sorted. Cells were treated with either 10 μM STI, 4 μM OHT, or both for 17 hours. TRAIL transcription was analyzed by real time PCR and normalized to HPRT. Graph presents fold TRAIL induction.
IKKβ activity in v-Abl transformed cells inactivates FoxO3a
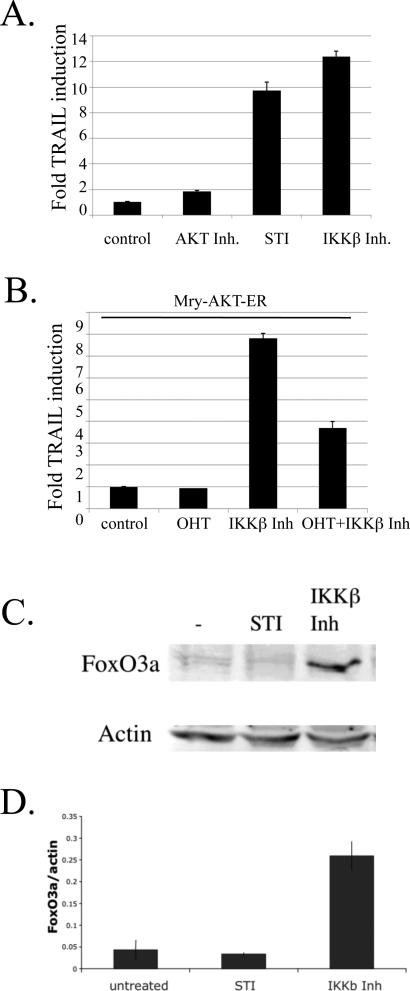
FoxoO3a is unique amongst FoxO family members in that it can be phosphorylated by the IκB kinase IKKβ, leading to its ubiquitination and degradation (Hu et al., 2004) (Huang and Tindall, 2007). This strategy for keeping FoxO3a inactive is used by a number of transformed cell lines that exhibit low basal levels of AKT activity (Hu et al., 2004). Active IKKβ is phosphorylated upon specific serine residues within the activation loop of the kinase (Hacker and Karin, 2006). Treatment of Abelson cells with an IKKβ inhibitor but not an AKT inhibitor led to an increase in TRAIL mRNA levels, along with a dramatic increase in apoptotic cells (Fig. 4a, supplemental Fig. 2). Combined treatment of cells with STI571 along with the IKKβ inhibitor did not have an additive effect on the upregulation of TRAIL transcription (data not shown). To determine if the increase in TRAIL message was due in part to FoxO3a activation, we treated our constitutively-active AKT-expressing cells with the IKKβ inhibitor in the presence or absence of the AKT inducer. We found that AKT activity blunted the IKKβ-inhibitor induced increase in TRAIL transcription (Fig. 4b). Additionally, the inhibition of IKKβ led to an approximately 6-fold increase in the amount of total FoxO3a protein in v-Abl transformed cells (Fig. 4c & 4d). To further test the link between FoxO3a and IKKβ, we utilized a FoxO1 and FoxO3a shRNA expression system previously described by our lab, which results in a partial knockdown of the FoxO protein levels (Amin and Schlissel, 2008). Neither shRNA construct affected the upregulation of TRAIL in response to STI571 or the IKKβ inhibition (data not shown), most likely due to the incomplete nature of the knockdown. Our results suggest that IKKβ plays a role in inactivating FoxO3a in Abelson cells, and that following IKKβ inhibition, TRAIL transcription is activated via the FOXO3a pathway. However, STI-571 treatment alone did not significantly increase the amount of FoxO3a protein. This implies that multiple mechanisms for controlling FoxO3a activation and protein levels exist within Abelson cells and that FoxO3a activation is not the only mechanism by with STI571 treatment upregulated TRAIL transcription..
