Abstract
The phytochemical curcumin, from the Indian spice turmeric, has many biological properties, including anti-inflammatory and anti-carcinogenic activities. We have examined the effects of curcumin on the rat C6 glioma cell line. Treated and control cells were analyzed by Hoechst 33342 dye and flow cytometry. We observed a decrease in the side population (SP) of C6 cells after daily curcumin treatment of the C6 cells. Direct incubation of curcumin to C6 cells during the Hoechst assay also decreased SP. Since SP has been associated with stem cell populations, curcumin may be a dietary phytochemical with potential to target cancer stem cells.
Keywords: C6 glioma, cancer stem cells, curcumin, side population
1. Introduction
The concept of cancer stem cells (CSCs) has generated research interests because of the availability of novel techniques. A CSC is a cell within a tumor that possesses the capacity of self-renewal and can give rise to the heterogeneous lineages of cancer cells that comprise the tumor. Such a CSC is also named a tumor-initiating cell. Experimentally, these cells result in tumor xenografts when transplanted into appropriate animal models, such as the nonobese diabetic (NOD) / severe combined immunodeficiency (SCID) mice [1-3]. However, it should be emphasized that the situation is dynamic; miscellaneous conditions including the tumor microenvironment influence outcome of CSCs and the cancer [4-6].
It has been suggested that conventional chemotherapy will kill most cells in a tumor, but leave the CSCs intact, resulting in development of resistance. This is caused by the capacity of CSCs. Sharing many of the properties of normal stem cells, they are relatively quiescent and resistant to therapeutic drugs, through mechanisms including the expression of ATP-binding cassette (ABC) transporters [7, 8]. Cancer drug resistance can be overcome by phytochemicals. Our in vitro studies have shown that curcumin, from turmeric, and epigallocatechin gallate (EGCG), from green tea, act synergistically with the conventional chemotherapeutic drug cisplatin towards drug-resistant ovarian cancer cell lines; similar findings with different tumors have been reported in vitro as well as in experimental animal models by many investigators [9, 10, and review in 11]. Since CSCs lead to drug resistance, phytochemicals may also be useful in targeting these cells [review in 12].
One functional assay for stem cells is based on the activity of ABC transport, yielding a side population (SP) that retains less of the Hoechst 33342 dye. First identified as a technique to isolate murine hematopoietic stem cells from bone marrow, SP has been observed in stem cells of other tissues, as well as in tumor cells [13-15]. Therefore, SP analysis can be used to identify CSCs [16, 17]. In this paper, we reported that SP of the rat C6 glioma can be inhibited by curcumin. This rat cell line is a model for studying cell growth and invasion, and SP has been shown to be present in C6 cells [18, 19]. The phytochemical curcumin, alias diferuloylmethane, is the active ingredient of the Indian spice turmeric (it gives the yellow color of curry dishes), which is produced from rhizome of the plant Curcuma longa. Curcumin has conferred various health promoting effects in vitro and in vivo [20-22]; and multiple clinical trials using this compound are in progress [23].
2. Materials and Methods
2.1. Cell culture
Rat C6 glioma cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing high glucose and pyruvate, with 10% fetal bovine serum plus antibiotics penicillin and streptomycin (serum from HyClone, others from Invitrogen GIBCO). Cells were maintained in T25 tissue culture flasks at 37°C in a humidified 5% CO2 atmosphere. Confluent cells were harvested by washing in phosphate-buffered saline (PBS) and followed by trypsinization (0.25% in EDTA) for subculture.
2.2. Curcumin treatment
Curcumin was dissolved in the solvent dimethyl sulfoxide (Sigma) to make a stock solution of 20 mM (Cayman Chemicals). It was then diluted to final concentrations in complete medium containing 0.15% (volume/volume) solvent. C6 cells harvested from a confluent T25 flask were split 1:4 in new flasks. After allowing cells to attach (about 4 hours), new medium was added with either solvent alone, or 5 μM curcumin in solvent. Concerning the stability of the phytochemical during incubation, medium with curcumin and solvent alone were changed daily. Once the flask became confluent, 1:2 splitting was performed. At different days of the experiment, the cells were harvested and the side population (SP) was analyzed. To ensure repeatability of curcumin treatment and reliability of the results, each independent experiment was performed two or more times.
2.3. Flow cytometry
For side population analysis, harvested C6 cells were adjusted to 106 cells/ml in prewarmed DMEM containing 2% fetal bovine serum and 10 mM HEPES buffer, and then incubated for 90 minutes in a 37°C water bath with Hoechst 33342 (at 2.5 μg/ml final concentration, from 1 mg/ml stock of the dye dissolved in water, from Invitrogen Molecular Probes) and with intermittent mixing. Control cells were given verapamil (50 μM final concentration, in 95% ethanol, from Sigma). At the end of incubation, cells were centrifuged in the cold and then adjusted to 3 × 106 cells/ml in PBS containing 2% fetal bovine serum and 10 mM HEPES [19, 24, 25].
Cytometric analysis was performed on a MoFlo CDP jet-in-air high-speed sorter (Beckman Coulter, Fullerton, CA). Hoechst 33342 dye was excited at 351 nm by UV laser and its fluorescence detected at two wavelengths using 450/20 (Hoechst blue) and 670/40 nm (Hoechst red) band-pass filters. A 510 nm long-pass dichroic filter was used to split the emission wavelengths. Dead cells were excluded from the logarithmic scale plot based on propidium iodide staining (final 2 μg/ml, from Sigma) [26].
