Introduction
Breast cancer is the most common malignancy in women and is responsible for one of every three cancers in women. Approximately 1.1 million cases are diagnosed worldwide every year with approximately a quarter million of these cases occurring in the USA (Jemalet et al., 2006). Approximately 41,000 women and 460 men are predicted to die from breast cancer each year in the USA. Thus, breast cancer is the one of the most common cancers occurring in women and certainly one of the most feared. The most difficult breast cancers to treat are those that have metastasized. Many patients undergo unnecessary systemic cancer therapy following the removal of a breast tumor because it is difficult to predict whether some tumors have metastasized and the presence of residual cancer initiating cells (CICs). Understanding the process of metastasis and identifying the signal transduction pathways responsible could lead to more effective treatment.
We have identified signaling pathways which play important roles in breast cancer drug resistance, namely the CaM-K, Ras/Raf/MEK/ERK and Ras/PI3K/PTEN/mTOR pathways. All of these pathways can be induced by mutations in upstream cytokine/growth factor receptors genes and the signals transmitted by these receptors are often regulated by Ras which is itself frequently mutated in human cancer. We hypothesize that these pathways play critical roles in breast cancer drug resistance, metastasis and influence the frequency of CICs. By combining small molecule inhibitors which specifically target components of these pathways with classical chemotherapy, we have observed synergistic effects in the induction of death in drug resistant breast cancer cells.
The population of drug resistant breast cells possess more cells with the characteristics of breast cancer CICs; they have higher frequencies of “side population (SP)” cells, CD44↑ CD24↓ cells and frequently give rise to mammospheres. We hypothesize that chemotherapeutic drugs such as doxorubicin activate these pathways. These events can lead to drug resistance and increased metastatic properties. In this manuscript, we will discuss approaches to target the CaM-K, Ras/Raf/MEK/ERK and Ras/PI3K/PTEN/Akt/mTOR signaling pathways, with the ultimate goal of making existing therapies more effective by eliminating the CICs.
CaM-Ks
The CaM-Ks are a group of related kinases activated in response to many different stimuli including: increased Ca2+ levels (Solderling, 1999), phosphatase inhibition, and oxidative stress (Howe et al., 2004). Oxidative stress and reactive oxygen species (ROS) can increase intracellular Ca2+, but they also are able to activate the CaM-Ks in the absence of increases in intracellular Ca2+ (Rodriguez-Mora et al., 2005a). CaM-KI and CaM-KII are expressed in many tissues, whereas the expression of CaM-KIV is more restricted. Multiple genes encode different isoforms of CaM-KII which are designated CaM-KII-α, -β, -δ, and –γ (Rodriguez-Mora et al., 2005b). Maximal activity of CaM-KI, CaM-KII and CaM-KIV requires phosphorylation. The mechanisms by which these enzymes are phosphorylated differs (Tokumistu et al., 1994; Racioppi and Means, 2008). CaM-KII undergoes autophosphorylation whereas CaM-KI and CaM-KIV are phosphorylated by CaM-KK. Once phosphorylated, these kinases retain catalytic activity even in the absence of increased intracellular Ca2+. Protein phosphatase 1 (PP1) and PP2A cleave the phosphate group from CaM-KII and CaM-KIV rendering them inactive (Park and Soderling, 1995; Strack et al., 1997; Westpha, 1998). Binding of Ca2+/calmodulin to CaM-KIV displaces PP2A from CaM-KIV (Racioppi and Means, 2008). Inactivation of these phosphatases is one mechanism by which ROS can activate these kinases.
CaM-K-induced cell signaling
CaM-Ks participate in the activation of multiple signaling pathways including Ras/Raf/MEK/ERK and Ras/PI3K/PTEN/Akt/mTOR (Yano et al., 1998;Solderling, 1999; Rodriguez-Mora et al., 2005a; Howe et al., 2006). ROS also induce ERK and Akt phosphorylation and this is partially under CaM-K control (LaHair et al., 2006) (see Figure 1). CaM-Ks contribute to the activation of the cAMP response element binding protein (CREB) transcription factor, which is also regulated by both the Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways. We and others have demonstrated that both the Ras/Raf/MEK/ERK and Ras/PI3K/PTEN/Akt/mTOR pathways are involved in breast cancer drug resistance (Faridi et al., 2003; Steelman et al, 2008a; Steelman et al., 2008b). CaM-KI, CaM-KII, and CaM-KIV phosphorylate CREB (Tokumitsu et al., 2005; Schneider et al., 2007; Takeda et al., 2007). These data not only demonstrate that CaM-Ks can influence CREB activity but also that they interact with additional signaling pathways involved in drug resistance and metastasis. The Ras/Raf/MEK/ERK and CaM-K pathways interact at multiple levels, including upstream Ras and Src-family tyrosine kinases. Furthermore we demonstrated that CaM-KII is involved in ROS-induced ERK activation in MCF-7 breast cancer cells, which is associated with chemotherapeutic drug resistance (Rodriguez-Mora et al., 2006b).
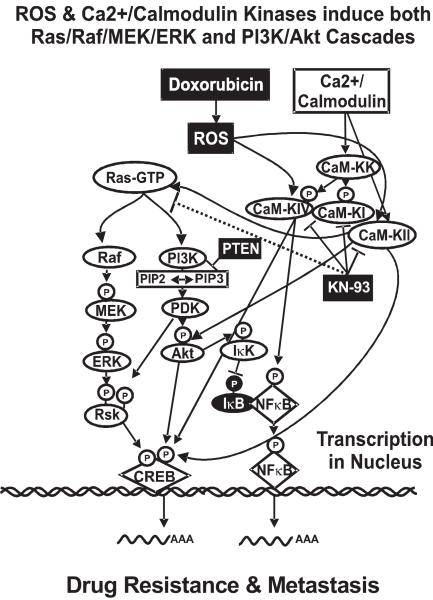
Figure 1. Overview of CaM-K-induced activation of signaling pathways. Legend.
ROS and Ca2+/calmodulin can activate the CaM-K cascade which in turn can activate the Raf/MEK/ERK and PI3K/Akt cascades, often via Ras. The CaM-Ks can be targeted by inhibitors such as KN-93 (inhibits CaM-KI, CaM-KII and CaM-KIV). Inhibition of the CaM-K cascade should result in inhibition of Raf/MEK/ERK and PI3K/Akt cascades. All the kinase cascades have multiple downstream targets, only CREB and NF-κB are shown for simplicity.
CaM-K roles in neoplasia
CaM-Ks are involved in the development of various cancers. Tumor cells of neuronal origin express different variants of the CaM-KII isoforms (γ); in contrast to normal cerebral cortex (α) (Tombes et al., 1997) suggesting that during the neuronal tumorogenesis process, the CaM-KII α isoform is turned off and other isoforms (γ) appear. CaM-KII prevents apoptosis of androgen-dependent prostate cancer cells upon androgen ablation and facilitates the progression to an androgen-independent state (Rokhlin et al., 2007). Hepatocellular carcinomas (HCC) usually develop during chronic liver disease, particularly chronic viral hepatitis. CaM-KK and CaM-KIV are expressed in HCC and detected at elevated levels in tumor tissues compared with normal hepatocytes (Tamura et al., 2000).
MCF-7 breast cancer cells express higher levels of CaM-KII activity when compared to non-transformed breast epithelial cells (Takahashi, 2001). MCF-7 cells, as well as many breast tumors, lack PP2A (Suzuki and Takahashi, 2003). Inhibition/ablation of PP2A activity leads to increases in CaM-KII activity (Howe et al., 2004). The reduction/elimination of PP2A activity may explain the increased CaM-KII activity observed in some breast cancer cells and also the reduced Ca2+ requirement that transformed cells have for cell cycle progression (Gardner et al., 2000; Whitfield, 1992). CaM-KIβ is upregulated during mammary development and it has been proposed that this upregulation may identify the epithelial cell subtype found in breast cancer (Whitfield, 1992; Gardner et al., 2000). Tamoxifen, an important anti estrogen-receptor drug used in hormonal therapy of breast cancer, reduces the incidence of breast cancer and also down-regulates CaM-KII activity (DaSilva et al., 2004). Thus the CaM-K pathway plays key roles in cancer and breast development.
Breast Cancer Metastasis, CICs and CaM-K
Hematogenous metastasis occurs when a cell moves from the primary tumor site to distant places in the body. This type of metastasis is associated with breast cancer as it moves to sites such as the brain, lungs, liver, and most commonly bone. Invasion is the initial step in metastatic behavior and involves the participation of a variety of proteins and cellular functions such as altered cell adhesion, proteolysis and motility. It is likely that both malignant and non-malignant cells contribute to this process at both the primary and secondary sites of tumor development. Metastasizing cells undergo an epithelial-mesenchymal like transition (EMT) (Gavert and Ben-Ze’ev, 2008). EMT is characterized by the loss of epithelial type phenotype including diminished cellular adhesion, decreased E- and β-cadherin expression and increased cellular motility. EMT is also associated with increased mesenchymal-like cellular phenotype which includes: loss of contact inhibition, reorganization of the actin cytoskeleton, increased motility, enhanced invasiveness and increased expression of vimentin and fibronectin. The Ras/Raf/MEK/ERK, Ras/PI3K/PTEN/Akt/mTOR, other signaling pathways and growth factors such as Wnt can also induce EMT. The Ras/Raf/MEK/ERK pathway activates the transcription factors Snail and Slug which are repressors of E-cadherin that can result in EMT (Come et al., 2006). Other transcription factors and growth factors such as Twist and transforming growth factor-β (TGF-β) are involved in EMT (Roberts et al., 2006). TGF-β and Ras cooperate in the induction of EMT. Cell motility or migration is required for the malignant cell to move from the tumor into the bloodstream and is often measured using migration and invasion assays such as the wound healing/scratch and Matrigel assays (Surmacz and Bartucci, 2004; Garcia et al., 2006; Filigheddu et al., 2007; Planas-Silva and Waltz, 2007; Woodward et al., 2007; Detry et al., 2008; Wang et al., 2008). Chemotaxis or motility of breast cancer cells can be stimulated by epidermal growth factor (EGF) (Garcia et al., 2006), insulin-like growth factor-I (IGF-1) (Surmacz and Bartucci, 2004), and additional growth factors (Filigheddu et al., 2007). The ability of estrogen to stimulate motility and EMT in breast cancer cells is controversial (Planas-Silva and Waltz, 2007).
CD24- cells express low levels of epithelial markers such as E- and β-cadherin and higher levels of meschymal markers such as vimentin and fibronectin. This phenotype is often associated with cells undergoing EMT and may be necessary for disruption of cell:cell junctions and actin cytoskeleton reorganization. These events may be associated with EMT and required for loss of contact inhibition and increased motility and invasiveness. Increased radios of CD44↑CD24↓ are often associated with CICs. Ras and TGF-β can drive EMT and potentially also CIC development (Morel et al., 2008).
CaM-KIIδ is another positive regulator of migration (House and Singer, 2008; Mercure et al., 2008). CaM-K inhibition by the small molecule CaM-K inhibitor, KN-93, suppressed human osteosarcoma cells from intratibial and subcutaneous tumor growth in an immunocompromised (nude) mouse model (Yuan et al., 2007). Inhibition of CaM-KII decreased membrane-bound Tiam1 and GTP-bound Rac1, which are involved in tumor growth in a variety of solid malignant neoplasms. Experimental metastasis and CIC generation, in some cases, is enhanced by constitutive activation of the Raf/MEK/ERK pathway (Webb et al., 1998; Morel et al., 2008). Akt also promotes cell survival, migration, invasion, and angiogenesis (Chen et al., 2008; Jiang and Liu, 2008) and decreased Akt expression reduces adhesion, motility in wound healing assays, and metastasis in vivo (Wang et al., 2008).
CaM-Ks can influence NF-κB activity which leads to enhanced motility and metastasis of breast cancer cells through repression of E-cadherin (Criswell and Arteaga, 2007). The expression of bone sialoprotein is under the control of the CREB and AP-1 transcription factors (Detry et al., 2008). CREB can promote metastasis by stimulating matrix metallo-proteinase-2 (MMP2) expression (Melnikova et al., 2006). Expression of a dominant negative (DN) form of CREB prevents metastasis of melanoma cells (Xie et al., 1997).
In summary, while CaM-Ks are not commonly thought as key therapeutic targets in cancer, it has become clear that they play important roles in the regulation of many downstream pathways which are frequently implicated in cancer. Moreover, CaM-Ks are often activated by ROS which are induced by many common chemotherapeutic drugs, thus their deregulation could serve to drive chemotherapeutic drug resistance and CIC formation.
Ras/Raf/MEK/ERK signaling pathway
Downstream of cytokine/growth factor receptors and in some cases the CaM-Ks, lies the Ras/Raf/MEK/ERK cascade, which consists primarily of a series of serine/threonine (S/T) and sometimes upstream tyrosine (Y) kinases (see below). Cytokines trigger and activate cell membrane receptors and the Ras/Raf/MEK/ERK pathway relays these messages from the cytoplasm to the nucleus to control gene expression. Furthermore, this pathway also regulates the activity of, and subcellular localization of, many apoptotic molecules which are normally localized and function in the mitochondrion.
After growth factor/cytokine/mitogen stimulation of the appropriate (cognate) receptor, a Shc adaptor protein becomes associated with the C-terminus of the specific growth factor receptor activated (e.g., vascular endothelial growth factor receptor [VEGFR], epidermal growth factor receptor [EGFR], insulin like growth factor-1 receptor [IGF-1R] and many others) (Avruch, 2007; Lee et al., 2008). Shc recruits the Grb2 protein and the SOS homolog protein, resulting in the loading of membrane-bound Ras with GTP (Rajalingam et al., 2007). Ras:GTP then recruits Raf to the membrane where it becomes activated, likely via a Src-family tyrosine kinase (Marais et al., 1995; Marais et al., 1997). At this point we will be somewhat generic, although it should be pointed out that both Ras and Raf are members of multi-gene families and there are three Ras members (Ki-Ras, N-Ras and Ha-Ras) (Rajalingam et al., 2007) and three Raf members (B-Raf, Raf-1 [a.k.a c-Raf] and A-Raf) (Marais et al., 1997; Mason et al., 1999). Raf is responsible for S/T phosphorylation of MEK1 (Xu et al., 1995). MEK1 phosphorylates ERK1 and 2 at specific T and Y residues (Xu et al., 1995). Activated ERK1 and ERK2 serine S/T kinases phosphorylate and activate a variety of substrates, including p90Rsk1 (Derijard et al., 1995; Xing et al., 1996; Cardon et al., 1998; Allan et al., 2003; McCubrey et al., 2007;). The number of ERK1,2 targets is easily in the hundreds (>600).
p90Rsk1 can activate the CREB transcription factor (Xing et al., 1996). Activated ERK can also phosphorylate B-Raf, Raf-1 and MEK1 which alter their activity. Depending upon the site phosphorylated on Raf-1, ERK phosphorylation can either enhance (Balan et al., 2006) or inhibit (Dougherty et al., 2005) Raf-1 activity. In contrast, when B-Raf (Brummer et al., 2003) or MEK1 (Catalanotti et al., 2009) are phosphorylated by ERK, their activity decreases. These phosphorylation events serve to alter the stability and/or activities of the proteins. Moreover, activated ERK can translocate to the nucleus and phosphorylate many additional transcription factors, such as Elk-1, CREB, Fos and Gata-1 (Deng and Karin, 1994; Davis, 1995; McCubrey et al., 2000; Aplin et al., 2001; Ponti et al., 2002; Tresini et al., 2001; Towatari et al., 2004), which bind promoters of many genes, including growth factor and cytokine genes that are important in promoting growth and preventing apoptosis of multiple cell types (Wang et al., 1994; Eblen et al., 2001; Adachi et al., 2002; Andrieuz et al., 2007). Under certain circumstances, aberrant regulation of this pathway can contribute to deregulated cellular proliferation which may lead to many abnormalities including, drug resistance and potentially result in the generation/propagation of CICs (Thomas et al., 1997; Deng et al., 2001; Blalock et al., 2003; Carter et al., 2003; Jia et al., 2003; Troppmair and Rapp, 2003). Thus there has been sustained interest in the targeting of this pathway to prevent various proliferative disorders and cancer.