Figure 4. IKKβ is active in v-Abl-transformed cells and inhibits FoxO3a activity.
a) 7G-S cells were cultured in the presence of 2 μM AKT inhibitor VIII, 10 μM STI571, or 5 μM IKKβ inhibitor III for 20 hours at which time TRAIL and HPRT transcripts were analyzed by real-time PCR. TRAIL transcripts were normalized to HPRT. Graph presents fold-induction of TRAIL.
b) myr-Akt-ER-transduced cells were cultured in the presence of 4 μM OHT, 5 μM IKKβ inhibitor III, or both for 17 hours at which time transcripts levels of TRAIL and HPRT were determined by real time PCR. Results indicate fold TRAIL induction.
c) Total cell lysates from 7G-S Abelson cells cultured in the presence of 10 μM STI or 10 μM IKKβ inhibitor III, and analyzed by Western blot for FoxO3a and actin.
d) The graph represents the intensity of the FoxO3a band normalized to the intensity of the actin band from two independent repetitions of the experiment shown part c, above.
NF-κB can activate TRAIL transcription in Abelson cells
Abelson transformed cells, unlike BCR-Abl transformed cells, exhibit low levels of NF-κB activity; upon v-Abl inhibition, NF-κB activity levels dramatically increase (Hakansson et al., 2008; Klug et al., 1994; Zou and Calame, 1999). Previously, our lab reported that overexpression of the NF-κB subunit RelA is toxic to v-Abl transformed cells, resulting in a 2-3 fold increase in the fraction of apoptotic cells (Sheehy and Schlissel, 1999). NF-κB itself has been linked to TRAIL transcription in Jurkat T cells. Given that blocking FoxO3a activation via AKT expression does not completely block the STI-571 induced TRAIL response (Fig. 3b), we tested whether in Abelson cells the activation of NF-κB downstream of STI-571 treatment induces TRAIL transcription.
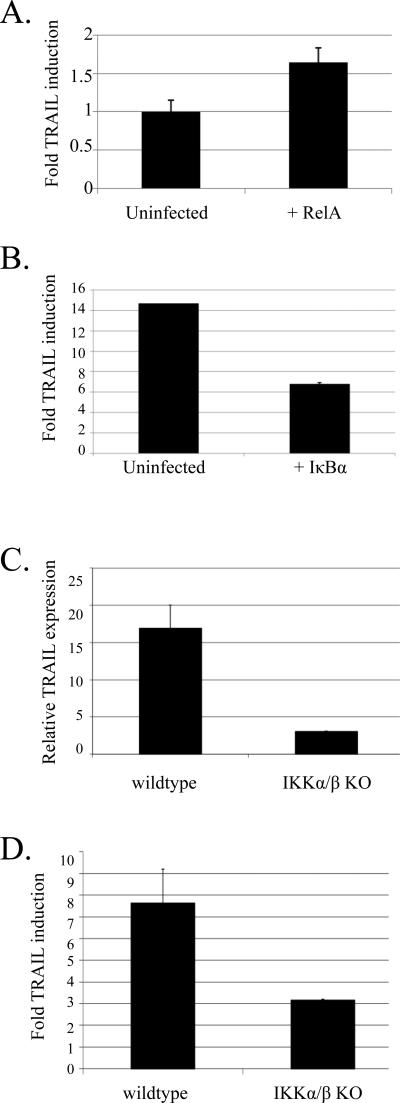
Ableson cells were retrovirally transduced with a RelA cDNA, sorted, and then assayed for TRAIL transcription. We found a 40% increase in TRAIL transcription in the RelA-overexpressing cells (Fig.5a). To block NF-κB induction after STI treatment, we expressed an N-terminally truncated version of IκBα (IκBαΔN), which is incapable of being degraded. IκBαΔN expression was capable of blocking the nuclear accumulation of RelA after STI-571 treatment as assayed by immunofluorescence (Supplementary Figure 3). IκBαΔN-expressing cells showed a 2-fold decrease in TRAIL transcription after STI treatment (Fig. 5b). To examine the requirement for NFκB in the upregulation of TRAIL, we examined the level of TRAIL message in 4 independently derived IKKα/β knockout Ableson-transformed cell lines in the presence or absence of STI-571. We found that in three out of four lines, the basal level of TRAIL transcription in untreated cells was significantly reduced (Fig. 5c) and upon STI-571 treatment, TRAIL upregulation was diminished (Fig. 5d). Together, these results suggest that NF-κB activity plays a role in the induction of TRAIL transcription after STI-571 treatment of Abelson cells and is responsible in large part for the basal levels of TRAIL transcription seen in v-Abl transformed cell lines. This is in contrast to BCR-Abl-transformed cells that show constitutive NF-κB activity in the absence of significant TRAIL expression (Hakansson et al., 2008; Zou and Calame, 1999).
