Abstract
The periaqueductal gray (PAG) is known to play a crucial role in pain modulation and has shown a strong interaction with anterior cingulate cortex in previous functional imaging studies. We investigated the intrinsic functional connectivity of PAG using resting fMRI data from 100 subjects. The results showed that PAG is functionally connected to ACC (rostral and pregenual ACC) and also rostral ventromedial medulla (RVM), forming a core ACC-PAG-RVM network for pain modulation even no pain stimulus is applied. The comparison between genders showed that for the contrast of female minus male, significant difference was observed at mid-cingulate cortex; for the contrast of male minus female, significant differences were observed at left medial orbital prefrontal cortex, and uncus; right insula /operculum and prefrontal cortex. We believe eluciation of this Intrinsic PAG network duing the resting state will enhance our physiological and pathological understanding of the development and maintenance of chronic pain states.
Keywords: periaqueductal gray, PAG, pain, fMRI, resting state, functional connectivity
Introduction
Interest in the role of the periaqueductal gray (PAG) in pain modulation has a long history. Previous studies in both animals [35,47] and patients [5,25] have shown that PAG stimulation can significantly inhibit behavioral responses to noxious stimuli. More recent research has identified an entire descending pain modulation system in which PAG plays a crucial role [19]. It is now believed that PAG receives direct projections from the hypothalamus and regions within the limbic forebrain such as anterior cingulate cortex (ACC) [8,19,24,34]. It is also known that PAG can modulate pain perception through brain stem structures, such as the rostral ventromedial medulla (RVM), that directly communicate with nociceptive neurons from the dorsal horn of the spinal cord [19,34].
Recently, functional connectivity fMRI (fcMRI) during resting state has drawn the attention of brain imaging investigators [7,20,45]. It is believed that Low-frequency components of the spontaneous functional MR imaging signal can provide information about the intrinsic functional and anatomical organization of the brain [9,31]. fcMRI has been used to identify the default network [23], the dorsal attention network [21] and the control network [16,55]. It has also been used to investigate the functional connectivity of specific neural structures including the anterior cingulate cortex [33], the thalamus [60], the hippocampus [27,28,56], the insula [51], the cerebellum [30,39],and the pain matrix (S1, S2 and insula) [29], which has significantly enhanced our understanding of the intrinsic functional and anatomical connections of these brain structures. More recently, researchers have started to investigator the functional connectivity differences between neuropathic pain patients and the healthy subjects [10–12], and found altered functional connectivity in chronic pain patients.
Despite the enhanced understanding of the role of PAG in pain modulation, our knowledge of the structural and functional connectivity of the PAG stems largely from animals studies. To our best knowledge, no studies have yet systemically assessed the intrinsic connectivity between the PAG and other brain regions at resting state. In addition to expanding our physiological and anatomical understanding of PAG, we believe this research is important on the following accounts. Firstly, accumulated evidence from recent brain imaging studies suggests that dysfunction of the central nervous system is involved in the pathophysiology of chronic pain [2,3]. Some investigators believe that dysfunction of the descending pain modulation system, particularly the PAG, may be involved in the development and maintenance of chronic pain states [2,43]. Thus, a better understanding of PAG’s physiological functional connectivity in healthy subjects during rest will not only deepen our understanding of pain perception / modulation and the development of chronic pain, but also ultimately help inform mechanism-based therapies for treating different types of acute and chronic pain [2,48,52]. Secondly, prior studies have also implicated a role for PAG in neuropsychiatric disturbances such as panic disorders and anxiety disorders [15,22,36,37]. This investigation of PAG’s resting coherence with other brain regions will thereby enhance our understanding of PAG’s role in mental disorders.
In a recent study using a relatively large sample size of 61 subjects, we have found that, compared with low intensity pain, high intensity heat pain stimuli applied to the right forearm can produce significantly stronger activation at ventrolateral PAG [29]. Previous reports have suggested that, of four subdivisions of PAG (dorsal, dorsolateral, ventrolateral, and medial), ventrolateral PAG is the only region at which injections of low doses of morphine produces antinociception [4]. Thus, we will use the area of the PAG observed to be most strongly activated by heat pain in our previous study [29] as the seed region to investigate the innate functional connectivity between the ventrolateral PAG and other brain regions.
