SUMMARY
Recent studies have found short-term adrenocortical benefits of early interventions for at-risk children. The current study evaluated the effects of the Family Bereavement Program on cortisol levels six years after the program. Parentally bereaved children were randomly assigned to the 12-week preventive intervention (n=78) or a self-study control (n=61) condition. Six years later (mean age 17.5), salivary cortisol levels were measured before and after a conflict discussion task conducted in late afternoon/early evening. The intervention group had significantly higher cortisol levels across the task compared to the control group, and lower cortisol was associated with higher externalizing symptoms. The group effect did not differ by age at the time of death, and the group difference remained significant after adjustment for pre-intervention mental health and current mental health symptoms. Results suggest that a family-focused intervention for parentally-bereaved youth may have prevented the development of attenuated cortisol secretion suggestive of dysregulation and associated with externalizing problems.
Keywords: parental loss, cortisol, intervention, bereavement, externalizing
A variety of early adverse experiences, such as child maltreatment, foster care, and parental loss, have been associated with dysregulated cortisol activity during childhood and later in life, representing an important link to long-term psychological and physical health (Gunnar, 2007). The sudden loss of a caregiver, one of the most traumatic events that can happen to a child, increases the long-term risk of psychological and physical disorder (Luecken, 2008). Neuroendocrine dysregulation represents a plausible pathway for the relations of early loss experiences with psychopathology and poor physical health outcomes.
Exposure to childhood adversity has been associated with various patterns of cortisol dysregulation, including higher or lower basal levels, exaggerated or attenuated stress responses, and abnormal patterns of diurnal secretion (Gunnar, 2007). Patterns of cortisol activity linked to psychopathology in lower risk populations may be indicative of positive functioning in higher risk populations (Cicchetti & Rogosch, 2007). Given the complexity in identifying an adaptive neurobiological profile for at-risk children, researchers have noted the importance of assessing multiple domains and current psychosocial functioning. A wide range of mental disorders have been linked to neuroendocrine dysregulation. One common finding has been an association between attenuated cortisol activity and externalizing problems (Shirtcliff et al., 2005).
The randomized experimental trial design is a powerful technique for demonstrating the potential for preventive interventions to mitigate the negative effects of adversity on the neuroendocrine system. Brotman and colleagues (2007) reported that preschool-aged siblings of delinquent youth randomized to a 6–8 month intervention exhibited higher cortisol immediately prior to a social challenge task relative to those in a control group. Fisher and colleagues (2007) found that children in foster care randomized to an intervention condition exhibited diurnal cortisol activity similar to that of non-maltreated children, whereas children in the control condition exhibited an increasingly flattened diurnal pattern. Similarly, children in foster care whose caregivers participated in an attachment intervention showed more typical diurnal patterns post-intervention than those in a control condition (Dozier et al., 2006). These studies provide evidence that, at least in the short-term, interventions with high risk children may prevent or reverse dysregulated patterns of cortisol activity.
Less is known about the potential long-term neurobiological effects of early preventive interventions with high risk populations. This study reports on cortisol activity in parentally bereaved youth who participated in a randomized controlled trial of the Family Bereavement Program (FBP), a 12-week family-based preventive intervention designed to change potentially modifiable risk and protective factors associated with adaptation after parental death (Sandler et al., 2003). The FBP was found to increase positive caregiver-child interactions, decrease youth exposure to stressful events, and increase youth positive coping skills immediately following the intervention, and reduce youth mental health problems at 11-month follow-up (Sandler et al., 2003). Given a growing literature finding attenuated cortisol in adolescents and adults exposed to chronic adversity earlier in life (Elzinga et al., 2008; Miller, Chen, &, Zhou, 2007) and associations between externalizing symptoms and attenuated cortisol (e.g., Shirtcliff et al., 2005), the hypothesis was tested that participation in the FBP would be associated with higher cortisol 6 years later, during late adolescence/young adulthood.
Methods
Participants
Families with a child aged 8–16 years were recruited from the Phoenix, Arizona metropolitan area through community presentations and mail solicitation. For complete recruitment and eligibility details, see Sandler et al. (2003). Participating families were randomly assigned to either the FBP (n=135) or a home-based self-study (control) condition (n=109). Of the 244 youth assigned to intervention or control, 218 completed the 6-year follow-up. To reduce caregiver response burden, a maximum of two offspring were randomly selected for cortisol sampling. Youth were ineligible for cortisol sampling if they reported less than three verbal contacts in the last month with the caregiver. Of the 190 eligible youth, 19 refused and 32 could not be scheduled within the appropriate time range (3–9 PM), resulting in a final sample size of 139. Attrition did not differ across intervention and control conditions in the final sample, and attrition status was not associated with baseline mental health, participant age, ethnicity, gender, months since parental death, or family income (all p’s > .10).
