Abstract
Cell and tissue responses to polymeric materials are orchestrated in part by the conformations of adsorbed plasma proteins. Thus, the chemical properties of a polymer membrane that govern protein adsorption behaviour can play an important role in determining the biological properties of tissue engineered scaffolds derived from that polymer. In this study, we explored the role of membrane thickness as a factor influencing cell adhesion and proliferation on chitosan membranes with and without covalently-attached glycosaminoglycans. Rat mesenchymal stem cells cultured on chitosan membranes of various thicknesses demonstrated significantly improved cell adhesion, spreading and proliferation as membrane thickness was increased. Hepatocytes displayed increased spreading on the substrate with increasing membrane thickness similar to MSCs. Increased thickness reduced the overall crystallinity of the membrane, and the data indicate that the improved cellular responses were likely due to enhanced adsorption of serum vitronectin, presumably due to reduced membrane crystallinity. These results demonstrate that membrane thickness is an important design variable that can be manipulated in chitosan-based scaffolds to achieve enhanced cell spreading, proliferation and function.
Keywords: chitosan, glycosaminoglycans, mesenchymal stem cells, membrane thickness, protein adsorption, crystallinity
INTRODUCTION
Challenges in biomaterial design for tissue engineering applications include achieving material properties that match the tissue to be replaced as well as inducing biological responses for enhanced cell attachment, proliferation and/or differentiation [1]. In the absence of any incorporated biological cues, the biological activity of a tissue engineering scaffold is indirectly determined by the physicochemical nature of the material. In particular, its physical properties, such as hydrophobicity, surface charge, topography, and stiffness may directly influence adsorption of adhesion proteins and subsequent cellular interactions [2–4]. Polymer crystallinity is another such factor, since biomaterials with high crystallinity have been reported to exhibit low affinity for cells when compared to their amorphous counterparts [5–8].
In our previous studies [9], we observed that mesenchymal stem cell (MSC) attachment, morphology, and proliferation was non-uniform on cast chitosan membranes, and consistently higher on the thicker regions of such cast membranes. Therefore, we postulated that the membrane thickness could be an important but neglected design variable in tissue engineering using scaffolds with membrane components. In this study, we evaluated morphological and proliferative responses as a function of the thickness of GAG-immobilized chitosan membranes, and investigated the dependence of microstructure and protein adsorption on membrane thickness.
MATERIALS AND METHODS
Materials
Heparin sodium USP (average MW of 10–12 kDa), heparan sulfate (average MW of 10–12 kDa) and dermatan sulfate (average MW of 28–32 kDa) from porcine intestinal mucosa were purchased from Celsus Laboratories (Cincinnati, OH). Dextran Sulfate (average MW 500kDa) was purchased from CarboMer, Inc (San Diego, CA). Chondroitin 4-sulfate (average MW of 20–30 kDa) from bovine trachea, chondroitin 6-sulfate (average MW of 50–60 kDa) from shark cartilage and chitosan from crab shells (medium MW of 450 kDa and 85% degree of deacetylation), penicillin-streptomycin, amphotericin B, Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), trypsin-EDTA solution, Hank’s buffered salt solution, Krebs-Ringer bicarbonate buffer, Percoll, glucagon, insulin, epidermal growth factor, L-proline, hydrocortisone, collagenase from Clostridium histolyticum and methylthiazolyldiphenyl-tetrazolium bromide (MTT) were all purchased from Sigma-Aldrich (St. Louis, MO). 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride (EDC) was purchased from BioChemika-Fluka (Allentown, PA). Safranin-O was purchased from EM Sciences (Cherry Hill, NJ).
