Abstract
The ability to investigate the electrophysiological properties of individual cells in acute brain tissue led to the discovery that many glial cells have the capacity to respond rapidly to neuronal activity. In particular, a distinct class of neuroglial cells known as NG2 cells, which exhibit many of the properties that have been described for glial subtypes such as complex cells, polydendrocytes, synantocytes and GluR cells, express ionotropic receptors for glutamate and GABA. In both gray and white matter, NG2 cells form direct synaptic junctions with axons, which enable transient activation of these receptors. Electrophysiological analyses have shown that these neuron-glia synapses exhibit all the hallmarks of ‘classical’ neuron-neuron synapses, including rapid activation, quantized responses, facilitation and depression, and presynaptic inhibition. Electron microscopy indicates that axons form morphologically distinct junctions at discrete sites along processes of NG2 cells, suggesting that NG2 cells are an overt target of axonal projections. AMPA receptors expressed by NG2 cells exhibit varying degrees of Ca2+ permeability, depending on the brain region and stage of development, and in white matter NG2 cells have also been shown to express functional NMDA receptors. Ca2+ influx through AMPA receptors following repetitive stimulation can trigger long term potentiation of synaptic currents in NG2 cells. The expression of receptors with significant Ca2+ permeability may increase the susceptibility of NG2 cells to excitotoxic injury. Future studies using transgenic mice in which expression of receptors can be manipulated selectively in NG2 cells have to define the functions of this enigmatic neuron-glia signaling in the normal and diseased CNS.
Keywords: Neuroglia, NG2 cell, synaptic transmission, neuron-glia synapses, AMPA receptors, GABAA receptors
1. Introduction
Ionotropic neurotransmitter receptors play well defined roles in neurons – they regulate excitability by allowing current flow and induce structural and functional plasticity by enabling Ca2+ influx. Despite the abundant evidence that these receptors are expressed by all major classes of glia, it is less clear what functions these receptors subserve in these diverse cell types. We are only beginning to understand the context under which these receptors are activated, what signals they produce and what consequences these responses have for glia and their environment. This is particularly true for a newly recognized distinct class of glial cells known as NG2 cells, as the roles of these abundant, widely distributed glial cells in the adult CNS remain largely undefined.
The application of the patch-clamp technique to cells in acute brain slices in the early 1990’s revealed that glial cells display a much greater physiological diversity then anticipated from previous electrophysiological analyses using “blind” recording with sharp microelectrodes or by analysis of glia in cell culture (Berger et al., 1991; Steinhäuser et al., 1992). Based on their distinct whole-cell current patterns, the terms “complex” and “passive” cells were introduced to qualitatively distinguish glial subtypes in grey matter (Steinhäuser et al., 1994). At this time, passive cells were classified as bona fide astrocytes because they expressed GFAP, exhibited dye coupling and possessed glutamate and GABA transporters. However, the identity of the complex cells remained nebulous. The observation that the resting conductance of complex cells appeared to increase during postnatal maturation, and the finding that they typically lacked GFAP, but often expressed S100β led to the conclusion that complex cells represented immature astrocytes (Bordey and Sontheimer, 1997; Kressin et al., 1995). Subsequent analyses of transgenic mice in which enhanced green fluorescent protein EGFP) is expressed under control of the GFAP promoter suggested that this assumption was incorrect, revealing that the hippocampus contained a distinct group of glial cells that shared some properties with astrocytes. Specifically, these and other studies provided evidence that the vast majority of cells with complex current patterns, which were later termed GluR cells because they express ionotropic glutamate receptors, do not represent immature astrocytes (Matthias et al., 2003; Wallraff et al., 2004), and are also found in the adult brain (Zhou et al., 2006).
In 2000, Bergles and colleagues demonstrated the existence of functional neuron-glia synapses in the brain (Bergles et al., 2000). The authors regarded the postsynaptic glia as oligodendrocyte precursor cells (OPCs), as they exhibited immunoreactivity to NG2, a chondroitin sulphate proteoglycan expressed by oligodendrocyte progenitors. These innervated glial cells shared many properties with GluR cells, including the expression of AMPA and GABAA receptors, lack of dye coupling and absence of glutamate transporters. Others have termed cells with similar morphological and functional properties synantocytes (Butt et al., 2002) or polydendrocytes (Nishiyama et al., 2002), as lineage tracing experiments suggest that not all NG2 cells develop into oligodendrocytes. There is now an emerging consensus in the field that i) NG2 cells and GluR/complex cells represent overlapping populations of glia (although the degree of overlap is still unclear) and ii) that NG2 cells represent a fourth class of neuroglial cells in the adult CNS (Nishiyama et al., 2009; Peters, 2004). Here, we will refer to these distinct glial cells as NG2 cells, although unpublished studies suggest the existence of complex cells that do not express this protein (Jennißen and Steinhäuser, unpublished observation). In this review, we discuss the morphological and functional properties of NG2 cells, with a special emphasis on their intriguing capability to form synapses with neurons.