No statistically significant differences were found between youth in the intervention and control groups on gender, ethnicity, age, gender of deceased parent, cause of death, time since the death, age at parental death, family income, baseline mental health, hormonal contraceptives, psychiatric medications, smoking status, or daily caffeine. On testing day, the groups did not differ in the time of testing, cigarettes smoked, exercise, caffeine, or time of the last meal.
Procedures
FBP Intervention
Caregivers and youth met in separate groups for twelve 2-hr sessions. The caregiver program focused on increasing positive caregiver-child relationships and effective discipline, and decreasing children’s exposure to stressful events. The youth program focused on increasing positive coping skills, promoting adaptive beliefs about why negative events occur, increasing self-esteem and self-efficacy, and reducing the perceived need to inhibit expressions of grief (for more detail, see Sandler et al., 2003).
Discussion Task
At the participants’ homes, adolescents/young adults and their caregivers participated in a 12-minute videotaped behavioral observation interaction in which they discussed a current problem common to late adolescence/young adulthood. Issues for discussion were selected based on responses to the Parent Issues Checklist (Prinz, Foster, Kent & O’Leary, 1979), completed just before the discussion task.
Cortisol Sampling
Sampling occurred on weekdays between 3–9 PM. Saliva samples, obtained with the Salivette device (Sarstedt, Rommelsdorf, Germany), were collected from adolescents/young adults before, immediately after, and at 15 and 30 -minutes after the discussion task. Samples were analyzed at Salimetrics (State College, PA) using high-sensitive enzyme immunoassay (range of sensitivity from .007 to 1.8 μg/dl; mean intra-and inter-assay coefficients of variation 4.13% and 8.89%). Removal of potential outliers (+/− 3 SD from the mean) did not significantly change the pattern of results, therefore all data were retained. Similarly, winsorizing potential outliers did not change the pattern or significance of results. Cortisol values were log-transformed to correct for deviations from normality.
Measures
A composite of caregiver and youth reports of youth internalizing and externalizing problems prior to assignment to intervention or control groups was used to assess pre-intervention psychosocial risk, using standardized scores of internalizing and externalizing symptoms on the Child Behavior Checklist (completed by caregiver; α’s = .87–.90), the Youth Self-Report (completed by child; α = .86), the Child Depression Inventory (completed by child; α = .87), and the Children’s Manifest Anxiety Scale-Revised (completed by child; α = .90) (Reynolds & Richmond, 1978; Achenbach, 1991b; Kovacs, 1992). As the CDI and R-CMAS were highly correlated (r = .65), a composite of the means of their standardized scores was used as the youth report of internalizing problems. Composite scores were constructed by standardizing the caregiver and youth report measures of internalizing problems or externalizing measures and then averaging them. Contemporaneous externalizing and internalizing symptoms were assessed at the 6-year follow-up with the Youth Self Report if the participant was 14–18 year old (α = .88–.90), or the Young Adult Self Report for participants over 18 (α = .87–.88; Achenbach, 1991a; 1997).
Data Analytic Strategy
Multilevel Linear Modeling was used to evaluate group differences in cortisol using the SPSS MIXED procedure, with repeated cortisol measures nested within subjects and subjects nested within families. A level 1 variable, cortisol sample order, modeled the pattern of responses (within-persons) over time. Group assignment served as the between-persons dimension, coded with the intervention group = ‘1’, control group = ‘0’. Time of day was included as a level 2 variable. Participant gender was included as a covariate for analyses involving internalizing or externalizing symptoms.
