Abstract
Nanomedicine is an emerging field of medicine which utilizes nanotechnology concepts for advanced therapy and diagnostics. This convergent discipline, which merges research areas such as chemistry, biology, physics, mathematics and engineering thus bridging the gap between molecular and cellular interactions, has a potential to revolutionize current medical practice. This review presents recent developments in nanomedicine research, which are poised to have an important impact on cardiovascular disease and treatment by improving therapy and diagnosis of such cardiovascular disorders as atherosclerosis, restenosis and myocardial infarction. Specifically, we discuss the use of nanoparticles for molecular imaging and advanced therapeutics, specially designed drug eluting stents and in vivo/ex vivo early detection techniques.
Introduction
Despite significant clinical advancements in the field, cardiovascular diseases (CVD), which include various disorders of blood vasculature and heart, as well as stroke, remain the leading cause of death in the United States. Based on the NIH and American Heart Association statistics, close to 80 million people in the U.S. suffer from CVD and more than 35% of American deaths are attributed to CVD (http://www.nhlbi.nih.gov; www.americanheart.org). The last disruptive technology to impact CVD occurred over a decade ago with the introduction of the coronary stent by Palmaz & Schatz—FDA approved in 1994. Since then, clinical medicine has relied upon new blockbuster therapeutics (statins, beta blockers, and diuretics) and refinements of surgical procedures such as percutaneous transluminal coronary angioplasty (PTCA), coronary artery bypass grafts (CABG) and stenting to treat CVD; however, current techniques for early detection and advanced therapies of CVD are limited and their efficiency in preventing the diseases is questionable.
By definition, nanotechnology involves the following interrelated constituents: nanoscale dimensions of the whole system or its vital components, man-made nature and the unique characteristics of new material that arise due to its nanoscopic size [1, 2]. In fact, nanotechnology represents a convergent discipline, in which the margins separating various research areas such as chemistry, biology, physics, mathematics and engineering, become blurred. Cardiovascular nanomedicine is likely to face and address current challenges in CVD and to improve detection and therapy by advancing ex vivo and in vivo biomarkers detection and imaging, as well as by directed/improved drug delivery and tissue regeneration [3].
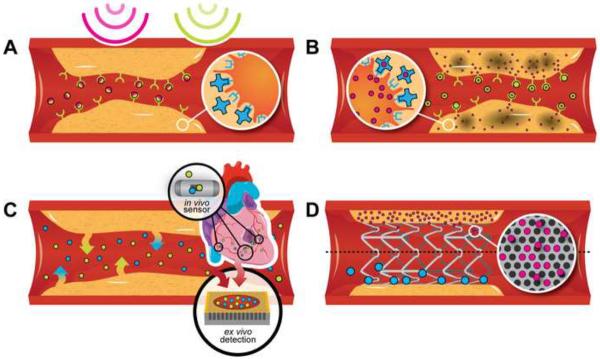
In this review we will summarize and discuss recent developments in the field of nanotechnology for the detection and therapy of CVD, focusing on nanoparticles, specially designed therapeutic and tissue regeneration devices, and in vivo/ex vivo early detection techniques (Figure 1).
Figure 1.
Schematic presentation of various nanotechnological approaches for advanced CVD diagnosis and therapy: Nanoparticles for (A) multimodal image contrast and (B) improved treatment of CVD can be targeted to immune cells or the specific ligands presented on the inflamed endothelium of the atherosclerotic plaque; (C) in vivo sensors implanted in the pericardial region or on one of the main blood vessels and techniques for ex vivo biomarker detection; (D) nanostructured drug/nanoparticles eluting stents.
Nanoparticles for advanced diagnostics and therapy of CVD
A variety of nanoparticle-based drug delivery systems have been and are being developed for applications in cancer, CVD and other conditions. These have different features and multiple-functionalities [2, 4–6], exhibiting differences in (i) sizes, ranging from few tens of nanometers (as for dendrimers, gold and iron-oxide nanoparticles) to few hundreds of nanometers (as for polymeric and lipid-based particles) to micron-sized particles; (ii) shapes, from the classical spherical particles to discoidal, hemispherical, cylindrical and conical; (iii) surface functionalizations, with a broad range of electrostatic charges and bio-molecule conjugations.
Use of nanocarriers for these conditions allows for local or directed delivery, prolonged effect of the drug, facilitated delivery into the target cells, reduction of the shear effects of the blood flow. In the course of development, atherosclerotic plaque and neointima display a variety of stage-specific molecules which can be used as targeting moieties in CVD (αvβ3-integrin, VCAM-1, YIGSR, etc.).
Nanoparticles for advanced diagnostics of CVD
One of the major focuses of application of nanotechnology for cardiovascular research has been the directed imaging and therapy of atherosclerosis, restenosis and over cardiovascular conditions. Nanoscale contrast agents have emerged as multifaceted modalities able to identify and characterize early disease stages prior to the development of gross disease manifestations, which can be detected by conventional clinical imaging techniques. Contrast generating nanomaterials for cardiovascular imaging include fluorescent, radioactive, paramagnetic, superparamagnetic, electron dense and light scattering particles (Table 1).
Table 1.