Previous studies [6,18,41,58,61] suggest that PAG activity tends to cohere with rACC during pain perception and modulation; we thus hypothesize that there is inherited functional connectivity between the PAG and ACC, even during resting state. Also, Loyd et al. [32] reported that morphine administration produces greater antinociception in males compared to females, and that the anatomical and physiological characteristics of the PAG is different between the two genders; as an exploratory aim, we will also investigate the gender differences in the functional connectivity of PAG.
Material and Methods
In total, data from 100 healthy right-handed subjects (50 male, ages 22 ± 3.2 years (mean ± SD)) are included in this study. All subjects were originally enrolled for an fMRI study on cognitive tasks; the resting state data used in this study was collected at the very beginning of each subject’s scan before any tasks were performed. Experiments were conducted with the written consent of each subject and approved by the Massachusetts General Hospital’s Institutional Review Board.
fMRI Data Acquisition and Analysis
All brain imaging was performed on a 3 Tesla Siemens whole body MRI System (47 axial slices with 4 mm thickness and 1 mm gap, TR = 3000 ms, TE = 30 ms). During scanning, participants were instructed to keep their eyes open and stay still during two 6-minute resting state scans.
Analytical methods replicated those employed in previous functional connectivity studies [21,28,29,56]. In brief, functional data were preprocessed to decrease image artifacts and between-slice timing differences, and to eliminate differences in odd/even slice intensity Data were then spatially smoothed using a Gaussian kernel of 6mm full-width at half-maximum and temporally filtered (0.009Hz<f<0.08Hz). Next, several spurious or nonspecific sources of variance were removed by regression of the following variables: (1) six movement parameters computed by rigid body translation and rotation during preprocessing, (2) mean whole brain signal, (3) mean brain signal within the lateral ventricles, and (4) the mean signal within a deep white matter ROI. Inclusion of the first temporal derivatives of these regressors within the linear model accounted for the time-shifted versions of spurious variance.
Next, a functional connectivity analysis produced coefficients for each previously defined seed-voxel correlation. Fisher’s r-to-z transformation was used to convert correlation maps into z maps. Group effects were tested with a random-effects analysis using a one sample t-test. The threshold was set at voxel-wise p = 0.05 corrected (family-wise error, FWE) with a minimum cluster extent of 10 contiguous voxels. To explore gender differences in the functional connectivity of PAG, a two-sample T test was performed comparing males and females. Due to the between group comparison, we will use a less conservative threshold of voxel-wise p = 0.001 uncorrected with a minimum cluster extent of 10 contiguous voxels.
In this study, we use the right ventrolateral PAG (peak 4 −26 −14, with 2mm radius) as our seed. The reason we chose this location as a seed is: 1) in a previous study, we found that increased levels of heat pain can evoke a significant fMRI signal increase at this region [29]; 2) it is located within the ventrolateral PAG, which is believed to be important for opioid antinociception [4].
Results
Of the 100 subjects used in this study, the average age (mean ± SD) for the 50 male and 50 female subjects was 22 ± 3.6 and 22 ± 3.6 respectively; there is no significant difference between the two groups (P = 0.82).
The functional connectivity results are shown in Table 1 and Figure 1. The results show predominantly positive functional connectivity between PAG and nearby brain structures including midbrain tegmentum, substantia nigra, raphe nucleus, striatum, globus pallidum, hypothalamus, thalamus and cerebellum. In addition, PAG also showed significant functional connectivity with certain distant brain regions including bilateral rostral anterior cingulate cortex (rACC), pregenual ACC (pACC), rostral ventromedial medulla (RVM), and right anterior insula.
Table 1.
