Abstract
Background:
Initiatives to improve early detection and access to HIV services have increased over time. We assessed the immune status of patients at initial presentation for HIV care from 1997-2007 in 13 US and Canadian clinical cohorts.
Methods:
We analyzed data from 44,491 HIV-infected patients enrolled in the North American – AIDS Cohort Collaboration on Research and Design. We identified first presentation for HIV care as the time of first CD4+ T-lymphocyte (CD4) measurement and excluded patients who prior to this date had HIV RNA measurements, evidence of antiretroviral exposure, or a history of AIDS-defining illness. Trends in mean CD4 count (measured as cells/mm3) and 95% confidence intervals ([,]) were determined using linear regression adjusted for age, gender, race/ethnicity, HIV transmission risk and cohort.
Results:
Median age at first presentation for HIV care increased over time (range 40-43 years, p<0.01), while the proportion of patients with injection drug use HIV transmission risk decreased (26% to 14%, p<0.01) and heterosexual transmission risk increased (16% to 23%, p<0.01). Median CD4 at presentation increased from 256 (IQR: 96-455) to 317 (IQR: 135-517) in 1997 to 2007 (p<0.01). The proportion with a CD4 count ≥350 at first presentation also increased from 1997 to 2007 (38% to 46%, p=<0.01). The estimated adjusted mean CD4 count increased at a rate of 6 [5, 7] per year.
Conclusion:
CD4 count at first presentation for HIV care has increased annually over the past 11 years, but has remained <350 cells/mm3, suggesting the urgent need for earlier HIV diagnosis and treatment.
Keywords: CD4 Lymphocyte Count, Delivery of Health Care / statistics & numerical data, HIV Infections / therapy, United States, Canada
BACKGROUND
Approximately 25% of the estimated 1.1 million Americans and 58,000 Canadians living with HIV infection are unaware they are infected [1, 2]. Among the estimated 56,000 new infections occurring each year in the US [3], over half are estimated to be transmitted by persons who are unaware of their HIV infection [4]. In the US from 2000 to 2006, rates for a history of ever being tested for HIV infection have remained stable at less than 40%; among persons with established risk factors for HIV during 2006 only 23% were tested [5]. In Canada in 2003, a general population survey found that 29% of women and 24% of men reported ever being tested for HIV [6]; in 1996, 71% of men who have sex with men (MSM), 62% of injection drug users (IDUs) and 51% of high-risk heterosexuals reported ever being tested, although these higher proportions may be due to testing for research participation [2]. Even among pregnant women in the US in 2006, only 61% had been tested despite long-standing recommendations and extensive efforts to incorporate routine opt-out testing into pregnancy care [5]. Similar guidelines for testing during pregnancy exist in Canada; however, prenatal HIV screening programs vary. Data from 2002 to 2006 show the proportion of pregnant women screened for HIV ranges from 60% in Manitoba to ≥95% in Alberta, Newfoundland and Labrador, and the Northwest Territories [2]. Persons who are unaware of their HIV status are unable to benefit from the advances made in HIV treatment, and late presentation for HIV care is associated with higher mortality even after receipt of antiretroviral therapy [7, 8], and a diminished recovery of CD4+ T-lymphocytes (CD4) [9]. Late presenters also have a prolonged opportunity to transmit HIV while unaware of their HIV infection.
To assess when individuals are entering HIV care, we analyzed the immune status in patients who newly presented for care over the past 11 years in the US and Canada. Given the improvements that have occurred in HIV therapy, and efforts to improve early diagnosis and treatment of HIV [10-12], our objective was to assess whether patients presented for HIV care earlier in the course of their HIV infection over time and to determine factors associated with a later presentation to HIV care.
METHODS
Study design and population
All patients were part of the North American Cohort Collaboration on Research and Design (NA-ACCORD), a regional group of the International Epidemiological Databases to Evaluate AIDS (IeDEA) project. The NA-ACCORD is a multisite collaboration of 8 interval and 14 clinical cohort studies with sites in the US and Canada [13]. Each cohort's participation in NA-ACCORD was approved by the respective local institutional review boards. Contributing cohorts have used standardized methods of data collection and have submitted demographic, treatment, clinical, laboratory, and vital status data on enrolled participants.
Inclusion criteria and variables of interest
Only participants from clinical cohorts were included in this study because our interest was in the degree of immunosuppression at first presentation for HIV clinical care; the interval research cohorts in the NA-ACCORD do not administer clinical care. All of the 14 clinical cohorts agreed to participate in this study although one was excluded because their study population enrollment criteria restricted to those in later stages of HIV disease. These 13 clinical cohorts have clinical sites in the following US states and territories and Canadian provinces: Alberta, Alabama, California, Colorado, Florida, Georgia, Illinois, Maryland, Michigan, Missouri, New York, North Carolina, Ohio, Ontario, Oregon, Pennsylvania, Quebec, Tennessee, Texas, Washington and Washington DC. Due to the geographic dispersion of these clinical cohorts where individuals are receiving their HIV care, the possibility that an individual is participating in more than one of the clinical cohorts is low.
We analyzed data from HIV-infected adults (≥18 years of age) who first presented for clinical care between January 1997 through December 2007, where first presentation for HIV clinical care was defined as the date (month and year) at which the first CD4 count was reported. The clinical cohorts of the NA-ACCORD are well-established, and have procedures to determine medical histories at first presentation for care. HIV-related treatments, laboratory results and diagnoses prior to enrollment are routinely recorded.
