Abstract
Atheroma formation and restenosis following percutaneous vascular intervention involve the growth and migration of vascular smooth muscle cells (SMCs) into neointimal lesions, in part due to changes in the extracellular matrix. While some clinical studies have suggested that, in comparison to non-diabetics, β3 integrin inhibition in diabetic patients confers protection from restenosis, little is known regarding the role of β3 integrin inhibition on SMC responses in this context. To understand the molecular mechanisms underlying integrin-mediated regulation of SMC function in diabetes, we examined SMC responses in diabetic mice deficient in integrin β3 and observed that the integrin was required for enhanced proliferation, migration and extracellular regulated kinase (ERK) activation. Hyperglycemia enhanced membrane recruitment and catalytic activity of PKCβ in an integrin β3-dependent manner. Hyperglycemia also promoted SMC filopodia formation and cell migration, both of which required αVβ3, PKCβ, and ERK activity. Furthermore, the integrin–kinase association was regulated by the αVβ3 integrin ligand thrombospondin and the integrin modulator Rap1 under conditions of hyperglycemia. These results suggest that there are differences in SMC responses to vascular injury depending on the presence or absence of hyperglycemia and that SMC response under hyperglycemic conditions is largely mediated through β3 integrin signaling
Keywords: intima, smooth muscle cells, hyperglycemia, restenosis, proliferation
1. Introduction
Vascular complications, including premature and accelerated atherosclerosis of the coronary, renal, cerebral, and peripheral arteries, are major causes of morbidity and mortality in diabetic patients (Beckman et al., 2002;Ceriello, 2004). Diabetic patients also display higher rates of formation of neointimal hyperplasia and its clinical correlate, restenosis, after percutaneous vascular interventions (Jimenez-Quevedo and Sabate, 2005;Karha and Bhatt, 2004). In the Diabetes Control and Complications Trial (DCCT), 1229 individuals (85%) with Type 1 diabetes had increased carotid intima–media thickness as compared with age- and sex-matched controls (Nathan et al., 2003), and similar findings have been observed in other clinical studies (Giannattasio et al., 2001;Yamasaki et al., 1994). Patients with type 2 diabetes also have greater carotid intima–media thickness when compared to their nondiabetic counterparts (Folsom et al., 1994;Wagenknecht et al., 1998). Similarly accelerated intimal hyperplasia has been observed in both type 1 and type 2 diabetic animal models (Jonas et al., 2005;Salzberg et al., 2006;Sasaki et al., 2008).
In response to vascular injury, such as that from atherosclerosis or vascular instrumentation, smooth muscle cells (SMC) residing in the vessel wall are stimulated to proliferate and migrate, resulting in the development of neointimal hyperplasia. Various changes in the vascular environment, including alterations in the extracellular matrix and in the basement membrane, promote the conversion of normally quiescent, non-motile SMCs into cells that rapidly grow and migrate (Hedin et al., 1999;Raines, 2000). The interaction of SMCs with their extracellular matrix is governed, in large part, by members of the integrin family of adhesion receptors (Moiseeva, 2001). Integrin αVβ3, in particular, may contribute to these events (Moiseeva, 2001;Stouffer et al., 1998;Stouffer and Smyth, 2003). The molecular mechanisms responsible for accelerated atherosclerosis and restenosis in diabetes are incompletely understood, although several factors have been proposed to play a role. Among these is activation of protein kinase C (Yan et al., 2006), which may occur in diabetes as a consequence of de novo synthesis of diacylglycerol (DAG), increases in reactive oxygen species, and/or production of cytokines. In the setting of hyperglycemia, de novo synthesis of DAG may be stimulated by the accumulation of the glycolytic intermediate glycerol-3-phosphate or by flux through the polyol pathway, which produces dihydroxyacetone phosphate that is converted sequentially to lysophosphatidic acid, phosphatidic acid, and finally DAG. In the SMC, DAG-responsive “conventional” PKC isoforms, such as PKC β and δ, are activated by hyperglycemia (Nakamura et al., 2001). Among the PKCβ isoforms, PKCβ II has been implicated as a key mediator of hyperglycemia-induced Raf/MEK/ERK signaling, and hyperglycemia-induced potentiation of SMC proliferation and migration (Campbell et al., 2004). However, the molecular mechanisms that regulate PKCβ in the setting of hyperglycemia are not known. Furthermore, the coupling of PKC activation to integrin-sensed changes in the ECM, such as those that occur following vascular injury, are not well understood.
Some (Lincoff et al., 1999;Marso et al., 1999), but not all clinical studies (Lincoff, 2003), have suggested that the combination of the integrin β3 antagonist abciximab, with stent implantation, results in a complementary decrease in adverse clinical events in diabetic patients, but not in non-diabetic patients. This includes reductions in the need for target vessel revascularization, which is a clinical marker for restenosis. These observations suggest that integrin β3 may play an important role in restenosis in the context of diabetes. Activation of the hexosamine pathway by glucose catabolism in vascular SMCs upregulates the production of thrombospondin-1 (TSP-1) (Raman et al., 2007;Stenina et al., 2003), an extracellular matrix protein that serves as a ligand for integrin αVβ3. Thus, a hyperglycemia-mediated increase in TSP-1 expression could activate integrin signaling through interaction with integrin αVβ3, and potentially result in SMC proliferation and migration to areas of vascular injury. In the present study, we examined whether integrin αVβ3 plays a unique role in the regulation of SMC responses to injury in the context of hyperglycemia, using cell culture systems, and then confirmed our findings in aortic tissue from diabetic mice.
2. Research Design and Methods
2.1. Reagents
PD98059 was from Calbiochem (Gibbstown, NJ, USA); U0126 from Promega (Madison, WI, USA); active PKCβII, and PKC lipid activator from Upstate (Danvers, MA, USA); cognate peptides to PKC isoforms from Santa Cruz Biotechnology (Santa Cruz, CA, USA); and the PKCβII-specific inhibitor [3-(1-(3-Imidazol-1-ylpropyl)-1H-indol-3-yl)-4-anilino-1H-pyrrole-2,5-dione] (Tanaka et al., 2004;Tanaka et al., 2006), platelet thrombospondin, streptozotocin (STZ) and the distintegrin and potent integrin antagonist echistatin from Sigma-Aldrich (St. Louis, MO, USA). Monoclonal (2C9.G2) and polyclonal antibodies to integrin β3 have been previously characterized (Beer and Coller, 1989;Smyth et al., 2000).
2.2. Mice
All procedures conformed to the recommendations of the "Guide for the Care and Use of Laboratory Animals" (Department of Health, Education, and Welfare publication number NIH 78-23, 1996), and were approved by the Institutional Animal Care and Use Committee. The generation of integrin β3-deficient (Itgβ3−/−), Rap1a−/− and Rap1b−/− mice have been described previously (Chrzanowska-Wodnicka et al., 2005;Hodivala-Dilke et al., 1999;Li et al., 2007). Thrombospondin-deficient mice (Thbs1−/− mice) were obtained from Jackson Research Laboratories (Bar Harbor, Maine, USA). PKCβ-deficient mice (Prkcb−/−) mice were generated as previously described (Leitges et al., 1996). Mice were maintained on a 14 h light and 10 h dark cycle, and were fed water and standard rodent chow (2018 Harlan Tekland Rodent Diet) ad libitum. Diabetes was induced at ~8 weeks of age, following the protocol of “Animal Models of Diabetic Complications Consortium”, using an intraperitoneal injection of 50 mg/kg of body weight STZ in a citrate buffer, pH 4.5, daily for 5 days. Blood sugars were monitored by lateral tail vein sample. Animals with a blood glucose level >300 mg/dl were considered diabetic. Experimental protocols were performed on mice that were diabetic for 2 weeks. Control animals were injected with citrate buffer only, and underwent protocols on the same schedule as the diabetic mice.
2.3. Arterial Injury
Arterial injury was performed and analyzed as previously described (Smyth et al., 2001). In brief, the femoral vessels were exposed by a longitudinal groin incision and viewed with the aid of a surgical microscope (Carl Zeiss). The distal portion of the femoral artery was encircled with an 8-0 nylon suture, a vascular clamp was placed proximally at the level of the inguinal ligament, and a 0.010-in (0.25-mm) diameter angioplasty guidewire (Advanced Cardiovascular Systems) was introduced into the arterial lumen through an arteriotomy made just distal to the suture. After release of the clamp, the guidewire was advanced to the level of the aortic bifurcation and immediately pulled back; this process was repeated 2 additional times to denude the endothelium. The guidewire was then removed, and the arteriotomy site was ligated by tying the previously placed suture. Animals were routinely monitored after surgery.
