Abstract
Background
Although alopecia is a commonly recognized problem affecting many captive Rhesus macaque colonies, there is no consensus as to the underlying etiology or appropriate course of management.
Methods and Results
We performed skin biopsies on a group of Rhesus macaques and demonstrate that alopecia is associated with superficial dermal perivascular mononuclear cell infiltrates and skin pathology consistent with chronic hypersensitivity dermatitis. Immunohistochemistry demonstrated that the inflammation is primarily composed of CD4+ cells admixed with histiocytes and mast cells. Inflammation is correlated with degree of alopecia. Further analysis in different groups of macaques revealed that animals born outdoors or infected with lung mites had reduced dermal inflammatory cell infiltrates and a lower incidence of alopecia.
Conclusions
These findings support a hypothesis that an altered housing status resulting in decreased pathogen burden in Rhesus macaque colonies may contribute to dermal immunophenotypic alterations and subsequent development of dermatitis with resultant alopecia.
Keywords: skin, macaque, hair-loss, dermatitis, allergy
Introduction
Alopecia is a problem affecting primate colonies worldwide with some reports estimating the incidence as high as 68% [1]. While common, the etiology of this condition is poorly understood with proposed causes to include endocrine diseases, nutritional deficiencies or imbalances, behavioral abnormalities, aging, immunologic disorders, and stress. There are few published studies aimed at identifying the cause of alopecia in non-human primates, specifically Rhesus macaques (Macaca mulatta). Several large surveys have been published implicating time of year, living space, stress, housing conditions, age, and sex of the animal while stressing that alopecia is a multifactorial problem with many contributing factors [1-3]. In addition, there are a number of individual case reports including alopecia areata [4], mutations in the hair loss gene [5], latex hypersensitivity [6], atopic dermatitis [7, 8], telogen effluvium [9], and seborrheic dermatitis [10]. Finally, some work has shown that dietary and/or nutritional imbalances can contribute to alopecia, especially zinc deficiency [11]. The long term impact of alopecia on animal welfare and as a potential confounder of experimental work is unknown.
Due to the overall lack of knowledge concerning its predominant etiology, and despite its frequency, alopecia remains a challenging diagnostic and treatment dilemma for laboratory animal veterinarians. It has been hypothesized that alopecia is the result of overgrooming or stereotypical trichotillomania due to boredom and/or stress [2]. As was emphasized in a recent review, there is often a tendency to relate hair loss to stress [12] and to treat it with various behavioral methods including additional environmental enrichment. However, this is probably an oversimplification of a multifactorial problem and as a result these methods do not always succeed. Animals may receive limited or ineffective therapeutic interventions. Additionally, while some animals show alopecia without other dermatologic manifestations, others demonstrate additional clinical signs including erythema, pruritis, scaling, or lichenification. This constellation of clinical findings suggest that a subset of animals become alopecic following repetitive bouts of dermal inflammation, pruritis, and mechanical hair removal as in a chronic hypersensitivity reaction.
Here, we present data on skin pathology and histomorphometric findings from biopsies of normal and alopecic Rhesus macaques and demonstrate alterations in the immunophenotypic composition of resident dermal immune cells. In addition we compare the inflammatory infiltrates to those of Cynomolgus macaques (Macaca fascicularis) raised outdoors and to Rhesus macaques subjected to different pathogen burdens. We propose that a cutaneous inflammatory reaction due to chronic hypersensitivity, and similar in histologic appearance to atopic dermatitis in man, is responsible for the observed clinical signs in a significant subset of Rhesus macaques. We propose that Cynomolgus macaques and some Rhesus macaques are partially protected due to a history of outdoor housing and hypothesize that this effect is potentially mediated by pathogen burden.
Materials and Methods
All animals that participated in this study were housed at the New England Primate Research Center (NEPRC) and maintained in accordance with the “Guide for the Care and Use of Laboratory Animals” of the Institute of Laboratory Animal Resources, National Research Council. The facility is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International and all work was approved by Harvard Medical School’s Standing Committee on Animals.
The NEPRC maintains a large breeding colony of Indian-origin Rhesus macaques in addition to a cohort of macaques who are active in a variety of research protocols. Virological testing is performed on a quarterly to semiannual basis for Simian Immunodeficiency virus (SIV), Simian T-lymphotropic virus type 1 (STLV-1), Herpes B virus (BV) and Simian Retrovirus Type-D (SRV-D) as previously described [13] and the breeding colony has been specific pathogen free from these agents along with Mycobacterium tuberculosis and Measles virus since 1988. All animals in the colony are monitored for alopecia on a monthly basis. A score is applied to each case using a scale of 0-5 in which Grade 0 is defined as no observable alopecia, grade 1 as alopecia involving less than 10% of the integument, grade 2 between 10 and 20% of the integument, grade 3 between 20 and 40% of the integument, grade 4 between 40 and 60% of the integument, and grade 5 greater than 60% of the integument. Each animal is scored during evaluation in their home cages.
From this list, a subset of 36 animals presenting with varying degrees of alopecia and with normal haircoats was selected and, over the course of 2-3 months, each animal was sedated with ketamine (10-15 mg/kg IM, Ketamine, FortDodge) for a full physical exam and bloodwork. Animals were selected based on alopecia history and in order to exclude animals with experimental histories that would interfere with this investigation or for whom the procedures in this study would interfere with ongoing experiments. Approximately 10 mL of blood was drawn from the femoral vein for a complete blood count (CBC), serum chemistry, thyroid hormone profile, and serum cortisol. CBCs were performed using a Hemavet HV1700FS Multispecies Hematology Instrument and chemistry profiles and thyroid hormone tests were sent to a diagnostic lab for testing (Idexx Laboratories, North Grafton, MA). Additionally, serum samples were sent for cortisol measurement (Yerkes National Primate Research Center’s Biomarkers Core Lab, Atlanta, GA) and, on a subset of animals, for IgE levels (Anilytics Incorporated, Gaithersburg, MD).