Several methods were used to eliminate patients who might have been receiving HIV care prior to their first recorded CD4 count. First, we excluded patients who were taking antiretroviral therapy or had an HIV RNA measurement recorded prior to the date of the first CD4 measurement. Second, we also excluded those patients who had an AIDS-defining diagnosis recorded more than 3 months prior to the first CD4 measurement. A period of 3 months prior to the first CD4 measurement was utilized to include those who may have had an AIDS-defining diagnosis at the time of HIV diagnosis and to exclude those who may have been seeking care elsewhere. Third, for each cohort, we excluded all individuals contributing data during the first year that the cohort contributed data to the NA-ACCORD who may have been patients previously in care but contributing data to a new cohort data capture system. Although these criteria might not completely exclude earlier presentation for HIV care (particularly presentation at clinical sites outside of the participating cohort) we believe that these criteria minimize the possibility that the patients in this study had presented for HIV care at an earlier time.
The first measured CD4 was our outcome of interest. The month and year in which the CD4 was measured were recorded. If there was more than one CD4 measurement in the first month at presentation for HIV care, we calculated the mean CD4 count for the month. Other information obtained at first presentation for care included self-reported date of birth, gender, race/ethnicity and HIV transmission risk group. Race/ethnicity was categorized as black, white, Latino and other/unknown. HIV transmission risk group was categorized as MSM, IDU, heterosexual, and other/unknown. Participants with both sexual and IDU transmission risk were categorized as IDU.
Statistical Analysis
Statistical comparisons of demographic and clinical characteristics across calendar dates were made using the Cochran-Armitage trend test for categorical variables (e.g. country of care, gender, race/ethnicity and HIV transmission risk group) or using the Cuzick trend test for continuous variables (e.g. age, CD4 count). We determined the median absolute CD4 count at first presentation for HIV clinical care annually from 1997 through 2007. Multivariate linear regression models were used to describe the annual trends in estimated mean CD4 count using a linear variable for year and adjusting for cohort demographic and risk characteristics; 95% confidence intervals ([,]) were also estimated using these models. Two-way interactions between calendar year and age, gender, race/ethnicity and HIV transmission group were considered. Nonlinearity of the relationship between calendar time and CD4 count at first presentation for HIV care was assessed by including a quadratic term for calendar year. Results with a two-sided p-value of <0.05 were considered statistically significant. Analyses were conducted using SAS, version 9.
RESULTS
A total of 67,961 patients receiving clinical care at one of the participating NA-ACCORD sites between 1997 and 2007 had complete date and CD4 measurement information. Of these, 21,983 (32%) had a prior history of antiretroviral therapy or HIV RNA results and 1,487 (2%) had an AIDS-defining diagnosis recorded more than 3 months prior to the first recorded CD4 count. Thus, our study population consisted of 44,491 HIV-infected individuals.
The characteristics of individuals who first presented for HIV care each year are shown in Table 1. Over time, the median age at first presentation increased (range: 40 – 43 years, p<0.01). The proportion of white patients decreased over time (from 30% in 1996 to 24% in 2007, p<0.01) while the proportion of black patients fluctuated, but remained higher than the proportion of white and Latino patients (p<0.01). The proportion of patients with IDU transmission risk decreased (26% in 1997 to 14% in 2007, p<0.01) and heterosexual transmission risk increased (16% in 1997 to 23% in 2007, p<0.01). There was a slight increase in the proportion of Canadian patients, however the proportion remained <10% over time (p<0.01).
Table 1.
Characteristics of N=44,491 participating patients in NA-ACCORD for HIV clinical care, by year at first presentation
| Total N= 44,491 |
1997 n= 4,479 |
1998 n= 4,412 |
1999 n= 4,857 |
2000 n= 5,262 |
2001 n= 4,258 |
2002 n= 4,063 |
2003 n= 3,688 |
2004 n= 3,773 |
2005 n= 3,486 |
2006 n= 3,354 |
2007 n= 2,859 |
p-valuea | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | |||||||||||||||||||||||||
| Median | 41 | 40 | 41 | 41 | 40 | 41 | 41 | 41 | 41 | 41 | 42 | 43 | <0.01 | ||||||||||||
| IQR | 34-48 | 34-47 | 35-47 | 34-47 | 34-47 | 34-48 | 34-48 | 34-49 | 34-49 | 34-48 | 34-49 | 34-50 | |||||||||||||
| Country | |||||||||||||||||||||||||
