Abstract
Cardiac tissue engineering typically utilizes protein-rich scaffolding materials and growth factors to improve cardiac tissue function in vitro and in vivo. The objectives of this preliminary study were (I) to investigate the potential of porcine small intestinal submucosa gel (SIS gel) in cardiac tissue engineering and (II) to compare the function of tissue based on either SIS gel or Matrigel, a tumor-derived benchmark material. Neonatal rat cardiac cells were combined with either SIS gel or Matrigel and cultured on porous elastomeric scaffolds composed of poly(glycerol sebacate) for 13 days. Tissue function was assessed by measuring contraction rates twice daily. Tissue morphology was compared qualitatively by H&E staining. Normalized troponin T expression (troponin T:DNA) was compared using image analysis. SIS gel constructs contracted at significantly higher rates than Matrigel constructs on days 8–11. Normalized troponin T expression was significantly higher in SIS gel constructs compared to Matrigel constructs. In summary, this research demonstrates that: (I) SIS gel can be used to create contractile engineered cardiac tissue; and (II) SIS gel produced engineered cardiac tissues with a more physiologic contraction rate and higher phenotypic protein expression based on basic in vitro examinations performed in this study.
Keywords: tissue engineering, porcine small intestinal submucosa, Matrigel, poly(glycerol sebacate), tissue engineering scaffolding materials
INTRODUCTION
Myocardial infarction affects millions throughout the world, increasing mortality and morbidity rates and consuming medical and financial resources.1 Patients suffering acute myocardial infarction have increased mortality rates and a greater risk of additional infarcts, stroke, and heart failure compared to the general population.1,2 The limited regenerative capacity of the myocardium3,4 and heart transplant shortages1 have spurred the investigation of potential therapies5 which focus on improving myocardial function by administering biomolecules,6–8 acellular materials,9,10 cells,11–14 or engineered tissue14–17 to native cardiac tissue. Various challenges accompany each of these approaches, including biomolecule degradation and depletion from the infarct site,18 the viability and integration of injected cells,19–21 and the development of scar tissue between engineered and native myocardium.22 Investigative therapies commonly employ naturally derived materials as one element of a treatment in combination with cells or synthetic materials, though natural materials alone may constitute a treatment.9,10,17
Naturally derived materials used in experimental or clinical treatment of infarcts include tumor-derived basement membrane matrix gel (Matrigel),13,15,23 alginate,9,24 collagen,14,25 laminin,26 fibrin,10,27,28 and decellularized extracellular matrix (ECM),17,29 all of which can enhance cell and tissue function in the myocardial infarct area. Only two of these materials, Matrigel and decellularized ECM, mimic the extracellular environment with an array of proteins and other biomolecules such as growth factors.30,31 However, there are concerns about Matrigel’s safety in potential clinical applications because its components are derived from basement membrane-producing Engelbreth-Holm-Swarm sarcomas32 and because Matrigel and basement membrane matrix are known to enhance tumorigenesis and tumor growth in vivo.33–36 These concerns have been substantiated by evidence that the phenotype of cancer cell lines becomes more invasive and malignant in the presence of Matrigel and growth factor-reduced Matrigel as compared to a gel form of decellularized ECM derived from porcine small intestinal submucosa (SIS gel).37 The purpose of this preliminary study was to compare simple metrics in engineered myocardium containing Matrigel or SIS gel, thereby determining the potential of SIS gel as an alternative to Matrigel in developing myocardial therapies. Selected metrics included contraction rate as an indicator of tissue function, tissue morphology to qualitatively assess cell distribution, and normalized troponin T expression (troponin T:DNA) as an indicator of cellular function since troponin T is one of the essential proteins for contractile function and an indicator of differentiation in cardiomyocytes.38,39
Porous foams composed of elastomeric and biodegradable poly(glycerol sebacate) (PGS) have previously shown promise in cardiac tissue engineering and were selected as a compliant substrate in this study.40,41 Scaffolds composed of PGS are elastic and reversibly deformable, and are thereby conducive to contracting cardiomyocytes and engineered myocardium.42 Other desirable properties of PGS include control of its mechanical properties, which can match those of native myocardium,43 the capacity to form a variety of geometries at the macro-44 and micro-scale,45 support of adhesion and phenotypic protein expression for a variety of primary cell types in vitro,46,47 and low inflammatory response and fibrotic encapsulation coupled with retention of mechanical strength during degradation in vivo.47,48
MATERIALS AND METHODS
Cardiac Cell Isolation
A mixed population of cardiac cells, including cardiomyocytes, was obtained from the hearts of 3-day-old neonatal rats (Sprague-Dawley) using a protocol similar to methods previously described,49 which conformed to the Guide for the Care and Use of Laboratory Animals. A single litter of neonates were anesthetized with 5% isoflurane gas, decapitated, and thoracotomized. Hearts were excised, minced into 1–2 mm3 pieces, rinsed in Hank’s Balanced Salt Solution (HBSS; Mediatech, Herndon, VA), placed in 0.06% trypsin (Mediatech) in HBSS, and dissociated overnight (24 h) on an orbital shaker at 4°C and 60 rpm. Trypsin was deactivated with 10% fetal bovine serum (FBS; Cambrex, Walkersville, MD) in Dulbecco’s modified Eagle’s medium (DMEM). The tissue and cell suspension was warmed to 37°C with mild agitation every 60 sec for 240 sec, centrifuged at 100×g for 60 sec, and the initial supernatant was discarded. Ten repetitions of adding 0.1% collagenase A (Roche, Indianapolis, IN), agitating at 60 rpm for 480 sec (240 sec for first repetition), and retaining the supernatant were completed. Supernatant was passed through a 100-μm cell strainer to remove tissue pieces and large cell aggregates before cell quantification using a hemacytometer and trypan blue. Cells were centrifuged at 1000×g for 240 sec and resuspended in culture medium, which contained DMEM, 10% FBS, 0.25% taurine (Alfa Aesar, Pelham, NH), and 50 μg/ml gentamicin (Mediatech).
