Abstract
Deep brain stimulation (DBS) is an established medical therapy for the treatment of movement disorders and shows great promise for several other neurological disorders. However, after decades of clinical utility the underlying therapeutic mechanisms remain undefined. Early attempts to explain the mechanisms of DBS focused on hypotheses that mimicked an ablative lesion to the stimulated brain region. More recent scientific efforts have explored the wide-spread changes in neural activity generated throughout the stimulated brain network. In turn, new theories on the mechanisms of DBS have taken a systems-level approach to begin to decipher the network activity. This review provides an introduction to some of the network based theories on the function and pathophysiology of the cortico-basal-ganglia-thalamo-cortical loops commonly targeted by DBS. We then analyze some recent results on the effects of DBS on these networks, with a focus on subthalamic DBS for the treatment of Parkinson's disease. Finally we attempt to summarize how DBS could be achieving its therapeutic effects by overriding pathological network activity.
Keywords: Neuromodulation, Neurostimulation, Parkinson's Disease, Essential Tremor, Dystonia, Epilepsy, Tourette's Syndrome, Obsessive Compulsive Disorder, Depression
INTRODUCTION
Deep brain stimulation (DBS) is a powerful clinical therapy for the treatment of numerous neurological and psychiatric disorders. The origins of clinical DBS date back to neurosurgical pioneers such as Hassler (1960) and Cooper (1980), and the advent of modern day DBS was principally spearheaded by Benabid et al. (1987). While decades have passed since the inception of DBS, and its clinical utility has grown exponentially, the underlying therapeutic mechanisms of chronic high frequency (~100 Hz) brain stimulation remain mysterious and controversial. This review attempts to explore the mechanisms of DBS from a network perspective, relying on the concept that disorders treated with DBS are fundamentally disorders of a specific brain network, as opposed to a specific neuron type, ion channel, or molecule (Llinas et al., 1999; DeLong and Wichmann, 2007). Our working hypothesis is that DBS interacts with the diseased network to eliminate or subdue the underlying pathological neural activity. This general hypothesis was actually the original proposition of Benabid et al. (1991), which later became known as “jamming” (Benabid et al., 1996). However, following years saw much of the scientific investigation on DBS mechanisms transition away from a systems-level perspective to focus on the cellular effects of stimulation near the electrode, with contentious debate over whether high frequency stimulation induced neural activation or inhibition (Lozano et al., 2002; McIntyre et al., 2004c). While that interesting question continues to be explored, we propose that it may not be the fundamental issue underlying the therapeutic mechanisms of DBS. Recent results suggest that changes in the underlying dynamics of the stimulated brain networks may represent the core mechanisms of DBS and that those basic dynamical changes can be achieved via activation, inhibition, or lesion. In turn, the goal of this review is to summarize some of the latest findings on DBS induced network activity in a quest to create a scientific definition for the term “jamming”.
CORTICO-BASAL-GANGLIA-THALAMO-CORTICAL NETWORK
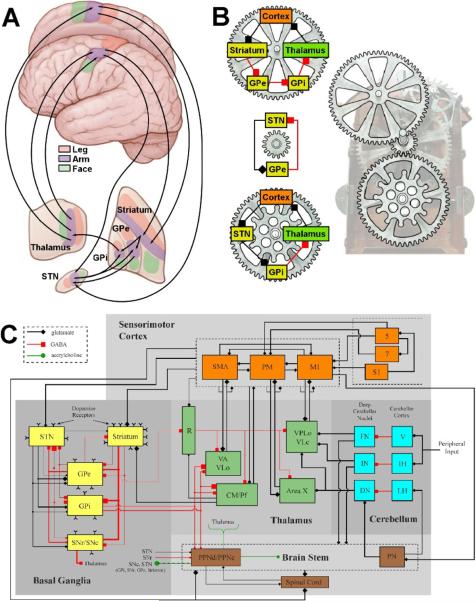
Currently, the most common form of clinical DBS is stimulation of the subthalamic region for the treatment of Parkinson's disease (PD). As such, this review will focus on subthalamic nucleus (STN) DBS and the basal ganglia (BG). However, we believe that the general concepts discussed in this review are applicable to all forms of DBS and that while subtle details may differ the fundamental mechanisms of DBS are consistent across all therapeutic applications. We propose that the first step in unlocking the mechanisms of DBS is to understand the brain circuits that are being stimulated (Fig 1).
Figure 1.
Network models of the motor circuit. A) Anatomical description of the somatotopically organized cortico-basal-ganglia-thalamo-cortical network. Arrows show some of the sub-circuits within the portion of the motor circuit concerned with the arm. B) Multiple loops exist within the network. Three examples loops are shown, each with a different number of nuclei involved and could be considered oscillators with different periods. Each loop also has at least one nucleus in common with one of the other loops. In turn, these loops interact with each other creating an interlocking system that is part of an even larger system. C) Detailed schematic of the sensorimotor network (adapted from Johnson et al. (2008)). Synaptic terminal shape (square, diamond, circle) signifies the type of neurotransmitter involved, whereas the size of the shape reflects the degree of axonal collateralization in the target nucleus. Within the basal ganglia, line thicknesses represent proportions of each type of projection neuron. Abbreviations are as follows for the cortex (M1: primary motor cortex, PM: premotor cortex, S1: primary somatosensory cortex, SMA: supplementary motor area); the basal ganglia (GPe: globus pallidus pars externa, GPi: globus pallidus pars interna, SNc: substantia nigra pars compacta, SNr: substantia nigra pars reticulata, STN: subthalamic nucleus); the thalamus (CM: centromedian nucleus, Pf: parafascicular nucleus, R: reticular formation of thalamus, VA: ventralis anterior, VLc: ventralis lateralis pars caudalis, VLo: ventralis lateralis pars oralis, VPLo: ventralis posterolateralis pars oralis); the cerebellum (DN: dentate nucleus, FN: fastigial nuclei, IH: intermediate hemisphere of cerebellum, IN: interposed nuclei, LH: lateral hemisphere of cerebellum, V: vermis); and the brain stem (PN: pontine nucleus, PPNc: caudal pedunculpontine nucleus, PPNd: dorsal pedunculopontine nucleus).
The BG consists of four interconnected nuclei: the striatum (caudate nucleus and putamen), globus pallidus (internus and externus), substantia nigra (pars compacta and pars reticulata) and subthalamic nucleus (Parent and Hazrati, 1995a,b). Traditional theories propose that two main pathways are present through the BG, the direct and indirect. Cortical information is transmitted through the direct and indirect pathways to the basal ganglia output nuclei, the globus pallidus pars interna (GPi) and the substantia nigra pars reticulata (SNr). Neurons from GPi and SNr project to the ventral motor and intralaminar nuclei of the thalamus, which project back to the frontal cortex and striatum, respectively (Fig. 1).
While we prefer to think of the network as a series of continuous loops that interact with each other (Fig. 1B), the striatum is commonly considered the main input structure of the basal ganglia sub-circuit. Glutamatergic projections from virtually all cortical areas converge onto striatal spiny projection neurons. The striatum also receives an important dopaminergic input from the substantia nigra pars compacta (SNc). The output of the striatum is transmitted by subpopulations of spiny neurons that project either directly to the output nuclei (GPi and SNr), or convey their information to the output nuclei via an indirect route. The striatal neurons that give rise to the indirect pathway project to the globus pallidus pars externa (GPe), which, in turn, project to the STN and then to the output nuclei of the BG (GPi and SNr).