2. Receptor expression by complex cells/NG2 glia
2.1 Ionotropic glutamate receptors
Earlier findings using acute hippocampal slices suggested expression of Ca2+ permeable kainate receptors by complex cells (Jabs et al., 1994; Steinhäuser et al., 1994). However indirect effects, through activation of neighbouring cells, could not be excluded in these and several subsequent in situ experiments. Under more defined experimental conditions, in which whole-cell patch-clamp recording of freshly isolated cells was combined with single-cell transcript analyses, data indicated that in the hippocampus these cells express functional AMPA receptors, but not kainate or NMDA receptors. The AMPA receptors expressed by these glial cells displayed an intermediate Ca2+ permeability which was conserved during postnatal development, although the receptor composition varied early after birth (Seifert et al., 1997b; Seifert and Steinhäuser, 1995). By postnatal day 5, a mosaic of Ca2+-permeable and less Ca2+-permeable AMPA receptors coexist in individual complex cells, while receptors with a more uniform, low divalent cationic permeability dominate in older cells (Seifert et al., 2003). In juvenile mouse hippocampus, the most thoroughly investigated brain region, these cells predominantly express the AMPA receptor subunits GluR2 and GluR4, while GluR3 is least abundant (Seifert et al., 1997a). Furthermore, combined pharmacological and molecular analyses demonstrated enhanced relative expression of GluR2 flip splice versions of the receptors and decreased inward rectification of AMPA receptor currents during early postnatal maturation of the hippocampus (Seifert et al., 2003). Similar properties have been reported for complex cells in human hippocampus where an alteration in GluR1 splicing was identified in epilepsy patients presenting with hippocampal sclerosis (Seifert et al., 2004). Together, these studies indicate that NG2 cells express a complex repertoire of AMPA receptor subunits that assemble to form glutamate receptors that exhibit rapid kinetics and an intermediate Ca2+ permeability.
Physiological studies indicate that Ca2+ permeable AMPA receptors are also present in NG2 cells of the cerebellum (Lin et al., 2005), which show even stronger inward rectification than in the hippocampus. In the corpus callosum, an opposite age-dependence of rectification of AMPA receptors has been found compared to that observed in the hippocampus: the current- to-voltage relationship of the responses shifted from linear at young ages to inwardly rectifying in adults (Ziskin et al., 2007). As inward rectification is an indication of AMPA receptors assembled without the GluR2 subunit, these findings suggest that there are age- and region-specific differences in Ca2+ permeability of AMPA receptors at NG2 cell synapses (cf. 3.2).
The Ca2+ permeability of AMPA receptors in NG2 cells could enable activation of intracellular signalling cascades in response to axonal firing. In the hippocampus, these receptors were reported to mediate long-term potentiation of neuron-NG2 cell synapses and to affect AMPA receptor trafficking (Ge et al., 2006) (cf. 3.2). However, whether synaptic activation of these receptors induces glial Ca2+ transients, which might potentially lead to the release of signalling molecules from NG2 cells, remains unknown. Sustained activation of glial AMPA receptors can lead to inhibition of Kir channels, due to receptor-mediated influx of Na+, which occludes the channels from the intracellular side (Schröder et al., 2002). This transient inhibition of IKir can combine with the cationic receptor current to boost depolarization of the cell during glutamate exposure.
2.2 Ionotropic GABA receptors
The analysis of GABA receptors in NG2 cells has attracted less attention. Several years ago slice recordings qualitatively identified functional GABA receptors in complex glial cells of the hippocampus and spinal cord (Bekar et al., 1999; Pastor et al., 1995; Steinhäuser et al., 1994). In addition to activation of a Cl− conductance, these reports provided evidence of a block of K+ outward currents upon GABA application, with the mechanism linking receptor activation and K+ channel inhibition remaining unknown. Recent findings indicate that in hippocampus, activation of GABAA receptors in NG2 cells leads to depolarization, due to the relatively high glial intracellular Cl− concentration ([Cl−]i) maintained by these cells (Lin and Bergles, 2004). Interestingly, by increasing the membrane conductance and by altering [Cl−]i, GABA application concomitantly decreased AMPA receptor currents, which may serve to fine tune the effects of these two excitatory inputs. Recently, Tong et al. (2009) described that GABAA receptor induced depolarization may increase [Ca2+]i in hippocampal NG2 cells. This process seems to require voltage gated Na+ channels and was mediated by activation of the Na+/Ca2+ exchanger, operating in the reversed mode. The subunit composition of GABAA receptors in NG2 cells is still largely unknown, although the slow kinetics and pharmacological properties of GABA responses in hippocampal NG2 cells suggest that these receptors contain the α5 subunit (Lin and Bergles, 2004). Whether NG2 cells in the corpus callosum possess GABAA receptors is unclear, as neither spontaneous or evoked GABA receptor mediated responses have been reported (Kukley et al., 2007; Ziskin et al., 2007).
