Abstract
Given the scope of the human immunodeficiency virus (HIV) pandemic, millions of people will be in need of chronic antiretroviral therapy (ART) for decades into the future. It is hoped that progress in prevention of HIV infection can be made, but there have been few successes in this effort thus far. At the same time, lifelong ART presents formidable problems. Therefore, while research must continue on improvements in prevention and treatment, future HIV research should now also seek to develop temporally contained therapies capable of eradicating HIV infection. This review will explore what is known about the mechanisms that restrain HIV expression and result in persistent, latent proviral infection, and what these mechanisms tell us about potential approaches towards eradication of HIV infection.
Introduction
With more than 7,000 new infections each day, the human immunodeficiency virus (HIV) pandemic remains a concern of utmost public health importance [1]. Although access to antiretroviral therapy (ART) is increasing, currently more people are infected every day than initiate ART. Further, despite highly effective antiretrovirals capable of reducing plasma viremia to less than 50 copies of HIV per milliliter (ml) in the millions of treated individuals [2], there is only a single report of an HIV-infected individual in whom HIV infection might have been cleared [3]. Virus quickly rebounds upon treatment interruption, thus life-long access and adherence to antiretrovirals are necessary to control viremia [4]. Over the long term, the burden of life-long ART in millions of patients across the world may not be sustainable.
HIV persistence is primarily due to the twin phenomenon of HIV to latently infect long-lived cells of the immune system and continued virus release from undefined reservoirs. For the past two decades, the majority of therapeutic research in the field has focused on developing vaccines and designing antiretrovirals to block specific steps in the virus life cycle. Only of late has there been a reawakening of interest in strategies to purge the latent reservoir of HIV with the goals of a drug-free remission of viremia and, ultimately, virus eradication. Recently, several researchers called for a broad collaboration between governments, institutional donors, academia, and the pharmaceutical industry to pursue anti-latency research similar to current initiatives for HIV vaccine research [5]. This review summarizes recent discoveries into the mechanisms that regulate HIV latency, efforts to define and reach still hidden viral reservoirs, as well as proposed strategies to eradicate HIV.
Persistent HIV infection
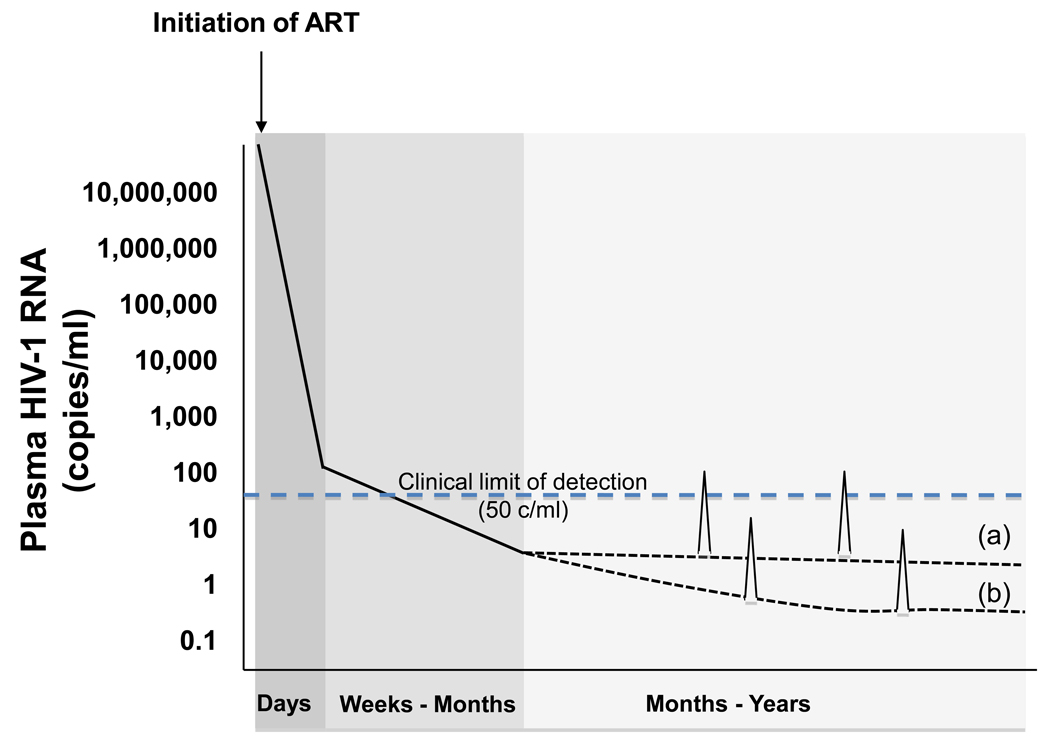
ART has been extremely effective at controlling viral replication in HIV infected individuals. However, persistent expression of HIV RNA (without evidence of full rounds of replication) can be detected in HIV-infected patients on durably successful ART by research assays in the plasma [6–7]. Recent studies have demonstrated that intensifying standard ART with an additional potent drug such as the non-nucleoside reverse transcriptase inhibitor efavirenz, the fusion inhibitor enfuvirtide, protease inhibitors lopinavir/ritonavir or atazanavir/ritonavir, or the HIV integrase inhibitor raltegravir does not reduce residual viremia in patients [8–10]. These studies suggest that eradication of HIV may not be achieved by antiretroviral drugs that block HIV replication and that additional efforts should be focused on purging the persistent latent viral reservoirs [Fig. 1].