Results of functional connectivity during resting state demonstrating positive and negative correlations between brain regions and seed region.
| Seed Regions |
Area (Brodmann Area) | Z score |
Number of voxels in cluster |
Peak coordinate (x y z) |
|---|---|---|---|---|
| Positive | Bilateral PAG and surrounding areas (midbrain tegmentum, substantia nigra, raphe nucleus, hypothalamus, striatum, globus pallidum, thalamus, cerebellum, hippocampus) |
Inf | 7427 | −2 −28 −8 |
| Bilateral rostral ventromedial medulla | Inf | 4 −34 −50 | ||
| Bilateral rACC (24 / 32) | 6.86 | 75 | 2 32 24 | |
| Bilateral pregenual ACC (24) | 5.90 | 33 | 4 38 6 | |
| Right anterior insula | 6.65 | 51 | 46 12 2 | |
| left cerebellum | 6.87 | 357 | −34 52 −36 | |
| Right cerebellum | 6.46 | 23 | 24 −40 −44 | |
| Negative | Right lateral orbital prefrontal cortex (11) | 6.85 | 73 | 42 52 −16 |
| Left lateralorbital prefrontal cortex (11) | 5.62 | 10 | −20 52 −16 | |
| left lateral orbital prefrontal cortex (47 / 11 /10) | 6.59 | 413 | −52 20 −12 | |
| Bilateral medial frontal cortex (6) | 6.36 | 49 | 2 −20 58 | |
| Left lateral prefrontal cortex (44) | 6.22 | 30 | −54 14 24 | |
| Left lateral prefrontal cortex (45) | 5.77 | 11 | −56 28 16 | |
| Left lateral prefrontal cortex (9) | 6.03 | 78 | −54 12 38 | |
| Right post-central gyrus (3/1/2) | 6.85 | 795 | 50 −22 58 | |
| Left post-central gyrus (3/1/2) | 6.81 | 535 | −46 −16 56 | |
| Left paracentral lobule ((4, 5) | 6.11 | 34 | −2 −38 64 | |
| Right inferior parietal lobule (40) | 6.01 | 11 | 40 −28 38 | |
| Left superior temporal gyrus (38) | 6.71 | 28 | −44 14 −20 | |
| Left middle temporal gyrus (21) | 6.28 | 25 | −60 −54 6 | |
| Right middle temporal gyrus (21) | 6.27 | 30 | 52 2 −14 | |
| Left middle temporal gyrus (21) | 6.04 | 77 | −52 −30 −8 | |
| Right middle occipital gyrus (19/39) | 7.44 | 284 | 52 −72 6 | |
| Left middle occipital gyrus (19/39) | 7.27 | 177 | −52 −72 6 | |
| Right middle occipital cortex (18) | 5.9 | 26 | 32 −100 4 | |
| Right posterior insula | 5.65 | 11 | 36 −12 18 | |
Note: The threshold was set to voxel-wise p< 0.05 with 10 continuous voxels. Peak coordinates refer to the MNI atlas
Figure 1.
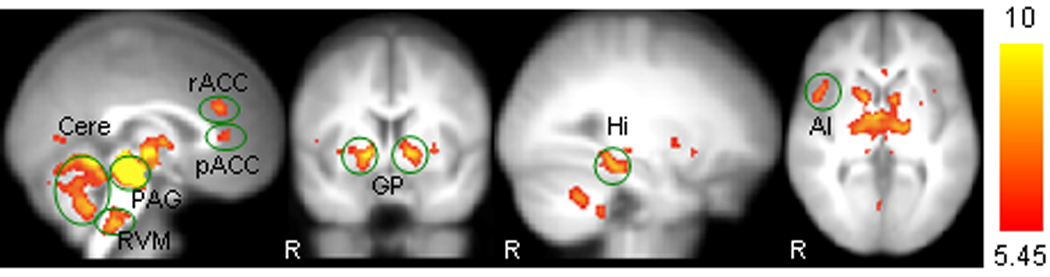
Representative brain regions showing positive functional connectivity with ventrolateral PAG: rACC, rostral ACC; pACC, pre-genual ACC; Cere, cerebellum; RVM, ventromedial medulla; GP, globus pallidum; Hi, hippocampus; AI, anterior insula. R indicates right side.