| United States, n % | 42,133 | 95% | 4,229 | 94% | 4,209 | 95% | 4,657 | 96% | 5,083 | 97% | 4,082 | 96% | 3,837 | 94% | 3,488 | 95% | 3,583 | 95% | 3,255 | 93% | 3,101 | 92% | 2,609 | 91% | <0.01 |
| Canada, n % | 2,358 | 5% | 250 | 6% | 203 | 5% | 200 | 4% | 179 | 3% | 176 | 4% | 226 | 6% | 200 | 5% | 190 | 5% | 231 | 7% | 253 | 8% | 250 | 9% | |
| Sex | |||||||||||||||||||||||||
| Male, n % | 36,155 | 81% | 3,843 | 86% | 3,716 | 84% | 3,886 | 80% | 4,013 | 76% | 3,407 | 80% | 3,296 | 81% | 3,027 | 82% | 3,044 | 81% | 2,811 | 81% | 2,724 | 81% | 2,388 | 84% | 0.31 |
| Female, n % | 8,336 | 19% | 636 | 14% | 696 | 16% | 971 | 20% | 1,249 | 24% | 851 | 20% | 767 | 19% | 661 | 18% | 729 | 19% | 675 | 19% | 630 | 19% | 471 | 16% | |
| Race | |||||||||||||||||||||||||
| White, n % | 10,255 | 23% | 1,345 | 30% | 1,213 | 27% | 1,064 | 22% | 911 | 17% | 957 | 22% | 899 | 22% | 716 | 19% | 711 | 19% | 903 | 26% | 848 | 25% | 688 | 24% | <0.01 |
| Black, n % | 18,745 | 42% | 2,014 | 45% | 2,032 | 46% | 2,202 | 45% | 2,447 | 47% | 1,805 | 42% | 1,651 | 41% | 1,197 | 32% | 1,400 | 37% | 1,484 | 43% | 1,367 | 41% | 1,146 | 40% | <0.01 |
| Latino, n % | 6,110 | 14% | 509 | 11% | 532 | 12% | 718 | 15% | 972 | 18% | 653 | 15% | 555 | 14% | 465 | 13% | 505 | 13% | 411 | 12% | 402 | 12% | 388 | 14% | 0.15 |
| Other/Unknown, n % | 9,381 | 21% | 611 | 14% | 635 | 14% | 873 | 18% | 932 | 18% | 843 | 20% | 958 | 24% | 1,310 | 36% | 1,157 | 31% | 688 | 20% | 737 | 22% | 637 | 22% | <0.01 |
| HIV Risk Group | |||||||||||||||||||||||||
| MSM, n % | 12,931 | 29% | 1,092 | 24% | 1,090 | 25% | 1,333 | 27% | 1,402 | 27% | 1,194 | 28% | 1,212 | 30% | 1,159 | 31% | 1,192 | 32% | 1,140 | 33% | 1,166 | 35% | 951 | 33% | <0.01 |
| IDU, n % | 8,615 | 19% | 1,173 | 26% | 1,158 | 26% | 1,121 | 23% | 1,188 | 23% | 844 | 20% | 691 | 17% | 557 | 15% | 529 | 14% | 501 | 14% | 450 | 13% | 403 | 14% | <0.01 |
| Heterosexual, n % | 10,272 | 23% | 734 | 16% | 848 | 19% | 1,120 | 23% | 1,451 | 28% | 1,076 | 25% | 960 | 24% | 852 | 23% | 967 | 26% | 813 | 23% | 780 | 23% | 671 | 23% | <0.01 |
| Other/Unknown, n % | 12,673 | 28% | 1,480 | 33% | 1,316 | 30% | 1,283 | 26% | 1,221 | 23% | 1,144 | 27% | 1,200 | 30% | 1,120 | 30% | 1,085 | 29% | 1,032 | 30% | 958 | 29% | 834 | 29% | <0.01 |
| CD4+ T-cell Count | |||||||||||||||||||||||||
| (cells/mm3) | |||||||||||||||||||||||||
| Median | 288 | 256 | 265 | 270 | 279 | 280 | 304 | 293 | 302 | 311 | 319 | 317 | <0.01 | ||||||||||||
| IQR | 107-486 | 96-455 | 87-468 | 96-458 | 104-486 | 97-482 | 120-495 | 112-498 | 115-497 | 119-502 | 129-509 | 135-517 | |||||||||||||
Abbreviations: NA-ACCORD= North American Cohort Collaboration on Research and Design, MSM=men who have sex with men, IDU =injection drug use
P-values calculated using Cochran-Armitage test for categorical variables or Cuzick's test for continuous variables.
The median CD4 count of the study population increased over time but the changes were within a range of 61 cells/mm3 over all 11 years (Figure 1). The percentage of patients presenting with a CD4 count ≥350 cells/mm3 increased from 38% in 1997 to 46% in 2007 (p<0.01) (Figure 1). The overall estimated annual change in mean CD4 count was 6 [5, 7] cells/mm3, adjusting for age, gender, race/ethnicity, transmission risk group and cohort. The quadratic term for calendar year was not significant (p=0.27), suggesting the relationship between calendar year and CD4 count at first presentation for HIV care was not U-shaped. However, there were significant interactions between the change in CD4 count per year with race/ethnicity (p=0.01) and with transmission risk group (p<0.01), but not gender (p=0.13).
Figure 1.
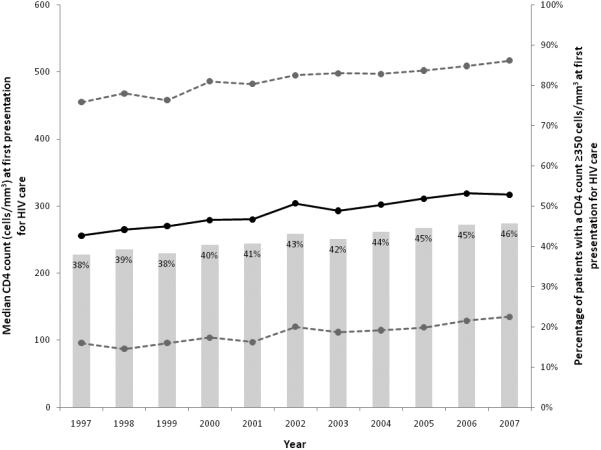
Median CD4 count (and interquartile range), and the percentage of patients with a CD4 count ≥350 cells/mm3, at first presentation for HIV clinical care, NA-ACCORD 1997-2007
Table 2 displays the results from the overall and stratified multivariate models. Although females had higher observed mean CD4 counts in 1997, the estimated mean increase in CD4 count per year among females was less than males (females: 5 [3, 7] cells/mm3 per year; males: 6 [5, 7] cells/mm3 per year). Latinos had the greatest annual increase in estimated mean CD4 count since 1997 (9 [7, 12] cells/mm3) and blacks had the smallest increase (5 [3, 7] cells/mm3). Latinos showed a lower observed (293 cells/mm3) mean CD4 count in 1997 compared with whites (328 cells/mm3) and blacks (305 cells/mm3). The estimated annual change in mean CD4 count at first presentation was 7 [6, 9] cells/mm3 among MSM, 5 [3, 7] cells/mm3 among IDU and 2 [0, 4] cells/mm3 among those with heterosexual transmission risk. MSM showed low observed mean CD4 counts in 1997 (303 cells/mm3) and those with IDU and heterosexual transmission risk had a slightly higher observed mean CD4 count in 1997 (334 and 328 cells/mm3, respectively).