Examples of contrast enhancing nanoparticles for imaging of CVD. CLIO - cross-linked iron oxide; PET - Positron emission tomography; SPECT- Single photon emission computed tomography; Gd-DTPA - Gadolinium chelated with diethylenetriamine pentetic acid; MRI- Magnetic Resonance Imaging; CT- Computed Tomography; NIRF- Near infrared fluorescence.
| Category | Agent (examples) | Imaging techniques | Refs. |
|---|---|---|---|
| Fluorescent | Quantum dots | Fluorescence tomography | [12] |
| Radioactive | 18F CLIO, 111In nanoparticles | PET, SPECT | [15] |
| Paramagnetic | Gd-DTPA | MRI | [17] |
| Superparamagnetic | Iron oxide nanoparticles | MRI | [21] |
| Electron dense | Gold or I-based nanoparticles | CT | [18,19,78,79] |
| Light scattering | Gold nanoshells | Optical coherent tomography | [80] |
| [81] | |||
| Photoacoustic | Colloidal nanobeacons | Photoacustic tomography | [16] |
| Multimodal | Copper-CLIO | PET, MRI, NIRF | [33, 82] |
| Perfluorocarbon nanoparticles | MRI, Molecuar imaging | ||
Cardiovascular imaging by magnetic resonance imaging (MRI) requires powerful magnetic fields and radiofrequency waves to generate images of internal structures. Energy changes in tissue in response to magnetic field are detected and the presence of contrast agents amplifies these changes. Three MR imaging techniques are T1, T2*, and off-resonance [7]. [E1] Off-resonance imaging relies on pulse sequences that excite and refocus off resonance water, leading to positive contrast[8]. Paramagnetic contrast agents, such as gadolinium chelates (e.g. gadolinium-diethylenetriaminopentaacetic acid, DTPA), enhance T1 contrast, resulting in bright contrast in MR images [9]. Manganese nanoparticles represent another, recently introduced, example of T1 enhancing contrast agent [10, 11]. Superparamagnetic contrast agents, such as iron oxide (IO) nanoparticles, predominately magnetite (Fe2O3/Fe3O4), typically enhance T2 contrast and produce dark contrast [8]. The technique of choice depends on the application and the weight of sensitivity, specificity and artifact minimization such as bright contrast originating from perivascular fat on atherosclerotic plaque images [9].
Nanoparticles have size-dependent imaging properties. For example, intrinsically fluorescent nanoparticles, known as quantum dots, emit light over a broad range from near-UV to mid-infrared. Increases in particle size are positively corelated to increases in emission wavelength [12]. Microparticle-based contrast agents for imaging include porous silicon particles that encapsulate abundant iron oxide nanoparticles in a single unit for enhanced contrast [13] These multistage particles, as well as other particles that are candidates for phagocytosis by macrophages, offer imaging of inflamed areas where macrophages accumulate, such as atherosclerotic plaque [14]. The feasibility of multi-modal imaging with nanoparticles containing multiple contrast agents, such as 18F-cross-linked iron oxide (18F-CLIO) nanoparticles [15, 16] has been demonstrated. 18F-CLIO agents consist of a cross-linked dextran shell formed on a superparamagnetic iron oxide (IO) core and functionalized with the radionuclide 18F. These particles can be detected with positron emission tomography, fluorescence molecular tomography, and MRI. A study by Chen et al [17] examined in vivo MRI contrast of vulnerable plaque high-density lipoprotein (rHDL) nanoparticles enriched with Gd-based amphiphiles and a targeting moiety to intraplaque macrophages (apolipoprotein E-derived lipopeptide, P2fA2). Data showed a significant enhancement in MRI signal of the atherosclerotic wall 24 h after the 50 μmol Gd/kg injection of rHDL-P2fA2 relative to administration of rHDL (90 vs. 53% enhancement, respectively).
Interesting set of studies explored the use of nanoliposomes as carriers for contrast agents such a iodine for MRI and computed tomography (CT)[E2] [18, 19].. These systems were shown to efficiently prevent a rapid clearance of the contrast agent from the body, significantly improving capabilities of total blood pool and cardiac imaging in animal models. As an example, with improved liposomal formulation of iodine, time–attenuation curves showed an initial enhancement of about 900 H in the aorta and the plateau levels of 800 H were achieved after two hours, indicating a high blood pool iodine concentration. These blood levels of liposomal iodine enabled excellent contrast discrimination between the myocardium and blood in the right and left ventricles, aorta, pulmonary trunk, and inferior vena cava with substantially lower liver and spleen contrast, as it is expected from the delayed clearance of the PEGylated liposomal iodine formulation via the reticulo-endothelial system. The long residence time at stable, high opacity s makes liposomal iodine a promising effective micro-CT agent for contrast enhancement within submillimeter vessels, and no significant renal clearance[18, 19] CT tomography represents another emerging field where nanoparticles were shown the capability to increase imaging contrast [20].
Currently FDA approved nanoparticles for imaging are limited to three IO formulations, AMI-121 (Ferumoxsil), OMP50, and AMI-25 (Feridex), targeted to the gastrointestinal tract and the liver and spleen. Injection of high doses of iron was shown to be nontoxic in the nanoparticle formulation due to slow release of free iron and assimilation into iron containing substances [21].
Targets for atherosclerotic plaque imaging include endothelia, macrophages [22], fibrin [23], collagen III [24], and markers of angiogenesis (Fig. 1A). Fibrin deposition is one of the earliest signs of plaque rupture, and fibrin, as well as tissue factor, are targets for imaging arterial thrombi by ultrasound [25] and magnetic resonance imaging [26]. One example of angiogenesis targeting is the use of nanoparticles conjugated to ligands that specifically interact with αvβ3-integrin [27].
Therapeutic and theranostic nanoparticles
Various nanotechnological applications are being investigated for treatment of atherosclerosis and restenosis, including nanocarriers for drug delivery (Fig. 1B) and devices such as mechanical stents, possessing nanoscale components (Fig. 1D), which will be discussed further. Among the drugs used to prevent restenosis are cytotoxics that inhibit smooth muscle cell growth (e.g. paclitaxel, cytarbine, etoposides, doxorubicin), PDGF receptor antagonists (e.g. tyrphostins), inhibitors of inflammatory response/immunomodulators (e.g. steroids, bisphosphonates, Cyclosporine A), and antibiotics (e.g. fumagillin). Other promising therapeutics affect specific gene targets, responsible for thrombosis or intimal hyperplasia (e.g. prostacyclin synthase and thymidine kinase). In the case of genetic materials and other biomolecules, their encapsulation in nanoparticles provides protection from enzymatic degradation and allows for prolonged release profiles. These therapeutic strategies have been recently thoroughly reviewed. [28, 29]
The main nanocarrier classes investigated as therapeutic and theranostic agents for restenosis are liposomes with different surface characteristics, polymeric nanoparticles and micelles, perfluorocarbon nano-emulsions and CLIO particles conjugated to therapeutic molecules [30–34]. Examples of these classes are given in Table 2.