Preparation of Chitosan Membranes
Membranes were prepared in 24-well tissue culture plates by air drying 50, 250 or 500 μl of sterile chitosan solution (1.5 wt% in 0.2 M acetic acid) in each well to form thin films containing 0.375, 1.875, or 3.75 mg of chitosan per cm2. Membrane thickness was measured using a micrometer. The chitosan membranes were derivatized with glycosaminoglycans (GAGs) including: heparin (HEP), heparan sulfate (HS), chondroitin 4-sulfate (C4S), chondroitin-6-sulfate (C6S), dermatan sulfate (DS) and the semi-synthetic GAG analog dextran sulfate (DxS). Membrane derivatizations were done at a density of 1 mg GAG per mg of chitosan. GAGs were covalently immobilized on the membranes as follows. Dried chitosan membranes were neutralized by washing with 0.2 M NaOH and followed by phosphate buffered saline (PBS). Each GAG solution (2.5, 5, 7.5, 12.5 and 25 mg/ml in PBS corresponding to membranes containing 0.375, 0.75, 1.125, 1.875, or 3.75 mg of chitosan per cm2) was mixed with an equal volume of EDC solution prepared at a concentration such that the molar ratio of EDC to GAG carboxyls in the reaction mixture was 10:1. The active intermediate was allowed to form for 15 minutes and the activated GAG solution (600 μl/well) was applied onto the chitosan membranes. The reaction was allowed to proceed with continuous gentle shaking for 24 hours then the GAG-EDC solution was removed and the membranes washed with three changes of PBS over 3 hours. Dextran sulfate was first modified with chloroacetic acid to form the carboxymethyl derivative by the method of Brunswick [10]. Carboxymethyl dextran sulfate was then covalently linked to the chitosan membranes using EDC as described above. The amount of GAG bound on the membranes was determined indirectly by measuring the concentration of GAG in the reaction solution and the wash solutions collected after the reaction was complete. GAG concentrations were measured using Safranin-O dye. Briefly, thirty microliters of GAG solution or wash solution was mixed with 240 μl Safranin-O (0.04 mg/ml in 50 mM sodium acetate buffer, pH 7.4) and the absorbance of the resulting solution was measured at 510 nm. The amount of GAG in milligrams in each solution was calculated using a standard curve prepared for each GAG and this value was subtracted from the amount of GAG initially applied to each membrane. The difference was reported as the amount of GAG bound on the membranes.
MSC Isolation and Culture
Mesenchymal stem cells were isolated from the bone marrow of adult male Sprague-Dawley rats as described elsewhere [11]. Briefly, the shafts of femurs and tibias were flushed with sterile PBS using an 18-gauge needle, and the aspirate was passed through a 70-μm nylon cell strainer. After centrifugation at 1500 rpm for 10 minutes and washing twice with sterile PBS, the cells were suspended in DMEM containing 10% FBS supplemented with 20 μg/ml streptomycin, 20 mU/ml penicillin, 2.5 μg/ml amphotericin B and plated into 75-cm2 tissue culture flasks. After 3–4 days, the medium was changed to remove the unattached cells and the adherent cells were grown to 80% confluency. The cells were then trypsinized and subcultured at a ratio of 1:4. The cells utilized in these experiments were between passage number 2 (P2) and 10 (P10).
For cell spreading and proliferation measurement experiments, MSCs (P2) were seeded on GAG-chitosan membranes at a density of 5,000 cells/cm2. Cells were allowed to attach for 4 days with a half volume medium change on day 2. Full medium changes were done every 2–3 days thereafter. The cultures were monitored via phase contrast microscopy. The captured images on day 3 of the culture period were used to measure the projected area of attached cells using the computer software SigmaScan Pro 5 (Systat Software, Inc., San Jose, CA) to determine the degree of cell spreading on the surfaces. On days 2 and 4 of the culture, wells were sacrificed to evaluate the metabolic activity of the cells via the MTT assay. Fifty microliters of 6 mg/ml MTT in culture medium was added to the wells and cells were incubated for 4 hours at 37°C. The culture medium was removed and the accumulated formazan crystals and cells were dissolved via an overnight incubation with 5% SDS solution, pH 2.0. The absorbance of this solution was measured at 540 nm using a spectrophotometer. During the exponential growth phase, the MTT metabolic activity kinetics between two time points, to and t, is given by the equation
where A is the measured absorbance of formazan, t denotes time in days and μA is the specific growth rate in day−1 based on metabolic activity [12].