Mutations at the Ras/Raf/MEK/ERK Pathway in Human Cancer
Mutations that lead to the expression of constitutively-active Ras proteins have been observed in approximately 20 to 30% of human cancers (Li et al., 2004; Rajalingam et al., 2007). These are often point mutations that alter Ras activity. Genome RAS amplification or overexpression of Ras, perhaps due to altered methylation of its promoter region, are also detected in some tumors (Rajalingam et al., 2007; Ciuffreda et al., 2009). In cholangiocarcinoma, KRAS gene mutations have been identified in 45% of examined tumors (Flotho et al., 1999; Goel et al., 2005; Dahl and Guidberg, 2007; Rajalingam et al., 2007; Inder et al., 2008). Ras mutations are present in up to 20% of AML (Neubauer et al., 1994; Kiyoi et al., 1999; Nakao et al., 1996; Beghini et al., 2000; Bacher et al., 2006;) are another major cause of activation of this cascade. The frequency of KRAS mutations is very high (~80%) in advanced pancreatic cancers (Leicht et al., 2007; Shaul and Seger, 2007; Rajalingam et al., 2007). Mutations that result in increased Ras activity often perturb the Raf/MEK/ERK cascade (Neubauer et al., 1994; Kiyoi et al., 1999; Nakao et al., 1996; Beghini et al., 2000). The majority of RAS mutations in humans occur in KRAS, which is followed by NRAS (Li et al., 2004; Avruch, 2007; Rajalingam et al., 2007; Dahl and Guidberg, 2007; Inder et al., 2008; Ciuffreda et al., 2009). The mutation rate at HRAS brings up a distant third. RAS mutations (e.g., HRAS) predominantly signal through PI3K, while KRAS mutations signal predominantly via Raf, while NRAS mutations will signal through both Raf and PI3K (Li et al., 2004; Avruch, 2007; Rajalingam et al., 2007; Dahl and Guidberg, 2007; Inder et al., 2008).
Prior to around 2003, it was believed that the RAF oncogenes were not frequently mutated in human cancer. There are three RAF genes in humans, ARAF, BRAF and CRAF encoding three distinct proteins with diverse and common functions. With the advent of improved methods of DNA sequencing, it was demonstrated that BRAF is frequently mutated in melanoma (27 to 70%), papillary thyroid cancer (36 to 53%), colorectal cancer (5 to 22%), cholangiocarcinoma (22%), ovarian cancer (30%), and a small minority of lung cancer patients (1-3%) (Tannapfel et al., 2003; Garnett and Marais, 2004; Davies et al., 2002; Fransen et al., 2004; Wan et al., 2004; Libra et al., 2006). BRAF mutation occurs in approximately 7% of all cancers (Tannapfel et al., 2003; Garnett and Marais, 2004). In contrast, CRAF and ARAF are not believed to be frequently mutated in human cancer. Mutations downstream of Raf in the Ras/Raf/MEK/ERK cascade have not been frequently detected in human cancer.
Ras/PI3K/PTEN/Akt/mTOR Pathway
Phosphatidylinositol-3-kinase (PI3K) is a heterodimeric protein with an 85-kDa regulatory subunit and a 110-kDa catalytic subunit, the isozyme most frequently discussed in the scientific literature is encoded by PIK3CA, however there are other isoforms. PI3K serves to phosphorylate a series of membrane phospholipids including PtdIns(4)P and PtdIns(4,5)P2, catalyzing the transfer of ATP-derived phosphate to the D-3 position of the inositol ring of membrane phosphoinositides, thereby forming the second messenger lipids PtdIns(3,4)P2 and PtdIns(3,4,5)P3. Most often, PI3K is activated via the binding of a ligand to its cognate receptor, whereby p85 associates with phosphorylated tyrosine residues on the receptor via an SH2 (Src-homology 2) domain. After association with the receptor, the p110 catalytic subunit then transfers phosphate groups to the aforementioned membrane phospholipids. It is these lipids, specifically PtdIns(3,4,5)P3, that attract a series of kinases to the plasma membrane thereby initiating the signaling cascade (Toker and Cantley, 1997).
Downstream of PI3K, is the primary effector molecule of the PI3K signaling cascade, Akt/PKB (protein kinase B). Akt was originally discovered as the cellular homologue of the transforming retrovirus AKT8 and as a kinase with properties similar to protein kinases A and C (Staal, 1987; Staal and Hartley, 1988). Akt contains an amino-terminal pleckstrin homology (PH) domain that serves to target the protein to the membrane for activation (Franke et al., 1995; Datta et al., 1995; Franke et al., 1997). Within its central region, Akt has a large kinase domain and is flanked on the carboxy-terminus by hydrophobic and proline-rich regions (Coffer and Woodgett, 1991; Bellacosa et al., 1991). Akt is activated via phosphorylation of two residues: T308 and S473.
The phosphotidylinositide-dependent kinases (PDKs) are responsible for activation of Akt. PDK1 is the kinase responsible for phosphorylation of T308 (Alessi et al., 1997). Akt is also be phosphorylated by the mTOR complex referred to as (Rapamycin-insensitive companion of mTOR/mLST8 complex) mTORC2 (see below) (Martelli et al., 2009, Plas and Thomas, 2009). Before its discovery, the activity responsible for this phosphorylation event was called PDK2. Phosphorylation of Akt is complicated as it is phosphorylated by a complex which lies downstream of itself. Moreover, it can be dephosphorylated by events mediated by p70S6K which also lies downstream of Akt (Plas and Thomas, 2009). Once activated, Akt leaves the cell membrane to phosphorylate intracellular substrates.
Negative regulation of the PI3K pathway is primarily accomplished through the action of the phosphatase and tensin homologue deleted on chromosome ten (PTEN) tumor suppressor protein. PTEN encodes a lipid and protein phosphatase whose primary lipid substrate is PtdIns(3,4,5)P3. The protein substrate(s) of PTEN are more varied, including focal adhesion kinase (FAK), the Shc exchange protein and the transcriptional regulators ETS-2 (Weng et al., 2002) and Sp1 (Kang-Park and Lee, 2003). PTEN may also negatively regulate the activation of the platelet-derived growth factor (PDGF) receptor (Mahimainathan and Choudhury, 2004).
mTOR is a 289-kDa S/T kinase (Ligresti et al., 2009). It regulates translation in response to nutrients/growth factors by phosphorylating components of the protein synthesis machinery, including p70S6K and eukaryotic initiation factor (eIF)-4E binding protein-1 (4EBP-1), the latter resulting in release of the eukaryotic initiation factor-4E eIF-4E, allowing eIF-4E to participate in the assembly of a translational initiation complex (Wendel et al., 2004). p70S6K, which can also be directly activated by PDK-1, phosphorylates the 40S ribosomal protein, S6, leading to active translation of mRNAs (Ligresti et al., 2009). Integration of a variety of signals (mitogens, growth factors, hormones) by mTOR assures cell cycle entry only if nutrients and energy are sufficient for cell duplication (Fingar and Blenis, 2004; Tokunaga et al., 2004).
Mutations of the PI3K/PTEN/Akt/mTOR Pathway in Human Cancer
The PI3K/PTEN/Akt/mTOR pathway is often aberrantly expressed in human cancer. Various components of this pathway are frequently mutated in human cancer (Steelman et al., 2008a; Steelman et al., 2008c; Ligresti et al., 2009). The gene encoding the catalytic subunit PI3K, PIK3CA, is one of the most commonly mutated genes in breast cancer (Ligresti et al., 2009). The PTEN gene is frequently mutated, silenced or deleted in many cancers including: glioblastoma, hepatocellular carcinoma (HCC), lung carcinoma, melanoma, endometrial carcinoma and prostate cancer (Easton and Houghton, 2006; Steelman et al., 2008c). The Akt gene it mutated in about 8-10% of breast cancer (Steelman et al., 2008c). Both the PI3K and Akt genes are amplified in certain cancers which increases the levels of these kinases. It has recently been demonstrated that there are other kinases besides Akt (e.g., the serum glucocorticoid kinase-3 [SGK3]) which may be activated in cells with mutant PIK3CA (Easton and Houghton, 2006). SGK3 is regulated by both PDK1 and potentially mTORC2.
Other downstream components of this pathway are mutated in human cancer. The tumor suppressor serine/threonine kinase 11 (STK1/LKB1), which activates the adenosine monophosphate (AMP)-dependent kinase (AMPK) that normally functions to inhibit mTOR via the tuberous sclerosis complex protein 2 (TSC2), is mutated in patients with Peutz-Jeghers syndrome (Shaw et al., 2004; Easton and Houghton, 2006). Patients with this syndrome display elevated levels of mTOR activity under low energy conditions. The tumor suppressor TSC2 gene is mutated in patients with tuberous sclerosis syndrome (Inoki et al., 2003; Liu et al., 2006). These TSC2-mutant patients have elevated mTOR activity under hypoxic conditions. Patients with TSC2 mutations have well-vascularized hamartomas and increased risk of renal ccll carcinoma (RCC) (Yeung et al., 1994; Kwiatkowski, 2003).
Small molecule inhibitors to PI3K, Akt and mTOR have been developed. The most well established and clinically investigated inhibitor, is Rapamycin, a mTOR inhibitor. Various modifications of Rapamycin have been developed and are generically referred to as Rapalogs. Various Rapalogs are being used to treat certain cancer patients, e.g., RCC patients as these tumors are highly dependent on mTOR signaling. Rapalogs are also being clinically tested with many other tumor types, including breast cancers. Rapalogs have even proven effective in the eliminations of CICs in certain experimental models.
Overview of Cancer Initiating Cells
Perhaps the Holy Grail in cancer therapy today, is the cancer stem cell (CSC), (a.k.a. the cancer-initiating cell, CIC). Tumors possess a minor fraction of CSC, which maintain the propagation of the disease. In many cancer types, it is difficult to completely eliminate the CIC and reoccurrence of the cancer usually occurs. This field dates to 1994 when Dick and colleagues disassociated what were initially called leukemia stem cells (LSCs) from the bulk of acute myeloid leukemia (AML) cells (Lapidot et al., 1994). The LSC nomenclature is still used sometimes today, however, some scientists argue that the LSC are not true stem cells, that is why it is perhaps more appropriate to refer to them as CICs. Immature LSCs reside within the CD34+CD38− subpopulation and are only a small fraction of the total leukemic blasts. Importantly, LSCs were able to transmit AML to non-obese diabetic-severe combined immunodeficient (NOD-SCID) mice and repopulate the bone marrow of the irradiated recipients (Bonnet and Dick, 1997). In contrast, CD34+CD38+ AML cells were not competent in engrafting in NOD-SCID mice. NOD-SCID mice were shown to be superior in comparison to SCID mice for the engraftment of AML and hence continue to be used extensively to investigate the differences between normal and LSC (Krause and Van Etten, 2007).
The work performed in the Dick laboratory, along with similar studies by other investigators led to the formulation of the cancer stem-cell hypothesis, which postulates that tumors are maintained by a small minority of stem-like cancer cells, which possess the capacity for indefinite self-renewal. Functionally, CICs are often defined by their capacity to transfer the malignancy to NOD-SCID mice. Since this initial discovery, CICs have been documented in several other cancers including solid tumors such as breast cancers (Krause and Van Etten, 2007). The studies on LSCs and CICs have revolutionized current perceptions regarding cancer occurrence and therapeutic options.
The genetic/epi-genetic mechanisms responsible for CICs remain under intense research. Many different types of events could occur which result in CICs including, point mutations, deletions, translocations, amplifications, methylation, abnormal microRNA expression and other events which must be propagated in the CIC, by various mechanisms including asynchronous cell division. Regardless of knowing the precise cell type where the original genetic mutation occurred, CICs likely pose both the key and barrier to discovering effective cancer therapies. Although CICs have the potential for self-renewal, they spend the majority of their time in the G0 phase of the cell cycle. This means that many chemotherapeutic drugs, which act on cycling cell populations are less effective on CICs than regular cancer cells, which are sometimes referred to as bulk cancer cells (BCS) (Ravandi and Estrov, 2006). The quiescent, non-cycling state of CICs may contribute to their resistance to conventional chemotherapeutics. Conventional chemotherapeutic drugs that target BCS have been shown to be ineffective in completely eradicating CICs. This quiescent nature of CICs may result in low rates of long-term remission and multi-drug resistance.
Targeting CICs Based on Altered Drug Transporter Expression
Cells develop drug resistance via a variety of mechanisms that include: decreased drug uptake, increased drug efflux, accelerated detoxifiation, defective apoptosis or altered expression of signaling pathways (Gal et al., 2006). ATP-dependant drug efflux has been linked to the increased expression of ATP-binding cassette (ABC) transporter proteins (de Jong-Peeters et al., 2007).
Within the ABC family of active transporters, at least forty-nine separate transmembrane proteins have been described (de Jong-Peeters et al., 2007). These transporters are expressed in almost all cells and play important roles in normal physiology by transporting a variety of nutrients and biologically active substances across cellular barriers. This family is divided into seven subfamilies: ABCA through ABCG. Several of these genes including ABCG1 and ABCB1 are expressed in immature CD34+CD38− sub-populations and are down-regulated upon differentiation into the CD34+CD38+ sub-population. Studies by de Grouw et al., have demonstrated that twenty-two ABC transporters were differentially expressed in AML LSCs versus AMLs and all were expressed at lower levels in CD34+CD38+ cells in comparison to CD34+CD38− cells (de Grouw et al., 2006). Of these sub-populations, ABCB1, ABCG2 and ABCC1 are the three primary multi-drug resistant genes that have been detected to be expressed most frequently in tumor cells. Included in this sub-group are MDR1 (multi-drug resistance-1), BCRP (breast cancer resistance protein) and multi-drug resistance association protein (MRP1) (de Jong-Peeters et al., 2007). The proteins involved in drug transport have been well documented to confer drug resistance by mediating the active efflux of many anti-cancer drugs (Krause and Van Etten, 2007).