Table 2.
Examples of nanocarriers for CVD therapy
| Nanocarrier | Example of agent | Experimental model | Outcomes | Refs. |
|---|---|---|---|---|
| Neutral liposomes | Bisphosphonates (clodronate, alendronate, etc.) | Injured rat carotid artery | Macrophage depletion, reduced inflammation | [30] |
| Cationic liposomes | Chloramphenicol acetyl transferase (CAT) encoding gene [31] | Balloon injured Yorkshir pig arthery, local delivery | Increased CAT expression | [31,32] |
| Vascular endothelial growth factor (VEGF) encoding viral vector | Clinical trial, patients with 60–99% stenosis in major artheries, local delivery through catheter | Significant improvement in myocardial perfusion | ||
| Hemaglutin virus of Japan (HVJ) liposomes | Tissue factor pathway inhibitor gene | Iliac artery of hyperlipidemic rabbit following angioplasty. Local delivery through catheter. | Reduction of intimal hyperplasia | [83] |
| Perfluorocarbon nanoparticles | Surface bound streptokinase, α3β integrins, others | Human plasma clots, hyperlipidemic animals | In vitro fibrinolysis, theranostic in vivo | [82] |
| Polyelectrolyte nanoparticles (RNAor polyvynil sulfate with polyethylene imine/DNA complex) | Gene encoding for urokinase plasminogen activator | Rat carotid artery | High transfection efficiency | [84] |
| Polymeric (PLA orPLGA) nanoparticles | AG-1295 and AGL-2043 | Balloon injured rat carotid artery | Inhibition of restenosis | [85, 86] |
Particles integrating diagnostic imaging and therapeutic components, or “theranostic” agents, gained much recent interest as a valuable advance for drug delivery [35]. Though this strategy is still in its infancy for CVD applications, it has numerous potential advantages, which are being extensively investigated in the field of cancer nanomedicine. Combining a diagnostic imaging moiety with a targeted therapeutic nanoparticle allows for precise, temporal and spatial monitoring of the therapeutic agent as well as treatment outcomes. Imaging capabilities of theranostic nanoparticles can serve to verify the delivery of an active compound to its intended site of action, monitor and quantify the efficacy of the therapeutics on the molecular or cellular level, design dosing regiments and identify the population of responders/non-responders for a specific therapy. As an example, a prolonged antiangiogenesis therapy was reported using theranostic αvβ3-integrin targeted paramagnetic nanoparticles in hyperlipidemic animals [33, 36, 37]. MRI data showing a reduction of 50% to 75% in neovascular signal for three weeks, corresponded well with histological evaluation, pointing toward the potential of this strategy for efficient antiangiogenic therapy, simultaneously evaluating plaque stability. Other example is a system based on fibrin-coated perfluorocarbon nanoparticles, which can be used for acoustic or MRI imaging with targeted thrombolysis [38].
There is also an opposite side of interaction of nanoparticles with blood vessel walls. Vascular endothelium could be a barrier and unwanted target for nanoparticles to be delivered to other organs [39]. In this aspect, cardiovascular diseases may affect the transport of nanoparticles across vessel wall, organelle-targeted delivery of nanoparticles and other effects of nanoparticles on vessel cells, which should be taken into account in future nanomedical research.
Devices for ex vivo and in vivo early detection of CVD indicators
Along with the development and adoption of novel strategies for treatment and prevention of CVD, efforts are being spent to apply nanotechnologies for ex-vivo and in-vivo detection of CVD signals (Fig. 1C). The ability to monitor for precursor signals of CVD could potentially reduce the large number of fatalities associated with the diseases. For example, monitoring thrombotic or hemorrhagic events could facilitate the diagnosis and treatment of stroke and embolisms. Moreover, the measurement of variations in the blood pressure, flow, and biomolecule or ion concentration can provide insight for the understanding of cardiovascular events.
Nanotechnology for ex vivo biomarkers harvesting and detection
Identification of biomarkers provides a powerful approach for screening, diagnosis, prognosis and therapeutic monitoring [40]. Addressing the underlying causes of CVD and improving the detection of early-stage disease will permit early intervention with more efficient disease management and significant decrease in premature mortality. Development of high performance “point of care” methods will improve the prognosis for patients suffering from CVD, by obtaining more sensitive, more specific, and faster assessment of diagnostic markers. Several publications have reported the interest of individual biomarkers of CVD to better identify high-risk population, including levels of C-reactive protein [41], B-type natriuretic peptide [42], fibrinogen [43], D-dimer [44], and homocysteine [45]. A study by Wang et al. reported the simultaneous measurement of several biomarkers to enhance risk stratification [46]. They concluded that the use of current biomarkers, even in a multi-detection approach, improves only moderately the standard assessment. Therefore, there is an urgent need for technological advances in biomarker strategies to improve the detection of current markers and to discover new biomarkers.
In medical applications, the use of nanotechnology presenting unique physical and chemical properties has the potential to dramatically advance the current diagnostic methods and provide innovative devices for more efficient molecular detection. Tunable nanoporous materials have been used to selectively harvest low molecular weight proteins, providing a unique opportunity to detect and identify new circulating biomarkers after fractionation of body fluids [47, 48]. Nanowires offer great potential for diagnosis by measuring pH variations or detecting trace amount of biological and chemical species [49]. Nanocantilevers can be used as multiple label-free assays for detection of DNA species or circulating protein biomarkers [50].
