Abstract
Recent research indicates that the orbital prefrontal cortex (PFo) represents stimulus valuations and that the amygdala updates these valuations. An exploration of how PFo and the amygdala interact could improve the understanding of both. PFo and the amygdala function cooperatively when monkeys choose objects associated with recently revalued foods. In other tasks, they function in opposition. PFo uses positive feedback to promote learning in object-reward reversal tasks, and PFo also promotes extinction learning. Amygdala function interferes with both kinds of learning. The amygdala underlies fearful responses to a rubber snake from the first exposure on, but PFo is necessary only after the initial exposure. The amygdala mediates an arousal response in anticipation of rewards, whereas PFo sometimes suppresses such arousal. A role for PFo in advanced cognition, for the amygdala in instinctive behavior, and for cortex-subcortex interactions in prioritizing behaviors provides one account for these findings.
Introduction
In no case is an animal activity to be interpreted in terms of higher psychological processes, if it can be fairly interpreted in terms of processes which stand lower in the scale of psychological evolution and development. [1, p. 59]
According to Morgan’s canon, quoted above, accounts of behavior lowest on the “scale of … evolution” should always prevail. But contemporary biology has resoundingly discredited the concept of an evolutionary scale, and Morgan’s canon too often leads to a psychology that differs little from behaviorism, if it does so at all. Although behaviorism is “all but dead” as a philosophical matter [2], many neuroscientists still become unduly suspicious when accounts of behavior invoke concepts beyond the behaviorist trinity of stimuli, responses and behavioral outcomes. Here we reject such skepticism, along with Morgan’s canon, in order to interpret recent findings on the orbital prefrontal cortex (PFo) and the amygdala in terms of advanced cognition and innate behaviors, as well as stimuli, responses and outcomes. By advanced cognition, we mean learned behaviors such as strategies, high-order rules and learning sets that transcend credible accounts in terms of the behaviorist trinity. By innate behaviors, we mean unlearned, inborn behaviors characteristic of a species, including unconditioned autonomic responses and valuations of biological importance.
After summarizing selected findings on the neural substrates of reward processing and affect in primates, we discuss them in three sections: one each on amygdala function, prefrontal function and cortex-subcortex interactions. As an example of cortex-subcortex interactions, we begin with a consideration of those between PFo and the amygdala.
Orbital prefrontal-amygdala interactions
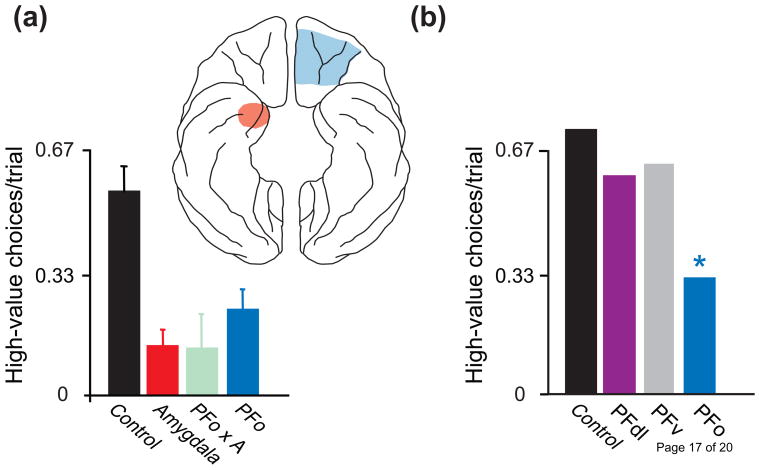
Recent findings have revealed both cooperative and contrasting functions of PFo and the amygdala. Results from the devaluation task point to a cooperative function. This task assesses an animal’s ability to link objects or other biologically neutral stimuli with reward value. Typically, the task begins when animals consume one kind of food to satiety, thus devaluing it. Later, they choose between a stimulus associated with the devalued food and a stimulus associated with a different food. Intact rats and monkeys avoid choosing stimuli associated with the devalued food, a finding called the devaluation effect; animals with amygdala lesions typically fail to show this effect [3,4,5,6]. For example, macaque monkeys with bilateral amygdala lesions (Fig. 1a, red) choose objects associated with the devalued food more often than controls (Fig. 1a, black). Bilateral PFo lesions (Fig. 1a, blue) and surgical disconnections that disrupt PFo-amygdala interactions (Fig. 1a, green) have the same effect, but damage elsewhere in prefrontal cortex does not [7•,8•] (Fig. 1b). Importantly, the monkeys with PFo and amygdala lesions perform poorly only when choosing between objects; when choosing between the two foods they successfully avoid the devalued one. In monkeys, reversible inactivation of the amygdala during the selective satiation procedure, not afterward, disrupts the devaluation effect [9]. This finding indicates a role for the amygdala in updating food values as animals approach satiety during feeding.
Figure 1.
Reinforcer devaluation. (a) Macaque monkeys chose between two objects, each associated with a different kind of food. Prior to the choice tests, one of the two foods had been devalued via selective satiation. Plot summarizes data from three different studies to show results from intact, control monkeys (pooled across studies) and monkeys with bilateral lesions of the amygdala [6], bilateral lesions of orbital prefrontal cortex (PFo), and crossed disconnection (illustrated in the inset) of the amygdala (A) from PFo (PFo × A). Each operated group differs significantly from controls. Error bars: SEM. (b) Results [7•,8•] from control monkeys and monkeys with bilateral lesions of the dorsolateral prefrontal cortex (area 46, PFdl), ventral prefrontal cortex (area 12, PFv), and PFo. *, significantly different from controls.