During the same anesthetic episode, skin biopsies were obtained from all 36 animals. Briefly, a small patch of skin was lightly shaved and prepped with betadine and alcohol. The area to be biopsied was marked and lidocaine was instilled subcutaneously. A 6 mm punch biopsy instrument was used to obtain biopsies from the affected areas. Samples were fixed in 10% neutral buffered formalin, embedded in paraffin and stained with H&E for evaluation by light microscopy. Samples were obtained from affected skin of animals with alopecia, when possible from the mid lumbar region to match the area sampled in the normal control animals. Historical samples from Cynomolgus macaques (n=7) and Pneumonyssus simicola (lung mite) infected Rhesus macaques (n=11) were chosen from the pathology archives of the NEPRC. These samples were collected at necropsy, fixed in 10% neutral buffered formalin and embedded in paraffin.
Immunohistochemistry for T cells (CD3), T helper cells (CD4), cytotoxic T cells (CD8), B cells (CD20), macrophages (Ham56, CD163), human leukocyte antigen (HLA-DR), and dendritic cells (DC-SIGN) was performed using an ABC immunostain technique on formalin fixed paraffin embedded tissues as previously described [14-16]. Briefly, 5 μm sections were deparaffinized in xylene, rehydrated in graded alcohol, and rinsed in tris-buffered saline. Sections were then incubated with 3% hydrogen peroxide for 5 minutes to block endogenous peroxidases and rinsed in tris-buffered saline for 5 minutes. Epitope retrieval was accomplished using heat or enzymatic methods (proteinase K) if necessary. Non-specific antigen binding was blocked by incubating slides for 10 minutes with a commercially available protein blocking solution (Dako Cytomation, Carpinteria, CA). Positive control tissue and irrelevant antibody controls were used in each run. Slides were then incubated with primary and secondary antibodies either overnight or for 30 minutes depending on the specific antibody. Color was developed using freshly prepared ABC Elite solution (Vectastain Elite ABC Kit, Vector Laboratories) followed by washing in tris-buffered saline. Diamino-benzidine-tetrahydrochloride-dihydrate (DAB) was applied as a chromogen. Sections were counterstained with Mayer’s hematoxylin, dehydrated through graded ethanol, cleared in xylene, and cover slipped.
To specifically highlight mast cells, toluidine blue staining was performed on formalin fixed paraffin embedded tissues. Five μm sections were deparaffinized in xylene, rehydrated in graded alcohol and rinsed in water. Sections were stained with filtered toluidine blue for 15 minutes and washed in tap water, dehydrated through graded ethanol, cleared in xylene and cover slipped.
Cellular infiltrates were quantified by one investigator (JAK) who was blinded to alopecia status at the time of counting. Labeled cells were counted in five 20× fields and these counts averaged. The resulting count was expressed as number of cells/mm of epidermis. Statistical analysis was performed using either GraphPad Prism v5.01 (GraphPad Software, San Diego, CA) or SigmaStat v3.10 (Systat Software, San Jose, CA).
Results
Overall demographics correlate age, female sex, and source colony with alopecia
Monthly alopecia monitoring of all of the Rhesus macaques excluding those of the main SPF breeding facility identified a large cohort of animals with alopecia. Forty-eight percent of Rhesus macaques had alopecia at some point in their history and 27% had a history of alopecia in the past twelve months. Most cases (79.4%) were Grade 2 or lower which is considered mild. Singly housed Cynomolgus macaques had significantly less alopecia with only 4/51 (7.8%) showing signs of alopecia (Fisher’s exact test, p < 0.001).
There was a significant difference between the ages of animals with and without alopecia. The mean age of the animals presenting with alopecia in the past year was 9.7 years compared with 7.4 years for the whole colony (t-test, p<0.001) and 6.2 for those animals with no history of alopecia (t-test, p<0.001).
Sex has been shown to correlate with alopecia in the past, with females being more susceptible than males. The overall sex distribution of the singly housed macaques in our colony is 46.2% female, 53.8% male. Of the singly housed animals with any history of alopecia, 44.4% were male and 55.6% female. Overall, 58.2% of singly housed females and 39.6% of singly housed males had alopecia. Females were significantly overrepresented compared to males in the group of animals with alopecia (Fisher’s exact test, p<0.0001). Some of this discrepancy may be because females are generally older than males with a mean age of 7.4 years compared to 5.5 years for males.
Animals from different sources were compared for any differences in prevalence. While the NEPRC has a large breeding colony, some animals are imported from other colonies. One of the largest groups comes from a source colony in which animals are housed outdoors in a natural environment before being moved indoors. Animals originating from this source, but currently housed under identical husbandry conditions to macaques bred at the NEPRC have an alopecia incidence of 22% while animals born at the NEPRC and raised exclusively indoors have an incidence of 56% (Fisher’s exact test, p < 0.0001). The average age of the group from the outside source colony is significantly lower than the group from the NEPRC (5.45 years compared to 7.63 years, t-test, p=0.0002) and these animals are almost exclusively males. A multiple logistic regression of the data showed that animal’s origin maintained statistical significance when corrected for age and sex and that animals from the outdoor colony were 38% as likely to develop alopecia as those from our colony (p<0.001, Odds Ratio 0.384). In this analysis, age maintained significance (p<0.001) and sex approached significance with an increased risk for females (p=0.063, odds ratio 1.463).
Viral serological status not associated with presence of alopecia
All animals housed at the NEPRC are tested for antibodies to BV, SRV-D, STLV-1, and SIV. In the main housing facility outside of the SPF breeding colony, there is a very low incidence of viral antibodies with 0.9% of animals testing positive for STLV, 1.4% for SRV-D, and 0.5% for BV. No animals are SIV positive. For those animals with alopecia, 1.1% were STLV-1 positive, 1.1% SRV-D positive and no animals were B virus positive. There were no significant differences using a Fisher’s exact test comparing serologic status to alopecia status.
Serology results were also available for all of the Cynomolgus macaques and 8/11 of the Pneumonyssus infected Rhesus macaques. No Cynomolgus macaques tested positive for any of the viruses. One of the Pneumonyssus infected Rhesus macaques tested positive for BV.
Physical exams reveal three main presentations of alopecia
Physical exams on affected animals revealed no major clinical conditions aside from alopecia. The animals selected for biopsy had three distinct patterns of alopecia. In the first, animals (n=5) had very localized alopecia on their forearms or lower legs which occasionally spread to their shoulders and/or thighs. The underlying skin showed varying degrees of erythema and lichenification. The second group of animals (n=1) had diffuse alopecia affecting large portions of the body and generally sparing the hair at the nape of the neck. The skin of this animal was essentially normal except for the hair loss. The third group of animals (n=19) had multifocal to diffuse alopecia affecting limbs and the torso. Their underlying skin tended to be erythematous and there were varying degrees of excoriation, lichenification, and scaling. The remaining 11 animals chosen for biopsy had normal haircoats. All groups were included in the final immunophenotypic analysis.