Figure 1. Decay of plasma viremia induced by current antiretroviral therapy (ART).
Initiation of potent ART results in a precipitous decline in measurable plasma viremia. Level of viremia drop below the level of detection of commonly available clinical assays (ca. 50 copies of HIV-1 RNA/ml) within weeks, but sometimes take up to 6 months to decline below the limit of detection. This initial phase of decay often occurs in a biphasic pattern, as illustrated here, but a monophasic decline can be seen with regimens that contain an integrase inhibitor. After this initial decline, the slope(s) of further declines in levels of plasma viremia appear to gradually approach zero. Stable production of low levels of viremia are detectable in a minority of patients with more sensitive research assays (a), and even lower levels may be established in most patient at levels that cannot even be detected with current research assays (b). Intermittent viremia (blips) may occur due to stochastic surges in viral production and/or variation in assay performance near its limit of detection, and may be detected in both patient populations.
Early in infection, HIV primarily infects activated CD4+ T cells. Infection of these cells is almost always productive and quickly leads to cell death. Once ART is initiated, studies of the kinetics of the decay of viremia illustrate multiple phases of decay. The initial two phases of decay of viremia has been long assumed to originate first from activated CD4 cells, and then from long-lived cells such as macrophages. However, in ART that includes an HIV integrase inhibitor only a single initial phase of decay is observed. This led to the suggestion that the second, slower phase of decay originates from cells with slow rates of cycling in which the kinetics of replication progress at slower rates [11].
Regardless, following the initial decay, which occurs over a period of a few months, there is a slower decay thought to represent depletion of virus in cells with a half-life of approximately 39 weeks. The final phase consists of a stable, low level of plasma viremia of approximately 1 to 5 copies per milliliter, observable in most patients with the use of research assays, for which there is no measurable rate of decay [6].
Resting memory CD4+ T cells are relatively resistant to HIV infection due to the intrinsic resting phenotype of the cell, which results in a lower efficiency of entry, reverse transcription, and integration in these cells [12]. Although the number of latently infected resting CD4+ T cells is very rare (less than 1 per million cells contain a replication competent integrated genome), the reservoir is rapidly generated early in infection [13]. Largely, seeding of this reservoir with virus is thought to occur when an activated CD4+ T cell is infected as it is transitioning to the resting, memory T cell state. Once the cell has obtained a resting phenotype, virus replication is further impeded due to multiple blocks to the HIV lifecycle that occur in resting CD4+ T cells. If HIV has already integrated into the DNA of the T cell, then the virus could then remain latently infected for the life of the cell. It has recently been proposed that this population of resting CD4+ memory cells containing latent, replication-competent virus might be expanded if the infected memory cell can undergo mitosis, a natural homeostatic process that is thought to preserve immunological memory, and can enter cell cycle without inducing viral replication and the destruction of the infected cell [14]. If this is so, then the stable frequency resting cell infection in patients on prolonged ART must reflect a balance between the extremely rare entry of a new proviral genome into the resting pool during ART, the activation of a resting memory cell by an immune stimulus, and the homeostatic proliferation of infected memory CD4+ cells.
Sequence analysis of HIV proviral DNA in resting CD4+ T cells has not uncovered evidence of viral evolution in this reservoir in patients who are stably suppressed with antiretroviral therapy [4, 7]. Phylogenetic analyses of proviral DNA from resting CD4+ T cells and plasma HIV RNA indicate that the persistent, low-level viremia in patients on ART might be released from some other cell type, as plasma sequences sometimes do not match proviral sequences from resting CD4+ T cells [15–16]. Nevertheless, patients who are stably suppressed on ART rarely develop resistance to antiretroviral drugs [17], suggesting that there may not be full rounds of ongoing viral replication in patients on ART, and that plasma viremia stems solely from virus that has been released from an unidentified cell or a clonal population of cells.
However, it should be mentioned that studies such as the ones cited above do have limitations, including only sampling virus from the periphery and the current sensitivity of virus detection assays (approximately 1 copy per ml [18]). One recent report does find evidence of viral evolution in such patients [19]. Thus, we are still not able to confidently settle the ongoing debate about whether there is continued viral replication on ART or if plasma viremia only represents virus released from stable reservoirs.