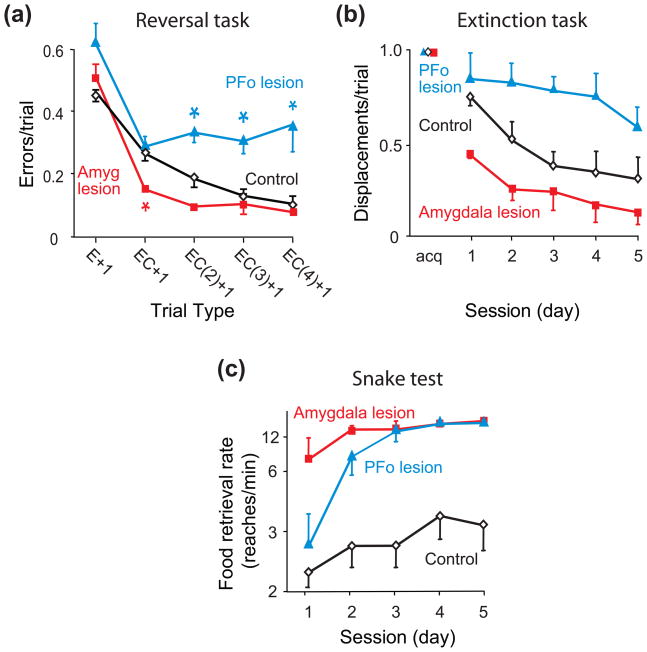
Other results point to contrasting roles for PFo and the amygdala. In the serial object-reversal learning task, macaque monkeys learn to choose a previously unrewarded object and to avoid a previously rewarded one. During this task, PFo and amygdala lesions both influence the use of positive feedback, but in opposite ways [10••]. PFo lesions cause a deficit in using positive feedback (Fig. 2a, blue), whereas amygdala lesions lead to an improvement (Fig. 2a, red). In contrast, monkeys with both kinds of lesions use negative feedback nearly normally [10••, cf. 11].
Figure 2.
Amygdala and PFo contributions to three behavioral tests in macaque monkeys. (a) Serial object-reversal learning [10••]. In this task, monkeys chose between two objects, and only one choice produced a food reward. The choice that did so changed periodically. Across a series of such reversals, monkeys with bilateral lesions of orbital prefrontal cortex (PFo) benefited less than controls after two, three or four correct (C) choices following an error (E) [EC(2)+1, EC(3)+1, and EC(4)+1 trial types, respectively]. Monkeys with bilateral amygdala (Amyg) lesions benefited more than controls when an error trial was followed by a single correct choice (EC+1 trial type). *, significantly different from controls. (b) Extinction. Monkeys first learned to displace a single object to obtain a food reward hidden underneath. After five consecutive days of consistently displacing the object to obtain food reward (acquisition, acq), object displacement failed to produce food over the next five days [12]. (c) Snake test. Food-retrieval rates when monkeys had to reach over a rubber snake to retrieve food [13]. Amygdala lesion group differed significantly from controls on all five sessions, whereas PFo group differed from controls on sessions 3–5 only. The fastest retrieval rates matched closely the times for reaching over neutral objects. Error bars: SEM.
Three additional tests also reveal contrasts in PFo and amygdala function: the extinction task, the snake test, and Pavlovian conditioning. Like serial object-reversal learning, the extinction task involves changes in object-reward associations. Initially, displacement of an object produces a reward, but the extinction task rescinds this association. In macaque monkeys, PFo lesions delay the extinction of object displacement (Fig. 2b, blue), whereas amygdala lesions speed extinction (Fig. 2b, red) [12]. These findings show that PFo function promotes this kind of learning and that amygdala function interferes with it.
In the snake test, macaque monkeys need to reach over either a neutral object or a rubber snake to retrieve food. Food-retrieval latency quantifies fear in the presence of the snake, which appears once per day in a series of trials administered every other day for five days. On trials with neutral objects, all monkeys retrieve the food quickly (data not shown). On ‘snake trials’, control monkeys retrieve the food slowly, if at all (Fig. 2c, black). After PFo lesions, monkeys reach for the food quickly (Fig. 2c, blue), as do monkeys with amygdala lesions (Fig. 2c, red) [13,14]. The important finding is this: for PFo lesions—but not for amygdala lesions—this abnormally fast food retrieval occurs only after an initial exposure to the frightening object.
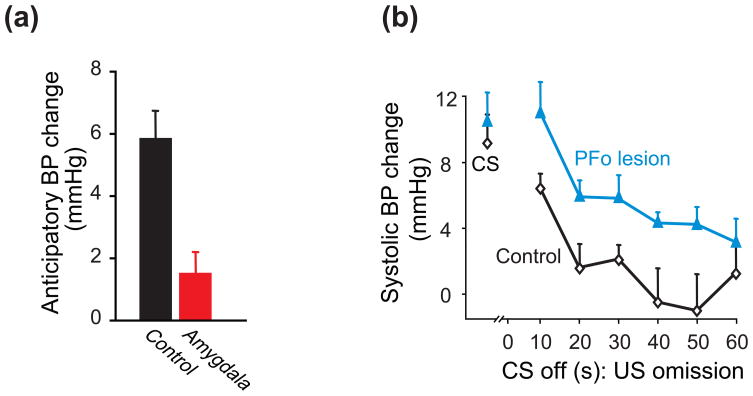
In Pavlovian conditioning, a conditioned stimulus (CS) predicts the occurrence of a future unconditioned stimulus (US). For example, the sight of food (CS) can indicate its later availability (US). In this situation, marmosets with amygdala lesions show impaired anticipatory autonomic responses to imminent food availability (Fig. 3a) [15]. In another example, a tone (CS) can indicate the future availability of a food reward (US). Then, on a probe test in which the CS stops and the predicted reward fails to appear, marmosets with PFo lesions maintain an abnormally high level of arousal (Fig. 3b) [16••].