For checking the direct effect of curcumin on Hoechst dye exclusion assay, untreated C6 cells were incubated with Hoechst 33342 together with curcumin (from 5 to 20 μM) and the effect on SP was analyzed.
For the sorting of SP and non-SP of C6 cells, cells were sorted into 5 ml tubes containing complete DMEM with serum. To ensure purity, a second sort was performed for the non-SP cells. To check the dynamics of SP and non-SP cells, the sorted cells were cultured in T25 flasks until confluence and then re-analyzed for Hoechst dye exclusion.
3. Results
3.1. Effect of curcumin on C6 cell growth and side population
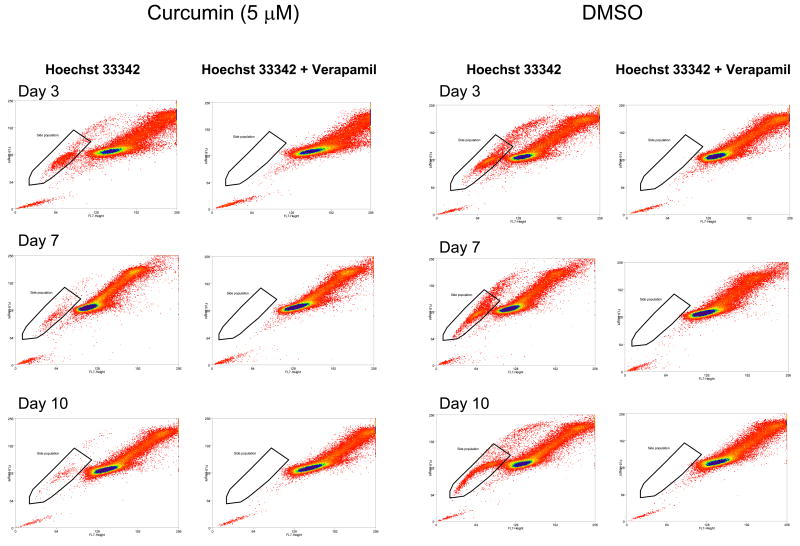
Under our experimental conditions with treatment of 5 μM curcumin, during rat C6 glioma growth, a depletion of SP was observed, detectable by the Hoechst 33342 dye exclusion assay. As shown in Fig. 1, a gradual decrease of SP was seen from day 3 to day 10, whereas the solvent control had no effect. As expectedly, verapamil, a calcium channel blocker and non-specific inhibitor of ABC transporters, inhibited SP generation. SP decreased from 3.61% on day 3, to 0.70% on day 7, and to 0.55% on day 10. Daily treatment of curcumin led to decrease of SP in C6 cells. However, at the concentration of curcumin used, we found no noticeable inhibition of C6 proliferation. Growth inhibition was observed at higher concentrations of curcumin, for example, a 20-fold decrease in cell number at 15 μM curcumin after 3 days of culture. The decision on 5 μM curcumin was based on our view of preventing growth inhibition and using the lowest concentration to detect SP decrease.
Fig.1.
Effect of curcumin on C6 cell growth and side population (SP). At days 3, 7 and 10, curcumin-treated and solvent-treated (control) cells were assayed for SP using Hoechst 33342 dye. Decrease in SP is seen only with the phytochemical-treated samples (as bracketed in graphs). SP is inhibited by control treatment with verapamil.
For C6 glioma cells, Platet et al. (2007) found that, if SP is defined by verapamil sensitivity, an additional cell population, ones with more DNA content, can be included and they named the cells “upper SP” [25]. We also detected “upper SP” in this study. Similar to SP, the “upper SP” is also decreased by 5 μM curcumin treatment (seen in Fig.1 as a “tail” of cells following the bracketed SP cells and this “upper SP” disappears after verapamil treatment).