Hepatocyte Isolation and Culture
Adult rat primary hepatocytes were isolated using the collagenase digestion method of Seglen [13] as modified by Dunn [14]. Male Sprague-Dawley rats weighing 175–200 g were anesthetized and the portal vein was cannulated for perfusion of the liver with 250 ml of Krebs Ringer bicarbonate buffer (KRB) supplemented with 1 mM EDTA followed by 250 ml of 0.5 mg/ml collagenase solution supplemented with calcium chloride. The solutions were kept at 37°C during perfusion. The liver was then dissected out, the capsule was removed and it was shaken gently in cold Hanks buffered saline solution (HBSS) to release the cells. The cell suspension was filtered through a nylon mesh with 100 μm pore size, after which the cells were washed with HBSS twice. The cell suspension was later centrifuged over isodensity Percoll to clear dead cells and debris. The pellet was washed one more time and resuspended in culture medium. Culture medium used consisted of DMEM supplemented with 10% (v/v) FBS, 18.52 μg/ml insulin, 7 μg/ml glucagon, 7.5 μg/ml hydrocortisone, 40 μg/ml L-proline, 20 μg/ml streptomycin, 20 mU/ml penicillin and 2.5 μg/ml amphotericin B. The isolations yielded around 150–350 million hepatocytes with a viability ranging from 86% to 95% evaluated by the trypan blue exclusion. Hepatocytes were seeded on unmodified chitosan and GAG-derivatized chitosan at a density of 1×105 cells/cm2. Hepatocytes cultured in collagen sandwich configuration served as positive control. Medium change and sampling was done every 24 hours after seeding. Hepatocyte morphology was evaluated using phase contrast microscopy. Albumin secretion was measured daily by ELISA.
Analysis of Membrane Structure with X-ray Diffraction
Chitosan solution was air dried in 10-cm Petri dishes to obtain membranes with varying thicknesses (1.125, 1.875, 3.75 mg chitosan/cm2). The membranes were neutralized with 20% ammonia solution and soaked in dH2O to completely hydrate the films. The membrane thickness was measured using a micrometer in the hydrated state. The crystalline structure of the membranes was analyzed using a Rigaku, Rotaflex X-ray diffractometer in which the high-intensity monochromatic Ni-filtered Cu Kα radiation was generated at 40 kV and 200mA. The scanning was done at 1.2 degrees/min with a step size of 0.03.
Measurement of Adsorbed Serum Adhesion Proteins on Membranes
Protein adsorption on the membranes was evaluated by an immunoassay for fibronectin and vitronectin. The GAG-modified chitosan membranes were incubated overnight with culture medium containing 10% FBS at 4°C. The membranes were washed with four changes of PBS over 2 hours. The primary antibodies were allowed to attach to the membranes for 2 hours and then washed three times with PBS over 30 minutes. Next, membranes were incubated with alkaline phosphatase-conjugated secondary antibodies for 2 hours. After three washes with PBS over 30 minutes, p-nitrophenyl phosphate (Sigma-Aldrich, St. Louis, MO) in Tris-buffer was added as the substrate and kept for 30 minutes. The reaction was stopped by addition of 3 M sodium hydroxide and the absorbance of the resultant solution was measured at 405 nm using a multiwell plate reader. The primary antibodies were rabbit anti-human fibronectin and monoclonal mouse anti-vitronectin, clone VIT-2, and the secondary antibodies were alkaline phosphatase conjugates of anti-mouse IgM and anti-rabbit IgG, respectively.
Statistical Analysis
The statistical analysis of data on GAG quantification, cell proliferation, and protein adsorption were performed using Student’s t-test with 95% confidence limit.