Figure 3.
Pavlovian conditioning in marmoset monkeys. (a) Over several trials, marmosets saw a highly valued food for 20 s before being allowed to consume it. In intact monkeys, systolic blood pressure (BP) increased during the 20-s anticipatory phase; the amygdala lesion group displayed significantly lower BP relative to controls [15]. The Measure: millimeters of mercury (Hg). (b) First, marmosets learned that a tone (the conditioned stimulus, CS) indicated the availability of food (the unconditioned stimulus, US) during CS presentation. On a subsequent probe trial, the tone stopped (CS off) and the predicted food failed to appear (US omission). Points to left show BP during tone presentation; the series of points to the right show BP as a function of time after CS offset on the probe trial [16••]. Error bars: SEM.
Amygdala function
The amygdala appears to link stimuli to innate behaviors, including autonomic responses. This idea accounts for the most well known amygdala function, fear conditioning, which involves associating an initially neutral stimulus, a CS, with innate (unconditioned) fear responses [17]. Can the amygdala results presented here be understood in that framework?
Like fear conditioning, the devaluation effect involves linking an initially neutral stimulus with an innate response: an unlearned valuation response. In this case, the valuation response reflects the decreased value of a food as animals consume it to satiety and includes the affective responses generated by that valuation. The valuation response is unlearned in the sense that animals do not have to learn that a food becomes less valuable with satiety or that they should produce a certain autonomic response on that basis. Because amygdala-PFo interactions underlie the choice of objects leading to high-value foods (Fig. 1a), and because the amygdala is only necessary during the devaluation (satiety) process [9], the amygdala appears to update representations of food value stored elsewhere, including PFo. We view such updated representations as memories of the instinctive valuation responses generated by the amygdala.
Indeed, updating valuations could be a general amygdala function. For instance, when response outcomes involve two or more foods, updated valuations depend on the basolateral amygdala [18], with the updating tied to each food’s specific sensory qualities. In contrast, the central nucleus of the amygdala conveys updated value in a more general sense, one independent of a food’s specific sensory qualities [19]. Updating can also explain the influence of rewarded stimuli on tests of Pavlovian-to-instrumental transfer (PIT). For central-nucleus dependent tasks, lesions of the nucleus accumbens core (but not shell) disrupt PIT [20], whereas for basolateral-dependent tasks, shell (but not core) lesions have that effect [21]. Updated valuations provided by the amygdala to the nucleus accumbens probably mediate these two effects. In another aspect of stimulus valuation, called salience or emotional attention (Fig. 4), the amygdala appears to enhance the processing of emotional facial expressions in visual cortex [22]. In accord with the foregoing discussion about PFo, this phenomenon could reflect another aspect of linking updated valuation responses, generated by the amygdala, to sensory representations elsewhere.
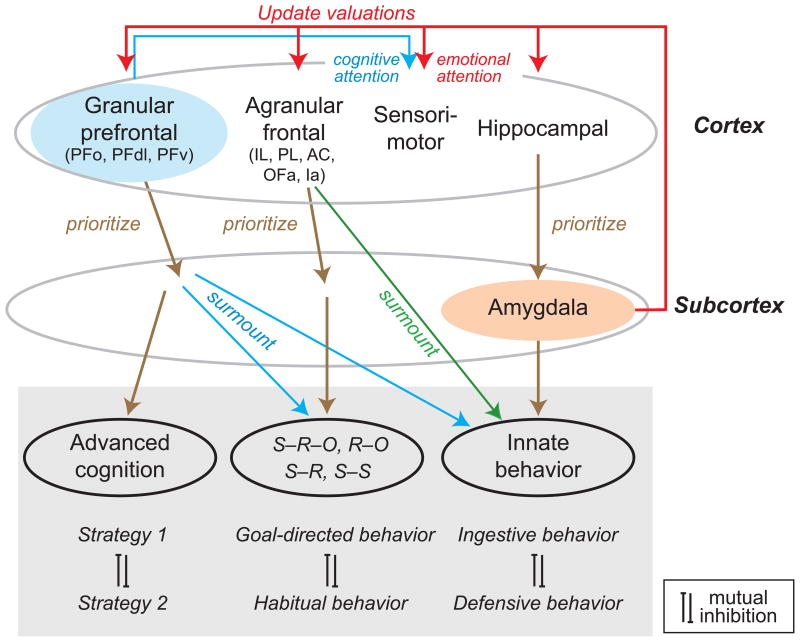
Figure 4.
Scheme of interactions among cortical (top row) and subcortical (middle row) structures. According to this scheme, the granular prefrontal cortex prioritizes learned strategies, rules and other aspects of advanced cognition; the agranular frontal cortex prioritizes behaviors based on learned associations among stimuli (S), responses (R), and outcomes (O); and the hippocampal cortex and amygdala prioritize innate behaviors. The granular prefrontal cortex also implements advanced cognition by suppressing conditioned behaviors when warranted, and both the granular and agranular parts of frontal cortex suppress innate behaviors when warranted. Cortical-field abbreviations from left to right: PFo, PFdl and PFv, orbital, dorsolateral and ventral prefrontal cortex, respectively; IL, infralimbic cortex; PL, prelimbic cortex; AC, anterior cingulate cortex; OFa, agranular orbitofrontal cortex; Ia, agranular insular cortex.