2.4. Smooth Muscle Cell Culture
Thoracic aortas were removed and the adventitia was gently removed under a surgical microscope. The endothelial cells were removed by wiping the inner (luminal) surface of the vessel with a sterile cotton swab. Mouse aortic SMCs were obtained by sequential digestion of the aortas with collagenase type II (Worthington, Lakewood, NJ, USA; 175 units/ml) followed by collagenase type II (175 units/ml) and elastase (Sigma-Aldrich, St. Louis, MO, USA; 0.5 mg/ml), which yielded 100,000 cells per aortas. Cells were grown in DMEM containing 5 mM (low) or 25 mM (high) glucose and 0.5 ng/ml EGF, 5 µg/ml insulin, 2 ng/ml β-FGF, 10% FBS, 100 units/ml penicillin, and 100 µg/ml streptomycin. SMC lineage was confirmed by immunostaining for α-actin (Sigma) in >99% of the cells. Experiments involving SMCs were performed using cells with a passage number ≤5.
2.5. Cell Proliferation
Cell proliferation was determined in 96 well plates by WST-1 assay kit (Biochain). WST-1 is cleaved by mitochondrial succinate-tetrazolium reductase in viable cells to form formazan dye. Production of formazan is proportional to the number of viable cells. After 2 hours of incubation in WST-1, absorbance at 450 nm was measured. Cell migration was performed either by using a scratch assay, or by placing cells (1.8 × 104/well) in the upper well of a chamber of a multiwell chamber (Neuroprobe Inc., Gaithersburg, MD, USA) with a polyvinylpyrrolidone-free polycarbonate filter (5 µm pore). The bottom chamber was filled with the indicated inhibitors. After a 12 hour incubation period, at 37°C, the migrated cells on the underneath surface of the filter were stained with Diff-Quik® (VWR Scientific Products, West Chester, PA, USA). Digital images of the membranes were obtained using a Nikon 80i microscope with a 20x objective (NA = 0.5). The total area (µm2) occupied by migrated cells was determined using Metamorph imaging software.
2.6. Electron Microscopy
Scanning electron micrographs were obtained of cells plated on 12-mm round coverslips. The cells were fixed with 2.5% glutaraldehyde in a 0.1 M sodium cacodylate buffer. The coverslips were treated with 1% osmium tetroxide in cacodylate buffer. The samples were dehydrated in a graded series of ethanol, critical point dried, (Balzers Union, Balzers, Liechtenstein, Germany), and then coated with 7 nm of gold-palladium by using a Hummer X sputter coater (Anatech Ltd., Springfield, VA, USA). All coverslips from a given experiment were critical point dried together in the same chamber and sputter coated together. Images were generally collected at a tilt angle of 42° by using a Cambridge S-200 scanning electron microscope attached to a digital camera (4 Pi Imaging digital system, Carl Zeiss SMT, Inc.). About 4 – 6 samples from separate experiments were analyzed by counting at lower magnification the number of filopodia per cell for 30–40 cells and scoring them as having <10, 10–100, or >100 filopodia.
2.7. Immunoblotting, Immunoprecipitation, and Enzyme Assays
SMCs were lysed in buffer (10 mM Tris-HCl pH 7.2, 1% Nonidet P-40, 158 mM NaCl, 1 mM EDTA, 50 mM NaF, 1 mM PMSF, 10 µg/ml aprotinin, 10 µg/ml leupeptin, 1 mM sodium orthovanadate, 10 mM sodium pyrophosphate), and lysates centrifuged at 16,000g for 10 min. Protein content in the lyaste was determined (BCA protein assay; Pierce, Rockford, IL, USA); subsequent experiments were performed with lysate with the indicated amount of protein. Immunoblotting (5 µg protein/well) was performed with antibodies against ERK1 and 2/phospho-ERK Thr202/Tyr204, Akt/phosphor-Akt Ser473, rabbit polyclonal to PKCα, PKCδ (Cell Signaling Technology, Danvers, MA, USA), PKC-ε, PKC-β, and actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) as a loading control. Secondary antibodies were conjugated with Alexa680 (Molecular Probes, Carlsbad, CA, USA) or IRdye800 (Rockland Immunochemicals, Gilbertsville, PA, USA), and were detected and quantified using the Odyssey infrared imaging system (LI-COR, Lincoln, NE, USA). Untransferred proteins in the gel were stained with Coomassie blue as an additional method to confirm equal protein loading.
For immunoprecipitation, antibodies (2.5 µg/ml) were incubated for 2 hours at 4°C with protein G-conjugated agarose beads (50 µl) and then washed five times with lysis buffer. Lysates (500 µl containing 700 µg protein) were incubated with 50 µl of beads overnight at 4°C.
The PKC kinase assay (TruLight™ Protein Kinase Cβ I/II Assay Kit –Calbiochem, Gibbstown, NJ, USA) was performed according to the manufacturer’s instructions. Cells were disrupted by sonication, and then PKCβ activity in the membrane pellet (5 µg protein) was assayed using TruLight™ Protein Kinase Cβ I/II Assay Kit. Emission was read at 490 nm following excitation at 450 nm (475-nm cutoff filter).
Rap activity was assayed by pull-down assay using RalGDS – RBD coupled to Ni21-NTA–agarose beads (Millipore, Danvers, MA, USA). This assay uses a GST-fusion protein containing the Rap1 binding domain (RBD) of human Ra1GDS to affinity precipitate active Rap1 (GTP-Rap1) from cell lysates. 40µl of the GST-Ra1GDS-RBD fusion protein coupled beads were incubated with cell lysates (700µg protein) at 4°C for 30–90 min with slight agitation. Following SDS-PAGE, Rap1 was detected by immunoblotting, using an anti-Rap1 monoclonal antibody (BD Transduction Laboratories, San Jose, CA, USA).
2.8. Transient Transfections
SMCs (passage 3; grown in 100 mm plates) were placed in serum and antibiotic-free medium (10% (v/v) FBS in DMEM) at least 2 h before transfection. The following siRNA oligonucleotides (Dharmacon, Lafayette, CO, USA) were used: Rap1a: CAGCUUUUAUGGAUGAUUA, UGGGAUAACUGAUUUCUAU, UGCAAUAGCAGUUAUAACA, and CAAUUAUACAGAAGAGCAU; Rap1b:GUUAACUGGUCCACGAGUA, GCUUGGAUCAGGUGGCGUU, CGACAUUUAACGAGUUACA, and UCCAGUAUCACGAGAGAUU; RASGRP2:CCGAAUAAUUAACCUCGAA, AUCCACAGCUAGUGCGCAU, CAUCACACAUUUCGUGCAU, and CCUACAAGUGGAAGCGGCA. siGLO Green Transfection Indicator was used as a qualitative indicator of delivery. Scrambled siRNA was used as a control. Cells were incubated with approximately 100 nM of siRNA and 1 µl/cm2 plate of X- tremeGENE siRNA Transfection Reagent (Roche, Indianapolis, IN, USA). Both siRNA and transfection reagent were diluted in OPTI-MEM medium (Gibco, Carlsbad, CA, USA). After 24 hours, the medium was changed to low or high glucose DMEM medium containing antibiotics (10% (v/v) FBS, 1% (m/v) penicillin and streptomycin), and the Rap pull-down assay was performed as described above.
2.9. Statistical Analysis
Unless otherwise stated, results are expressed as mean ± standard error of the mean. In vitro studies were repeated a minimum of three times, and the results were analyzed by student t-test, Mann-Whitney U test, or ANOVA, as indicated. Statistical significance within strains was determined using ANOVA with multiple pair-wise comparisons. Statistical analysis was performed using Sigma-STAT software, version 3.5 (Systat Software Inc. San Jose, CA, USA). A p-value of less than 0.05 was considered significant.