3.2. Effect of curcumin on Hoechst dye exclusion assay
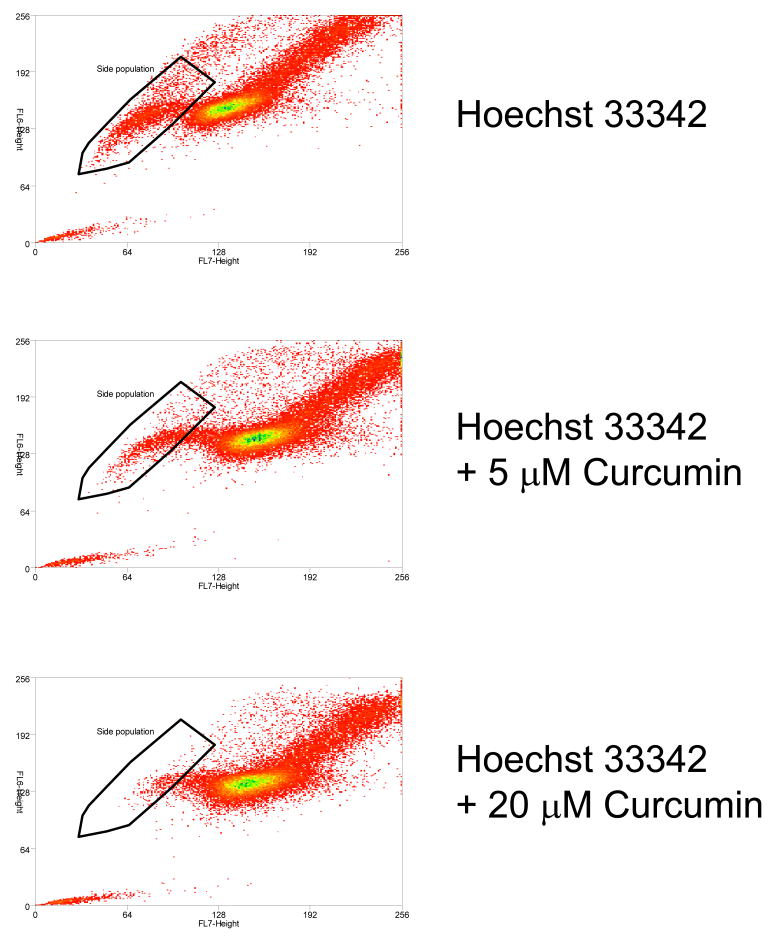
To check the direct effect of curcumin on Hoechst 33342 dye exclusion assay, different concentrations of curcumin were co-incubated with the dye during the assay. As shown in Fig.2, curcumin had a direct effect on dye exclusion activity, as the SP was decreased by treatment of curcumin (from 2.68% at 5 μM to 1.32% at 20 μM). The control, verapamil, as expected, also inhibited SP generation (data not shown).
Fig.2.
Direct effect of curcumin on the Hoechst dye assay. Addition of curcumin during the assay decreases side population (SP), and the decrease is dose-dependent.
3.3. Dynamics of C6 side population and non-side population cells
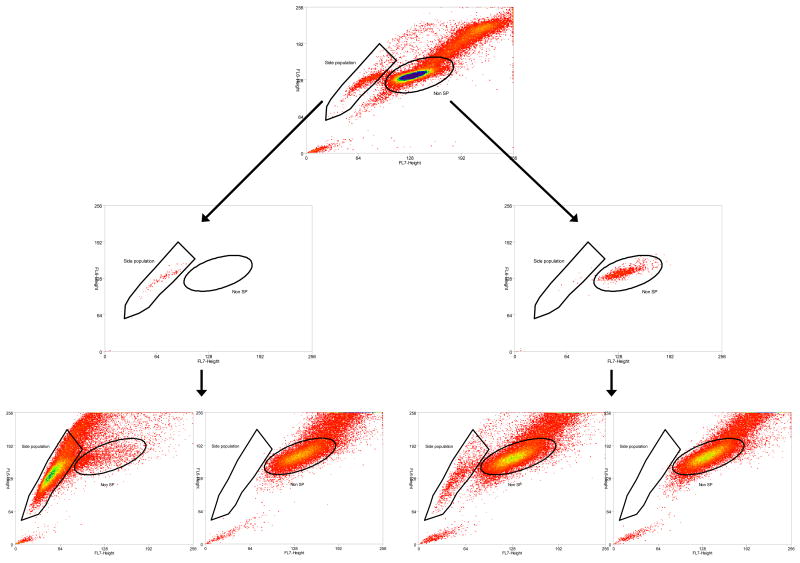
The sorted C6 SP and non-SP cells were cultured (104 cells in T25 flask). Once confluent, they were subjected to the Hoechst assay again. As shown in Fig.3, the sorted SP yielded both SP and non-SP, although the majority of the cells maintained SP characteristics. Similarly, the non-SP also yielded both, although the majority remained non-SP. This finding indicated the dynamics of C6 cells under our experimental conditions. We had also cultured the sorted cells in 96 well tissue culture plates and treated them with curcumin. Preliminary results showed both SP and non-SP cells (300 cells per well or less) were equally susceptible to curcumin (5 μM or less) (data not shown).
Fig. 3.
Dynamics of C6 side population (SP) and non-SP cells. SP and non-SP cells were sorted, cultured and then re-sorted. The top graph shows original SP and non-SP distribution. The middle graphs show cleanliness of sorting (sorted SP on the left and non-SP on the right). SP and twice-sorted non-SP cells were cultured and the re-sorting results are presented in the bottom graphs. Most SP and non-SP cells maintain their original sorted phenotype, although C6 dynamics is observed. Whereas verapamil addition depletes the SP, SP gives rise to both SP and non-SP (two graphs on the left), and non-SP gives rise to both non-SP and SP (two graphs on the right).
4. Discussion
We report here that curcumin, at low concentration (5 μM), inhibits the generation of SP of C6 glioma cells. In addition, at this concentration there is minimal effect on cell growth. Our result confirms findings in a previous publication which notes that both 5 and 10 μM curcumin have no effect on C6 cell viability, whereas inhibitory effect is seen at 15, 25 and 50 μM [27]. These authors also demonstrate curcumin inhibition of AP-1 and NFκB signaling pathways in glioblastoma cells. However, inhibition of C6 growth by curcumin at 5 μM has been observed by another group, who also report activation of anti-oxidative enzymes glutathione S-transferase A5, heme oxygenase 1, and others [28]. The discrepancy may be due to the source of curcumin. We have noticed that the condition of curcumin is crucial for its activity, and oxidized curcumin (as shown by color change from yellow to brown) is highly toxic to C6 cells.