Significant negative functional connectivity was observed in brain regions including bilateral orbital prefrontal cortex, medial frontal cortex, postcentral gyrus, middle temporal gyrus, middle occipital gyrus; left lateral prefrontal cortex, paracentral lobule superior temporal gyrus, right inferior parietal lobule, and posterior insula Figure 2.
Figure 2.
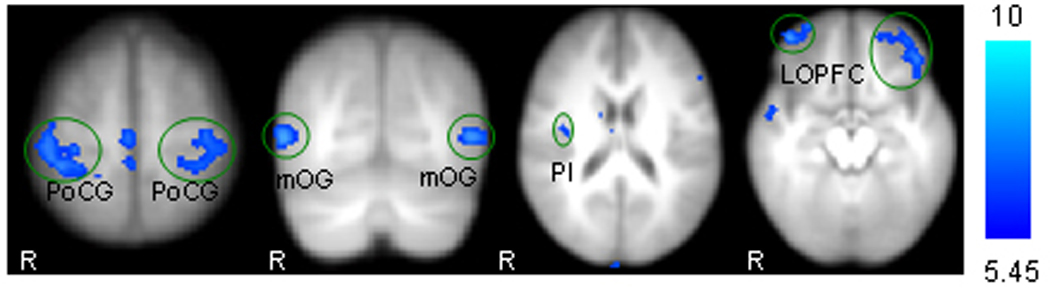
Representative brain regions showing negative functional connectivity with ventrolateral PAG: PoCG, post-central gyrus; mOG, middle occipital gyrus; PI, posterior insula; LOPFC, lateral orbital prefrontal cortex. R indicates right side.
The comparison between the two genders only showed mild differences (Table 2). For the contrast of female minus male, significant differences were observed at mid-cingulate cortex. For the contrast of male minus female, significant differences were observed at left medial orbital prefrontal cortex, and uncus; right insula /operculum and prefrontal cortex.
Table 2.
Results of functional connectivity difference between genders
| Comparison | Area (Brodmann Area) | Z score |
Number of voxels in cluster |
Peak coordinate (x y z) |
|---|---|---|---|---|
| F > M | Bilateral midcingulate cortex (24) | 3.49 | 25 | 0 −8 44 |
| M > F | Right insula / operculum | 3.95 | 36 | 50 −10 4 |
| Left medial orbital prefrontal cortex (10) | 3.69 | 17 | −16 70 −2 | |
| Right prefrontal cortex (10) | 3.40 | 16 | 14 72 18 | |
| Left uncus (34) | 3.42 | 21 | −12 6 −14 | |
Note: The threshold was set to voxel-wise p < 0.001 uncorrected with 10 continuous voxels. Peak coordinates refer to the MNI atlas. F indicates female; M indicates male.
Discussion
In this study, we investigated functional connectivity between ventrolateral PAG and other brain regions during resting state in 100 healthy normal subjects. Results showed that PAG activity is significantly correlated with surrounding brain regions such as midbrain tegmentum, substantia nigra, raphe nucleus, thalamus, striatum, pallidum, hypothalamus, hippocampus, and cerebellum, as well as distant brain regions including bilateral rACC, pACC, RVM, and right anterior insula.
As hypothesized, we found a significant intrinsic functional coherence between PAG and ACC (rACC and pACC) during resting state which provides direct evidence that PAG and ACC form a core intrinsic functional system. We believe elucidation of this ACC-PAG network will further facilitate our understanding of the pathology of chronic pain. For instance, in a recent study, Jensen and colleagues [26] found that compared with healthy control subjects, fibromyalgia syndrome patients (FMS) failed to respond to pain provocation in rACC. We speculate this decreased activity in rACC of FMS patients may further trigger dysfunction (low activity of PAG) of the pain descending inhibition system. Consistent with results from previous studies [19], we also found significant coherence between the PAG and RVM. It is known that PAG’s modulation function depends on its descending projection to RVM, which is involved in both the inhibiting and facilitating of pain perception. As such, we believe that dysfunction of the intrinsic functional connectivity of ACC-PAG-RVM may be part of the pathological basis of chronic pain.