Figure 5. RelA expression activates TRAIL transcription.
a) PD31 cells were infected with a retrovirus expressing RelA and sorted based on a marker of infection. The infected and uninfected cells were grown in culture for 3 days and then analyzed for relative levels of TRAIL transcripts by real time PCR. The graph presents fold TRAIL induction upon RelA expression normalized for HPRT levels. Error bars are the standard deviation of triplicate samples. Results are representative of 3 similar experiments.
b) 7G-S cells were infected with IκB-ΔN and sorted. Cells were treated with 10 μM STI for 17 hours and then analyzed for TRAIL and HPRT transcription via real time PCR. Graph represents the fold induction of STI treated infected and uninfected cells as compared to untreated cells. Error bars are the standard deviation of triplicate samples. Results representative of 3 similar experiments.
c) Wildtype or IKKα/β knockout cell lines were analyzed for TRAIL and HPRT transcription via real time PCR. Graph represents the level of TRAIl transcription normalized to HPRT transcripts. Results are representative of three out of four independently derived cell lines.
d) Wildtype or IKKα/β knockout cell lines were treated with 2.5 μM STI571 for 24 hours and then analyzed for TRAIL and HPRT transcription via real time PCR. Graph represents the fold induction of STI treated infected and uninfected cells as compared to untreated cells. Error bars are the standard deviation of triplicate samples. Results representative of 3 out of 4 cell lines tested.
DISCUSSION
Cells are protected from transformation by a variety of mechanisms that respond to aberrant growth signals by activating apoptosis. The human fusion oncoprotein BCR-Abl activates proliferation while avoiding apoptosis by suppressing TRAIL transcription via FoxO3a inactivation (Ghaffari et al., 2003; Kikuchi et al., 2007; Komatsu et al., 2003). We set out to probe the role of TRAIL suppression in transformation of murine pro-B cells by v-Abl. We found that the inhibition of v-Abl kinase activity results in an increase in TRAIL transcription (Fig. 1a). In addition, disruption of signaling through the TRAIL receptor by expression of a FADD-DN construct delays the onset of apoptosis in STI-571-treated Abelson cells, albeit not as completely as the expression of a Bcl-xL transgene. High levels of the anti-apoptotic members of the Bcl-2 family were previously reported to protect Abelson cells from apoptosis (Banerjee and Rothman, 1998; Chen et al., 1994; Cory et al., 2003). Taken together, this implies that TRAIL-induced apoptosis in Abelson cells works via a Type II mechanism (Zhang and Fang, 2005).
The FADD-DN construct had an unexpected affect on the cell cycle status of Abelson transfectants. Treatment of v-Abl transformed pro-B cells with STI-571 results in a strong G1 arrest. We found that the onset of G1 arrest was delayed in cells expressing the FADD-DN construct. This effect was not seen with the Bcl-xL transgene, and thus cannot be simply explained by a delay in the activation of apoptosis. In addition to its role in apoptosis, FADD has been shown to play a role in cell proliferation (Park et al., 2005). Peripheral T cells from FADD knockout mice show a defect in co-stimulation-induced proliferation (Zhang et al., 1998; Zhang et al., 2001). In thymocytes, FADD-DN increased negative selection, indicating that FADD signaling can also promote survival and proliferation (Newton et al., 1998). In our Abelson cell system, FADD-DN had the opposite effect, promoting cycling after oncogene inhibition. This implies that in Abelson cells, FADD may play a negative role in the cell cycle. The biochemical basis of this surprising observation remains to be determined.