Table 2.
Observed mean CD4 count in 1997 and 2007 and estimated mean annual change in CD4 count at first presentation for HIV clinical care, NA-ACCORD 1997-2007
| Observed mean CD4 count (standard deviation) | Estimated change in CD4 count (95%CI) per year (1997-2007)a |
|||
|---|---|---|---|---|
| 1997 | 2007 | |||
| Overallb | 307 (259) | 360 (283) | 6 | (5, 7) |
|
Models stratified by sexc |
||||
| Male | 300 (255) | 353 (278) | 6 | (5, 7) |
| Female | 349 (281) | 395 (303) | 5 | (3, 7) |
|
Models stratified by race/ethnicityd |
||||
| White | 328 (271) | 382 (280) | 6 | (5, 8) |
| Black | 305 (261) | 328 (279) | 5 | (3, 7) |
| Latino | 293 (246) | 383 (301) | 9 | (7, 12) |
| Other/Unknown | 281 (235) | 380 (276) | 7 | (5, 9) |
|
Models stratified by HIV transmission risk groupe |
||||
| MSM | 303 (255) | 374 (263) | 7 | (6, 9) |
| IDU | 334 (260) | 358 (311) | 5 | (3, 7) |
| Heterosexual | 328 (279) | 337 (284) | 2 | (0, 4) |
| Other/Unknown | 280 (249) | 363 (289) | 8 | (7, 10) |
Abbreviations: NA-ACCORD= North American Cohort Collaboration on Research and Design, CI=confidence interval, MSM=men who have sex with men, IDU=injection drug user
Estimated mean CD4 count, estimated change in CD4 count per year from 1996-2007 and 95% confidence intervals from multivariate linear regression models.
Adjusted for age, gender, race/ethnicity, HIV transmission risk group and cohort.
Adjusted for age, race/ethnicity, HIV transmission risk group and cohort.
Adjusted for age, gender, HIV transmission risk group and cohort.
Adjusted for age, gender, race/ethnicity and cohort.
Patients who were older had lower CD4 counts at first presentation for HIV care, with an average decrease of 24 [−27, −21] cells/mm3 at the time of first presentation for care over 11 years, adjusting for gender, race/ethnicity, transmission risk group and cohort. The interaction of age and calendar time on CD4 count at first presentation for HIV care was borderline statistically significant (p=0.05).
After stratifying by cohort, n=6 cohorts showed a significant increase in estimated mean annual change in CD4 count at presentation (range: 5 to 8 cells/mm3) and n=7 cohorts showed no difference in estimated mean CD4 count over time (Figure 2). Cohort 3 had a borderline significant decrease of 5 cells/mm3 per year. Participants in cohort 3 had the highest mean CD4 count in 1997, 78% were black and 53% reported heterosexual transmission risk – all factors potentially contributing to this decrease.
Figure 2.
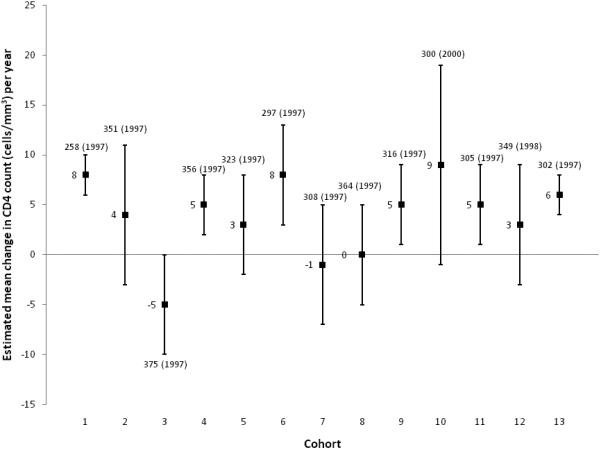
Observed mean CD4 count (year) and estimateda mean annual change in CD4 count and 95% confidence interval at first presentation for HIV clinical care, by cohort, NA-ACCORD, 1997 -2007
DISCUSSION
Since the mid-1990's, public health efforts have focused on identifying HIV infection at an early stage, which should imply an increase in patients presenting for HIV care earlier in the course of disease. Our data from multiple clinical sites across the US and Canada indicate that although CD4 count at presentation has increased since 1997, most patients continue to first present for HIV care with a CD4 count below 350 cells/mm3, the level at which initiation of antiretroviral therapy is currently recommended by multiple major national guideline committees for persons living in developed countries [14-18]. Stratifying out results by cohort demonstrated our findings were not an artifact of the combined data set. Knowing that within-patient variation in CD4 measurements is approximately 25% with increased variation at lower CD4 counts, our estimated mean annual increase in the CD4 count is likely of little clinical relevance [19].