Alopecia not associated with clinical pathology changes consistent with hormonal, nutritional, or endocrine diseases
The 36 animals chosen for more in depth examination represented a variety of alopecia scores (eleven normal, six Grade 1, five Grade 2, seven Grade 3, five Grade 4, two Grade 5). Complete blood counts and serum chemistry profiles performed on each of these animals showed no major abnormalities (table 1). Correlations that were observed between blood chemistry or CBC data and alopecia score did not point to a specific underlying etiology and values that were correlated with alopecia fell within the normal range. Serum cortisol levels were performed on each animal and showed neither a significant difference between animals with and without alopecia (mean alopecia, 20.68 and no alopecia, 19.52; p=0.349) nor a correlation to alopecia score (r=−0.1469, p=0.4223).
Table 1.
Summary of blood chemistry results by alopecia status.
| Normal | Alopecia | P value1 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Correlation Coeff.2 |
P value2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| ALT | 44.1 | 31.0 | 0.070 | 22.0 | 38.6 | 30.6 | 25.3 | 51.5 | −0.098 | 0.575 |
| AST | 47.6 | 30.0 | 0.001 | 28.7 | 36.8 | 31.3 | 23.5 | 26.0 | −0.477 | 0.004 |
| Alb | 6.3 | 3.6 | 0.078 | 3.7 | 3.4 | 3.7 | 3.1 | 4.2 | −0.185 | 0.289 |
| TP | 6.6 | 6.6 | 0.402 | 6.7 | 6.6 | 6.5 | 6.6 | 6.9 | 0.038 | 0.828 |
| Glob | 3.1 | 3.0 | 0.393 | 3.0 | 3.2 | 2.8 | 3.4 | 2.7 | −0.060 | 0.731 |
| Tbili | 0.2 | 0.2 | 0.274 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | −0.006 | 0.975 |
| BUN | 15.6 | 15.7 | 0.491 | 14.0 | 16.4 | 16.7 | 14.5 | 17.5 | 0.099 | 0.570 |
| Cr | 0.95 | 0.75 | 0.007 | 0.7 | 0.7 | 0.9 | 0.6 | 0.8 | −0.332 | 0.052 |
| Chol | 147.5 | 259.4 | 0.088 | 348.3 | 288.0 | 270.7 | 128.3 | 143.5 | 0.001 | 0.994 |
| Glu | 49.7 | 60.3 | 0.022 | 65.0 | 63.0 | 58.6 | 59.8 | 46.5 | 0.102 | 0.559 |
| Ca | 9.7 | 9.2 | 0.012 | 9.6 | 9.2 | 9.2 | 8.3 | 9.5 | −0.519 | 0.001 |
| Ph | 3.2 | 3.7 | 0.091 | 4.4 | 3.7 | 3.8 | 3.1 | 3.1 | −0.021 | 0.906 |
| Cl | 104.1 | 104.2 | 0.482 | 101.2 | 108.4 | 103.3 | 101.5 | 111.5 | 0.092 | 0.599 |
| K | 3.6 | 3.8 | 0.233 | 4.1 | 4.1 | 3.8 | 3.1 | 3.7 | −0.131 | 0.453 |
| Na | 141.3 | 139.6 | 0.291 | 138.7 | 144.0 | 136.7 | 136.8 | 147.0 | −0.058 | 0.743 |
| TG | 149.5 | 130.6 | 0.344 | 122.0 | 163.6 | 183.4 | 57.3 | 52.0 | −0.158 | 0.394 |
| TT4 | 3.6 | 4.4 | 0.099 | 3.9 | 5.1 | 4.6 | 5.0 | n/a | 0.377 | 0.112 |
| fT4 | 0.6 | 0.7 | 0.176 | 0.9 | 0.7 | 0.5 | 0.7 | n/a | −0.058 | 0.777 |
| Cortisol | 20.68 | 19.52 | 0.349 | 25.44 | 17.16 | 15.94 | 19.86 | 25.85 | −0.1469 | 0.422 |
= unpaired t-test
= Pearson correlation
Serum IgE levels were tested on 4 animals without alopecia and 12 animals with alopecia (three Grade 1, two Grade 2, three Grade 3, two Grade 4, and two Grade 5). The mean for animals with alopecia was higher than that for animals without alopecia, but this was not statistically significant (mean alopecia, 2.87 and no alopecia 1.148; p=0.112). No correlation was found between IgE levels and alopecia score (r=0.1306 and p=0.6296). Two animals had total IgE levels more than one standard deviation from the mean and in the group as a whole there were many animals with total IgE levels <1.0 ng/mL and many with levels >3.5ng/mL but only two animals between those values. One animal had an IgE level of 8.19 ng/mL and this animal also had the highest number of CD3+ cells in the group. Total serum IgE levels were correlated positively with total WBC count (Pearson correlation, r=0.7690, p=0.0093) and globulins (Pearson correlation, r=0.6231, p=0.0050).
Measurements of thyroid hormone levels were conducted on a majority of these animals to determine if hypothyroidism was responsible for any of the cases of alopecia (table 1). Neither free T4 nor total T4 values showed a significant association with alopecia status, and there was no correlation between alopecia score and thyroid function.