3. Results
3.1. Mice lacking integrin β3 are partially protected from diabetes-accelerated formation of neointima
Enhanced atherosclerosis and restenosis following percutaneous vascular injury occur with diabetes. In both humans and rodents, diabetes has been associated with enhanced intimal hyperplasia. The goal of this work was to investigate the molecular mechanisms involved in diabetic vascular responses to injury by integrating results obtained in vascular tissue of diabetic animals with findings in SMCs cultured under hyperglycemic conditions.
Mice were made diabetic by injecting STZ, which destroys beta cells of the pancreatic islets. Two weeks following the induction of diabetes, femoral artery injury was performed to elicit the development of intimal hyperplasia as previously described (Smyth et al., 2001). In comparison with normal glycemic controls, diabetic mice displayed a 1.5 ± 0.1 fold increase in intimal area, a 1.7 ± 0.20 fold increase in intima/media ratio, and a corresponding 33% reduction in luminal area following endoluminal denudation of the femoral artery. To determine if integrin β3 is required for diabetes-accelerated intimal hyperplasia, injury was performed in diabetic Itgβ3−/− mice. Diabetic Itgβ3−/− mice were partially protected from enhanced injury-induced development of neointimal hyperplasia (Figure 1A). In comparison with diabetic wild-type mice, diabetic Itgβ3−/− mice had smaller intimal areas, lower intima/media ratios and larger luminal areas (Table 1). There was not a statistically significant difference between WT and Itgβ3−/− mice in the onset (~ 7 days after STZ administration), duration (14 days), and the extent of hyperglycemia following STZ treatment. Average blood glucose two weeks after STZ-treatment was 518 ± 29 mg/dl in wild-type mice and 494 ± 33 mg/dl in Itgβ3−/− mice. Our group previously reported that non-diabetic wild-type mice and Itgβ3−/− mice developed a similar degree of neointimal hyperplasia in this arterial injury model (Smyth et al., 2001). Because our new results imply that integrin β3 may play a specific role in accelerated SMC proliferation and migration in the context of hyperglycemia, we sought to determine the mechanism(s) and signaling pathways involved.
Figure 1. Hyperglycemia promotes SMC growth, proliferation, and the development of intimal hyperplasia in an integrin β3-dependent manner.
Representative combined Masson elastic stained sections of femoral arteries of diabetic wild-type (WT) or Itgβ3−/− four weeks after wire-induced endothelial denuduation (A; 20x). Isolated murine SMCs (20 000 cells) were cultured in euglycemic (5 mM; open bars) or hyperglycemic (25 mM; dark bars) conditions with the indicated inhibitors for 48 hours. Cell proliferation was measured by WST-1 assay kit, and the results are presented as mean ± SE from three independent experiments (B). Migration of SMCs was performed in euglycemic (5 mM; open bar) or hyperglycemic (25 mM; dark bars) conditions with the indicated inhibitors. Inhibitors used were as follows: PKCβ (3-(1-(3-Imidazol-1-ylpropyl)-1H-indol-3-yl)-4-anilino-1H-pyrrole-2,5-dione, 5nM), anti-β3 antibody (10 µg/ml), control antibody (10 µg/ml). Cell migration was measured as described in Methods, and the results are presented as mean ± SE from three independent experiments (C). *P < 0.001 as compared to corresponding control.
Table 1.
Hyperglycemia promotes the development of intimal hyperplasia in an integrin β3-dependent manner.
| Genotype | Number of vessels |
Intima (µm2) | Media (µm2) | I/M Ratio | Lumen (µm2) |
|---|---|---|---|---|---|
| WT (DM) | 10 | 21561 ± 1266 | 9784 ± 1736 | 2.40 ± 0.30 | 6501 ± 1059 |
| Itgβ3−/− (DM) | 10 | 15000 ± 1378 | 15600 ± 2182 | 1.00 ± 0.08 | 17400 ± 1288 |
| WT(DM) vs Itgβ3−/−(DM) |
P = 0.008 | P = 0.070 | P = 0.002 | P = 0.001 | |
| WT(ctl) | 16 | 14600±2800 | 9400 ±600 | 1.45±0.2 | 21 000±3100 |
| Itgβ3−/−(ctl) | 16 | 12700±1000 | 9000±800 | 1.3±0.1 | 24 600 ±2700 |
| WT(ctl) vs Itgβ3−/−(ctl) |
P = 0.75 | P = 0.064 | P = 0.60 | P = 0.70 |
Areas (mean ± SE) for intima, media, and lumen of wild-type (WT) or Itgβ3−/− mice are provided. For comparison, control (ctl) values from an earlier publication are included (Smyth et al., 2001).
3.2. Hyperglycemic enhancement of SMC proliferation and migration is dependent on integrin β3
To examine the role of β3 integrin signaling in the context of hyperglycemia, we investigated the effects of hyperglycemia on proliferation, migration, and signaling in primary cultured mouse aortic SMCs. We confirmed findings in vivo by examining aortas from diabetic mice where we could selectively study SMC responses by removing the vascular endothelium following isolation. As others have previously reported (Wang and Li, 2009;Li et al., 2006;Villeneuve et al., 2008;Reddy et al., 2008), murine aortic SMCs cultured under hyperglycemic conditions (25 mM; corresponding to a blood glucose level of ~455 mg/dl) displayed enhanced proliferative and migratory responses compared to cells cultured in euglycemic conditions (5 mM; corresponding to a blood glucose level of ~91 mg/dl). Hyperglycemia resulted in a 2.3 ± 0.04 fold increase in cell number over a 48 hour period (Figure 1B and 1C). This was not simply the result of a change in osmolarity; incubation with 25 mM mannitol had no effect on SMC proliferation. Unlike SMC proliferation under euglycemic conditions, hyperglycemia-enhanced proliferation was partially attenuated by an antibody inhibitor to integrin β3 and was also lower in cells from Itgβ3−/− mice (Figure 1B and 1C). PKC has been implicated as both an upstream activator and downstream effector of integrin signaling and a mediator of hyperglycemic SMC responses. We examined the effects of pharmacologic inhibition of PKC on SMC proliferation and found that a PKCβ inhibitor (Tanaka et al., 2004;Tanaka et al., 2006) reduced proliferation under both normal glycemic and hyperglycemic conditions (Figure 1B).
Hyperglycemia also promoted SMC migration in a manner requiring both integrin β3 and PKCβ activity (Figure 1C). Interestingly, we observed that the enhanced migratory capacity of SMCs exposed to hyperglycemic conditions was associated with dramatic morphological changes, most notably ~10 fold increase in protrusions of plasma membrane extensions from the periphery and dorsal surface of the cells (Figure 2) (Table 2). These structures are likely to be filopodia, which could play an important role in hyperglycemia-induced migration. We found that hyperglycemia-induced formation of the filopodia-like structures was abolished by a PKCβ inhibitor and by antibody inhibition of integrin β3, but was not affected by a control antibody (Figure 2).
Figure 2. Hyperglycemia triggers dorsal and lateral filopodial-like projections in SMCs.
Electron micrographs of adherent SMCs cultured in euglycemic (5 mM) or hyperglycemic (25 mM) conditions with the indicated inhibitors. Inhibitors used were as follows: PKCβ(3-(1-(3-Imidazol-1-ylpropyl)-1H-indol-3-yl)-4-anilino-1H-pyrrole-2,5-dione, 5nM), anti-β3 antibody (10µg/ml), control antibody (10µg/ml). The photomicrographs are representative of results obtained in five experiments. The white arrow head (→) points to the lateral filapodia and black arrow head(→) points to the dorsal filapodia. Bar denotes 2µm.
Table 2.
Hyperglycemia triggers filopodial-like projections in SMCs.
| mannitol | 5 mM glucose |
25 mM glucose | |||
|---|---|---|---|---|---|
| <10 | <10 | DMSO | PKCβ inh | Anti-β3 Ab | control Ab |
| >100 | <10 | <10 | <10 | ||
| P=0.829 | P=0.001 | P =0.75 | P=0.861 | P=0.001 | |
For each condition, the number of projections in 30 – 40 cells in lower power fields was counted and scored as <10, 10 – 100, or >100. P≤0.001 vs glucose.