Cell Seeding and Culture
Porous poly(glycerol sebacate) (PGS) foams with a thickness of 2.0 mm and porosity of ~90% were fabricated as previously described.50 Six disk-like scaffolds with a diameter of 7.94 mm and an approximate pore volume of 89 mm3 were punched from the foams. Scaffolds were soaked in a series of agitated baths (60 rpm): 75%, 50%, and 25% ethanol (45 min each), phosphate-buffered saline without calcium or magnesium (PBS; twice for 45 min each), and 10% FBS in DMEM (24 h). One scaffold was placed in each well of a non-tissue culture-treated polystyrene six-well plate. Excess medium was removed from scaffolds using low-pressure vacuum immediately prior to cell seeding.
The mixed cardiac cell population was resuspended in 1005 μl of culture medium and divided into volumes of 500, 500, and 5 μl. The 5 μl volume was used to assess cell viability and seeding density. The 500 μl volumes were mixed with 125 μl of either Matrigel (BD Biosciences, Bedford, MA) or SIS gel (Cook Biotech, West Lafayette, IN) by repeated slow aspirations with a pipette similar to cell pellet resuspension. Each scaffold received 89 μl of unset gel suspension (71 μl of cell suspension mixed with 18 μl of either Matrigel or SIS gel). Seeded scaffolds were placed in an incubator at 37°C for 60 min to allow for gel crosslinking before submersion in 10 ml culture medium. Cell seeding was the same for all constructs regardless of gel type. The relative protein content of Matrigel and SIS gel were assessed by measuring the wet and dry mass of small gel samples (nominal wet mass of 79 ± 22 mg) prior to and following lyophilization for 24 h. SIS gel and Matrigel were used as supplied. Five ml of medium was exchanged for fresh culture medium every fourth day.
Engineered Tissue Evaluation
Engineered cardiac tissue function was assessed by quantifying contraction rates of constructs during a 13-day culture period followed by end-point histological analysis. Contraction rates were observed using a dissection microscope and manually recorded twice daily (separation >3 h, observation window <15 min). If a construct was contracting asynchronously then the contraction rate of the largest synchronous area was recorded.
At the conclusion of the culture period constructs were rinsed twice with PBS, fixed in 10% neutral buffered formalin, and snap-frozen in optimal cutting temperature compound (Sakura Finetek USA, Torrance, CA). Constructs were cryosectioned perpendicular to the scaffold’s flat surfaces at a thickness of 10 μm. Cell and protein distribution within constructs was investigated by staining with hematoxylin and eosin.
Cardiomyocyte function and differentiation and myocardium formation were investigated by immunostaining with fluorophore-labeled antibodies against troponin T followed by DNA staining. Cryosections were permeabilized with 0.1% Triton X-100 (Sigma-Aldrich, St. Louis, MO) for 5 min at 20°C, rinsed twice with PBS, incubated with 5% normal donkey serum (NDS; Sigma-Aldrich) for 60 min at 37°C, incubated with primary mouse monoclonal anti-cardiac troponin T antibodies (Lab Vision Corporation, Fremont, CA) diluted at 1:50 in 1% NDS for 40 min at 37°C, rinsed twice with PBS, incubated with secondary donkey anti-mouse antibodies conjugated with Alexa Fluor 488 (Invitrogen Corporation, Carlsbad, CA) diluted at 1:50 in 1% NDS for 40 min at 37°C, and rinsed twice with PBS. Tissue sections were then incubated with Hoechst 33258 (Invitrogen) diluted at 1:1000 in PBS for 10 min at 37°C, rinsed twice with PBS, rinsed once with deionized water, and covered with mounting medium and coverslips.
Slides were stored at 4°C and imaged using a Nikon TE-2000U inverted microscope equipped with a 4-megapixel Diagnostics Spot Flex digital camera. One or two non-overlapping images representative of each tissue section were used to quantify fluorescence. Images were taken at 100X or 150X and magnification did not vary significantly between groups. Fluorescence intensity was analyzed using ImageJ version 1.38× by splitting RGB channels and quantifying pixels with >50% intensity on the green channel for troponin T (Alexa Fluor 488) or the blue channel for DNA (Hoechst 33258). To remove bias error in sampling, pixel counts were normalized by gain and exposure, which were not significantly different between groups.
Statistics
All groups were compared using one-way analysis of variance with Tukey post-hoc tests for equal sample sizes or Tukey-Kramer post-hoc tests where sample sizes varied. All graphical representations of data show mean ± standard deviation.
RESULTS
Cell Viability, Cell Seeding Density, and Gel Protein Content
As determined using a hemacytometer, a total of 5.2×106 cells per engineered cardiac tissue construct were seeded. Cell viability was approximately 69%, translating to a live cell seeding density of 3.6×106 cells per construct. No extraneous cells were observed on six-well plate surfaces during the course of cell culture. Initial cell densities within the scaffolds were therefore 5.3×104 total cells per mm3 or 3.6×104 live cells per mm3. Construct diameter was qualitatively observed to decrease during culture, and mean diameter at the conclusion of culture did not differ significantly between SIS gel and Matrigel constructs (6.4 ± 0.4 mm and 6.1 ± 0.3 mm respectively; original diameter 7.94 mm). The dry mass of SIS gel and Matrigel as a percentage of initial wet mass were respectively 7.5 ± 0.4% and 2.7 ± 0.1% (n = 3 for each gel type).