Mechanisms of proviral latency
Numerous transcriptional and post-transcriptional blocks to HIV replication in resting CD4+ T cells have been identified [Fig. 2]. A major transcriptional block is the lack or sequestering of activating transcription factors and co-factors in resting CD4+ T cells. For example, low levels of NFAT, cytoplasmic localization of NF-κB p50-p65, and the sequestration of the positive transcription elongation factor (P-TEFb) by HEXIM have been described in detail [20]. The site of virus integration can also have profound effects upon HIV transcriptional expression. Although, HIV primarily integrates into genomic regions of euchromatin characterized by active transcription [21], occasionally the virus may integrate into heterochromatic regions of limited transcriptional activity. Furthermore, virus that integrates into euchromatin can be subject to transcriptional interference by up or down stream cellular promoters [22–23].
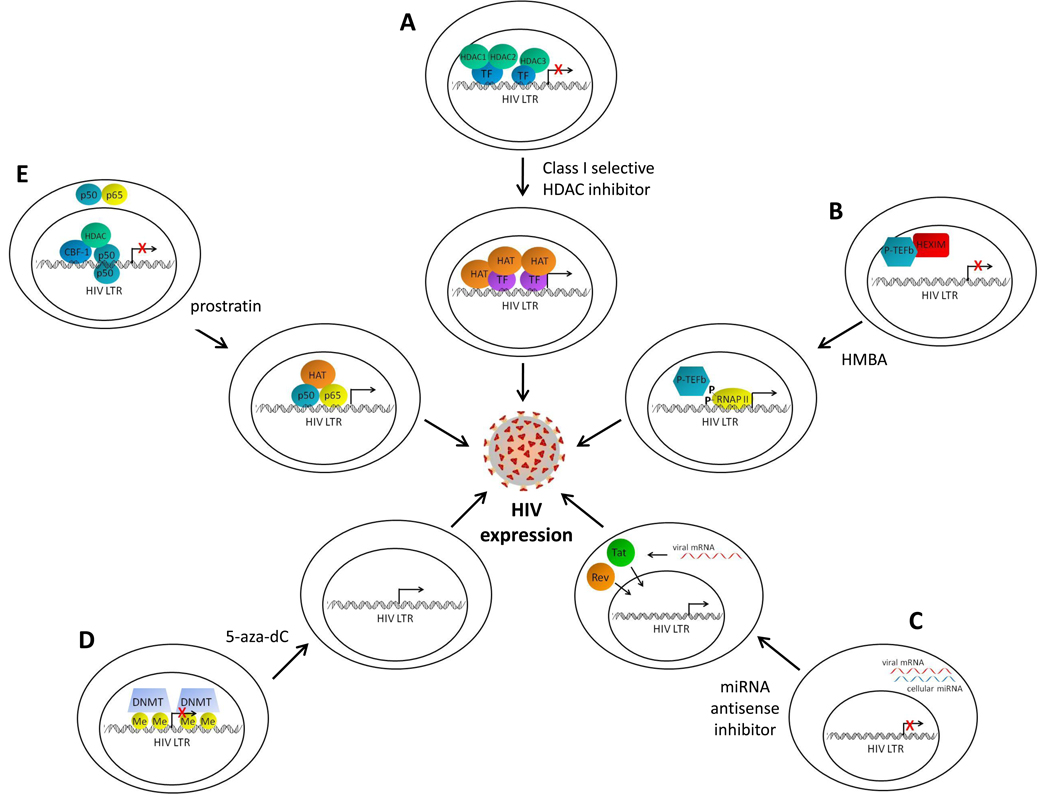
Figure 2. Strategies to purge the latent reservoir.
(a) Class I selective histone deacetylase (HDAC) inhibitors inactivate HDACs 1, 2, and 3 at the human immunodeficiency virus (HIV) long-terminal repeat promoter (LTR). This allows unopposed activity of histone acetyltransferases (HAT), recruited to the LTR by transcription factors (TF), resulting in acetylation of histones at nucleosomes near the site of initiation of LTR transcription, and HIV expression. (b) Hexamethylene bisacetamide (HMBA) induces the release of positive transcription elongation factor (P-TEFb) from HMBA-induced protein 1 (HEXIM), leading to phosphorylation of RNA polymerase II (RNAP II) at the LTR and subsequent processive HIV transcription. (c) Cellular microRNAs (miRNA) in resting CD4+ T cells prevent the translation of HIV mRNA. Inhibition of these miRNAs with antisense miRNA inhibitors permits translation of the HIV proteins Tat and Rev, which translocate to the nucleus and facilitate HIV expression. (d) Cytosine residues at the LTR can be methylated by DNA methyltransferases (DNMT), a phenomenon associated with transcriptional silencing. The DNA methylation inhibitor 5-aza-2’ deoxycytidine (5-aza-dC) removes the methylation marks and can stimulate HIV expression. (e) Complexes at the enhancer sites of the LTR, such as C-promoter binding factor-1 (CBF-1), or the homodimeric p50 form of NF-κB may recruit HDACs during latency. In resting CD4+ T cells, the activating NF-κB heterodimer p50-p65 is sequestered in the cytoplasm. Prostratin induces the nuclear translocation of p50-p65 and the recruitment of HATs to the NF-κB binding sites on the LTR, resulting in HIV expression.