MDR1 overexpression specifically often causes resistance to amphipathic drugs such as paclitaxel and anthracyclines, including doxorubicin (de Grouw et al., 2006). Additionally, its expression is higher in patients with secondary leukemias as compared with those in primary disease states (Ross, 2000). In response, several drugs have been examined that block and compete with the Pgp (MDR1) mediated drug efflux. Unfortunately, the treatment options with the current MDR1 inhibitors have thus far been ineffective (de Fisher and Sikic, 1995; Ross, 2000; Ross, 2004; Grouw et al., 2006; de Jong-Peeters et al., 2007). Second generation MDR modulators have yielded similar results (Greenberg et al., 2004). This may result from MDR1 not being the unique transporter the cell uses against chemotherapeutic drugs and suggests that additional agents that inhibit multiple transporter and other modes of drug-resistance would be more effective. Upon examination of the expression of various ABC transporters, a high redundancy was observed in both normal and early AML cells (de Grouw et al., 2006). These results suggest that current MDR modulators may be ineffective due to the presence of multiple transporters, which can efflux the same drug, thus making targeting of a single transporter ineffective. Additionally, it is possible that irrelevant transporters were inhibited or there were pharmacokinetic interactions between the chemotherapeutic agent and the ABC transporter inhibitor. Third generation MDR modulators that are more powerful are currently being developed and examined in clinical settings (de Jonge-Peeters et al., 2007).
This ability to efflux many drugs has been practically exploited in the isolation of CICs. Many ABC transporters expressed in CICs efflux the fluorescent dyes Hoechst-33342 and rhodamine 123. This is in contrast to non CICs, which retain the dyes. After Hoechst-33342 staining and flow cytometric analysis, a large percentage of CICs have been shown to reside in the side population (SP) cells. Additionally these SP cells were found in the bone marrow of over 80% of AML patients and many other cancer patients (de Jonge-Peeters et al., 2007).
The PI3K/PTEN/Akt/mTOR has been determined to be consistently activated in AML cell lines and strongly contributes to the proliferation, survival and drug resistance (Martelli et al., 2006). Additionally, its upregulation has been specifically observed in LSCs transplanted in NOD-SCID mice and shown to provide an anti-apoptotic response (Xu et al., 2005). While this upregulation is associated with increased drug resistance and a poor prognosis, a surprising new study demonstrated the upregulation of this pathway is actually a favorable factor in de novo cases of AML (Tamburini et al., 2007). This is perhaps because the upregulation of this pathway moves immature leukemic cells, including LSCs, into S phrase and therefore makes them more susceptible to certain chemotherapeutic agents, especially those targeting actively replicating cells (Martelli et al., 2007). This has also been observed in certain experimental situations in PTEN-knock-out mice (see below).
Furthermore, inhibitors of mTOR show promise in CIC therapy. These inhibitors used in conjunction with conventional therapies induce apoptosis and specifically have been shown to reduce the abundance of CICs (Krause and Van Etten, 2007). Upstream of Akt, there also exists the tumor suppressor PTEN, which has been a further point of intervention as it is frequently mutated or silenced in human cancer (Martelli et al., 2007).
Additionally, recent evidence indicates a relationship between the PI3K/PTEN/Akt/mTOR pathway and expression of the multidrug resistance-associated protein (MRP1), another ABC transporter protein that is also linked with multidrug resistance. Upregulation of this pathway was found to increase levels of the MRP1 protein (Tazzari et al., 2007). The data suggests a p53-dependant mechanism of MRP1 since the inhibition of MRP1 was linked with a concurrent increase in p53 levels. Inhibition of the ubiquitous ligase, MDM-2, with Nutlin-3a increases p53 stability and promotes apoptosis of leukemia which are WT at p53 (Rortul et al., 2003; Xu et al., 2003; Kojima et al., 2006a; Kojima et al., 2006b; Pederson-Bjergaard et al., 2006; Pluta et al., 2006; Pederson-Bjergaard et al., 2006; Pluta et al., 2006; Kojima et al., 2007; Janz et al., 2007; Lehman et al., 2007; Doepfner et al., 2007; Longo et al., 2007; Steelman et al., 2008a). p53 has been shown to be an important target in cancer. Additionally, the connection between MRP1 and this pathway show another possible reason for multidrug resistance in CICs.
This connection between multidrug resistance with the PI3K/PTEN/mTOR/Akt pathway also substantiates the potential of targeting this cascade. Studies using inhibitors to PI3K such as Wortmannin and LY294002 in combination with conventional chemotherapeutic drugs demonstrated an increased sensitivity of the cells to more readily undergo apoptosis (Bortul et al., 2003; Xu et al., 2003). Furthermore, since normal progenitor cells are less affected by these inhibitors, the potential to selectively target CICs and reduce toxicity is a possibility (Martelli et al., 2007; McCubrey et al., 2008b).
Targeting CICs by Inhibition the PI3K Pathway—Importance of PTEN
Recent advances have highlighted extensive phenotypic and functional similarities between normal stem cells and cancer stem cells. This raises the question of whether disease therapies can be developed to eliminate CICs without eliminating normal stem cells.
Components of the PI3K pathway, including PTEN, Akt and mTOR are critical regulators of both normal stem cell function and tumorigenesis. Intriguingly, inactivation of some pathway components, like PTEN has opposite effects on normal hematopoietic stem cells and CICs. Therefore, mechanistic differences between normal stem cells and cancer stem cells can thus be targeted to deplete cancer stem cells without damaging normal stem cells.
PTEN functions as a negative regulator of the PI3K pathway which has crucial roles in cell proliferation, survival, differentiation and migration. Pten is the most frequently mutated gene in human cancers, and is inactivated by a variety of mechanisms (Di Cristofano and Pandolfi, 2000; Yilmaz et al., 2006). Conditional deletion of Pten tumor suppressor gene in bone marrow HSCs causes their short-term expansion, while long-term decline leads to enhanced level of HSC activation (Yilmaz et al., 2006). Pten-deficient HSCs engraft normally in recipient mice, but have an impaired ability to sustain hematopoietic reconstitution, reflecting the dysregulation of their cell cycle and decreased retention in the bone marrow niche. Pten deficiencies have no discernable effect on HSCs differentiation or survival; however, after 3 week of Pten deletion HSCs became depleted. Thus, Pten has essential roles in restricting the activation of HSCs, in lineage fate determination, and in the prevention of leukemogenesis (Yilmaz et al., 2006; Zhang et al., 2006).
In contrast to this requirement for Pten in maintenance of HSCs, CICs arose and expanded in number after Pten deletion. The CICs were transplantable and could be enriched among cells that expressed HSC markers. Most mice died with AML and ALL within 6 weeks of Pten deletion (Yilmaz et al., 2006). Furthermore the CICs were determined to be sensitive to the mTOR inhibitor Rapamycin, while the normal HSCs were not.
It was demonstrated that Pten deletion in prostate epithelium progenitor cells led to senescence response by a p53–mediated mechanism (Zhang et al., 2006). An analogous mechanism could explain the response of HSCs following Pten deletion; moreover mutations that occur during the progression of Pten-deficent cells to leukemia, such as p53, could bypass the senescence response in LSCs. Further investigations could elucidate whether Pten deletion in HSCs induces a senescence response, and whether the p53 pathway might be involved in this mechanism.
Pten deletion leads to increased activation of Akt and mTOR, the mammalian target of rapamycin. Administration of rapamycin, a potent and specific inhibitor of mTOR, to Pten-deleted mice eliminate LSCs and maintained the health of mice, but it also rescued the depletion of Pten-deficient HSCs. Pten-deficient HSCs could provide long-term multilineage reconstitution of irradiated mice as long as the mice continued to be treated with rapamycin. This demonstrates that some effects of Pten deletion were mediated by increased mTOR activation. Therefore CICs could be eliminated by targeting mTOR without compromising normal stem cells.
Using a murine lymphoma model, Lowe and colleagues (Wendel et al., 2004) demonstrated that Akt promotes tumorigenesis and drug resistance by disrupting apoptosis, and that disruption of Akt signaling using the mTOR inhibitor rapamycin reverses chemoresistance in lymphomas expressing Akt, but not in those tumors in which the apoptotic machinery is compromised. Treatment of these mice with doxorubicin together with rapamycin, resulted in a strong tumor remission, lasting more than 60 days (Wendel et al., 2004). Moreover, further evidence suggest that the transcription factor NF-κB functions as an important tumor survival factor by conferring rapamycin-resistance and that concomitant inhibition of NF-κB and mTOR increases cancer cells death (Ghosh et al., 2006). Therefore, rapamycin and its analogues may also result in synergistic interactions with other agents.
These results provide further in vivo validation for a strategy to reverse drug resistance in human cancers and highlight the potential role of translational deregulation in oncogenesis, resistance and CICs, shedding light on the importance of cancer therapy based on tumor genotype.
Targeting Leukemia Stem Cells by Inhibition the Raf/MEK/ERK Pathway
Another well-studied pathway is the Raf/MEK/ERK kinase cascade. It is overexpressed in over 70% of cases of AML (McCubrey et al., 2007). Drugs which target proteins in this pathway are currently being extensively studied with the belief that targeting one point of this pathway will have monumental effects on the entire pathway and its numerous downstream targets. Small molecule weight inhibitors, which target Ras, Raf and MEK, have been developed and recently reviewed (McCubrey et al., 2008). Some of the downstream targets of ERK play critical roles in metastasis. Thus the targeting of this pathway may be an effective approach in cancer therapy.
Materials & Methods
Cell Lines and Growth Factors
Cells were maintained in a humidified 5% CO2 incubator with RPMI-1640 [(RPMI) Invitrogen, Carlsbad, CA, USA] supplemented with 10% fetal bovine serum (FBS) (Atlanta Biologicals, Atlanta, GA, USA).
Analysis of Cell Sensitivity to Anticancer Agents
The sensitivities of the cells to doxorubicin, paclitaxel, daunorubicin, cisplatin or 5-flurouracil were investigated by characterizing the effects of these agents on proliferation as described (Steelman et al 2008b) Inhibitory concentration 50% (IC50) is defined in this context as the drug dose that causes the cells to proliferate at a rate that is half as rapid as cells incubated in the absence of drugs as determined by MTT assays as described (Steelman et al., 2008b).
In order to determine the importance of certain signaling pathways, cells were treated with: the CaM-K inhibitor KN-93, the inactive analogue KN-92, the MEK inhibitor PD0325901, the mTOR inhibitor Rapamycin. These inhibitors were dissolved in dimethyl-sulfoxide (DMSO). In order to ascertain the drug resistance of the cells, they were treated with different concentrations of doxorubicin, paclitaxel, docetaxel, 5-flurouracil, berberine, isoliquiritigenin and 4 hydroxytamoxifen (4HT).
Growth and Isolation of CIC Cells
Bulk tumor cells (BTS) (MCF-7, T47D & MDA-MB-231 cells) were grown for at least two days and the supernatants were collected which contains the CIC fraction. The CICs were collected each time before the BTS were trypsinized. The CIC population was purified from cellular debris present in the supernatants. The supernatants were centrifuged at 400g at 20°C for 10 minutes and then the pellet was resuspended in 2ml of RPMI 1640 and then layered on top of 5ml of Ficoll in a 15ml conical tube which was centrifuged at 400g-600g at 20°C for 30 minutes. Dead cells remained on the bottom of the centrifuge tube and the CICs were contained in the interphase. The CICs were transferred to a new tube and washed twice with cold PBS and centrifuged at 400g at 20°C for 10 minutes. The CICs were then resuspended in phenol red free DMEM/F-12 (50/50) (Cellgro), containing 20 ng/ml EGF and 20 ng/ml basic fibroblast growth factor (bFGF) (Sigma) and standard antibiotics. CICs were recovered from both drug sensitive and resistant MCF-7, T47D and MDA-MB-231 cells. 25nM doxorubicin was included in the culture medium of the CICs isolated from doxorubicin resistant cells. CICs were fed fresh media once a week.
Western blot analysis
Western blots were performed with antibodies specific for phospho or total MEK, ERK, Akt, GSK-3β, p53 as we have previously described. (McCubrey et al., 2008). All antibodies used in this study were purchased from Cell Signaling (Beverly, MA, USA).
Results and Discussion
Isolation of Drug Resistant MCF-7, T47D and MDA-MB-231 Breast Cancer Cells
Drug resistance remains a significant problem in breast cancer therapy as well as in the treatment of many other types of cancer. Developing novel approaches to overcome breast cancer drug resistance could significantly enhance current therapeutic approaches and improve patient care.
We isolated doxorubicin resistant (DoxR) MCF-7, T47D and MDA-MB-231 cells by culturing them in the presence of either 10 or 25 nM doxorubicin for over 2 months. MCF7/DoxR, T47D/DoxR and MDA-MB-231/DoxR cells have increased IC50s to three different drugs examined: doxorubicin, etoposide, and paclitaxel (Table 1).
Table 1. IC50s of Drug Sensitive and Resistant Breast Cells [nM]1.
| Drug [IC50] | MCF-7/ DoxS |
MCF-7/ DoxR |
T47D/ DoxS |
T47D/ DoxR |
MDA- MB- 231/DoxS |
MDA- MB- 231/DoxR |
|---|---|---|---|---|---|---|
| Doxorubicin | 10 nM | 300 nM | 20 nM | 300 nM | 260 nM | 800 nM |
| Fold Increase | - | 30X↑ | - | 15X↑ | - | 3X↑ |
| Etoposide | 700 nM | 5000 nM | 10,000 | >50,000 | 15,000 | >50,000 |
| Fold Increase | - | 7X↑ | >5X↑ | >3X↑ | ||
| Paclitaxel | 7 nM | 200 nM | 6 nM | 300 nM | 5 nM | >300 nM |
| Fold Increase | 29X↑ | 50X↑ | >60X↑ |
MTT analysis was performed with 3 different chemotherapeutic drugs on 3 different drug sensitive and resistant cell lines. Determined by plating 5,000 cells/well in 96 well plates in phenol red free RPMI 1640 + 10% FBS. Serial 2-fold dilutions (n=12 dilutions) at 8 wells per each drug concentration were added after 1 day. MTT analysis was performed after 4 additional days of incubation and results were normalized to untreated cells.
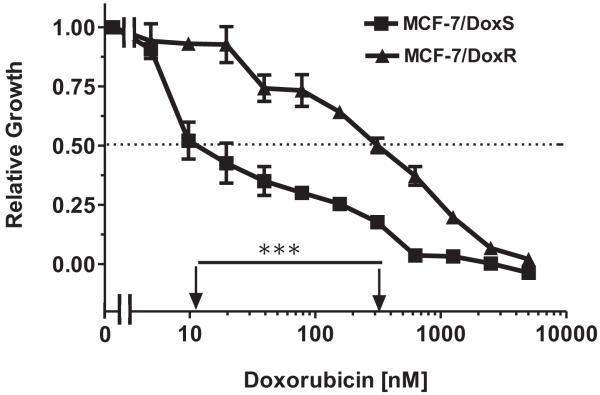
We have chosen doxorubicin (Adriamycin) as the chemotherapeutic drug to concentrate on since it is often administered to breast cancer patients and it produced the most significant killing curves. An example of an MTT curve with doxorubicin with MCF-7/DoxS and MCF-7/DoxR cells is presented in Figure 2.