Duration of Contractions and Contraction Rates
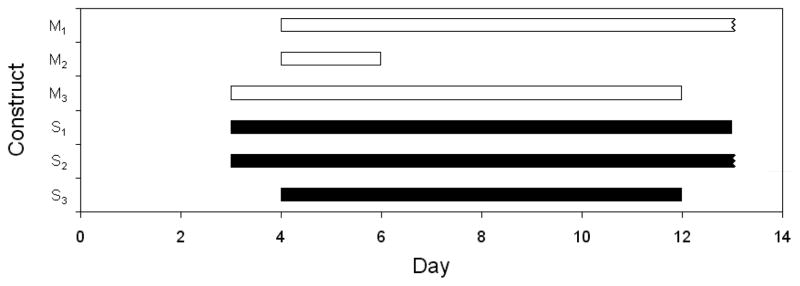
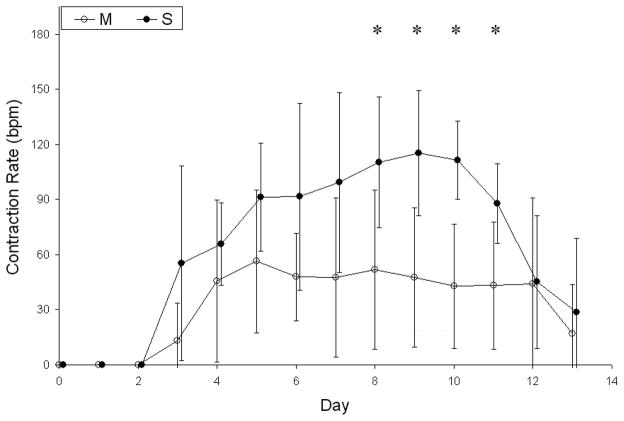
Engineered tissue constructs began contracting on days 3 or 4, and one construct from each group was still contracting at the time of snap-freezing on day 13 (Fig. 1). There were no significant differences in the time between cell seeding and initial contractions, the duration of contractions, or the time between cell seeding and final contractions for constructs containing Matrigel or SIS gel. The mean contraction rate of SIS gel constructs was significantly higher than the mean contraction rate of Matrigel constructs on days 8, 9, 10, and 11 and generally higher throughout the culture period (Fig. 2).
Figure 1.
Timeline showing contractile period of engineered cardiac tissue containing Matrigel (outlined) or porcine SIS gel (filled). Engineered tissue constructs began contracting on day 3 or day 4 and continued for up to 10 days. One construct from each group was still contracting at the time of snap-freezing on day 13. No significant differences were observed between constructs containing Matrigel (M1–3) or SIS gel (S1–3) for the following parameters: the time between cell seeding and initial contractions; the duration of contractions; and the time between cell seeding and final contractions.
Figure 2.
Contraction rates of engineered tissue constructs containing SIS gel (S; n = 3) were approximately twice those of constructs containing Matrigel (M; n = 3) between day 6 and day 11. *SIS gel constructs contracted at rates that were significantly closer to native rat myocardium55 compared to Matrigel constructs on days 8–11 (p < 0.01 on days 9 and 10, p < 0.05 on days 8 and 11, one-way analysis of variance with Tukey post-hoc test). Statistical comparison of groups was completed separately for each discrete time point. Time points for the two groups are staggered slightly for clearer presentation. Contraction rates are expressed in beats per minute (bpm).
Histology and Immunohistochemistry
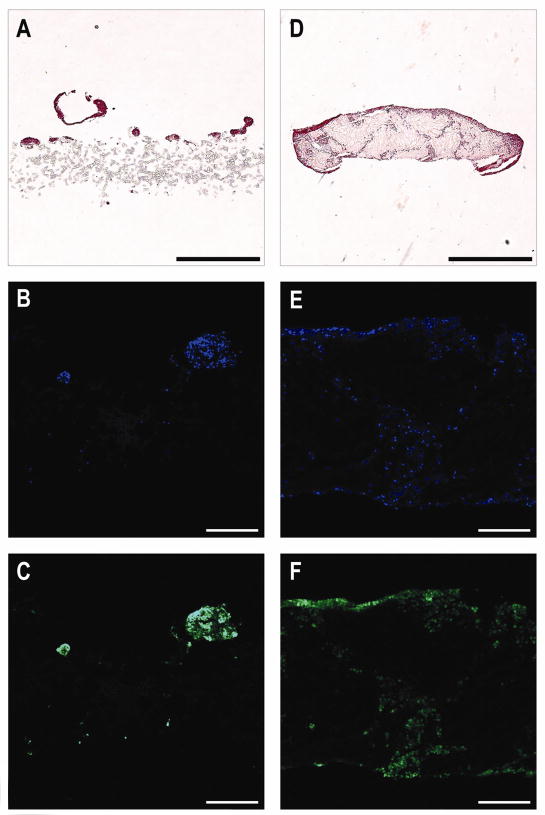
Cells in engineered cardiac tissue were concentrated near the upper surface of the scaffolds and distributed throughout the remainder of the scaffold as indicated by staining of the cross-sections with hematoxylin and eosin or with Hoechst to locate cell nuclei and antibodies against troponin T to highlight cells likely to be contractile (Fig. 3). Contractile proteins and nuclei appeared to collocate well. Microscopy images indicated that cells appeared to be more evenly distributed in the SIS gel constructs. Matrigel constructs appeared to display increased cell clustering and tissue discontinuities (Fig. 3A–C) in contrast to SIS gel constructs, which showed more continuous tissue with more diffuse cells (Fig. 3D–F).
Figure 3.
Representative cell and protein distributions in engineered cardiac tissue sectioned perpendicular to the disk-shaped scaffold. (A–C) Matrigel constructs appeared to display increased cell clustering and tissue discontinuities. (D–F) SIS gel constructs showed more continuous tissue with more diffuse cells and less cell clustering. Cell and protein distributions were indicated by staining with hematoxylin and eosin (A and D). Cells containing DNA (B and E) and troponin T (C and F) showed greater concentrations at the tissue-medium interface. Constructs containing SIS gel appeared to have higher cell densities in the core of the tissue. Separation of tissue and scaffold commonly occurred during snap-freezing (D–F). Scale bars are 1.0 mm (A and D) or 250 μm (B–C and E–F).