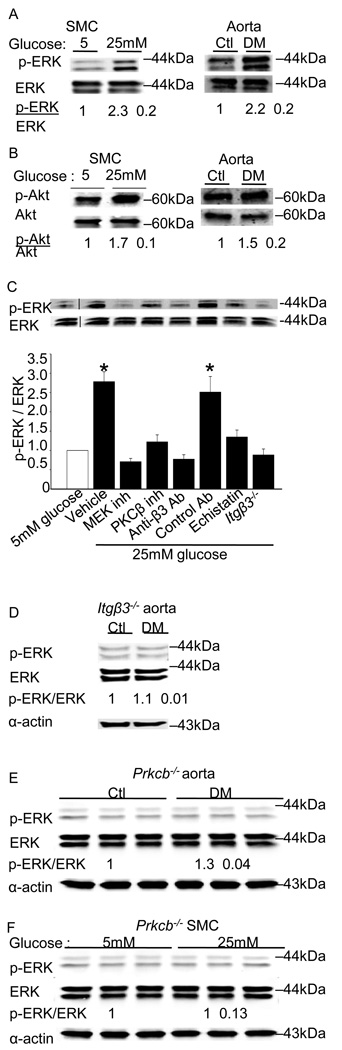
In accordance with the observation of enhanced proliferation in the context of hyperglycemia, ERK activity was 2.3 ± 0.2 fold higher in SMCs cultured in 25 mM versus 5 mM glucose and 2.2 ± 0.2 fold higher in aortas from diabetic versus control mice (Figure 3A). In SMCs cultured in hyperglycemic conditions, a 1.7 ± 0.1 fold-increase in Akt activity was observed. Aortas from the diabetic mice also had a 1.5 ± 0.2 fold increase in Akt activity over that observed in the control aortas. Hyperglycemia-induced ERK activation was attenuated by PKCβ and integrin β3 inhibitors, and was reduced in the SMCs of Itgβ3−/− mice (Figure 3C) and in aortas (Figure 3E) and cells (Figure 3F) from mice lacking PKCβ (Prkbc−/−). Unlike hyperglycemia-induced ERK activation, the activation of Akt did not appear to depend on integrin β3, as Akt activity was not significantly affected by integrin inhibition or by genetic deficiency of integrin β3 (Online Supplemental Figure I).
Figure 3. Hyperglycemia triggers PKCβ and integrin β3-dependent signaling in vitro and in vivo.
ERK activation (A) and Akt activation (B) was measured by immunoblotting aorta or SMCs as described in Methods. The results are presented as the level of phosphorylated/total protein normalized to results obtained in euglycemic conditions and are graphed as the mean ± SE from eight independent experiments. ERK activation was measured in SMCs cultured under euglycemic (5 mM) or hyperglycemic (25mM) conditions in the presence of the indicated inhibitors. The results are presented as the level of phosphorylated/total protein normalized to results obtained with 5 mM glucose and are graphed as the mean ± SE from three independent experiments (C). ERK activation was measured by immunoblotting aorta (D) from the Itgβ3−/− mice. ERK activation was measured by immunoblotting aorta (E) or SMCs (F) from the Prkcb−/− mice as described in Methods. The results are presented as the level of phosphorylated/total protein normalized to results obtained in euglycemic conditions and are graphed as the mean ± SE from three independent experiments. Membrane associated PKCβ activity in SMCs was measured as described in Methods, and mean ± SE from three independent experiments are graphed (G). Integrin β3-immunoprecipitates from cells cultured in 5 mM or 25 mM glucose were probed for PKCβ by immunoblotting using a pan-PKCβ antibody (H). Cells were cultured in the presence of the indicated inhibitors for 24 hours. Inhibitors used were as follows: ERK (PD98059, 10µM), MEK (U0126, 10µM), PKCβ (3-(1-(3-Imidazol-1-ylpropyl)-1H-indol-3-yl)-4-anilino-1H-pyrrole-2,5-dione, 5nM), integrin β3 (echistatin, 10µM), anti-β3 antibody (10µg/ml), control antibody (10µg/ml). The fold changes (mean ± SE from three experiments) in the level of PKCβ in the immunoprecipitates relative to results obtained in 5 mM glucose are graphed. *P<0.001 as compared to corresponding control.
3.3. PKCβ associates with integrin β3 under conditions of hyperglycemia; and integrin ligation is required for enhanced PKCβ activity
Next, we examined the effects of hyperglycemia on the expression and activity of PKCβ and αVβ3. No difference in the levels of integrin β3 protein was observed in SMCs cultured under normal and hyperglycemic conditions. Furthermore, integrin β3 protein expression in aortas was similar from control and diabetic mice (Online Supplemental Figure II). SMCs cultured under conditions of hyperglycemia had a 2.6 ± 0.2-fold increase in PKCβ levels (Online Supplemental Figure II), and a >10-fold increase in membrane-associated PKC activity measured using an in vitro substrate phosphorylation assay (Figure 3G). The increase in PKCβ membrane activity was dependent on β3 integrins; it was blocked by anti-β3 antibodies and did not occur in SMCs from Itgβ3−/− mice (Figure 3H). Co-immunoprecipitation was used to determine whether hyperglycemia promoted a physical association between integrin β3 and PKCβ. SMCs cultured under hyperglycemic conditions demonstrated a 4.3 ± 0.2 fold increased association of PKCβ with integrin β3 compared with SMCs cultured under euglycemic conditions. Similarly, aortas from diabetic mice showed a 2.5 ± 0.3 fold increased association of PKCβ with integrin β3 compared with euglycemic controls. No difference in the amount of immunoprecipitated integrin β3 was observed under these conditions. The association was specific, in that PKCβ was detected in immunoprecipitates from both monoclonal and polyclonal antibodies to integrin β3 but not in immunoprecipitates using a control antibody. In addition, integrin β3 was detected in PKCβ-immunoprecipitates. Finally, inhibitors of PKCβ and integrin β3 reduced PKCβ levels in anti-integrin β3-immunoprecipitates (Figure 3H). Together, these findings suggest that both integrin ligation and PKC activation are required for PKC - integrin β3 interactions. No association of integrin β3 with PKCα, PKCδ, or PKCε was observed by immunoprecipitation (Online Supplemental Figure III).
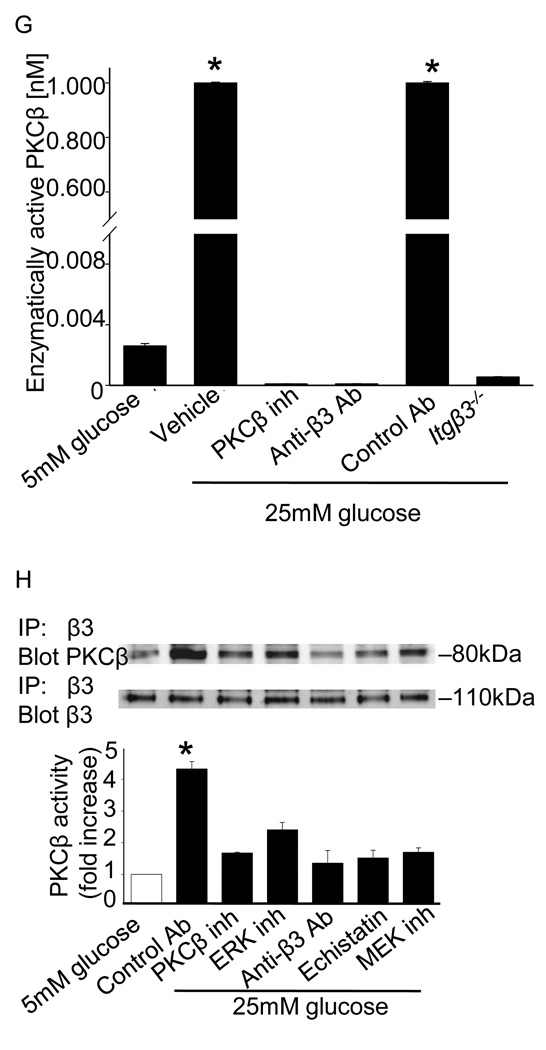
Previous reports indicate that thrombospondin 1 (TSP-1) expression is regulated by glucose and may be an important link between diabetes and accelerated vascular responses (Stouffer et al., 1998;Raman et al., 2007;Stenina et al., 2003). Because TSP-1 is an integrin β3 ligand, we sought to determine whether upregulation of TSP-1 might promote the association of αVβ3 with PKCβ and trigger down-stream signaling. As previously reported (Stenina et al., 2003;Maile et al., 2007), we detected a 4.3 ± 0.1 fold increase in TSP-1 expression in SMCs cultured under hyperglycemic conditions and a 4.1 ± 0.4 fold increase in TSP-1 expression in diabetic aortas (Figure 4A). In SMCs cultured under normal glycemic conditions, the addition of purified TSP-1 dose-dependently stimulated proliferation (data not shown) and ERK activity in a PKCβ and integrin dependent manner (Figure 4B), with a maximal effect observed at 20 µg/ml TSP-1. A similar response was observed at 10µg/ml TSP-1 (Online Supplemental Figure IV). PKCβ inhibition reduced ERK activity to levels observed in the absence of TSP-1, whereas MEK inhibition essentially abolished ERK activity (Figure 4B).