Several smaller regional studies have also found that patients present late in the course of HIV infection. Among 1,209 patients who newly presented for care in an infectious diseases clinic in Alabama, 41% had already progressed to AIDS [20]. In South Carolina from 2001-05, 41% of AIDS cases were diagnosed within 1 year of HIV diagnosis [21], in North Carolina from 2000-03, 50% had a CD4 count <200 cells/mm3 at first presentation for HIV care [22], in Washington DC from 1997-2006, 66% of AIDS cases were diagnosed within one year of HIV diagnosis [23], and in Maryland the CD4 count at first presentation for HIV care declined from 371 to 276 cells/mm3 from 1990 to 2006 [24]. A Centers for Disease Control and Prevention (CDC) analysis of data collected in 33 US states from 2001-03 found a median CD4 count within 12 months of diagnosis of HIV-infection of only ~170 cells/mm3 [25]. In a population of primarily Canadian Aboriginals newly-diagnosed patients with HIV from 1998-2003, the median CD4 count was 330 cells/mm3; 28% had a CD4 count <200 cells/mm3 at diagnosis and median time to care was 27 days [26]. Another study from Calgary, Canada found that 39% of patients first presented with a CD4 count <200 cells/mm3 and had care costs that were 200% higher than for patients who presented with higher CD4 counts [27]. In addition to age, gender, and race/ethnicity [20, 22, 24, 26], these studies have also shown associations between CD4 count at first presentation and insurance type [20] and nonmetropolitan residence [26] in multivariate analyses; we were unable to systematically measure these variables in our participating cohorts.
Over time, the demography of the HIV epidemic in the US and Canada has shifted [2, 28]. Although the greatest proportion of persons diagnosed with HIV/AIDS remain MSM, the proportions of persons diagnosed with HIV/AIDS who are women, who are of minority race/ethnicity, and who have as their principal HIV transmission risk factor IDU or heterosexual contact, have increased. US national surveillance data from 2000-2003 demonstrated that late testers (persons who had their first positive HIV test within one year before diagnosis of AIDS) were significantly more likely to be younger (aged 18-29 years), black or Latino, and to have been infected through heterosexual contact [29]. In Canada from 1997-2004, various studies report younger individuals (age 25-34 years), and those who are higher-risk for acquiring HIV infection (i.e. MSM, IDUs, ≥2 partners in the last year among heterosexuals) were more likely to be tested [2]. We did not see this shift to younger patients at time of presentation, nor significantly greater percentages among Latino HIV-infected patients. However, black participants had the smallest mean annual increase in CD4 count at first presentation of any racial group. Similarly, the estimated mean annual increase in CD4 count among those with heterosexual transmission risk was dramatically lower than other risk groups. National surveillance data coupled with our findings suggest the number of blacks and high-risk heterosexuals entering into care at a later stage of disease will grow; however, the impact might be diminished with the expansion of routine HIV testing that would likely result in earlier diagnosis and entry into care.
The public health implications for our findings are clear: 1) delayed diagnosis reduces survival; and 2) individuals entry into HIV care had lower CD4 counts than the guideline for antiretroviral therapy initiation. A study from the Netherlands found that mortality after starting antiretroviral therapy could be reduced by 20% if patients were to present for HIV care with a CD4 count ≥400 cells/mm3 [8]. Current guidelines recommend starting antiretroviral therapy before the CD4 count reaches 350 cells/mm3, and recent data from across the US and Canada suggests that initiating therapy at even higher CD4 levels improves survival [30]. This underscores the importance of identifying infection and engaging in HIV care at a earlier stage than is occurring currently.
A delay in presentation for treatment not only increases the chance of clinical disease progression for that patient but also increases the risk of ongoing transmission. Early effective antiretroviral treatment can lower circulating HIV-1 RNA levels, thereby decreasing the risk of HIV transmission [31-33]. Patients who learn they are infected with HIV may reduce their HIV-1 RNA with the effective antiretroviral treatment and reduce their risk-taking behavior, consequently resulting in reduced risk of transmitting HIV to others [34].
Our results found no significant differences in CD4 trends over time based by gender. Some women were likely tested as part of pre-natal screening (pregnant women are not excluded from these clinical care cohorts), and it is possible the CD4 count at which they presented for care was higher than the CD4 count at which non-pregnant women were tested and subsequently entered care. As the circumstances under which women were tested and subsequently entered care were unknown, we were unable to determine differences in CD4 counts at first presentation for care for these two groups of women.
There is clearly a need for earlier HIV diagnosis and intervention. Relying on clinical indicators for HIV infection to prompt testing is inadequate. A study from a large managed care organization in California indicated that only 22% of patients in medical care had at least one of eight clinical indicators suggested in the literature as reasons to test for HIV within one year of being diagnosed [35]. A recent study among US veterans suggested that delayed presentation for HIV care is not a result of lack of medical care access for this population [36].
HIV testing is entering a new era as practice guidelines have recently changed to address the need for increased, earlier testing and with linkage to care. In September 2006, the CDC released Revised Recommendations for HIV Testing of Adults, Adolescents, and Pregnant Women in Health-Care Settings [37]. These advised routine HIV screening in health-care settings of adults and adolescents aged 13-64 years and of all pregnant women with prior notification and opt-out allowed. Separate informed consent, a barrier to HIV testing [38, 39], is no longer recommended. More recently, the American College of Medicine released recommendations for universal HIV screening [40]. If we are to make any impact on improving the health of HIV-infected persons and reducing the spread of the virus, public health officials, hospital administrators, and clinicians will need to adopt these recommendations into practice and change policies (e.g., legal requirements for informed consent for HIV testing and elimination of the need for HIV pretest counseling in most US states and Canadian provinces) to ensure that more persons are screened and referred for early treatment.
In summary, between 1997 and 2007 in the US and Canada, there was little improvement in the immunologic stage of HIV infection among patients entering HIV care. Patients presented with relatively low CD4 counts, at a stage of disease where the effectiveness of treatment is reduced and providing an extended opportunity to transmit HIV to others. These data provide strong evidence that implementation of new strategies for earlier HIV testing and effective linkage into care are urgently needed.