A high specificity immunoassay-based diagnostics device combining impedimetric analysis nanoelectrodes and microfluidics has been developed to measure the whole blood concentration of D-dimer, a recognized biomarker of increased blood clotting activity in deep vein thrombosis (DVT) [51]. This device could improve the accuracy and reliability of early assessment of patients with risk of DVT. Other lab-on-a-chip approaches have been developed in combination with nanotechnology to improve the sensitivity and accuracy of biomarkers detection. Associated to electrocardiogram (ECG) analysis, a saliva-based biomarker test within a lab-on-a-chip platform exceeded the screening capacity of ECG alone, providing a rapid screening for acute myocardial infarction (AMI) patients [52]. Another study demonstrated the use of a rapid fluorophore mediated immuno-sensing system for simultaneous quantification of four cardiac markers in AMI patients. This technology is based on micro-electro-mechanical system (MEMS) and nanoparticle reagents that increase the sensitivity of the detection [53].
Isoforms of troponins are structural proteins that are unique to cardiac myocytes (cardiac troponin I and T). These proteins are tissue specific and the immuno-detection of their cardiac forms has become a standard in the diagnosis of myocardial infarction [54]. A clinical report using a novel ultrasensitive nanoparticle assay for cardiac troponin I demonstrated the ability to detect pg/mL concentrations of the protein in serum, revealing a significant increase of the sensitivity of detection and providing a promising earlier detection of myocardial injury [55]. Using engineered viral nanoparticles combining troponin antibodies and nickel nanohairs, a different study has reported detection limit of troponin in human serum with six to seven orders of magnitude lower than conventional immuno-assays [56].
In vivo sensors for CVD
Myocardial ischemia is the deadliest form of CVD and affects millions of people causing large numbers of fatalities. Several studies have focused on the development of nanosensors for in-situ rapid detection of ions such as K+, H+, Na+ and Ca2+ and demonstrated the role of K+ and H+ ions activity as potential indicators of the onset of acute myocardial ischemia [57]. The in vivo analysis was conducted by performing an epicardial and arterial implantation of the sensors. Flexible nanoelectrode sensors for K+ were also developed to address the mechanism of ischemic heart disease [58]. In addition, a multi-nanosensor silicon needle was developed in vitro for the detection of myocardial ischemia during cardiac surgery by employing the technology of field effect transistors (FET) [59]. Nanosensors were also developed for the in vitro detection and analysis of real-time sodium concentration during action potentials [60] in HEK PN1 cells. The flux of Na+ ions across the cell membrane plays a fundamental role in the generation of action potentials and regulation of membrane excitability in cells such as cardiomyocytes. Diseases such as long QT syndrome and heart failure are correlated to an alteration of sodium channel function. Functionalized nanowires were developed for real-time detection of Ca2+ ions important in the context of CVD [49]. Ca2+ ions are known for activating biological process such as muscle contraction, protein secretion, cell death and development.
Nanosensors are in development for the detection of other molecules which play an important role in the cardiovascular system physiology as well. Near-infrared fluorescence sensors for NO were developed with single-walled carbon nanotube technology [61]. A porphyrinic nanosensor for in-situ measurement of nitric oxide in endothelial cells or in beating heart allowed understanding the effect of hypertension and ischemia/reperfusion on the release of NO [62]. In2O3 nanowire-based FET sensors were employed as lab-on-a-chip devices for detection of oxidized low density lipoprotein (oxLDL) cholesterol [63] which is considered a biomarker for acute heart attack in patients with coronary artery disease (CAD).
Other studies have explored the employment of nanotechnology for monitoring physical-mechanical parameters such as pressure and blood flow as potential indexes of CVD.
A large number of bio-MEMS pressure sensors have been developed in-vitro for the in-situ measurement of blood pressure [64] such as an implantable device for telemetric real time monitoring of BP with potential to diagnose myocardial infarction [65]. Additionally, wireless bio-MEMs sensors for continuous monitoring of blood flow in-situ open opportunities for surveillance strategies to detect stenosis and to prevent impending graft failure.
Nanotechnology for therapeutic and tissue regeneration devices
The utility offered by nanotechnology for cardiovascular device applications is being primarily investigated as an enhancement of stent technology. The integration of nanotechnology into stent design has provided novel strategies for drug delivery from mesoporous substrates and enhanced biocompatibility from nano-textured surfaces. The classical challenges of deploying stents in an attempt to revascularize pathologically narrowed arteries are, in-stent restenosis as the result of intimal hyperplasia[66], and late stage thrombosis mediated by activated platelets[67]. When clinical advancements were thought to be exhausted through variations of stent geometry, the next degree of device sophistication established the stent as a drug delivery platform. Soon industry giants such as Boston Scientific, Johnson & Johnson, Medtronic, and Guidant began manufacturing drug eluting stents (DES) that released such drugs as paclitaxel and sirolimus to exploit their anti-proliferative effects. DES have demonstrated lower incidence of restenosis six months post procedure compared to their bare metal counterparts, however recent longer term studies have raised major concern over the long term benefit of DES [68–71]. The anti-proliferative nature of the eluted drugs inhibit the cell cycle thus prohibiting normal vessel remodeling that results in the integration of the stent within the vessel wall. The exposed structure of the stent as the result of incomplete neointimal coverage facilitates thrombus formation which leads to increased mortality due to late stage thrombosis[69]. This clinical outcome is commonly observed after the early cessation of dual antiplatelet therapy (aspirin/clopidogrel) [70].