Figure 2. IC50 analysis of Doxorubicin Sensitive and Resistant Breast Cancer Cells.
MTT analysis was performed with drug sensitive and resistant MCF-7 cell lines. Determined by plating 5,000 cells/well in 96 well plates in phenol red free RPMI 1640 + 10% FBS. Serial 2-fold dilutions (n=12 dilutions) at 8 wells per each drug concentration were added after 1 day. MTT analysis was performed after 4 additional days of incubation and results were normalized to untreated cells. Symbols (■, squares = unselected cells), (▲, triangles = doxorubicin selected cells). Arrows indicate where the IC50 can be estimated. The statistical significance of the IC50s differences were examined by the unpaired t test and are indicated with *** (P <0.001). In those cases where the error bars and not visible, the standard deviation was contained within the size of the error bars. These experiments were repeated over 6 times with 3 different drug resistant pools from each cell line and similar results were observed.
Analysis of Signaling Pathways Activated in Breast Cancer Cells in Response to Doxorubicin
Although there are many different biochemical mechanisms which can result in or contribute to chemotherapeutic drug resistance (e.g., increased expression or altered activity of drug transporters, loss of tumor suppressor gene products and other mechanisms), we have focused our efforts on the analysis of signal transduction pathways which may be altered after chemotherapeutic drug to determine what some of the biochemical consequences are after chemotherapy of breast cancer.
As stated above, the CaM-K pathway is often activated after doxorubicin treatment. We have determined that the CaM-KIV is an isoform that is expressed in breast cancer cells. The CaM-K pathway may contribute to chemotherapeutic drug resistance, by activation of Ras, which can activated both the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR signaling pathways.
The Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR signaling pathways have been reported to be involved in drug resistance in many different tumor types (Steelman et al., 2008b; McCubrey et al., 2006; McCubrey et al., 2006a]. Components of these pathways may be mutated in human cancer which can contribute to abnormal proliferation as well as drug resistance. For example, the PI3K p110 catalytic subunit gene (PIK3CA) gene is mutated in MCF-7 and T47D cells and the BRAF and KRAS genes are mutated in MDA-MB-231 cells (Kozma et al., 1987; Hollestelle et al., 2007). However, these cells are not normally considered as being drug resistant, at least the vast majority of the bulk cancer cells (BCS) presnt in in the populations of these cell lines.
Previously, we have determined that abnormal expression of either the Raf/MEK/ERK or PI3K/PTEN/Akt/mTOR pathways can result in breast cancer drug resistance (Weinstein et al., 2001; Steelman et al., 2008b). However, as stated above, these breast cancer cell lines contain mutations which can contribute to their drug resistance. A key question arises as to how these pathways may be further induced by chemotherapeutic drugs which leads to their elevated expression, as these pathways are frequently active after chemo- and radio-therapy. A key connection between chemotherapeutic drugs and the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways is the CaM-K cascade which when activated by the ROS induced by chemotherapeutic drugs can result in Ras activation which can activate both Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways. Thus we chose to investigate the role of the CaM-K pathway in drug resistance as it can be induced by ROS which are generated after doxorubicin treatment. ROS may lead to activation of both the Raf/MEK/ERK and PI3K/Akt pathways by the common mediators CaM-Ks and Ras (Figure 1).
The effects of doxorubicin on the induction of CaM-KIV, Akt, and MEK/ERK expression and activation were examined by western blot analysis in T47D cells (Table 2). Doxorubicin induced the expression of CaM-KIV in a dose-dependent fashion. 67-fold more CaM-KIV was detected in the 25 nM doxorubicin treated T47D cells than in untreated or 10 nM doxorubicin treated cells. 25 nM Doxorubicin treatment also resulted in elevated levels of activated Akt, MEK and ERK expression (Table 2). High levels of p53 were constitutively detected in T47D cells as they have mutant p53 alleles.
Table 2. Induction of CaM-K and Activated MEK/ERK and Akt Expression by Doxorubicin1.
| Fold Induction after 24 hr Treatment with: | |||
|---|---|---|---|
| Treatment→ | 0 nM Doxorubicin | 10 nM Doxorubicin | 25 nM Doxorubicin |
| Protein Examined↓ | |||
| CaM-KIV | 1 | 0.7X | 67X |
| P-Akt (p473) | 1 | 1 | 2.1X |
| T-Akt | 1 | 1 | 0.9 |
| P-MEK | 1 | 1.5X | 3.4X |
| T-MEK | 1 | 1 | 0.8 |
| P-ERK | 1 | 1.5X | 1.5X |
| T-ERK | 1 | 1 | 1.2 |
| p53 | 1 | 1 | 1.2 |
T47D cells were cultured with the indicated concentrations of doxorubicin for 24 hrs and then western blot analysis was performed with antibodies which recognize phosphorylated (activated) Akt, MEK, and ERK proteins, as well as antibodies which recognize total CaM-KIV, Akt, MEK, ERKs and p53 proteins. The fold-differences in expression were determined by densitometric scanning of the gels followed by normalization to the 0 nM doxorubicin treated samples.
We have previously shown that the CaM-K pathway plays a key role in the response to ROS generated by doxorubicin and H2O2 (Rodriguez-Mora et al., 2005; Jemal et al., 2005; Rodriguez-Mora et al., 2006). We therefore investigated whether there was increased expression of different CaM-K isoforms in the drug resistant breast cancer cells. In the drug resistant cells, we determined that there were higher levels of CaM-KIV expression than in the drug sensitive cells. In contrast, there were relative equal levels of CaM-KII expressed in drug sensitive and resistant cells.
Effects of CaM-K inhibitors on breast cancer cells
Since we observed that doxorubicin induced CaM-KIV expression in T47D cells and we detected elevated CaM-KIV expression in drug resistant cells, we examined the effects the CaM-K inhibitor KN-93 on the sensitivity of drug resistant and sensitive breast cancer cells (Table 3). KN-93 suppresses CaM-KI, CaM-KII and CaM-KIV (Figure 1). The most dramatic results were observed with drug resistant T47D cells as the drug resistant cells were extremely sensitive to KN-93. As a control, we also examined the effects of the inactive KN-92 analogue. KN-92 did not inhibit the growth of the breast cancer cells. These results suggest involvement of the CaM-K pathway in the drug resistance of these breast cancer cells.
Table 3. Effect of CaM-K Inhibitors on Drug Resistant and Sensitive Breast Cancer Cells1.
| IC50→ | |||||
|---|---|---|---|---|---|
| Treatment→ | KN-92 (active) |
KN-92 (inactive analogue) |
Doxorubicin | 500 nM KN- 93 + Doxorubicin |
500 nM KN- 92 + Doxorubicin |
| Cells↓ | |||||
| T47D/DoxS | 5000 nM | >50,000 nM | 20 nM | 4 nM | 40 nM |
| T47D/DoxR | 2500 nM | >50,000 nM | 200 nM | 4 nM | 150 nM |
Drug sensitive and resistant T47D breast cells were treated with various concentrations of the CaM-K inhibitor, KN-93, the inactive analogue KN-92, doxorubicin or with different doses of doxorubicin and a constant, suboptimal (500 nM) dose of either KN-93 or KN-92. MTT analysis was performed in RPMI-1640 medium containing 10% FBS as described in Table 1.
Ability of CaM-K Inhibitors to Increase the Sensitivity to Doxorubicin
To determine whether the CaM-K inhibitors could synergize with doxorubicin in inducing cell death, a suboptimal concentration of the CaM-K inhibitor (500 nM KN-93) was added with different doses of doxorubicin (Table 3). KN-93 synergized with doxorubicin in the drug resistant cells. Essentially, addition of the suboptimal dose of KN-93 completely eliminated the drug resistance to T47D cells, suggesting the involvement of the CaM-K pathway in the resistance to doxorubicin in these cells. Results with T47D cells are presented in Table 3. Similar results were obtained with MCF-7 and MDA-MB-231 cells. In contrast, the structurally-related, but inactive, KN-92 compound did not synergize with doxorubicin to inhibit the growth of the breast cancer cells. The degree of synergy was calculated with the Calcusyn program. Combination indexes (CI) <1 indicates synergy, CI > 1 indicates antagonism. With dose of doxorubicin greater than 10 nM, strong synergy between doxorubicin and the CaM-K inhibitor KN-93 were observed. In contrast at lower doses of doxorubicin, antagonism between doxorubicin and the CaM-K inhibitor was observed.
Lack of Requirement of Functional p53 Pathway in Sensitization to Doxorubicin by CaM-K Inhibitors
In cells with wild type (WT) p53, doxorubicin is normally a potent inducer of p53. Induction of the p53 pathway by doxorubicin could potentially synergize with CaM-K inhibitors to induce cell death. However, we observed the effects of the CaM-K inhibitor were not dependent on the p53 gene status, as they occurred in cells with either WT p53 (MCF-7) or mutant p53 (T47D and MDA-MB-231). Doxorubicin is known to induce cell death in cells which have defective p53 genes (p53-independent cell death mediated by doxorubicin) (McCubrey et al., 2008a). Our results indicate that the interactions between doxorubicin and the CaM-K inhibitors appear to be p53-independent.
Analysis of Potential Downstream Pathways Regulated by CaM-K Activation
Previously, we have shown that the CaM-K pathway can regulate Raf/MEK/ERK and PI3K/Akt activation (Franklin et al., 2000; Howe et al., 2002; Jemal et al., 2005; Rodriguez-Mora et al., 2006; LaHair et al., 2006). We have also determined that ectopic expression of either constitutively active (CA) Raf-1 or dominant negative (DN) PTEN (which leads to higher levels of activated Akt), confers drug resistance to breast cancer cells (Weinstein-Oppenheimer et al., 2001; Davis et al., 2003; Steelman et al., 2008). As both ERK and Akt have been implicated in breast cancer drug resistance, the levels of activated ERK and Akt were examined in the drug sensitive and resistant cells after doxorubicin treatment by western blot analysis (Table 4). Higher levels of activated Akt (2.3 to 3.5-fold) were detected in the T47D cells resistant to 25 nM doxorubicin. Increased levels (2.7 to 3.2-fold) of the downstream Akt target, GSK-3β (S9-phosphorylated) were observed in the T47D cells resistant to 25 nM doxorubicin. Doxorubicin induced ERK in the drug sensitive T47D cells (2.6 to 3.4-fold) and approximately 3-10-fold higher levels of activated ERK were detected in the drug resistant T47D cells than in the corresponding drug sensitive cells (comparison of similarly treated cells) (Table 4). The drug resistant T47D cells expressed higher levels of active Akt and phosphorylated (inactivated) GSK-3β than the drug sensitive T47D cells. In addition higher levels of active ERK were detected in the drug resistant T47D cells than in the drug sensitive cells. As a control, the levels of p53 were examined in this p53 mutant cell line and the p53 levels were high in the absence and presence of doxorubicin, however, the levels of p53 actually did increase upon addition of doxorubicin. Thus doxorubicin is still increasing p53 levels, probably via transcriptional and post-translational mechanisms in cells with mutant p53. These experiments differed from those presented in Table 2, as the results presented in Table 4 were obtained with drug sensitive and resistant T47D cells while the resulted presented in Table 2 were obtained from drug sensitive T47D cells.
Table 4. Elevated MEK/ERK and Akt Expression in Doxorubicin-Resistant Cells1.
| Cell line resistance properties→ |
T47D/DoxS | T47D/DoxR (10 nM) |
T47D/DoxR (25 nM) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Fold Induction after 24 hr Treatment with: | |||||||||
| Treatment→ | 0 nM Dox |
10 nM Dox |
25 nM Dox |
0 nM Dox |
10 nM Dox |
25 nM Dox |
0 nM Dox |
10 nM Dox |
25 nM Dox |
| Protein Examined↓ |
|||||||||
| P-Akt | 1 | 1.2X | 1.2X | 1.5X | 1.2X | 1.2X | 2.3X | 3X | 3.5X |
| T-Akt | 1 | 0.9X | 0.8X | 0.8X | 0.7X | 0.7X | 1.0X | 1.1X | 1X |
| P-GSK-3β | 1 | 1X | 1X | 1.3X | 1.8X | 1.9X | 3.1X | 2.7X | 3.2X |
| T-GSK-3β | 1 | 1.4X | 1.2X | 1.1X | 1.2X | 1.4X | 1.3X | 1.1X | 1.1X |
| P-ERK | 1 | 2.6X | 3.4X | 10X | 11.4X | 11.6X | 6.4X | 8.1X | 9.1X |
| T-ERK | 1 | 1X | 1.1X | 1X | 1X | 1.1X | 0.9X | 0.9X | 0.8X |
| p53 | 1 | 1.6X | 1.6X | 0.8X | 0.8X | 0.9X | 1.5X | 1.7X | 1.8X |
Doxorubicin sensitive (DoxS) and resistant (DoxR) T47D cells selected in either 10 nM or 25 nM doxorubicin were collected after trypsin treatment, and plated in 100 mm dishes with RPMI + 10 % FBS for 24 hrs. The cells were then treated with 0, 10 or 25 nM doxorubicin for 24 hrs. Lysates were then prepared and western blot analysis performed. The fold-differences in expression were normalized to the 0 nM doxorubicin treatment in the DoxS cells are presented above each lane and were determined as described in Table 2.
Role of CaM-K in Control of ERK Expression
The effects of CaM-K inhibition on activation of ERK following either FBS or doxorubicin treatment were examined in drug sensitive and resistant MCF-7 cells (Table 5). Treatment of FBS-depleted (charcoal stripped to remove E2) cells with either FBS or doxorubicin resulted in the induction of active ERK. 2.8-fold more ERK was detected in the drug sensitive cells after FBS stimulation, while 69-fold more ERK was detected after similar stimulation of the drug resistant cells. A higher fold induction of activated ERK was detected after doxorubicin treatment of drug resistant cells (60-fold) than drug sensitive cells (1.5-fold).