A simplified explanation of BG function is commonly provided by the rate theory which laid much of the original groundwork for the network analysis of movement disorders (Albin et al., 1989; Alexander et al., 1990). The rate theory proposes that by virtue of the neurotransmitters and base line activity of the neurons in the cortico-basal-ganglia-thalamo-cortical network, modulation of the direct and indirect pathways produces functionally opposite effects in the thalamic neurons receiving BG output. Corticostriatal neurons, thalamocortical neurons and neurons of the STN are excitatory, utilizing glutamate as a neurotransmitter. All other neurons in the network are inhibitory using GABA as their main neurotransmitter. Under resting conditions, the activity of the output neurons of the striatum is low compared to that of tonically active neurons in the GPe and STN. Activation of the corticostriatal pathway leads to increased firing of striatal projection neurons. Increased activity of the direct pathway (striatum → GPi/SNr) leads to inhibition of the output nuclei (GPi and SNr). A reduction in tonic activity of the neurons in GPi/SNr leads to a reduction in the inhibition of neurons in the thalamus. In contrast, activation of the traditional indirect pathway (striatum → GPe → STN → GPi/SNr) leads to the opposite functional effect on the thalamus. Increased activity of the striatal output neurons inhibits the tonically active neurons in the GPe. Inhibition of the neurons in GPe disinhibits neurons in the STN. Increased activity of the excitatory neurons of the STN leads to increased firing of neurons in GPi and SNr. An increase in the tonic activity of the neurons in GPi and SNr leads to an increase in the inhibition of neurons in the thalamus. It should be noted that this is a highly simplified view of the workings of the BG, ignoring numerous additional pathways (e.g. hyperdirect pathway – direct cortical input to STN), nuclei (e.g. peduncluopontine nucleus), and synaptic interactions (Parent and Hazrati, 1995a,b; Smith et al., 1998; Pahapill and Lozano, 2000; Nambu, 2004) (Fig. 1C). However, the general framework provides an excellent starting point and a conceptual guide to preliminary network analysis.
FUNCTIONAL IMAGING
Research modalities such as functional magnetic resonance imaging (fMRI) or positron emission tomography (PET) represent excellent tools to investigate brain networks. However, when interpreting the results from functional imaging experiments it is important to remember that the brain activity changes are not direct measures of neural activity and that the activated regions are most indicative of changes in afferent input to that region, not necessarily efferent output (Logothetis et al., 2001; Lin et al., 2008). Nonetheless, functional imaging does provide a unique opportunity to observe systems-level changes in the network activity of human subjects implanted with DBS devices.
Functional imaging experiments performed during DBS has shown that therapeutic stimulation, in all forms tested, generates metabolic and blood flow changes throughout the brain (Perlmutter and Mink, 2006). The first functional imaging investigation of DBS was performed by Limousin et al. (1998) to compare subthalamic DBS and globus pallidus DBS for the treatment of PD. They and many subsequent others, have shown changes in both cortical and sub-cortical brain regions during DBS. Recently, PET studies from the Eidelberg laboratory have shown that suppression of their Parkinson's disease related spatial covariance patterns are a common feature of dopaminergic therapy, STN lesioning, and STN DBS (Trost et al., 2006; Asanuma et al., 2006). Several PET studies of STN DBS have also concluded that therapeutic stimulation drives STN output, inducing metabolic activation of the STN region and pallidum (Hilker et al., 2008); thereby increasing regional cerebral blood flow (rCBF) in thalamus and midbrain while decreasing rCBF in frontal cortical areas (Hershey et al., 2003; Payoux et al., 2004; Grafton et al., 2006; Karimi et al., 2008). Similarly, PET studies of DBS for neuropsychiatric disorders show network wide changes in the cortico-striato-thalamo-cortical circuit, albeit through limbic and prefrontal territories (Rauch et al., 2006; Mayberg et al., 2005).
In general, fMRI has higher spatial and temporal resolution than PET. Additionally, fMRI is more easily integrated with other MRI datasets such as diffusion tensor imaging and high-resolution anatomic imaging of lead placement. Consequently, fMRI is ideally suited to individual subject analysis, and direct comparison of experimental data with patient-specific DBS computer models (McIntyre et al., 2008). However, due to safety concerns the number of DBS fMRI studies has been limited (Rezai et al., 1999; Jech et al., 2001; Stefurak et al., 2003; Arantes et al., 2006; Phillips et al., 2006). The general consensus from the available fMRI studies is that STN DBS generates activation throughout the network, with activation of the globus pallidus and thalamus being common across most patients.
The available data from functional imaging during DBS make a compelling argument that there is much more to the effects of DBS than just what happens around the electrode. However, functional imaging results only provide an indirect measure of neural activity changes. In turn, a systems-level description of the effects of DBS must integrate additional pieces of information, such as electrophysiological recordings of stimulation induced neural activity.
ELECTROPHYSIOLOGICAL INVESTIGATION
Neural recordings examining the effects of DBS have been conducted on brain slices, anesthetized or awake animals, and human patients. Of particular interest to understanding the network effects of DBS are in vivo microelectrode recordings performed in nuclei downstream from the site of stimulation in human patients (Pralong et al., 2003; Galati et al., 2006; Montgomery, 2006), and non-human primates (Hashimoto et al., 2003; Anderson et al., 2003; Kita et al., 2005; Xu et al., 2008; Johnson et al., 2009). The Vitek laboratory demonstrated that neuronal activity in GPe and GPi increased (Hashimoto et al., 2003), while the pallidal receiving area of thalamus decreased (Xu et al., 2008), in response to therapeutic STN DBS of two parkinsonian monkeys. In addition to changes in firing rate, pallidal neurons had a consistent pattern of response to STN DBS with peaks of increased activity in the post-stimulus time histogram occurring at 3 ms and 6.5 ms. Though individual cells were not necessarily entrained to every pulse of the stimulus, the precise pattern and latency of the overall responses resulted in regularization of GPe and GPi activity. During therapeutically ineffective stimulation, the overall firing rate and pattern of GPi activity did not change significantly. These results suggest that therapeutic STN DBS activates subthalamopallidal projections and changes the discharge pattern of GP neurons. That is, the activity pattern shifts from one that is irregular to one that is more regular and organized by the stimulus timing (Hahn et al., 2008; Dorval et al., 2008). Similarly, neurons in the pallidal receiving area of thalamus reduced their rate and became more periodic and regular with a shift in oscillatory activity from low to high frequencies (Xu et al., 2008).
Several studies in human PD patients, where recordings in downstream nuclei were possible, further support the concept that DBS resets the neural output of the stimulated nucleus (Pralong et al., 2003; Galati et al., 2006; Montgomery et al., 2006). For example, Galati et al. (2006) recorded an increase in firing frequency and more regularity in the firing pattern of SNr neurons during therapeutic STN DBS. The underlying concept was also supported by Montgomery (2006) who reported a reduction in thalamic neuronal activity 3.5–5 ms following a stimulus pulse during GPi DBS, consistent with orthodromic activation of GPi output leading to inhibition of thalamic neurons.
Reviewing the experimental data it would appear that STN DBS causes downstream excitation when glutamatergic STN axons are activated; inhibition, when GABAergic GPi axons are activated; or a combination of excitation and inhibition, when polysynaptic pathways are involved. For example, the GPi response to STN DBS is influenced by the direct excitatory STN-GPi projections and an indirect inhibitory STN-GPe-GPi pathway. The role of antidromic axonal activation also needs to be considered, such as the activation of afferent cortical projections to STN (Ashby et al., 1999; Li et al., 2007; Gradinaru et al., 2009), as well as activation of GPe to STN projections which also have axon collaterals to GPi (Kita et al., 2005). To further complicate matters, it is likely that some synaptic terminals are unable to maintain high frequency release of neurotransmitter over long periods of time due to synaptic fatigue (Urbano et al., 2002). The end result is a complex pattern of excitatory and inhibitory effects which modulate not only local BG activity but the cortico-basal-ganglia-thalamo-cortical network as a whole. However, the specific role of these neural activity changes in the alleviation of parkinsonian motor signs remains to be determined.