Once integrated, HIV is subject to the same epigenetic mechanisms of transcriptional regulation as host DNA. Certain histone modifying enzymes have been shown to maintain HIV latency including histone deacetylases (HDACs), which deacetylate lysine residues on histone tails, creating a more condensed, repressive chromatin environment and removing important docking signals for activating transcription factors [24]. The histone methyltransferases EZH2 and SUV39h1 have also been reported to regulate HIV latency in cell-line models [25–26]. In addition to histone modifications, integrated proviral genomes have been shown to be methylated on cytosine residues of DNA surrounding the virus promoter in regions referred to as CpG islands [27–28]. Cytosine DNA methylation of promoters is associated with transcriptional silencing and a general resistance to activating signals.
Post-transcriptional mechanisms regulate HIV latency as well. Resting cells have low expression of polypyrimidine tract binding protein (PTB), a factor involved in export of HIV RNA to the cytoplasm. Over expression of PTB in resting CD4+ T cells from HIV infected patients leads to increased transcription of latent HIV ex vivo [29]. This induction of HIV gene expression is presumably due to increasing the export and eventual translation of the viral proteins Tat and Rev, which are important regulators of HIV RNA transcription and nuclear export, respectively.
Five cellular microRNAs (miRNAs) enriched in resting CD4+ T cells were demonstrated to inhibit translation of HIV proteins [30]. Like PTB, these miRNAs prevent nuclear accumulation of the HIV proteins Tat and Rev, thus inhibiting HIV gene expression and RNA export. Thus, resting cells provide a particularly unfavorable environment for HIV replication and this can result in silencing of integrated HIV proviral genomes in this population of cells.
In addition to possessing numerous blocks to replication, resting CD4+ T cells contribute to the persistence of HIV due to their intrinsic stability and processes of antigen-driven and homeostatic proliferation. Resting CD4+ T cells include both naïve and memory cell populations. Naïve CD4+ T cells have not been exposed to antigen and rarely contain integrated HIV proviral DNA. When a naïve CD4+ T cell comes into contact with its corresponding antigen, it will become an activated CD4+ T cell and proliferate. These activated CD4+ T cells are permissive to HIV and quickly die following infection. Some activated CD4+ T cells will revert to a resting state and become memory CD4+ T cells that are ready to respond should they encounter the same antigen again. Activated CD4+ T cells that become infected as they are transitioning to a resting memory phenotype are likely to be the source of latent HIV in this population of cells [31].
Memory CD4+ T cells can be further divided into various functional subsets based on surface expression markers and these subsets include central memory (TCM), transitional memory (TTM), effector memory, and terminally differentiated memory cells, the latter of which do not persist for long periods of time and do not enter the persistent memory pool. A recent study that examined the prevalence of HIV proviral DNA within these subpopulations observed that TCM cells possess the highest rate of latent HIV in patients with high CD4 counts [14]. In addition to being extremely long-lived cells, this population can propagate the latent reservoir via antigen-driven proliferation. However, patients with low CD4 counts possess a larger latent reservoir in TTM cells, which persist through homeostatic proliferation driven by interleukin-7 (IL-7). Thus, multiple mechanisms of immune cell stability and proliferation contribute the persistence of the latent HIV reservoir in resting memory CD4+ T cells.
Models for evaluating anti-latency therapeutic approaches
Linked to the study of causes of HIV latency is the evaluation of strategies to target virus eradication. Theoretically, anti-latency strategies would purge the latent reservoir by inducing transcriptional activation of latent virus. Infected cells could undergo apoptosis following virus expression, and antiretroviral therapy would prevent infection of new cells. Antiviral immune responses might also aid in the clearance of infected cells, and prevent the spread of viral infection. Until recently, latency research has primarily made use of numerous cell-line models where integrated HIV proviral DNA is transcriptionally silent. However, there have been advances in developing more clinically relevant systems for anti-latency therapy evaluation.
Outgrowth assays using resting CD4+ T cells harvested from HIV-infected patients on ART has been a useful too for the evaluation of anti-latency approaches [32–33]. Resting cells are exposed to potential HIV-inducing compounds and maintained in limiting dilution cultures over a two and a half week period along with CCR5-expressing, stimulated PBMCs to permit spread of reactivated latent virus. Virus outgrowth is measured at the end of the culture period by HIV gag p24 antigen ELISA and the number of infected resting CD4+ T cells represented in the initial cell pool is calculated using a maximum likelihood analysis.