Table 5. Role of CaM-K Expression in ERK Activation1.
| Pre- Treatment (1hr) |
DMSO | DMSO | DMSO | KN-93 | KN-93 | KN-93 |
|---|---|---|---|---|---|---|
| Post- Treatment (1 hr) |
PBS | FBS | Dox | PBS | FBS | Dox |
| Fold Stimulation in Doxorubicin Sensitive Cells | ||||||
| P-ERK | 1X | 2.8X | 1.5X | 1X | 3.3X | 0.1X |
| T-ERK | 1X | 1X | 1.2X | 1X | 1X | 1.4X |
| Fold Stimulation in Doxorubicin Resistant Cells | ||||||
| P-ERK | 1X | 69X | 60X | 1X | 2.5X | 1.1X |
| T-ERK | 1X | 0.9X | 0.9X | 1X | 1.4X | 0.7X |
DoxS and DoxR breast cancer cells cells were collected after trypsin treatment and plated out in RPMI + 10% FBS in 100 mm dishes to allow adherence. 24 hrs later the monolayers were washed twice with PBS and then phenol red free RPMI + 0.5% charcoal stripped FBS was added to the monolayers. The cells were incubated for an additional 48 hrs and then treated with either DMSO or 10 μM CaM-K inhibitor KN-93 for 1 hour before treatment with PBS, 10% FBS or 100 nM doxorubicin for 1 hr. Lysates were then collected and western blots performed and probed with the indicated antibodies. The fold-differences in expression are normalized to the PBS treatment and were determined as described in Table 2.
In the drug sensitive cells, CaM-K suppression prevented induction of ERK approximately 15-fold in response to doxorubicin but not in response to FBS (right panel) (when compared to DMSO treated cells). In contrast in the drug resistant cells, CaM-K inhibition resulted in decreased ERK induction in response to either FBS (approximately 28-fold) or doxorubicin (55-fold) treatment (when compared to DMSO treated cells). These results suggest both that drug sensitive and resistant cells were utilizing the CaM-K pathway to activate ERK in response to doxorubicin treatment. Moreover, these results suggested that the ability of serum growth factors, present in FBS, to induce ERK in the drug resistant cells, but not in the drug sensitive cells, was dependent upon the CaM-K pathway.
Characterization of CICs Isolated From Drug Resistant Breast Cancer Cell Lines
We have estimated the size of the CIC component in the three different breast cancer cell lines: MCF-7, T47D and MDA-MB-231 based on Hoechst dye exclusion, CD44+/CD24−/low expression and their sensitivity to doxorubicin. Doxorubicin sensitive and resistant MCF-7, MDA-MB-231, and T47D cells were maintained in a humidified 5% CO2 incubator at 37°C. We noticed that there were often cells present in the doxorubicin treated cultures which resembled CICs based on their non-adherence, mammosphere formation and increased drug resistance phenotypes (see below).
Increased Drug Resistance in Breast Cancer CICs
The sensitivities of the CIC recovered from the doxorubicin resistant cells were compared with doxorubicin resistant and sensitive MCF-7, T47D, and MDA-MB-231 cells (Table 7). The CIC recovered from drug resistant cells were very resistant to doxorubicin.
Table 7. Increased Drug Resistance in CIC Cells1.
| Cell Line↓ | Doxorubcin IC50 | Fold Increase in IC50 in DoxoR vs Unselected |
Fold Increase in IC50 in CIC vs Doxo Resistant |
Fold Increase in IC50 in CIC vs Unselected |
|---|---|---|---|---|
| MCF-7/DoxS | 15 nM | - | - | - |
| MCF-7/DoxR | 250 nM | 17X | - | - |
| MCF-7/DoxR CIC | 4000 nM | - | 26X | 267X |
| T47D/DoxS | 20 nM | - | - | - |
| T47D/DoxR | 80 nM | 4X | - | - |
| T47D/DoxR CIC | 350 nM | - | 4.4X | 18X |
| MDA-MB-231 DoxS |
220 nM | - | - | - |
| MDA-MB-231 DoxR |
1000 nM | 4.5X | - | - |
| MDA-MB-231 DoxR CIC |
>10,000 nM | - | >10X | >45X |
The sensitivities of CIC populations isolated from MCF-7, T47D, and MDA-MB-231 cells to doxorubicin were examined by MTT as described in Table 1. These experiments were repeated over 3 times with 3 different pools of CICs isolated from drug resistant cells and similar results were observed. These experiments were performed in RPMI containing 10% FBS.
CIC cells are reported to be resistant to many different therapeutic approaches. It is important to determine novel methods of targeting CICs. We decided to examine the effects of various different chemotherapeutic and hormonal drugs and signal transduction inhibitors on CICs in comparison to drug resistant cells and BTS. We performed these experiments in the presence of low concentrations of FBS (0.5 % FBS) as our CICs are normally cultured in low concentrations of EGF and FGF. All of the cell growth experiments in this Table 8 were performed on the same day with the same batches of the three different types of cells.
Table 8. Differences in IC50s between MCF-7 DoxS, DoxR and DoxR CICs1.
| Drug | MCF-7/Dox | MCF-7/DoxR | Fold Change in | MCF-7/DoxR | Fold Change |
|---|---|---|---|---|---|
| IC50 | IC50 | IC50 in DoxR vs DoxS |
CIC IC50 |
in IC50 in CIC vs DoxR |
|
| Doxorubicin | 7 nM | 40 Nm | 5.7 X↑ | 800 nM | 20 X↑ |
| Paclitaxel | 0.3 nM | 0.6 Nm | 2 X↑ | 1.8 nM | 3 X↑ |
| Docetaxel | 0.8 nM | 1 nM | 1.3X | 3 nM | 3 X↑ |
| Isoliquiritigenin | 3 nM | 1500 Nm | 500 X↑ | 2000 nM | 1.3 |
| 5-Fluoruracil | 3 nM | 200 nM | 67 X↑ | 30 nM | 67 X↓ |
| 4HT (Tamoxifen) | 0.8 nM | 1.4 nM | 1.8 X↑ | 70 nM | 50 X↑ |
| Berberine | 8 nM | 800 nM | 100 X↑ | 2.5 nM | 320 X↓ |
| MEK Inh. (PD0325901) |
60 nM | 4000 nM | 67 X↑ | 60 nM | 67 X↓ |
| mTOR Inh. (Rapamycin) |
>0.05 nM | 18 nM | 360 X↑ | >0.05 nM | 360 X↓ |
| CaM-K Inh (KN93) |
20 nM | 2500 nM | 125 X↑ | 10 nM | 250 X↓ |
| Inactive CaM-K Inh (KN92) |
2000 nM | 2000 nM | 1X | 2000 nM | 1X |
MTT analysis was performed on the three different types of MCF-7 cells in RPMI + 0.5 % FBS and harvested after 4 days as described in Table 2.
We observed that the CICs isolated from the drug resistant MCF-7 cells (MCF7/DoxR CIC) were indeed more resistant to doxorubicin (Table 8), paclitaxel, 4HT than either the MCF7/DoxS or MCF7/DoxR cells. Furthermore the MCF7/DoxR CIC cells were more resistant to docetaxel and isoliquiritigenin (data not presented). Isoliquiritigenin is a phytoestrogen, and can act (in part) by binding to the estrogen receptor and inhibiting growth (Maggiolini et al., 2002; Ye et al., 2009). MCF-7/DoxS cells were more sensitive to 5-flurouracil (5-FU) than either MCF7/DoxR CIC or MCF7/DoxR cells. Interestingly, the MCF7/DoxR CIC were more sensitive to 5-FU than the MCF7/DoxR cells. It was important to examine the effects of 5-FU as it is used to treat metastatic breast cancer patients (Geisler et al., 2003). These results are summarized in Table 8.
Some of the most interesting results were observed with berberine, a natural product isolated from the flower is named Coptis, also know as Gold Thread (Liu et al., 2009). Berberine is used in traditional Chinese medicine to treat a wide variety of diseases from diabetes to cancer and has recently been investigated in breast cancer (Kim et al., 2008; Kim et al., 2009; Liu et al., 2009). Intriguingly, berberine suppressed the growth of the MCF7/DoxR CIC at lower concentrations than either MCF7/DoxS or MCF7/DoxR (Table 8). Furthermore while we have determined that isoliquiritigenin was more effective on ER+ cells (MCF-7) than ER− cells (MDA-MB-231), berberine was also effective on drug resistant ER-MDA-MB-231 cells.
We next examined the effects of small molecule signal transduction inhibitors on the cells to determine if they exhibited differences in their sensitivities to targeted therapy. Interesting the MCF7/DoxR CICs were as sensitive to the MEK inhibitor PD0325901 as the parental MCF7/DoxS cells. The doxorubicin resistant MCF7/DoxR cells were resistant to this MEK inhibitor when they were cultured in the presence of 0.5 % FBS.
We also examined the effects of targeting either the CaM-K (Table 8) or PI3K/Akt/mTOR with the KN-93 or Rapamycin inhibitors. The MCF7/DoxR CIC cells were more sensitive to the CaM-K and mTOR inhibitors than either the MCF7/DoxS or MCF7/DoxR cells.
As a control, the effects of the structurally-related but inactive CaM-K inhibitor KN92 were examined on these three types of MCF-7 cells (Table 8), the same day as all the other experiments were performed. Importantly and strikingly, this inactive compound did not show any differences between the three different types of MCF-7 cells, indicating that the effects that we were observing were specific to inhibition of particular pathways by the different types of signal transduction inhibitors, chemotherapeutic and hormonal based drugs.
In summary, our results on breast cancer CICs reproduce and extend what others have observed, as the CICs were resistant to common chemotherapeutic and hormonal based drugs normally used to treat breast cancer patients (e.g, doxorubicin, paclitaxel, docetaxel, 5FU, 4HT). However, our results demonstrate that the CICs are more sensitive to certain drugs (e.g., berberine) and signal transduction inhibitors (e.g., CaM-K and mTOR inhibitors) than either drug sensitive or drug resistant cells, implicating the targets of berberine, CaM-K and mTOR may be important in eliminating the CICs.
Summary
Breast cancer is one of the most common cancers and affects nearly 1 in 7 women. We have demonstrated that targeting the CaM-K, Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways may be a novel approach to treat drug resistant breast cancer and eliminate cancer stem cells. Common chemotherapeutic drugs, such as doxorubicin, induce the CaM-K pathway which in turn, leads to activation of anti-apoptotic pathways such as Raf/MEK/ERK and PI3K/Akt. Some drug resistant breast cancers exhibited increased expression of CaM-KIV. CaM-K inhibitors synergized with doxorubicin to induce the death of all drug resistant breast cancers examined. Since CaM-Ks are known to result in activation of the Raf/MEK/ERK and PI3K/Akt pathways, we investigated the roles that these pathways exert in breast cancer drug resistance. CaM-K inhibitors suppressed ERK activation in response to doxorubicin in both drug sensitive and resistant cells. CaM-K inhibitors also suppressed ERK activation in response to FBS in the drug resistant cells suggesting dependence on the CaM-K pathway for proliferation. Both the Raf/MEK/ERK and PI3K/Akt pathways are involved in breast cancer drug resistance as they were detected at elevated, activated levels in the drug resistant cells and introduction of constitutively activated forms of Raf-1 and Akt-1 resulted in drug resistance. Drug resistant CICs were often hypersensitive to MEK and mTOR inhibitors, implicating important roles of these pathways in drug resistance. In summary, targeting these pathways may enhance therapy of drug resistant breast cancer and eliminate CICs.
Breast cancer therapy is often limited by the occurrence of drug resistance which may be due to the re-emergence of CICs. The studies outlined in this proposal may identify a potentially novel role for CaM-Ks in drug resistance and metastasis and may lead to improved approaches to treat breast tumors by eliminating CICs. Our proposed studies are highly innovative as we will determine the involvement of the CaM-K pathway in breast cancer drug resistance, metastasis and CIC formation. Similar approaches have not been previously performed. Our studies may result in the discovery of novel methods to treat breast cancer by targeting the CaM-K pathway in combination with currently used and approved chemotherapeutic regimens to eliminate CICs which may be responsible for both drug resistance and metastasis.
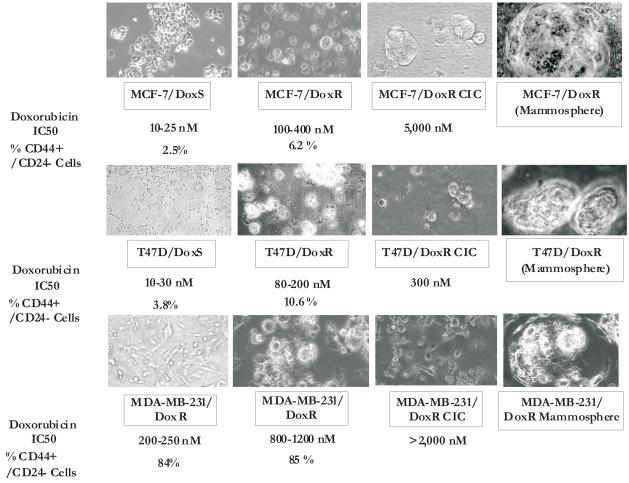
Figure 3. Morphology of DoxS, DoxR and DoxR CIC cells from MCF-7, T47D, and MDA-MB-231 Cells, Their percent CD44+/CD24−/low and Doxorubicin IC50.
With the doxorubicin resistant cells on the far right panels, we attempted to photograph the frequently observed masses of non-adherent cells which resemble mammospheres. Also included in this figure is a summary of their CD44+/CD24−/low expression and their doxorubicin IC50s.