This study shows that curcumin inhibits SP of C6 glioma cells. SP is due to the action of the ABC transporter ABCG2, alias breast cancer resistance protein (BCRP) [29]. Curcumin is the most potent modulator of ABCG2 activity, although it can also act on ABCB1 (P-glycoprotein) and ABCC1 (multidrug resistance protein 1, MRP1) [see 30]. In L1210 murine leukemia cells, curcumin down-regulates the multidrug resistance gene mdr1b by inhibiting the NFκB pathway [31]. In Caco-2 human colon adenocarcinoma cells, curcumin and other phytochemicals can induce ABCG2 mRNA expression [32]. In cells transfected with ABCG2 expression vector and in MCF-7 human breast cancer cells, curcumin inhibits drug transport via the ABCG2 transporter although curcumin itself is not transported [30]. Curcumin reverses drug resistance of ABCG2-expressing cells. Compounds such as mitoxantrone, topotecan and doxorubicin are substrates of ABCG2, and non-toxic concentration (5 μM) of curcumin increases drug sensitivity by 3- to 8-fold [30]. Compounds such as verapamil are inhibitors of ABCG2. Verapamil (5 μM) and curcumin (25 μM) have additive chemosensitizing effect on doxorubicin cytotoxicity of drug-resistant HEp2 human laryngeal carcinoma cells [33]. Verapamil, calcium channel blocker and non-specific inhibitor of ABC transporters, decreases intracellular calcium, and curcumin can do the same. A combination of verapamil (40 μM) and curcumin (60 μM) decreases intracellular calcium ion concentration by 2-fold after 10 hour treatment of human COLO205 colorectal carcinoma cells (from 285 nM to 141 nM) [34]. The inhibitory effect on ABCG2 by curcumin is still observable in its metabolite tetrahydrocurcumin [35]. Moreover, curcumin modulation of ABCG2 drug transport activity has been demonstrated ex vivo in isolated rat brain capillaries and in vivo in mice [36]. Thus, our observation that curcumin inhibits Hoechst dye exclusion assay is in agreement with these results. Furthermore, we extend the known curcumin effect on ABCG2 to C6 glioma cells and demonstrate its inhibition of SP, thus suggesting a potential therapeutic role of curcumin in brain tumor treatment.
Curcumin decreases both SP and “upper SP” of C6 glioma cells (see Fig. 1). It has been suggested that the “upper SP” cell population corresponds to SP cycling cells or SP polyploid cells [37]. This may be a feature of fast-growing cell lines. In our experience, the rat C6 glioma cells grow much faster than the human ovarian cancer cell lines (such as A2780 and SKOV3) [9, 10]. In addition, under both cell culture (5 μM curcumin) and Hoechst dye assay (20 μM curcumin) conditions, although there is a decrease of total SP after curcumin treatment, a residual SP remains. Reason for the persistence of residual SP is obscure at the moment and needs further study. Possible causes may be ABCG2 isoforms, or other transporters that can efflux the dye but are unaffected by curcumin. Although the dye assay has been adapted from normal stem cells to cancer stem cells (CSCs), it should be emphasized that differences between the two stem cells must be appreciated, because CSCs are genetically and epigenetically unstable [37]. Methods other than Hoechst dye exclusion assay might be used to complement or confirm the cancer stem cell features of SP cells. One that comes to mind is the functional assay of aldehyde dehydrogenase isoform 1 (ALDH1) for normal stem cells and CSCs [38].
Whereas SP enriches CSCs, it has been found that both ABCG2 positive and negative cells are tumorigenic [39]. This may be related to the dynamics of SP and non-SP, especially with respect to the rat C6 glioma cell line. We found that sorted non-SP can give rise to SP. This result agrees with those reported by Platet et al. (2007) but not with those by Kondo et al. (2004) [19, 25]. We used the same medium as Platet et al. (2007), DMEM with fetal bovine serum, and our results agree with theirs. They also found that non-SP cells can give rise to SP. In addition, they showed that C6 glioma SP is enriched with abcg2 (alias bcrp1) mRNA expression by reverse transcription-polymerase chain reaction (RT-PCR), and suggested fluctuation of SP and non-SP phenotype of C6 glioma cells [25]. Thus, there is a dynamic state of C6 CSCs. Using clonal analysis, it has been demonstrated that most C6 cells are CSCs [40, 41]. However, using serum-free medium and growth factors for sphere cultures, and nestin as markers for cytometric analysis, Zhou et al. (2009) reported 4.02% for CSCs for the C6 cells and 4.24% CSCs for the C6 xenografts [42].