3. Neuron-glia synaptic signalling
3.1 Evidence for direct synapses
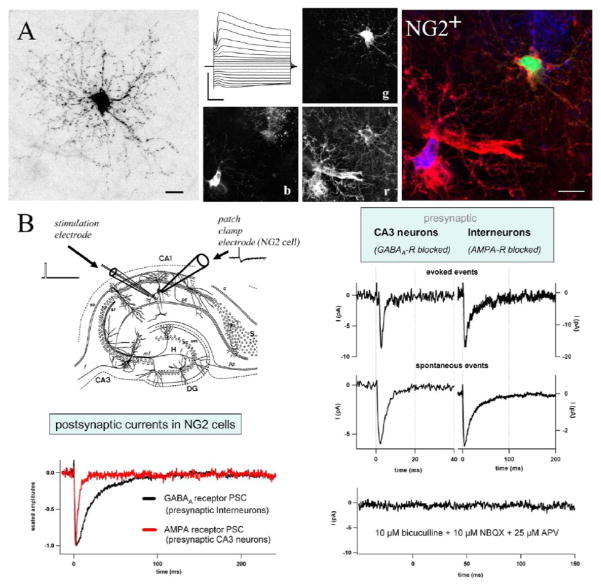
It was generally assumed that receptors in glial cells are present to detect increases in ambient transmitter levels, such as those induced during periods of intense activity or trauma, or indirectly through spillover of transmitter from neuronal synapses, in cases where glial membranes are positioned adjacent to the synaptic cleft. While such mechanisms may contribute to receptor activation in cells such as astroglia (astrocytes and Bergmann glia) (Bergles et al., 1997; Clark and Barbour, 1997) that extend fine lamellae that surround synapses, biophysical studies of transmitter dynamics at excitatory synapses indicate that glutamate levels decline rapidly through diffusion and dilution after fusion of transmitter-laden vesicles at synapses containing single active zones (Clements et al., 1992; Diamond and Jahr, 1997), suggesting that activation of low affinity AMPA receptors would be minimal in NG2 cells if the source of glutamate was from neighbouring synapses. Indeed, AMPA receptors are more likely to be desensitized by chronic exposure to glutamate than activated (Trussell and Fischbach, 1989). Nevertheless, whole-cell recordings from NG2 cells in hippocampal slices isolated acutely from rodent brain revealed that both AMPA and GABAA receptors are subject to transient rather than tonic activation in these cells (Fig. 1) (Bergles et al., 2000; Jabs et al., 2005; Lin and Bergles, 2004). The duration of these brief receptor-mediated currents induced by stimulation of Schaffer collateral-commissural axons were comparable to those produced at neuronal synapses through vesicular fusion, reaching a peak in the case of AMPA receptor currents in a few hundred microseconds and decaying with a time constant of slightly more than one millisecond at room temperature (Fig. 1). As the rate of receptor activation is dependent on ligand concentration, the rapid activation of these low affinity receptors suggests that they were exposed to a high concentration of glutamate. The only mechanism that has been described to produce such transients in brain is through fusion of vesicles loaded with glutamate with the plasma membrane. Subsequent studies have shown that these glial responses exhibit all the hallmarks of events produced at synapses. They occur with minimal delay after action potentials are triggered in surrounding axons; they can be reliably evoked by a single action potential; spontaneous events are visible when action potentials are prevented with tetrodotoxin; the smallest evoked responses have time courses similar to the spontaneous events; they facilitate in response to repetitive stimulation; and event amplitudes fluctuate from trial to trial, as expected from the stochastic nature of vesicle release (Fig. 1) (Paukert and Bergles, 2006). Moreover, AMPA receptor currents can be induced repeatedly for more than one hour with repetitive stimulation (Ziskin et al., 2007), indicating that such signalling is likely to take place at stable junctions where terminals possess all the machinery necessary to release, recycle and refill vesicles with glutamate.
Figure 1.
Properties of synaptic inputs to NG2 cells in an acute slices of hippocampus from juvenile transgenic hGFAP/EGFP mice. (A) The morphology of NG2 cells was visualized by Texas Red dextran-filling during whole-cell recording. Subsequent confocal analysis and 2D maximum projection shows the extensive arborization of their processes. Note the typical nodules appearing as dots all along the fine processes (bar 10 μm). A typical current pattern is given in the middle panel. Current responses were evoked by de- and hyperpolarizing the membrane between +20 and −160 mV (holding potential −80mV, 10 mV steps, bars 1 nA, 10 ms). Post-recording immunostaining and triple fluorescence confocal analysis were applied to test for NG2 immunoreactivity. The middle panel shows three separated colour channels of one confocal plane. Texas Red dextran labelling is given in green (g), NG2 immunoreactivity in red (r), and EGFP expression in blue (b). The superimposed RGB picture (right) shows the membrane-associated distribution of NG2 immunoreactivity of the recorded cell (yellow details). (B) Postsynaptic currents were recorded from an NG2 cell upon short single pulse stimulation. At least two types of presynaptic neurons, glutamatergic CA3 pyramidal neurons and GABAergic interneurons, innervate hippocampal NG2 cells. Corresponding glial postsynaptic currents can be distinguished by their current kinetics and their pharmacological profiles. Modified from Jabs et al. (2005).
Evoked and spontaneous AMPA receptor-mediated currents with characteristics similar to those observed in the hippocampus also have been recorded from NG2 cells in other grey matter regions, such as the cerebellum (Lin et al., 2005), cortex (Chittajallu et al., 2004) and brainstem (Muller et al., 2009), as well in white matter regions such as the corpus callosum (Kukley et al., 2007; Ziskin et al., 2007) and cerebellar white matter (Karadottir et al., 2005) that are largely devoid of neuronal dendrites. These studies suggest that synaptic signalling is a conserved property of this class of glia, and that this form of rapid neuron-glia communication is widespread in the brain. Inputs to these cells arise from axons that innervate neighbouring neurons; that is, they do not appear to be the target of a unique set of axons. For example, NG2 cells form synapses with Schaffer collateral axons of CA3 neurons in the CA1 region of the hippocampus (Bergles et al., 2000), with granule cells in the dentate gyrus (Mangin et al., 2008), with climbing fibres and parallel fibres in the molecular layer of the cerebellum (Lin et al., 2005), with axons of globular bushy cells of the cochlear nucleus in the medial nucleus of the trapezoid body (Muller et al., 2009), and with axons of cortical neurons in the corpus callosum (Ziskin et al., 2007). Although it is possible that these cells are influenced by long-range neuromodulatory projections (Papay et al., 2004; Papay et al., 2006), the predominant input, in terms of their ability to induce transmembrane ion flow, arises from projections that are not exclusive to NG2 cells. Indeed, paired recordings from Purkinje cells and NG2 cells in the cerebellum demonstrated that the same climbing fibres that innervate Purkinje cells also make synapses with NG2 cells (Lin et al., 2005), and ultrastructural analysis of NG2 cell processes in the hippocampus revealed single boutons that formed both axo-dendritic synapses and axon-NG2 cell synaptic junctions (Bergles et al., 2000).