Table 6. Increase in CD44+/CD24-low Phenotype and Mammospheres in Drug Resistant Breast Cancer Cells1.
| % CD44+/ CD24-low |
Fold Increase in Drug Resistant Cells. |
Increased Presence of Mammospheres in Drug Resistant Cells. |
|
|---|---|---|---|
| MCF-7/DoxS | 2.2% | ||
| MCF-7/DoxR | 5.3% | 2.4 X | Yes |
| T47D/DoxS | 3.5% | ||
| T47D/DoxR | 10.2% | 2.9 X | Yes |
| MDA-MB-231 DoxS | 84% | ||
| MDA-MB-231 DoxR | 85% | 1X (no increase) | Yes |
The percentage of CD44+/CD24−/low cells was determined by FACS analysis in doxorubicin sensitive and resistant breast cancer cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi T, Kar S, Wang M, Carr BI. Transient and sustained ERK phosphorylation and nuclear translocation in growth control. J Cell Physiol. 2002;192:151–9. doi: 10.1002/jcp.10124. [DOI] [PubMed] [Google Scholar]
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–9. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- Allan LA, Morrice N, Brady S, Magee G, Pathak S, Clarke PR. Inhibition of caspase-9 by phosphorylation at Thr125 by ERK MAPK. Nature Cell Biol. 2003;5:647–54. doi: 10.1038/ncb1005. [DOI] [PubMed] [Google Scholar]
- Andrieux LO, Fautrei A, Bessard A, Guillouzo A, Baffet G, Langouët S. GATA-1 is essential in EGF-meditated induction of nucleotide excision repair activity and ERCCI expression through ERK2 in human hepatoma cells. Cancer Res. 2007;67:2114–23. doi: 10.1158/0008-5472.CAN-06-3821. [DOI] [PubMed] [Google Scholar]
- Aplin AE, Stewart SA, Assoian RK, Juliano RL. Integrin-mediated adhesion regulates ERK nuclear translocation and phosphorylation of Elk-1. J Cell Biol. 2001;153:273–82. doi: 10.1083/jcb.153.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avruch J. MAP kinase pathways: The first twenty years. Biochem Biophys Acta. 2007;1773:1150–61. doi: 10.1016/j.bbamcr.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacher U, Haferlach T, Schoch C, Kern W, Schnittger S. Implications of NRAS mutations in AML: a study of 2502 patients. Blood. 2006;107:3847–53. doi: 10.1182/blood-2005-08-3522. [DOI] [PubMed] [Google Scholar]
- Balan V, Leicht DT, Zhu J, Balan K, Kaplun A, Singh-Gupta V, et al. Identification of novel in vivo Raf-1 phosphorylation sites mediating positive feedback Raf-1 regulation by extracellular signal-regulated kinase. Mol Biol Cell. 2006;17:141–53. doi: 10.1091/mbc.E04-12-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JE. Early transplantation to a normal microenvironment prevents the development of Steel hematopoietic stem cell defects. Exp Hematol. 1997;25:542–7. [PubMed] [Google Scholar]
- Beghini A, Peterlongo P, Ripamonti CB, Larizza L, Cairoli R, Morra E, et al. c-Kit mutations in core binding factor leukemias. Blood. 2000;95:726–7. [PubMed] [Google Scholar]
- Bellacosa A, Testa JR, Staal SP, Tsichlis PN. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science. 1991;254:274–7. doi: 10.1126/science.254.5029.274. [DOI] [PubMed] [Google Scholar]
- Blalock WL, Navolanic PM, Steelman LS, Shelton JG, Moye PW, Lee JT, et al. Requirement for the PI3K/Akt pathway in MEK1-mediated growth and prevention of apoptosis: Identification of an Achilles heel in leukemia. Leukemia. 2003;17:1058–67. doi: 10.1038/sj.leu.2402925. [DOI] [PubMed] [Google Scholar]
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–7. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- Bortul R, Tazzari PL, Cappellini A, Tabellini G, Billi AM, Bareggi R, et al. Constitutively active Akt1 protects HL60 leukemia cells from TRAIL-induced apoptosis through a mechanism involving NF-κB activation and cFLIP(L) up-regulation. Leukemia. 2003;17:379–89. doi: 10.1038/sj.leu.2402793. [DOI] [PubMed] [Google Scholar]
- Brummer T, Naegele H, Reth M, Misawa Y. Identification of novel ERK-mediated feedback phosphorylation sites at the C-terminus of B-Raf. Oncogene. 2003;22:8823–34. doi: 10.1038/sj.onc.1207185. [DOI] [PubMed] [Google Scholar]
- Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, et al. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–21. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- Carter BZ, Milella M, Tsao T, McQueen T, Schober WD, Hu W, et al. Regulation and targeting of antiapoptotic XIAP in acute myeloid leukemia. Leukemia. 2003;17:2081–9. doi: 10.1038/sj.leu.2403113. [DOI] [PubMed] [Google Scholar]
- Catalanotti F, Reyes G, Jesenberger V, Galabova-Kovacs G, de Matos Simoes R, Carugo O, et al. A Mek1-Mek2 heterodimer determines the strength and duration of the Erk signal. Nat Struct Mol Biol. 2009;16:294–3. doi: 10.1038/nsmb.1564. [DOI] [PubMed] [Google Scholar]
- Chen BP, Fraser C, Reading C, Murray L, Uchida N, Galy A, et al. Cytokine-mobilized peripheral blood CD34 + Thy-1 +Lin-human hematopoietic stem cells as target cells for transplantation-based gene therapy. Leukemia. 1995;9:S17–S25. [PubMed] [Google Scholar]
- Cheng GZ, Zhang W, Wang LH. Regulation of cancer cell survival, migration, and invasion by Twist: AKT2 comes to interplay. Cancer Res. 2008;68:957–60. doi: 10.1158/0008-5472.CAN-07-5067. [DOI] [PubMed] [Google Scholar]
- Ciuffreda L, McCubrey JA, Milella M. Cytoplasmic signaling intermediates PI3K/PTEN/Akt/mTOR and Raf/MEK/ERK pathways as therapeutic targets for anti-cancer and anti-angiogenesis treatments. Current Signal Transduction Therapy. 2009 In Press. [Google Scholar]
- Coffer PJ, Woodgett JR. Molecular cloning and characterisation of a novel putative protein-serine kinase related to the cAMP-dependent and protein kinase C families. Eur J Biochem. 1991;201:475–81. doi: 10.1111/j.1432-1033.1991.tb16305.x. [DOI] [PubMed] [Google Scholar]
- Come C, Magnino F, Bibeau F, DeSanta BP, Becker KF, Theillet C, et al. Snail and slug play distinct roles during breast carcinoma progression. Clin Cancer Res. 2006;12:5395–402. doi: 10.1158/1078-0432.CCR-06-0478. [DOI] [PubMed] [Google Scholar]
- Criswell TL, Arteaga CL. Modulation of NFkappaB activity and E-cadherin by the type III transforming growth factor beta receptor regulates cell growth and motility. J Biol Chem. 2007;282:32491–500. doi: 10.1074/jbc.M704434200. [DOI] [PubMed] [Google Scholar]
- Da Silva ID, Dias-Netto E, Villanova FE, Yamamoto L, Baracat EC, Lima GR, Gebrim LH. Tamoxifen down-regulates CaMKII messenger RNA levels in normal human breast tissue. Clin Exp Obstet Gynecol. 2004;31:204–8. [PubMed] [Google Scholar]
- Dahl C, Guidberg P. The genome and epigenome of malignant melanoma. APMIS. 2007;115:1161–76. doi: 10.1111/j.1600-0463.2007.apm_855.xml.x. [DOI] [PubMed] [Google Scholar]
- Datta K, Franke TF, Chan TO, Makris A, Yang SI, Kaplan DR, et al. AH/PH domain-mediated interaction between Akt molecules and its potential role in Akt regulation. Mol Cell Biol. 1995;15:2304–10. doi: 10.1128/mcb.15.4.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- Davis JM, Navolanic PM, Weinstein-Oppenheimer CR, Steelman LS, Hu W, Konopleva M, et al. Raf-1 and Bcl-2 induce distinct and common pathways that contribute to breast cancer drug resistance. Clin Cancer Res. 2003;9:1161–70. [PubMed] [Google Scholar]
- Davis RJ. Transcriptional regulation by MAP kinases. Mol Reprod Dev. 1995;42:459–67. doi: 10.1002/mrd.1080420414. [DOI] [PubMed] [Google Scholar]
- de Grouw EP, Raaijmakers MH, Boezeman JB, van der Reijden BA, van de Locht LT, de Witte TJ, et al. Preferential expression of a high number of ATP binding cassette transporters in both normal and leukemic CD34+CD38− cells. Leukemia. 2006;20:750–4. doi: 10.1038/sj.leu.2404131. [DOI] [PubMed] [Google Scholar]
- de Jonge-Peeters SD, Kuipers F, de Vries EG, Vellenga E. ABC transporter expression in hematopoietic stem cells and the role in AML drug resistance. Crit Rev Oncol Hematol. 2007;62:214–26. doi: 10.1016/j.critrevonc.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Deng T, Karin M. c-Fos transcriptional activity stimulated by H-Ras-activated protein kinase distinct from JNK and ERK. Nature. 1994;371:171–5. doi: 10.1038/371171a0. [DOI] [PubMed] [Google Scholar]
- Deng X, Kornblau SM, Ruvolo PP, May WS., Jr. Regulation of Bcl2 phosphorylation and potential significance for leukemic cell chemoresistance. J Natl Cancer Inst Monogr. 2001;28:30–7. doi: 10.1093/oxfordjournals.jncimonographs.a024254. [DOI] [PubMed] [Google Scholar]
- Dérijard B, Raingeaud J, Barrett T, Wu IH, Han J, Ulevitch RJ, et al. Independent human MAP kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267:682–5. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- Detry C, Lamour V, Castronovo V, Bellahcene A. CREB-1 and AP-1 transcription factors JunD and Fra-2 regulate bone sialoprotein gene expression in human breast cancer cells. Bone. 2008;42:422–31. doi: 10.1016/j.bone.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Di Cristofano A, Pandolfi PP. The multiple roles of PTEN in tumor suppression. Cell. 2000;100:387–90. doi: 10.1016/s0092-8674(00)80674-1. [DOI] [PubMed] [Google Scholar]
- Doepfner KT, Spertini O, Arcaro A. Autocrine insulin-like growth factor-1 signaline promotes growth and survival of human acute myeloid leukemia cells via the phosphoinositide 3-kinase/Akt pathway. Leukemia. 2007;21:1921–30. doi: 10.1038/sj.leu.2404813. [DOI] [PubMed] [Google Scholar]
- Dougherty MK, Muller J, Ritt DA, Zhou M, Zhou XZ, Copeland TD, et al. Regulation of Raf-1 by direct feedback phosphorylation. Mol Cell. 2005;17:215–24. doi: 10.1016/j.molcel.2004.11.055. [DOI] [PubMed] [Google Scholar]
- Easton JB, Houghton PJ. mTOR and cancer therapy. Oncogene. 2006;25:6436–46. doi: 10.1038/sj.onc.1209886. [DOI] [PubMed] [Google Scholar]
- Eblen ST, Catling AD, Assanah MC, Weber MJ. Biochemical and biological functions of the N-terminal, noncatalytic domain of extracellular signal-regulated kinase 2. Mol Cell Biol. 2001;21:249–59. doi: 10.1128/MCB.21.1.249-259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faridi J, Wang L, Endemann G, Roth RA. Expression of constitutively active Akt-3 in MCF-7 breast cancer cells reverses the estrogen and tamoxifen responsivity of these cells in vivo. Clin Cancer Res. 2003;9:2933–9. [PubMed] [Google Scholar]
- Filigheddu N, Cutrupi S, Porporato PE, Riboni F, Baldanzi G, Chianale F, et al. Diacylglycerol kinase is required for HGF-induced invasiveness and anchorage-independent growth of MDA-MB-231 breast cancer cells. Anticancer Res. 2007;27:1489–92. [PubMed] [Google Scholar]
- Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 2004;23:3151–71. doi: 10.1038/sj.onc.1207542. [DOI] [PubMed] [Google Scholar]
- Fisher GA, Sikic BI. Clinical studies with modulators of multidrug resistance. Hematol Oncol Clin North Am. 1995;9:363–82. [PubMed] [Google Scholar]
- Flotho S, Valcamonica S, Mach-Pascual S, Schmahl G, Corral L, Ritterbach J, et al. RAS mutations and clonality analysis in children with juvenile myelomonocytic leukemia (JMML) Leukemia. 1999;13:32–7. doi: 10.1038/sj.leu.2401240. [DOI] [PubMed] [Google Scholar]
- Franke TF, Kaplan DR, Cantley LC, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665–8. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- Franke TF, Yang SI, Chan TO, Datta K, Kazlauskas A, Morrison DK, et al. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–36. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- Franklin RA, Atherfold PA, McCubrey JA. Calcium-induced ERK activation in human T lymphocytes occurs via p56(Lck) and CaM-kinase. Mol Immunol. 2000;37:675–83. doi: 10.1016/s0161-5890(00)00087-0. [DOI] [PubMed] [Google Scholar]
- Fransén K, Klintenäs M, Osterström A, Dimberg J, Monstein HJ, Söderkvist P. Mutation analysis of the BRAF, ARAF and RAF-1 genes in human colorectal adenocarcinomas. Carcinogenesis. 2004;25:527–33. doi: 10.1093/carcin/bgh049. [DOI] [PubMed] [Google Scholar]
- Gal H, Amariglio N, Trakhtenbrot L, Jacob-Hirsh J, Margalit O, Avigdor A, et al. Gene expression profiles of AML derived stem cells; similarity to hematopoietic stem cells. Leukemia. 2006;20:2147–54. doi: 10.1038/sj.leu.2404401. [DOI] [PubMed] [Google Scholar]
- Garcia R, Franklin RA, McCubrey JA. EGF induces cell motility and multi-drug resistance gene expression in breast cancer cells. Cell Cycle. 2006;5:2820–6. doi: 10.4161/cc.5.23.3535. [DOI] [PubMed] [Google Scholar]
- Gardner HP, Ha SI, Reynolds C, Chodosh LA. The CaM kinase, PNCK, is spatially and temporally regulated during murine mammary gland development and may identify an epithelial cell subtype involved in breast cancer. Cancer Res. 2000;60:5571–7. [PubMed] [Google Scholar]
- Garnett MJ, Marais R. Guilty as charged: B-Raf is a human oncogene. Cancer Cell. 2004;6:313–9. doi: 10.1016/j.ccr.2004.09.022. [DOI] [PubMed] [Google Scholar]
- Gavert N, Ben-Ze’ev A. Epithelial-mesenchymal transition and the invasive potential of tumors. Trends Mol Med. 2008;9:9. doi: 10.1016/j.molmed.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Tergaonkar V, Rothlin CV, Correa RG, Bottero V, Bist P, et al. Essential role of tuberous sclerosis genes TSC1 and TSC2 in NF-kappaB activation and cell survival. Cancer Cell. 2006;10:215–26. doi: 10.1016/j.ccr.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Giesler S, Borresen-Dale AL, Johnsen H, Aas T, Giesler J, Akslen LA, et al. TP53 gene mutations predict the response to neoadjuvant treatment with 5-fluorouracil and mitomycin in locally advanced breast cancer. Clin Caner Res. 2003;9:5582–8. [PubMed] [Google Scholar]
- Goel VK, Lazar AJ, Warneke CL, Redston MS, Haluska FG. Examination of mutations in B-Raf, N-Ras and PTEN in primary cutataneous melanoma. J Inv Dermatology. 2005;126:154–60. doi: 10.1038/sj.jid.5700026. [DOI] [PubMed] [Google Scholar]