Elucidating the intrinsic functional connectivity between ACC and PAG may also deepen our understanding of mood disorders. Prior studies have associated the rACC with lateral and accessory basal nuclei of the amygdala and implicated a role for the rACC during emotional / motivational processing [13] and positively valenced events such as happiness [57]. Further studies report the region’s involvement in mood disorders including depression [14,17] and post-traumatic stress disorder (PTSD) [49,50]. In addition, PAG is also significantly connected with hypothalamus, a key region of Hypothalamic–pituitary–adrenal (HPA) axis. It is known that the HPA axis is also involved in the mood disorder such as stress and anxiety, major depression and sleep disorders [1,40]; thus, PAG demonstrates another pathway for involvement in mood and emotional regulation. Considering the last two findings together, we speculate that PAG may be involved in the mood / emotion regulation through two pathways: the rACC, amygdala and prefrontal cortex and the HPA-axis. Further study on these two pathways could considerably advance our understanding of mental disorders. Also, this overlap between chronic pain physiology and the brain regions associated with mood disorders may explain the high degree of co-morbidity between chronic pain and mental disorders [59].
Our data showed a strong functional coherence between PAG and brain regions immediately surrounding it. This pattern is very similar to prior observations [37] reporting that as electric shock grew closer, brain responses shifted from rACC to PAG and surrounding areas including thalamus, striatum, pallidum, hypothalamus, and cerebellum (see original Figure 2 in [37] for details). In addition, Price [44] suggested that neuroanatomical systems including hypothalamus, thalamus , ventral pallidum and anterior insula may be involved in the related functions of fear and discernment of the consequences of one's actions, as both are related to a system for control and modulation of visceral functions stretching from the spinal cord. This system is important for the control of emotions and plays a central role in the ability to discern the consequences of one's actions and make appropriate behavioral choices.
In this study, we also found significant functional connectivity between the PAG and anterior insula, a key region in pain process. This result is consistent with a recent publication in which the authors found that the functional connectivity between the anterior insula and PAG before a sensory event reflects the susceptibility to a subsequent noxious stimulus being perceived as painful or non-painful [42]. In addition, we believe the finding [42] also demonstrates the physiological significance of the functional connectivity among brain pain matrix.
Previous studies suggest that morphine produces a significantly greater degree of analgesia in males in comparison to females; some investigators believe that the sexually dimorphic function of the PAG-RVM circuit is involved in this process [32]. Unfortunately, we did not find significant differences in the PAG-RVM functional connectivity between males and females. We speculate that the reason lies in the fact that the sexual-dimorphism of PAG-RVM function exists at the molecular level, thus this difference can not be detected by MRI technique. Nevertheless, we found that females showed more connectivity with mid-cingulate cortex, a region believed to be involved in the affective aspect of pain [46]; males showed more connectivity with left medial orbital prefrontal cortex, uncus, right insula /operculum and prefrontal cortex. We believe these findings will shed some new light on our understanding of the gender difference in pain perception and modulation.
In addition to positive functional connectivity, we also found that PAG is negatively associated with brain regions including bilateral orbital prefrontal cortex, medial frontal cortex, postcentral gyrus, middle temporal gyrus, middle occipital gyrus, left lateral prefrontal cortex, paracentral lobule superior temporal gyrus, right inferior parietal lobule, and posterior insula. However, as interpretation of negative functional connectivity findings are still widely debated in the literature [38], a further study clarifying the meaning of these result is needed.
In a recent report, Mobbs and colleagues [36] investigated the neural organization of two adaptive responses to potential and imminent danger, postencounter and circa-strike defensive, using PPI analysis. They found that during the circa-strike condition, subjects showed increased coupling between the midbrain and mid-dorsal ACC and decreased coupling with the sgACC, amygdala, and hippocampus. Greater activity was observed in the right pregenual ACC for high compared with low probability of capture during circa-strike threat. This region showed decreased coupling with the amygdala, insula, and ventromedial prefrontal cortex. Although their study used different methods and studied a different condition, we believe that their results corroborate our findings, particularly our discovery of the functional connection between the PAG and ACC.
