Abstract
Nlrc4 is a member of the Nod-like receptors (NLRs), a family of cytosolic receptors involved in sensing bacterial molecules. NLRs are a group of proteins containing spans of leucine-rich repeats that senses bacterial factors within the eukaryotic cytosol. The recognition of bacterial factors provokes the formation of the inflammasome complex which includes specific NLRs. The inflammasome is responsible for caspase-1 activation which leads to the cleavage and maturation of inflammatory cytokines such as IL-1β and IL-18. Nlrc4 was considered to be a devoted flagellin sensor in eukaryotic cells. However, studies using a variety of pathogens such as Salmonella, Legionella, Shigella and Pseudomonas at high bacterial burdens revealed that Nlrc4 can mediate caspase-1 activation independent of bacterial flagellin. On the other hand, new reports showed that Nlrc4 can restrict bacterial infection independently of caspase-1. Therefore, Nlrc4 maybe involved in sensing more than one bacterial molecule and may participate in several immune complexes.
1. Introduction
The recognition of conserved microbial structures known as PAMP (pathogen associated molecular patterns) is accomplished by membrane bound Toll-like receptors (TLRs) and cytoplasmic NOD (nucleotide oligomerization domain) like receptors (NLRs) (Lamkanfi et al., 2007a). There are 23 NLR in human genome, while mouse genome contains about 34 NLR-encoding genes (Amer, 2009). NLRs include NALPs (NACHT-, LRR-, and pyrin domain-containing proteins), Nlrc4 (Ipaf, CLAN or CARD12), and NAIPs (neuronal apoptosis inhibitory proteins) (Ting et al., 2008). Upon sensing of PAMPs, the NLRs interact with members of the inflammasome complex. Consequently, inflammasomes are named after the specific NLR involved such as the NLRP1, NLRP3, NLRC4, AIM2 and the pyrin inflammasomes (Lamkanfi et al., 2007a).
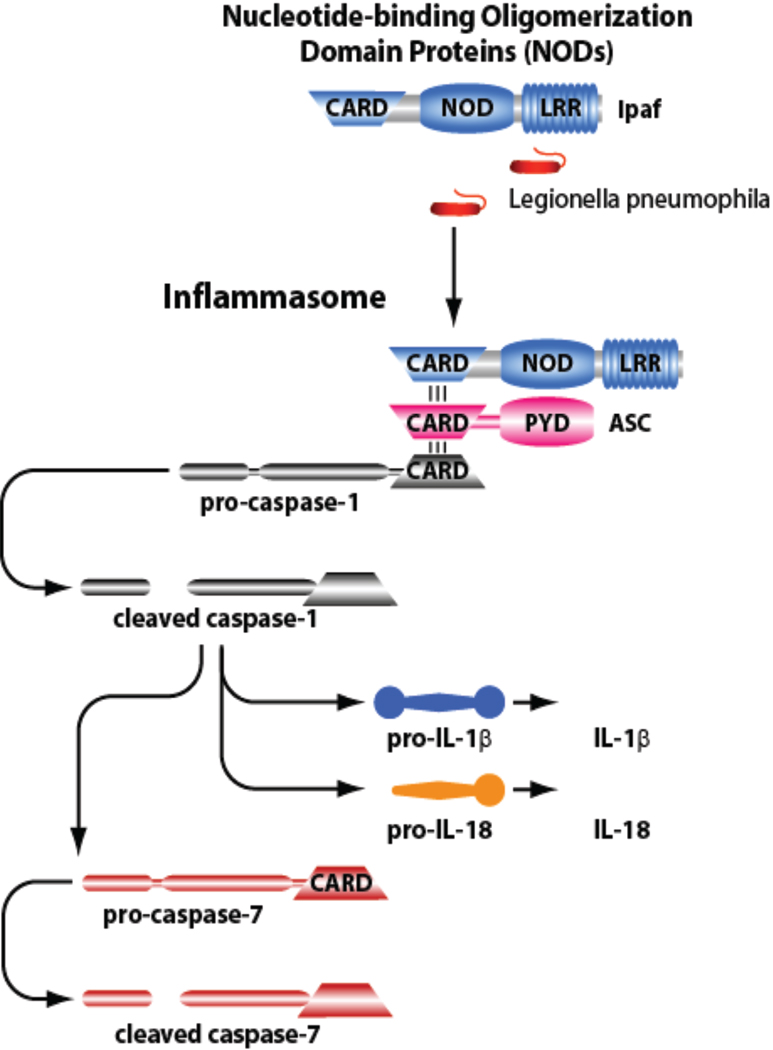
Assembly of the inflammasome complex leads to the cleavage and activation of procaspase-1 (Figure 1). Once activated, caspase-1 promotes the proteolytic maturation and activation of IL-1β, IL-18 and caspase-7 and the deactivation of IL-33 (Lamkanfi et al., 2007a). Active caspase-1 also mediates pyroptotic cell death (Bergsbaken et al., 2009).