- Greenberg PL, Lee SJ, Advani R, Tallman MS, Sikic BI, Letendre L, et al. Mitoxantrone, etoposide, and cytarabine with or without valspodar in patients with relapsed or refractory acute myeloid leukemia and high-risk myelodysplastic syndrome: a phase III trial (E2995) J Clin Oncol. 2004;22:1078–86. doi: 10.1200/JCO.2004.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollestelle A, Elstrodt F, Nagel JHA, Kallemeijn WW, Schutte M. Phosphatidylinositol-3-OH kinase or Ras pathway mutati9ons in human breast cancer cell lines. Mol Cancer Res. 2007;5:195–201. doi: 10.1158/1541-7786.MCR-06-0263. [DOI] [PubMed] [Google Scholar]
- House SJ, Singer HA. CaMKII-delta isoform regulation of neointima formation after vascular injury. Arterioscler Thromb Vasc Biol. 2008;28:441–7. doi: 10.1161/ATVBAHA.107.156810. [DOI] [PubMed] [Google Scholar]
- Howe CJ, LaHair MM, Maxwell JA, Lee JT, Robinson PJ, Rodriguez-Mora O, et al. Participation of the calcium/calmodulin-dependent kinases in hydrogen peroxide-induced Ikappa B phosphorylation in human T lymphocytes. J Biol Chem. 2002;277:30469–76. doi: 10.1074/jbc.M205036200. [DOI] [PubMed] [Google Scholar]
- Howe CJ, Lahair MM, McCubrey JA, Franklin RA. Redox regulation of the calcium/calmodulin-dependent protein kinases. J Biol Chem. 2004;279:44573–81. doi: 10.1074/jbc.M404175200. [DOI] [PubMed] [Google Scholar]
- Inder K, Harding A, Plowman SJ, Philips MR, Parton RG, Hancock JF. Activation of the MAPK module from different spatial locations generates distinct system outputs. Mol Biol Cell. 2008;19:4776–84. doi: 10.1091/mbc.E08-04-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;15:577–90. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- Janz M, Stühmer T, Vassilev LT, Bargou RC. Pharmacologic activation of p53-dependent and p53-independent apoptotic pathways in Hodgkin/Reed-Sternberg cells. Leukemia. 2007;21:772–9. doi: 10.1038/sj.leu.2404565. [DOI] [PubMed] [Google Scholar]
- Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- Jia W, Yu C, Rahmani M, Krystal G, Sausville EA, Dent P, et al. Synergistic antileukemic interactions between 17-AAG and UCN-01 involve interruption of RAF/MEK- and AKT-related pathways. Blood. 2003;102:1824–32. doi: 10.1182/blood-2002-12-3785. [DOI] [PubMed] [Google Scholar]
- Jiang BH, Liu LZ. AKT signaling in regulating angiogenesis. Curr Cancer Drug Targets. 2008;8:19–26. doi: 10.2174/156800908783497122. [DOI] [PubMed] [Google Scholar]
- Kang-Park S, Lee YI. PTEN modulates insulin-like growth factor II (IGF-II)-mediated signaling; the protein phosphatase activity of PTEN downregulates IGF-II expression in hepatoma cells. FEBS Lett. 2003;545:203–8. doi: 10.1016/s0014-5793(03)00535-0. [DOI] [PubMed] [Google Scholar]
- Kim JB, Lee KM, Ko E, Han W, Lee JE, Shin I, et al. Berberine inhibits growth of the breast cancer cell lines MCF-7 and MDA-MB-231. Planta Med. 2008;74:39–42. doi: 10.1055/s-2007-993779. [DOI] [PubMed] [Google Scholar]
- Kim JB, Ko E, Han W, Sahin I, Park SY, Noh DY. Berberine diminishes the side population and ABCG2 transporter expression in MCF-7 breast cancer cells. Planta Med. 2008;74:1693–700. doi: 10.1055/s-0028-1088313. [DOI] [PubMed] [Google Scholar]
- Kiyoi H, Naoe T, Nakano Y, Yokota S, Minami S, Miyawaki S, et al. Prognostic implication of FLT3 and N-RAS gene mutations in acute myeloid leukemia. Blood. 1999;93:3074–80. [PubMed] [Google Scholar]
- Kojima K, Knopleva M, Samudio IJ, Shikami M, Cabbreira-Hansen M, McQueen T, et al. MDM2 antagonists induce p53-dependent apoptosis in AML: implications for leukemia therapy. Blood. 2005;106:3150–9. doi: 10.1182/blood-2005-02-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima K, Konopleva M, McQueen T, O’Brien S, Plunkett W, Andreeff M. Mdm2 inhibitor Nutlin-3a induces p53-mediated apoptosis by transcription-dependent and transcription-independent mechanism and may overcome Atm-mediated resistance to fludarabine in chronic lymphocytic leukemia. Blood. 2006a;108:993–1000. doi: 10.1182/blood-2005-12-5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima K, Konopleva M, Samudio IJ, Schober WD, Bornmann WG, Andreeff M. Concomitant inhibition of MDM2 and Bcl-2 protein function synergistically induce mitochondrial apoptosis in AML. Cell Cycle. 2006b;5:2778–6. doi: 10.4161/cc.5.23.3520. [DOI] [PubMed] [Google Scholar]
- Kojima K, Konopleva M, Samudio IJ, Ruvolo V, Andreeff M. Mitogen-activated protein kinase inhibiton enhances nuclear proapoptotic function of p53 in acute myelogenous leukemia cells. Cancer Res. 2007;67:3210–9. doi: 10.1158/0008-5472.CAN-06-2712. [DOI] [PubMed] [Google Scholar]
- Kozma SC, Bogaard ME, Buser K, Saurer SM, Bos JL, Groner B, et al. The human c-Kirsten ras gene is activated by a novel mutation in condon 13 in the breast carcinoma cell line MDA-MB231. Nucleic Acids Res. 1987;15:5963–71. doi: 10.1093/nar/15.15.5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause DS, Van Etten RA. Right on target: eradicating leukemic stem cells. Trends Mol Med. 2007;13:470–81. doi: 10.1016/j.molmed.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski DJ. Tuberous sclerosis: from tubers to mTOR. Ann Human Genet. 2003;67:87–96. doi: 10.1046/j.1469-1809.2003.00012.x. [DOI] [PubMed] [Google Scholar]
- Lahair MM, Howe CJ, Rodriguez-Mora O, McCubrey JA, Franklin RA. Molecular pathways leading to oxidative stress-induced phosphorylation of Akt. Antioxid Redox Signal. 2006;8:1749–56. doi: 10.1089/ars.2006.8.1749. [DOI] [PubMed] [Google Scholar]
- Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–8. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- Lee JT, Lehmann BD, Terrrian DM, Chappell WH, Stivala F, Libra M, et al. Targeting prostate cancer based on signal transduction and cell cycle pathways. Cell Cycle. 2008;7:1745–62. doi: 10.4161/cc.7.12.6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman BD, McCubrey JA, Jefferson HS, Paine MS, Chappell WH, Terrian DM. A dominant role for p53-dependent cellular senescence in radiosensitization of human prostate cancer cells. Cell Cycle. 2007;6:595–605. doi: 10.4161/cc.6.5.3901. [DOI] [PubMed] [Google Scholar]
- Leicht DT, Balan V, Kaplun A, Singh-Gupta V, Kaplun L, Dobson M, et al. Raf kinases: function, regulation and role in human cancer. Biochem Biophys Acta. 2007;1773:1196–213. doi: 10.1016/j.bbamcr.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Zhu T, Guan KL. Transformation potential of Ras isoforms correlates with activation of phosphatidylinositol 3-kinase but not ERK. J Biol Chem. 2004;279:37398–406. doi: 10.1074/jbc.M405730200. [DOI] [PubMed] [Google Scholar]
- Liu J, He C, Zhou K, Wang J, Kang JX. Coptis extracts enhance the anticancer effect of estrogen receptor antagonists on human breast cancer cells. BBRC. 2009;78:174–8. doi: 10.1016/j.bbrc.2008.10.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libra M, Malaponte G, Navolanic PM, Gangemi P, Bevelacqua V, Proietti L, et al. Analysis of BRAF mutation in primary and metastatic melanoma. Cell Cycle. 2006;4:968–70. [Google Scholar]
- Ligresti G, Militello L, Steelman LS, Cavallaro A, Basile F, Nicoletti F, et al. PIK3CA mutations in human solid tumors. Cell Cycle. 2009;8:1352–8. doi: 10.4161/cc.8.9.8255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Cash TP, Jones RG, Keith B, Thompson CB, Simon MC. Hypoxia-induced energy stress regulates mRNA translation and cell growth. Mol Cell. 2006;21:521–31. doi: 10.1016/j.molcel.2006.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo PG, Laurenti L, Gobessi S, Petlickovski A, Pelosi M, Chiusolo P, et al. The Akt signaling pathway determines the different proliferative capacity of chronic lymphocytic leukemia B-cells from patients with progressive and stable disease. Leukemia. 2007;21:110–20. doi: 10.1038/sj.leu.2404417. [DOI] [PubMed] [Google Scholar]
- Mangiolini M, Statti G, Vivacqua A, Gabriele S, Rago V, Loisso M, et al. J Steroid Biochem Mol Biol. 2001;82:315–22. doi: 10.1016/s0960-0760(02)00230-3. [DOI] [PubMed] [Google Scholar]
- Mahimainathan L, Choudhury GG. Inactivation of platelet-derived growth factor receptor by the tumor suppressor PTEN provides a novel mechanism of action of the phosphatase. J Biol Chem. 2004;279:15258–68. doi: 10.1074/jbc.M314328200. [DOI] [PubMed] [Google Scholar]
- Marais R, Light Y, Paterson HF, Marshall CJ. Ras recruits Raf-1 to the plasma membrane for activation by tyrosine phosphorylation. EMBO J. 1995;14:3136–45. doi: 10.1002/j.1460-2075.1995.tb07316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marais R, Light Y, Paterson HF, Mason CS, Marshall CJ. Differential regulation of Raf-1, A-Raf, and B-Raf by oncogenic ras and tyrosine kinases. J Biol Chem. 1997;272:4378–83. doi: 10.1074/jbc.272.7.4378. [DOI] [PubMed] [Google Scholar]
- Martelli AM, Nyakern M, Tabellini G, Bortul R, Tazzari PL, Evangelisti C, et al. Phosphoinositide 3-kinase/Akt signaling pathway and its therapeutical implications for human acute myeloid leukemia. Leukemia. 2006;20:911–28. doi: 10.1038/sj.leu.2404245. [DOI] [PubMed] [Google Scholar]
- Martelli AM, Tazzari PL, Evangelisti C, Chiarini F, Blalock WL, Billi AM, et al. Targeting the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin module for acute myelogenous leukemia therapy: from bench to bedside. Curr Med Chem. 2007;14:2009–23. doi: 10.2174/092986707781368423. [DOI] [PubMed] [Google Scholar]
- Martelli AM, Evangelisti C, Chirini F, et al. Targeting the PI3K/AKT/mTOR signaling network in acute myelogenous leukemia. Expert Opinion Investigational Drugs. 2009 doi: 10.1517/14728220903136775. In Press. [DOI] [PubMed] [Google Scholar]
- Mason CS, Springer CJ, Cooper RG, Superti-Furga G, Marshall CJ, Marais R. Serine and tyrosine phosphorylations cooperate in Raf-1, but not B-Raf activation. EMBO J. 1999;18:2137–48. doi: 10.1093/emboj/18.8.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubrey JA, May WS, Duronio V, Mufson A. Serine/threonine phosphorylation in cytokine signal transduction. Leukemia. 2000;14:9–21. doi: 10.1038/sj.leu.2401657. [DOI] [PubMed] [Google Scholar]
- McCubrey JA, Abrams SL, Ligresti G, Misaghian N, Wong ET, Basecke J, et al. Involvement of p53 and Raf/MEK/ERK pathways in hematopoietic drug resistance. Leukemia. 2008a;22:2080–90. doi: 10.1038/leu.2008.207. [DOI] [PubMed] [Google Scholar]
- McCubrey JA, Steelman LS, Abrams SL, Bertrand FE, Ludwig DE, Basecke J, et al. Targeting survival cascades induced by activation of Ras/Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways for effective leukemia therapy. Leukemia. 2008b;22:708–22. doi: 10.1038/leu.2008.27. [DOI] [PubMed] [Google Scholar]
- McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263–84. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubrey JA, Steelman LS, Abrams SL, Lee JT, Chang F, Bertrand FE, et al. Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt pathways in malignant transformation and drug resistance. Adv Enzyme Regul. 2006;46:249–79. doi: 10.1016/j.advenzreg.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Melnikova VO, Mourad-Zeidan AA, Lev DC, Bar-Eli M. Platelet-activating factor mediates MMP-2 expression and activation via phosphorylation of cAMP-response element-binding protein and contributes to melanoma metastasis. J Biol Chem. 2006;281:2911–22. doi: 10.1074/jbc.M508683200. [DOI] [PubMed] [Google Scholar]
- Mercure MZ, Ginnan RPD, Singer HA. CaM Kinase II-{delta}2 Dependent regulation of vascular smooth muscle cell polarization and migration. Am J Physiol Cell Physiol. 2008;2:2. doi: 10.1152/ajpcell.90638.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel A-P, Lievre M, Thomas C, Hinkal G, Ansieau S, Puisieuz A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 2008;3:2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao M, Yokota S, Iwai T, Kaneko H, Horiike S, Kashima K, et al. Internal tandem duplication of the FLT3 gene found in acute myeloid leukemia. Leukemia. 1996;10:1911–8. [PubMed] [Google Scholar]
- Neubauer A, Dodge RK, George SL, Davey FR, Silver RT, Schiffer CA, et al. Prognostic importance of mutations in the ras proto-oncogenes in de novo acute myeloid leukemia. Blood. 1994;83:1603–11. [PubMed] [Google Scholar]
- Park I, Soderling T. Activation of Ca2+/calmodulin-dependent protein kinase (CaM-kinase) IV by CaM-kinase kinase in Jurkat T lymphocytes. J Biol Chem. 1995;270:30464–9. doi: 10.1074/jbc.270.51.30464. [DOI] [PubMed] [Google Scholar]
- Pederson-Bjergaard J, Christiansen DH, Desta F, Andersen MK. Alternative genetic pathways and cooperating genetic abnormalities in the pathogenesis of therapy-related myelodysplasia and acute myeloid leukemia. Leukemia. 2006;20:1943–9. doi: 10.1038/sj.leu.2404381. [DOI] [PubMed] [Google Scholar]
- Planas-Silva MD, Waltz PK. Estrogen promotes reversible epithelial-to-mesenchymal-like transition and collective motility in MCF-7 breast cancer cells. J Steroid Biochem Mol Biol. 2007;104:11–21. doi: 10.1016/j.jsbmb.2006.09.039. [DOI] [PubMed] [Google Scholar]
- Plas DR, Thomas G. Tubers and tumors: rapamycin therapy for benign and malignant tumors. Current Opinion in Cell Biol. 2009;21:230–6. doi: 10.1016/j.ceb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Pluta A, Nyman U, Joseph B, Robak T, Zhivotovsky B, Smolewski P. The role of p73 in hematological malignancies. Leukemia. 2006;20:757–66. doi: 10.1038/sj.leu.2404166. [DOI] [PubMed] [Google Scholar]
- Ponti C, Gibellini D, Boin F, Melloni E, Manzoli FA, Cocco L, et al. Role of CREB transcription factor in c-fos activation in natural killer cells. Eur J Immunol. 2002;32:3358–65. doi: 10.1002/1521-4141(200212)32:12<3358::AID-IMMU3358>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Racioppi L, Means AR. Calcium/calmodulin-dependent kinase IV in immune and inflammatory responses: novel routes for an ancient traveller. Trends Immunol. 2008;29:600–7. doi: 10.1016/j.it.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Rajalingam K, Schreck R, Rapp UR, Albert S. Ras oncogenes and their downstream targets. Biochem Biophys Acta. 2007;1773:1177–96. doi: 10.1016/j.bbamcr.2007.01.012. S. [DOI] [PubMed] [Google Scholar]