ACKNOWLEDGEMENTS
We are grateful to all patients, physicians, investigators, and staff involved in the NA-ACCORD. This work was supported by grants from the National Institutes of Health: U01-AI069918, U10-AA013566, U01-AI31834, U01-AI34989, U01-AI34993, U01-AI34994, U01-AI35004, U01-AI35039, U01-AI35040, U01-AI35041, U01-AI35042, U01-AI35043, U01-AI37613, U01-AI37984, U01-AI38855, U01-AI38858, U01-AI42590, U01-AI68634, U01-AI68636, U01-HD32632, M01-RR00071, M01-RR00079, M01-RR00083, M01-RR00722, P30-AI27757, P30-AI27767, P30-AI50410, P30-AI54999, R01-DA04334, R01-DA12568, R01-MH54907, R24-AI067039, Z01-CP010176, , N02-CP55504, R01-DA11602, AI-69432, K01-AI071754, R01-AA16893, K24-DA00432, K23-AI610320, R01-AI069434 and the Agency for Healthcare Research and Quality: HS 290-01-0012
Disclosures:
KNA, MMK, SJG, AGA, ACJ, JTB, SBR, RJB, JNM, LPJ, TRS, GDK, SN, LMC, JJG, SEV, HMC, BL, AMF, and RGM declare they have no conflict of interest.
MSS has received consulting fees from Ardea Biosciences, Avexa, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck, Monogram Biosciences, Pain Therapeutics, Panacos, Pfizer, Progenics, Roche Laboratories, Tibotec, Tobira Therapeutics, and Vicro and research support from Achillion Pharmaceuticals, Avexa, Boehringer-Ingelheim, GlaxoSmithKline, Merck, Panacos, Pfizer, Progenics, Theratechnologies, and Tibotec; SGD received consulting fees from GlaxoSmithKline, Roche, Gilead, and Boehringer-Ingelheim and grant support from Merck, Gilead, Bristol-Myers Squibb, and Pfizer; JJE has received consulting fees from Tibotec, Bristol-Myers Squibb, Merck, GlaxoSmithKline, and Pfizer, lecture fees from Roche, Bristol-Myers Squibb, Tibotec, and Merck, and grant support from GlaxoSmithKline, Merck, and Boehringer-Ingelheim; MJG has received consulting fees from Gilead, GlaxoSmithKline, Abbott, Merck, Boehringer-Ingelheim, Tibotec, and Pfizer and grant support from GlaxoSmithKline, Abbott, Canadian Institutes of Health Research, Tibotec, and Pfizer; RSH has received grant support from Merck; MBK has received consulting fees from GlaxoSmithKline, Abbott, Pfizer, and Boehringer-Ingelheim, lecture fees from Abbott, Gilead, Tibotec, Bristol-Myers Squibb, and GlaxoSmithKline and research support from Canadian Institutes of Health Research/ Fonds de la recherche en santé du Québec, Canadian HIV Trials Network, Ontario HIV Treatment Network, and Schering Plough Canada; ARR has received consulting and lecture fees from GlaxoSmithKline, Abbott, Merck, Pfizer, Bristol Myers Squibb, Gilead and Tibotec and grant support from GlaxoSmithKline, Tibotec, Boehringer-Ingelheim, Abbott, Merck, Pfizer, and Roche; MAH has received grant support from Gilead, Abbott, and Bristol-Myers Squibb; MJS has received grant support from Pfizer, Merck, Gilead, Universitywide AIDS Research Program, and Community Benefit/Kaiser Permanente; KAG has received consulting fees from Tibotec and grant support from Johns Hopkins University Richard Ross Award; RDM has received consulting fees from Bristol-Myers Squibb and GlaxoSmithKline, lecture fees from Gilead, and grant support from Pfizer, Merck, and Gilead; ACC has received consulting fees from Merck, Pfizer, and GlaxoSmithKline, equity ownership/stock options in Bristol-Myers Squibb and Abbott, and grant support from Schering-Plough, Tibotec-Virco, Gilead, Koronis, and Merck; CAB has received consulting fees from GlaxoSmithKline, Pfizer, Merck, and Achillion, and grant support from Gilead; BR has received consulting fees from Gilead and Bristol-Myers Squibb, lecture fees from Bristol-Myers Squibb, and grant support from STERIS. Dr. Brooks, as a CDC employee, notes “The findings and conclusions in this report are those of the authors and do not necessarily represtent the official position of the Centers for Disease Control and Prevention.