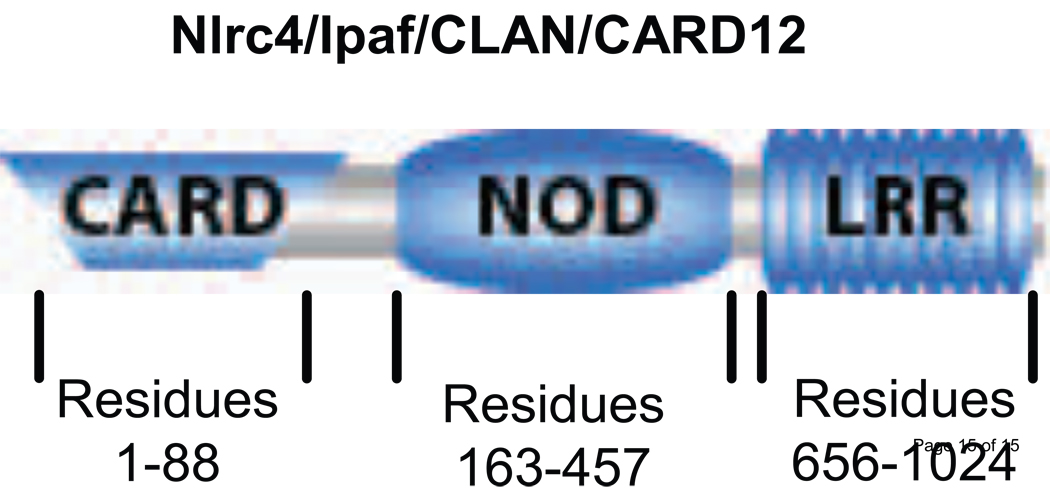
Figure 1.
Schematic representation of the structure of Nlrc4. The positions of the first and last residues for the different domains of Nlrc4 are indicated.
Nlrc4 was identified in 2001 as a novel member of the NLR family (Damiano et al., 2001; Poyet et al., 2001). Studies showed that Nlrc4 is the sensor for bacterial flagellin during infection with L. pneumophila, Salmonella, and Pseudomonas. However, new reports are emerging showing that Nlrc4 mediates caspase-1 activation independently of bacterial flagellin in mice. Studies in human cells show that Nlrc4 mediates restriction of bacterial infections independently of flagellin or caspase-1 (Vinzing et al., 2008). These new data suggest that Nlrc4 is involved in sensing PAMPs other than flagellin and contributing to complexes other than the typical caspase-1 inflammasome. The new data suggest the existence of a flagellin-independent Nlrc4 inflammasome confirming that NLRs may not be dedicated bacterial sensors after all. Here we will discuss the new findings supporting the role of Nlrc4 in sensing flagellated and non-flagellated pathogens.
2. Structure
Nlrc4 is encoded by a genomic locus on human chromosome 2 p21-p22 (Damiano et al., 2001). The transcript of this locus is 3.3 kb that forms an open reading frame for 1024 amino acid residue with a predicted molecular mass of 113 kDa (Geddes et al., 2001). Like all NLR, Nlrc4 consists of three domains N Terminal CARD domain (residues 1-88), this domain is the effector domain that can interact with CARD of ASC as well as CARD of caspase-1 (Figure 2). A central NACHT domain (residues 163-457), which shows distinct motifs including ATP/GTPase, specific P-loop, and magnesium binding motif. This domain functions as nucleotide binding and oligomerization domain (NBD). C-terminal Leucine Rich Repeats (LRR) (residues 656-1024) contains at least 13 LRR motifs (Figure 2). The LRR domain detects upstream signal resulting in the activation of Nlrc4 (Poyet et al., 2001). Deletion of this domain from Nlrc4 results in a constitutively active protein. Although CARD truncated NLRC4 is inactive, CARD motif alone show slight activity. So both CARD and NBD are crucial for Nlrc4 activity.
Figure 2.
The Nlrc4 inflammasome is composed of Ipaf, ASC, and caspase-1. Nlrc4 accepts ASC then caspase-1 via their CARD domains. Then, caspase-1 is activated and activates IL-1β, IL-18 and caspase-7.