Established cancer cell lines are useful for study of CSCs because they are not potentially contaminated with tissue stem cells, as may occur in primary tumors [43]. However, it is known that SP can be influenced by different microenvironmental factors, such as degrees of confluency, serum and oxygen levels, as well as the presence of chemotherapeutic drugs (as demonstrated in other cancer cell lines including MCF-7) [44]. Culture medium without serum but with growth factor supplementation, may give better yield of CSCs. Kondo et al. (2004) have used medium with basic fibroblast growth factor (bFGF) and platelet-derived growth factor (PDGF) for their sorted C6 SP cells, and showed that only the SP can give rise to both SP and non-SP cells [19]. It has been reported that primary human glioblastoma cells cultured in bFGF and epidermal growth factor (EGF) retain more CSC phenotype [45]. Thus, we predict that we may have a different result with the non-SP cell culture if we substitute fetal bovine serum with growth factors. Indeed, differential treatment of C6 cells yield cultures with low or high fractions of CSCs (serum versus growth factors, monolayer versus spheres), and tumor xenografts indicate that the C6 CSCs contribute to tumor angiogenesis [46].
To our knowledge, our use of curcumin to deplete SP is the first demonstration of applying a dietary phytochemical to CSCs. Application of other phytochemicals, especially from medicinal plants, to target CSCs has been investigated in several cases. Parthenolide (PTL), from the feverfew plant (Tanacetum parthenium) is commonly used in herbal medicine, but also targets human acute myelogenous leukemia stem cells [47]. PTL is an inhibitor of NFκB, it also acts on human breast cancer MCF-7 CSCs [48]. Screening in silico for compounds with similar effects on gene expression as PTL has revealed others, including celastrol, which is found in thunder god vine (Tripterygium wilfordii), and used in Chinese herbal medicine [49]. Another compound, berberine, from plants such as goldenseal (Hydrastis canadensis), and the Chinese goldthread (Coptis chinensis), also used in herbal medicine, targets MCF-7 SP cells [50]. For glioblastoma, cyclopamine from the plant corn lily (Veratrum californicum), has been shown to target CSCs [51]. Of course it should be noted that cyclopamine is a teratogen and may cause holoprosencephaly (alias cyclopia).
There are different approaches to attack CSCs and brain tumors, ranging from conventional therapy, differentiation and anti-angiogenic therapy, to specific targeting of SP cells and CSCs [52]. It has recently been reported that the standard treatment of gliomas, temozolomide, increases SP in glioma cells [53]. Therefore, there is an urgent need to search for novel therapy against CSCs and SP cells. Dietary phytochemicals, especially ones already shown to be active against drug-resistant cancer cells, may be potentially valuable in targeting these tumor-initiating cells [11, 12].
Among the various phytochemicals, curcumin may have applications in brain tumor therapy. In experimental models with mice using a soluble formulation of curcumin (dimethyl sulfoxide, the same solvent as this study), curcumin injected at the tail vein crosses the blood-brain barrier and kills tumor cells inside the brain [54]. This investigation becomes the first in vivo demonstration of the anti-carcinogenic and anti-metastatic activity of curcumin in the brain [54]. It is well known that curcumin has multiple cellular targets and affects many cellular pathways. We attempt to summarize the sites of action of curcumin in cancer cells in a concluding figure (Fig. 4) [20-23, 55-58].
Fig. 4.
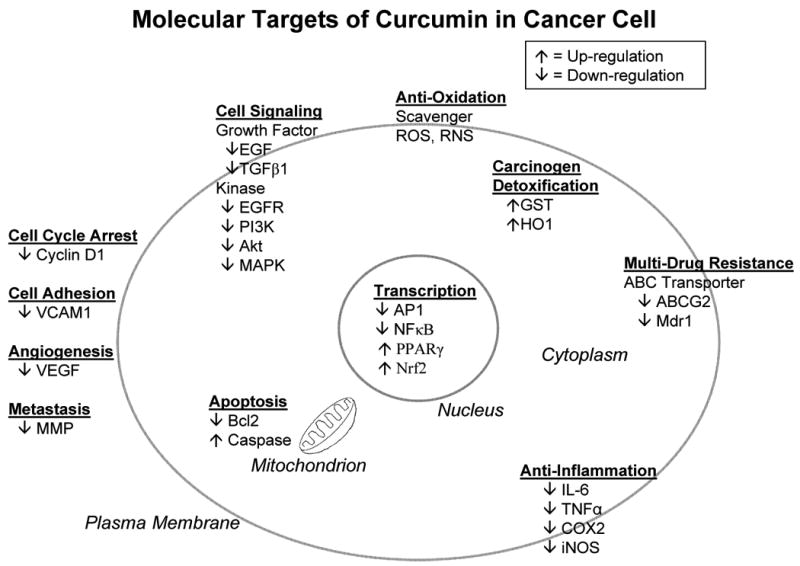
Sites of action of curcumin on cancer cells. Curcumin acts on multiple cellular targets involved in tumor initiation, promotion and progression. In anti-oxidation, curcumin scavenges free radicals. ROS, reactive oxygen species; RNS, reactive nitrogen species. In carcinogen detoxification, curcumin induces phase II enzymes. GST, glutathione S-transferase; HO1, heme oxygenase 1. In multi-drug resistance, curcumin inhibits ABC transporters. ABCG2, breast cancer resistance protein; Mdr 1, P-glycoprotein. Curcumin acts on cell signaling pathways. EGF, epidermal growth factor; TGFβ1, transforming growth factor β1; EGFR, epidermal growth factor receptor; PI3K, phosphoinositide 3-kinase; Akt, protein kinase B; MAPK, mitogen-activated protein kinase. Curcumin modulates transcription factor activities. AP1, activating protein 1; NFκB, nuclear factor kappa B; PPARγ, peroxisome proliferator-activated receptor γ; Nrf2, nuclear factor erythroid 2-related factor 2. Curcumin is antiinflammatory. IL-6, interleukin-6; TNFα, tumor necrosis factor α; COX2, cyclooxygenase-2; iNOS, inducible nitric oxide synthase. Curcumin induces apoptosis. Bcl2, B-cell lymphoma protein 2; Caspase, cysteine-aspartic acid protease. Curcumin modulates the cell cycle and cell adhesion. VCAM1, vascular cell adhesion molecule 1. Curcumin inhibits angiogenesis and metastasis. VEGF, vascular endothelial growth factor; MMP, matrix metalloproteinase. Curcumin effect is shown as up- or down-regulation. Only major targets and selected examples are presented. For simplicity and clarity purposes, pathway interactions and crosstalks are ignored. However, intracellular (nuclear, cytosolic, mitrochondrial, plasma membrane) and extracellular (secreted, cell-cell interaction, cell migration) localizations of molecular targets are illustrated in the schematic diagram.