Resting CD4+ T cell infection also occurs in rhesus macaques infected with simian immunodeficiency virus (SIV), suggesting that this model system may be one means of exploring anti-latency therapies [34]. However, studies of proviral latency mechanisms have not been performed in this model. Thus, it remains to be determined whether the blocks to SIV expression in the latent reservoir of macaques are the same as those in the resting CD4+ T cells of humans. Importantly, humanized mouse models have been established that show promise as a model for anti-latency research. These models include bone marrow-liver-thymus mice [35] and humanized (hu-) Rag2−/−γc−/− mice [36]. We recently demonstrated that plasma viremia can be suppressed below the limit of detection following treatment of HIV-infected hu-Rag2−/−γc−/− mice with combination antiretroviral therapy [37]. When ART is interrupted, HIV levels quickly rebound and depletion of CD4+ T cells resumes. These findings give hope that humanized mouse models may soon be utilized to study HIV latency and investigate potential HIV eradication strategies.
Approaches to target HIV proviral latency in resting CD4+ T cells
Even though other reservoirs may exist, targeting latent virus in resting CD4+ T cells is an important step in eradicating HIV. Once this population of infected cells is eliminated it may permit easier identification and study of other reservoirs. Chemical compounds that target transcription factors and co-factors that are either limiting or sequestered in resting CD4+ T cells have been proposed as anti-latency therapeutics (reviewed in [38]). These include drugs such as prostratin, which induces nuclear accumulation of the activating NF-kB heterodimer p50-p65 and hexamethylbisacetamide (HMBA), which promotes the release of p-TEFb from HEXIM leading to transcriptional activation of the HIV promoter (Figure 2). Prostratin has been shown to induce the expression of HIV in both human cell and animal model systems, but hurdles in pre-clinical safety and toxicity testing have thus far prevented the approval of this compound for further testing in humans [39–41].
HDACs associate with the integrated proviral HIV promoter and inhibition of HDAC enzymatic activity leads to outgrowth of latent virus from resting CD4+ T cells of HIV-infected patients (reviewed in [42]). More recently we have demonstrated that the class I HDACs 1, 2, and 3 may be particularly important to maintaining HIV latency (Figure 2). These HDACs associate with the HIV promoter in cell line models of latency, are expressed in the nuclei of resting CD4+ T cells, and selective chemical inhibition of HDACs 1, 2, and 3 can induce ex vivo viral outgrowth from the resting CD4+ T cells of HIV infected individuals better than the non-selective HDAC inhibitor valproic acid. In contrast, inhibitors selective for certain members of the class II HDACs, including HDACs 4, 5, 7, and 9, do not induce outgrowth of latent HIV [33, 43].
Histone methyltransferases and DNA methyltransferases have also been shown to contribute to HIV transcriptional repression in cell line models of latency. In recent studies from two groups, exposure of latently infected cell lines to the DNA cytosine methylation inhibitor 5-aza-2’ deoxycytidine (5-aza-dC) led to induction of HIV expression (Figure 2) [27–28]. However these investigators and others, had reported in earlier studies that DNA methylation was relatively unimportant in LTR regulation. Further, it is clear that not all integrated HIV genomes are heavily methylated at the promoter in resting CD4+ T cells obtained from patients [27]. Therefore, additional strategies may need to be employed to purge all latent HIV genomes in this population of cells. Strategies that target multiple transcriptional blocks, such as combining inhibitors to both selective class I HDACs and specific histone or DNA methyltransferases, may provide an even more effective induction of latent HIV [44]. But it appears that studies in animal models, or even human clinical studies, will be required to definitively answer these questions.
Alternatively, as HATs and HDACs regulate the recruitment and activity of methyltransferases [45] it is also possible that sufficiently potent and appropriately targeted HDAC inhibitors may be sufficient to induce the expression of latent HIV. Indeed, even in heavily methylated promoters, the most effective inducing agent was found to be the potent HDAC inhibitor suberoylanilide hydroxamic acid (SAHA) [27].
Specific cellular miRNAs present a post-transcriptional block to HIV expression in resting CD4+ T cells. Ex vivo exposure of resting CD4+ T cells from HIV-infected patients to 2’-O-methyl-oligoribonucleotide antisense inhibitors of these specific miRNAs leads to latent virus outgrowth without T cell activation (Figure 2) [30]. Thus, anti-miRNA inhibitors are another potential anti-latency therapeutic. Delivery systems for anti-miRNA inhibitors in humans do not currently exist. However, development of such delivery systems is an area of active research [46–49]. Consequently, anti-miRNA inhibitors may be a potential therapeutic in the future. More research is warranted into the effects of inhibiting these specific miRNAs on the host as a single miRNA can regulate hundreds of target RNA molecules.