3. Biological functions
3.1 Nlrc4 and Shigella
Shigella flexneri (Shigella) infection induces caspase-1 activation, IL-1β processing and cell death in macrophages. These responses require a functional bacterial type III secretion system and the host NLR Nlrc4 (Schroeder et al., 2007; Suzuki et al., 2007). Interestingly, caspase-1 activation by Shigella is independent of flagellin but requires a bacterial protein secreted by the type III secretion system called IpaB. Caspase-1 can also be activated by lipopolysaccharide moiety released from the bacteria (Suzuki et al., 2007). Notably, in the absence of Nlrc4, autophagy is detected in Shigella infected macrophages. It is possible that autophagy is induced independently of Nlrc4 but only apparent when pyropotosis is abolished in macrophages lacking Nlrc4. Alternatively, it is possible that Nlrc4 suppresses autophagy by an unkown mechanism.
3.2 Nlrc4 and Salmonella typhimurium
Infection of macrophages with Salmonella typhimurium (Salmonella) results in the activation of caspase-1 in an Nlrc4 dependent manner (Franchi et al., 2006). Active caspase-1 cleaves pro-IL1β and IL18, and induces cell death and caspase-7 activation (Lamkanfi et al., 2008; Damiano et al., 2004). Overexpression of Nlrc4 in human cell lines restricted Salmonella during moderate levels of infection. On the other hand, overexpression of Nlrc4 predisposes macrophages to cell death upon exposure to large burdens of Salmonella (Damiano et al., 2001). These findings suggest that the role of Nlrc4 differs according to the bacterial burden. The activation of caspase-1 requires functional Salmonella Pathogenicity Island 1 (SPI1) encoding type III secretion system (T3SS). There are several proteins secreted by this system, however, none of them is implicated in caspase-1 activation (Ehrbar et al., 2002; Lostroh and Lee, 2001). On the other hand, purified Salmonella flagellin delivered into the cell by lyposomes or by pore forming proteins induced Nlrc4 inflammasome recruitment and subsequent activation of caspase-1 (Sun et al., 2007; Amer et al., 2006). So how could T3SS deliver flagellin into host cytosol? It is hypothesized that flagellin monomers leak from bacterial cytoplasm into host cytosol through T3SS with the transfer of other virulence factors (Sun et al., 2007).
3.3 Nlrc4 and Legionella pneumophila
Macrophages derived from most mouse strains activate caspase-1 in response to Legionella pneumophila (L. pneumophila) infection. Caspase-1 activation is accompanied with restriction of intracellular L. pneumophila growth whereas macrophages derived from Nlrc4 or caspase-1 knockout mice allow L. pneumophila growth (Amer, 2009). At physiological (low) levels of bacterial burden, caspase-1 activation is dependent on bacterial flagellin and on host Nlrc4. The activation of caspase-1 by L. pneumophila also requires a functional bacterial Dot/Icm type IV secretion system (Amer, 2009; Amer et al., 2006). Similar to Salmonella, caspase-7 is activated down stream of caspase-1 during L. pneumophila infection (Lamkanfi et al., 2008). However, Naip5 (Birc1e) is also essential for restriction of L. pneumophila (Amer, 2009). Naip5 is one of the NLRs containing 3 BIR motifs as signaling domain (Lamkanfi et al., 2007a). A/J derived macrophages which express mutant Naip5 cannot restrict L. pneumophila infection, although they do activate caspase-1 in response to infection (Akhter et al., 2009; Lamkanfi et al., 2007b). However, the activation of caspase-7 in A/J derived macrophages (expressing mutant Naip5) is lacking, suggesting that Naip5 is involved in caspase-7 activation downstream of the Nlrc4 inflammasome (Akhter et al., 2009; Amer, 2009). Therefore, inflammasome complexes may harbor more than one NLR once activated. On the other hand, at high bacterial burdens, L. pneumophila mutants lacking flagellin still activated caspase-1, but independently of Nlrc4 (Case et al., 2009). Taken together, it seems that NLRC4 mediate caspase-1 activation during physiological levels of infection but when the eukaryotic cell encounters high bacterial burdens, Nlrc4 is dispensable for capsase-1 activation.
In primary human-derived macrophages, caspase-1 is not activated in response to L. pneumophila. Yet, the ablation of human Nlrc4 by gene silencing lead to enhanced growth of flagellated bacteria (Vinzing et al., 2008). These data suggest that Nlrc4 restricts L. pneumophila infection in human-derived macrophages independently of caspase-1 (Akhter et al., 2009) .