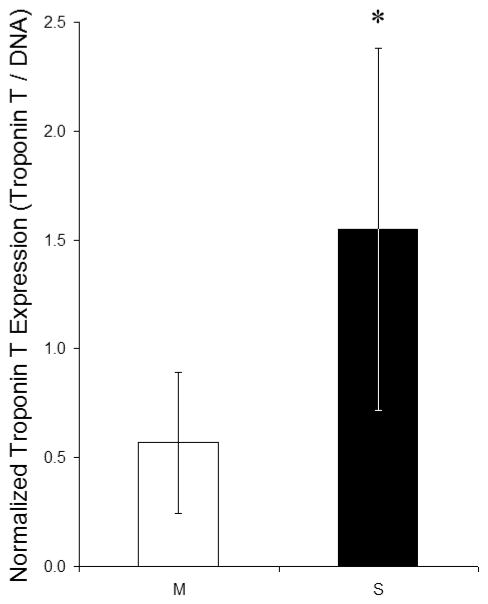
Semi-quantitative analysis of staining intensity revealed a significantly higher ratio of troponin T protein expression to DNA content in SIS gel constructs compared to Matrigel constructs (Fig. 4). The fluorescence intensity cut-off could be varied from 12.5% to full intensity without altering statistical significance.
Figure 4.
The normalized troponin T protein expression in engineered cardiac tissue was determined by comparing the fluorescence intensities of immunolabeled troponin T and DNA; only pixels with at least 50% maximum intensity were included, although an intensity cut-off of 12.5% to full intensity could be used without altering statistical significance. *The normalized troponin T expression was significantly higher in SIS gel constructs (S; n = 7) compared to Matrigel constructs (M; n = 5) (p < 0.05, one-way analysis of variance with Tukey-Kramer post-hoc tests). Non-specific Hoechst (DNA) fluorescence intensities in controls containing only Matrigel or SIS gel in PGS scaffolds were <10% for Matrigel and <1% for SIS gel compared to experimental groups, and non-specific troponin T fluorescence intensities in controls were <20% for Matrigel and <1% for SIS gel compared to experimental groups.
DISCUSSION
In this study, a single pool of rat neonatal cardiac cells was divided and used to populate identical PGS scaffolds in combination with either Matrigel or SIS gel. The engineered cardiac tissue samples, or constructs, were seeded with 3.6×104 live cells per mm3, a density that is within an order of magnitude of typical healthy rat myocardium (~1×105 cells per mm3).51 The density of live cells likely increased during the culture period due to tissue compaction evident from decreased construct diameters, though apoptosis resulting from diffusion limitations in static culture may have mitigated increasing cell densities. The escalation and magnitudes of contractile rates observed for engineered cardiac tissues in this study were similar to other engineered myocardium (~2.0 Hz after ~7 days).52–54 It is also noteworthy that the contraction rate of SIS gel constructs more closely approached native neonatal rat myocardium.55
In this study, cardiac cells functioned differently depending on the composition of the matrix to which they were exposed in culture (Fig. 2 and 3), though the ability of the cells to organize into contractile tissue appeared to be unaffected by gel type (Fig. 1). Some of the morphological and functional differences observed between Matrigel and SIS gel constructs may be attributed to gel protein composition. The principal structural protein of adult myocardium is collagen, and of the total collagen in the myocardium ~85% is type I and ~11% is type III.56 The roles of collagens I and III contrast greatly: collagen I is thicker and provides stiffness and structural support during loading, while collagen III is thinner and provides flexibility and elastic recovery.48 The composition of Matrigel has been studied widely, and its key contents include laminin, collagen IV, heparan sulfate proteoglycans (HSPGs), proteases such as matrix metalloproteinases (MMPs), transforming growth factor-β1 (TGF- β1), basic fibroblast growth factor (bFGF), epidermal growth factor (EGF), insulin-like growth factor 1, and platelet-derived growth factor.30,57 Components of Matrigel are similar to those of basement membrane.32 In contrast, a recent study showed that SIS has little similarity to basement membrane in its composition.58 Known contents of SIS include collagens I, III, IV, and VII, HSPGs, bFGF, vascular endothelial growth factor, and low levels of TGF-β1.31,58 Based on high concentrations of collagens I and III in myocardium and SIS but not in Matrigel, it is likely that SIS provides a more ideal in vitro extracellular environment for cardiac cells than Matrigel.
Greater tissue compaction and adhesion to PGS scaffolds qualitatively observed in Matrigel relative to SIS gel constructs was likely attributable to the high laminin content of Matrigel, which would aid in cell adhesion and the ability of cells to apply tensile forces to the surrounding matrix.26 The high laminin content of Matrigel and the considerable anchorage-dependence of cardiomyocytes may explain the excellent performance of Matrigel in cardiac tissue engineering. Matrigel and other basement membrane extracts from Engelbreth-Holm-Swarm sarcomas have most notably been used to enhance cardiomyocyte function in vitro and develop methodologies for myocardial tissue engineering by Zimmermann et al.,54,55,59 Kofidis et al.,13,25,60 and others.52
In comparison, SIS contains collagens I and III as well as the basement membrane-associated collagens IV and VII.31 More uniform cell distribution and greater expression of the contractile protein troponin T per cell (normalized by Hoechst nuclear staining intensity) in SIS gel constructs (Fig. 3 and 4) were likely a result of ECM that promoted sufficient cell adhesion while more closely resembling the in vivo cardiac extracellular environment. Cardiac ECM may be the ideal biomaterial for cardiac tissue engineering,61 although this idea is diametrically opposed by the limited regenerative capacity of mammalian myocardium3,4 and promising in vivo myocardial regeneration demonstrated by non-cardiac ECM.29,62 In fact, the ability of acellular SIS sheets to improve left ventricular function and revascularization after infarct has recently been demonstrated.39
Differences in the concentration and activity level of TGF-β1 offer a partial explanation for differences between contraction rates and compaction of Matrigel and SIS gel constructs, with other growth factors and divergent cell densities and functionality likely to also contribute. Higher levels of TGF-β1 may have caused the significantly lower contraction rates observed in Matrigel constructs compared to their SIS gel counterparts.63 Faster contraction in SIS gel constructs could accelerate the release of TGF-β1 from the gel and its consumption by cardiac cells, amplifying differences in contraction rates at later time points during culture (Fig. 2). Decreasing contraction rates after day 10 were likely caused by cardiomyocyte dedifferentiation as previously observed in in vitro co-culture.64 Apparently stronger adhesion of Matrigel constructs to the PGS scaffolds as well as differences in cell distribution and clustering (Fig. 3) may be at least partially explained by the combined effect of varying concentrations of TGF-β1, platelet-derived growth factor, and other growth factors within the different gels.65
The results of this study suggest that further investigation of SIS gel as a scaffolding material for cardiac tissue engineering is warranted. Future studies seeking to compare engineered myocardium containing decellularized ECM such as Matrigel, SIS gel, or others may achieve more physiologic culture conditions through electrical stimulation23 and tissue perfusion66,67 during in vitro culture, possibly using channeled or vascularized scaffolds and increased oxygenation.41 More biomimetic culture conditions would reduce apoptosis, enabling higher cell densities and thicker constructs. Due to different growth factor content in the two gels, in vivo testing would include comparative evaluation of angiogenesis, vasculogenesis, and oncogenesis (tumorigenesis). Serum-free culture and non-controversial cell sources are also necessary for translation to clinical applications.