RESULTS
In this study, we investigated the influence of GAG enhanced chitosan film thickness on cell spreading and proliferation and explored the membrane microstructure and subsequent changes in protein adsorption properties as a possible mechanism for this effect.
MSC Morphology and Spreading
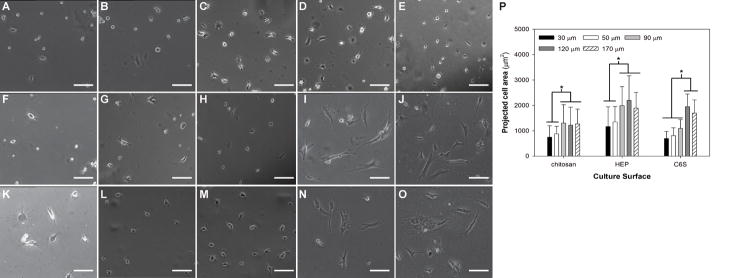
MSCs were cultured on chitosan and GAG-immobilized membranes for 3 days before images were captured. MSC morphology displayed differences depending on the underlying membrane thickness. In general, MSCs were round on thinner membranes becoming less round and assuming spindle shape as the membrane thickness was increased (Figure 1). The phase contrast images clearly show that as the membrane thickness increased from 50 to 90 μm, the extent of cell spreading increased on chitosan and HEP-chitosan membranes (Figure 1B,C and 1L,M) whereas the increase in cell spreading was observed when the membrane thickness was increased from 90 to 120 μm on C6S-chitosan membranes (Figure H,I). The projected cell area was measured to be significantly higher on thicker membranes (90, 120 and 170 μm) when compared to thin chitosan membranes (30 and 50 μm) on chitosan only and HEP-modified membranes. However, the degree of cell spreading was found to be independent of chitosan membrane thickness above 90 μm. On C6S-modified membranes, the projected area didn’t increase when the membrane thickness was increased above 120 μm (Figure 1P).
Figure 1.
Effect of membrane thickness on MSC morphology on day 3 of culture. MSCs were cultured on chitosan only (A–E), C6S-modified (F–J) and heparin modified (K–O) membranes of different thicknesses 30 μm (A,F and K) 50 μm (B, G and L), 90 μm (C, H and M), 120 μm (D, I and N) and 170 μm (E, J and O). Cell spreading was assessed by measurement of projected cell area (P) (*p<0.01). Scale bars represent 100 μm.
MSC Proliferation
The effect of membrane thickness on MSC growth on GAG augmented chitosan was investigated by evaluating the reduction of MTT to formazan crystals over 4 hours. The results indicate that the rate of growth increased as the membrane thickness was increased for chitosan and all the GAGs studied, indicating a direct effect of thickness on proliferation independent of GAG immobilization (Figure 2). Notably on the HEP-chitosan membrane, the increase displayed saturation when the membrane thickness was increased above 120 μm. The proliferation rate was the lowest on chitosan-only membranes for all thicknesses except for HEP on low thickness (30 μm) membranes.
Figure 2.

MSC proliferation rate as a function of membrane thickness. MSC proliferation increased with increasing membrane thickness, *p < 0.05 for all the GAG types studied.