Alopecia is associated with a mononuclear cell dermatitis, acanthosis, and hyperkeratosis
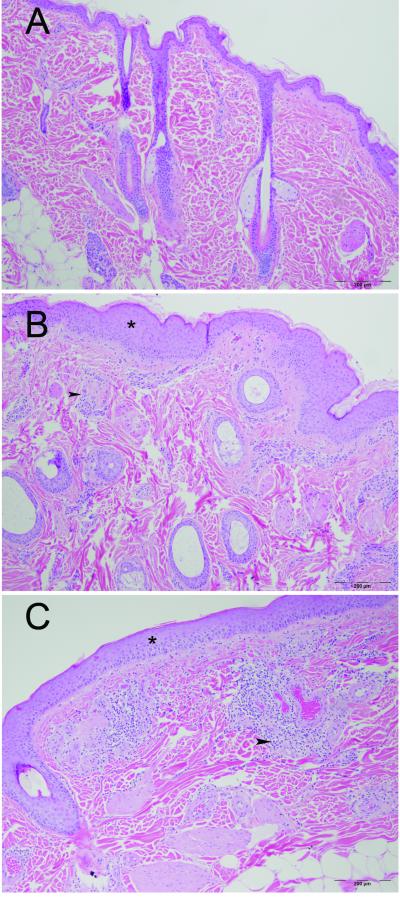
Skin biopsies from the affected animals showed a spectrum of changes ranging from mild perivascular mononuclear cell dermatitis with few other changes to more severe perivascular dermatitis with mild to moderate acanthosis and hyperkeratosis, edema and disorganization of the reticular dermis and occasional infiltration of hair bulbs by mononuclear cells (figure 1).
Figure 1. Photomicrographs of normal, moderately affected, and severely affected lumbar skin from three Rhesus macaques.
A) Normal skin with thin epidermis, scant perivascular mononuclear cell infiltrates and normal hair follicles. B) Skin from an animal with grade 3 alopecia showing acanthosis (*) with increased perivascular infiltrates (arrowhead) composed of predominantly lymphocytes, histiocytes, and mast cells. Numerous hair follicles are present. C) Skin from an animal with grade 5 alopecia showing acanthosis (*) with a marked perivascular infiltrate (arrowhead) composed of lymphocytes, mast cells, histiocytes and numerous eosinophils. Vessels are mildly distended and there is mild vascular stasis in the region. There is a reduction in the number of hair follicles in this section. H&E stained sections.
The perivascular mononuclear cell infiltrate within the superficial dermis was composed of lymphocytes, macrophages, scattered mast cells and, in a few sections, eosinophils. In some sections, the infiltrate extended away from blood vessels into the dermal connective tissue and individual inflammatory cells occasionally infiltrated the epidermis. The reticular dermis was variably disorganized in affected animals compared to the biopsies from normal animals and some sections showed dermal edema. Additionally, there was a variable degree of proliferation and/or dilatation of the small blood vessels in the dermis in biopsies from affected animals.
Alopecia is associated with increased numbers of perivascular mast cells, lymphocytes, and histiocytes
Based on these findings, a series of immunohistochemical and metachromatic stains were performed on the skin biopsies to characterize the inflammatory cell infiltrate (table 2). The stains targeted a variety of inflammatory cell types with emphasis on lymphocytes, histiocytes, and mast cells. This immunophenotypic analysis revealed statistically significant differences between animals with and without alopecia. The number of mast cells (mean alopecia, 14.79 cells/mm and no alopecia, 9.93 cells/mm; t-test, p=0.0118), CD3+ lymphocytes (mean alopecia, 25.36 cells/mm and no alopecia 13.41 cells/mm; p=0.0209), CD163+ histiocytes (mean alopecia, 12.54 cells/mm and no alopecia, 8.0 cells/mm; p=0.0302), and DC-SIGN+ cells (mean alopecia, 11.06 and no alopecia, 7.00; p=0.0326) were found to be statistically different between the two groups (table 2, figure 2). Further analysis revealed that the number of mast cells (Pearson correlation, r=0.4081; p=0.0135) CD163+ macrophages (r=0.3587; p=0.0372), and CD3+ lymphocytes (r=0.4668; p=0.0041) in biopsies from affected areas correlated positively with alopecia score (table 2).
Table 2.
Summary of immunohistochemical and metachromatic staining results by alopecia score.
| No Alopecia | Alopecia | P value1 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Corr. Coeff.2 |
P Value2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Toluidine Blue | 9.9 | 14.8 | 0.012 | 11.5 | 14.1 | 16.9 | 18.2 | 10.3 | 0.4081 | 0.0135 |
| CD3 | 13.4 | 25.4 | 0.021 | 22.7 | 19.9 | 19.6 | 31.9 | 50.8 | 0.4668 | 0.0041 |
| CD20 | 0.9 | 1.6 | 0.059 | 1.9 | 1.3 | 1.3 | 2.1 | 1.0 | 0.1680 | 0.3274 |
| CD4 | 22.4 | 26.3 | 0.206 | 21.0 | 27.2 | 23.4 | 30.9 | 35.6 | 0.2621 | 0.1283 |
| CD8 | 7.2 | 12.9 | 0.059 | 13.5 | 15.7 | 9.3 | 13.1 | 15.9 | 0.1878 | 0.2727 |
| HAM56 | 16.3 | 15.9 | 0.443 | 16.7 | 14.0 | 16.5 | 13.5 | 21.8 | 0.0065 | 0.9698 |
| CD163 | 8.0 | 12.5 | 0.030 | 11.8 | 11.2 | 11.1 | 16.4 | 13.6 | 0.3587 | 0.0372 |
| HLA-DR | 24.7 | 29.9 | 0.163 | 25.5 | 34.0 | 30.0 | 28.9 | 39.4 | 0.1977 | 0.2623 |
| DC-SIGN | 7.0 | 11.1 | 0.033 | 10.1 | 12.4 | 11.4 | 12.0 | 7.0 | 0.2165 | 0.2047 |
= unpaired t-test
= Pearson correlation
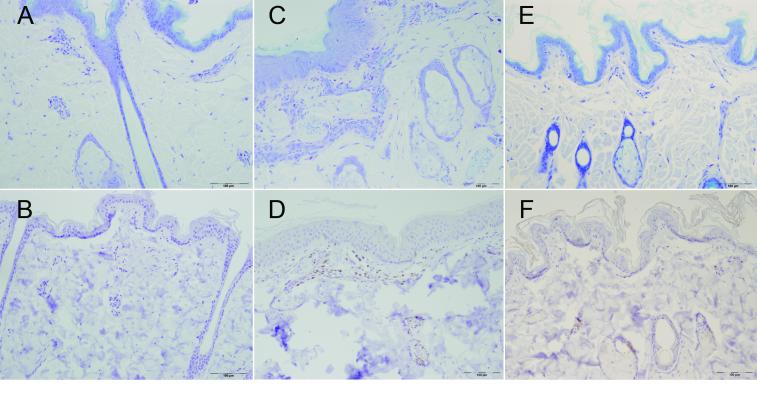
Figure 2. Phenotypic characterization of skin biopsies.