CONCLUSION
We have demonstrated that SIS gel-based cardiac tissue can be engineered in vitro with contractile rates and durations and contractile protein expression comparable to tissue containing Matrigel. Advantages of engineered myocardium based on SIS gel include: (1) the absence of tumor-derived biomaterials; (2) in vitro contraction rate closer to that of myocardium in vivo; and (3) increased expression of troponin T, a key intracellular protein in the contractile machinery of cardiomyocytes. The duration of contractions in SIS gel or Matrigel constructs appeared to be similar in both groups. These data demonstrate the potential of SIS gel as a scaffolding material for creating functional engineered cardiac tissue.
Acknowledgments
This work was supported by a Cook Biotech seed grant and NIH Training Grant 998687184. The authors thank Cook Biotech, Inc., especially Michael C. Hiles, Michelle Arthur, and Ginny Mahn, for providing the SIS gel used in this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heart Disease and Stroke Statistics- 2008 Update. Dallas: American Heart Association; 2008. [Google Scholar]
- 2.Heidenreich PA, McClellan M. Trends in treatment and outcomes for acute myocardial infarction: 1975–1995. Am J Med. 2001;110:165–74. doi: 10.1016/s0002-9343(00)00712-9. [DOI] [PubMed] [Google Scholar]
- 3.Parmacek MS, Epstein JA. Pursuing cardiac progenitors: regeneration redux. Cell. 2005;120:295–8. doi: 10.1016/j.cell.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 4.Von Harsdorf R, Poole-Wilson PA, Dietz R. Regenerative capacity of the mycoardium: Implications for treatment of heart failure. Lancet. 2004;363:1306–13. doi: 10.1016/S0140-6736(04)16006-6. [DOI] [PubMed] [Google Scholar]
- 5.Baumgartner WA, Burrows S, del Nido PJ, Gardner TJ, Goldberg S, Gorman RC, Letsou GV, Mascette A, Michler RE, Puskas JD, Rose EA, Rosengart TK, Sellke FW, Shumway SJ, Wilke N National Heart, Lung, and Blood Institute Working Group on Future Direction in Cardiac Surgery. Recommendations of the National Heart, Lung, and Blood Institute Working Group on Future Direction in Cardiac Surgery. Circulation. 2005;111:3007–13. doi: 10.1161/CIRCULATIONAHA.104.530154. [DOI] [PubMed] [Google Scholar]
- 6.Frigerio M, Roubina E. Drugs for left ventricular remodeling in heart failure. Am J Cardiol. 2005;96:10L–8L. doi: 10.1016/j.amjcard.2005.09.060. [DOI] [PubMed] [Google Scholar]
- 7.Ohtsuka M, Takano H, Zou Y, Toko H, Akazawa H, Qin Y, Suzuki M, Hasegawa H, Nakaya H, Komuro I. Cytokine therapy prevents left ventricular remodeling and dysfunction after myocardial infarction through neovascularization. FASEB J. 2004;18:851–3. doi: 10.1096/fj.03-0637fje. [DOI] [PubMed] [Google Scholar]
- 8.Loot AE, Roks AJ, Henning RH, Tio RA, Suurmeijer AJ, Boomsma F, van Gilst WH. Angiotensin-(1–7) attenuates the development of heart failure after myocardial infarction in rats. Circulation. 2002;105:1548–50. doi: 10.1161/01.cir.0000013847.07035.b9. [DOI] [PubMed] [Google Scholar]
- 9.Landa N, Miller L, Feinberg MS, Holbova R, Shachar M, Freeman I, Cohen S, Leor J. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation. 2008;117:1388–96. doi: 10.1161/CIRCULATIONAHA.107.727420. [DOI] [PubMed] [Google Scholar]
- 10.Christman KL, Fok HH, Sievers RE, Fang Q, Lee RJ. Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction. Tissue Eng. 2004;10:403–9. doi: 10.1089/107632704323061762. [DOI] [PubMed] [Google Scholar]
- 11.Drexler H, Meyer GP, Wollert KC. Bone-marrow-derived cell transfer after ST-elevation myocardial infarction: lessons from the BOOST trial. Nat Clin Pract Cardiovasc Med. 2006;3:S65–8. doi: 10.1038/ncpcardio0407. [DOI] [PubMed] [Google Scholar]
- 12.Patel AN, Geffner L, Vina RF, Saslavsky J, Urschel HC, Jr, Kormos R, Benetti F. Surgical treatment for congestive heart failure with autologous adult stem cell transplantation: a prospective randomized study. J Thorac Cardiovasc Surg. 2005;130:1631–8. doi: 10.1016/j.jtcvs.2005.07.056. [DOI] [PubMed] [Google Scholar]
- 13.Kofidis T, Lebl DR, Martinez EC, Hoyt G, Tanaka M, Robbins RC. Novel injectable bioartificial tissue facilitates targeted, less invasive, large-scale tissue restoration on the beating heart after myocardial injury. Circulation. 2005;112:I173–7. doi: 10.1161/CIRCULATIONAHA.104.526178. [DOI] [PubMed] [Google Scholar]
- 14.Chachques JC, Trainini JC, Lago N, Masoli OH, Barisani JL, Cortes-Morichetti M, Schussler O, Carpentier A. Myocardial assistance by grafting a new bioartificial upgraded myocardium (MAGNUM clinical trial): one year follow-up. Cell Transplant. 2008;16:927–34. doi: 10.3727/096368907783338217. [DOI] [PubMed] [Google Scholar]