What governs the formation of synapses with NG2 cells? Developmental studies indicate that synaptic inputs can be first recorded from these glial cells during the first week of life in rats and mice, and NG2 cells in mature tissue exhibit even more robust synaptic activity (Bergles et al., 2000), indicating that innervation of these cells closely parallels the maturation of synaptic connections between surrounding neurons. Given the small size and symmetrical shape of NG2 cells (Fig. 1), it is possible that these cells simply form synaptic junctions with a random selection of axons in their vicinity. Indeed, paired recordings from interneurons in the dentate gyrus and nearby “satellite” NG2 cells revealed that cells located within 200 μm typically received glutamatergic input from the same presynaptic neurons (Mangin et al., 2008). The density of neuron-NG2 cell synapses seems to vary considerably among brain regions. Hippocampal NG2 cells bear only a few synapses (7–19 per cell; Bergles et al., 2000). However, in the cerebellum (Lin et al., 2005) and in white matter (Kukley et al., 2007; Ziskin et al., 2007) single NG2 cells may form hundreds of synaptic contacts. It is unclear whether neurons select particular NG2 cells with which to form synapses, or vice versa. At present, the molecular mechanisms that guide the formation of glutamatergic and GABAergic synapses with NG2 cells have not been determined.
One of the most unexpected findings regarding glutamate receptor signalling in NG2 cells is that axon-glial synapses are also found in white matter. NG2 cells are slightly more abundant in white matter (Dawson et al., 2003) such as the corpus callosum, where their processes extend parallel to axons. Measurements of the conduction velocity of axons that form synapses with NG2 cells in the corpus callosum indicate that they preferentially receive input from unmyelinated axons (Kukley et al., 2007; Ziskin et al., 2007). As unmyelinated axons make up less than 30% of axons in the corpus callosum, it suggests that this pattern is unlikely to arise from random association. The specificity of these connections is surprising, as several anatomical studies have suggested that NG2 cells contact nodes of Ranvier along myelinated axons (Butt et al., 1999; Huang et al., 2005), where presumably glutamate could also be released. If NG2 cells initiate the formation of these junctions, the larger exposed area afforded by unmyelinated axons may increase the likelihood of synapse formation.
3.2 Diversity among NG2 cell synapses
Glutamaterigic synaptic currents are remarkably similar among NG2 cells in different brain regions, exhibiting small amplitudes and rapid kinetics (Chittajalluet al., 2004; Karram et al., 2008), and both evoked and spontaneous glutamatergic events are blocked by selective AMPA receptor antagonists (Fig. 1). Despite physiological evidence for expression of NMDA receptors by NG2 cells in white matter (Karadottir et al., 2005; Ziskin et al., 2007), there is little evidence for the contribution of NMDA receptors to synaptic responses in either white matter or gray matter. The inability of vesicular release of glutamate to reliably engage NMDA receptors at these neuron-glia junctions may indicate that these receptors are located primarily extrasynaptically; however, the summed NMDA receptor response induced by activation of receptors over the entire cell in response to exogenous agonists is very small in NG2 cells when compared to NMDA receptor mediated currents in neurons, suggesting that the density of these receptors is very low. Thus, it is possible that the summed response from a restricted group of synapses may simply fail to exceed detection threshold. This aspect of NG2 cell synaptic function may vary significantly between brain regions, as NMDA receptor-mediated currents have not been described in NG2 cells in gray matter and complex cells in the hippocampus lack NMDA receptor transcripts (see section 2.1). Future studies will be necessary to determine the extent of NMDA receptor expression among NG2 cells in different brain regions, the mechanisms that regulate their expression, and the physiological or pathological conditions under which they become activated.
Axo-glial synapses apparently share an exclusive reliance on AMPA receptors for transduction, but the properties of AMPA receptors that underlie these currents also vary among brain regions (see section 2.1). Although the functional implications of these differences have not been determined, Ca2+ influx through AMPA receptors following repetitive stimulation can trigger enhancement of synaptic currents in hippocampal NG2 cells (Ge et al., 2006), indicating that Ca2+ dependent mechanisms of potentiation also exist at axo-glial synapses.