SPIKE TRAIN ANALYSES
An important step toward understanding network activity is to attempt to decipher the code used by the individual elements of the network. Neurons in the BG and thalamus are known to modulate their activity via changes in firing rate, bursting, and oscillation (Kaneoke and Vitek, 1996). For example, both bursting and oscillation are known to increase in PD patients (Starr et al., 2005; Priori et al., 2004; Brown, 2003) and parkinsonian monkeys (Wichmann and Soares, 2006; Wichmann et al., 1999; Bergman et al., 1994). In addition, drug therapies (Kuhn et al., 2006; Doyle, 2005) and DBS (Hahn et al., 2008; Kuhn et al., 2008) have been shown to reduce these abnormal patterns in correlation with improvement of motor symptoms. Unfortunately, the functional role of bursting and oscillation in BG neurons remains unclear.
In attempts to move beyond traditional measures of neural coding and employ advanced tools from the signal processing world, spike trains have been analyzed for entropy (Strong et al., 1998) and fractal structure (Rodriquez et al., 2003; Goldberger et al., 2002). These measures have been used to explore the effects of apomorphine in Parkinson's patients (Rasouli et al., 2006), DBS in parkinsonian non-human primates (Dorval et al., 2008), and dopamine depletion in rats (Cruz et al., 2009). These measures detect patterns in a time series that reflect a structure deeper than that observed in traditional spike and burst rates. Entropy is an indication of variability, based on the concept that some amount of variability in a time series is necessary to encode information (Strong et al., 1998; de Ruyter van Steveninck et al., 1997). Fractal dimension is an indication of the mathematical property of self-similarity, scale invariance or long range correlation (Rodriquez et al., 2003; Goldberger et al., 2002). The scaling exponent obtained in fractal analysis is an indication of the statistical properties or variability of a spike train. Rasouli et al. (2006) showed an increase in scaling exponent and therefore an improvement in long-range persistent correlation in pallidum following administration of apomorphine. Cruz et al. (2009) found that dopamine depleted rats had a decrease in network entropy compared to controls. Dorval et al. (2008) showed a decrease in entropy in BG and thalamus during therapeutic STN DBS. These analyses suggest that a more regular spike pattern is associated with therapeutic benefit.
Unfortunately, single unit spike train analysis (rate, bursting, entropy, scaling exponent, etc.) is only a small-sample diagnostic tool for probing the network mechanisms of DBS. Moreover, interpretation of results can be difficult, even when significant differences are found. Unlike sensory systems where clear input-output relationships can be defined, we only have a limited understanding of the physiological function or neural code for individual spike trains in the cortico-basal-ganglia-thalamo-cortical network. For example, entropy does not measure the meaning of a spike train, but merely its theoretical ability to encode information. For the information carried in a string of spikes to have significance, the neuronal targets of that spike train must have the ability to use the information as presented. Similarly, fractal characteristics only indicate a statistical regularity, or lack of regularity, in a spike train. Neither entropy nor fractal analysis can actually define the mechanisms of DBS, but these metrics could represent useful tools to direct hypotheses as understanding of the overall network grows.
SPIKE TRAIN AS PART OF A NETWORK
Neural activity patterns contribute to behavior only in the context of the networks in which they are found. Pathological changes observed in spike trains recorded from single cells may be due to modifications of intrinsic properties of those cells or to the network as a whole. We propose that most of those changes result from network interactions and this general concept is integral to the rate model of PD (Albin et al., 1989; Alexander et al., 1990). In the rate model, BG output is defined by a balance between the direct and indirect pathways. As such, movement disorders are proposed to result from an imbalance between the direct and indirect pathways. However, this simplified representation is complicated by the presence of STN which receives input directly from cortex (Nambu et al., 2000), excites both GPe and GPi, and is itself inhibited by GPe (Fig. 1). In turn, attempting to explain BG network activity with only modulation of average firing rate has failed to explain numerous experimental and clinical findings (Pessiglione et al., 2005), including the observation that both increases (STN DBS) and decreases (lesion) of GPi rate have similar therapeutic effects (Montgomery, 2007).
More recently, the role of the BG has been considered within the context of a larger, closed loop network including thalamocortical and corticostriatal connections (Li et al., 2007; Leblois et al., 2006; Rubin and Terman, 2004; Rubchinsky et al., 2003) (Fig. 1). Movement disorders can be viewed as resulting from the corruption or spurious generation of sequential messages representing motor commands. For example, the rate model proposes that increased activity in GPi causes increased inhibition in thalamus and blocks the flow of motor information. However from the perspective of a closed loop network, movement disorders may result from subtle shifts in the dynamics of the loop activity. Under such a model the pathology lies not in suppression of any particular group of cells, but in corrupted spatiotemporal patterns of neuronal activity distributed across the entire network such that the normal flow of activity through the network is interrupted.
Closed network loops involving the motor BG, while not explicitly demonstrated to exist from a given cell back to itself, can be inferred from data on the somatotopical arrangement of the nuclei involved in the loop and the known internuclear connections (Kelly and Strick, 2004). Whether discrete or continuous, this collection of loops can be thought of as being organized in channels related to a body map (Alexander and Crutcher 1990) or motor program components (Rubchinsky et al., 2003; Mink, 1996). In either case, the question arises, where is information relevant to the generation of movements read off these loops? Where is the command for a movement inserted into these loops? Minimal data exist that would answer these questions with clarity. However, the identification of motor loops has led to the finding that segregation between loops breaks down in the low dopamine state (Pessiglione, et al. 2005, Goldberg, et al. 2002). As a result, activity in cortex, striatum and pallidum is more synchronous with reduced selectivity. Computational models that demonstrate loss of selectivity between loops emphasize the importance of network dynamics in this phenomenon (Leblois et al., 2006; Humphries et al., 2006; Rubchinsky et al., 2003).
CHARACTERIZING NETWORK ACTIVITY
The acquisition and analysis of network activity is complicated by numerous experimental difficulties. First, networks consist of multiple elements so that recording activity in single cells only reveals partial information about the network. Second, networks such as the cortico-basal-ganglia-thalamo-cortical loops are spatially extended in both a local and global sense. Some information about local or regional activity can be obtained from field recordings (i.e. LFP or EEG) or functional imaging (i.e. PET or fMRI). However, with the advent of large-scale simultaneous microelectrode recordings systems we are on the verge of being able to acquire massive amount of electrophysiological information throughout the network during stimulation while the experimental animal performs a task (Shi et al., 2006). One limitation of such an approach is the overwhelming amount of data generated, but novel hardware and computational methods are currently under development for just such investigations (Buzsaki, 2004).
With large-scale recordings in hand we will be faced with the daunting task of making sense of it all. One interesting concept of network function is the possibility that resonance is used to accentuate important patterns (Montgomery, 2007). The cortico-basal-ganglia-thalamo-cortical network consists of many different anatomical loops, and each loop could be considered to be an oscillator (Fig. 1B). The time in which it takes the output to travel around the loop and return is the period of oscillation. Resonance is a property of the dynamics of oscillators such that excitation at a period that is compatible with the natural period of the oscillator produces a much greater effect than stimulation at other non-compatible rates (Strogatz, 2001).
The loops of the cortico-basal-ganglia-thalamo-cortical network and recurrent loops in the local microcircuitry of the cortex, basal ganglia, and thalamus can each be thought of as oscillators with resonant frequencies (Fig. 1). Unfortunately it is difficult to describe feed forward recurrence in networks with inhibitory nodes along the loop. This is particularly true of loops in the cortico-basal-ganglia-thalamo-cortical network, since striatopallidal and pallidofugal synapses are inhibitory on their respective targets (Fig. 1). One possibility that preserves the oscillator model is that GABAergic synapses can be conceptually excitatory if inhibition is consistently followed by post inhibitory rebound excitation. This concept has been well documented in thalamic relay neurons, and appears in most neurons that express a strong hyperpolarization activated cation current.