The intrinsic properties of stability and proliferation in memory CD4+ T cells present major obstacles to the eradication of HIV with current antiretroviral therapies. The latent HIV reservoir in patients who possess high CD4 T cell counts primarily exists in TCM cells. These cells can persist for decades and integrated virus can be propagated following stimulation with antigen. Patients who are treated late following initial HIV infection typically have low CD4 T cell counts and high levels of immune activation. In these patients, latent HIV proviral DNA is primarily detected in TTM cells [14]. Depletion of CD4+ T cells correlates with plasma IL-7 levels and IL-7 promotes the survival and proliferation of TTM cells. Thus, the process of homeostatic proliferation is potentially a key mediator of persistent latent HIV in the TTM cells of patients with low CD4 counts. Anti-IL-7 therapies have been proposed as a potential means to purge this specific latent reservoir by preventing proliferation of infected TTM cells [14].
Although theoretically HIV expression in infected cells will lead to cell death within 1 to 2 days either by viral lysis or immune surveillance, it has been proposed that the addition of the glutathione-synthesis inhibitor buthionine sulfoximine (BSO) to HDAC inhibitors could accelerate the apoptosis of cells that are producing virus [50]. Expression of HIV would decrease the level of glutathione creating a pro-oxidant cellular environment, stimulating HIV transcription. The combination of BSO and HDAC inhibitors permitted a more potent induction of virus expression in latent cell line models at lower concentrations than required when HDAC inhibitors were used alone. Additionally, increased apoptosis of cells following virus induction was observed following the combined treatment, presumably due to the inability of infected cells to increase glutathione levels. These findings suggest one strategy of inducing virus expression and apoptosis of infected cells, while simultaneously decreasing the concentrations of HDAC inhibitors required to induce virus expression, thereby decreasing toxicity to uninfected cells.
Concluding remarks
Chronic, lifelong antiretroviral therapy may be needed for decades into the future to prevent AIDS in the millions of HIV-infected people, and to control the spread of the HIV pandemic. Several mechanisms that might contribute to the establishment and maintenance of latent proviral infection have been described, yet it seems most likely that several populations of cells that are persistently infected will require unique therapeutic approaches. Several strategies, as described above, have already emerged from our current understanding of persistent HIV infection. However, obviously no approach has yet been practical or successful. Developing the ability to eradicate established HIV infection will require a prolonged scientific commitment, further discoveries in the basic mechanisms of HIV persistence, the development of new model systems to test therapeutic approaches, and careful but innovative translational studies.
Acknowledgments
This work was supported by National Institutes of Health grants RO1 AI045297 and U19AI082608 to D.M.M and T32 AI 07001-33 to K.S.K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
KSK and ADK report no conflict of interest. DMM reports research funding and honoraria from Merck Research Laboratories, who market an HDAC inhibitor for oncology therapy, and a patent application for the use of HDAC inhibitors in HIV therapy.
References
- 1.UNAIDS. 2008 Report on the global AIDS epidemic. 2008. [Google Scholar]
- 2.Hermankova M, et al. Analysis of human immunodeficiency virus type 1 gene expression in latently infected resting CD4+ T lymphocytes in vivo. J Virol. 2003;77(13):7383–7392. doi: 10.1128/JVI.77.13.7383-7392.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hutter G, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360(7):692–698. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 4.Joos B, et al. HIV rebounds from latently infected cells, rather than from continuing low-level replication. Proc Natl Acad Sci U S A. 2008;105(43):16725–16730. doi: 10.1073/pnas.0804192105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richman DD, et al. The challenge of finding a cure for HIV infection. Science. 2009;323(5919):1304–1307. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- 6.Palmer S, et al. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A. 2008;105(10):3879–3884. doi: 10.1073/pnas.0800050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nottet HS, et al. HIV-1 can persist in aged memory CD4+ T lymphocytes with minimal signs of evolution after 8.3 years of effective highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2009;50(4):345–353. doi: 10.1097/QAI.0b013e318197eb04. [DOI] [PubMed] [Google Scholar]