3.3 Morphological evidence for direct neuron-NG2 cell synaptic junctions
Although there have been sporadic reports of synaptic-like or synaptoid contacts between axons and glial cells, anatomical studies have not described the consistent innervation of glial cells in the mature CNS. This discrepancy between physiological studies and the anatomical record may reflect difficulty in identifying the processes of NG2 cells in electron micrographs (EMs); indeed, it is only recently that NG2 cells were recognized as a distinct class of glial cells with unique ultrastructural characteristics (Peters, 2004; Reyners et al., 1982). It is also likely that these junctions evaded detection because they are relatively rare and less distinct than neuronal synapses. Nevertheless, EM-level analysis of physiologically-identified NG2 cells revealed the presence of anatomically distinct junctions between axons and NG2 cell membranes that exhibit many features of traditional synapses, including rigid alignment and consistent spacing of axonal and NG2 cell membranes over a restricted area, the presence of electron dense material in the space where these junctions are formed, and localization of small clear vesicles and mitochondria in region of the axon near the junction (Bergles et al., 2000; Lin et al., 2005; Ziskin et al., 2007). Moreover, confocal microscopic analysis has shown the accumulation of vesicular glutamate transporter 1 (VGLUT1)- and synaptophysin-immunoreactive puncta adjacent to the processes of NG2 cells in the corpus callosum, in regions where MAP2-immunoreactive dendrites were absent (Ziskin et al., 2007). These findings suggest that NG2 cells are a direct target of innervation, and support the hypothesis that AMPA receptor signalling occurs at discrete locations, rather than diffusely over the surface of these glial cells.
In accordance with these data, preliminary evidence suggests that AMPA receptors are not expressed uniformly over the surface of NG2 cells, but rather are clustered into discrete patches along their processes. Freeze-fracture immunolabeling of brain tissue from transgenic mice in which NG2 cells can be unambiguously identified through labeling with anti-GFP antibodies, show clusters of AMPA receptor-immunoreactive intramembrane particles along NG2 cell processes (Huck et al. 2008), and functional mapping of AMPA receptors using two-photon uncaging of MNI-L-glutamate has shown the presence of “hot spots” along NG2 cell processes where AMPA receptors are enriched (M. Paukert and Dwight Bergles, unpublished observations). Together, these anatomical studies indicate that rapid glutamatergic signalling between axons and NG2 cells occurs at bona fide synaptic junctions.
4. Functions and future studies
The transient activation of ionotropic receptors in NG2 cells at synapses provides one mechanism by which this population of cells can monitor ongoing activity in surrounding axons. The rapid kinetics of these events provide distinct advantages for following the timing of action potentials, rather than summed activity over extended periods, as would be more likely if metabotropic receptors were used for transduction. Moreover, this mechanism for communication has an inherently low threshold, as individual action potentials can induce quantal glutamate release, and spontaneous vesicular release occurs in the absence of activity. Several recent studies have shown that some NG2 cells in both white and gray matter can generate Na+-dependent spikes reminiscent of action potentials in response to current injection (Chittajallu et al., 2004; Karadottir et al., 2008; Ge et al., 2009), although the question of excitability within the NG2 cell population has not been thoroughly examined. If synaptic currents trigger spikes, they could promote Na+ influx, inhibit K+ channels and affect cellular development (Gallo et al., 1996). Such activity could also facilitate the activation of other voltage-gated channels to induce secretion of growth factors, neurotransmitters, or factors to influence the growth of axons. However, it is has not yet been shown that neuronal input under physiological conditions produces Ca2+ transients in these cells. NG2 cells possess several alternative pathways which might serve to induce intracellular Ca2+ transients, including Ca2+ permeable AMPA receptors (cf. 2.1) and voltage gated Ca2+ channels (Akopian et al., 1996), which at least in cultured cells can be activated through depolarizing GABAA receptor-mediated responses (Kirchhoff and Kettenmann, 1992). Combined electrophysiological and Ca2+ imaging analyses in situ will help address the contribution of ionotropic receptors to Ca2+ dynamics in NG2 cells.
As NG2 cell processes do not extend for more than 100 μm (Fig. 1), and these cells are not coupled via gap junctions (Bergles et al., 2000; Wallraff et al., 2004), the consequences of NG2 cell activation would be expected to manifest locally. At present, the incidence of Na+ spikes among NG2 cells in various brain regions at different times of development, the ability of synaptic inputs to induce these events, and the consequences of this excitability have not been determined.
Genetic fate mapping studies indicate that in white matter, a majority of NG2 cells differentiate into oligodendrocytes. In contrast, most NG2 cells in adult grey matter maintain their phenotype and only a minority differentiate into oligodendrocytes or astrocytes (Dimou et al., 2008; Rivers et al., 2008; Zhu et al., 2008). It is possible that synaptic signalling serves to alert white matter NG2 cells to the state of myelination of nearby axons, inducing proliferation or differentiation if certain patterns of activity are observed. The involvement of glutamatergic signalling in NG2 cell development is supported by in vitro studies (Gallo et al., 1996; Yuan et al., 1998), where glutamate receptor signalling also has been shown to influence the growth and migration of progenitors (Gudz et al., 2006). In addition, GABAA receptor activation has also been shown to influence NG2 cell migration in cultured tissue explants (Tong et al., 2009). Thus, it is possible that both glutamatergic and GABAergic synaptic input, by promoting an increase in [Ca2+]i, regulate the migration of NG2 cells during development and after brain injury.
It has been reported that NG2 cells have the capacity to differentiate into neurons and astrocytes in vitro when exposed to certain growth factors (Kondo and Raff, 2000). These findings raise the possibility that subpopulations of NG2 cells may act as multipotent progenitors with capacity for cell replacement. Further analysis using genetic techniques under both physiological and pathological conditions will help us to understand the potential of these cells to undergo further differentiation and to assess whether neuronal activity plays a role in this process.