Leblois et al. (2006) demonstrated that the recurrent loops of the cortico-basal-gangliathalamo-cortical network, while differentiating between the parallel loops of the direct (cortex → striatum → GPi/SNr) and hyperdirect (cortex → STN → GPi/SNr) pathways, can be viewed as a combination of positive and negative feedback loops. They describe a stable action selection mechanism based on competition between the parallel loops. Their model effectively combines the intrinsic rate of neural activity in individual nuclei with the polarity of the positive and negative feedback loops to describe a dynamic network, or oscillator, whose rate is made to vary based on transient, selective input representing motor commands. However, the focus is not on the time it takes a signal (one spike) to leave a given neuron, traverse the loop and return. Instead, the elements of each set of loops settle into a stable pattern of rates and no particular relationship between the timing of individual spikes is identified. Resonant effects in this case would not be achieved due to the dynamic properties of the loop, but perhaps by temporal summation of inputs at a particular point of the loop. In any case, models of this type represent the building blocks necessary to begin to characterize higher level network functions that are lost with single spike train analyses.
DISRUPTING THE DISRUPTION
Near the turn of the century Llinas et al. (1999) identified the concept of network dysrhythmia, pathological resonant interaction between cortical and sub-cortical structures, as the root cause of multiple neurological disorders. Specific emphasis on the network dynamics of PD has identified increased low frequency oscillations and enhanced neural synchrony in the beta band (13–30 Hz) throughout the cortico-basal-ganglia-thalamo-cortical network as likely contributors to the debilitating behavioral effects of PD (Hutchison et al., 2004). Additional support for the importance of beta band activity in PD is that this measure of pathological network dynamics can be reversed by therapeutic treatments (Kuhn et al., 2008; Ray et al., 2008; Bronte-Stewart et al., 2009). Interestingly, population activity in the motor cortex of normal subjects commonly exhibit oscillations in the beta frequency range that diminish during movements. These beta oscillations are normally not transmitted through the cortico-basal-ganglia-thalamo-cortical network to the BG. However, beta oscillations are prominent in the BG of parkinsonian monkeys, suggesting that the striatum, in the low dopamine state, cannot properly filter out cortical input at beta frequency (Gatev and Wichmann, 2008; Courtemanche et al., 2003; Bergman et al., 1994). This concept is reinforced by the observation that enhanced coherence between BG and cortex correlates with parkinsonian symptoms and is reduced by therapeutically effective treatments (Goldberg et al. 2004; Devos et al., 2004). Taken together these results demonstrate the role of network dynamics in parkinsonism and highlight the importance of the entire network in the generation of pathological activity.
Acceptance of PD as a network disorder (DeLong and Wichmann, 2007), enables explanation of why application of DBS to any of node of the network can disrupt the underlying pathological network dynamics and provide therapeutic benefit. While the STN is the most popular target, it has been well documented that stimulation of pallidum, thalamus, or cortex can improve parkinsonian symptoms. But, why are some stimulation locations better than others? Theoretical studies have shown that multiple neuron types (i.e. projection neurons, fibers of passage, afferent inputs) surrounding the electrode are directly stimulated by therapeutic DBS (McIntyre et al., 2007). However, the goal of DBS should be to maximize stimulation of the target neuron types with minimal activation of the non-target neuron types, using the least amount of energy possible. We hypothesize that the target neuron types for the treatment of PD are those directly connected to sub-loops of the cortico-basal-ganglia-thalamo-cortical network explicitly associated with motor function. Luckily the network is somatotopically organized and segregated between motor and non-motor pathways (Alexander et al., 1990; Kelly and Strick, 2004) (Fig. 1A). Plus, the anatomical structure of the system provides specific brain regions where multiple loops converge in a focused area, and one such area is the subthalamic region. Therefore, it is not surprising that a well placed electrode in the subthalamic region can generate excellent clinical outcomes with the lowest stimulation power requirements.
DBS of the subthalamic region is likely to directly activate a wide range of different neuron types including (but not limited to) STN projection neuron axons, GPi fibers of passage, SNc fibers of passage, and cortical afferent to STN (Miocinovic et al., 2006; Lee et al., 2006; Li et al., 2007). Recent results suggest that direct activation of cortical afferents to STN may be highly important in the beneficial effects of STN DBS (Li et al., 2007; Grandinaru et al., 2009). Li et al. (2007) showed that DBS induced antidromic spikes in Layer V pyramidal cells triggered a dampened oscillation of local field potentials in cortex with a resonant frequency around 120 Hz. This antidromic activation spread excitation through a rich collateral network of adjacent pyramidal cells, as well as neurons in more superficial layers. The spreading excitation eventually returned to antidromically activated cells to generate the resonance in the local field potential. Grandinaru et al. (2009) then demonstrated with optogenetics and solid-state optics that direct activation of cortical afferents projecting to the STN region was explicitly associated with therapeutic benefit. Taken together these studies suggest that by accessing the hyperdirect pathway via STN DBS it is possible to specifically interface with diffuse, but functionally related, cortical circuits in a way that regularizes the overall cortico-basal-ganglia-thalamo-cortical network.
PROBING NETWORK DYNAMICS WITH DBS
Potentially, the key to understanding the network mechanisms of DBS is to use DBS itself to manipulate the network. Dynamical properties of the cortico-basal-ganglia-thalamo-cortical network can be assessed experimentally via alterations in the DBS parameters settings. The stimulation parameters establish the spatiotemporal properties of the electric field associated with each pulse (McIntyre et al., 2004b). The applied field interacts with surrounding neurons and alters their action potential signaling (McIntyre et al. 2004a; Miocinovic et al., 2006; Johnson and McIntyre, 2008). These stimulated neurons then interact with the underlying network activity and depending on the stimulation parameter settings can result in either beneficial or deleterious behavioral effects. In either case DBS represents a tool to interact with network activity and enhance our understanding of the system.
Preliminary studies have explored the possibility that the interpulse interval corresponding to therapeutic DBS frequencies is compatible with the characteristic period of the cortico-basal-ganglia-thalamo-cortical oscillator loop or nested loops within this network (Montgomery and Gale, 2008; Montgomery, 2007; Grill et al., 2004) (Fig. 1). That is, optimal DBS frequencies are resonant frequencies of the network. However, key missing details remain in the quantitative description of the specific pathways that have latencies that are both consistent in their timing and commensurate with the optimal stimulation period. Nonetheless, variation of stimulus timing represents one approach to evaluate resonance effects in the network. For example, periodic stimulation is needed for resonance to occur. By adjusting the period of stimulation and quantifying the observed effect it may be possible to determine the natural period of a network oscillator. It is generally accepted that at lower frequencies DBS therapeutic benefit is reduced and that peak benefit is achieved at frequencies above ~100Hz. However, for a true resonance effect one would anticipate that benefit would drop off for frequencies greater than that corresponding to the natural period of the network oscillator. Instead, for frequencies greater than ~100Hz the beneficial effects of DBS typically saturate or decline only slightly. One possible explanation for this discrepancy in the case of STN DBS is that there are multiple natural periods related to parkinsonism, as there are multiple network loops passing through the STN (Fig. 1), and higher frequency (300 Hz) oscillatory activity can be recorded there (Foffani et al., 2003).