Acknowledgments
This study was supported in part by grants from the Department of Defense (to DF), the Cancer Institute of New Jersey (to TC), and the American Institute for Cancer Research and the National Institutes of Health (to MMC). RN was the recipient of funds from the Aresty Research Center for Undergraduates at Rutgers University. Flow cytometry core facility is a shared resource between the Cancer Institute of New Jersey and the Environmental and Occupational Health Sciences Institute (EOHSI) of University of Medicine and Dentistry of New Jersey (UMDNJ) and Rutgers University. We thank Ms. Rajvi V. Patel for her help in manuscript preparation. We are grateful to Dr. Debra L. Laskin for her advice and support.
Footnotes
Conflict of interest statement: None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 2.Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23:675–699. doi: 10.1146/annurev.cellbio.22.010305.104154. [DOI] [PubMed] [Google Scholar]
- 3.Vermeulen L, Sprick MR, Kemper K, Stassi G, Medema JP. Cancer stem cells--old concepts, new insights. Cell Death Differ. 2008;15:947–958. doi: 10.1038/cdd.2008.20. [DOI] [PubMed] [Google Scholar]
- 4.Shipitsin M, Polyak K. The cancer stem cell hypothesis: in search of definitions, markers, and relevance. Lab Invest. 2008;88:459–463. doi: 10.1038/labinvest.2008.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosen JM, Jordan CT. The increasing complexity of the cancer stem cell paradigm. Science. 2009;324:1670–1673. doi: 10.1126/science.1171837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcion E, Naveilhan P, Berger F, Wion D. Cancer stem cells: beyond Koch's postulates. Cancer Lett. 2009;278:3–8. doi: 10.1016/j.canlet.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Donnenberg VS, Donnenberg AD. Multiple drug resistance in cancer revisited: the cancer stem cell hypothesis. J Clin Pharmacol. 2005;45:872–877. doi: 10.1177/0091270005276905. [DOI] [PubMed] [Google Scholar]
- 8.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 9.Chan MM, Fong D, Soprano KJ, Holmes WF, Heverling H. Inhibition of growth and sensitization to cisplatin-mediated killing of ovarian cancer cells by polyphenolic chemopreventive agents. J Cell Physiol. 2003;194:63–70. doi: 10.1002/jcp.10186. [DOI] [PubMed] [Google Scholar]
- 10.Chan MM, Soprano KJ, Weinstein K, Fong D. Epigallocatechin-3-gallate delivers hydrogen peroxide to induce death of ovarian cancer cells and enhances their cisplatin susceptibility. J Cell Physiol. 2006;207:389–396. doi: 10.1002/jcp.20569. [DOI] [PubMed] [Google Scholar]
- 11.Chan MM, Fong D. Overcoming drug resistance by phytochemicals. In: Mehta K, Siddik ZH, editors. Drug Resistance in Cancer Cells. Springer; New York: 2009. pp. 317–344. [Google Scholar]
- 12.Kawasaki BT, Hurt EM, Mistree T, Farrar WL. Targeting cancer stem cells with phytochemicals. Mol Interv. 2008;8:174–184. doi: 10.1124/mi.8.4.9. [DOI] [PubMed] [Google Scholar]
- 13.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino BP. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 15.Hirschmann-Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel U, Goodell MA, Brenner MK. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci U S A. 2004;101:14228–14233. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hadnagy A, Gaboury L, Beaulieu R, Balicki D. SP analysis may be used to identify cancer stem cell populations. Exp Cell Res. 2006;312:3701–3710. doi: 10.1016/j.yexcr.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 17.Wu C, Alman BA. Side population cells in human cancers. Cancer Lett. 2008;268:1–9. doi: 10.1016/j.canlet.2008.03.048. [DOI] [PubMed] [Google Scholar]
- 18.Grobben B, De Deyn PP, Slegers H. Rat C6 glioma as experimental model system for the study of glioblastoma growth and invasion. Cell Tissue Res. 2002;310:257–270. doi: 10.1007/s00441-002-0651-7. [DOI] [PubMed] [Google Scholar]
- 19.Kondo T, Setoguchi T, Taga T. Persistence of a small subpopulation of cancer stem-like cells in the C6 glioma cell line. Proc Natl Acad Sci U S A. 2004;101:781–786. doi: 10.1073/pnas.0307618100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin JK. Molecular targets of curcumin. Adv Exp Med Biol. 2007;595:227–243. doi: 10.1007/978-0-387-46401-5_10. [DOI] [PubMed] [Google Scholar]