Hepatocyte Morphology and Function
In order to determine whether the effect of chitosan membrane thickness on cell attachment and proliferation was not exclusive to MSCs, we cultured primary adult rat hepatocytes on GAG-modified chitosan membranes of varying thicknesses. The chitosan membrane thicknesses of 30, 50 and 90 μm were selected because in our previous studies, these membrane thicknesses were found to support hepatocyte function best (unpublished). In addition, our previous work showed that primary adult rat hepatocytes displayed higher hepatic function on dextran sulfate modified chitosan compared to other GAGs (heparin or chondroitin 6-sulfate). Hepatocytes displayed increased spreading on the substrate with increasing membrane thickness similar to MSCs. As the membrane thickness was increased, spheroids became smaller and spreading of individual cells was visible around the spheroids (Figure 3B,C). The spheroid size was measured to be smaller on 50- and 90-μm membranes when compared to 30-μm membranes with statistical significance (Figure 3D). The effect on dextran sulfate-chitosan membranes was similar but more pronounced. While hepatocytes exhibited spheroid formation on the thinnest membrane (30 μm), hepatocytes attached and spread well on the dextran sulfate-chitosan membranes at membrane thicknesses 50 and 90 μm (Figures 3E–G) resembling cultures on collagen gel controls (Figure 3H). In order to quantify hepatocyte function, albumin secretion as a function of time in culture was quantified for cells cultured on chitosan and chitosan-DxS membranes (Figure 3I). Even though albumin secretion increased with time in culture, it didn’t show significant dependence on the membrane thickness neither on chitosan nor chitosan-DxS membranes.
Figure 3.
Effect of membrane thickness on primary adult rat hepatocyte morphology on day 3 of culture. (A–C) Chitosan only membranes, (D) spheroid size on chitosan only membranes, (E–G) DxS-modified membranes of different thicknesses (A and E) 30 μm, (B and F) 50 μm, and (C and G) 90 μm. (I) Albumin secretion by hepatocytes on chitosan and chitosan-DxS membranes. Scale bars represent 100 μm.
Immobilized GAGs on Chitosan
In order to explore whether the behavior of the cells on GAG-modified surfaces is determined by the differences in the levels of GAGs immobilized on the membranes, surfaces were analyzed to quantify immobilized GAG as a function of membrane thickness (Figure 4). Initially, GAGs were applied on chitosan membranes at GAG to chitosan ratio of 1 mg/mg. The amount of immobilized HEP, C6S and DxS was found to increase linearly with increasing membrane thickness as expected.
Figure 4.
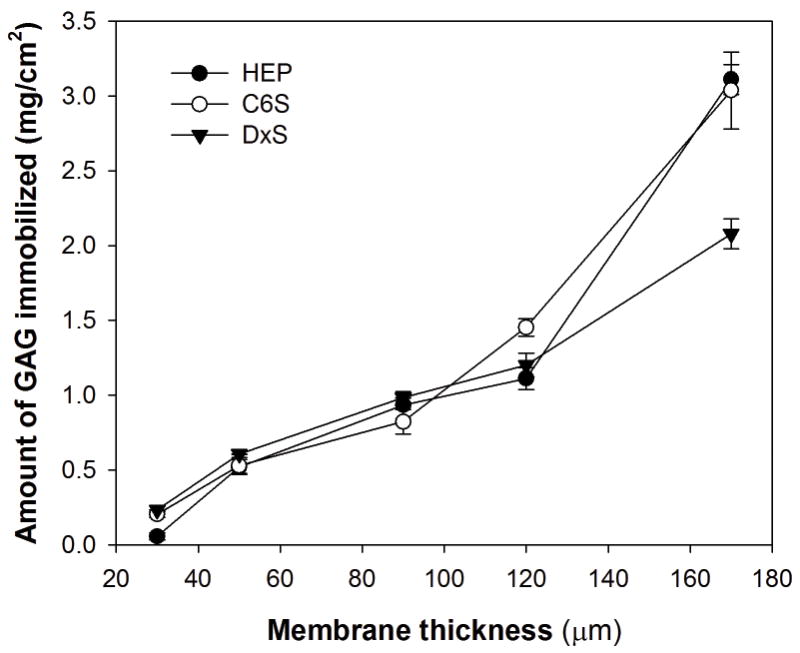
Effect of membrane thickness on the amount of GAG immobilized. The initial GAG to chitosan ratio was 1 mg/mg. p < 0.05 for all the GAGs studied.