Periodic pulse trains are the clinical standard for DBS; however, the statistical properties of the interpulse interval have also been varied in experimental and computational settings. Birdno et al. (2007; 2008) introduced randomness into the interpulse timing of high frequency DBS and evaluated its effects on controlling tremor. They found that as irregularity of DBS trains increased, they became less effective at controlling tremor, suggesting that the therapeutic effects of DBS are dependent not only on the average frequency of DBS, but also on the temporal spacing of DBS pulses. Conversely, when Ma and Wichmann (2004) stimulated the STN of a normal monkey with bursty spike trains recorded from the STN of a parkinsonian monkey, the normal monkey generated parkinsonian symptoms. However, when Baker et al. (2008) stimulated the globus pallidus of a parkinsonian monkey using pulses grouped in stereotyped regular bursts, performance on a reaching task was indistinguishable from traditional DBS. In turn, there appears to be much to learn about the effects of stimulus pulse timing in the cortico-basal-ganglia-thalamo-cortical network.
COMPUTATIONAL MODELING
Neurostimulation modeling provides a controlled testing ground to evaluate the effects of DBS at the electrode-tissue interface, as well as at the single neuron and neural network levels (McIntyre et al., 2007). We propose that computational models will play an increasingly important role in deciphering the network effects of DBS, as interpretation of the experimental data will require advanced tools to help identify the core features of the system. However, large-scale neural network models are inherently difficult to constrain and parameterize. The development of realistic network models will require in vivo microelectrode recordings acquired simultaneously from multiple sites in the brain with and without DBS. It will also be especially important to acquire this data in the context of a behavioral task to identify differences between the network at rest and the network in action. Effective execution of this process will require synergistic interaction between systems neurophysiologists and computational neuroscientists, as much of the necessary experimental and computational data does not currently exist. However, a number of preliminary computational studies do allow for early insights into how models can be used to address the network mechanisms of DBS (Montgomery and Baker, 2000; Tass, 2003; Rubin and Terman, 2004; Grill et al., 2004; Hahn et al., 2005; Feng et al., 2007; Shils et al., 2008). Based on results from simplified neural network models, Montgomery and Baker (2000) hypothesized that the stimulation-induced neural activity represents a noise source that disrupts pathological bursting behavior of the parkinsonian basal ganglia; thereby improving information transfer from the basal ganglia to the cortex. This general concept has been further supported by more detailed theoretical analyses (Rubin and Terman, 2004; Guo et al., 2008) and represents an excellent starting point for hypothesis generation on the interaction between various loops of the network in relation to the concepts of resonance discussed above.
SUMMARY
DBS represents an amazingly powerful research tool to interrogate brain networks. Stimulation can be adjusted as various aspects of network activity are measured in an attempt to understand both the underlying pathophysiology of neurological disorders, as well as the therapeutic mechanism of DBS. So far such analysis has predicted that therapeutic DBS is associated with reduced bursting activity, reduced variability in spiking, and with the overriding of disruptive oscillations. Further, these general predictions appear to hold true throughout the various nodes of the network. However, it remains unclear which particular dynamic features of network activity are directly linked with therapeutic DBS outcomes. During stimulation the networks are not necessarily restored to pre-pathological states, but rather to some third state that allows for function to improve relative to the diseased state, but may not necessarily be normal. One is still led to ask, what is the functional importance of the normal network, and why does manipulating the diseased network with DBS make patients better? Nonetheless, taking into consideration available data from imaging, electrophysiology, and modeling it appears that the one definition of the term “jamming” could be - stimulation induced resetting of network oscillatory patterns such that resonance at the stimulation frequency regularizes neural firing patterns across the cortico-basal-ganglia-thalamo-cortical circuit.
ACKNOWLDEGEMENTS
This work was supported by grants from National Institutes of Health (R01 NS047388, R01 NS059736). The authors thank Joe Kanasz for artistic contributions to the figure.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- Anderson KE, Mullins J. Behavioral changes associated with deep brain stimulation surgery for Parkinson's disease. Curr Neurol Neurosci Rep. 2003;3:306–313. doi: 10.1007/s11910-003-0007-z. [DOI] [PubMed] [Google Scholar]
- Arantes PR, Cardoso EF, Barreiros MA, Teixeira MJ, Goncalves MR, Barbosa ER, Sukwinder SS, Leite CC, Amaro E., Jr. Performing functional magnetic resonance imaging in patients with Parkinson's disease treated with deep brain stimulation. Mov Disord. 2006;21:1154–1162. doi: 10.1002/mds.20912. [DOI] [PubMed] [Google Scholar]
- Asanuma K, Tang C, Ma Y, Dhawan V, Mattis P, Edwards C, Kaplitt MG, Feigin A, Eidelberg D. Network modulation in the treatment of Parkinson's disease. Brain. 2006;129:2667–2678. doi: 10.1093/brain/awl162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby P, Kim YJ, Kumar R, Lang AE, Lozano AM. Neurophysiological effects of stimulation through electrodes in the human subthalamic nucleus. Brain. 1999;122(Pt 10):1919–1931. doi: 10.1093/brain/122.10.1919. [DOI] [PubMed] [Google Scholar]
- Baker KB, Zhang J, Xu W, Bynum E, Minnich J, Vitek JL. Soc. Neurosci. Abstr. Washington, DC: 2008. The effect of train rate and pattern on motor behavior during deep brain stimulation of the globus pallidus internus in a non-human primate model of Parkingson's disease; p. 139.5. [Google Scholar]
- Benabid AL, Pollak P, Gervason C, Hoffmann D, Gao DM, Hommel M, Perret JE, de Rougemont J. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet. 1991;337:403–406. doi: 10.1016/0140-6736(91)91175-t. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Pollak P, Louveau A, Henry S, de Rougemont J. Combined (thalamotomy and stimulation) stereotactic surgery of the VIM thalamic nucleus for bilateral Parkinson disease. Appl Neurophysiol. 1987;50:344–346. doi: 10.1159/000100803. [DOI] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol. 1994;72:507–520. doi: 10.1152/jn.1994.72.2.507. [DOI] [PubMed] [Google Scholar]
- Birdno MJ, Cooper SE, Rezai AR, Grill WM. Pulse-to-pulse changes in the frequency of deep brain stimulation affect tremor and modeled neuronal activity. J Neurophysiol. 2007;98:1675–1684. doi: 10.1152/jn.00547.2007. [DOI] [PubMed] [Google Scholar]