- Ravandi F, Estrov Z. Eradication of leukemia stem cells as a new goal of therapy in leukemia. Clin Cancer Res. 2006;12:340–4. doi: 10.1158/1078-0432.CCR-05-1879. [DOI] [PubMed] [Google Scholar]
- Roberts AB, Tian F, DaCosta Byfield S, Stuelten C, Ooshima A, Saika S, et al. Smad3 is key to TGF-β-mediated epithelial-to-mesenchymal transition, fibrosis, tumor suppression and metastasis. Cytokine & Growth Factor Reviews. 2006;17:19–27. doi: 10.1016/j.cytogfr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Mora OG, LaHair MM, McCubrey JA, Franklin RA. CaM-KI and CaM-KK Participate in the control of cell cycle progression in MCF-7 human breast cancer cells. Cancer Res. 2005a;65:5408–16. doi: 10.1158/0008-5472.CAN-05-0271. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Mora O, LaHair MM, Howe CJ, McCubrey JA, Franklin RA. Calcium/calmodulin-dependent protein kinases as potential targets in cancer therapy. Expert Opin Ther Targets. 2005b;9:791–808. doi: 10.1517/14728222.9.4.791. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Mora OG, Lahair MM, Evans MJ, Kovacs CJ, Allison RR, Sibata CH, et al. Inhibition of the CaM-kinases augments cell death in response to oxygen radicals and oxygen radical inducing cancer therapies in MCF-7 human breast cancer cells. Cancer Biol Ther. 2006;5:1022–30. doi: 10.4161/cbt.5.8.2910. [DOI] [PubMed] [Google Scholar]
- Rokhlin OW, Taghiyev AF, Bayer KU, Bumcrot D, Koteliansk VE, Glover RA, et al. Calcium/Calmodulin-Dependent Kinase II plays an important role in prostate cancer cell survival. Cancer Biol Ther. 2007;6:5. doi: 10.4161/cbt.6.5.3975. [DOI] [PubMed] [Google Scholar]
- Ross DD. Modulation of drug resistance transporters as a strategy for treating myelodysplastic syndrome. Best Pract Res Clin Haematol. 2004;17:641–51. doi: 10.1016/j.beha.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Ross DD. Novel mechanisms of drug resistance in leukemia. Leukemia. 2000;14:467–73. doi: 10.1038/sj.leu.2401694. [DOI] [PubMed] [Google Scholar]
- Schneider M, Spanagel R, Zhang SJ, Bading H, Klugmann M. Adeno-associated virus (AAV)-mediated suppression of Ca2+/calmodulin kinase IV activity in the nucleus accumbens modulates emotional behaviour in mice. BMC Neurosci. 2007;8:105. doi: 10.1186/1471-2202-8-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul YD, Seger R. The MEK/ERK cascade: from signaling specificity to diverse functions. Biochem Biophys Acta. 2007;1773:1213–27. doi: 10.1016/j.bbamcr.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Bordeesy N, Manning B, Lopez L, Kosmatka M, DePinto RA. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6:91–4. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Soderling TR. The Ca-calmodulin-dependent protein kinase cascade. Trends Biochem Sci. 1999;24:232–6. doi: 10.1016/s0968-0004(99)01383-3. [DOI] [PubMed] [Google Scholar]
- Staal SP, Hartley JW. Thymic lymphoma induction by the AKT8 murine retrovirus. J Exp Med. 1988;167:1259–64. doi: 10.1084/jem.167.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal SP. Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: amplification of AKT1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci USA. 1987;84:5034–7. doi: 10.1073/pnas.84.14.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steelman LS, Abrams SL, Whelan J, Bertrand FE, Ludwig DE, Basecke J, et al. Contributions of the Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and JAK/STAT Pathways to Leukemia. Leukemia. 2008a;22:686–7. doi: 10.1038/leu.2008.26. [DOI] [PubMed] [Google Scholar]
- Steelman LS, Navolanic PN, Sokolosky M, Taylor JR, Lehmann BD, Chappell WH, et al. Suppression of PTEN function increases breast cancer chemotherapeutic drug resistance while conferring sensitivity of mTOR inhibitors. Oncogene. 2008b;27:4086–95. doi: 10.1038/onc.2008.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steelman LS, Stadelman KM, Chappell WH, Horn S, Basecke J, Cervello M, et al. Akt as a therapeutic target in cancer. Exp Opin Therapeutic Targets. 2008c;12:1139–65. doi: 10.1517/14728222.12.9.1139. [DOI] [PubMed] [Google Scholar]
- Strack S, Barban MA, Wadzinski BE, Colbran RJ. Differential inactivation of postsynaptic density-associated and soluble Ca2+/calmodulin-dependent protein kinase II by protein phosphatases 1 and 2A. J Neurochem. 1997;68:2119–28. doi: 10.1046/j.1471-4159.1997.68052119.x. [DOI] [PubMed] [Google Scholar]
- Surmacz E, Bartucci M. Role of estrogen receptor alpha in modulating IGF-I receptor signaling and function in breast cancer. J Exp Clin Cancer Res. 2004;23:385–94. [PubMed] [Google Scholar]
- Suzuki K, Takahashi K. Reduced expression of the regulatory A subunit of serine/threonine protein phosphatase 2A in human breast cancer MCF-7 cells. Int J Oncol. 2003;23:1263–8. [PubMed] [Google Scholar]
- Takahashi K. The linkage between beta1 integrin and the actin cytoskeleton is differentially regulated by tyrosine and serine/threonine phosphorylation of beta1 integrin in normal and cancerous human breast cells. BMC Cell Biol. 2001;2:23. doi: 10.1186/1471-2121-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda H, Kitaoka Y, Hayashi Y, Kumai T, Munemasa Y, Fujino H, et al. Calcium/calmodulin-dependent protein kinase II regulates the phosphorylation of CREB in NMDA-induced retinal neurotoxicity. Brain Res. 2007;1184:306–15. doi: 10.1016/j.brainres.2007.09.055. [DOI] [PubMed] [Google Scholar]
- Tamburini J, Elie C, Bardet V, Chapuis N, Park S, Broet P, et al. Constitutive Phosphoinositide-3kinase/AKT activation represents a favourable prognostic factor in de novo AML patients. Blood. 2007;110:1025–8. doi: 10.1182/blood-2006-12-061283. [DOI] [PubMed] [Google Scholar]
- Tamura N, Tai Y, Sugimoto K, Kobayashi R, Konishi R, Nishioka M, et al. Enhanced expression and activation of Ca(2+)/calmodulin-dependent protein kinase IV in hepatocellular carcinoma. Cancer. 2000;89:1910–6. doi: 10.1002/1097-0142(20001101)89:9<1910::aid-cncr6>3.3.co;2-m. [DOI] [PubMed] [Google Scholar]
- Tannapfel A, Sommerer F, Benicke M, Katalinic A, Uhlmann D, Witzigmann H, et al. Mutations of the BRAF gene in cholangiocarcinoma but not in hepatocellular carcinoma. Gut. 2003;52:706–12. doi: 10.1136/gut.52.5.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazzari PL, Cappellini A, Ricci F, Evangelisti C, Papa V, Grafone T, et al. Multidrug resistance-associated protein 1 expression is under the control of the phosphoinositide 3 kinase/Akt signal transduction network in human acute myelogenous leukemia blasts. Leukemia. 2007;21:427–38. doi: 10.1038/sj.leu.2404523. [DOI] [PubMed] [Google Scholar]
- Thomas RS, Tymms MJ, McKinlay LH, Shannon MF, Seth A, Kola I. ETS1, NFκB and AP1 synergistically transactivate the human GM-CSF promoter. Oncogene. 1997;14:2845–55. doi: 10.1038/sj.onc.1201125. [DOI] [PubMed] [Google Scholar]
- Toker A, Cantley LC. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature. 1997;387:673–6. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- Tokumitsu H, Brickey DA, Glod J, Hidaka H, Sikela J, Soderling TR. Activation mechanisms for Ca2+/calmodulin-dependent protein kinase IV. Identification of a brain CaM-kinase IV kinase. J Biol Chem. 1994;269:28640–7. [PubMed] [Google Scholar]
- Tokumitsu H, Hatano N, Inuzuka H, Sueyoshi Y, Yokokura S, Ichimura T, et al. Phosphorylation of Numb family proteins. Possible involvement of Ca2+/calmodulin-dependent protein kinases. J Biol Chem. 2005;280:35108–18. doi: 10.1074/jbc.M503912200. [DOI] [PubMed] [Google Scholar]
- Tokunaga C, Yoshino K, Yonezawa K. mTOR integrates amino acid- and energy-sensing pathways. Biochem Biophys Res Commun. 2004;313:443–6. doi: 10.1016/j.bbrc.2003.07.019. [DOI] [PubMed] [Google Scholar]
- Tombes RM, Krystal GW. Identification of novel human tumor cell-specific CaMK-II variants. Biochim Biophys Acta. 1997;1355:281–92. doi: 10.1016/s0167-4889(96)00141-3. [DOI] [PubMed] [Google Scholar]
- Towatari M, Ciro M, Ottolenghi S, Tsuzuki S, Enver T. Involvement of the mitogen-activated protein kinase in the cytokine regulated phosphorylation of transcription factor GATA-1. Hematol J. 2004;5:262–72. doi: 10.1038/sj.thj.6200345. [DOI] [PubMed] [Google Scholar]
- Tresini M, Lorenzini A, Frisoni L, Allen RG, Cristofalo VJ. Lack of Elk-1 phosphorylation and dysregulation of the extracellular regulated kinase signaling pathway in senescent human fibroblast. Exp Cell Res. 2001;269:287–300. doi: 10.1006/excr.2001.5334. [DOI] [PubMed] [Google Scholar]
- Troppmair J, Rapp UR. Raf and the road to cell survival: A tale of bad spells, ring bearers and detours. Biochem Pharmacol. 2003;66:1341–5. doi: 10.1016/s0006-2952(03)00483-0. [DOI] [PubMed] [Google Scholar]
- Wan PT, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–67. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- Wang CY, Bassuk AG, Boise LH, Thompson CB, Bravo R, Leiden JM. Activation of the granulocyte-macrophage colony-stimulating factor promoter in T cells requires cooperative binding of Elf-1 and AP-1 transcription factors. Mol Cell Biol. 1994;14:1153–9. doi: 10.1128/mcb.14.2.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wan W, Sun R, Liu Y, Sun X, Ma D, et al. Reduction of Akt2 expression inhibits chemotaxis signal transduction in human breast cancer cells. Cell Signal. 2008;15:15. doi: 10.1016/j.cellsig.2007.12.023. [DOI] [PubMed] [Google Scholar]
- Webb CP, Van Aelst L, Wigler MH, Woude GF. Signaling pathways in Ras-mediated tumorigenicity and metastasis. Proc Natl Acad Sci USA. 1998;95:8773–8. doi: 10.1073/pnas.95.15.8773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein-Oppenheimer CR, Henriquez-Roldan CF, Davis JM, Navolanic PM, Saleh OA, Steelman LS, et al. Role of the Raf signal transduction cascade in the in vitro resistance to the anticancer drug doxorubicin. 2001;7:2898–907. [PubMed] [Google Scholar]
- Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S, et al. Survival signalling by Akt and elF4E in oncogenesis and cancer therapy. Nature. 2004;428:332–7. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- Weng LP, Brown JL, Baker KM, Ostrowski MC, Eng C. PTEN blocks insulin-mediated ETS-2 phosphorylation through MAP kinase, independently of the phosphoinositide 3-kinase pathway. Hum Mol Genet. 2002;11:1687–96. doi: 10.1093/hmg/11.15.1687. [DOI] [PubMed] [Google Scholar]
- Westphal RS, Anderson KA, Means AR, Wadzinski BE. A signaling complex of Ca2+-calmodulin-dependent protein kinase IV and protein phosphatase 2A. Science. 1998;280:1258–61. doi: 10.1126/science.280.5367.1258. [DOI] [PubMed] [Google Scholar]
- Whitfield JF. Calcium signals and cancer. Crit Rev Oncogene. 1992;3:55–90. [PubMed] [Google Scholar]
- Woodward JK, Holen I, Coleman RE, Buttle DJ. The roles of proteolytic enzymes in the development of tumour-induced bone disease in breast and prostate cancer. Bone. 2007;41:912–27. doi: 10.1016/j.bone.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Xie S, Price JE, Luca M, Jean D, Ronai Z, Bar-Eli M. Dominant-negative CREB inhibits tumor growth and metastasis of human melanoma cells. Oncogene. 1997;15:2069–75. doi: 10.1038/sj.onc.1201358. [DOI] [PubMed] [Google Scholar]
- Xing J, Ginty DD, Greenberg ME. Coupling of the Ras-MAPK pathway to gene activation by Rsk2, a growth factor regulated CREB kinase. Science. 1996;273:959–63. doi: 10.1126/science.273.5277.959. [DOI] [PubMed] [Google Scholar]
- Xu Q, Simpson SE, Scialla TJ, Bagg A, Carroll M. Survival of acute myeloid leukemia cells requires PI3 kinase activation. Blood. 2003;102:972–80. doi: 10.1182/blood-2002-11-3429. [DOI] [PubMed] [Google Scholar]
- Xu Q, Thompson JE, Carroll M. mTOR regulates cell survival after etoposide treatment in primary AML cells. Blood. 2005;106:4261–8. doi: 10.1182/blood-2004-11-4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Robbins D, Frost J, Dang A, Lange-Carter C, Cobb MH. MEKK1 phosphorylates MEK1 and MEK2 but does not cause activation of mitogen-activated protein kinase. Proc Natl Acad Sci USA. 1995;92:6808–12. doi: 10.1073/pnas.92.15.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano S, Tokumitsu H, Soderling TR. Calcium promotes cell survival through CaM-K kinase activation of the protein-kinase-B pathway. Nature. 1998;396:584–7. doi: 10.1038/25147. [DOI] [PubMed] [Google Scholar]
- Ye L, Gho WM, Chan FL, Chen S, Leung LK. Dietary administration of the licorice flavonoid isoliquiritigenin deters the growth of MCF-7 cells overexpressing aromatase. Int J Cancer. 2009;124:1028–36. doi: 10.1002/ijc.24046. [DOI] [PubMed] [Google Scholar]
- Yeung RS, Xiao G, Jin F, Lee W, Testa JR, Knudson AG. Predisposition to renal carcinoma in the eker rat is determined by germline mutation of the tuberous sclerosis 2 (TSC2) Proc Natl Acad Sci USA. 1994;91:11433–6. doi: 10.1073/pnas.91.24.11413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H, et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–82. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- Yuan K, Chung LW, Siegal GP, Zayzafoon M. alpha-CaMKII controls the growth of human osteosarcoma by regulating cell cycle progression. Lab Invest. 2007;87:938–50. doi: 10.1038/labinvest.3700658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Grindley JC, Yin T, Jayasinghe S, He XC, Ross JT, et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441:518–22. doi: 10.1038/nature04747. [DOI] [PubMed] [Google Scholar]