Increasing evidence suggests that NG2 cells are physiologically and morphologically diverse (Chittajallu et al., 2004; Karadottir et al., 2008; Karram et al., 2008), and that only some of these cells may serve as progenitors. The development of new transgenic mouse lines in which fluorescent proteins (Karram et al., 2008; Ziskin et al., 2007) and Cre recombinase (Rivers et al., 2008; Zhu et al., 2008) are selectively expressed under control of the NG2 promoter will allow direct manipulation of NG2 cell properties and analysis of the outcomes in vivo. Such studies will undoubtedly help define the functions of these enigmatic glial cells under both physiological and pathological conditions.
Acknowledgments
Work of the authors is supported by Deutsche Forschungsgemeinschaft (grants SPP 1172 SE 774/3 to CS, SFB/TR3 C1, C3 to CS), the European Commission (FP7-202167 NeuroGLIA to CS), and the National Institutes of Health (NS051509 to DEB).
Abbreviations
- EGFP
enhanced green fluorescent protein
- EM
electron micrograph
- [Cl−]i
intracellular Cl− concentration
- OPC
oligodendrocyte precursor cell
- VGLUT
vesicular glutamate transporter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akopian G, Kressin K, Derouiche A, Steinhäuser C. Identified glial cells in the early postnatal mouse hippocampus display different types of Ca2+ currents. Glia. 1996;17:181–194. doi: 10.1002/(SICI)1098-1136(199607)17:3<181::AID-GLIA1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Bekar LK, Jabs R, Walz W. GABAa receptor agonists modulate K+ currents in adult hippocampal glial cells in situ. Glia. 1999;26:129–138. [PubMed] [Google Scholar]
- Berger T, Schnitzer J, Kettenmann H. Developmental changes in the membrane current pattern, K+ buffer capacity, and morphology of glial cells in the corpus callosum slices. J Neurosci. 1991;11:3008–3024. doi: 10.1523/JNEUROSCI.11-10-03008.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DE, Dzubay JA, Jahr CE. Glutamate transporter currents in Bergmann glial cells follow the time course of extrasynaptic glutamate. Proc Natl Acad Sci USA. 1997;94:14821–14825. doi: 10.1073/pnas.94.26.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DE, Roberts JDB, Somogyi P, Jahr CE. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187–191. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- Bordey A, Sontheimer H. Postnatal development of ionic currents in rat hippocampal astrocytes in situ. J Neurophysiol. 1997;78:461–477. doi: 10.1152/jn.1997.78.1.461. [DOI] [PubMed] [Google Scholar]
- Butt AM, Duncan A, Hornby MF, Kirvell SL, Hunter A, Levine JM, Berry M. Cells expressing the NG2 antigen contact nodes of Ranvier in adult CNS white matter. Glia. 1999;26:84–91. [PubMed] [Google Scholar]
- Butt AM, Kiff J, Hubbard P, Berry M. Synantocytes: New functions for novel NG2 expressing glia. J Neurocytol. 2002;31:551–565. doi: 10.1023/a:1025751900356. [DOI] [PubMed] [Google Scholar]
- Chittajallu R, Aguirre A, Gallo V. NG2-positive cells in the mouse white and grey matter display distinct physiological properties. J Physiol. 2004;561:109–122. doi: 10.1113/jphysiol.2004.074252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BA, Barbour B. Currents evoked in Bergmann glial cells by parallel fibre stimulation in rat cerebellar slices. J Physiol (Lond) 1997;502:335–350. doi: 10.1111/j.1469-7793.1997.335bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements JD, Lester RAJ, Tong G, Jahr CE, Westbrook GL. The time course of glutamate in the synaptic cleft. Science. 1992;258:1498–1501. doi: 10.1126/science.1359647. [DOI] [PubMed] [Google Scholar]
- Dawson MRL, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNs. Mol Cell Neurosci. 2003;24:476–488. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- Diamond JS, Jahr CE. Transporters buffer synaptically released glutamate on a submillisecond time scale. J Neurosci. 1997;17:4672–4687. doi: 10.1523/JNEUROSCI.17-12-04672.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimou L, Simon C, Kirchhoff F, Takebayashi H, Gotz M. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J Neurosci. 2008;28:10434–10442. doi: 10.1523/JNEUROSCI.2831-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V, Zhou JM, McBain CJ, Wright P, Knutson PL, Armstrong RC. Oligodendrocyte progenitor cell proliferation and lineage progression are regulated by glutamate receptor-mediated K+ channel block. J Neurosci. 1996;16:2659–2670. doi: 10.1523/JNEUROSCI.16-08-02659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge WP, Yang XJ, Zhang Z, Wang HK, Shen W, Deng QD, Duan S. Long-term potentiation of neuron-glia synapses mediated by Ca2+-permeable AMPA receptors. Science. 2006;312:1533–1537. doi: 10.1126/science.1124669. [DOI] [PubMed] [Google Scholar]