3.4 Nlrc4 and Pseudomonas aerugenosa
Pseudomonas aerugenosa (P. aeruginosa) is a pathogenic bacterium which causes marked induction of inflammatory cytokines such as IL-1β and IL-18 in host tissues. Alveolar macrophages infected with P. aerugenosa activate caspase-1 in an Nlrc4 dependent mechanism (Sutterwala et al., 2007). Type III secretion system is required for virulence of P. aeruginosa and is critical for caspase-1 activation through Nlrc4 (Sutterwala et al., 2007). None of the four known virulence factors (Exo Y, ExoS, Exo T, ExoU) secreted by this system is implicated in activation of caspase-1. However, during low bacterial burden, flagellin is involved in caspase-1 activation during P. aeruginosa infection. Similar to L. pneumophila, at high levels of bacterial infection, P. aeruginosa mutants lacking flagellin are still capable of activating caspase1. Yet, unlike L. pneumophila the activation of caspase-1 by P. aeruginosa flagellin mutants is still dependent on Nlrc4 (Sutterwala et al., 2007). These data confirm the possibility that Nlrc4 can sense bacterial molecules other than flagellin.
4. Medical disorders
The dis-regulation of the innate immune system appears crucial for the pathogenesis of many autoimmune and inflammatory diseases (Lamkanfi et al., 2007a). Variation in Nlrc4 plays a role in diseases such as Kawasaki disease and atopic dermatitis. Kawasaki disease is characterized by a marked activation of the immune system with elevations of serum proinflammatory cytokines and chemokines at acute phase (Ikeda et al., 2009). The major complications of this disease are vasculitis, damage of the coronary artery, myocardial infarction, and sudden death (Ikeda et al., 2009). The recent study by Ikeda et al showed that Nlrc4 is one of five genes up-regulated in acute phase of Kawasaki disease and that the innate immune response plays a major role in the pathogenesis and pathophysiology of Kawasaki disease. On the other hand, atopic dermatitis is a chronic skin disease characterized by excessive immune reactions to ubiquitous antigens (Macaluso et al., 2007). Gene-gene interaction studies present evidence for an interaction between the promoter SNPs in the Nlrc4 and NALP1 genes (Macaluso et al., 2007). Taken together, these findings reveal that Nlrc4 is not only involved in detecting flagellin but also in sensing danger signals and if dis-regulated, it will lead to diseases.
Acknowledgements
We apologize to colleagues whose work was not cited owing to space limitations. We thank Tim Eubank for the figures. Work in Dr. Amer’s Laboratory is supported by grants from The National Institute of Health, grants number R01HL094586 and R21AI083871 and from the American Lung Association. DHA is supported by The Egyptian cultural and educational bureau for joint supervision program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akhter A, Gavrilin MA, Frantz L, Washington S, Ditty C, Limoli D, et al. Caspase-7 activation by the Nlrc4/Ipaf inflammasome restricts Legionella pneumophila infection. PLoS Pathogens. 2009;5:e1000361. doi: 10.1371/journal.ppat.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amer A, Franchi L, Kanneganti TD, Body-Malapel M, Ozoren N, Brady G, et al. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J Biol Chem. 2006;281:35217–35223. doi: 10.1074/jbc.M604933200. [DOI] [PubMed] [Google Scholar]
- Amer AO. Modulation of caspases and their non-apoptotic functions by Legionella pneumophila. Cell. Microbiol. 2009 doi: 10.1111/j.1462-5822.2009.01401.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nature Reviews. Microbiology. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case CL, Shin S, Roy CR. Asc and Ipaf Inflammasomes direct distinct pathways for caspase-1 activation in response to Legionella pneumophila. Infection & Immunity. 2009;77:1981–1991. doi: 10.1128/IAI.01382-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiano JS, Newman RM, Reed JC. Multiple roles of CLAN (caspase-associated recruitment domain, leucine-rich repeat, and NAIP CIIA HET-E, and TP1-containing protein) in the mammalian innate immune response. Journal of Immunology. 2004;173:6338–6345. doi: 10.4049/jimmunol.173.10.6338. [DOI] [PubMed] [Google Scholar]