A role for the amygdala in innate valuation responses could also account for results from the reversal, extinction and snake tests. In the first two cases, amygdala function contributes to linking neutral objects with positive affective responses; in the third case it contributes to linking a frightening object to negative affective responses. The reversal [10••,11] and extinction [11,12] tasks invite responses to objects linked to positive affective responses, which reflect experience prior to the change in object-reward contingencies that occurs in both tasks. Removal of the amygdala could enhance task performance (Fig. 2a,b, red) by making it easier for the monkeys to avoid objects that would, were the amygdala intact, produce positive affective responses. This idea also accounts for the finding, in rats, that basolateral amygdala lesions abolish reversal impairments induced by lesions of the agranular orbitofrontal cortex [23]. In the snake test, disrupting the linkage of stimuli with negative affective responses leads to faster, relatively fearless food retrieval (Fig. 2c, red).
Prefrontal function
The granular prefrontal cortex (PFg) stores knowledge about ordered sequences of events and actions played out over various time frames [24]. This knowledge plays a central role in acquiring strategies, learning sets and high-order rules, along with other aspects of advanced cognition [see ref. 25 for a consideration of high- vs. low-order rules]. Accordingly, PFg lesions cause deficits in behaviors guided by high-order rules and strategies [7•,26,27,28••], as well as learning sets [29•], which involve the use of rules and strategies to speed new learning. PFg functions both in new learning about events and actions distributed over time, as well as the recall of rules and strategies based on these events [29•,30••,31••,32].
PFo is a key part of PFg. It represents valuations of biological importance [33,34,35], which object representations can access via multimodal sensory inputs to PFo. For objects, inputs from inferotemporal and perirhinal cortex play an important role in creating these linkages. PFo also receives direct inputs from the amygdala, as well as relatively direct inputs from olfactory, gustatory and visceral cortex. Thus, inputs to PFo have a unique pattern of convergence that makes PFo ideal for linking stimuli to outcomes. There is, however, no reason to assume that its functions are limited to linking predicted outcomes with simple representations, such as those for sensory stimuli or stimulus sequences. In advanced cognition, an analogous process could create linkages between outcomes and high-order rules [36], strategies or learning sets. Can this idea account for the PFo results presented here?
In the snake test, monkeys with PFo lesions perform fearfully, like intact monkeys, on the first exposure to the rubber ‘snake’, but later act relatively fearlessly, like monkeys with amygdala lesions (Fig. 2c, blue). We think that this could occur because PFo learns about the outcome of response strategies in the overall testing situation, as opposed to learning about stimulus-outcome associations on a trial-by-trial basis. According to the present account, both PFo-lesioned and intact monkeys avoid the ‘snake’ on the first exposure because of the risk involved and the associated affective response (as signaled by the intact amygdala). The first ‘snake trial’ teaches intact monkeys that they can obtain a reward with only a modest cost in terms of delay and still avoid the frightening object. Thus, they forego taking food on subsequent ‘snake trials’ because they know that food can be obtained on a safe, neutral-object trial that will soon follow. Monkeys with PFo lesions never learn this more sophisticated response strategy and so their behavior is dominated by simpler factors, which lead to delay intolerance and rapid food retrieval, ‘snake’ or no ‘snake’.
PFo enhances the use of positive feedback to make correct choices after object-reward reversals (Fig. 2a, blue), and this finding, too, might be explained in terms of rules, strategies and learning set. We think that PFo uses positive feedback to evaluate and implement a form of learning set called a reversal set: the ability to shift choices quickly between two objects that depends on experience with previous reversals. Indeed, object valuations based on prior experience interfere with performance immediately after reversals, and a reversal set helps overcome this problem. This idea complements a previous one about the anterior cingulate cortex (AC). In a study of action-reward reversals, as opposed to the object-reward reversals discussed so far, Kennerley et al. [37] found that AC lesions impair the use of positive feedback to stay with correct actions. It also complements two other findings about PFo: PFo lesions disrupt choices among three objects when reward follows a dynamic, stochastic schedule [38••] and they also cause impairments in switching between well-learned rules [28••]. Taking these results together, we think that reward feedback in PFo (and AC) performs several functions. It guides the evaluation of stimuli (PFo) and actions (AC), but it also guides the evaluation of response rules such as a reversal set. Aversive feedback, also available in PFo [34], could function similarly, but not through response inhibition, as generally assumed. Instead, the greater importance of positive feedback vs. negative (nonreward) feedback in the reversal task [10••] argues against response inhibition and perseveration as useful concepts in understanding PFo function. As usually construed, failures of response inhibition result from a deficit in using negative feedback to switch from a given response, thus causing perseveration. Instead, we think that PFo contributes to the assignment of value to the reversal set, along with each of the choice objects.
PFo-mediated cognition could also account for results from the extinction task (Fig. 2b) and Pavlovian conditioning (Fig. 3b). Knowledge about the overall testing situation would speed “extinction” once intact monkeys learn that food availability does not follow object displacement. The distinction here is between knowing that reward will not appear and the suppression of a reflex-like stimulus-response association. Monkeys with PFo lesions would, according to this account, persistently displace the object (Fig. 2b) because they lack certain knowledge. In this case, for example, monkeys with PFo lesions would be unable to apply a rule such as “unrewarded objects remain unrewarded.” Similarly, for Pavlovian conditioning, we think that PFo stores knowledge about the many circumstances that do not involve rewards. Recall that in the experiment described above, a tone CS predicts reward, which becomes available during tone presentation. In a probe trial, however, the tone turns off and the reward fails to appear. According to this account, PFo suppresses anticipatory autonomic arousal after 10–20 s, once monkeys recognize the low-value context signaled by the absence of both the CS and the predicted reward. PFo lesions cause a more persistent arousal (Fig. 3b) because of the loss of knowledge about this low-value context.