NA-ACCORD Participating cohorts (representatives):
AIDS Link to the IntraVenous Experience (Gregory Kirk)
Adult AIDS Clinical Trials Group Longitudinal Linked Randomized Trials (Constance Benson, Ronald Bosch, Ann Collier)
HAART Observational Medical Evaluation and Research (Robert Hogg, Richard Harrigan, Julio Montaner)
HIV Outpatient Study (John T. Brooks)
HIV Research Network (Kelly Gebo)
Johns Hopkins HIV Clinical Cohort (Richard Moore)
John T. Carey Special Immunology Unit Patient Care and Research Database, Case Western Reserve University (Benigno Rodriguez)
Kaiser Permanente Northern California (Michael Horberg, Michael Silverberg)
Longitudinal Studies of Ocular Complications of AIDS (LSOCA) (Jennifer E. Thorne)
Multicenter Hemophilia Cohort Study–II (James Goedert)
Multicenter AIDS Cohort Study (Lisa Jacobson)
Montreal Chest Institute Immunodeficiency Service Cohort (Marina Klein)
Ontario HIV Treatment Network Cohort Study (Sean Rourke, Anita Rachlis)
Southern Alberta Clinic Cohort (John Gill)
Studies of the Consequences of the Protease Inhibitor Era (Steven Deeks, Jeff Martin)
University of Alabama at Birmingham 1917 Clinic Cohort (Michael Saag , Michael Mugavero, James Willig)
University of North Carolina, Chapel Hill HIV Clinic Cohort (Joseph Eron, Sonia Napravnik)
University of Washington HIV Cohort (Mari Kitahata)
Veterans Aging Cohort Study (Amy Justice, David Fiellin)
Vanderbilt-Meharry CFAR Cohort (Timothy Sterling, Sam Stinette, Peter Rebeiro, David Haas)
Women's Interagency HIV Study (Stephen Gange, Kathryn Anastos)
Footnotes
Executive Committee: Richard Moore, Michael Saag, Stephen Gange, Mari Kitahata, Rosemary McKaig, Aimee Freeman
Epidemiology/Biostatistics Core: Stephen Gange, Alison Abraham, Bryan Lau, Keri Althoff, Jinbing Zhang
Data Management Core: Mari Kitahata, Stephen Van Rompaey, Eric Webster, Brenda Simon
Administrative Core: Richard Moore, Aimee Freeman, Carol Lent
REFERENCES
- 1.Campsmith ML, Rhodes P, Hall HI, Green T. HIV Prevalence Estimates - United States, 2006. MMWR Weekly. 2008 Oct 3;57(39):1073–6. [PubMed] [Google Scholar]
- 2.Public Health Agency of Canada. HIV/AIDS Epi Updates, November 2007 Surveillance and Risk Assessment Division, Centre for Infectious Disease Prevention and Control, Public Health Agency of Canada. 2007 [Google Scholar]
- 3.Hall HI, Song R, Rhodes P, et al. Estimation of HIV incidence in the United States. JAMA. 2008 Aug 6;300(5):520–9. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention Estimates of new HIV infections in the United States. CDC HIV/AIDS Fact Sheet. 2008 August 3; [cited 2009 Oct 12];Available from: URL: http://www.cdc.gov/hiv/topics/surveillance/incidence.htm.
- 5.Centers for Disease Control and Prevention Persons Tested for HIV - United States. MMWR Weekly. 2008 Aug 8;57(31):845–9. [PubMed] [Google Scholar]
- 6.Choudhri Y, Cule S. Factors associated with testing for HIV among females and males in Canada. Can J Infect Dis Med Microbiol. 2009;17(Suppl A):304. [Google Scholar]
- 7.Paltiel AD, Walensky RP, Schackman BR, et al. Expanded HIV screening in the United States: effect on clinical outcomes, HIV transmission, and costs. Ann Intern Med. 2006 Dec 5;145(11):797–806. doi: 10.7326/0003-4819-145-11-200612050-00004. [DOI] [PubMed] [Google Scholar]
- 8.Smit C, Hallett TB, Lange J, Garnett G, de WF. Late entry to HIV care limits the impact of anti-retroviral therapy in The Netherlands. PLoS One. 2008;3(4):e1949. doi: 10.1371/journal.pone.0001949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robbins GK, Spritzler JG, Chan ES, et al. Incomplete reconstitution of T cell subsets on combination antiretroviral therapy in the AIDS Clinical Trials Group protocol 384. Clin Infect Dis. 2009 Feb 1;48(3):350–61. doi: 10.1086/595888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention . HIV counseling, testing and referral standards and guidelines. US Department of Health and Human Services, Public Health Service; Atlanta, GA: 1994. [Google Scholar]
- 11.Revised guidelines for HIV counseling, testing, and referral MMWR Recomm Rep. 2001 Nov 9;50(RR-19):1–57. [PubMed] [Google Scholar]
- 12.Krentz HB, Gill MJ. Cost of medical care for HIV-infected patients within a regional population from 1997 to 2006. HIV Med. 2008 Oct;9(9):721–30. doi: 10.1111/j.1468-1293.2008.00613.x. [DOI] [PubMed] [Google Scholar]
- 13.Gange SJ, Kitahata MM, Saag MS, et al. Cohort profile: the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) Int J Epidemiol. 2007 Apr;36(2):294–301. doi: 10.1093/ije/dyl286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panel on Antiretroviral Guidelines for Adults and Adolescents Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. US Department of Health and Human Services. 2008 [cited 2009 Nov 5];1-139. Available from: URL: http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf.