Photomicrographs are from a macaque with normal skin (A and B) a macaque with moderate alopecia (C and D) and a Cynomolgus macaque (E and F) showing increased mast cells in the alopecic Rhesus macaque (C) compared with normal Rhesus and Cynomolgus skin (A and E) and increased CD3+ lymphocytes (D) compared with normal Rhesus and Cynomolgus skin (B and F). Top row, Toluidine blue staining mast cell granules purple and bottom row CD3 immunostain using avidin-biotin-peroxidase method with DAB counterstain.
To determine if there were any correlations between the different inflammatory markers and the number of mast cells, a Pearson correlation comparing each inflammatory cell type to the number of mast cells was performed. CD4+ cells (Pearson correlation, r=0.3641, p=0.0290), CD163 (r=0.4105, p=0.0159), HLA-DR (r=0.3946, p=0.0209), and DC-SIGN (r=0.4567, p=0.0051) were correlated positively to the number of mast cells. Total serum IgE was correlated positively with CD20 cell counts (Pearson correlation, r=0.7050, p=0.0023) and DC-SIGN cell counts (Pearson correlation, r=0.6634, p=0.0051).
Rhesus macaques infected with lung mites and Cynomolgus macaques show reduced dermal inflammation
Necropsy samples from Cynomolgus macaques and P. simicola-infected Rhesus macaques were chosen from the archives of the NEPRC and stained with toluidine blue, CD3, CD4, CD8, CD20, and CD163 for comparison to alopecic macaques. Because these samples are from archived tissue, the coat condition of these animals could not be evaluated as part of the present study, however Cynomolgus macaques at our facility have a very low incidence of alopecia.
Comparing Cynomolgus macaques to all Rhesus macaques (including animals with and without alopecia) revealed significantly lower numbers of mast cells (mean Cynomolgus, 8.1 cells/mm and Rhesus 13.3 cells/mm; t-test, p=0.0024), CD3+ cells (mean Cynomolgus, 11.8 cells/mm and mean Rhesus, 21.7 cells/mm; p=0.0032), CD4+ cells (mean Cynomolgus, 7.8 cells/mm and Rhesus, 25.1 cells/mm; p<0.0001), CD8+ cells (mean Cynomolgus, 2.1 cells/mm and Rhesus, 11.1 cells/mm; p<0.0001) and CD20+ cells (mean Cynos, 0.69 cells/mm and Rhesus 1.4 cells/mm; p=0.0074) in Cynomolgus macaques compared with Rhesus macaques (table 3, figure 2). Similarly, there were significantly lower numbers of CD3+ cells (mean mite infected, 11.8 cells/mm and non-infected 21.7 cells/mm; p=0.0014), CD4+ cells (mean mite infected, 8.6 cells/mm and non-infected, 25.1 cells/mm; p<0.0001), CD8+ cells (mean mite infected, 4.4 cells/mm and non-infected, 11.14 cells/mm; p=0.001), and CD20+ (mean mite infected, 0.5 cells/mm and non-infected, 1.4 cells/mm; p=0.0011) cells in P. simicola infected macaques when compared to other macaques (table 3).
Table 3.
Summary of IHC and metachromatic staining comparing cynomolgus macaques and macaques infected with P. simicola mites to all other Rhesus macaques evaluated.
| Cynomolgus | Pneumonyssus | Rhesus | |
|---|---|---|---|
| Mast cells/mm | 8.1 (0.0024) | 12.4 (0.3289) | 13.3 |
| CD3/mm | 11.8 (0.0032) | 11.78 (0.0014) | 21.7 |
| CD4/mm | 7.8 (0.0001) | 8.6 (0.0001) | 25.1 |
| CD8/mm | 2.1 (0.0001) | 4.4 (0.001) | 11.1 |
| CD20/mm | 0.7 (0.0074) | 0.5 (0.0011) | 1.4 |
| CD163/mm | 8.2 (0.099) | 15.4 (0.059) | 11.3 |
P values for t-test in parentheses show significant differences when compared to all Rhesus monkeys for most of the cell types examined (boldface).
To determine if the differences between Cynomolgus macaques and Rhesus macaques were due to species differences or other causes, Cynomolgus macaques were compared to Rhesus macaques with normal haircoats. In this analysis, CD4+ cells (mean Cynomolgus, 7.8 and mean Rhesus, 22.4; p=0.0189) and CD8+ cells (mean Cynomolgus, 2.1 and mean Rhesus, 7.2; p=0.0105) were significantly different, but other measurements were not. When Cynomolgus macaques were compared to P. simicola infected macaques, there were only significant differences for mast cells (mean Cynomolgus, 8.1 and mean mite-infected, 12.4; t-test, p = 0.0323) and CD163+ cells (mean Cynomolgus, 8.2 and mite-infected, 15.4; t-test p = 0.0143).
Discussion
In this report we demonstrate imunophenotypic differences in resident dermal immune cells between alopecic and non alopecic Rhesus macaques. Alopecia was associated with increased numbers of mast cells, CD3+ lymphocytes, CD163+ histiocytes, and DC-SIGN+ cells. Additionally, biopsies from alopecic animals showed pathologic changes such as acanthosis, hyperkeratosis, and mild dermal edema which, together with the immunophenotypic differences, are consistent with a chronic hypersensitivity reaction or atopic dermatitis-like disease. These findings indicate that alopecia may be an important phenotype to consider when selecting animals for experimental work. Alterations in dermal inflammatory cell populations may affect responses to intradermal or subcutaneously administered vaccinations or drugs and further work is required to determine if these differences impact localized or systemic immune responses.
In other species, including man, chronic hypersensitivity reactions (food allergy, contact dermatitis, certain insect hypersensitivities) and atopic dermatitis, are associated with superficial perivascular mononuclear cell infiltrates and increased numbers of mast cells though the exact nature of the lesion often depends on the stage of disease. Eosinophils are variably recognized, even in acute cases [17-19]. In man, histopathologic findings for atopic dermatitis generally range from spongiosis and ballooning degeneration with a lymphocytic and histiocytic perivascular infiltrate in the acute stage to epidermal hyperplasia and hyperkeratosis with dermal infiltrates of lymphocytes, macrophages and mast cells in more chronic lesions [19]. Histological evaluation of human tissue has shown that the mononuclear perivascular infiltrate in patients with both atopic dermatitis and allergic contact dermatitis consists primarily of CD3+ and CD4+ memory t-cells and that there is an increase in the number of dermal Langerhan’s cells [19, 20]. This is consistent with the findings of this study but additional work will need to be done to differentiate atopic dermatitis from other chronic hypersensitivity disorders.