- Ge WP, Zhou W, Luo Q, Jan LY, Jan YN. Dividing glial cells maintain differentiated properties including complex morphology and functional synapses. PNAS. 2009;106:328–333. doi: 10.1073/pnas.0811353106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudz TI, Komuro H, Macklin WB. Glutamate stimulates oligodendrocyte progenitor migration mediated via an alphav integrin/myelin proteolipid protein complex. J Neurosci. 2006;26:2458–2466. doi: 10.1523/JNEUROSCI.4054-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JK, Phillips GR, Roth AD, Pedraza L, Shan W, Belkaid W, Mi S, Fex-Svenningsen A, Florens L, Yates JR, III, Colman DR. Glial membranes at the node of Ranvier prevent neurite outgrowth. Science. 2005;310:1813–1817. doi: 10.1126/science.1118313. [DOI] [PubMed] [Google Scholar]
- Huck JHJ, Kang SH, Roberts JD, Fukazawa Y, Shigemoto R, Bergles DE, Somogyi P. Clustering of AMPA type glutamate receptors on NG2 expressing glial cells and response to excitotoxic hippocampal lesion. 6th FENS Forum abstract; 2008. p. 216.6. [Google Scholar]
- Jabs R, Kirchhoff F, Kettenmann H, Steinhäuser C. Kainate activates Ca2+-permeable glutamate receptors and blocks voltage-gated K+ currents in glial cells of mouse hippocampal slices. Pflugers Arch. 1994;426:310–319. doi: 10.1007/BF00374787. [DOI] [PubMed] [Google Scholar]
- Jabs R, Pivneva T, Hüttmann K, Wyczynski A, Nolte C, Kettenmann H, Steinhäuser C. Synaptic transmission onto hippocampal glial cells with hGFAP promoter activity. J Cell Sci. 2005;118:3791–3803. doi: 10.1242/jcs.02515. [DOI] [PubMed] [Google Scholar]
- Karadottir R, Cavelier P, Bergersen LH, Attwell D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005;438:1162–1166. doi: 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadottir R, Hamilton NB, Bakiri Y, Attwell D. Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat Neurosci. 2008;11:450–456. doi: 10.1038/nn2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karram K, Goebbels S, Schwab M, Jennissen K, Seifert G, Steinhäuser C, Nave KA, Trotter J. NG2-expressing cells in the nervous system revealed by the NG2-EYFP-knockin mouse. Genesis. 2008;46:743–757. doi: 10.1002/dvg.20440. [DOI] [PubMed] [Google Scholar]
- Kirchhoff F, Kettenmann H. GABA triggers a [Ca2+]i increase in murine precursor cells of the oligodendrocyte lineage. Eur J Neurosci. 1992;4:1049–1058. doi: 10.1111/j.1460-9568.1992.tb00131.x. [DOI] [PubMed] [Google Scholar]
- Kondo T, Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 2000;289:1754–1757. doi: 10.1126/science.289.5485.1754. [DOI] [PubMed] [Google Scholar]
- Kressin K, Kuprijanova E, Jabs R, Seifert G, Steinhäuser C. Developmental regulation of Na+ and K+ conductances in glial cells of mouse hippocampal brain slices. Glia. 1995;15:173–187. doi: 10.1002/glia.440150210. [DOI] [PubMed] [Google Scholar]
- Kukley M, Capetillo-Zarate E, Dietrich D. Vesicular glutamate release from axons in white matter. Nat Neurosci. 2007;10:311–320. doi: 10.1038/nn1850. [DOI] [PubMed] [Google Scholar]
- Lin SC, Bergles DE. Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nat Neurosci. 2004;7:24–32. doi: 10.1038/nn1162. [DOI] [PubMed] [Google Scholar]
- Lin SC, Huck JH, Roberts JD, Macklin WB, Somogyi P, Bergles DE. Climbing Fiber Innervation of NG2-Expressing Glia in the Mammalian Cerebellum. Neuron. 2005;46:773–785. doi: 10.1016/j.neuron.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Mangin JM, Kunze A, Chittajallu R, Gallo V. Satellite NG2 Progenitor Cells Share Common Glutamatergic Inputs with Associated Interneurons in the Mouse Dentate Gyrus. J Neurosci. 2008;28:7610–7623. doi: 10.1523/JNEUROSCI.1355-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthias K, Kirchhoff F, Seifert G, Hüttmann K, Matyash M, Kettenmann H, Steinhäuser C. Segregated expression of AMPA-type glutamate receptors and glutamate transporters defines distinct astrocyte populations in the mouse hippocampus. J Neurosci. 2003;23:1750–1758. doi: 10.1523/JNEUROSCI.23-05-01750.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller J, Reyes-Haro D, Pivneva T, Nolte C, Schaette R, Lubke J, Kettenmann H. The principal neurons of the medial nucleus of the trapezoid body and NG2(+) glial cells receive coordinated excitatory synaptic input. J Gen Physiol. 2009;134:115–127. doi: 10.1085/jgp.200910194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Watanabe M, Yang Z, Bu J. Identity, distribution, and development of polydendrocytes: NG2-expressing glial cells. J Neurocytol. 2002;31:437–455. doi: 10.1023/a:1025783412651. [DOI] [PubMed] [Google Scholar]
- Papay R, Gaivin R, Jha A, McCune DF, McGrath JC, Rodrigo MC, Simpson PC, Doze VA, Perez DM. Localization of the mouse alpha1A-adrenergic receptor (AR) in the brain: alpha1AAR is expressed in neurons, GABAergic interneurons, and NG2 oligodendrocyte progenitors. J Comp Neurol. 2006;497:209–222. doi: 10.1002/cne.20992. [DOI] [PubMed] [Google Scholar]