We next examined what role FoxO3a inhibition plays in Abelson cells. By utilizing a tamoxifen-inducible versions of constitutively active constructs, we were able to show that FoxO3a activity but not that of a related family member leads to a large increase in TRAIL transcription as well as a G1 arrest of Abelson cells. A similar result has been reported for BCR-Abl-transformed cells (Kikuchi et al., 2007). Individual knockout mice for each of the mammalian FoxO members showed that the proteins had numerous non-redundant functions (Hosaka et al., 2004). However, in our hands, expression of FoxO3a(A3) is capable of transcriptionally upregulating the FoxO1 target gene Rag1 in Abelson cells (Amin and Schlissel, 2008). That a similar redundancy does not exist at the TRAIL locus highlights the specific role played by FoxO3a in promoting cell death in both mouse and human leukemias.
Our data show that both FoxO3a and NF-κB play a role in the activation of TRAIL transcription after STI-571 treatment (Fig. 6). We found that the inhibition of either pathway could not completely block TRAIL transcription, which supports the idea of multiple pathways converging on the activation of TRAIL. In BCR-Abl-transformed cells, the suppression of TRAIL transcription is mediated via the phosphorylation-based inhibition of FoxO3a (Ghaffari et al., 2003; Kikuchi et al., 2007). Our data shows that in addition to this inhibition, a second pathway is active within Abelson cells to inhibit FoxO3a. In tumors that lack AKT activity, IKKβ phosphorylates FoxO3a at a unique site not shared by the other FoxO family members, resulting in the degradation of FoxO3a (Hu et al., 2004). We found that inhibition of IKKβ induced TRAIL transcription within Abelson cells and increased the total protein levels of FoxO3a in the absence of v-Abl inhibition. However, STI-571 treatment alone did not significantly change overall FoxO3a protein levels (Fig. 4d). Though IKKβ remains active when v-Abl is inhibited (as evidenced by NF-κB activation, see below), the loss of inhibitory AKT-family phosphorylation may drive existing FoxO3a into the nucleus, resulting in TRAIL upregulation in the absence of a change in FoxO3a protein levels. We found that an incomplete knockdown of FoxO3a protein did not affect the magnitude of TRAIL upregulation after IKKβ inhibition or STI571 treatment. This indicates that the inactivation of cellular FoxO3a by AKT family members may play a more biologically important role in the inhibition of TRAIL upregulation than the control of the total protein levels via IKK-degradation. In Abelson cells, but not BCR-Abl transformed cells, multiple pathways apparently lead to the inactivation of FoxO3a, and the outcome - inactivation or nuclear translocation – relies upon the combination of these multiple inputs (Fig. 6). Further experiments are required to separate the involvement of these two pathways in Abelson transformed cells.
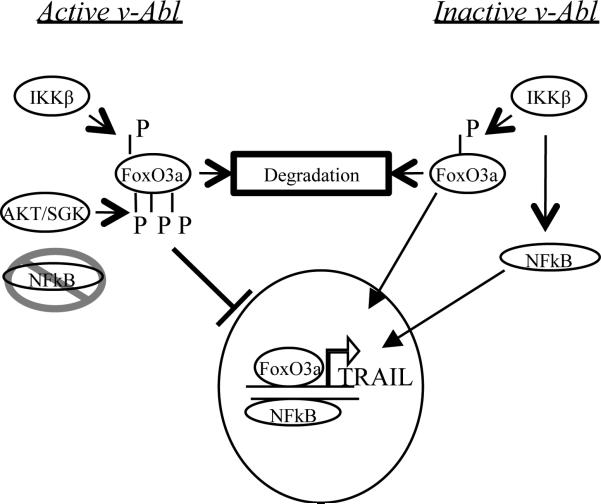
Figure 6. A Model for TRAIL activation in v-Abl transformed cells.