- Birdno MJ, Kuncel AM, Dorval AD, Turner DA, Grill WM. Tremor varies as a function of the temporal regularity of deep brain stimulation. Neuroreport. 2008;19:599–602. doi: 10.1097/WNR.0b013e3282f9e45e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronte-Stewart H, Barberini C, Koop MM, Hill BC, Henderson JM, Wingeier B. The STN beta-band profile in Parkinson's disease is stationary and shows prolonged attenuation after deep brain stimulation. Exp Neurol. 2009;215:20–28. doi: 10.1016/j.expneurol.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Brown P. Oscillatory nature of human basal ganglia activity: relationship to the pathophysiology of Parkinson's disease. Mov Disord. 2003;18:357–363. doi: 10.1002/mds.10358. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Large-scale recording of neuronal ensembles. Nat Neurosci. 2004;7:446–451. doi: 10.1038/nn1233. [DOI] [PubMed] [Google Scholar]
- Cooper IS, Upton AR, Amin I. Reversibility of chronic neurologic deficits. Some effects of electrical stimulation of the thalamus and internal capsule in man. Appl Neurophysiol. 1980;43:244–258. doi: 10.1159/000102263. [DOI] [PubMed] [Google Scholar]
- Courtemanche R, Fujii N, Graybiel AM. Synchronous, focally modulated beta-band oscillations characterize local field potential activity in the striatum of awake behaving monkeys. J Neurosci. 2003;23:11741–11752. doi: 10.1523/JNEUROSCI.23-37-11741.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz AV, Mallet N, Magill PJ, Brown P, Averbeck BB. Effects of dopamine depletion on network entropy in the external globus pallidus. J Neurophysiol. 2009;102:1092–1102. doi: 10.1152/jn.00344.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruyter van Steveninck RR, Lewen GD, Strong SP, Koberle R, Bialek W. Reproducibility and variability in neural spike trains. Science. 1997;275:1805–1808. doi: 10.1126/science.275.5307.1805. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64:20–24. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- Devos D, Labyt E, Derambure P, Bourriez JL, Cassim F, Reyns N, Blond S, Guieu JD, Destée A, Defebvre L. Subthalamic nucleus stimulation modulates motor cortex oscillatory activity in Parkinson's disease. Brain. 2004;127:408–419. doi: 10.1093/brain/awh053. [DOI] [PubMed] [Google Scholar]
- Dorval AD, Russo GS, Hashimoto T, Xu W, Grill WM, Vitek JL. Deep brain stimulation reduces neuronal entropy in the MPTP-primate model of Parkinson's disease. J Neurophysiol. 2008;100:2807–2818. doi: 10.1152/jn.90763.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle LM, Kuhn AA, Hariz M, Kupsch A, Schneider GH, Brown P. Levodopa-induced modulation of subthalamic beta oscillations during self-paced movements in patients with Parkinson's disease. Eur J Neurosci. 2005;21:1403–1412. doi: 10.1111/j.1460-9568.2005.03969.x. [DOI] [PubMed] [Google Scholar]
- Feng XJ, Greenwald B, Rabitz H, Shea-Brown E, Kosut R. Toward closed-loop optimization of deep brain stimulation for Parkinson's disease: concepts and lessons from a computational model. J Neural Eng. 2007;4:L14–21. doi: 10.1088/1741-2560/4/2/L03. [DOI] [PubMed] [Google Scholar]
- Foffani G, Priori A, Egidi M, Rampini P, Tamma F, Caputo E, Moxon KA, Cerutti S, Barbieri S. 300-Hz subthalamic oscillations in Parkinson's disease. Brain. 2003;126:2153–2163. doi: 10.1093/brain/awg229. [DOI] [PubMed] [Google Scholar]
- Galati S, Mazzone P, Fedele E, Pisani A, Peppe A, Pierantozzi M, Brusa L, Tropepi D, Moschella V, Raiteri M, Stanzione P, Bernardi G, Stefani A. Biochemical and electrophysiological changes of substantia nigra pars reticulata driven by subthalamic stimulation in patients with Parkinson's disease. Eur J Neurosci. 2006;23:2923–2928. doi: 10.1111/j.1460-9568.2006.04816.x. [DOI] [PubMed] [Google Scholar]
- Gatev P, Wichmann T. Interactions between cortical rhythms and spiking activity of single basal ganglia neurons in the normal and parkinsonian state. Cereb Cortex. 2008;19:1330–1344. doi: 10.1093/cercor/bhn171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JA, Boraud T, Maraton S, Haber SN, Vaadia E, Bergman H. Enhanced synchrony among primary motor cortex neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine primate model of Parkinson's disease. J Neurosci. 2002;22:4639–4653. doi: 10.1523/JNEUROSCI.22-11-04639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JA, Rokni U, Boraud T, Vaadia E, Bergman H. Spike synchronization in the cortex/basal-ganglia networks of Parkinsonian primates reflects global dynamics of the local field potentials. J Neurosci. 2004;24:6003–6010. doi: 10.1523/JNEUROSCI.4848-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberger AL, Amaral LA, Hausdorff JM, Ivanov P, Peng CK, Stanley HE. Fractal dynamics in physiology: alterations with disease and aging. Proc Natl Acad Sci U S A. 2002;99(Suppl 1):2466–2472. doi: 10.1073/pnas.012579499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324:354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafton ST, Turner RS, Desmurget M, Bakay R, Delong M, Vitek J, Crutcher M. Normalizing motor-related brain activity: subthalamic nucleus stimulation in Parkinson disease. Neurology. 2006;66:1192–1199. doi: 10.1212/01.wnl.0000214237.58321.c3. [DOI] [PubMed] [Google Scholar]
- Grill WM, Snyder AN, Miocinovic S. Deep brain stimulation creates an informational lesion of the stimulated nucleus. Neuroreport. 2004;15:1137–1140. doi: 10.1097/00001756-200405190-00011. [DOI] [PubMed] [Google Scholar]
- Guo Y, Rubin JE, McIntyre CC, Vitek JL, Terman D. Thalamocortical relay fidelity varies across subthalamic nucleus deep brain stimulation protocols in a data-driven computational model. J Neurophysiol. 2008;99:1477–1492. doi: 10.1152/jn.01080.2007. [DOI] [PubMed] [Google Scholar]
- Hahn PJ, Lee DC, Russo GS, Vitek JL, McIntyre CC. Soc. Neurosci. Abstr. Washington D.C.: 2005. Effects of deep brain stimulation on a model of subthalamopallidal network activity; p. 331.6. [Google Scholar]
- Hahn PJ, Russo GS, Hashimoto T, Miocinovic S, Xu W, McIntyre CC, Vitek JL. Pallidal burst activity during therapeutic deep brain stimulation. Exp Neurol. 2008;211:243–251. doi: 10.1016/j.expneurol.2008.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci. 2003;23:1916–1923. doi: 10.1523/JNEUROSCI.23-05-01916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassler R, Riechert T, Mundinger F, Umbach W, Ganglberger JA. Physiological observations in stereotaxic operations in extrapyramidal motor disturbances. Brain. 1960;83:337–350. doi: 10.1093/brain/83.2.337. [DOI] [PubMed] [Google Scholar]
- Hershey T, Revilla FJ, Wernle AR, McGee-Minnich L, Antenor JV, Videen TO, Dowling JL, Mink JW, Perlmutter JS. Cortical and subcortical blood flow effects of subthalamic nucleus stimulation in PD. Neurology. 2003;61:816–821. doi: 10.1212/01.wnl.0000083991.81859.73. [DOI] [PubMed] [Google Scholar]
- Hilker R, Voges J, Weber T, Kracht LW, Roggendorf J, Baudrexel S, Hoevels M, Sturm V, Heiss WD. STN-DBS activates the target area in Parkinson disease: an FDG-PET study. Neurology. 2008;71:708–713. doi: 10.1212/01.wnl.0000312380.01852.77. [DOI] [PubMed] [Google Scholar]
- Humphries MD, Stewart RD, Gurney KN. A physiologically plausible model of action selection and oscillatory activity in the basal ganglia. J Neurosci. 2006;26:12921–12942. doi: 10.1523/JNEUROSCI.3486-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison WD, Dostrovsky JO, Walters JR, Courtemanche R, Boraud T, Goldberg J, Brown P. Neuronal oscillations in the basal ganglia and movement disorders: evidence from whole animal and human recordings. J Neurosci. 2004;24:9240–9243. doi: 10.1523/JNEUROSCI.3366-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jech R, Urgosik D, Tintera J, Nebuzelsky A, Krasensky J, Liscak R, Roth J, Ruzicka E. Functional magnetic resonance imaging during deep brain stimulation: a pilot study in four patients with Parkinson's disease. Mov Disord. 2001;16:1126–1132. doi: 10.1002/mds.1217. [DOI] [PubMed] [Google Scholar]