Cortex-subcortex interactions
PFo and amygdala interactions occur in the context of cortex-subcortex interactions, generally (Fig. 4). According to Petrovich and Swanson [39], hippocampus-amygdala interactions prioritize the expression of competing innate behaviors, such as feeding and defensive behaviors. This function could explain the role of hippocampus in enabling snake fear on the second and later exposures [40] and in choice behavior based on an animal’s current motivational state (e.g., hungry vs. thirsty) [41,42]. Hippocampus-amygdala interactions can be viewed as one aspect of cortical-subcortical interactions.
According to the scheme presented in Figure 4, the agranular frontal cortex interacts with subcortical structures to prioritize behaviors based on learned associations among stimuli, responses and outcomes and overrides innate behaviors that also compete for control of behavior (Fig. 4, green) [43]. As an example of the latter, the matching-to-position task requires rats to surmount an innate tendency to avoid recently exploited foraging sites. Intact, control rats learn to surmount that instinctive tendency, but lesions of the medial agranular frontal cortex disrupt this learning [44].
Medial frontal cortex also prioritizes competing conditioned behaviors (Fig. 4, brown). Rats with AC lesions show deficits when two or more stimuli vie for control of behavior [45,46] and when two or more actions compete based on cost-benefit tradeoffs [47,48,49]. Another medial frontal area, prelimbic cortex (PL), biases behavioral control toward goal-directed (R–O) associations, whereas infralimbic cortex (IL) biases behavior toward outcome-independent, S–R associations (habits) [50]. These lesions limit the ability of rats to switch nimbly between competing behaviors. Inactivation of PL + IL cortex does not, however, affect the ability of rats to switch between two other competing conditioned responses, one based on extrinsic coordinates (e.g., “go North” ) and the other based on intrinsic coordinates (e.g., “go left” ). Such inactivation does, however, disrupt the ability of rats to use the most recently learned response 24 hours later [51•], which reflects a problem in retaining competing behaviors. Other parts of the agranular frontal cortex also bias the selection of competing behaviors [52,53,54,55]. For example, inactivating the agranular orbitofrontal cortex (OFa) leads to poor retention of reversals within (but not between) coordinate frames [56••].
Figure 4 also depicts PFg, which includes PFo, as prioritizing behaviors that depend on advanced cognition, such as competing high-order rules and strategies, as well as competing internal vs. sensory influences over behavior [57,58]. Consistent with a role in advanced cognition, the areas composing PFg are primate innovations, whereas most if not all mammals share the agranular frontal areas mentioned above [59]. PFo probably mediates its prioritizing function in part via corticostriatal signals that establish task sets [60].
In addition to prioritizing advanced behaviors, PFg also functions to surmount behaviors that depend on conditioned associations among stimuli, responses and outcomes, and also to surmount instinctive behaviors (Fig. 4, blue). For example, to develop a learning set monkeys surmount conditioned associations by using various strategies, including knowing what information to retain in working memory [29•,61].
Conclusion
The findings featured here point to both cooperating and contrasting functions of PFo and the amygdala. Cooperation arises when PFo depends on the value-updating function of the amygdala, whereas contrasting functions reflect, among other things, PFo’s role in surmounting behaviors that depend on the amygdala. In rejecting Morgan‘s canon, we have discussed PFo-amygdala interactions in terms of both advanced cognition and innate behaviors, in addition to conditioned associations. Likewise, future research should embrace accounts of animal behavior that extend beyond the behaviorist trinity of stimuli, responses and outcomes.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Mental Health. We thank Dr. Matthew Shapiro and Dr. Peter Rudebeck for their comments on an earlier version of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Morgan CL. An Introduction to Comparative Psychology. 2. London: W. Scott; 1903. [Google Scholar]
- 2.Bunge M. Philosophical Dictionary. New York: Prometheus; 2003. [Google Scholar]
- 3.Hatfield T, Han J, Conley M, Gallagher M, Holland P. Neurotoxic lesions of basolateral, but not central, amygdala interfere with Pavlovian second-order conditioning and reinforcer devaluation effects. J Neurosci. 1996;16:5256–5265. doi: 10.1523/JNEUROSCI.16-16-05256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malkova L, Gaffan D, Murray EA. Excitotoxic lesions of the amygdala fail to produce impairment in visual learning for auditory secondary reinforcement but interfere with reinforcer devaluation effects in rhesus monkeys. J Neurosci. 1997;17:6011–6020. doi: 10.1523/JNEUROSCI.17-15-06011.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balleine BW, Killcross AS, Dickinson A. The effect of lesions of the basolateral amygdala on instrumental conditioning. J Neurosci. 2003;23:666–675. doi: 10.1523/JNEUROSCI.23-02-00666.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Izquierdo A, Murray EA. Selective bilateral amygdala lesions in rhesus monkeys fail to disrupt object reversal learning. J Neurosci. 2007;27:1054–1062. doi: 10.1523/JNEUROSCI.3616-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7•.Baxter MG, Gaffan D, Kyriazis DA, Mitchell AS. Ventrolateral prefrontal cortex is required for performance of a strategy implementation task but not reinforcer devaluation effects in rhesus monkeys. Eur J Neurosci. 