- 8.Dinoso JB, et al. Treatment intensification does not reduce residual HIV-1 viremia in patients on highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 2009;106(23):9403–9408. doi: 10.1073/pnas.0903107106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones J, McMahon D, Wiegand A, Kearney M, Palmer S, McNulty S, Metcalf J, Coffin J, Mellors J, Maldarelli F. No decrease in residual viremia during raltegravir intensification in patients on standard ART in 16th Conference on Retroviruses and Opportunistic Infections; Montreal, Canada. 2009. [Google Scholar]
- 10.Lehrman G, et al. Depletion of latent HIV-1 infection in vivo: a proof-of-concept study. Lancet. 2005;366(9485):549–555. doi: 10.1016/S0140-6736(05)67098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murray JM, et al. Antiretroviral therapy with the integrase inhibitor raltegravir alters decay kinetics of HIV, significantly reducing the second phase. Aids. 2007;21(17):2315–2321. doi: 10.1097/QAD.0b013e3282f12377. [DOI] [PubMed] [Google Scholar]
- 12.Dai J, et al. Human immunodeficiency virus integrates directly into naive resting CD4+ T cells but enters naive cells less efficiently than memory cells. J Virol. 2009;83(9):4528–4537. doi: 10.1128/JVI.01910-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siliciano JD, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 2003;9(6):727–728. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 14.Chomont N, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15(8):893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sahu GK, et al. Low-level plasma HIVs in patients on prolonged suppressive highly active antiretroviral therapy are produced mostly by cells other than CD4 T-cells. J Med Virol. 2009;81(1):9–15. doi: 10.1002/jmv.21366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brennan TP, et al. Analysis of human immunodeficiency virus type 1 viremia and provirus in resting CD4+ T cells reveals a novel source of residual viremia in patients on antiretroviral therapy. J Virol. 2009;83(17):8470–8481. doi: 10.1128/JVI.02568-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kieffer TL, et al. Genotypic analysis of HIV-1 drug resistance at the limit of detection: virus production without evolution in treated adults with undetectable HIV loads. J Infect Dis. 2004;189(8):1452–1465. doi: 10.1086/382488. [DOI] [PubMed] [Google Scholar]
- 18.Palmer S, et al. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 2003;41(10):4531–4536. doi: 10.1128/JCM.41.10.4531-4536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiu C, et al. Identification of ongoing human immunodeficiency virus type 1 (HIV-1) replication in residual viremia during recombinant HIV-1 poxvirus immunizations in patients with clinically undetectable viral loads on durable suppressive highly active antiretroviral therapy. J Virol. 2009;83(19):9731–9742. doi: 10.1128/JVI.00570-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coiras M, et al. Understanding HIV-1 latency provides clues for the eradication of long-term reservoirs. Nat Rev Microbiol. 2009;7(11):798–812. doi: 10.1038/nrmicro2223. [DOI] [PubMed] [Google Scholar]
- 21.Han Y, et al. Resting CD4+ T cells from human immunodeficiency virus type 1 (HIV-1)-infected individuals carry integrated HIV-1 genomes within actively transcribed host genes. J Virol. 2004;78(12):6122–6133. doi: 10.1128/JVI.78.12.6122-6133.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han Y, et al. Orientation-dependent regulation of integrated HIV-1 expression by host gene transcriptional readthrough. Cell Host Microbe. 2008;4(2):134–146. doi: 10.1016/j.chom.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lenasi T, Contreras X, Peterlin BM. Transcriptional interference antagonizes proviral gene expression to promote HIV latency. Cell Host Microbe. 2008;4(2):123–133. doi: 10.1016/j.chom.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sadowski I, Lourenco P, Malcolm T. Factors controlling chromatin organization and nucleosome positioning for establishment and maintenance of HIV latency. Curr HIV Res. 2008;6(4):286–295. doi: 10.2174/157016208785132563. [DOI] [PubMed] [Google Scholar]
- 25.Marban C, et al. Recruitment of chromatin-modifying enzymes by CTIP2 promotes HIV-1 transcriptional silencing. Embo J. 2007;26(2):412–423. doi: 10.1038/sj.emboj.7601516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedman J, Pearson R, Karn J. Direct evidence that histone trimethylation is required for HIV proviral silencing during the establishment of latency. in 16th Conference on Retroviruses and Opportunistic Infections; Montreal, Canada. CROI; 2009. [Google Scholar]
- 27.Blazkova J, et al. CpG methylation controls reactivation of HIV from latency. PLoS Pathog. 2009;5(8):e1000554. doi: 10.1371/journal.ppat.1000554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kauder SE, et al. Epigenetic regulation of HIV-1 latency by cytosine methylation. PLoS Pathog. 2009;5(6):e1000495. doi: 10.1371/journal.ppat.1000495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lassen KG, et al. Nuclear retention of multiply spliced HIV-1 RNA in resting CD4+ T cells. PLoS Pathog. 2006;2(7):e68. doi: 10.1371/journal.ppat.0020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang J, et al. Cellular microRNAs contribute to HIV-1 latency in resting primary CD4+ T lymphocytes. Nat Med. 2007;13(10):1241–1247. doi: 10.1038/nm1639. [DOI] [PubMed] [Google Scholar]