When v-Abl is active (left side), low levels of NF-κB activity exist. FoxO3a is phosphorylated by IKKβ, leading to degradation, as well as by AKT/SGK, preventing nuclear translocation of the basal level of the protein. The inactivation of both NF-κB and FoxO3a results in low TRAIL transcription. Upon v-Abl inhibition (right side), NF-κB is activated downstream of the IKK complex, leading to TRAIL activation. Simultaneously, though still being kept at a low total protein level via IKKβ phosphorylation, cellular Foxo3a is activated by the removal of the inhibitory phosphorylation and translocates to the nucleus, activating TRAIL transcription.
Simultaneously with the activation of TRAIL transcription via FoxO3a, we found that NF-κB itself can promote TRAIL transcription within Abelson cells. This is in contrast to BCR-Abl transformed cells which have constitutive NF-κB activity in the absence of significant TRAIL transcription (Hakansson et al., 2008; Zou and Calame, 1999).
It has been shown that in Abelson cells, NF-κB is largely inactive; its activity can be induced by either LPS treatment (Sen and Baltimore, 1986) or v-Abl inactivation (Klug et al., 1994). Upon STI treatment of Abelson cells, in addition to the activation of FoxO3a, the activation of NF-κB leads to an increase in TRAIL transcription and the induction of apoptosis within these cells. Interestingly, although the basal activity of NFκB is low within Abelson cells, we found that completely blocking NFκB activation in an IKKα/β knockout cell line severely reduced the basal level of TRAIL transcription (Fig. 5c). The remaining low levels of transcription, along with the STI571-induced increase, may be a result of FoxO3a activation. In the absence of IKKβ activity, FoxO3a levels may increase to the point that they overwhelm the AKT-based inactivation system.
Our data support a role for IKKβ in the suppression of TRAIL transcription that is independent of its role in the IKK-complex in Abelson transformed cells. Cellular roles for the individual IKKs independent of their ability to activate NF-κB have been reported (Perkins, 2007). Accordingly, Abelson cells must activate mechanisms downstream of IKK activation to circumvent the activation of NF-κB. One possibility is a v-Abl-mediated stabilization of IkBα, an activity that has been reported for c-Abl (Kawai et al., 2002).
TRAIL expression promotes the G1 arrest and apoptosis of Abelson cells after STI-571 treatment. To block TRAIL transcription, Abelson cells block both FoxO3a as well as NF-κB activation. Unlike cells that express BCR-Abl, v-Abl transformed Abelson cells use multiple pathways to inhibit FoxO3a, highlighting the incompatibility of both NFκB and FoxO3a activity and their continued proliferation and survival, as well as the different mechanisms by which various Abelson oncogenes avoid TRAIL-induced apoptosis.