Total serum IgE values were not correlated with alopecia score in this study which is not unexpected given results from man and dog with atopic dermatitis in which total serum IgE is a relatively poor diagnostic test in adults due to the high variability of IgE levels in the normal population as a result of genetic factors, housing, race, and vaccine history [21]. However, our population is much more uniform with fewer differences in these factors and so we might expect less variability. It is, therefore, interesting that in our small sample size there was a high level of variability with animals stratifying into two distinct groups; those with IgE < 1.0 ng/mL and those with IgE > 3.5 ng/mL which may indicate that some of the animals in the high IgE group were in a more “allergic state” than the other animals. Further work to determine a normal range for rhesus macaques should be pursued.
In human atopic dermatitis there is immune dysregulation characterized by a shift from a Th1 phenotype to a Th2 phenotype with altered production of a variety of cytokines including IL4, IL5, IL10, IL13, and IL17 and downregulation of antimicrobial peptides in the skin. This leads to increased susceptibility to a variety of microorganisms including Staphyloccocus aureus, Malassezia furfur, Herpes simplex virus, and Poxvirus [22-25]. It will be important to determine if similar alterations are observed in alopecic Rhesus macaques. If this proves to be the case, then the effects on research could be profound. For example, it has been proposed that HIV replicates preferentially in Th2 type cells and it is documented that IL4 can enhance HIV replication [26] and that IL4 is upregulated in lymph nodes of SIV infected macaques early in infection [27]. In this case, a systemic switch to a Th2 phenotype and upregulation of IL4 prior to SIV infection in the Rhesus macaque SIV model caused by a systemic atopic condition would undoubtedly affect the immune system’s response to the virus and any research pertaining to that response.
Our findings suggest that housing history and pathogen burden may impact the development of dermal inflammatory cell infiltrates and alopecia. We demonstrate that animals born indoors at the NEPRC have a much higher risk of developing alopecia when compared to animals originating from outdoor colonies and that Cynomolgus macaques and P. simicola-infected Rhesus macaques have demonstrably lower levels of dermal inflammation compared with all other Rhesus macaques. We propose that the reduced incidence of alopecia in macaques born in an outdoor environment and the reduced inflammatory cell infiltrate in Cynomolgus macaques and lung mite infected Rhesus macaques is in part due to the housing history of these animals; with a history of outdoor housing and infection with lung mites providing protection from future development of alopecia, respectively. Likely, this is one of many contributing factors to the development of alopecia and individual variation may make this single factor more or less of a contributor in different animals.
Other surveys of large colonies of Rhesus macaques have found a similar association between housing status and coat condition. One main difference is that, in those reports, animals were housed outdoors continually, whereas our animals currently live indoors but have a history of outdoor housing. Several behavioral issues appear to play a prominent role in the development of alopecia, including overgrooming and foraging opportunity. Interestingly, in one report showing increased coat condition in outdoor housed animals, fecal cortisol metabolites were negatively correlated with alopecia status [2]. In our study, there were no bloodwork changes that one might expect with stress, such as neutrophilia, lymphopenia, or hyperglycemia. Additionally, serum cortisol levels were not significantly different between animals with and without alopecia. It is also our experience that not all animals with alopecia display other behavioral abnormalities and that some have clinical signs including pruritis that may be associated with underlying dermal disease.
Several studies have noted that poor coat condition is a seasonal phenomenon, with animals having the worst hair coats in winter and spring compared to summer and autumn [2, 3]. As suggested by these authors, a number of factors may contribute to this including pregnancy, humidity, and sunlight/day length. Seasonality is noted in both indoor and outdoor housed animals suggesting that sunlight played a limited role.
Females in other reports tend to not only show higher incidences of alopecia overall, consistent with our findings, but also marked seasonality in their alopecia relating to pregnancy status; with increased alopecia during late gestation and increased alopecia with increasing age [3]. The animals in our study were all singly housed, so none were pregnant, but we did show an increases alopecia risk with increasing age of the animal. Additionally, many of the females in our study had prior pregnancies which may have contributed to female alopecia in our study.
The other factor contributing to seasonality may be humidity. It is our experience that humidity even in indoor enclosures varies markedly with season in a temperate environment, and this could explain some of the observed seasonality in primate colonies. It is known that exacerbations of atopic dermatitis in people occur more frequently in winter months [28] due to reduced environmental humidity, skin dryness, irritation, and reduced barrier function of the epidermis. A similar mechanism could be at work in nonhuman primate colonies.
In man, a link has been made between various atopic diseases including dermatitis, allergic rhinitis, and asthma, and previous exposure to allergens and infectious agents [29-31]. The so-called “hygiene hypothesis” posits that early exposure to a variety of pathogens reduces the risk of atopic diseases later in life. Protection from atopic diseases was initially associated with family size and birth order [31] but additional data have pointed to the protective effects of early exposure to daycare [32], exposure to farms [33], and a number of infectious diseases, especially those of the gastrointestinal system [29, 30, 34, 35]. It follows that a reduction in pathogen exposure in Western, industrialized nations has led to a corresponding increase in allergic and autoimmune diseases over the past 30 years [29]. There are a number of proposed mechanisms to explain this phenomenon, but one commonality is alteration in the balance between Th1, Th2, and Treg phenotypes. In one of these mechanisms, coevolution of man and various bacterial and helminthic organisms led to immune tolerance of these organisms with increased expression of dendritic cells and regulatory T cells and resultant suppression of the immune system. As Western countries have eliminated many of these common infections, underlying immunoregulatory dysfunction has been exposed and this has led to the observed increased incidence of allergic and autoimmune diseases [30, 36].