2009;29:2049–2059. doi: 10.1111/j.1460-9568.2009.06740.x. The authors found that bilateral lesions of PFo in macaques disrupted the devaluation effect, but PFv lesions had no such effect. Conversely, PFv lesions but not PFo lesions caused deficits in strategy implementation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8•.Baxter MG, Gaffan D, Kyriazis DA, Mitchell AS. Dorsolateral prefrontal lesions do not impair tests of scene learning and decision-making that require frontal-temporal interaction. Eur J Neurosci. 2008;28:491–499. doi: 10.1111/j.1460-9568.2008.06353.x. The authors found that bilateral lesions of the PFdl in macaques had no effect on strategy implementation, scene learning or reinforcer devaluation. These data together with data in [7•] indicate a functional specialization within prefrontal cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wellman LL, Gale K, Malkova L. GABAA-mediated inhibition of basolateral amygdala blocks reward devaluation in macaques. J Neurosci. 2005;25:4577–4586. doi: 10.1523/JNEUROSCI.2257-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10••.Rudebeck PH, Murray EA. Amygdala and orbitofrontal cortex lesions differentially influence choices during object reversal learning. J Neurosci. 2008;28:8338–8343. doi: 10.1523/JNEUROSCI.2272-08.2008. The authors studied serial object-reversal learning in macaques and found opposing effects of amygdala and PFo lesions. Monkeys with PFo lesions benefited less than controls from correctly performed trials after an error and, contrary to a widely held view, showed little or no perseveration or loss of behavioral inhibition. Monkeys with amygdala lesions benefited more than controls from positive feedback. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clarke HF, Robbins TW, Roberts AC. Lesions of the medial striatum in monkeys produce perseverative impairments during reversal learning similar to those produced by lesions of the orbitofrontal cortex. J Neurosci. 2008;28:10972–10982. doi: 10.1523/JNEUROSCI.1521-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Izquierdo A, Murray EA. Opposing effects of amygdala and orbital prefrontal cortex lesions on the extinction of instrumental responding in macaque monkeys. Eur J Neurosci. 2005;22:2341–2346. doi: 10.1111/j.1460-9568.2005.04434.x. [DOI] [PubMed] [Google Scholar]
- 13.Izquierdo A, Suda RK, Murray EA. Comparison of the effects of bilateral orbital prefrontal cortex lesions and amygdala lesions on emotional responses in rhesus monkeys. J Neurosci. 2005;25:8534–8542. doi: 10.1523/JNEUROSCI.1232-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rushworth MF, Behrens TE, Rudebeck PH, Walton ME. Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behaviour. Trends Cogn Sci. 2007;11:168–176. doi: 10.1016/j.tics.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Braesicke K, Parkinson JA, Reekie Y, Man MS, Hopewell L, Pears A, Crofts H, Schnell CR, Roberts AC. Autonomic arousal in an appetitive context in primates: a behavioural and neural analysis. Eur J Neurosci. 2005;21:1733–1740. doi: 10.1111/j.1460-9568.2005.03987.x. [DOI] [PubMed] [Google Scholar]
- 16••.Reekie YL, Braesicke K, Man MS, Roberts AC. Uncoupling of behavioral and autonomic responses after lesions of the primate orbitofrontal cortex. Proc Natl Acad Sci USA. 2008;105:9787–9792. doi: 10.1073/pnas.0800417105. The authors combined autonomic measures with Pavlovian conditioning procedures in marmoset monkeys and studied the effect of bilateral PFo lesions. Compared to controls, lesioned monkeys were slower to adapt their blood-pressure responses to reversals of stimulus reward associations and to the absence of a predicted reward. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LeDoux J. The amygdala. Curr Biol. 2007;17:R868–R874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Johnson AW, Gallagher M, Holland PC. The basolateral amygdala is critical to the expression of Pavlovian and instrumental outcome-specific reinforcer devaluation effects. J Neurosci. 2009;29:696–704. doi: 10.1523/JNEUROSCI.3758-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corbit LH, Balleine BW. Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of Pavlovian-instrumental transfer. J Neurosci. 2005;25:962–970. doi: 10.1523/JNEUROSCI.4507-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall J, Parkinson JA, Connor TM, Dickinson A, Everitt BJ. Involvement of the central nucleus of the amygdala and nucleus accumbens core in mediating Pavlovian influences on instrumental behaviour. Eur J Neurosci. 2001;13:1984–1992. doi: 10.1046/j.0953-816x.2001.01577.x. [DOI] [PubMed] [Google Scholar]
- 21.Corbit LH, Muir JL, Balleine BW. The role of the nucleus accumbens in instrumental conditioning: Evidence of a functional dissociation between accumbens core and shell. J Neurosci. 2001;21:3251–3260. doi: 10.1523/JNEUROSCI.21-09-03251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vuilleumier P, Driver J. Modulation of visual processing by attention and emotion: windows on causal interactions between human brain regions. Philos Trans Roy Soc Lond B Biol Sci. 2007;362:837–855. doi: 10.1098/rstb.2007.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stalnaker TA, Franz TM, Singh T, Schoenbaum G. Basolateral amygdala lesions abolish orbitofrontal-dependent reversal impairments. Neuron. 2007;54:51–58. doi: 10.1016/j.neuron.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 24.Wood JN, Grafman J. Human prefrontal cortex: processing and representational perspectives. Nat Rev Neurosci. 2003;4:139–147. doi: 10.1038/nrn1033. [DOI] [PubMed] [Google Scholar]