Supplementary Material
REFERENCES
- Abelson HT, Rabstein LS. Lymphosarcoma: virus-induced thymic-independent disease in mice. Cancer Res. 1970;30:2213–2222. [PubMed] [Google Scholar]
- Advani AS, Pendergast AM. Bcr-Abl variants: biological and clinical aspects. Leuk Res. 2002;26:713–720. doi: 10.1016/s0145-2126(01)00197-7. [DOI] [PubMed] [Google Scholar]
- Alt FW, Yancopoulos GD, Blackwell TK, Wood C, Thomas E, Boss M, Coffman R, Rosenberg N, Tonegawa S, Baltimore D. Ordered rearrangement of immunoglobulin heavy chain variable region segments. EMBO J. 1984;3:1209–1219. doi: 10.1002/j.1460-2075.1984.tb01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin RH, Schlissel MS. Foxo1 directly regulates the transcription of recombination-activating genes during B cell development. Nat Immunol. 2008;9:613–622. doi: 10.1038/ni.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baetu TM, Kwon H, Sharma S, Grandvaux N, Hiscott J. Disruption of NF-kappaB signaling reveals a novel role for NF-kappaB in the regulation of TNF-related apoptosis-inducing ligand expression. J Immunol. 2001;167:3164–3173. doi: 10.4049/jimmunol.167.6.3164. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Rothman P. IL-7 reconstitutes multiple aspects of v-Abl-mediated signaling. J Immunol. 1998;161:4611–4617. [PubMed] [Google Scholar]
- Biggs WH, 3rd, Meisenhelder J, Hunter T, Cavenee WK, Arden KC. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci U S A. 1999;96:7421–7426. doi: 10.1073/pnas.96.13.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a). Mol Cell Biol. 2001;21:952–965. doi: 10.1128/MCB.21.3.952-965.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Wang J. Initiator caspases in apoptosis signaling pathways. Apoptosis. 2002;7:313–319. doi: 10.1023/a:1016167228059. [DOI] [PubMed] [Google Scholar]
- Chen YY, Wang LC, Huang MS, Rosenberg N. An active v-abl protein tyrosine kinase blocks immunoglobulin light-chain gene rearrangement. Genes Dev. 1994;8:688–697. doi: 10.1101/gad.8.6.688. [DOI] [PubMed] [Google Scholar]
- Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22:8590–8607. doi: 10.1038/sj.onc.1207102. [DOI] [PubMed] [Google Scholar]
- Deng Y, Lin Y, Wu X. TRAIL-induced apoptosis requires Bax-dependent mitochondrial release of Smac/DIABLO. Genes Dev. 2002;16:33–45. doi: 10.1101/gad.949602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derudder E, Cadera EJ, Vahl JC, Wang J, Fox CJ, Zha S, van Loo G, Pasparakis M, Schlissel MS, Schmidt-Supprian M, et al. Development of immunoglobulin lambda-chain-positive B cells, but not editing of immunoglobulin kappa-chain, depends on NF-kappaB signals. Nat Immunol. 2009;10:647–654. doi: 10.1038/ni.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S, Zimmermann J, Lydon NB. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2:561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- Fang W, Rivard JJ, Ganser JA, LeBien TW, Nath KA, Mueller DL, Behrens TW. Bcl-xL rescues WEHI 231 B lymphocytes from oxidant-mediated death following diverse apoptotic stimuli. J Immunol. 1995;155:66–75. [PubMed] [Google Scholar]
- Ghaffari S, Jagani Z, Kitidis C, Lodish HF, Khosravi-Far R. Cytokines and BCR-ABL mediate suppression of TRAIL-induced apoptosis through inhibition of forkhead FOXO3a transcription factor. Proc Natl Acad Sci U S A. 2003;100:6523–6528. doi: 10.1073/pnas.0731871100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green PL, Kaehler DA, Bennett LM, Risser R. Multiple steps are required for the induction of tumors by Abelson murine leukemia virus. J Virol. 1989;63:1989–1994. doi: 10.1128/jvi.63.5.1989-1994.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci STKE. 20062006:re13. doi: 10.1126/stke.3572006re13. [DOI] [PubMed] [Google Scholar]