Figure 4. Role for the β3 integrin ligand thrombospondin (TSP-1) in the effects of hyperglycemia on SMCs.
Immunoblots from aortas isolated from control or diabetic mice or SMCs cultured under euglycemic (5 mM) or hyperglycemic (25 mM) conditions. The fold increase in TSP-1 levels relative to the values in euglycemic conditions are presented as the mean ± SE from three experiments (A). Exposure of SMCs cultured under euglycemic conditions to TSP-1 triggers ERK activation in a PKCβ and integrin β3-dependent manner. The results are presented as the level of phosphorylated/total protein normalized to results obtained in euglycemic conditions and are graphed as the mean ± SE from three independent experiments (B). Diabetic mice lacking TSP-1 (Thbs1−/−) do not upregulate ERK activation; similarly, Thbs1−/− SMCs do not upregulate ERK activation when cultured in 25 mM glucose (C). No increase in PKCβ is observed in integrin β3-immunoprecipitates from aortas of diabetic Thbs1−/− mice (D) or Thbs1−/−cells cultured in 25 mM glucose (E). The addition of TSP-1 to SMCs cultured in euglycemic conditions (5 mM) promotes the association of PKCβ with integrin β3 (F). Inhibitors used were as follows: MEK (U0126, 10µM), PKCβ (3-(1-(3-Imidazol-1-ylpropyl)-1H-indol-3-yl)-4-anilino-1H-pyrrole-2,5-dione, 5nM), integrin (echistatin, 10µM) and anti-β3 antibody (10µg/ml), control antibody (10µg/ml). Results are representative of those obtained in 3 independent experiments. *P<0.001 as compared to corresponding control.
The effects of hyperglycemia on aortas and SMCs from Thbs1−/− mice were examined to determine if TSP-1 contributed to enhanced responses of SMCs to hyperglycemia in vivo and promoted the association between integrin β3 and PKCβ. No increase in ERK activity was observed in Thbs1−/− mice in response to STZ-induction of diabetes or in Thbs1−/− SMCs cultured under hyperglycemic conditions (Figure 4C), although the level of ERK activity was higher in Thbs1−/− aortas and cells than in wild-type controls at baseline. In aortas from diabetic animals and in hyperglycemic cells, the absence of TSP-1 reduced the association of integrin β3 and PKCβ (Figure 4D and Figure 4E). Moreover, under conditions of euglycemia, the association of PKCβ with integrin β3 was promoted by the addition of TSP-1 to SMCs (Figure 4F). Together, these results suggest that the upregulation of TSP-1 expression under hyperglycemic conditions may promote the association of integrin β3 with PKCβ and thereby trigger downstream signaling events that enhance proliferation and migration.
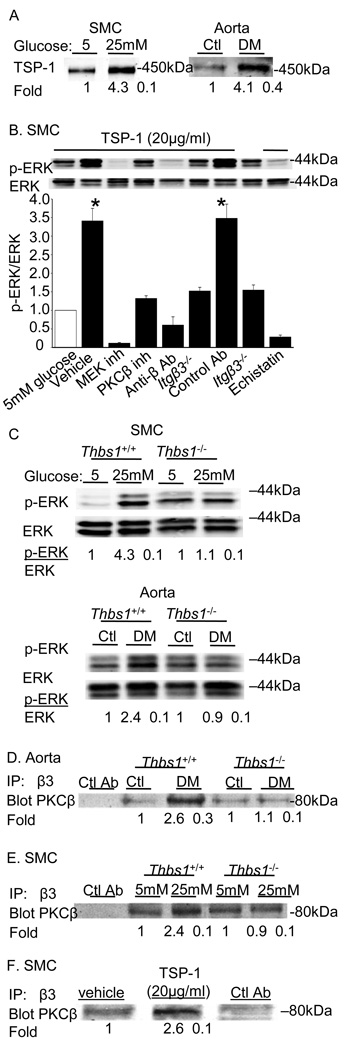
3.4. Rap1 regulates enhanced association of PKCβ and integrin β3 during hyperglycemia
The results described above suggest that TSP-1 is a potential upstream regulator of integrin – PKCβ interactions. We sought to determine if there were additional molecular regulators of these interactions. Members of the Rap1 family are attractive candidate mediators because these small GTPases can be activated by elevated DAG levels through Cal-DAG GEF and have been implicated as regulators of both PKC and integrin β3 activity. In particular, Rap1 promotes integrin affinity, integrin dependent adhesion, and ERK-activation in vascular cells and may be a key mediator of angiogenesis (Carmona et al., 2009;Chrzanowska-Wodnicka et al., 2008;Yan et al., 2008). Cells cultured under hyperglycemic conditions had a 3-fold elevation in Rap1 activity, as measured by pull-down assay (Figure 5A) that was not dependent on PKCβ (Online Supplemental Figure V). Down regulation of CalDAG-GEF or Rap1a and Rap1b by RNA-dependent gene silencing (RNAi) reduced hyperglycemia-mediated activation of Rap1 by 68% (Figure 5A), and reduced the association of integrin β3 and PKCβ by 85% (Figure 5B) without affecting the integrin expression levels. Unlike the association of αVβ3 and PKCβ observed in aortas from diabetic wild-type mice, in aortas isolated from Rap1b−/− mice there was no increase in PKCβ levels in anti-integrin β3-immunoprecipitates (Figure 5C). In aortas from diabetic Rap1a−/− there was a partial reduction in PKCβ levels in anti-integrin β3-immunoprecipitates (Figure 5C). Finally, the silencing of CalDAG-GEF or Rap1a and Rap1b by RNAi attenuated activation of ERK in SMCs cultured under hyperglycemic conditions (Figure 5D).
Figure 5. Hyperglycemia-induced activation of Rap1 promotes the association of Itgβ3 and PKCβ.
SMCs cultured under hyperglycemic conditions have elevated active Rap1 as measured by pull-down assay described in the Methods section (A). The activation of Rap1 by hyperglycemia could be attenuated by targeting Rap1 expression with siRNA to Rap1a and Rap1b or to the Rap1 CalDAG-GEF (A). Loss of Rap1 activation reduces the association of PKCβ with integrin β3 immunoprecipitates (B). The association of PKCβ with integrin β3 is not observed in aortas from diabetic Rap1a−/− and Rap1b−/− mice (C). Downregulation of Rap1 signaling attenuates ERK activation in SMC cultured in hyperglycemic conditions (D). (E) TSP-1 induced ERK activation was abolished in Rap1b−/− SMC cells. Results are representative of those obtained in 3 – 5 independent experiments.