- 15.Yildirim Y, Naito H, Didié M, Karikkineth BC, Biermann D, Eschenhagen T, Zimmermann WH. Development of a biological ventricular assist device: preliminary data from a small animal model. Circulation. 2007;116:I16–23. doi: 10.1161/CIRCULATIONAHA.106.679688. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu T, Sekine H, Yang J, Isoi Y, Yamato M, Kikuchi A, Kobayashi E, Okano T. Polysurgery of cell sheet grafts overcomes diffusion limits to produce thick, vascularized myocardial tissues. FASEB J. 2006;20:708–10. doi: 10.1096/fj.05-4715fje. [DOI] [PubMed] [Google Scholar]
- 17.Kochupura PV, Azeloglu EU, Kelly DJ, Doronin SV, Badylak SF, Krukenkamp IB, Cohen IS, Gaudette GR. Tissue-engineered myocardial patch derived from extracellular matrix provides regional mechanical function. Circulation. 2005;112:I144–9. doi: 10.1161/CIRCULATIONAHA.104.524355. [DOI] [PubMed] [Google Scholar]
- 18.Davis ME, Hsieh PC, Grodzinsky AJ, Lee RT. Custom design of the cardiac microenvironment with biomaterials. Circ Res. 2005;97:8–15. doi: 10.1161/01.RES.0000173376.39447.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–8. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 20.Bel A, Messas E, Agbulut O, Richard P, Samuel JL, Bruneval P, Hagege AA, Menasche P. Transplantation of autologous fresh bone marrow into infarcted myocardium: A word of caution. Circulation. 2003;108(Suppl 1):II247–52. doi: 10.1161/01.cir.0000089040.11131.d4. [DOI] [PubMed] [Google Scholar]
- 21.Muller-Ehmsen J, Whittaker P, Kloner RA, Dow JS, Sakoda T, Long TI, Laird PW, Kedes L. Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J Mol Cell Cardiol. 2002;34:107–16. doi: 10.1006/jmcc.2001.1491. [DOI] [PubMed] [Google Scholar]
- 22.Miyagawa S, Sawa Y, Sakakida S, Taketani S, Kondoh H, Memon IA, Imanishi Y, Shimizu T, Okano T, Matsuda H. Tissue cardiomyoplasty using bioengineered contractile cardiomyocyte sheets to repair damaged myocardium: their integration with recipient myocardium. Transplantation. 2005;80:1586–95. doi: 10.1097/01.tp.0000181163.69108.dd. [DOI] [PubMed] [Google Scholar]
- 23.Radisic M, Park H, Shing H, Consi T, Schoen FJ, Langer R, Freed LE, Vunjak-Novakovic G. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc Natl Acad Sci U S A. 2004;101:18129–34. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leor J, Aboulafia-Etzion S, Dar A, Shapiro L, Barbash IM, Battler A, Granot Y, Cohen S. Bioengineered cardiac grafts: A new approach to repair the infarcted myocardium. Circulation. 2000;102:III56–61. doi: 10.1161/01.cir.102.suppl_3.iii-56. [DOI] [PubMed] [Google Scholar]
- 25.Kutschka I, Chen IY, Kofidis T, Arai T, von Degenfeld G, Sheikh AY, Hendry SL, Pearl J, Hoyt G, Sista R, Yang PC, Blau HM, Gambhir SS, Robbins RC. Collagen matrices enhance survival of transplanted cardiomyoblasts and contribute to functional improvement of ischemic rat hearts. Circulation. 2006;114:I167–73. doi: 10.1161/CIRCULATIONAHA.105.001297. [DOI] [PubMed] [Google Scholar]
- 26.Baar K, Birla R, Boluyt MO, Borschel GH, Arruda EM, Dennis RG. Self-organization of rat cardiac cells into contractile 3-D cardiac tissue. FASEB J. 2005;19:275–7. doi: 10.1096/fj.04-2034fje. [DOI] [PubMed] [Google Scholar]
- 27.Furuta A, Miyoshi S, Itabashi Y, Shimizu T, Kira S, Hayakawa K, Nishiyama N, Tanimoto K, Hagiwara Y, Satoh T, Fukuda K, Okano T, Ogawa S. Pulsatile cardiac tissue grafts using a novel three-dimensional cell sheet manipulation technique functionally integrates with the host heart, in vivo. Circ Res. 2006;98:705–12. doi: 10.1161/01.RES.0000209515.59115.70. [DOI] [PubMed] [Google Scholar]
- 28.Ye Q, Zund G, Benedikt P, Jockenhoevel S, Hoerstrup SP, Sakyama S, Hubbell JA, Turina M. Fibrin gel as a three dimensional matrix in cardiovascular tissue engineering. Eur J Cardiothorac Surg. 2000;17:587–91. doi: 10.1016/s1010-7940(00)00373-0. [DOI] [PubMed] [Google Scholar]
- 29.Badylak SF, Kochupura PV, Cohen IS, Doronin SV, Saltman AE, Gilbert TW, Kelly DJ, Ignotz RA, Gaudette GR. The use of extracellular matrix as an inductive scaffold for the partial replacement of functional myocardium. Cell Transplant. 2006;15:S29–40. doi: 10.3727/000000006783982368. [DOI] [PubMed] [Google Scholar]
- 30.Vukicevic S, Kleinman HK, Luyten FP, Roberts AB, Roche NS, Reddi AH. Identification of multiple active growth factors in basement membrane Matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp Cell Res. 1992;202:1–8. doi: 10.1016/0014-4827(92)90397-q. [DOI] [PubMed] [Google Scholar]