- 31.Han Y, et al. Experimental approaches to the study of HIV-1 latency. Nat Rev Microbiol. 2007;5(2):95–106. doi: 10.1038/nrmicro1580. [DOI] [PubMed] [Google Scholar]
- 32.Archin N, Espeseth A, Parker D, Cheema M, Hazuda D, Margolis DM. Expression of Latent HIV Induced by the Potent HDAC Inhibitor Suberoylanilide Hydroxamic Acid. AIDS Research and Human Retroviruses. 2009;25(2):207–212. doi: 10.1089/aid.2008.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Archin NM, et al. Expression of latent human immunodeficiency type 1 is induced by novel and selective histone deacetylase inhibitors. Aids. 2009;23(14):1799–1806. doi: 10.1097/QAD.0b013e32832ec1dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen A, et al. Resting CD4+ T lymphocytes but not thymocytes provide a latent viral reservoir in a simian immunodeficiency virus-Macaca nemestrina model of human immunodeficiency virus type 1-infected patients on highly active antiretroviral therapy. J Virol. 2003;77(8):4938–4949. doi: 10.1128/JVI.77.8.4938-4949.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun Z, et al. Intrarectal transmission, systemic infection, and CD4+ T cell depletion in humanized mice infected with HIV-1. J Exp Med. 2007;204(4):705–714. doi: 10.1084/jem.20062411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang L, Kovalev GI, Su L. HIV-1 infection and pathogenesis in a novel humanized mouse model. Blood. 2007;109(7):2978–2981. doi: 10.1182/blood-2006-07-033159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choudhary SK, et al. Suppression of human immunodeficiency virus type 1 (HIV-1) viremia with reverse transcriptase and integrase inhibitors, CD4+ T-cell recovery, and viral rebound upon interruption of therapy in a new model for HIV treatment in the humanized Rag2−/−{gamma}c−/− mouse. J Virol. 2009;83(16):8254–8258. doi: 10.1128/JVI.00580-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bowman MC, Archin NM, Margolis DM. Pharmaceutical approaches to eradication of persistent HIV infection. Expert Rev Mol Med. 2009;11:e6. doi: 10.1017/S1462399409000970. [DOI] [PubMed] [Google Scholar]
- 39.Kulkosky J, et al. Prostratin: activation of latent HIV-1 expression suggests a potential inductive adjuvant therapy for HAART. Blood. 2001;98(10):3006–3015. doi: 10.1182/blood.v98.10.3006. [DOI] [PubMed] [Google Scholar]
- 40.Korin YD, et al. Effects of prostratin on T-cell activation and human immunodeficiency virus latency. J Virol. 2002;76(16):8118–8123. doi: 10.1128/JVI.76.16.8118-8123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brooks DG, et al. Molecular characterization, reactivation, and depletion of latent HIV. Immunity. 2003;19(3):413–423. doi: 10.1016/s1074-7613(03)00236-x. [DOI] [PubMed] [Google Scholar]
- 42.Quivy V, De Walque S, Van Lint C. Chromatin-associated regulation of HIV-1 transcription: implications for the development of therapeutic strategies. Subcell Biochem. 2007;41:371–396. [PubMed] [Google Scholar]
- 43.Keedy KS, et al. A limited group of class I histone deacetylases acts to repress human immunodeficiency virus type 1 expression. J Virol. 2009;83(10):4749–4756. doi: 10.1128/JVI.02585-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Redel L, et al. HIV-1 regulation of latency in the monocyte-macrophage lineage and in CD4+ T lymphocytes. J Leukoc Biol. 2009 Oct 2; doi: 10.1189/jlb.0409264. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 45.Cervoni N, Szyf M. Demethylase activity is directed by histone acetylation. J Biol Chem. 2001;276(44):40778–40787. doi: 10.1074/jbc.M103921200. [DOI] [PubMed] [Google Scholar]
- 46.Krutzfeldt J, et al. Silencing of microRNAs in vivo with 'antagomirs'. Nature. 2005;438(7068):685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 47.Zimmermann TS, et al. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441(7089):111–114. doi: 10.1038/nature04688. [DOI] [PubMed] [Google Scholar]
- 48.Peer D, et al. Systemic leukocyte-directed siRNA delivery revealing cyclin D1 as an anti-inflammatory target. Science. 2008;319(5863):627–630. doi: 10.1126/science.1149859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peer D, Shimaoka M. Systemic siRNA delivery to leukocyte-implicated diseases. Cell Cycle. 2009;8(6):853–859. doi: 10.4161/cc.8.6.7936. [DOI] [PubMed] [Google Scholar]
- 50.Savarino A, et al. "Shock and kill" effects of class I-selective histone deacetylase inhibitors in combination with the glutathione synthesis inhibitor buthionine sulfoximine in cell line models for HIV-1 quiescence. Retrovirology. 2009;6:52. doi: 10.1186/1742-4690-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]