- Hakansson P, Nilsson B, Andersson A, Lassen C, Gullberg U, Fioretos T. Gene expression analysis of BCR/ABL1-dependent transcriptional response reveals enrichment for genes involved in negative feedback regulation. Genes Chromosomes Cancer. 2008;47:267–275. doi: 10.1002/gcc.20528. [DOI] [PubMed] [Google Scholar]
- Henson ES, Johnston JB, Gibson SB. The role of TRAIL death receptors in the treatment of hematological malignancies. Leuk Lymphoma. 2008;49:27–35. doi: 10.1080/10428190701713655. [DOI] [PubMed] [Google Scholar]
- Hosaka T, Biggs WH, 3rd, Tieu D, Boyer AD, Varki NM, Cavenee WK, Arden KC. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc Natl Acad Sci U S A. 2004;101:2975–2980. doi: 10.1073/pnas.0400093101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang F, Yang JY, Zou Y, Bao S, Hanada N, Saso H, et al. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117:225–237. doi: 10.1016/s0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]
- Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120:2479–2487. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- Hueber AO, Zornig M, Bernard AM, Chautan M, Evan G. A dominant negative Fas-associated death domain protein mutant inhibits proliferation and leads to impaired calcium mobilization in both T-cells and fibroblasts. J Biol Chem. 2000;275:10453–10462. doi: 10.1074/jbc.275.14.10453. [DOI] [PubMed] [Google Scholar]
- Kabra NH, Kang C, Hsing LC, Zhang J, Winoto A. T cell-specific FADD-deficient mice: FADD is required for early T cell development. Proc Natl Acad Sci U S A. 2001;98:6307–6312. doi: 10.1073/pnas.111158698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai H, Nie L, Yuan ZM. Inactivation of NF-kappaB-dependent cell survival, a novel mechanism for the proapoptotic function of c-Abl. Mol Cell Biol. 2002;22:6079–6088. doi: 10.1128/MCB.22.17.6079-6088.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi S, Nagai T, Kunitama M, Kirito K, Ozawa K, Komatsu N. Active FKHRL1 overcomes imatinib resistance in chronic myelogenous leukemia-derived cell lines via the production of tumor necrosis factor-related apoptosis-inducing ligand. Cancer Sci. 2007;98:1949–1958. doi: 10.1111/j.1349-7006.2007.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug CA, Gerety SJ, Shah PC, Chen YY, Rice NR, Rosenberg N, Singh H. The v-abl tyrosine kinase negatively regulates NF-kappa B/Rel factors and blocks kappa gene transcription in pre-B lymphocytes. Genes Dev. 1994;8:678–687. doi: 10.1101/gad.8.6.678. [DOI] [PubMed] [Google Scholar]
- Komatsu N, Watanabe T, Uchida M, Mori M, Kirito K, Kikuchi S, Liu Q, Tauchi T, Miyazawa K, Endo H, et al. A member of Forkhead transcription factor FKHRL1 is a downstream effector of STI571-induced cell cycle arrest in BCR-ABL-expressing cells. J Biol Chem. 2003;278:6411–6419. doi: 10.1074/jbc.M211562200. [DOI] [PubMed] [Google Scholar]
- Kruyt FA. TRAIL and cancer therapy. Cancer Lett. 2008;263:14–25. doi: 10.1016/j.canlet.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Lee JY, Huerta-Yepez S, Vega M, Baritaki S, Spandidos DA, Bonavida B. The NO TRAIL to YES TRAIL in cancer therapy (review). Int J Oncol. 2007;31:685–691. [PubMed] [Google Scholar]
- Lewis S, Rosenberg N, Alt F, Baltimore D. Continuing kappa-gene rearrangement in a cell line transformed by Abelson murine leukemia virus. Cell. 1982;30:807–816. doi: 10.1016/0092-8674(82)90285-9. [DOI] [PubMed] [Google Scholar]
- Marshall AJ, Fleming HE, Wu GE, Paige CJ. Modulation of the IL-7 dose-response threshold during pro-B cell differentiation is dependent on pre-B cell receptor expression. J Immunol. 1998;161:6038–6045. [PubMed] [Google Scholar]