As in developed and industrialized nations, indoor housed primates in our colony are no longer exposed to the wide array of bacterial, parasitic, and viral agents that monkeys have coevolved with. In contrast, animals bred in an outdoor environment are exposed to a greater variety of pathogenic and nonpathogenic organisms. Contact with soil saprophytes and wild animals would provide opportunities for pathogen exposure in outdoor housed animals. Following the hygiene hypothesis, these animals would be expected to have a lower incidence of allergic and autoimmune diseases, including atopic dermatitis. While we did not find a correlation between virologic status and alopecia, it is more likely that specific gastrointestinal commensals play a more prominent role.
It will be important to determine whether a subset of monkeys can be treated for their inflammatory disease and regrow a normal hair coat. We have had some success treating pruritic animals with antihistamines (Loratadine) and omega-3 fatty acid supplements but not enough time has elapsed to gauge hair regrowth. We have also treated individuals with systemic prednisone with some success but large scale treatment has not been attempted due to possible side effects. Cyclosporine and topical anti-inflammatory drugs have shown promise in previous case reports but there are obstacles to both of these treatments including systemic immunosuppression and the risk to handlers of frequent topical drug administration [7, 8].
It is likely that there are a multitude of factors contributing to alopecia in macaque colonies with no one factor causing every case and, correspondingly, no single husbandry or treatment option to cure every case. Our results suggest that outdoor housing contributes to the decreased risk of alopecia in outdoor raised animals due to a decreased pathogen burden. But, behavioral differences due to the increased enrichment of outdoor housing compared to indoor housing, temperature or humidity differences in indoor and outdoor environments, exposure to sunlight, or other, yet unidentified factors, probably contribute to the different alopecia rates in these two groups as well. Additionally, it is likely that individual genetic variation will cause some animals to be more susceptible to hypersensitivity disorders, others to behavioral disorders, and still others to the multitude of other conditions that can cause alopecia.
We conclude that alterations in the immunophenotypic composition of resident immune cells differs between normal Rhesus macaques and those with generalized alopecia and that there is evidence supporting reduced pathogen burden in Rhesus macaque colonies as an underlying cause. Future work should clarify underlying immune dysregulation in this group of animals and effects that this may have on research.
Acknowledgements/Funding
The authors would like to thank Audra Hachey, Nicole Monts de Oca, and Christine Pearson for their technical assistance with immunohistochemistry and Elaine Roberts, John Tappan, Ernest Neale, Matthew Beck, Lisa Bezokas, James Ingersoll, and Leah Makaron for their assistance with animal procedures. This work was supported by NIH/ NCRR Grant P51 RR000168.
References
- 1.Steinmetz HW, Kaumanns W, Neimeier KA, Kaup FJ. Dermatologic investigation of alopecia in rhesus macaques (Macaca mulatta) J Zoo Wildl Med. 2005;36:229–238. doi: 10.1638/04-054.1. [DOI] [PubMed] [Google Scholar]
- 2.Steinmetz HW, Kaumanns W, Dix I, Heistermann M, Fox M, Kaup FJ. Coat condition, housing condition and measurement of faecal cortisol metabolites--a non-invasive study about alopecia in captive rhesus macaques (Macaca mulatta) J Med Primatol. 2006;35:3–11. doi: 10.1111/j.1600-0684.2005.00141.x. [DOI] [PubMed] [Google Scholar]
- 3.Beisner BA, Isbell LA. Factors influencing hair loss among female captive rhesus macaques (Macaca mulatta) Applied Animal Behaviour Science. 2009;119:91–100. [Google Scholar]
- 4.Beardi B, Wanert F, Zoller M, Freyschmidt-Paul P, Bodemer W, Kaup FJ. Alopecia areata in a rhesus monkey (Macaca mulatta) J Med Primatol. 2007;36:124–130. doi: 10.1111/j.1600-0684.2007.00212.x. [DOI] [PubMed] [Google Scholar]
- 5.Ahmad W, Ratterree MS, Panteleyev AA, Aita VM, Sundberg JP, Christiano AM. Atrichia with papular lesions resulting from mutations in the rhesus macaque (Macaca mulatta) hairless gene. Lab Anim. 2002;36:61–67. doi: 10.1258/0023677021911777. [DOI] [PubMed] [Google Scholar]
- 6.Macy JD, Jr., Huether MJ, Beattie TA, Findlay HA, Zeiss C. Latex sensitivity in a macaque (Macaca mulatta) Comp Med. 2001;51:467–472. [PubMed] [Google Scholar]
- 7.Ovadia S, Wilson SR, Zeiss CJ. Successful cyclosporine treatment for atopic dermatitis in a rhesus macaque (Macaca mulatta) Comp Med. 2005;55:192–196. [PubMed] [Google Scholar]
- 8.Torreilles SLL, Richard H, Felt Stephen A., McClure Diane E. Tacrolimus Ointment: a Novel and Effective Topical Treatment of Localized Atopic Dermatitis in a Rhesus Macaque (Macaca mulatta) Journal of the American Association for Laboratory Animal Science. 2009;48:307–311. [PMC free article] [PubMed] [Google Scholar]
- 9.Horenstein VD, Williams LE, Brady AR, Abee CR, Horenstein MG. Age-related diffuse chronic telogen effluvium- type alopecia in female squirrel monkeys (Saimiri boliviensis boliviensis) Comp Med. 2005;55:169–174. [PubMed] [Google Scholar]
- 10.Newcomer CE, Fox JG, Taylor RM, Smith DE. Seborrheic dermatitis in a rhesus monkey (Macaca mulatta) Lab Anim Sci. 1984;34:185–187. [PubMed] [Google Scholar]
- 11.Swenerton H, Hurley LS. Zinc deficiency in rhesus and bonnet monkeys, including effects on reproduction. J Nutr. 1980;110:575–583. doi: 10.1093/jn/110.3.575. [DOI] [PubMed] [Google Scholar]