- Damiano JS, Stehlik C, Pio F, Godzik A, Reed JC. CLAN, a novel human CED-4-like gene. Genomics. 2001;75:77–83. doi: 10.1006/geno.2001.6579. [DOI] [PubMed] [Google Scholar]
- Ehrbar K, Mirold S, Friebel A, Stender S, Hardt WD. Characterization of effector proteins translocated via the SPI1 type III secretion system of Salmonella typhimurium. Ijmm International Journal of Medical Microbiology. 2002;291:479–485. doi: 10.1078/1438-4221-00156. [DOI] [PubMed] [Google Scholar]
- Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozoren N, Jagirdar R, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages.[see comment] Nature Immunology. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- Geddes BJ, Wang L, Huang WJ, Lavellee M, Manji GA, Brown M, et al. Human CARD12 is a novel CED4/Apaf-1 family member that induces apoptosis. Biochem Biophys Res Commun. 2001;284:77–82. doi: 10.1006/bbrc.2001.4928. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Yamaguchi K, Tanaka T, Mizuno Y, Hijikata A, Ohara O, et al. Unique activation status of peripheral blood mononuclear cells at acute phase of Kawasaki disease. Clin Exp Immunol. 2009 doi: 10.1111/j.1365-2249.2009.04073.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkanfi M, Kanneganti TD, Franchi L, Nunez G. Caspase-1 inflammasomes in infection and inflammation. Journal of Leukocyte Biology. 2007a;82:220–225. doi: 10.1189/jlb.1206756. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Amer A, Kanneganti TD, Munoz-Planillo R, Chen G, Vandenabeele P, et al. The Nod-like receptor family member Naip5/Birc1e restricts Legionella pneumophila growth independently of caspase-1 activation. J Immunol. 2007b;178:8022–8027. doi: 10.4049/jimmunol.178.12.8022. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Kanneganti TD, Van Damme P, Vanden Berghe T, Vanoverberghe I, Vandekerckhove J, et al. Targeted peptidecentric proteomics reveals caspase-7 as a substrate of the caspase-1 inflammasomes. Mol Cell Proteomics. 2008;7:2350–2363. doi: 10.1074/mcp.M800132-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lostroh CP, Lee CA. The Salmonella pathogenicity island-1 type III secretion system. Microbes & Infection. 2001;3:1281–1291. doi: 10.1016/s1286-4579(01)01488-5. [DOI] [PubMed] [Google Scholar]
- Macaluso F, Nothnagel M, Parwez Q, Petrasch-Parwez E, Bechara FG, Epplen JT, Hoffjan S. Polymorphisms in NACHT-LRR (NLR) genes in atopic dermatitis. Exp. Dermatol. 2007;16:692–698. doi: 10.1111/j.1600-0625.2007.00589.x. [DOI] [PubMed] [Google Scholar]
- Poyet JL, Srinivasula SM, Tnani M, Razmara M, Fernandes-Alnemri T, Alnemri ES. Identification of Ipaf, a human caspase-1-activating protein related to Apaf-1. J Biol Chem. 2001;276:28309–28313. doi: 10.1074/jbc.C100250200. [DOI] [PubMed] [Google Scholar]
- Schroeder GN, Jann NJ, Hilbi H. Intracellular type III secretion by cytoplasmic Shigella flexneri promotes caspase-1-dependent macrophage cell death. Microbiology. 2007;153:2862–2876. doi: 10.1099/mic.0.2007/007427-0. [DOI] [PubMed] [Google Scholar]
- Sun YH, Rolan HG, Tsolis RM. Injection of flagellin into the host cell cytosol by Salmonella enterica serotype Typhimurium. Journal of Biological Chemistry. 2007;282:33897–33901. doi: 10.1074/jbc.C700181200. [DOI] [PubMed] [Google Scholar]
- Sutterwala FS, Mijares LA, Li L, Ogura Y, Kazmierczak BI, Flavell RA. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J Exp Med. 2007;204:3235–3245. doi: 10.1084/jem.20071239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Franchi L, Toma C, Ashida H, Ogawa M, Yoshikawa Y, et al. Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS Pathogens. 2007;3:e111. doi: 10.1371/journal.ppat.0030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting JP, Lovering RC, Alnemri ES, Bertin J, Boss JM, Davis BK, et al. The NLR gene family: a standard nomenclature. Immunity. 2008;28:285–287. doi: 10.1016/j.immuni.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinzing M, Eitel J, Lippmann J, Hocke AC, Zahlten J, Slevogt H, et al. NAIP and Ipaf control Legionella pneumophila replication in human cells. J Immunol. 2008;180:6808–6815. doi: 10.4049/jimmunol.180.10.6808. [DOI] [PubMed] [Google Scholar]