4. Discussion
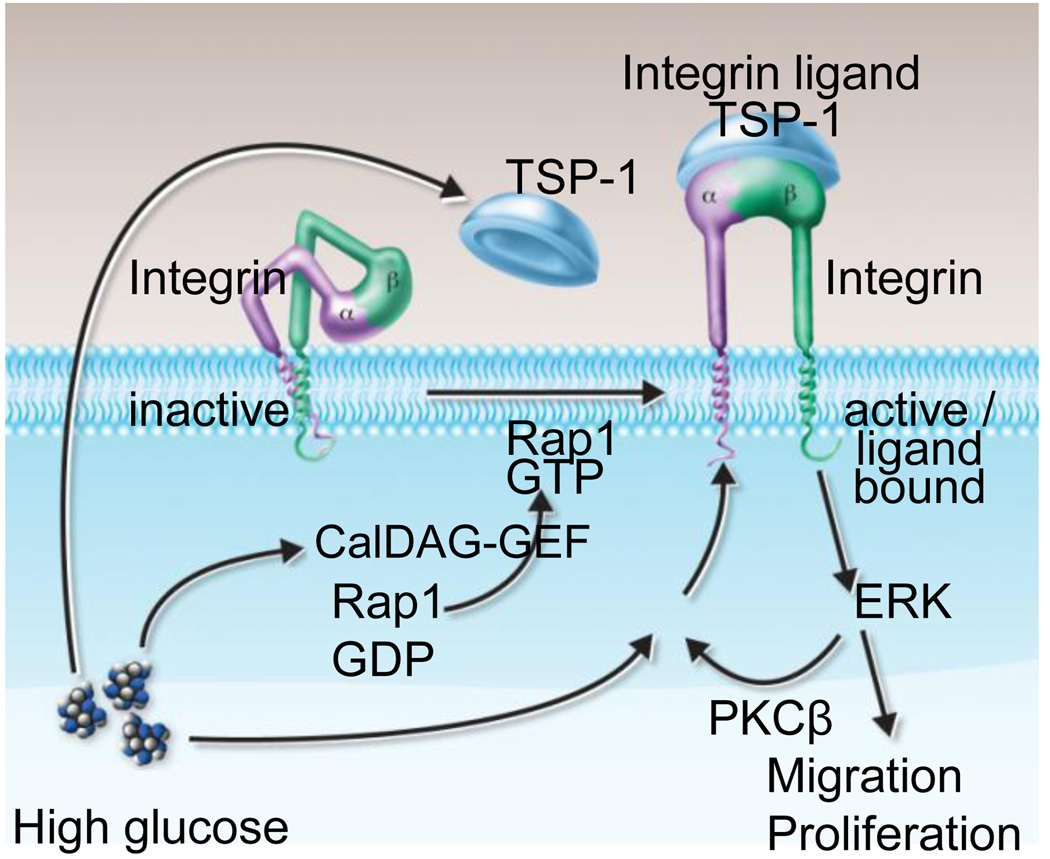
In this study, we report that the development of intimal hyperplasia following femoral artery denudation is accelerated in diabetic mice in an integrin β3-dependent manner. This finding augments our previous report that both wild-type and Itgb3−/− mice develop a similar degree of intimal hyperplasia in this model (Smyth et al., 2001), though others have reported a reduction in neointima formation in Itgb3−/− mice in a ligation injury model (Choi et al., 2004). Our results suggest a benefit from integrin β3 antagonism in the context of vascular injury and diabetes with SMC responses playing a dominant role. We examined the role of integrin αVβ3 in signaling responses in aortas from both euglycemic and hyperglycemic mice and in SMCs cultured under euglycemic or hyperglycemic conditions. Our findings indicate that 1) hyperglycemia enhances SMC proliferation and migration in an αVβ3- and PKCβ-dependent manner, 2) αVβ3 is required for enhanced membrane activity of PKCβ under conditions of hyperglycemia, and 3) hyperglycemia promotes an association between PKCβ and αVβ3. The association of PKCβ and αVβ3 is enhanced by TSP-1 production and requires activation of the small GTPAse Rap1 in the setting of hyperglycemia. These results suggest the presence of a signaling loop in which elevations in glucose, perhaps via DAG and/or calcium and CalDAG-GEF, activate Rap1 to promote αVβ3 ligation and membrane association of PKCβ, which in turn regulates downstream signaling events such as ERK activation that are required for SMC proliferation and migration (Figure 6). Others have reported that Rap1 interacts with the Rap1-GTP-interacting adaptor molecule (RIAM) to promote talin-dependent integrin activation (Lee et al., 2009). Although we did not specifically interrogate this pathway, it may be involved in Rap1-mediated pathways in the context of hyperglycemia.
Figure 6.
Model illustrating mechanism underlying glucose-mediated integrin activation leading to proliferation and migration of vascular smooth muscle cell and that may result in enhanced neointimal formation under diabetic condition.
Activation of members of the PKC family is a key signaling event that occurs in the setting of hyperglycemia (Yan et al., 2006;Nakamura et al., 2001). Further, PKC inhibitors attenuate a number of vascular abnormalities associated with diabetes in animal models (Clarke and Dodson, 2007;Das Evcimen and King, 2007;Tuttle and Anderson, 2003;Zhou et al., 2006). Hyperglycemia also increases the expression of PKCβII isoforms in vascular SMCs, and PKCβII is required for glucose-potentiated PI3K and ERK activity and SMC chemotaxis (Campbell et al., 2004). Integrin-specific signaling pathways control PKC activity in other cellular contexts as well (Buensuceso et al., 2005;Mostafavi-Pour et al., 2003). For example, occupancy of the major platelet integrin αVβ3 promotes a RACK1-dependent association of PKCβ with integrin that is required for integrin-dependent platelet spreading (Buensuceso et al., 2005). Our results in vascular SMCs suggest that the association of αVβ3 and PKCβ may be similarly affected by integrin ligation, and that hyperglycemia may drive this association by simultaneously enhancing the activity of the integrin regulator Rap1 and stimulating the production of the integrin ligand TSP-1. Although we did not specifically examine a role for RACK1 in promoting the association of PKCβ and αVβ3, RACK1 is an attractive candidate to mediate the interactions, given its ability to bind to integrin β cytoplasmic tails via WD 5– 7 repeats (Buensuceso et al., 2005;Buensuceso et al., 2001;Liliental and Chang, 1998), and to bind directly to PKCβ via its WD3 and WD6 repeats (Ron et al., 1994;Schechtman and Mochly-Rosen, 2001).
Observations in both in vitro and in preclinical models have supported a role for αVβ3 in the development of neointima in the context of atherosclerosis and restenosis (Stouffer et al., 1998;Stouffer and Smyth, 2003;Choi et al., 2004;Kokubo et al., 2007). Indeed, in at least 15 animal models, αVβ3 inhibitors demonstrated significant attenuation of intimal hyperplasia (Kokubo et al., 2007). Moreover, the EPIC trial of the β3-integrin receptor antagonist abciximab, in the setting of percutaneous coronary intervention (PCI), demonstrated reduced long-term complications and the need for repeat interventions (a marker of restenosis) in humans. Long-term data (≥ 5 years) from the EPIC trial continued to show a persistent mortality benefit from abciximab (Topol et al., 2002); however, a number of other trials looking at abciximab’s effects on restenosis have failed to demonstrate an effect (Lincoff, 2003). These results have promoted some speculation that the role of αVβ3 in regulating SMC response may be context-specific (Kokubo et al., 2007). Indeed, as described above abciximab may decrease clinical and angiographic restenosis after PCI and stent placement in diabetic patients (Marso et al., 1999;Lincoff, 2003).
The contribution of hyperglycemia in regulating the development of intimal hyperplasia has been examined in a number of preclinical models. In animals, elevations in blood glucose have been associated with heightened formation of neointima. Our results in a preclinical model of type 1 diabetes indicate that changes in signaling molecules occur within weeks of hyperglycemia. In cultured SMCs, exposure to high glucose alone is sufficient to promote the same molecular mechanisms. In patients with diabetes who are expected to have cyclic elevations in blood glucose and other metabolic abnormalities, additional factors, including signaling through growth factor receptors are likely to contribute to SMC responses (Maile et al., 2007). In conclusion, our results support the concept of fundamental differences in diabetic responses to injury and provide insight into a unique role that the Rap1- αVβ3 nexus may play in this context. Given the availability of drugs that target αVβ3, our findings may have implications for strategies to prevent the cardiovascular complications of diabetes.