- 31.Hurst RE, Bonner RB. Mapping of the distribution of significant proteins and proteoglycans in small intestinal submucosa by fluorescence microscopy. J Biomater Sci Polym Ed. 2001;12:1267–79. doi: 10.1163/156856201753395798. [DOI] [PubMed] [Google Scholar]
- 32.Orkin RW, Gehron P, McGoodwin EB, Martin GR, Valentine T, Swarm R. A murine tumor producing a matrix of basement membrane. J Exp Med. 1977;145:204–20. doi: 10.1084/jem.145.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sethi T, Rintoul RC, Moore SM, MacKinnon AC, Salter D, Choo C, Chilvers ER, Dransfield I, Donnelly SC, Strieter R, Haslett C. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: A mechanism for small cell lung cancer growth and drug resistance in vivo. Nat Med. 1999;5:662–8. doi: 10.1038/9511. [DOI] [PubMed] [Google Scholar]
- 34.Grant DS, Kibbey MC, Kinsella JL, Cid MC, Kleinman HK. The role of basement membrane in angiogenesis and tumor growth. Pathol Res Pract. 1994;190:854–63. doi: 10.1016/S0344-0338(11)80989-1. [DOI] [PubMed] [Google Scholar]
- 35.Bonfil RD, Vinyals A, Bustuoabad OD, Llorens A, Benavides FJ, Gonzalez-Garrigues M, Fabra A. Stimulation of angiogenesis as an explanation of Matrigel-enhanced tumorigenicity. Int J Cancer. 1994;58:233–9. doi: 10.1002/ijc.2910580215. [DOI] [PubMed] [Google Scholar]
- 36.Fridman R, Kibbey MC, Royce LS, Zain M, Sweeney M, Jicha DL, Yannelli JR, Martin GR, Kleinman HK. Enhanced tumor growth of both primary and established human and murine tumor cells in athymic mice after coinjection with Matrigel. J Natl Cancer Inst. 1991;83:769–74. doi: 10.1093/jnci/83.11.769. [DOI] [PubMed] [Google Scholar]
- 37.Hurst RE, Kyker KD, Bonner RB, Bowditch RD, Hemstreet GP., 3rd Matrix-dependent plasticity of the malignant phenotype of bladder cancer cells. Anticancer Res. 2003;23:3119–28. [PMC free article] [PubMed] [Google Scholar]
- 38.Parmacek MS, Solaro RJ. Biology of the troponin complex in cardiac myocytes. Prog Cardiovasc Dis. 2004;47:159–76. doi: 10.1016/j.pcad.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 39.Tan MY, Zhi W, Wei RQ, Huang YC, Zhou KP, Tan B, Deng L, Luo JC, Li XQ, Xie HQ, Yang ZM. Repair of infarcted myocardium using mesenchymal stem cell seeded small intestinal submucosa in rabbits. Biomaterials. 2009;30:3234–40. doi: 10.1016/j.biomaterials.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Radisic M, Park H, Martens TP, Salazar-Lazaro JE, Geng W, Wang Y, Langer R, Freed LE, Vunjak-Novakovic G. Pre-treatment of synthetic elastomeric scaffolds by cardiac fibroblasts improves engineered heart tissue. J Biomed Mater Res A. 2008;86:713–24. doi: 10.1002/jbm.a.31578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Radisic M, Park H, Chen F, Salazar-Lazzaro JE, Wang Y, Dennis R, Langer R, Freed LE, Vunjak-Novakovic G. Biomimetic approach to cardiac tissue engineering: oxygen carriers and channeled scaffolds. Tissue Eng. 2006;12:2077–91. doi: 10.1089/ten.2006.12.2077. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Ameer GA, Shappard BJ, Langer R. A tough biodegradable elastomer. Nat Biotechnol. 2002;20:602–6. doi: 10.1038/nbt0602-602. [DOI] [PubMed] [Google Scholar]
- 43.Chen QZ, Bismarck A, Hansen U, Junaid S, Tran MQ, Harding SE, Ali NN, Boccaccini AR. Characterisation of a soft elastomer poly(glycerol sebacate) designed to match the mechanical properties of myocardial tissue. Biomaterials. 2008;29:47–57. doi: 10.1016/j.biomaterials.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 44.Crapo PM, Gao J, Wang Y. Seamless tubular poly(glycerol sebacate) scaffolds: High-yield fabrication and potential applications. J Biomed Mater Res A. 2008;86:354–63. doi: 10.1002/jbm.a.31598. [DOI] [PubMed] [Google Scholar]
- 45.Bettinger CJ, Weinberg EJ, Kulig KM, Vacanti JP, Wang Y, Borenstein JT, Langer R. Three-dimensional microfluidic tissue-engineering scaffolds using a flexible biodegradable polymer. Adv Mater. 2006;18:165–9. doi: 10.1002/adma.200500438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao J, Ensley AE, Nerem RM, Wang Y. Poly(glycerol sebacate) supports the proliferation and phenotypic protein expression of primary baboon vascular cells. J Biomed Mater Res A. 2007;83:1070–5. doi: 10.1002/jbm.a.31434. [DOI] [PubMed] [Google Scholar]
- 47.Sundback CA, Shyu JY, Wang Y, Faquin WC, Langer RS, Vacanti JP, Hadlock TA. Biocompatibility analysis of poly(glycerol sebacate) as a nerve guide material. Biomaterials. 2005;26:5454–64. doi: 10.1016/j.biomaterials.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 48.Wang Y, Kim YM, Langer R. In vivo degradation characteristics of poly(glycerol sebacate) J Biomed Mater Res A. 2002;66:192–7. doi: 10.1002/jbm.a.10534. [DOI] [PubMed] [Google Scholar]