Membrane Microstructure
We hypothesized that the increases in cell spreading (for hepatocytes and MSCs) and MSC proliferation rate with membrane thickness would correlate with microstructural changes in the membrane as the thickness was increased. In order to test the hypothesis, the membranes were analyzed for crystalline structure by X-ray diffraction (XRD). Membranes were prepared by casting and air drying chitosan solutions to form membranes that are 90, 120 and 170 μm thick. According to the XRD spectra, chitosan membranes of all thicknesses display two peaks at 2θ = 10° and 2θ = 20° characteristic to chitosan [15]. In addition, the spectra for 170 μm and 120μm membranes showed a broad peak around 2θ = 26.3° which was only marginally present in the 90 μm membrane (Figure 5). The presence of such a peak suggests a significant increase in the amorphous fraction with increasing thickness [16].
Figure 5.

XRD Spectra of chitosan membranes as a function of membrane thickness.
Adsorption of Serum Proteins
We further hypothesized that the effect of increased amorphous fraction in membranes on cell spreading and proliferation was through its effect on the adsorption of serum adhesion proteins, vitronectin and fibronectin. To evaluate this effect, membranes were incubated overnight with medium containing 10% FBS and the relative levels of adsorbed fibronectin and vitronectin were determined using an immunoassay. The results show that the level of vitronectin binding increased proportionally with increasing membrane thickness and statistical analysis revealed that the amount of vitronectin adsorbed on 30 and 170 μm membranes were significantly different. The level of vitronectin adsorption on HEP reached saturation at a membrane thickness of 120 μm (Figure 6A), exactly paralleling the effect of increased thickness on MSC proliferation on HEP-modified chitosan (Figure 2). On the other hand, the level of fibronectin binding displayed a different trend being the lowest on the membrane with medium thickness (120 μm). The highest (170 μm) and lowest (30 μm) thickness membranes had comparable levels of fibronectin binding (Figure 6B).
Figure 6.
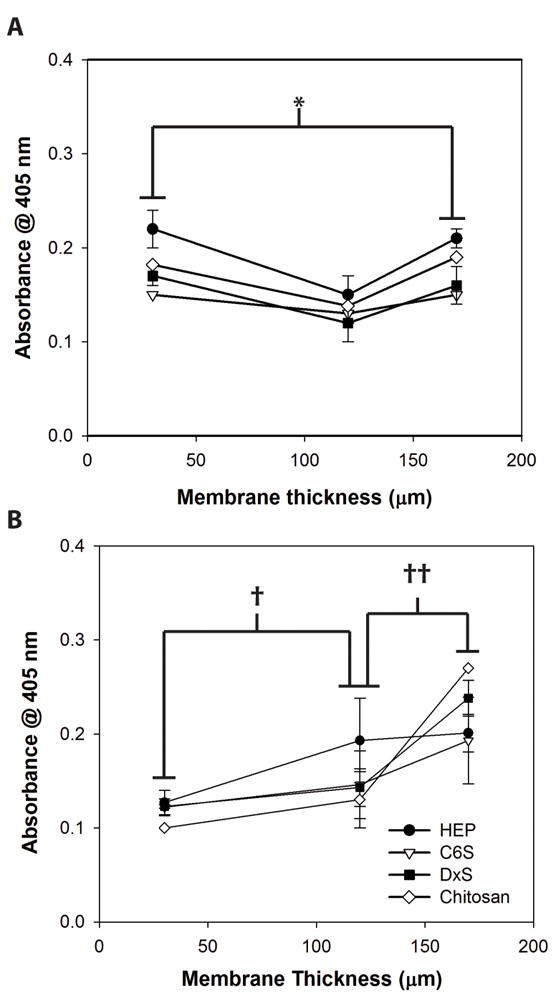
Adsorption of serum proteins on GAG modified chitosan (A) fibronection, (B) vitronectin. *†‡p < 0.05 for all GAGs studied.