Results
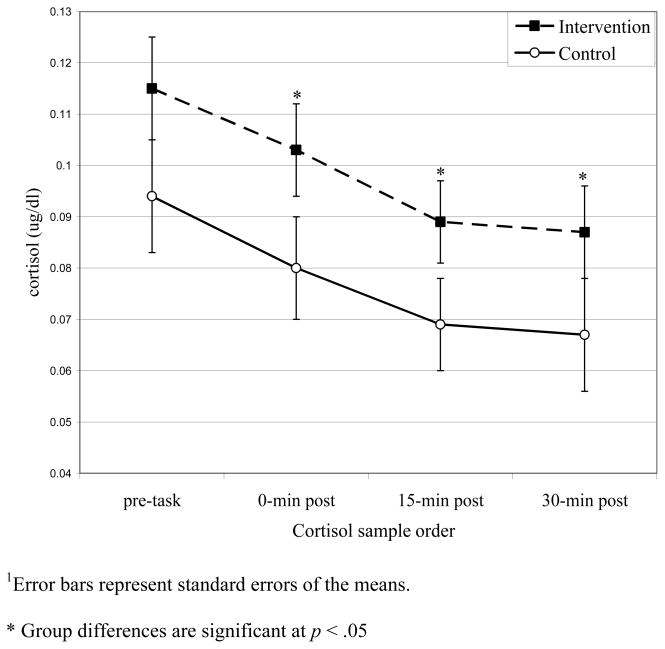
The main effect of group on cortisol was significant (β = 0.092; 95% Confidence Interval [CI], 0.011 – 0.174; F(1,135) = 5.1, p = .026; Cohen’s d = .39). The intervention group showed significantly higher cortisol across the task (see Figure 1), but did not differ in the pattern of response over time (p = .98). The intervention group’s cortisol was significantly higher immediately after the task, p = .021, 15 minutes after the task, p = .021, and 30 minutes after the task, p = .048. The main effect remained significant after adjusting for gender, age, age at the time of parental death, family income, hormonal contraceptive use, caffeine intake, exercise, smoking status, time of last meal, use of medications, pre-intervention psychosocial risk, and contemporaneous internalizing and externalizing symptoms.
Figure 1.
Intervention group difference in cortisol1
Cortisol levels were not associated with pre-intervention psychosocial risk (p = .27) or contemporaneous internalizing symptoms (p = .90). However, lower cortisol levels were significantly associated with higher contemporaneous externalizing symptoms, (β = −0.054; 95% Confidence Interval [CI], −0.093 – −0.014; p = .008; ηp2 = .15). The group by externalizing symptoms interaction was not a significant predictors of cortisol (p = .55), suggesting the impact of intervention on cortisol did not differ by level of current externalizing symptoms.
Post hoc analyses considered if the age at which parental death occurred would moderate the impact of intervention on cortisol levels six years later. The group by age at parental death interaction was not a significant predictor of cortisol, (β =−0.0018; 95% Confidence Interval [CI], −0.035 – 0.032; p = .92). Similarly, the group by age at intervention interaction did not predict cortisol (β =−0.007; 95% Confidence Interval [CI], −0.041 – 0.028, p = .71.)
Discussion
The present study extends previous research on the short-term neurobiological effects of preventive interventions by evaluating cortisol activity in parentally bereaved children six years after participation in a preventive intervention. Adolescents and young adults randomized to the FBP condition exhibited significantly higher cortisol collected across four samples in the late afternoon/early evening compared to youth in the control group. The intervention effects on cortisol did not differ by participant age at the time of parental death. In addition, lower cortisol levels were associated with elevated externalizing symptoms. These results suggest that a family-based preventive intervention for parentally-bereaved children may have prevented the development of a pattern of adrenocortical activity often associated with externalizing problems.
The current findings are consistent with shorter-term studies (Dozier et al., 2006; Brotman et al., 2007; Fisher et al., 2007), which suggest that with effective early intervention the potential adrenocortical dysregulation associated with early adversity may be preventable. Given the complexity of neuroendocrine alterations associated with early adversity, the interpretation of higher cortisol in FBP participants is not straightforward. One way to address this issue is to consider associated affective pathology. The findings that youth in the control condition had lower cortisol, and lower cortisol was associated with higher externalizing symptoms, suggest that for these at-risk youth, higher cortisol levels may be indicative of better mental health.
There are several limitations of these analyses. First, cortisol levels were not assessed pre-intervention; however, individuals were randomly assigned to condition, and no evidence was found for significant pre-intervention differences that could explain differences in cortisol six years later. Therefore, baseline individual differences in cortisol should be unconfounded with group assignment. However, we cannot demonstrate longitudinal changes in cortisol activity, limiting interpretation to a group difference 6 years post-intervention. Second, the trial did not include non-bereaved children. We are unable to compare responses to the task with those from non-bereaved youth. Third, although cortisol was sampled before and after discussion of a current conflict, stress reactivity was not evident. Anticipatory cortisol increases may have occurred across the sample. Alternatively, the task may not have been stressful or novel enough to elicit a clear stress response, or both groups may have been displaying blunted reactivity. Others have found that parent-child conflict discussion tasks are associated with declining cortisol rather than reactivity (Klimes-Dougan et al., 2001; Kobac, Zajac, & Levine, 2009). These tasks may have unique effects on the child’s cortisol response, perhaps reflective of parental/social regulation (Adam, Klimes-Dougan & Gunnar, 2007). Fourth, there was a wide age range at recruitment (8–16 years old). While there was no evidence of age-related differences in the intervention effect, a larger sample size may have more power to detect age-related effects. Finally, lower cortisol was associated with higher externalizing symptoms, but a causal interpretation cannot be drawn (i.e., symptoms may precede or follow attenuated cortisol).