- Papay R, Gaivin R, McCune DF, Rorabaugh BR, Macklin WB, McGrath JC, Perez DM. Mouse alpha1B-adrenergic receptor is expressed in neurons and NG2 oligodendrocytes. J Comp Neurol. 2004;478:1–10. doi: 10.1002/cne.20215. [DOI] [PubMed] [Google Scholar]
- Pastor A, Chvátal A, Syková E, Kettenmann H. Glycine- and GABA-activated currents in identified glial cells of the developing rat spinal cord slice. Eur J Neurosci. 1995;7:1188–1198. doi: 10.1111/j.1460-9568.1995.tb01109.x. [DOI] [PubMed] [Google Scholar]
- Paukert M, Bergles DE. Synaptic communication between neurons and NG2+ cells. Curr Opin Neurobiol. 2006;16:515–521. doi: 10.1016/j.conb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Peters A. A fourth type of neuroglial cell in the adult central nervous system. J Neurocytol. 2004;33:345–357. doi: 10.1023/B:NEUR.0000044195.64009.27. [DOI] [PubMed] [Google Scholar]
- Reyners H, Gianfelici dR, Maisin JR. The beta astrocyte: a newly recognized radiosensitive glial cell type in the cerebral cortex. J Neurocytol. 1982;11:967–983. doi: 10.1007/BF01148311. [DOI] [PubMed] [Google Scholar]
- Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;11:1392–1401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder W, Seifert G, Hüttmann K, Hinterkeuser S, Steinhäuser C. AMPA receptor-mediated modulation of inward rectifier K+ channels in astrocytes of mouse hippocampus. Mol Cell Neurosci. 2002;19:447–458. doi: 10.1006/mcne.2001.1080. [DOI] [PubMed] [Google Scholar]
- Seifert G, Hüttmann K, Schramm J, Steinhäuser C. Enhanced relative expression of glutamate receptor 1 flip AMPA receptor subunits in hippocampal astrocytes of epilepsy patients with Ammon’s horn sclerosis. J Neurosci. 2004;24:1996–2003. doi: 10.1523/JNEUROSCI.3904-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert G, Rehn L, Weber M, Steinhäuser C. AMPA receptor subunits expressed by single astrocytes in the juvenile mouse hippocampus. Mol Brain Res. 1997a;47:286–294. doi: 10.1016/s0169-328x(97)00059-4. [DOI] [PubMed] [Google Scholar]
- Seifert G, Steinhäuser C. Glial cells in the mouse hippocampus express AMPA receptors with an intermediate Ca2+ permeability. Eur J Neurosci. 1995;7:1872–1881. doi: 10.1111/j.1460-9568.1995.tb00708.x. [DOI] [PubMed] [Google Scholar]
- Seifert G, Weber M, Schramm J, Steinhäuser C. Changes in splice variant expression and subunit assembly of AMPA receptors during maturation of hippocampal astrocytes. Mol Cell Neurosci. 2003;22:248–258. doi: 10.1016/s1044-7431(03)00039-3. [DOI] [PubMed] [Google Scholar]
- Seifert G, Zhou M, Steinhäuser C. Analysis of AMPA receptor properties during postnatal development of mouse hippocampal astrocytes. J Neurophysiol. 1997b;78:2916–2923. doi: 10.1152/jn.1997.78.6.2916. [DOI] [PubMed] [Google Scholar]
- Steinhäuser C, Berger T, Frotscher M, Kettenmann H. Heterogeneity in the membrane current pattern of identified glial cells in the hippocampal slice. Eur J Neurosci. 1992;4:472–484. doi: 10.1111/j.1460-9568.1992.tb00897.x. [DOI] [PubMed] [Google Scholar]
- Steinhäuser C, Jabs R, Kettenmann H. Properties of GABA and glutamate responses in identified glial cells of the mouse hippocampal slice. Hippocampus. 1994;4:19–36. doi: 10.1002/hipo.450040105. [DOI] [PubMed] [Google Scholar]
- Tong XP, Li XY, Zhou B, Shen W, Zhang ZJ, Xu TL, Duan S. Ca2+ signaling evoked by activation of Na+ channels and Na+/Ca2+ exchangers is required for GABA-induced NG2 cell migration. J Cell Biol. 2009;186:113–128. doi: 10.1083/jcb.200811071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussell LO, Fischbach GD. Glutamate receptor desensitization and its role in synaptic transmission. Neuron. 1989;3:209–218. doi: 10.1016/0896-6273(89)90034-2. [DOI] [PubMed] [Google Scholar]
- Wallraff A, Odermatt B, Willecke K, Steinhäuser C. Distinct types of astroglial cells in the hippocampus differ in gap junction coupling. Glia. 2004;48:36–43. doi: 10.1002/glia.20040. [DOI] [PubMed] [Google Scholar]
- Yuan X, Eisen AM, McBain CJ, Gallo V. A role for glutamate and its receptors in the regulation of oligodendrocyte development in cerebellar tissue slices. Development. 1998;125:2901–2914. doi: 10.1242/dev.125.15.2901. [DOI] [PubMed] [Google Scholar]
- Zhou M, Schools GP, Kimelberg HK. Development of GLAST(+) astrocytes and NG2(+) glia in rat hippocampus CA1: Mature astrocytes are electrophysiologically passive. J Neurophysiol. 2006;95:134–143. doi: 10.1152/jn.00570.2005. [DOI] [PubMed] [Google Scholar]
- Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135:145–157. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]
- Ziskin JL, Nishiyama A, Rubio M, Fukaya M, Bergles DE. Vesicular release of glutamate from unmyelinated axons in white matter. Nat Neurosci. 2007;10:321–330. doi: 10.1038/nn1854. [DOI] [PMC free article] [PubMed] [Google Scholar]