- Mattioni T, Louvion JF, Picard D. Regulation of protein activities by fusion to steroid binding domains. Methods Cell Biol. 1994;43(Pt A):335–352. doi: 10.1016/s0091-679x(08)60611-1. [DOI] [PubMed] [Google Scholar]
- Mauro MJ, Druker BJ. STI571: targeting BCR-ABL as therapy for CML. Oncologist. 2001;6:233–238. doi: 10.1634/theoncologist.6-3-233. [DOI] [PubMed] [Google Scholar]
- Modur V, Nagarajan R, Evers BM, Milbrandt J. FOXO proteins regulate tumor necrosis factor-related apoptosis inducing ligand expression. Implications for PTEN mutation in prostate cancer. J Biol Chem. 2002;277:47928–47937. doi: 10.1074/jbc.M207509200. [DOI] [PubMed] [Google Scholar]
- Muljo SA, Schlissel MS. A small molecule Abl kinase inhibitor induces differentiation of Abelson virus-transformed pre-B cell lines. Nat Immunol. 2003;4:31–37. doi: 10.1038/ni870. [DOI] [PubMed] [Google Scholar]
- Newton K, Harris AW, Bath ML, Smith KG, Strasser A. A dominant interfering mutant of FADD/MORT1 enhances deletion of autoreactive thymocytes and inhibits proliferation of mature T lymphocytes. Embo J. 1998;17:706–718. doi: 10.1093/emboj/17.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SM, Schickel R, Peter ME. Nonapoptotic functions of FADD-binding death receptors and their signaling molecules. Curr Opin Cell Biol. 2005;17:610–616. doi: 10.1016/j.ceb.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Nakamura N, Sansal I, Bergeron L, Sellers WR. A novel mechanism of gene regulation and tumor suppression by the transcription factor FKHR. Cancer Cell. 2002;2:81–91. doi: 10.1016/s1535-6108(02)00086-7. [DOI] [PubMed] [Google Scholar]
- Roth W, Reed JC. Apoptosis and cancer: when BAX is TRAILing away. Nat Med. 2002;8:216–218. doi: 10.1038/nm0302-216. [DOI] [PubMed] [Google Scholar]
- Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME. Two CD95 (APO-1/Fas) signaling pathways. Embo J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer U, Voloshanenko O, Willen D, Walczak H. TRAIL: a multifunctional cytokine. Front Biosci. 2007;12:3813–3824. doi: 10.2741/2354. [DOI] [PubMed] [Google Scholar]
- Sen R, Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986;47:921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- Sheehy AM, Schlissel MS. Overexpression of RelA causes G1 arrest and apoptosis in a pro-B cell line. J Biol Chem. 1999;274:8708–8716. doi: 10.1074/jbc.274.13.8708. [DOI] [PubMed] [Google Scholar]
- Sheridan JP, Marsters SA, Pitti RM, Gurney A, Skubatch M, Baldwin D, Ramakrishnan L, Gray CL, Baker K, Wood WI, et al. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 1997;277:818–821. doi: 10.1126/science.277.5327.818. [DOI] [PubMed] [Google Scholar]
- Xu G, Shi Y. Apoptosis signaling pathways and lymphocyte homeostasis. Cell Res. 2007;17:759–771. doi: 10.1038/cr.2007.52. [DOI] [PubMed] [Google Scholar]
- Zhang J, Cado D, Chen A, Kabra NH, Winoto A. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature. 1998;392:296–300. doi: 10.1038/32681. [DOI] [PubMed] [Google Scholar]
- Zhang J, Kabra NH, Cado D, Kang C, Winoto A. FADD-deficient T cells exhibit a disaccord in regulation of the cell cycle machinery. J Biol Chem. 2001;276:29815–29818. doi: 10.1074/jbc.M103838200. [DOI] [PubMed] [Google Scholar]
- Zhang J, Winoto A. A mouse Fas-associated protein with homology to the human Mort1/FADD protein is essential for Fas-induced apoptosis. Mol Cell Biol. 1996;16:2756–2763. doi: 10.1128/mcb.16.6.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Fang B. Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther. 2005;12:228–237. doi: 10.1038/sj.cgt.7700792. [DOI] [PubMed] [Google Scholar]
- Zou X, Calame K. Signaling pathways activated by oncogenic forms of Abl tyrosine kinase. J Biol Chem. 1999;274:18141–18144. doi: 10.1074/jbc.274.26.18141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.