DISCUSSION
We have demonstrated that membrane thickness has a significant positive effect on the MSC growth and proliferation. We have also showed that increasing the membrane thickness improved primary rat hepatocyte attachment and spreading indicating that the effect was not limited to MSCs. This effect was present on unmodified as well as GAG-enhanced chitosan membranes
It was hypothesized that the changes in the membrane microstructure changed the protein binding properties of the membranes, which subsequently enhanced cell spreading and proliferation as the membrane thickness was increased. In support of this hypothesis, the analysis of membrane crystalline structure with XRD revealed the presence of an amorphous region in thicker membranes (Figure 5). This is an expected finding, since during the membrane casting/air drying process, polymer chains align with the plane of the underlying substrate, thus generating a fairly high degree of crystallinity. However, the alignment effect declines with polymer chain distance from the substrate plane. Thus thicker membranes that have more material further away from the casting surface would have an overall lower level of crystallinity.
Furthermore, our finding that MSC spreading and proliferation increased with decreasing membrane crystallinity is supported by several examples that demonstrate increased cell adhesion and proliferation on less crystalline materials. For example, it has been shown for osteoblast precursor cells that amorphous hydroxyapatite substrates enhanced initial cell attachment compared to highly crystalline hydroxyapatite [5, 6]. In addition, it was reported that fibroblast attachment decreased when a more crystalline carboxymethyl-chitin membrane was used instead of a less crystalline carboxymethyl-chitosan film as the culture substrate [7]. Polymer surface crystallinity was also found to affect the endochondral ossification of rabbit articular cartilage chondrocytes [8].
We demonstrated that the changes in membrane crystallinity as the thickness was increased subsequently altered the protein adsorption properties of the membranes. The relative level of adsorbed vitronectin was found to increase with increasing membrane thickness; however, fibronectin binding showed a different trend with reduced adsorption on medium thickness membranes which suggests a difference in the mechanism of binding and its dependence on crystallinity for the two proteins. The specific growth rate as a function of membrane thickness was well correlated with the relative levels of adsorbed vitronectin for all GAG-immobilized chitosan membranes. It has also been suggested that decreased surface crystallinity is associated with increased protein adsorption [17] on materials like polypropylene [18], polyamide sheets [19] and PEG coatings [20] supporting our results.
This study demonstrated that membrane thickness is a significant factor in cell spreading and proliferation in chitosan membranes enhanced with various GAGs. Further, we established that increased thickness reduces crystallinity of the membrane, and the superiority is possibly explained by enhanced adsorption of serum adhesion protein vitronectin, presumably due to reduced crystallinity. These results demonstrate that membrane thickness is a design variable that can be modified in construction of membrane scaffolds for enhanced cell attachment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ratner BD, Bryant SJ. Biomaterials: where we have been and where we are going. Annu Rev Biomed Eng. 2004;6:41. doi: 10.1146/annurev.bioeng.6.040803.140027. [DOI] [PubMed] [Google Scholar]
- 2.Wang YX, Robertson JL, Spillman WB, Jr, Claus RO. Effects of the chemical structure and the surface properties of polymeric biomaterials on their biocompatibility. Pharm Res. 2004;21:1362. doi: 10.1023/b:pham.0000036909.41843.18. [DOI] [PubMed] [Google Scholar]