- 25.Wise SP, Murray EA, Gerfen CR. The frontal cortex - basal ganglia system in primates. Crit Rev Neurobiol. 1996;10:317–356. doi: 10.1615/critrevneurobiol.v10.i3-4.30. [DOI] [PubMed] [Google Scholar]
- 26.Gaffan D, Easton A, Parker A. Interaction of inferior temporal cortex with frontal cortex and basal forebrain: Double dissociation in strategy implementation and associative learning. J Neurosci. 2002;22:7288–7296. doi: 10.1523/JNEUROSCI.22-16-07288.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bussey TJ, Wise SP, Murray EA. The role of ventral and orbital prefrontal cortex in conditional visuomotor learning and strategy use in rhesus monkeys (Macaca mulatta) Behav Neurosci. 2001;115:971–982. doi: 10.1037//0735-7044.115.5.971. [DOI] [PubMed] [Google Scholar]
- 28••.Buckley MJ, Mansouri FA, Hoda H, Mahboubi M, Browning PG, Kwok SC, Phillips A, Tanaka K. Dissociable components of rule-guided behavior depend on distinct medial and prefrontal regions. Science. 2009;325:52–58. doi: 10.1126/science.1172377. The authors examined the effects of bilateral lesions of different parts of PFg on macaque monkeys’ abilities to switch between two rules, match-to-color and match-to-shape, cued only by reward or non-reward feedback. Lesions of PFo impaired the ability of monkeys to switch between these rules. [DOI] [PubMed] [Google Scholar]
- 29•.Browning PGF, Easton A, Gaffan D. Frontal-temporal disconnection abolishes object discrimination learning set in macaque monkeys. Cereb Cortex. 2007;17:859–864. doi: 10.1093/cercor/bhk039. The authors show that functionally decoupling the frontal cortex from the inferotemporal cortex abolishes visual discrimination learning set in macaques. Learning set is the rapid learning that develops when monkeys acquire experience in making such discriminations. After the lesions, monkeys returned to the same slow learning rate they showed prior to developing the learning set. [DOI] [PubMed] [Google Scholar]
- 30••.Browning PG, Gaffan D. Prefrontal cortex function in the representation of temporally complex events. J Neurosci. 2008;28:3934–3940. doi: 10.1523/JNEUROSCI.0633-08.2008. The authors functionally decoupled the frontal and inferotemporal cortex in macaques either by crossed surgical disconnection or by bilateral transection of a fiber bundle connecting them. Both lesions caused a severe impairment in acquiring simple visual discriminations involving a two-item sequence of stimuli, but not in acquiring discriminations involving two simultaneously presented stimuli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31••.Browning PGF, Gaffan D. Global retrograde amnesia but selective anterograde amnesia after frontal-temporal disconnection in monkeys. Neuropsychologia. 2008;46:2494–2502. doi: 10.1016/j.neuropsychologia.2008.04.012. The authors functionally decoupled the inferotemporal and prefrontal cortex and studied both simultaneous and temporally extended visual discriminations. The lesion disrupted retention of both, but impaired acquisition of only the latter. These findings show that frontal-temporal interaction plays a general role in retrieving visual memories and a specific role in acquisition of visual memories of events that play out over an extended time frame. [DOI] [PubMed] [Google Scholar]
- 32.Wilson CRE, Gaffan D, Mitchell AS, Baxter MG. Neurotoxic lesions of ventrolateral prefrontal cortex impair object-in-place scene memory. Eur J Neurosci. 2007;25:2514–2522. doi: 10.1111/j.1460-9568.2007.05468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Padoa-Schioppa C. Range-adapting representation of economic value in the orbitofrontal cortex. J Neurosci. 2009;29:14004–14014. doi: 10.1523/JNEUROSCI.3751-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morrison SE, Salzman CD. The convergence of information about rewarding and aversive stimuli in single neurons. J Neurosci. 2009;29:11471–11483. doi: 10.1523/JNEUROSCI.1815-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kennerley SW, Dahmubed AF, Lara AH, Wallis JD. Neurons in the frontal lobe encode the value of multiple decision variables. J Cogn Neurosci. 2008;21:1162–1178. doi: 10.1162/jocn.2009.21100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaffan D. Hippocampus: memory, habit and voluntary movement. Philos Trans Roy Soc Lond B Biol Sci. 1985;308:87–99. doi: 10.1098/rstb.1985.0012. [DOI] [PubMed] [Google Scholar]
- 37.Kennerley SW, Walton ME, Behrens TE, Buckley MJ, Rushworth MF. Optimal decision making and the anterior cingulate cortex. Nat Neurosci. 2006;9:940–947. doi: 10.1038/nn1724. [DOI] [PubMed] [Google Scholar]
- 38••.Rudebeck PH, Behrens TE, Kennerley SW, Baxter MG, Buckley MJ, Walton ME, Rushworth MF. Frontal cortex subregions play distinct roles in choices between actions and stimuli. J Neurosci. 2008;28:13775–13785. doi: 10.1523/JNEUROSCI.3541-08.2008. The authors studied the effects of lesions of the PFo and anterior cingulate cortex in macaques on learning object reward and action reward associations. Unlike previous studies, which used deterministic stimulus reward associations, in this study the associations were stochastic. PFo lesions produced a deficit in stimulus-based but not action-based learning, whereas anterior cingulate lesions had the opposite effect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Rev. 2001;38:247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- 40.Chudasama Y, Wright KS, Murray EA. Hippocampal lesions in rhesus monkeys disrupt emotional responses but not reinforcer devaluation effects. Biol Psychiat. 2008;63:1084–1091. doi: 10.1016/j.biopsych.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 41.Kennedy PJ, Shapiro ML. Retrieving memories via internal context requires the hippocampus. J Neurosci. 2004;24:6979–6985. doi: 10.1523/JNEUROSCI.1388-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stouffer EM, White NM. Neural circuits mediating latent learning and conditioning for salt in the rat. Neurobiol Learn Memory. 2006;86:91–99. doi: 10.1016/j.nlm.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 43.Murray EA, Wise SP, Rhodes SEV. What can different brains do with reward? In: Gottfried JA, editor. The Neurobiology of Sensation and Reward. Boca Raton, FL: Taylor & Francis; 2009. [Google Scholar]