Supplementary Material
Acknowledgments
We thank Jessica Caicedo and Paul Mueller for animal husbandry, Kirk McNaughton for assistance with histology, and Robert Bagnell for assistance with electron microscopy. This work was supported by grants HL074219 andHL078663 from NIH (S.S.S.), a Beginning Grant-in-Aid from the American Heart Association (M.P), grant 0950118G from the American Heart Association (MW), and CA10867 from NIH (LQ). A portion of this work was presented in abstract form at the American Heart Association Scientific Sessions in 2007.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- Beer J, Coller BS. Evidence that platelet glycoprotein IIIa has a large disulfide-bonded loop that is susceptible to proteolytic cleavage. J Biol Chem. 1989;264:17564–17573. [PubMed] [Google Scholar]
- Buensuceso CS, Obergfell A, Soriani A, Eto K, Kiosses WB, Arias-Salgado EG, et al. Regulation of outside-in signaling in platelets by integrin-associated protein kinase C beta. J Biol Chem. 2005;280:644–653. doi: 10.1074/jbc.M410229200. [DOI] [PubMed] [Google Scholar]
- Buensuceso CS, Woodside D, Huff JL, Plopper GE, O'Toole TE. The WD protein Rack1 mediates protein kinase C and integrin-dependent cell migration. J Cell Sci. 2001;114:1691–1698. doi: 10.1242/jcs.114.9.1691. [DOI] [PubMed] [Google Scholar]
- Campbell M, Allen WE, Sawyer C, Vanhaesebroeck B, Trimble ER. Glucose-potentiated chemotaxis in human vascular smooth muscle is dependent on cross-talk between the PI3K and MAPK signaling pathways. Circ Res. 2004;95:380–388. doi: 10.1161/01.RES.0000138019.82184.5d. [DOI] [PubMed] [Google Scholar]
- Carmona G, Gottig S, Orlandi A, Scheele J, Bauerle T, Jugold M, et al. Role of the small GTPase Rap1 for integrin activity regulation in endothelial cells and angiogenesis. Blood. 2009;113:488–497. doi: 10.1182/blood-2008-02-138438. [DOI] [PubMed] [Google Scholar]
- Ceriello A. Impaired glucose tolerance and cardiovascular disease: the possible role of post-prandial hyperglycemia. Am Heart J. 2004;147:803–807. doi: 10.1016/j.ahj.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Choi ET, Khan MF, Leidenfrost JE, Collins ET, Boc KP, Villa BR, et al. Beta3-integrin mediates smooth muscle cell accumulation in neointima after carotid ligation in mice. Circulation. 2004;109:1564–1569. doi: 10.1161/01.CIR.0000121733.68724.FF. [DOI] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Kraus AE, Gale D, White GC, 2nd, Vansluys J. Defective angiogenesis, endothelial migration, proliferation, and MAPK signaling in Rap1b-deficient mice. Blood. 2008;111:2647–2656. doi: 10.1182/blood-2007-08-109710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Smyth SS, Schoenwaelder SM, Fischer TH, White GC., 2nd Rap1b is required for normal platelet function and hemostasis in mice. J Clin Invest. 2005;115:680–687. doi: 10.1172/JCI22973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke M, Dodson PM. PKC inhibition and diabetic microvascular complications. Best Pract Res Clin Endocrinol Metab. 2007;21:573–586. doi: 10.1016/j.beem.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Das Evcimen N, King GL. The role of protein kinase C activation and the vascular complications of diabetes. Pharmacol Res. 2007;55:498–510. doi: 10.1016/j.phrs.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Folsom AR, Eckfeldt JH, Weitzman S, Ma J, Chambless LE, Barnes RW, et al. Relation of carotid artery wall thickness to diabetes mellitus, fasting glucose and insulin, body size, and physical activity. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Stroke. 1994;25:66–73. doi: 10.1161/01.str.25.1.66. [DOI] [PubMed] [Google Scholar]
- Giannattasio C, Failla M, Grappiolo A, Gamba PL, Paleari F, Mancia G. Progression of large artery structural and functional alterations in Type I diabetes. Diabetologia. 2001;44:203–208. doi: 10.1007/s001250051600. [DOI] [PubMed] [Google Scholar]
- Hedin U, Roy J, Tran PK, Lundmark K, Rahman A. Control of smooth muscle cell proliferation--the role of the basement membrane. Thromb Haemost. 1999;82 Suppl 1:23–26. [PubMed] [Google Scholar]
- Hodivala-Dilke KM, McHugh KP, Tsakiris DA, Rayburn H, Crowley D, Ullman-Cullere M, et al. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest. 1999;103:229–238. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Quevedo P, Sabate M. Percutaneous coronary revascularization in diabetics: from balloon angioplasty to drug-eluting stents. Expert Rev Cardiovasc Ther. 2005;3:635–646. doi: 10.1586/14779072.3.4.635. [DOI] [PubMed] [Google Scholar]
- Jonas M, Edelman ER, Groothuis A, Baker AB, Seifert P, Rogers C. Vascular neointimal formation and signaling pathway activation in response to stent injury in insulin-resistant and diabetic animals. Circ Res. 2005;97:725–733. doi: 10.1161/01.RES.0000183730.52908.C6. [DOI] [PubMed] [Google Scholar]
- Karha J, Bhatt DL. Percutaneous coronary intervention in diabetics. Rev Endocr Metab Disord. 2004;5:277–285. doi: 10.1023/B:REMD.0000032417.33194.09. [DOI] [PubMed] [Google Scholar]
- Kokubo T, Uchida H, Choi ET. Integrin alpha(v)beta(3) as a target in the prevention of neointimal hyperplasia. J Vasc Surg. 2007;45 Suppl A:A33–A38. doi: 10.1016/j.jvs.2007.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Lim CJ, Puzon-McLaughlin W, Shattil SJ, Ginsberg MH. RIAM activates integrins by linking talin to ras GTPase membrane-targeting sequences. J Biol Chem. 2009;284:5119–5127. doi: 10.1074/jbc.M807117200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitges M, Schmedt C, Guinamard R, Davoust J, Schaal S, Stabel S, et al. Immunodeficiency in protein kinase cbeta-deficient mice. Science. 1996;273:788–791. doi: 10.1126/science.273.5276.788. [DOI] [PubMed] [Google Scholar]
- Li SL, Reddy MA, Cai Q, Meng L, Yuan H, Lanting L, et al. Enhanced proatherogenic responses in macrophages and vascular smooth muscle cells derived from diabetic db/db mice. Diabetes. 2006;55:2611–2619. doi: 10.2337/db06-0164. [DOI] [PubMed] [Google Scholar]
- Li Y, Yan J, De P, Chang HC, Yamauchi A, Christopherson KW, 2nd, et al. Rap1a null mice have altered myeloid cell functions suggesting distinct roles for the closely related Rap1a and 1b proteins. J Immunol. 2007;179:8322–8331. doi: 10.4049/jimmunol.179.12.8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liliental J, Chang DD. Rack1, a receptor for activated protein kinase C, interacts with integrin beta subunit. J Biol Chem. 1998;273:2379–2383. doi: 10.1074/jbc.273.4.2379. [DOI] [PubMed] [Google Scholar]
- Lincoff AM. Important triad in cardiovascular medicine: diabetes, coronary intervention, and platelet glycoprotein IIb/IIIa receptor blockade. Circulation. 2003;107:1556–1559. doi: 10.1161/01.cir.0000055653.52489.e9. [DOI] [PubMed] [Google Scholar]
- Lincoff AM, Califf RM, Moliterno DJ, Ellis SG, Ducas J, Kramer JH, et al. Complementary clinical benefits of coronary-artery stenting and blockade of platelet glycoprotein IIb/IIIa receptors. Evaluation of Platelet IIb/IIIa Inhibition in Stenting Investigators. N Engl J Med. 1999;341:319–327. doi: 10.1056/NEJM199907293410503. [DOI] [PubMed] [Google Scholar]
- Maile LA, Capps BE, Ling Y, Xi G, Clemmons DR. Hyperglycemia alters the responsiveness of smooth muscle cells to insulin-like growth factor-I. Endocrinology. 2007;148:2435–2443. doi: 10.1210/en.2006-1440. [DOI] [PubMed] [Google Scholar]
- Marso SP, Lincoff AM, Ellis SG, Bhatt DL, Tanguay JF, Kleiman NS, et al. Optimizing the percutaneous interventional outcomes for patients with diabetes mellitus: results of the EPISTENT (Evaluation of platelet IIb/IIIa inhibitor for stenting trial) diabetic substudy. Circulation. 1999;100:2477–2484. doi: 10.1161/01.cir.100.25.2477. [DOI] [PubMed] [Google Scholar]