- 12.Novak MA, Meyer JS. Alopecia: possible causes and treatments, particularly in captive nonhuman primates. Comp Med. 2009;59:18–26. [PMC free article] [PubMed] [Google Scholar]
- 13.Daniel MD, Letvin NL, Sehgal PK, Schmidt DK, Silva DP, Solomon KR, Hodi FS, Jr., Ringler DJ, Hunt RD, King NW, et al. Prevalence of antibodies to 3 retroviruses in a captive colony of macaque monkeys. Int J Cancer. 1988;41:601–608. doi: 10.1002/ijc.2910410421. [DOI] [PubMed] [Google Scholar]
- 14.Yearley JH, Pearson C, Carville A, Shannon RP, Mansfield KG. SIV-associated myocarditis: viral and cellular correlates of inflammation severity. AIDS Res Hum Retroviruses. 2006;22:529–540. doi: 10.1089/aid.2006.22.529. [DOI] [PubMed] [Google Scholar]
- 15.Yearley JH, Pearson C, Shannon RP, Mansfield KG. Phenotypic variation in myocardial macrophage populations suggests a role for macrophage activation in SIV-associated cardiac disease. AIDS Res Hum Retroviruses. 2007;23:515–524. doi: 10.1089/aid.2006.0211. [DOI] [PubMed] [Google Scholar]
- 16.Mansfield KG, Veazey RS, Hancock A, Carville A, Elliott M, Lin KC, Lackner AA. Induction of disseminated Mycobacterium avium in simian AIDS is dependent upon simian immunodeficiency virus strain and defective granuloma formation. Am J Pathol. 2001;159:693–702. doi: 10.1016/S0002-9440(10)61740-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller GH, Kirk RW, Scott DW, Miller WH, Griffin CE. Muller & Kirk’s small animal dermatology. W.B. Saunders; Philadelphia: 2001. [Google Scholar]
- 18.Maxie MG, Jubb KVF. Pathology of domestic animals. Elsevier Saunders; Edinburgh ; New York: 2007. [Google Scholar]
- 19.Leung DY. Atopic dermatitis: the skin as a window into the pathogenesis of chronic allergic diseases. J Allergy Clin Immunol. 1995;96:302–318. doi: 10.1016/s0091-6749(95)70049-8. quiz 319. [DOI] [PubMed] [Google Scholar]
- 20.Bangert C, Friedl J, Stary G, Stingl G, Kopp T. Immunopathologic features of allergic contact dermatitis in humans: participation of plasmacytoid dendritic cells in the pathogenesis of the disease? J Invest Dermatol. 2003;121:1409–1418. doi: 10.1111/j.1523-1747.2003.12623.x. [DOI] [PubMed] [Google Scholar]
- 21.DeBoer DJ, Hillier A. The ACVD task force on canine atopic dermatitis (XVI): laboratory evaluation of dogs with atopic dermatitis with serum-based “allergy” tests. Vet Immunol Immunopathol. 2001;81:277–287. doi: 10.1016/s0165-2427(01)00304-x. [DOI] [PubMed] [Google Scholar]
- 22.Incorvaia C, Frati F, Verna N, D’Alo S, Motolese A, Pucci S. Allergy and the skin. Clin Exp Immunol. 2008;153(Suppl 1):27–29. doi: 10.1111/j.1365-2249.2008.03718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nomura I, Goleva E, Howell MD, Hamid QA, Ong PY, Hall CF, Darst MA, Gao B, Boguniewicz M, Travers JB, Leung DY. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol. 2003;171:3262–3269. doi: 10.4049/jimmunol.171.6.3262. [DOI] [PubMed] [Google Scholar]
- 24.Ogg G. Role of T cells in the pathogenesis of atopic dermatitis. Clin Exp Allergy. 2009;39:310–316. doi: 10.1111/j.1365-2222.2008.03146.x. [DOI] [PubMed] [Google Scholar]
- 25.Jung T, Stingl G. Atopic dermatitis: therapeutic concepts evolving from new pathophysiologic insights. J Allergy Clin Immunol. 2008;122:1074–1081. doi: 10.1016/j.jaci.2008.09.042. [DOI] [PubMed] [Google Scholar]
- 26.Montaner LJ, Gordon S. TH2 downregulation of macrophage HIV-1 replication. Science. 1995;267:538–539. doi: 10.1126/science.7824955. [DOI] [PubMed] [Google Scholar]
- 27.Zou W, Lackner AA, Simon M, Durand-Gasselin I, Galanaud P, Desrosiers RC, Emilie D. Early cytokine and chemokine gene expression in lymph nodes of macaques infected with simian immunodeficiency virus is predictive of disease outcome and vaccine efficacy. J Virol. 1997;71:1227–1236. doi: 10.1128/jvi.71.2.1227-1236.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elias PM, Hatano Y, Williams ML. Basis for the barrier abnormality in atopic dermatitis: outside-inside-outside pathogenic mechanisms. J Allergy Clin Immunol. 2008;121:1337–1343. doi: 10.1016/j.jaci.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–920. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 30.Rook GA. Review series on helminths, immune modulation and the hygiene hypothesis: the broader implications of the hygiene hypothesis. Immunology. 2009;126:3–11. doi: 10.1111/j.1365-2567.2008.03007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strachan DP. Hay fever, hygiene, and household size. Bmj. 1989;299:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Celedon JC, Wright RJ, Litonjua AA, Sredl D, Ryan L, Weiss ST, Gold DR. Day care attendance in early life, maternal history of asthma, and asthma at the age of 6 years. Am J Respir Crit Care Med. 2003;167:1239–1243. doi: 10.1164/rccm.200209-1063OC. [DOI] [PubMed] [Google Scholar]
- 33.Braback L, Hjern A, Rasmussen F. Trends in asthma, allergic rhinitis and eczema among Swedish conscripts from farming and non-farming environments. A nationwide study over three decades. Clin Exp Allergy. 2004;34:38–43. doi: 10.1111/j.1365-2222.2004.01841.x. [DOI] [PubMed] [Google Scholar]
- 34.Flohr C, Pascoe D, Williams HC. Atopic dermatitis and the ’hygiene hypothesis’: too clean to be true? Br J Dermatol. 2005;152:202–216. doi: 10.1111/j.1365-2133.2004.06436.x. [DOI] [PubMed] [Google Scholar]
- 35.von Mutius E. Allergies, infections and the hygiene hypothesis--the epidemiological evidence. Immunobiology. 2007;212:433–439. doi: 10.1016/j.imbio.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Jackson JA, Friberg IM, Little S, Bradley JE. Review series on helminths, immune modulation and the hygiene hypothesis: immunity against helminths and immunological phenomena in modern human populations: coevolutionary legacies? Immunology. 2009;126:18–27. doi: 10.1111/j.1365-2567.2008.03010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]