- 15.Hammer SM, Eron JJ, Jr., Reiss P, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA. 2008 Aug 6;300(5):555–70. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 16.Montaner JSG, IAS-USA Therapeutic guidelines: Antiretroviral treament of adult HIV infection. British Columbia Centre for Excellence in HIV/AIDS. 2009 [Google Scholar]
- 17.Clumeck N, Pozniak A, Raffi F. European AIDS Clinical Society (EACS) guidelines for the clinical management and treatment of HIV-infected adults. HIV Med. 2008 Feb;9(2):65–71. doi: 10.1111/j.1468-1293.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- 18.Gazzard BG. British HIV Association Guidelines for the treatment of HIV-1-infected adults with antiretroviral therapy 2008. HIV Med. 2008 Oct;9(8):563–608. doi: 10.1111/j.1468-1293.2008.00636.x. [DOI] [PubMed] [Google Scholar]
- 19.Hughes MD, Stein DS, Gundacker, HM, Valentine FT, Phair JP, Volberding PA. Wihtin-subject variation in CD4 lymphocyte count in asymptomatic human immunodeficiency virus infection: implications for patient monitoring. J Infect Dis. 1994 Jan;169(1):28–36. doi: 10.1093/infdis/169.1.28. [DOI] [PubMed] [Google Scholar]
- 20.Krawczyk CS, Funkhouser E, Kilby JM, Kaslow RA, Bey AK, Vermund SH. Factors associated with delayed initiation of HIV medical care among infected persons attending a southern HIV/AIDS clinic. South Med J. 2006 May;99(5):472–81. doi: 10.1097/01.smj.0000215639.59563.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Missed opportunities for earlier diagnosis of HIV infection--South Carolina, 1997-2005 MMWR Morb Mortal Wkly Rep. 2006 Dec 1;55(47):1269–72. [PubMed] [Google Scholar]
- 22.Gay CL, Napravnik S, Eron JJ., Jr Advanced immunosuppression at entry to HIV care in the southeastern United States and associated risk factors. AIDS. 2006 Mar 21;20(5):775–8. doi: 10.1097/01.aids.0000216380.30055.4a. [DOI] [PubMed] [Google Scholar]
- 23.Castel A, Jolaosho T, Woolfork M, Kuo I, West T, Greenberg A. Late to test: Concurrent HIV/AIDS diagnoses in a city with high AIDS prevalence. 543 ed. Washington DC: 2007. 2008. [Google Scholar]
- 24.Keruly JC, Moore RD. Immune status at presentation to care did not improve among antiretroviral-naive persons from 1990 to 2006. Clin Infect Dis. 2007 Nov 15;45(10):1369–74. doi: 10.1086/522759. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention . HIV/AIDS surveillance supplemental report, 2005. 2. Vol. 11. Centers for Disease Control and Prevention; Atlanta: 2005. Reported CD4+ T-lymphocyte results for adults and adolescents with HIV/AIDS - 33 states, 2005. (Report No.). [Google Scholar]
- 26.Plitt SS, Mihalicz D, Singh AE, Jayaraman G, Houston S, Lee BE. Time to testing and accessing care among a population of newly diagnosed patients with HIV with a high proportion of Canadian Aboriginals, 1998-2003. AIDS Patient Care STDS. 2009 Feb;23(2):93–9. doi: 10.1089/apc.2007.0238. [DOI] [PubMed] [Google Scholar]
- 27.Krentz HB, Auld MC, Gill MJ. The high cost of medical care for patients who present late (CD4 <200 cells/microL) with HIV infection. HIV Med. 2004 Mar;5(2):93–8. doi: 10.1111/j.1468-1293.2004.00193.x. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention . HIV/AIDS surveillance report, 2008. Centers for Disease Control and Prevention; Atlanta: 2008. [Google Scholar]
- 29.Late versus early testing of HIV--16 Sites United States, 2000-2003. MMWR Morb Mortal Wkly Rep. 2003 Jun 27;52(25):581–6. [PubMed] [Google Scholar]
- 30.Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009 Apr 30;360(18):1815–26. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia PM, Kalish LA, Pitt J, et al. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. Women and Infants Transmission Study Group. N Engl J Med. 1999 Aug 5;341(6):394–402. doi: 10.1056/NEJM199908053410602. [DOI] [PubMed] [Google Scholar]
- 32.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000 Mar 30;342(13):921–9. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 33.Modjarrad K, Chamot E, Vermund SH. Impact of small reductions in plasma HIV RNA levels on the risk of heterosexual transmission and disease progression. AIDS. 2008 Oct 18;22(16):2179–85. doi: 10.1097/QAD.0b013e328312c756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. J Acquir Immune Defic Syndr. 2005 Aug 1;39(4):446–53. doi: 10.1097/01.qai.0000151079.33935.79. [DOI] [PubMed] [Google Scholar]
- 35.Klein D, Hurley LB, Merrill D, Quesenberry CP., Jr Review of medical encounters in the 5 years before a diagnosis of HIV-1 infection: implications for early detection. J Acquir Immune Defic Syndr. 2003 Feb 1;32(2):143–52. doi: 10.1097/00126334-200302010-00005. [DOI] [PubMed] [Google Scholar]
- 36.Gandhi NR, Skanderson M, Gordon KS, Concato J, Justice AC. Delayed presentation for human immunodeficiency virus (HIV) care among veterans: a problem of access or screening? Med Care. 2007 Nov;45(11):1105–9. doi: 10.1097/MLR.0b013e3181271476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006 Sep 22;55(RR-14):1–17. [PubMed] [Google Scholar]
- 38.Fincher-Mergi M, Cartone KJ, Mischler J, Pasieka P, Lerner EB, Billittier AJ. Assessment of emergency department health care professionals' behaviors regarding HIV testing and referral for patients with STDs. AIDS Patient Care STDS. 2002 Nov;16(11):549–53. doi: 10.1089/108729102761041100. [DOI] [PubMed] [Google Scholar]
- 39.Lyons MS, Lindsell CJ, Ledyard HK, Frame PT, Trott AT. Emergency department HIV testing and counseling: an ongoing experience in a low-prevalence area. Ann Emerg Med. 2005 Jul;46(1):22–8. doi: 10.1016/j.annemergmed.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 40.Qaseem A, Snow V, Shekelle P, Hopkins R, Jr., Owens DK. Screening for HIV in health care settings: a guidance statement from the American College of Physicians and HIV Medicine Association. Ann Intern Med. 2009 Jan 20;150(2):125–31. doi: 10.7326/0003-4819-150-2-200901200-00300. [DOI] [PubMed] [Google Scholar]