- Moiseeva EP. Adhesion receptors of vascular smooth muscle cells and their functions. Cardiovasc Res. 2001;52:372–386. doi: 10.1016/s0008-6363(01)00399-6. [DOI] [PubMed] [Google Scholar]
- Mostafavi-Pour Z, Askari JA, Parkinson SJ, Parker PJ, Ng TT, Humphries MJ. Integrin-specific signaling pathways controlling focal adhesion formation and cell migration. J Cell Biol. 2003;161:155–167. doi: 10.1083/jcb.200210176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura J, Kasuya Y, Hamada Y, Nakashima E, Naruse K, Yasuda Y, et al. Glucose-induced hyperproliferation of cultured rat aortic smooth muscle cells through polyol pathway hyperactivity. Diabetologia. 2001;44:480–487. doi: 10.1007/s001250051646. [DOI] [PubMed] [Google Scholar]
- Nathan DM, Lachin J, Cleary P, Orchard T, Brillon DJ, Backlund JY, et al. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med. 2003;348:2294–2303. doi: 10.1056/NEJMoa022314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines EW. The extracellular matrix can regulate vascular cell migration, proliferation, and survival: relationships to vascular disease. Int J Exp Pathol. 2000;81:173–182. doi: 10.1046/j.1365-2613.2000.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman P, Krukovets I, Marinic TE, Bornstein P, Stenina OI. Glycosylation mediates up-regulation of a potent antiangiogenic and proatherogenic protein, thrombospondin-1, by glucose in vascular smooth muscle cells. J Biol Chem. 2007;282:5704–5714. doi: 10.1074/jbc.M610965200. [DOI] [PubMed] [Google Scholar]
- Reddy MA, Villeneuve LM, Wang M, Lanting L, Natarajan R. Role of the lysine-specific demethylase 1 in the proinflammatory phenotype of vascular smooth muscle cells of diabetic mice. Circ Res. 2008;103:615–623. doi: 10.1161/CIRCRESAHA.108.175190. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ron D, Chen CH, Caldwell J, Jamieson L, Orr E, Mochly-Rosen D. Cloning of an intracellular receptor for protein kinase C: a homolog of the beta subunit of G proteins. Proc Natl Acad Sci U S A. 1994;91:839–843. doi: 10.1073/pnas.91.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzberg SP, Filsoufi F, Anyanwu A, von Harbou K, Karlof E, Carpentier A, et al. Increased neointimal formation after surgical vein grafting in a murine model of type 2 diabetes. Circulation. 2006;114:I302–I307. doi: 10.1161/CIRCULATIONAHA.105.001339. [DOI] [PubMed] [Google Scholar]
- Sasaki N, Yamashita T, Takaya T, Shinohara M, Shiraki R, Takeda M, et al. Augmentation of vascular remodeling by uncoupled endothelial nitric oxide synthase in a mouse model of diabetes mellitus. Arterioscler Thromb Vasc Biol. 2008;28:1068–1076. doi: 10.1161/ATVBAHA.107.160754. [DOI] [PubMed] [Google Scholar]
- Schechtman D, Mochly-Rosen D. Adaptor proteins in protein kinase C-mediated signal transduction. Oncogene. 2001;20:6339–6347. doi: 10.1038/sj.onc.1204778. [DOI] [PubMed] [Google Scholar]
- Smyth SS, Reis ED, Zhang W, Fallon JT, Gordon RE, Coller BS. Beta(3)-integrin-deficient mice but not P-selectin-deficient mice develop intimal hyperplasia after vascular injury: correlation with leukocyte recruitment to adherent platelets 1 hour after injury. Circulation. 2001;103:2501–2507. doi: 10.1161/01.cir.103.20.2501. [DOI] [PubMed] [Google Scholar]
- Smyth SS, Tsakiris DA, Scudder LE, Coller BS. Structure and function of murine alphaIIbbeta3 (GPIIb/IIIa): studies using monoclonal antibodies and beta3-null mice. Thromb Haemost. 2000;84:1103–1108. [PubMed] [Google Scholar]
- Stenina OI, Krukovets I, Wang K, Zhou Z, Forudi F, Penn MS, et al. Increased expression of thrombospondin-1 in vessel wall of diabetic Zucker rat. Circulation. 2003;107:3209–3215. doi: 10.1161/01.CIR.0000074223.56882.97. [DOI] [PubMed] [Google Scholar]
- Stouffer GA, Hu Z, Sajid M, Li H, Jin G, Nakada MT, et al. Beta3 integrins are upregulated after vascular injury and modulate thrombospondin- and thrombin-induced proliferation of cultured smooth muscle cells. Circulation. 1998;97:907–915. doi: 10.1161/01.cir.97.9.907. [DOI] [PubMed] [Google Scholar]
- Stouffer GA, Smyth SS. Effects of thrombin on interactions between beta3-integrins and extracellular matrix in platelets and vascular cells. Arterioscler Thromb Vasc Biol. 2003;23:1971–1978. doi: 10.1161/01.ATV.0000093470.51580.0F. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Sagawa S, Hoshi J, Shimoma F, Matsuda I, Sakoda K, et al. Synthesis of anilino-monoindolylmaleimides as potent and selective PKCbeta inhibitors. Bioorg Med Chem Lett. 2004;14:5171–5174. doi: 10.1016/j.bmcl.2004.07.061. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Sagawa S, Hoshi J, Shimoma F, Yasue K, Ubukata M, et al. Synthesis, SAR studies, and pharmacological evaluation of 3-anilino-4-(3-indolyl) maleimides with conformationally restricted structure as orally bioavailable PKCbeta-selective inhibitors. Bioorg Med Chem. 2006;14:5781–5794. doi: 10.1016/j.bmc.2006.05.033. [DOI] [PubMed] [Google Scholar]
- Topol EJ, Lincoff AM, Kereiakes DJ, Kleiman NS, Cohen EA, Ferguson JJ, et al. Multi-year follow-up of abciximab therapy in three randomized, placebo-controlled trials of percutaneous coronary revascularization. Am J Med. 2002;113:1–6. doi: 10.1016/s0002-9343(02)01145-2. [DOI] [PubMed] [Google Scholar]
- Tuttle KR, Anderson PW. A novel potential therapy for diabetic nephropathy and vascular complications: protein kinase C beta inhibition. Am J Kidney Dis. 2003;42:456–465. doi: 10.1016/s0272-6386(03)00741-8. [DOI] [PubMed] [Google Scholar]
- Villeneuve LM, Reddy MA, Lanting LL, Wang M, Meng L, Natarajan R. Epigenetic histone H3 lysine 9 methylation in metabolic memory and inflammatory phenotype of vascular smooth muscle cells in diabetes. Proc Natl Acad Sci U S A. 2008;105:9047–9052. doi: 10.1073/pnas.0803623105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenknecht LE, D'Agostino RB, Jr, Haffner SM, Savage PJ, Rewers M. Impaired glucose tolerance, type 2 diabetes, and carotid wall thickness: the Insulin Resistance Atherosclerosis Study. Diabetes Care. 1998;21:1812–1818. doi: 10.2337/diacare.21.11.1812. [DOI] [PubMed] [Google Scholar]
- Wang S, Li Y. Expression of constitutively active cGMP-dependent protein kinase inhibits glucose-induced vascular smooth muscle cell proliferation. Am J Physiol Heart Circ Physiol. 2009;297:H2075–H2083. doi: 10.1152/ajpheart.00521.2009. [DOI] [PubMed] [Google Scholar]
- Yamasaki Y, Kawamori R, Matsushima H, Nishizawa H, Kodama M, Kajimoto Y, et al. Atherosclerosis in carotid artery of young IDDM patients monitored by ultrasound high-resolution B-mode imaging. Diabetes. 1994;43:634–639. doi: 10.2337/diab.43.5.634. [DOI] [PubMed] [Google Scholar]
- Yan J, Li F, Ingram DA, Quilliam LA. Rap1a is a key regulator of fibroblast growth factor 2-induced angiogenesis and together with Rap1b controls human endothelial cell functions. Mol Cell Biol. 2008;28:5803–5810. doi: 10.1128/MCB.00393-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan SF, Harja E, Andrassy M, Fujita T, Schmidt AM. Protein kinase C beta/early growth response-1 pathway: a key player in ischemia, atherosclerosis, and restenosis. J Am Coll Cardiol. 2006;48:A47–A55. doi: 10.1016/j.jacc.2006.05.063. [DOI] [PubMed] [Google Scholar]
- Zhou W, Wang XL, Lamping KG, Lee HC. Inhibition of protein kinase Cbeta protects against diabetes-induced impairment in arachidonic acid dilation of small coronary arteries. J Pharmacol Exp Ther. 2006;319:199–207. doi: 10.1124/jpet.106.106666. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.