- 21.Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Curcumin and cancer: an “old-age” disease with an “age-old” solution. Cancer Lett. 2008;267:133–164. doi: 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 22.Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008;269:199–225. doi: 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Strimpakos AS, Sharma RA. Curcumin: preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid Redox Signal. 2008;10:511–545. doi: 10.1089/ars.2007.1769. [DOI] [PubMed] [Google Scholar]
- 24.Montanaro F, Liadaki K, Schienda J, Flint A, Gussoni E, Kunkel LM. Demystifying SP cell purification: viability, yield, and phenotype are defined by isolation parameters. Exp Cell Res. 2004;298:144–154. doi: 10.1016/j.yexcr.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 25.Platet N, Mayol JF, Berger F, Hérodin F, Wion D. Fluctuation of the SP/non-SP phenotype in the C6 glioma cell line. FEBS Lett. 2007;581:1435–1440. doi: 10.1016/j.febslet.2007.02.071. [DOI] [PubMed] [Google Scholar]
- 26.Sales-Pardo I, Avendaño A, Martinez-Muñoz V, García-Escarp M, Celis R, Whittle P, Barquinero J, Domingo JC, Marin P, Petriz J. Flow cytometry of the Side Population: tips & tricks. Cell Oncol. 2006;28:37–53. doi: 10.1155/2006/536519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dhandapani KM, Mahesh VB, Brann DW. Curcumin suppresses growth and chemoresistance of human glioblastoma cells via AP-1 and NFkappaB transcription factors. J Neurochem. 2007;102:522–538. doi: 10.1111/j.1471-4159.2007.04633.x. [DOI] [PubMed] [Google Scholar]
- 28.Panchal HD, Vranizan K, Lee CY, Ho J, Ngai J, Timiras PS. Early anti-oxidative and anti-proliferative curcumin effects on neuroglioma cells suggest therapeutic targets. Neurochem Res. 2008;33:1701–1710. doi: 10.1007/s11064-008-9608-x. [DOI] [PubMed] [Google Scholar]
- 29.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino BP. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 30.Chearwae W, Shukla S, Limtrakul P, Ambudkar SV. Modulation of the function of the multidrug resistance-linked ATP-binding cassette transporter ABCG2 by the cancer chemopreventive agent curcumin. Mol Cancer Ther. 2006;5:1995–2006. doi: 10.1158/1535-7163.MCT-06-0087. [DOI] [PubMed] [Google Scholar]
- 31.Choi BH, Kim CG, Lim Y, Shin SY, Lee YH. Curcumin down-regulates the multidrug-resistance mdr1b gene by inhibiting the PI3K/Akt/NF kappa B pathway. Cancer Lett. 2008;259:111–118. doi: 10.1016/j.canlet.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Ebert B, Seidel A, Lampen A. Phytochemicals induce breast cancer resistance protein in Caco-2 cells and enhance the transport of benzo[a]pyrene-3-sulfate. Toxicol Sci. 2007;96:227–236. doi: 10.1093/toxsci/kfl147. [DOI] [PubMed] [Google Scholar]
- 33.Harbottle A, Daly AK, Atherton K, Campbell FC. Role of glutathione S-transferase P1, P-glycoprotein and multidrug resistance-associated protein 1 in acquired doxorubicin resistance. Int J Cancer. 2001;92:777–783. doi: 10.1002/ijc.1283. [DOI] [PubMed] [Google Scholar]
- 34.Chen YC, Kuo TC, Lin-Shiau SY, Lin JK. Induction of HSP70 gene expression by modulation of Ca2+ ion and cellular p53 protein by curcumin in colorectal carcinoma cells. Mol Carcinog. 1996;17:224–234. doi: 10.1002/(SICI)1098-2744(199612)17:4<224::AID-MC6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 35.Limtrakul P, Chearwae W, Shukla S, Phisalphong C, Ambudkar SV. Modulation of function of three ABC drug transporters, P-glycoprotein (ABCB1), mitoxantrone resistance protein (ABCG2) and multidrug resistance protein 1 (ABCC1) by tetrahydrocurcumin, a major metabolite of curcumin. Mol Cell Biochem. 2007;296:85–95. doi: 10.1007/s11010-006-9302-8. [DOI] [PubMed] [Google Scholar]
- 36.Shukla S, Zaher H, Hartz A, Bauer B, Ware JA, Ambudkar SV. Curcumin inhibits the activity of ABCG2/BCRP1, a multidrug resistance-linked ABC drug transporter in mice. Pharm Res. 2009;26:480–487. doi: 10.1007/s11095-008-9735-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayol JF, Loeuillet C, Hérodin F, Wion D. Characterisation of normal and cancer stem cells: one experimental paradigm for two kinds of stem cells. Bioessays. 2009;31:993–1001. doi: 10.1002/bies.200900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Douville J, Beaulieu R, Balicki D. ALDH1 as a functional marker of cancer stem and progenitor cells. Stem Cells Dev. 2008;18:1–9. doi: 10.1089/scd.2008.0055. [DOI] [PubMed] [Google Scholar]
- 39.Patrawala L, Calhoun T, Schneider-Broussard R, Zhou J, Claypool K, Tang DG. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2- cancer cells are similarly tumorigenic. Cancer Res. 2005;65:6207–6219. doi: 10.1158/0008-5472.CAN-05-0592. [DOI] [PubMed] [Google Scholar]