It will be important to replicate these findings to better understand the health significance of higher cortisol in bereaved children who receive intervention. Associations between cortisol and symptomatology are complicated and likely influenced by context as well as developmental transitions (e.g., Shirtcliff & Essex, 2008). At this developmental stage, lower cortisol appears to be a marker of concurrent symptomatology, but future studies that investigate the implications of lower cortisol in late adolescence/young adulthood for future psychological and physical health will be important. A critical theoretical issue that should be investigated in replication studies as well concerns the pathways that mediate the impact of early intervention programs on neurobiological systems across development. Given that the FBP was designed to target multiple aspects of functioning (including problematic as well as positive outcomes), these pathways are likely to involve a complicated pattern of program effects on multiple mediators such as stress exposure or protective factor enhancement, rather than just the absence of psychopathology.
Research has clearly demonstrated the detrimental mental and physical health risks associated with childhood adversity, and neuroendocrine dysregulation is commonly theorized to represent an underlying pathway linking adversity to long-term health. In the present study, a family-focused intervention for parentally bereaved children provided in childhood was associated with higher cortisol six years later relative to a control group of bereaved youth. Across the sample, lower cortisol was associated with higher externalizing symptoms. Strengths of this study include the use of a randomized experimental design and an evidence-based intervention with documented mental health benefits. Although preliminary, these findings may further understanding of the potential biological as well as psychological health benefits of early preventive interventions for at-risk children.
Table 1.
Sample Characteristics1
| Total | Intervention | Control | |
|---|---|---|---|
| Participants (N) | 139 | 78 | 61 |
| Gender (N) | |||
| Male | 81 | 42 | 39 |
| Female | 58 | 36 | 22 |
| Age (M, SD) | 17.5 (2.4) | 17.7 (2.6) | 17.2 (2.1) |
| Ethnicity (N) | |||
| Hispanic | 23 | 16 | 7 |
| Anglo/Caucasian | 85 | 47 | 38 |
| African American | 9 | 3 | 6 |
| Native American | 6 | 2 | 4 |
| Asian/Pacific Islander | 2 | 2 | 0 |
| Other | 13 | 8 | 5 |
| Oral contraceptive use (N) | 7 | 5 | 2 |
| Smoked in last 12 months (N) | 63 | 36 | 27 |
| Psychiatric medication use | 10 | 6 | 4 |
| Age at parental death (M, SD) | 10.4 (2.5) | 10.7 (2.7) | 10.1 (2.3) |
| Baseline risk composite | .01 (.71) | .014 (.82) | .0057 (.55) |
| Internaliz. symptoms (current) | 3.54 (1.4 | 3.53 (1.4) | 3.56 (1.4) |
| Externaliz. Symptoms (current) | 3.72 (1.1) | 3.60 (1.2) | 3.87 (0.90) |
chi-square and t-tests revealed no significant group differences in any of the above demographic comparisons
Acknowledgments
We appreciate critical reviews provided by Michelle Little, Ph.D. and David MacKinnon, Ph.D.