- 44.Dias R, Aggleton JP. Effects of selective excitotoxic prefrontal lesions on acquisition of nonmatching- and matching-to-place in the T-maze in the rat: differential involvement of the prelimbic-infralimbic and anterior cingulate cortices in providing behavioural flexibility. Eur J Neurosci. 2000;12:4457–4466. doi: 10.1046/j.0953-816x.2000.01323.x. [DOI] [PubMed] [Google Scholar]
- 45.Cardinal RN, Parkinson JA, Lachenal G, Halkerston KM, Rudarakanchana N, Hall J, Morrison CH, Howes SR, Robbins TW, Everitt BJ. Effects of selective excitotoxic lesions of the nucleus accumbens core, anterior cingulate cortex, and central nucleus of the amygdala on autoshaping performance in rats. Behav Neurosci. 2002;116:553–567. doi: 10.1037//0735-7044.116.4.553. [DOI] [PubMed] [Google Scholar]
- 46.Cardinal RN, Parkinson JA, Marbini HD, Toner AJ, Bussey TJ, Robbins TW, Everitt BJ. Role of the anterior cingulate cortex in the control over behavior by Pavlovian conditioned stimuli in rats. Behav Neurosci. 2003;117:566–587. doi: 10.1037/0735-7044.117.3.566. [DOI] [PubMed] [Google Scholar]
- 47.Walton ME, Bannerman DM, Rushworth MF. The role of rat medial frontal cortex in effort-based decision making. J Neurosci. 2002;22:10996–11003. doi: 10.1523/JNEUROSCI.22-24-10996.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schweimer J, Hauber W. Involvement of the rat anterior cingulate cortex in control of instrumental responses guided by reward expectancy. Learn Mem. 2005;12:334–342. doi: 10.1101/lm.90605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rudebeck PH, Walton ME, Smyth AN, Bannerman DM, Rushworth MF. Separate neural pathways process different decision costs. Nat Neurosci. 2006;9:1161–1168. doi: 10.1038/nn1756. [DOI] [PubMed] [Google Scholar]
- 50.Killcross S, Coutureau E. Coordination of actions and habits in the medial prefrontal cortex of rats. Cereb Cortex. 2003;13:400–408. doi: 10.1093/cercor/13.4.400. [DOI] [PubMed] [Google Scholar]
- 51••.Rich EL, Shapiro ML. Prelimbic/infralimbic inactivation impairs memory for multiple task switches, but not flexible selection of familiar tasks. J Neurosci. 2007;27:4747–4755. doi: 10.1523/JNEUROSCI.0369-07.2007. The authors trained rats to perform according to one of two different conditioned responses in a plus maze: one based on extrinsic coordinates, the other based on intrinsic coordinates. Muscimol inactivation of PL+ IL did not impair the acquisition of learning in the second coordinate frame, but did impair memory for it 24 hours later, when the rats were drug free. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burke KA, Takahashi YK, Correll J, Brown PL, Schoenbaum G. Orbitofrontal inactivation impairs reversal of Pavlovian learning by interfering with ‘disinhibition’ of responding for previously unrewarded cues. Eur J Neurosci. 2009;30:1941–1946. doi: 10.1111/j.1460-9568.2009.06992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winstanley CA, Theobald DE, Cardinal RN, Robbins TW. Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. J Neurosci. 2004;24:4718–4722. doi: 10.1523/JNEUROSCI.5606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kheramin S, Body S, Mobini S, Ho MY, Velazquez-Martinez DN, Bradshaw CM, Szabadi E, Deakin JF, Anderson IM. Effects of quinolinic acid-induced lesions of the orbital prefrontal cortex on inter-temporal choice: a quantitative analysis. Psychopharmacol (Berl) 2002;165:9–17. doi: 10.1007/s00213-002-1228-6. [DOI] [PubMed] [Google Scholar]
- 55.Kesner RP, Gilbert PE. The role of the agranular insular cortex in anticipation of reward contrast. Neurobiol Learn Mem. 2007;88:82–86. doi: 10.1016/j.nlm.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56••.Young JJ, Shapiro ML. Double dissociation and hierarchical organization of strategy switches and reversals in the rat PFC. Behav Neurosci. 2009;123:1028–1035. doi: 10.1037/a0016822. The authors inactivated the OFa and PL+IL in rats and studied conditioned responses in extrinsic and intrinsic coordinate frames. Neither inactivation affected the acquisition or performance of the tasks. When rats were tested 24 hours later, however, prior PL+IL inactivations impaired switches between but not within frames, whereas prior OFa inactivations did the opposite, although the impairment disappeared after repeated reversals. Thus activity in PL+IL and OFa during learning contributed to later retention. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith CD, Burgess PW. Functional specialization within rostral prefrontal cortex (Area 10): A meta-analysis. J Cogn Neurosci. 2006;18:932–948. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- 58.Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wise SP. Forward frontal fields: phylogeny and fundamental function. Trends Neurosci. 2008;31:599–608. doi: 10.1016/j.tins.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aron AR, Watkins L, Sahakian BJ, Monsell S, Barker RA, Robbins TW. Task-set switching deficits in early-stage Huntington’s disease: Implications for basal ganglia function. J Cogn Neurosci. 2003;15:629–642. doi: 10.1162/089892903322307357. [DOI] [PubMed] [Google Scholar]
- 61.Murray EA, Gaffan D. Prospective memory in the formation of learning sets by rhesus monkeys (Macaca mulatta) J Exp Psychol Anim Behav Process. 2006;32:87–90. doi: 10.1037/0097-7403.32.1.87. [DOI] [PubMed] [Google Scholar]