- 40.Zheng X, Shen G, Yang X, Liu W. Most C6 cells are cancer stem cells: evidence from clonal and population analyses. Cancer Res. 2007;67:3691–3697. doi: 10.1158/0008-5472.CAN-06-3912. [DOI] [PubMed] [Google Scholar]
- 41.Shen G, Shen F, Shi Z, Liu W, Hu W, Zheng X, Wen L, Yang X. Identification of cancer stem-like cells in the C6 glioma cell line and the limitation of current identification methods. In Vitro Cell Dev Biol Anim. 2008;44:280–289. doi: 10.1007/s11626-008-9115-z. [DOI] [PubMed] [Google Scholar]
- 42.Zhou XD, Wang XY, Qu FJ, Zhong YH, Lu XD, Zhao P, Wang DH, Huang QB, Zhang L, Li XG. Detection of cancer stem cells from the C6 glioma cell line. J Int Med Res. 2009;37:503–510. doi: 10.1177/147323000903700226. [DOI] [PubMed] [Google Scholar]
- 43.Kondo T. Stem cell-like cancer cells in cancer cell lines. Cancer Biomark. 2007;3:245–250. doi: 10.3233/cbm-2007-34-508. [DOI] [PubMed] [Google Scholar]
- 44.Tavaluc RT, Hart LS, Dicker DT, El-Deiry WS. Effects of low confluency, serum starvation and hypoxia on the side population of cancer cell lines. Cell Cycle. 2007;6:2554–2562. doi: 10.4161/cc.6.20.4911. [DOI] [PubMed] [Google Scholar]
- 45.Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, Park JK, Fine HA. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 46.Folkins C, Shaked Y, Man S, Tang T, Lee CR, Zhu Z, Hoffman RM, Kerbel RS. Glioma tumor stem-like cells promote tumor angiogenesis and vasculogenesis via vascular endothelial growth factor and stromal-derived factor 1. Cancer Res. 2009;69:7243–7251. doi: 10.1158/0008-5472.CAN-09-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guzman ML, Rossi RM, Karnischky L, Li X, Peterson DR, Howard DS, Jordan CT. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood. 2005;105:4163–4169. doi: 10.1182/blood-2004-10-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou J, Zhang H, Gu P, Bai J, Margolick JB, Zhang Y. NF-kappaB pathway inhibitors preferentially inhibit breast cancer stem-like cells. Breast Cancer Res Treat. 2008;111:419–427. doi: 10.1007/s10549-007-9798-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hassane DC, Guzman ML, Corbett C, Li X, Abboud R, Young F, Liesveld JL, Carroll M, Jordan CT. Discovery of agents that eradicate leukemia stem cells using an in silico screen of public gene expression data. Blood. 2008;111:5654–5662. doi: 10.1182/blood-2007-11-126003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim JB, Ko E, Han W, Shin I, Park SY, Noh DY. Berberine diminishes the side population and ABCG2 transporter expression in MCF-7 breast cancer cells. Planta Med. 2008;74:1693–1700. doi: 10.1055/s-0028-1088313. [DOI] [PubMed] [Google Scholar]
- 51.Bar EE, Chaudhry A, Lin A, Fan X, Schreck K, Matsui W, Piccirillo S, Vescovi AL, DiMeco F, Olivi A, Eberhart CG. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells. 2007;25:2524–2533. doi: 10.1634/stemcells.2007-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee da Y, Gutmann DH. Cancer stem cells and brain tumors: uprooting the bad seeds. Expert Rev Anticancer Ther. 2007;7:1581–1590. doi: 10.1586/14737140.7.11.1581. [DOI] [PubMed] [Google Scholar]
- 53.Bleau AM, Hambardzumyan D, Ozawa T, Fomchenko EI, Huse JT, Brennan CW, Holland EC. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell. 2009;4:226–235. doi: 10.1016/j.stem.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Purkayastha S, Berliner A, Fernando SS, Ranasinghe B, Ray I, Tarig H, Banarjee P. Curcumin blocks brain tumor formation. Brain Res. 2009;1266:130–138. doi: 10.1016/j.brainres.2009.01.066. [DOI] [PubMed] [Google Scholar]
- 55.Aggarwal BB, Surh YJ, Shishodia S, editors. Advances in Experimental Medicine and Biology. Vol. 595. Springer; New York: 2007. The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease. [Google Scholar]
- 56.Sa G, Das T. Anti-cancer effects of curcumin: cycle of life and death. Cell Div. 2008;3:14. doi: 10.1186/1747-1028-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: From ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65:1631–1652. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ravindran J, Prasad S, Aggarwal BB. Curcumin and cancer cells: how many ways can curry kill tumor cells selectively? AAPS J. 2009;11:495–510. doi: 10.1208/s12248-009-9128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]