In this study, we did not find significant functional connectivity between the amygdala and PAG at the threshold we set. However, when the threshold was lowered to voxelwise p < 0.001 uncorrected, significant functional connectivity between bilateral amygdala and PAG was observed. It is known that amygdala is an important region in the pain descending inhibition system and plays a role in emotional processing and regulation. We speculate that this relative weak linkage between the PAG and amygdala may due to the fact that the subjects were neither in pain nor perform any emotional processing during resting state, thus the intrinsic coherence between the two region is not as important as ACC. Further study is required at this point.
Although it is well accepted in the field to use the coordinates from a previous fMRI study to locate the seed [23,53,56], one potential concern is that the results from our seed region may not be representative of the connectivity at other parts of ventrolateral PAG. To further address this concern, we chose another coordinate (2 −28 −8) of right ventrolateral PAG, based on the NMI template, and performed an identical functional analysis using this seed as an independent coordinate to validate our findings. Results showed that PAG activity is significantly correlated with surrounding brain regions (including tegmentum, substantia nigra, raphe nucleus, thalamus, striatum, pallidum, hypothalamus, and cerebellum) and distant brain regions (including bilateral rACC and RVM) at the threshold we set. When we lower the threshold to voxelwise p < 0.001 uncorrected, the result is very similar to the results we obtained using our original seed, which provides further support for our results.
One potential limitaiton for this study is that we didn’t use a cardiac gated MRI acquisition, which reduces the effect of cardic pulsation on the variance of voxels around the brain stem. However, our global signal regression during the preprocessing stage is proven to reduce the correlation due to physiological origin [54]. Thus, we do not believe physiological noise significantly influenced our results.
In summary, we found that PAG is intrinsicly connected with brain regions such as rACC, pACC and RVM. Eluciation of this ACC-PAG-RVM network has the potential to enhance our understanding of the physiological and pathological development and maintenance of chronic pain states and mood disorders. In addition, PAG is also connected to several important regions for visceral control and may play a central role in the ability to discern the consequences of one's actions and make appropriate behavioral choices.
Acknowledgements
NIH (NCCAM) K01 AT003883 and R21AT004497 to JK. Brain Genomics Superstruct Project and the laboratory of Dr. Randy Buckner for functional MRI data. M01-RR-01066 and UL1 RR025758-01 for Clinical Research Center Biomedical Imaging Core from National Center for Research Resources (NCRR), P41RR14075 for Center for Functional Neuroimaging Technologies from NCRR and the MIND Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Antonijevic I. HPA axis and sleep: identifying subtypes of major depression. Stress. 2008;11:15–27. doi: 10.1080/10253890701378967. [DOI] [PubMed] [Google Scholar]
- 2.Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog Neurobiol. 2009;87:81–97. doi: 10.1016/j.pneurobio.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci. 2008;28:1398–1403. doi: 10.1523/JNEUROSCI.4123-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci. 1994;17:379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 5.Baskin DS, Mehler WR, Hosobuchi Y, Richardson DE, Adams JE, Flitter MA. Autopsy analysis of the safety, efficacy and cartography of electrical stimulation of the central gray in humans. Brain Res. 1986;371:231–236. doi: 10.1016/0006-8993(86)90358-6. [DOI] [PubMed] [Google Scholar]
- 6.Bingel U, Lorenz J, Schoell E, Weiller C, Buchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120:8–15. doi: 10.1016/j.pain.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 7.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 8.Brooks J, Tracey I. From nociception to pain perception: imaging the spinal and supraspinal pathways. J Anat. 2005;207:19–33. doi: 10.1111/j.1469-7580.2005.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cauda F, D'Agata F, Sacco K, Duca S, Cocito D, Paolasso I, Isoardo G, Geminiani G. Altered resting state attentional networks in diabetic neuropathic pain. J Neurol Neurosurg Psychiatry. 2009 doi: 10.1136/jnnp.2009.188631. [DOI] [PubMed] [Google Scholar]