In light of the challenges of drug eluting stents, nanotechnology is currently being applied to stent design to improve clinical outcomes. Nanoporous platforms have demonstrated the ability to provide controlled drug release profiles over a predetermined period of time; such nanoporous technology has been applied to cancer applications [4]. At the present time, investigators are exploring the utility of nanoporous stent surfaces of: aluminum oxide to deliver tacrolimus [72], carbon-carbon nanoparticle matrixes for the elution of paclitaxel [73], and gold [74] or titanium oxide[75] for the delivery of various therapies. To ameliorate the problem of impaired vessel revascularization mediated by the physical presence of stents, surface nanotexturing is being investigated to enhance endothelial cell interaction with stent surfaces. Studies suggest that nanoscale roughness/topography on nickel titanium [67] and hydroxyapatite [76] substrates may mimic the natural structure of vascular tissue and improve cell adhesion and subsequently enhance endothelialization of stent struts and articulations for the reduction of thrombosis[77]. Although speculative, it is logical to anticipate that the next evolutionary stent advancement may be realized through the combinatory integration of nano-porous stent surfaces for the controlled, time release of anti-proliferative agents with nanotextured features to promote vessel endothelialization to lower the incidence of restenosis and occurrence of late stage mortality attributed to thrombosis.
Conclusions and perspectives
In the developed countries, CVD represent an enormous burden on the healthcare system and economy, being the leading cause of death and morbidity. This becomes even more important considering the relentless tendency of higher representation of geriatric and obese population. Rapid evolution of fields such as genetics, proteomics, molecular and cellular biology, material science and bioengineering, make nanotechnology, which bridges the gap between interactions on the molecular and microsopic levels, one of the major potential players in the progress of CVD treatment and detection. Though still in very early developmental (“embryonic”) stages, cardiovascular nanomedicine is likely to meet the high demand for the breakthrough innovation in the CVD therapy and diagnosis, taking an advantage of the nanotechnological solutions developed for other medical applications, mostly oncology, where therapeutic nanocarriers currently occupy a significant therapeutic niche. Different from the conventional molecular therapeutics, nanomedicine enables design of multicomponent, multitasking, multimodular agents which can simultaneously and precisely detect and treat the disease. As an example, we can envision smart nano-sensors integrated in existing implants such as defibrillators, stents or pace makers that may trigger warning, or perhaps acute release of drug, if required. Another nanomedical solution for CVD could be projected for vulnerable plaque, where “click-chemistry” or highly controlled crosslinking strategies that can target and “secure” the plaque prior to subsequent AMI without danger of occluding the vessel can be utilized. In summary, here we gave a brief overview on the current developments in cardiovascular nanomedicine with a great potential impact; however, we believe that these pale comparing to the future opportunities for application of nanotechnology for treatment and diagnosis of CVD.
Table 3.
Nanotechnology based in vivo CVD sensors
| Sensor Targets | Technology | Applications | Refs. |
|---|---|---|---|
| K+, H+ ions | Field Effect Transistor (FET) | Myocardial Ischemia | [57–59] |
| Na+ ions | Fluorescent Nanosensors | QT Syndrome, Heart Failure | [60] |
| Ca2+ ions | Boron-doped Silicon Nanowires (SiNWs) | Multiple CVD | [49] |
| Nitric Oxide | Single-walled Carbon Nanotube (SWNT) | Hypertension, | [61] |
| oxLDL | Phorphyrinic Nanosensor | Ischemia/Reperfusion | [62] |
| Cholesterol | In2O3 nanowire-based FET | Acute Heart Attack | [63] |
| Blood pressure | Piezoelectric-BioMEMS | Pressure Monitoring, | [64] |
| Chip Embedded Flexible Packaging (CEFP) | Myocardial Infarction Stenosis in Heart Bypass | [65] | |
| Blood Flow | Piezoelectric-BioMEMS | Grafts | |
ACKNOWLEDGMENTS
Matt Landry is gratefully recognized for his artistry in the preparation of Figure 1. The authors acknowledge a financial support from the following sources: DODW81XWH-09-1-0212, DODW81XWH-07-2-0101; NASA NNJ06HE06A; NIH RO1CA128797, NIH – R33 CA122864, NIH U54CA143837 and State of Texas, Emerging Technology Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Theis T, et al. nan'o.tech.nol'o.gy n. Nat Nanotechnol. 2006;1(1):8–10. doi: 10.1038/nnano.2006.77. [DOI] [PubMed] [Google Scholar]
- 2.Riehemann K, et al. Nanomedicine--challenge and perspectives. Angew Chem Int Ed Engl. 2009;48(5):872–97. doi: 10.1002/anie.200802585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kong DF, Goldschmidt-Clermont PJ. Tiny solutions for giant cardiac problems. Trends Cardiovasc Med. 2005;15(6):207–11. doi: 10.1016/j.tcm.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5(3):161–71. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 5.Peer D, et al. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2(12):751–60. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 6.Ferrari M. Nanogeometry: beyond drug delivery. Nat Nanotechnol. 2008;3(3):131–2. doi: 10.1038/nnano.2008.46. [DOI] [PubMed] [Google Scholar]