Role of Funding Source: Support for this research was provided by NIMH R01 Grant MH49155 and P30 MH0686856 to conduct the six-year follow-up evaluation of the Family Bereavement Program. NIMH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflicts of Interest, financial or otherwise: None
Luecken made substantial contributions to the conception and design, acquisition of data, analysis and interpretation of data, drafting and critical revision of the manuscript, statistical analyses, and obtaining funding. Hagan made substantial contributions to the analysis and interpretation of data, and to the drafting and critical revision of the manuscript. Sandler made substantial contributions to the conception and design, acquisition of data, analysis and interpretation of data, critical revision of the manuscript, and obtaining funding. Tein made substantial contributions to the conception and design, acquisition of data, analysis and interpretation of data, critical revision of the manuscript, statistical analyses, and obtaining funding. Ayers made substantial contributions to the conception and design, acquisition of data, analysis and interpretation of data, critical revision of the manuscript, and obtaining funding. Wolchik made substantial contributions to the conception and design, acquisition of data, analysis and interpretation of data, critical revision of the manuscript, and obtaining funding.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach TM. Manual for the Young Adult Self-Report and Young Adult Behavior Checklist. University of Vermont, Department of Psychiatry; Burlington, VT: 1997. [Google Scholar]
- Achenbach TM. Manual for the Youth Self-Report and 1991 Profile. University of Vermont, Department of Psychology; Burlington, VT: 1991a. [Google Scholar]
- Achenbach TM. Manual for the Child Behavior Checklist and 1991 Profile. University of Vermont, Department of Psychology; Burlington, VT: 1991b. [Google Scholar]
- Adam EK, Klimes-Dougan B, Gunnar M. Social regulation of the adrenocortical response to stress in infants, children, and adolescents: Implications for psychopathology and education. In: Coch D, Dawson G, Fischer K, editors. Human behavior and the developing brain: Atypical development. Guilford Press; New York: 2007. [Google Scholar]
- Brotman LM, Gouley KK, Huang KY, Kamboukos D, Fratto C, Pine DS. Effects of a psychosocial family-based preventive intervention on cortisol response to a social challenge in preschoolers at high risk for antisocial behavior. Arch Gen Psychiatry. 2007;64:1172–1179. doi: 10.1001/archpsyc.64.10.1172. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Personality, adrenal steroid hormones, and resilience in maltreated children: A multilevel perspective. Dev Psychopathol. 2007;19:787–809. doi: 10.1017/S0954579407000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozier M, Peloso E, Lindhiem O, Gordon MK, Manni M, Sepulveda S, Ackerman J, Bernier A, Levine S. Developing evidence-based interventions for foster children: An example of a randomized clinical trial with infants and toddlers. J Soc Issues. 2006;62:767–785. [Google Scholar]
- Elzinga BM, Roelofs K, Tollenaar MS, Bakvis P, van Pelt J, Spinhoven P. Diminished cortisol responses to psychosocial stress associated with lifetime adverse events: A study among healthy subjects. Psychoneuroendocrinology. 2008;33:227–237. doi: 10.1016/j.psyneuen.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Stoolmiller M, Gunnar MR, Burraston BO. Effects of a therapeutic intervention for foster preschoolers on diurnal cortisol activity. Psychoneuroendocrinology. 2007;32:892–905. doi: 10.1016/j.psyneuen.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR. Stress effects on the developing brain. In: Romer D, Walker EF, editors. Adolescent Psychopathology and the Developing Brain. Oxford University Press; New York, NY: 2007. [Google Scholar]
- Klimes-Dougan B, Hastings PD, Granger DA, Usher BA, Zahn-Waxler C. Adrenocortical activity in at-risk and normally developing adolescents: individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Dev Psychopathol. 2001;13(3):695–719. doi: 10.1017/s0954579401003157. [DOI] [PubMed] [Google Scholar]
- Kobak R, Zajac K, Levine S. Cortisol and antisocial behavior in early adolescence: the role of gender in an economically disadvantaged sample. Dev Psychopathol. 2009;21(2):579–91. doi: 10.1017/S0954579409000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M. Children’s Depression Inventory Manual. North Tonawanda, NY: Multi-Health Systems; 1992. [Google Scholar]
- Luecken LJ. Long-term consequences of parental death in childhood: Physiological and psychological manifestations. In: Stroebe M, Hansson RO, Schut H, Stroebe W, editors. Handbook of Bereavement Research and Practice: 21st Century Perspectives. American Psychological Association Press; Washington, DC: 2008. [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Prinz RJ, Foster S, Kent RN, O’Leary KD. Multivariate assessment of conflict in distressed and nondistressed mother-adolescent dyads. J Appl Behav Anal. 1979;12:691–700. doi: 10.1901/jaba.1979.12-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CR, Richmond BO. What I Think and Feel: A revised measure of children’s manifest anxiety. J Abnorm Child Psychol. 1978;6:271–280. doi: 10.1007/BF00919131. [DOI] [PubMed] [Google Scholar]
- Sandler IN, Ayers TS, Wolchik SA, Tein JY, Kwok OM, Haine RA, Twohey JL, Suter J, Lin K, Padgett-Jones S, Lutzke JR, Cole E, Kriege GM. The Family Bereavement Program: Efficacy evaluation of a theory-based prevention program for parentally bereaved children and adolescents. J Consult Clin Psychol. 2003;71:587–600. doi: 10.1037/0022-006x.71.3.587. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Essex MJ. Concurrent and longitudinal associations of basal and diurnal cortisol with mental health symptoms in early adolescence. Dev Psychobiol. 2008;50(7):690–703. doi: 10.1002/dev.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]