- 11.Cauda F, Sacco K, D'Agata F, Duca S, Cocito D, Geminiani G, Migliorati F, Isoardo G. Low-frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in diabetic neuropathic pain. BMC Neurosci. 2009;10:138. doi: 10.1186/1471-2202-10-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cauda F, Sacco K, Duca S, Cocito D, D'Agata F, Geminiani GC, Canavero S. Altered resting state in diabetic neuropathic pain. PLoS One. 2009;4:e4542. doi: 10.1371/journal.pone.0004542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Critchley HD. The human cortex responds to an interoceptive challenge. Proc Natl Acad Sci U S A. 2004;101:6333–6334. doi: 10.1073/pnas.0401510101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davidson RJ, Lewis DA, Alloy LB, Amaral DG, Bush G, Cohen JD, Drevets WC, Farah MJ, Kagan J, McClelland JL, Nolen-Hoeksema S, Peterson BS. Neural and behavioral substrates of mood and mood regulation. Biol Psychiatry. 2002;52:478–502. doi: 10.1016/s0006-3223(02)01458-0. [DOI] [PubMed] [Google Scholar]
- 15.Del-Ben CM, Graeff FG. Panic disorder: is the PAG involved? Neural Plast. 2009;2009:108135. doi: 10.1155/2009/108135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48:813–829. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- 18.Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Buchel C. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009;63:533–543. doi: 10.1016/j.neuron.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 19.Fields H. State-dependent opioid control of pain. Nat Rev Neurosci. 2004;5:565–575. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- 20.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 21.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graeff FG, Del-Ben CM. Neurobiology of panic disorder: from animal models to brain neuroimaging. Neurosci Biobehav Rev. 2008;32:1326–1335. doi: 10.1016/j.neubiorev.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 23.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: Specificity, recruitment and plasticity. Brain Res Rev. 2009;60:214–225. doi: 10.1016/j.brainresrev.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hosobuchi Y, Adams JE, Linchitz R. Pain relief by electrical stimulation of the central gray matter in humans and its reversal by naloxone. Science. 1977;197:183–186. doi: 10.1126/science.301658. [DOI] [PubMed] [Google Scholar]
- 26.Jensen KB, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SC, Choy E, Giesecke T, Mainguy Y, Gracely R, Ingvar M. Evidence of dysfunctional pain inhibition in Fibromyalgia reflected in rACC during provoked pain. Pain. 2009;144:95–100. doi: 10.1016/j.pain.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 27.Kahn I, Andrews-Hanna JR, Vincent JL, Snyder AZ, Buckner RL. Distinct cortical anatomy linked to subregions of the medial temporal lobe revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:129–139. doi: 10.1152/jn.00077.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kong J, Gollub RL, Polich G, Kirsch I, Laviolette P, Vangel M, Rosen B, Kaptchuk TJ. A functional magnetic resonance imaging study on the neural mechanisms of hyperalgesic nocebo effect. J Neurosci. 2008;28:13354–13362. doi: 10.1523/JNEUROSCI.2944-08.2008. PMCID: PMC2649754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kong J, Loggia ML, Zyloney C, Tu P, Laviolette P, Gollub RL. Exploring the brain in pain: Activations, deactivations and their relation. Pain. 2010;148:257–267. doi: 10.1016/j.pain.2009.11.008. PMID: 20005043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krienen FM, Buckner RL. Segregated Fronto-Cerebellar Circuits Revealed by Intrinsic Functional Connectivity. Cereb Cortex. 2009 doi: 10.1093/cercor/bhp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu H, Buckner RL, Talukdar T, Tanaka N, Madsen JR, Stufflebeam SM. Task-free presurgical mapping using functional magnetic resonance imaging intrinsic activity. J Neurosurg. 2009 doi: 10.3171/2008.10.JNS08846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loyd DR, Murphy AZ. The role of the periaqueductal gray in the modulation of pain in males and females: are the anatomy and physiology really that different? Neural Plast. 2009;2009:462879. doi: 10.1155/2009/462879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage. 2007;37:579–588. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 34.Mason P. Deconstructing endogenous pain modulations. J Neurophysiol. 2005;94:1659–1663. doi: 10.1152/jn.00249.2005. [DOI] [PubMed] [Google Scholar]