- 49.Rashid ST, Salacinski HJ, Hamilton G, Seifalian AM. The use of animal models in developing the discipline of cardiovascular tissue engineering: A review. Biomaterials. 2004;25:1627–37. doi: 10.1016/s0142-9612(03)00522-2. [DOI] [PubMed] [Google Scholar]
- 50.Gao J, Crapo PM, Wang Y. Macroporous elastomeric scaffolds with extensive micropores for soft tissue engineering. Tissue Eng. 2006;12:917–25. doi: 10.1089/ten.2006.12.917. [DOI] [PubMed] [Google Scholar]
- 51.Mandarim-de-Lacerda CA, Pereira LM. Numerical density of cardiomyocytes in chronic nitric oxide synthesis inhibition. Pathobiology. 2000;68:36–42. doi: 10.1159/000028113. [DOI] [PubMed] [Google Scholar]
- 52.Zhao YS, Wang CY, Li DX, Zhang XZ, Qiao Y, Guo XM, Wang XL, Dun CM, Dong LZ, Song Y. Construction of a unidirectionally beating 3-dimensional cardiac muscle construct. J Heart Lung Transplant. 2005;24:1091–7. doi: 10.1016/j.healun.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 53.Okamura S, Suzuki A, Johkura K, Ogiwara N, Harigaya M, Yokouchi T, Sasaki K. Formation of the biopulsatile vascular pump by cardiomyocyte transplants circumvallating the abdominal aorta. Tissue Eng. 2002;8:201–11. doi: 10.1089/107632702753724978. [DOI] [PubMed] [Google Scholar]
- 54.Zimmermann WH, Melnychenko I, Eschenhagen T. Engineered heart tissue for regeneration of diseased hearts. Biomaterials. 2004;25:1639–47. doi: 10.1016/s0142-9612(03)00521-0. [DOI] [PubMed] [Google Scholar]
- 55.Zimmermann WH, Melnychenko I, Wasmeier G, Didie M, Naito H, Nixdorff U, Hess A, Budinsky L, Brune K, Michaelis B, Dhein S, Schwoerer A, Ehmke H, Eschenhagen T. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12:452–8. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- 56.Jugdutt BI. Ventricular remodeling after infarction and the extracellular collagen matrix: when is enough enough. Circulation. 2003;108:1395–403. doi: 10.1161/01.CIR.0000085658.98621.49. [DOI] [PubMed] [Google Scholar]
- 57.Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15:378–86. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 58.Brown B, Lindberg K, Reing J, Stolz DB, Badylak SF. The basement membrane component of biologic scaffolds derived from extracellular matrix. Tissue Eng. 2006;12:519–26. doi: 10.1089/ten.2006.12.519. [DOI] [PubMed] [Google Scholar]
- 59.Zimmermann WH, Fink C, Kralisch D, Remmers U, Weil J, Eschenhagen T. Three-dimensional engineered heart tissue from neonatal rat cardiac myocytes. Biotechnol Bioeng. 2000;68:106–14. [PubMed] [Google Scholar]
- 60.Kofidis T, de Bruin JL, Hoyt G, Lebl DR, Tanaka M, Yamane T, Chang CP, Robbins RC. Injectable bioartificial myocardial tissue for large-scale intramural cell transfer and functional recovery of injured heart muscle. J Thorac Cardiovasc Surg. 2004;128:571–8. doi: 10.1016/j.jtcvs.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 61.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–21. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 62.Badylak S, Obermiller J, Geddes L, Matheny R. Extracellular matrix for myocardial repair. Heart Surg Forum. 2003;6:E20–6. doi: 10.1532/hsf.917. [DOI] [PubMed] [Google Scholar]
- 63.Kubin T, Tomars M, Fach C, Hein S, Bramlage P, Shim GJ, Scholz D, Kostin S, Zimmermann R, Elsasser A, Schaper W, Schaper J. Transforming growth factor-beta1 downregulates beating frequency and remodeling of cultured rat adult cardiomyocytes. Cell Tissue Res. 2005;321:57–66. doi: 10.1007/s00441-005-1125-5. [DOI] [PubMed] [Google Scholar]
- 64.Dispersyn GD, Geuens E, Ver Donck L, Ramaekers FC, Borgers M. Adult rabbit cardiomyocytes undergo hibernation-like dedifferentiation when co-cultured with cardiac fibroblasts. Cardiovasc Res. 2001;51:230–40. doi: 10.1016/s0008-6363(01)00326-1. [DOI] [PubMed] [Google Scholar]
- 65.Cheng M, Park H, Engelmayr GC, Moretti M, Freed LE. Effects of regulatory factors on engineered cardiac tissue in vitro. Tissue Eng. 2007;13:2709–19. doi: 10.1089/ten.2006.0414. [DOI] [PubMed] [Google Scholar]
- 66.Radisic M, Yang L, Boublik J, Cohen RJ, Langer R, Freed LE, Vunjak-Novakovic G. Medium perfusion enables engineering of compact and contractile cardiac tissue. Am J Physiol. 2004;286:H507–16. doi: 10.1152/ajpheart.00171.2003. [DOI] [PubMed] [Google Scholar]
- 67.Kofidis T, Lenz A, Boublik J, Akhyari P, Wachsmann B, Mueller-Stahl K, Hofmann M, Haverich A. Pulsatile perfusion and cardiomyocyte viability in a solid three-dimensional matrix. Biomaterials. 2003;24:5009–14. doi: 10.1016/s0142-9612(03)00429-0. [DOI] [PubMed] [Google Scholar]