- 7.Smith RC, McCarthy S. Physics of magnetic resonance. J Reprod Med. 1992;37(1):19–26. [PubMed] [Google Scholar]
- 8.Cunningham CH, et al. Positive contrast magnetic resonance imaging of cells labeled with magnetic nanoparticles. Magn Reson Med. 2005;53(5):999–1005. doi: 10.1002/mrm.20477. [DOI] [PubMed] [Google Scholar]
- 9.Sosnovik DE, Nahrendorf M, Weissleder R. Magnetic nanoparticles for MR imaging: agents, techniques and cardiovascular applications. Basic Res Cardiol. 2008;103(2):122–30. doi: 10.1007/s00395-008-0710-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan D, et al. Sensitive and efficient detection of thrombus with fibrin-specific manganese nanocolloids. Chem Commun (Camb) 2009;(22):3234–6. doi: 10.1039/b902875g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan D, et al. Ligand-directed nanobialys as theranostic agent for drug delivery and manganese-based magnetic resonance imaging of vascular targets. J Am Chem Soc. 2008;130(29):9186–7. doi: 10.1021/ja801482d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michalet X, et al. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307(5709):538–44. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serda RE, Godin B, Tasciotti E, Liu X, Ferrari M. Mitotic trafficking of silicon microparticles. Nanoscale. 2009;1(2):250–259. doi: 10.1039/b9nr00138g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kooi ME, et al. Accumulation of ultrasmall superparamagnetic particles of iron oxide in human atherosclerotic plaques can be detected by in vivo magnetic resonance imaging. Circulation. 2003;107(19):2453–8. doi: 10.1161/01.CIR.0000068315.98705.CC. [DOI] [PubMed] [Google Scholar]
- 15.Devaraj NK, et al. 18F labeled nanoparticles for in vivo PET-CT imaging. Bioconjug Chem. 2009;20(2):397–401. doi: 10.1021/bc8004649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nahrendorf M, et al. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation. 2008;117(3):379–87. doi: 10.1161/CIRCULATIONAHA.107.741181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen W, et al. Incorporation of an apoE-derived lipopeptide in high-density lipoprotein MRI contrast agents for enhanced imaging of macrophages in atherosclerosis. Contrast Media Mol Imaging. 2008;3(6):233–42. doi: 10.1002/cmmi.257. [DOI] [PubMed] [Google Scholar]
- 18.Kao CY, et al. Long-residence-time nano-scale liposomal iohexol for X-ray-based blood pool imaging. Acad Radiol. 2003;10(5):475–83. doi: 10.1016/s1076-6332(03)80055-7. [DOI] [PubMed] [Google Scholar]
- 19.Mukundan S, Jr., et al. A liposomal nanoscale contrast agent for preclinical CT in mice. AJR Am J Roentgenol. 2006;186(2):300–7. doi: 10.2214/AJR.05.0523. [DOI] [PubMed] [Google Scholar]
- 20.Pan D, et al. Detecting vascular biosignatures with a colloidal, radio-opaque polymeric nanoparticle. J Am Chem Soc. 2009;131(42):15522–7. doi: 10.1021/ja906797z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corot C, et al. Recent advances in iron oxide nanocrystal technology for medical imaging. Adv Drug Deliv Rev. 2006;58(14):1471–504. doi: 10.1016/j.addr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 22.Amirbekian V, et al. Detecting and assessing macrophages in vivo to evaluate atherosclerosis noninvasively using molecular MRI. Proc Natl Acad Sci U S A. 2007;104(3):961–6. doi: 10.1073/pnas.0606281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Botnar RM, et al. In vivo magnetic resonance imaging of coronary thrombosis using a fibrin-binding molecular magnetic resonance contrast agent. Circulation. 2004;110(11):1463–6. doi: 10.1161/01.CIR.0000134960.31304.87. [DOI] [PubMed] [Google Scholar]
- 24.Cyrus T, et al. MR three-dimensional molecular imaging of intramural biomarkers with targeted nanoparticles. J Cardiovasc Magn Reson. 2006;8(3):535–41. doi: 10.1080/10976640600580296. [DOI] [PubMed] [Google Scholar]
- 25.Lanza GM, et al. In vitro characterization of a novel, tissue-targeted ultrasonic contrast system with acoustic microscopy. J Acoust Soc Am. 1998;104(6):3665–72. doi: 10.1121/1.423948. [DOI] [PubMed] [Google Scholar]
- 26.Morawski AM, et al. Targeted nanoparticles for quantitative imaging of sparse molecular epitopes with MRI. Magn Reson Med. 2004;51(3):480–6. doi: 10.1002/mrm.20010. [DOI] [PubMed] [Google Scholar]
- 27.Winter PM, et al. Molecular imaging of angiogenesis in early-stage atherosclerosis with alpha(v)beta3-integrin-targeted nanoparticles. Circulation. 2003;108(18):2270–4. doi: 10.1161/01.CIR.0000093185.16083.95. [DOI] [PubMed] [Google Scholar]
- 28.Brito L, Amiji M. Nanoparticulate carriers for the treatment of coronary restenosis. Int J Nanomedicine. 2007;2(2):143–61. [PMC free article] [PubMed] [Google Scholar]
- 29.Buxton DB. Nanomedicine for the management of lung and blood diseases. Nanomed. 2009;4(3):331–9. doi: 10.2217/nnm.09.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Danenberg HD, et al. Macrophage depletion by clodronate-containing liposomes reduces neointimal formation after balloon injury in rats and rabbits. Circulation. 2002;106(5):599–605. doi: 10.1161/01.cir.0000023532.98469.48. [DOI] [PubMed] [Google Scholar]
- 31.Stephan D, et al. Direct gene transfer in the rat kidney in vivo. Arch Mal Coeur Vaiss. 1997;90(8):1127–30. [PubMed] [Google Scholar]
- 32.Hedman M, et al. Safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and in-stent restenosis and in the treatment of chronic myocardial ischemia: phase II results of the Kuopio Angiogenesis Trial (KAT) Circulation. 2003;107(21):2677–83. doi: 10.1161/01.CIR.0000070540.80780.92. [DOI] [PubMed] [Google Scholar]