- Johnson MD, McIntyre CC. Quantifying the neural elements activated and inhibited by globus pallidus deep brain stimulation. J Neurophysiol. 2008;100:2549–2563. doi: 10.1152/jn.90372.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MD, Miocinovic S, McIntyre CC, Vitek JL. Mechanisms and targets of deep brain stimulation in movement disorders. Neurotherapeutics. 2008;5:294–308. doi: 10.1016/j.nurt.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MD, Vitek JL, McIntyre CC. Pallidal stimulation that improves parkinsonian motor symptoms also modulates neuronal firing patterns in primary motor cortex in the MPTP-treated monkey. Exp Neurol. 2009;219:359–362. doi: 10.1016/j.expneurol.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneoke Y, Vitek JL. Burst and oscillation as disparate neuronal properties. J Neurosci Methods. 1996;68:211–223. doi: 10.1016/0165-0270(96)00081-7. [DOI] [PubMed] [Google Scholar]
- Karimi M, Golchin N, Tabbal SD, Hershey T, Videen TO, Wu J, Usche JW, Revilla FJ, Hartlein JM, Wernle AR, Mink JW, Perlmutter JS. Subthalamic nucleus stimulation-induced regional blood flow responses correlate with improvement of motor signs in Parkinson disease. Brain. 2008;131:2710–2719. doi: 10.1093/brain/awn179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Macro-architecture of basal ganglia loops with the cerebral cortex: use of rabies virus to reveal multisynaptic circuits. Prog Brain Res. 2004;143:449–459. doi: 10.1016/s0079-6123(03)43042-2. [DOI] [PubMed] [Google Scholar]
- Kita H, Tachibana Y, Nambu A, Chiken S. Balance of monosynaptic excitatory and disynaptic inhibitory responses of the globus pallidus induced after stimulation of the subthalamic nucleus in the monkey. J Neurosci. 2005;25:8611–8619. doi: 10.1523/JNEUROSCI.1719-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn AA, Kempf F, Brucke C, Gaynor Doyle L, Martinez-Torres I, Pogosyan A, Trottenberg T, Kupsch A, Schneider GH, Hariz MI, Vandenberghe W, Nuttin B, Brown P. High-frequency stimulation of the subthalamic nucleus suppresses oscillatory beta activity in patients with Parkinson's disease in parallel with improvement in motor performance. J Neurosci. 2008;28:6165–6173. doi: 10.1523/JNEUROSCI.0282-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn AA, Kupsch A, Schneider GH, Brown P. Reduction in subthalamic 8–35 Hz oscillatory activity correlates with clinical improvement in Parkinson's disease. Eur J Neurosci. 2006;23:1956–1960. doi: 10.1111/j.1460-9568.2006.04717.x. [DOI] [PubMed] [Google Scholar]
- Leblois A, Boraud T, Meissner W, Bergman H, Hansel D. Competition between feedback loops underlies normal and pathological dynamics in the basal ganglia. J Neurosci. 2006;26:3567–3583. doi: 10.1523/JNEUROSCI.5050-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Blaha CD, Harris BT, Cooper S, Hitti FL, Leiter JC, Roberts DW, Kim U. Dopamine efflux in the rat striatum evoked by electrical stimulation of the subthalamic nucleus: potential mechanism of action in Parkinson's disease. Eur J Neurosci. 2006;23:1005–1014. doi: 10.1111/j.1460-9568.2006.04638.x. [DOI] [PubMed] [Google Scholar]
- Li S, Arbuthnott GW, Jutras MJ, Goldberg JA, Jaeger D. Resonant antidromic cortical circuit activation as a consequence of high-frequency subthalamic deep-brain stimulation. J Neurophysiol. 2007;98:3525–3537. doi: 10.1152/jn.00808.2007. [DOI] [PubMed] [Google Scholar]
- Limousin P, Krack P, Pollak P, Benazzouz A, Ardouin C, Hoffmann D, Benabid AL. Electrical stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med. 1998;339:1105–1111. doi: 10.1056/NEJM199810153391603. [DOI] [PubMed] [Google Scholar]
- Lin TP, Carbon M, Tang C, Mogilner AY, Sterio D, Beric A, Dhawan V, Eidelberg D. Metabolic correlates of subthalamic nucleus activity in Parkinson's disease. Brain. 2008;131:1373–1380. doi: 10.1093/brain/awn031. [DOI] [PubMed] [Google Scholar]
- Llinás RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: A neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci U S A. 1999;96:15222–15227. doi: 10.1073/pnas.96.26.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Lozano AM, Dostrovsky J, Chen R, Ashby P. Deep brain stimulation for Parkinson's disease: disrupting the disruption. Lancet Neurol. 2002;1:225–231. doi: 10.1016/s1474-4422(02)00101-1. [DOI] [PubMed] [Google Scholar]
- Ma Y, Wichmann T. Soc. Neurosci. Abstr. San Diego, CA: 2004. Disruption of motor performance by basal ganglia stimulation; p. 416.2. [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- McIntyre CC, Hahn PJ, Lowe MJ, Phillips MD. Soc. Neurosci. Abstr. Washington, DC: 2008. Integration of patient-specific network models and fMRI activation during deep brain stimulation; p. 641.2. [Google Scholar]
- McIntyre CC, Grill WM, Sherman DL, Thakor NV. Cellular effects of deep brain stimulation: model-based analysis of activation and inhibition. J Neurophysiol. 2004a;91:1457–1469. doi: 10.1152/jn.00989.2003. [DOI] [PubMed] [Google Scholar]
- McIntyre CC, Mori S, Sherman DL, Thakor NV, Vitek JL. Electric field and stimulating influence generated by deep brain stimulation of the subthalamic nucleus. Clin Neurophysiol. 2004b;115:589–595. doi: 10.1016/j.clinph.2003.10.033. [DOI] [PubMed] [Google Scholar]
- McIntyre CC, Savasta M, Kerkerian-Le Goff L, Vitek JL. Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clin Neurophysiol. 2004c;115:1239–1248. doi: 10.1016/j.clinph.2003.12.024. [DOI] [PubMed] [Google Scholar]
- McIntyre CC, Miocinovic S, Butson CR. Computational analysis of deep brain stimulation. Expert Rev Med Devices. 2007;4:615–622. doi: 10.1586/17434440.4.5.615. [DOI] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Miocinovic S, Parent M, Butson CR, Hahn PJ, Russo GS, Vitek JL, McIntyre CC. Computational analysis of subthalamic nucleus and lenticular fasciculus activation during therapeutic deep brain stimulation. J Neurophysiol. 2006;96:1569–1580. doi: 10.1152/jn.00305.2006. [DOI] [PubMed] [Google Scholar]
- Montgomery EB., Jr. Effects of GPi stimulation on human thalamic neuronal activity. Clin Neurophysiol. 2006;117:2691–2702. doi: 10.1016/j.clinph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Montgomery EB., Jr. Basal ganglia physiology and pathophysiology: a reappraisal. Parkinsonism Relat Disord. 2007;13:455–465. doi: 10.1016/j.parkreldis.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Montgomery EB, Jr., Baker KB. Mechanisms of deep brain stimulation and future technical developments. Neurol Res. 2000;22:259–266. doi: 10.1080/01616412.2000.11740668. [DOI] [PubMed] [Google Scholar]
- Montgomery EB, Jr., Gale JT. Mechanisms of action of deep brain stimulation(DBS) Neurosci Biobehav Rev. 2008;32:388–407. doi: 10.1016/j.neubiorev.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Nambu A. A new dynamic model of the cortico-basal ganglia loop. Prog Brain Res. 2004;143:461–466. doi: 10.1016/S0079-6123(03)43043-4. [DOI] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Hamada I, Kita H, Imanishi M, Akazawa T, Ikeuchi Y, Hasegawa N. Excitatory cortical inputs to pallidal neurons via the subthalamic nucleus in the monkey. J Neurophysiol. 2000;84:289–300. doi: 10.1152/jn.2000.84.1.289. [DOI] [PubMed] [Google Scholar]