- 35.Mayer DJ, Wolfle TL, Akil H, Carder B, Liebeskind JC. Analgesia from electrical stimulation in the brainstem of the rat. Science. 1971;174:1351–1354. doi: 10.1126/science.174.4016.1351. [DOI] [PubMed] [Google Scholar]
- 36.Mobbs D, Marchant JL, Hassabis D, Seymour B, Tan G, Gray M, Petrovic P, Dolan RJ, Frith CD. From threat to fear: the neural organization of defensive fear systems in humans. J Neurosci. 2009;29:12236–12243. doi: 10.1523/JNEUROSCI.2378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mobbs D, Petrovic P, Marchant JL, Hassabis D, Weiskopf N, Seymour B, Dolan RJ, Frith CD. When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science. 2007;317:1079–1083. doi: 10.1126/science.1144298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Reilly JX, Beckmann CF, Tomassini V, Ramnani N, Johansen-Berg H. Distinct and Overlapping Functional Zones in the Cerebellum Defined by Resting State Functional Connectivity. Cereb Cortex. 2009 doi: 10.1093/cercor/bhp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 41.Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia-- imaging a shared neuronal network. Science. 2002;295:1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- 42.Ploner M, Lee MC, Wiech K, Bingel U, Tracey I. Prestimulus functional connectivity determines pain perception in humans. Proc Natl Acad Sci U S A. 107:355–360. doi: 10.1073/pnas.0906186106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- 44.Price JL. Free will versus survival: brain systems that underlie intrinsic constraints on behavior. J Comp Neurol. 2005;493:132–139. doi: 10.1002/cne.20750. [DOI] [PubMed] [Google Scholar]
- 45.Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- 46.Rainville P. Brain mechanisms of pain affect and pain modulation. Curr Opin Neurobiol. 2002;12:195–204. doi: 10.1016/s0959-4388(02)00313-6. [DOI] [PubMed] [Google Scholar]
- 47.Reynolds DV. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science. 1969;164:444–445. doi: 10.1126/science.164.3878.444. [DOI] [PubMed] [Google Scholar]
- 48.Schweinhardt P, Lee M, Tracey I. Imaging pain in patients: is it meaningful? Curr Opin Neurol. 2006;19:392–400. doi: 10.1097/01.wco.0000236620.89710.63. [DOI] [PubMed] [Google Scholar]
- 49.Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, Peters PM, Metzger LJ, Dougherty DD, Cannistraro PA, Alpert NM, Fischman AJ, Pitman RK. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61:168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- 50.Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- 51.Taylor KS, Seminowicz DA, Davis KD. Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp. 2008 doi: 10.1002/hbm.20705. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55:377–391. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 53.Tu P, Buckner RL, Zollei L, Dyckman KA, Goff DC, Manoach DS. Reduced functional connectivity in a right-hemisphere network for volitional ocular motor control in schizophrenia. Brain. 2010;133:625–637. doi: 10.1093/brain/awp317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic Functional Connectivity As a Tool For Human Connectomics: Theory, Properties, and Optimization. J Neurophysiol. 2009 doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol. 2006;96:3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- 57.Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wagner K, Frings L, Quiske A, Unterrainer J, Schwarzwald R, Spreer J, Halsband U, Schulze-Bonhage A. The reliability of fMRI activations in the medial temporal lobes in a verbal episodic memory task. Neuroimage. 2005;28:122–131. doi: 10.1016/j.neuroimage.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 59.Wiech K, Tracey I. The influence of negative emotions on pain: behavioral effects and neural mechanisms. Neuroimage. 2009;47:987–994. doi: 10.1016/j.neuroimage.2009.05.059. [DOI] [PubMed] [Google Scholar]
- 60.Zhang D, Snyder AZ, Shimony JS, Fox MD, Raichle ME. Noninvasive Functional and Structural Connectivity Mapping of the Human Thalamocortical System. Cereb Cortex. 2009 doi: 10.1093/cercor/bhp182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, Nichols TE, Stohler CS. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci. 2005;25:7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