- 33.Lanza GM, et al. Nanomedicine opportunities for cardiovascular disease with perfluorocarbon nanoparticles. Nanomed. 2006;1(3):321–9. doi: 10.2217/17435889.1.3.321. [DOI] [PubMed] [Google Scholar]
- 34.Jaffer FA, Libby P, Weissleder R. Optical and multimodality molecular imaging: insights into atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29(7):1017–24. doi: 10.1161/ATVBAHA.108.165530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cyrus T, et al. Intramural delivery of rapamycin with alphavbeta3-targeted paramagnetic nanoparticles inhibits stenosis after balloon injury. Arterioscler Thromb Vasc Biol. 2008;28(5):820–6. doi: 10.1161/ATVBAHA.107.156281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winter PM, et al. Antiangiogenic synergism of integrin-targeted fumagillin nanoparticles and atorvastatin in atherosclerosis. JACC Cardiovasc Imaging. 2008;1(5):624–34. doi: 10.1016/j.jcmg.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winter PM, et al. Endothelial alpha(v)beta3 integrin-targeted fumagillin nanoparticles inhibit angiogenesis in atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26(9):2103–9. doi: 10.1161/01.ATV.0000235724.11299.76. [DOI] [PubMed] [Google Scholar]
- 38.Marsh JN, et al. Fibrin-targeted perfluorocarbon nanoparticles for targeted thrombolysis. Nanomed. 2007;2(4):533–43. doi: 10.2217/17435889.2.4.533. [DOI] [PubMed] [Google Scholar]
- 39.Lukyanenko V. Delivery of nano-objects to functional sub-domains of healthy and failing cardiac myocytes. Nanomed. 2007;2(6):831–46. doi: 10.2217/17435889.2.6.831. [DOI] [PubMed] [Google Scholar]
- 40.Vasan RS. Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation. 2006;113(19):2335–62. doi: 10.1161/CIRCULATIONAHA.104.482570. [DOI] [PubMed] [Google Scholar]
- 41.Danesh J, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350(14):1387–97. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 42.Wang TJ, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350(7):655–63. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 43.Danesh J, et al. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA. 2005;294(14):1799–809. doi: 10.1001/jama.294.14.1799. [DOI] [PubMed] [Google Scholar]
- 44.Cushman M, et al. Fibrinolytic activation markers predict myocardial infarction in the elderly. The Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 1999;19(3):493–8. doi: 10.1161/01.atv.19.3.493. [DOI] [PubMed] [Google Scholar]
- 45.Mangoni AA, Jackson SH. Homocysteine and cardiovascular disease: current evidence and future prospects. Am J Med. 2002;112(7):556–65. doi: 10.1016/s0002-9343(02)01021-5. [DOI] [PubMed] [Google Scholar]
- 46.Wang TJ, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355(25):2631–9. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 47.Gaspari M, et al. Nanoporous surfaces as harvesting agents for mass spectrometric analysis of peptides in human plasma. J Proteome Res. 2006;5(5):1261–6. doi: 10.1021/pr050417+. [DOI] [PubMed] [Google Scholar]
- 48.Luchini A, et al. Smart hydrogel particles: biomarker harvesting: one-step affinity purification, size exclusion, and protection against degradation. Nano Lett. 2008;8(1):350–61. doi: 10.1021/nl072174l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cui Y, et al. Nanowire nanosensors for highly sensitive and selective detection of biological and chemical species. Science. 2001;293(5533):1289–92. doi: 10.1126/science.1062711. [DOI] [PubMed] [Google Scholar]
- 50.McKendry R, et al. Multiple label-free biodetection and quantitative DNA-binding assays on a nanomechanical cantilever array. Proc Natl Acad Sci U S A. 2002;99(15):9783–8. doi: 10.1073/pnas.152330199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McMurray AA, et al. A novel point of care diagnostic device: impedimetric detection of a biomarker in whole blood. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:115–8. doi: 10.1109/IEMBS.2007.4352236. [DOI] [PubMed] [Google Scholar]
- 52.Floriano PN, et al. Use of saliva-based nano-biochip tests for acute myocardial infarction at the point of care: a feasibility study. Clin Chem. 2009;55(8):1530–8. doi: 10.1373/clinchem.2008.117713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang J, et al. Mini sensing chip for point-of-care acute myocardial infarction diagnosis utilizing micro-electro-mechanical system and nano-technology. Adv Exp Med Biol. 2009;645:101–7. [PubMed] [Google Scholar]
- 54.Jaffe AS, et al. It's time for a change to a troponin standard. Circulation. 2000;102(11):1216–20. doi: 10.1161/01.cir.102.11.1216. [DOI] [PubMed] [Google Scholar]
- 55.Wilson SR, et al. Detection of myocardial injury in patients with unstable angina using a novel nanoparticle cardiac troponin I assay: observations from the PROTECT-TIMI 30 Trial. Am Heart J. 2009;158(3):386–91. doi: 10.1016/j.ahj.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 56.Park JS, et al. A highly sensitive and selective diagnostic assay based on virus nanoparticles. Nat Nanotechnol. 2009;4(4):259–64. doi: 10.1038/nnano.2009.38. [DOI] [PubMed] [Google Scholar]
- 57.Vogt S, et al. Efficacy of ion-selective probes in early epicardial in vivo detection of myocardial ischemia. Physiol Meas. 2004;25(6):N21–6. doi: 10.1088/0967-3334/25/6/n02. [DOI] [PubMed] [Google Scholar]
- 58.Ji T, et al. In vitro evaluation of flexible pH and potassium ion-sensitive organic field effect transistor sensors. Applied Physics Letters. 2008;92(23):233304. [Google Scholar]
- 59.Errachid A, et al. New technology for multi-sensor silicon needles for biomedical applications. Sensors and Actuators B: Chemical. 2001;78:279–284. [Google Scholar]