- Pahapill PA, Lozano AM. The pedunculopontine nucleus and Parkinson's disease. Brain. 2000;123:1767–1783. doi: 10.1093/brain/123.9.1767. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev. 1995a;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Res Brain Res Rev. 1995b;20:128–154. doi: 10.1016/0165-0173(94)00008-d. [DOI] [PubMed] [Google Scholar]
- Payoux P, Remy P, Damier P, Miloudi M, Loubinoux I, Pidoux B, Gaura V, Rascol O, Samson Y, Agid Y. Subthalamic nucleus stimulation reduces abnormal motor cortical overactivity in Parkinson disease. Arch Neurol. 2004;61:1307–1313. doi: 10.1001/archneur.61.8.1307. [DOI] [PubMed] [Google Scholar]
- Perlmutter JS, Mink JW. Deep brain stimulation. Annu Rev Neurosci. 2006;29:229–257. doi: 10.1146/annurev.neuro.29.051605.112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessiglione M, Guehl D, Rolland AS, Francois C, Hirsch EC, Feger J, Tremblay L. Thalamic neuronal activity in dopamine-depleted primates: evidence for a loss of functional segregation within basal ganglia circuits. J Neurosci. 2005;25:1523–1531. doi: 10.1523/JNEUROSCI.4056-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips MD, Baker KB, Lowe MJ, Tkach JA, Cooper SE, Kopell BH, Rezai AR. Parkinson disease: pattern of functional MR imaging activation during deep brain stimulation of subthalamic nucleus--initial experience. Radiology. 2006;239:209–216. doi: 10.1148/radiol.2391041990. [DOI] [PubMed] [Google Scholar]
- Pralong E, Debatisse D, Maeder M, Vingerhoets F, Ghika J, Villemure JG. Effect of deep brain stimulation of GPI on neuronal activity of the thalamic nucleus ventralis oralis in a dystonic patient. Neurophysiol Clin. 2003;33:169–173. doi: 10.1016/j.neucli.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Priori A, Foffani G, Pesenti A, Tamma F, Bianchi AM, Pellegrini M, Locatelli M, Moxon KA, Villani RM. Rhythm-specific pharmacological modulation of subthalamic activity in Parkinson's disease. Exp Neurol. 2004;189:369–379. doi: 10.1016/j.expneurol.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Rasouli G, Rasouli M, Lenz FA, Verhagen L, Borrett DS, Kwan HC. Fractal characteristics of human Parkinsonian neuronal spike trains. Neuroscience. 2006;139:1153–1158. doi: 10.1016/j.neuroscience.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Dougherty DD, Malone D, Rezai A, Friehs G, Fischman AJ, Alpert NM, Haber SN, Stypulkowski PH, Rise MT, Rasmussen SA, Greenberg BD. A functional neuroimaging investigation of deep brain stimulation in patients with obsessive-compulsive disorder. J Neurosurg. 2006;104:558–565. doi: 10.3171/jns.2006.104.4.558. [DOI] [PubMed] [Google Scholar]
- Ray NJ, Jenkinson N, Wang S, Holland P, Brittain JS, Joint C, Stein JF, Aziz T. Local field potential beta activity in the subthalamic nucleus of patients with Parkinson's disease is associated with improvements in bradykinesia after dopamine and deep brain stimulation. Exp Neurol. 2008;213:108–113. doi: 10.1016/j.expneurol.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Rezai AR, Lozano AM, Crawley AP, Joy ML, Davis KD, Kwan CL, Dostrovsky JO, Tasker RR, Mikulis DJ. Thalamic stimulation and functional magnetic resonance imaging: localization of cortical and subcortical activation with implanted electrodes. Technical note. J Neurosurg. 1999;90:583–590. doi: 10.3171/jns.1999.90.3.0583. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Pereda E, Gonzalez J, Abdala P, Obeso JA. Neuronal activity in the substantia nigra in the anaesthetized rat has fractal characteristics. Evidence for firing-code patterns in the basal ganglia. Exp Brain Res. 2003;151:167–172. doi: 10.1007/s00221-003-1442-4. [DOI] [PubMed] [Google Scholar]
- Rubchinsky LL, Kopell N, Sigvardt KA. Modeling facilitation and inhibition of competing motor programs in basal ganglia subthalamic nucleus-pallidal circuits. Proc Natl Acad Sci U S A. 2003;100:14427–14432. doi: 10.1073/pnas.2036283100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin JE, Terman D. High frequency stimulation of the subthalamic nucleus eliminates pathological thalamic rhythmicity in a computational model. J Comput Neurosci. 2004;16:211–235. doi: 10.1023/B:JCNS.0000025686.47117.67. [DOI] [PubMed] [Google Scholar]
- Shi LH, Luo F, Woodward DJ, Chang JY. Basal ganglia neural responses during behaviorally effective deep brain stimulation of the subthalamic nucleus in rats performing a treadmill locomotion test. Synapse. 2006;59:445–457. doi: 10.1002/syn.20261. [DOI] [PubMed] [Google Scholar]
- Smith Y, Bevan MD, Shink E, Bolam JP. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience. 1998;86:353–387. doi: 10.1016/s0306-4522(98)00004-9. [DOI] [PubMed] [Google Scholar]
- Starr PA, Rau GM, Davis V, Marks WJ, Jr., Ostrem JL, Simmons D, Lindsey N, Turner RS. Spontaneous pallidal neuronal activity in human dystonia: comparison with Parkinson's disease and normal macaque. J Neurophysiol. 2005;93:3165–3176. doi: 10.1152/jn.00971.2004. [DOI] [PubMed] [Google Scholar]
- Stefurak T, Mikulis D, Mayberg H, Lang AE, Hevenor S, Pahapill P, Saint-Cyr J, Lozano A. Deep brain stimulation for Parkinson's disease dissociates mood and motor circuits: a functional MRI case study. Mov Disord. 2003;18:1508–1516. doi: 10.1002/mds.10593. [DOI] [PubMed] [Google Scholar]
- Strogatz SH. Nonlinear Dynamics And Chaos: With Applications To Physics, Biology, Chemistry, And Engineering. Addison-Wesley, Reading, Mass; 1994. [Google Scholar]
- Strong SP, Koberle R, de Ruyter van Steveninck RR, Bialek W. Entropy and information in neural spike trains. Phys Rev Lett. 1998;80:197–200. [Google Scholar]
- Tass PA. A model of desynchronizing deep brain stimulation with a demand-controlled coordinated reset of neural subpopulations. Biol Cybern. 2003;89:81–88. doi: 10.1007/s00422-003-0425-7. [DOI] [PubMed] [Google Scholar]
- Trost M, Su S, Su P, Yen RF, Tseng HM, Barnes A, Ma Y, Eidelberg D. Network modulation by the subthalamic nucleus in the treatment of Parkinson's disease. Neuroimage. 2006;31:301–307. doi: 10.1016/j.neuroimage.2005.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbano FJ, Leznik E, Llinas RR. Cortical activation patterns evoked by afferent axons stimuli at different frequencies: an in vitro voltage-sensitive dye imaging study. Thalamus Rel Syst. 2002;1:371–378. [Google Scholar]
- Wichmann T, Bergman H, Starr PA, Subramanian T, Watts RL, DeLong MR. Comparison of MPTP-induced changes in spontaneous neuronal discharge in the internal pallidal segment and in the substantia nigra pars reticulata in primates. Exp Brain Res. 1999;125:397–409. doi: 10.1007/s002210050696. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Soares J. Neuronal firing before and after burst discharges in the monkey Basal Ganglia is predictably patterned in the normal state and altered in parkinsonism. J Neurophysiol. 2006;95:2120–2133. doi: 10.1152/jn.01013.2005. [DOI] [PubMed] [Google Scholar]
- Xu W, Russo GS, Hashimoto T, Zhang J, Vitek JL. Subthalamic nucleus stimulation modulates thalamic neuronal activity. J Neurosci. 2008;28:11916–11924. doi: 10.1523/JNEUROSCI.2027-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]