- 3.Wilson CJ, Clegg RE, Leavesley DI, Pearcy MJ. Mediation of biomaterial-cell interactions by adsorbed proteins: a review. Tissue Eng. 2005;11:1. doi: 10.1089/ten.2005.11.1. [DOI] [PubMed] [Google Scholar]
- 4.Roach P, Eglin D, Rohde K, Perry CC. Modern biomaterials: a review - bulk properties and implications of surface modifications. J Mater Sci Mater Med. 2007;18:1263. doi: 10.1007/s10856-006-0064-3. [DOI] [PubMed] [Google Scholar]
- 5.Balasundaram G, Sato M, Webster TJ. Using hydroxyapatite nanoparticles and decreased crystallinity to promote osteoblast adhesion similar to functionalizing with RGD. Biomaterials. 2006;27:2798. doi: 10.1016/j.biomaterials.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Yang Y, Dennison D, Ong JL. Protein adsorption and osteoblast precursor cell attachment to hydroxyapatite of different crystallinities. Int J Oral Maxillofac Implants. 2005;20:187. [PubMed] [Google Scholar]
- 7.Wongpanit P, Sanchavanakit N, Pavasant P, Supaphol P, Tokura S, Rujiravanit R. Preparation and characterization of microwave-treated carboxymethyl chitin and carboxymethyl chitosan films for potential use in wound care application. Macromol Biosci. 2005;5:1001. doi: 10.1002/mabi.200500081. [DOI] [PubMed] [Google Scholar]
- 8.Zheng Z, Bei FF, Tian HL, Chen GQ. Effects of crystallization of polyhydroxyalkanoate blend on surface physicochemical properties and interactions with rabbit articular cartilage chondrocytes. Biomaterials. 2005;26:3537. doi: 10.1016/j.biomaterials.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 9.Uygun BE, Stojsih S, Matthew H. Effects of immobilized glycosaminoglycans on the proliferation and differentiation of mesenchymal stem cells. Tissue Engineering. 2009 doi: 10.1089/ten.tea.2008.0405. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiernan JA. Theory and Practice. London: Arnold; 1999. Histological and Histochemical Methods. [Google Scholar]
- 11.Javazon EH, Colter DC, Schwarz EJ, Prockop DJ. Rat marrow stromal cells are more sensitive to plating density and expand more rapidly from single-cell-derived colonies than human marrow stromal cells. Stem Cells. 2001;19:219. doi: 10.1634/stemcells.19-3-219. [DOI] [PubMed] [Google Scholar]
- 12.Bailey JE, Ollis DF. Biochemical Engineering Fundamentals. Singapore: McGraw-Hill; 1986. [Google Scholar]
- 13.Seglen PO. Hepatocyte suspensions and cultures as tools in experimental carcinogenesis. J Toxicol Environ Health. 1979;5:551. doi: 10.1080/15287397909529766. [DOI] [PubMed] [Google Scholar]
- 14.Dunn JC, Tompkins RG, Yarmush ML. Long-term in vitro function of adult hepatocytes in a collagen sandwich configuration. Biotechnol Prog. 1991;7:237. doi: 10.1021/bp00009a007. [DOI] [PubMed] [Google Scholar]
- 15.Samuels RJ. Solid-State Characterization of The Structure of Chitosan Films. J Polym Sci Pt B-Polym Phys. 1981;19:1081. [Google Scholar]
- 16.Alexander L. X-ray Diffraction methods in polymer science. London: John Wiley & Sons Inc; 1970. [Google Scholar]
- 17.Chen S, Yu F, Yu Q, He Y, Jiang S. Strong resistance of a thin crystalline layer of balanced charged groups to protein adsorption. Langmuir. 2006;22:8186. doi: 10.1021/la061012m. [DOI] [PubMed] [Google Scholar]
- 18.Kawamoto N, Mori H, Yui N, Terano M. Mechanistic aspects of blood-contacting properties of polypropylene surfaces--from the viewpoint of macromolecular entanglement and hydrophobic interaction via water molecules. J Biomater Sci Polym Ed. 1998;9:543. doi: 10.1163/156856298x00037. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka H, Mori H, Nitta KH, Terano M, Yui N. Improved blood compatibility of drawn polyamide sheets. J Biomater Sci Polym Ed. 1996;8:211. doi: 10.1163/156856296x00255. [DOI] [PubMed] [Google Scholar]
- 20.Alcantar NA, Aydil ES, Israelachvili JN. Polyethylene glycol-coated biocompatible surfaces. J Biomed Mater Res. 2000;51:343. doi: 10.1002/1097-4636(20000905)51:3<343::aid-jbm7>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]