- 60.Dubach JM, et al. Visualizing sodium dynamics in isolated cardiomyocytes using fluorescent nanosensors. Proc Natl Acad Sci U S A. 2009;106:16145–16150. doi: 10.1073/pnas.0905909106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim J-H, et al. The rational design of nitric oxide selectivity in single-walled carbon nanotube near-infrared fluorescence sensors for biological detection. Nat Chem. 2009;1(6):473–481. doi: 10.1038/nchem.332. [DOI] [PubMed] [Google Scholar]
- 62.Sato M, et al. Cell-based indicator to visualize picomolar dynamics of nitric oxide release from living cells. Anal Chem. 2006;78(24):8175–82. doi: 10.1021/ac061791b. [DOI] [PubMed] [Google Scholar]
- 63.Rouhanizadeh M, et al. Differentiation of oxidized low density lipoproteins by nanosensors. Sensors and Actuators B: Chemical. 2006;114:788–798. [Google Scholar]
- 64.Kovacs GTA. Micromachined Transducers Handbook. McGraw Hill; New York: 1998. [Google Scholar]
- 65.Shin KH, et al. Flexible wireless pressure sensor module. Sensors and Actuators A. 2005;123–124:30–35. [Google Scholar]
- 66.Hoffmann R, et al. Patterns and mechanisms of in-stent restenosis. A serial intravascular ultrasound study. Circulation. 1996;94(6):1247–54. doi: 10.1161/01.cir.94.6.1247. [DOI] [PubMed] [Google Scholar]
- 67.Samaroo HD, Lu J, Webster TJ. Enhanced endothelial cell density on NiTi surfaces with sub-micron to nanometer roughness. Int J Nanomedicine. 2008;3(1):75–82. doi: 10.2147/ijn.s2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kastrati A, et al. Analysis of 14 trials comparing sirolimus-eluting stents with bare-metal stents. N Engl J Med. 2007;356(10):1030–9. doi: 10.1056/NEJMoa067484. [DOI] [PubMed] [Google Scholar]
- 69.Lagerqvist B, et al. Long-term outcomes with drug-eluting stents versus bare-metal stents in Sweden. N Engl J Med. 2007;356(10):1009–19. doi: 10.1056/NEJMoa067722. [DOI] [PubMed] [Google Scholar]
- 70.Mauri L, et al. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med. 2007;356(10):1020–9. doi: 10.1056/NEJMoa067731. [DOI] [PubMed] [Google Scholar]
- 71.Stone GW, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007;356(10):998–1008. doi: 10.1056/NEJMoa067193. [DOI] [PubMed] [Google Scholar]
- 72.Wieneke H, et al. Synergistic effects of a novel nanoporous stent coating and tacrolimus on intima proliferation in rabbits. Catheter Cardiovasc Interv. 2003;60(3):399–407. doi: 10.1002/ccd.10664. [DOI] [PubMed] [Google Scholar]
- 73.Bhargava B, et al. A novel paclitaxel-eluting porous carbon-carbon nanoparticle coated, nonpolymeric cobalt-chromium stent: evaluation in a porcine model. Catheter Cardiovasc Interv. 2006;67(5):698–702. doi: 10.1002/ccd.20698. [DOI] [PubMed] [Google Scholar]
- 74.Erlebacher J, et al. Evolution of nanoporosity in dealloying. Nature. 2001;410(6827):450–3. doi: 10.1038/35068529. [DOI] [PubMed] [Google Scholar]
- 75.Ayon AA, et al. Drug loading of nanoporous TiO2 films. Biomed Mater. 2006;1(4):L11–5. doi: 10.1088/1748-6041/1/4/L01. [DOI] [PubMed] [Google Scholar]
- 76.Liu DM, Yang Q, Troczynski T. Sol-gel hydroxyapatite coatings on stainless steel substrates. Biomaterials. 2002;23(3):691–8. doi: 10.1016/s0142-9612(01)00157-0. [DOI] [PubMed] [Google Scholar]
- 77.Caves JM, Chaikof EL. The evolving impact of microfabrication and nanotechnology on stent design. J Vasc Surg. 2006;44(6):1363–8. doi: 10.1016/j.jvs.2006.08.046. [DOI] [PubMed] [Google Scholar]
- 78.Kim D, et al. Antibiofouling polymer-coated gold nanoparticles as a contrast agent for in vivo X-ray computed tomography imaging. J Am Chem Soc. 2007;129(24):7661–5. doi: 10.1021/ja071471p. [DOI] [PubMed] [Google Scholar]
- 79.Hyafil F, et al. Noninvasive detection of macrophages using a nanoparticulate contrast agent for computed tomography. Nat Med. 2007;13(5):636–41. doi: 10.1038/nm1571. [DOI] [PubMed] [Google Scholar]
- 80.Pan D, et al. Molecular photoacoustic tomography with colloidal nanobeacons. Angew Chem Int Ed Engl. 2009;48(23):4170–3. doi: 10.1002/anie.200805947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Loo C, et al. Nanoshell-enabled photonics-based imaging and therapy of cancer. Technol Cancer Res Treat. 2004;3(1):33–40. doi: 10.1177/153303460400300104. [DOI] [PubMed] [Google Scholar]
- 82.Kaneda MM, et al. Perfluorocarbon nanoemulsions for quantitative molecular imaging and targeted therapeutics. Ann Biomed Eng. 2009;37(10):1922–33. doi: 10.1007/s10439-009-9643-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yin X, et al. HVJ-AVE liposome-mediated Tissue Factor Pathway Inhibitor (TFPI) gene transfer with recombinant TFPI (rTFPI) irrigation attenuates restenosis in atherosclerotic arteries. Int J Cardiol. 2009;135(2):245–8. doi: 10.1016/j.ijcard.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 84.Zaitsev S, et al. Polyelectrolyte nanoparticles mediate vascular gene delivery. Pharm Res. 2004;21(9):1656–61. doi: 10.1023/b:pham.0000041462.19131.08. [DOI] [PubMed] [Google Scholar]
- 85.Fishbein I, et al. Local delivery of platelet-derived growth factor receptor-specific tyrphostin inhibits neointimal formation in rats. Arterioscler Thromb Vasc Biol. 2000;20(3):667–76. doi: 10.1161/01.atv.20.3.667. [DOI] [PubMed] [Google Scholar]
- 86.Banai S, et al. Locally delivered nanoencapsulated tyrphostin (AGL-2043) reduces neointima formation in balloon-injured rat carotid and stented porcine coronary arteries. Biomaterials. 2005;26(4):451–61. doi: 10.1016/j.biomaterials.2004.02.040. [DOI] [PubMed] [Google Scholar]