Abstract
Phosphoinositide 3-kinase γ (PI3Kγ) is a critical mediator of directional cell movement. Here, we sought to characterize the role of PI3Kγ in mediating the different steps of PMN trafficking in the lung.
In a murine model of LPS-induced lung injury, PMN migration into the different lung compartments was determined in PI3Kγ gene-deficient (PI3Kγ−/−) and wildtype mice. Bone marrow chimeras were created to characterize the role of PI3Kγ on hematopoietic vs. non-hematopoietic cells. A small molecule PI3Kγ inhibitor was tested in vitro and in vivo.
PMN adhesion to the pulmonary endothelium and transendothelial migration into the lung interstitium was enhanced in PI3Kγ−/− mice. However, transepithelial migration into the alveolar space was reduced in these mice. When irradiated PI3Kγ−/− mice were reconstituted with bone marrow from wildtype mice, migratory activity into the alveolar space was restored partially. A small molecule PI3Kγ inhibitor reduced chemokine-induced PMN migration in vitro when PMNs or epithelial cells but not when endothelial cells were treated. The inhibitor also reduced LPS-induced PMN migration in vivo.
We conclude that PI3Kγ is required for transepithelial but not for transendothelial migration in LPS-induced lung injury. Inhibition of PI3Kγ activity may be effective at curbing excessive PMN infiltration in lung injury.
Keywords: Acute lung injury, chemotaxis, inflammation, Polymorphonuclear leukocytes, transmigration
Introduction
Recruitment of polymorphonuclear leukocytes (PMNs) to inflamed tissues is an essential requirement of the innate immune response but can lead to organ damage when excessive and uncontrolled. In the lung, excessive PMN infiltration can result in acute lung injury (ALI) and acute respiratory distress syndrome (ARDS), a condition that can follow pneumonia, acid aspiration, major trauma, or sepsis and causes approximately 75,000 deaths per year in the United States alone [1]. Depletion of PMNs curbs experimental lung damage [2] but is not desirable in most patients because it induces impaired host defense. Although the pathogenicity of PMNs in ALI/ARDS has been impressively demonstrated, molecular mechanisms underlying PMN trafficking in the lung remain poorly understood [3]. This may explain why, to this day, there is no strategy to modulate PMN infiltration in humans and no therapy available for ALI/ARDS beyond mechanical ventilation and other supportive approaches [4]. The mortality of ARDS remains high at 35–40% [5].
Pulmonary infiltration with inflammatory leukocytes is initiated by activation of circulating PMNs, resulting in altered mechanical properties and enhanced migratory activity [6]. Initial contact between PMNs and pulmonary endothelium requires adhesion molecules in some ARDS models [7], but not in others [8]. Once activated PMNs adhere to the pulmonary capillaries, additional steps are required to initiate transendothelial migration into the lung interstitium and transepithelial migration into the alveolar space, including activation of chemokine receptors and structural re-arrangement of adhesion molecules [9]. Cytoskeletal reorganization of PMNs, endothelial and epithelial cells is a prerequisite to facilitate directional movement of leukocytes to the lung. The family of class I phosphoinositide 3-kinases (PI3Ks) are isoforms of heterodimeric lipid-modifying proteins that are involved in the regulation of numerous cell functions including cell growth, proliferation, adhesion, motility and survival [10]. PI3Kγ is a class IB PI3K, consisting of a p110γ catalytic subunit and a 101-kD regulatory subunit (p101). PI3Kγ signaling is found downstream of different receptor types including G protein-coupled chemokine receptors. Activation of chemokine receptors leads to the release of the G protein subunit βγ that associates with the p110 adaptor protein and initiates translocation of PI3Kγ to the cell membrane where it mediates the phosphorylation of posphatidylinositol (PI) 3,4-bisphosphate to PI 3,4,5-trisphosphate [11]. PI 3,4,5-trisphosphate is an essential mediator of cell orientation and directional cell movement [12], thus making PI3Kγ a promising target in leukocyte-dependent inflammatory diseases [13].
Involvement of PI3Kγ in acute lung injury has been implicated but study results have been ambiguous. In a model of ventilator-induced lung injury, PI3Kγ gene-deficient (PI3Kγ−/−) mice exhibited improved lung mechanics and reduced formation of hyaline membranes while release of chemotactic cytokines in the lung was unaltered [14]. In the same model, others demonstrated an attenuation in the activation of nuclear factor-κB in inflammatory cells and a decrease in the release of inflammatory cytokines in mice pretreated with an non-selective PI3K inhibitor [15]. In contrast, PI3Kγ−/− mice were more susceptible to acute lung injury induced by intraperitoneal administration of Escherichia coli [16] or intratracheal application of pneumococcal virulence factor pneumolysin [17]. Pretreatment with the non-selective PI3K inhibitor wortmannin increased serum levels of proinflammatory cytokines and increased mortality in another sepsis model [18]. In different models of acute lung injury, PMN recruitment and infiltration into the lungs of PI3Kγ−/− mice was found to be attenuated [19], increased [16] or similar [17] to wildtype mice. It is important to recognize that in all these studies, single steps of PMN trafficking in the lung were not differentiated.
Functional expression of PI3Kγ in endothelial cells has recently been demonstrated and suggested to mediate selectin-dependent adhesion of leukocytes [19]. Whether PI3Kγ on pulmonary microvascular endothelial or epithelial cells is involved in adhesion or transmigration is unknown.
The current study was designed to elucidate the role of PI3Kγ for the different steps of PMN trafficking in the lung, i.e. recruitment from the peripheral blood and adherence to the pulmonary capillaries, transendothelial migration into the lung interstitium, and transepithelial migration into the alveolar space. We used gene-deficient mice and a selective small-molecule inhibitor to block PI3Kγ function in vitro and in vivo. We created bone marrow chimeras to study PI3Kγ effects on hematopoietic versus non-hematopoietic cells. Our results demonstrate a specific role of PI3Kγ in transepithelial neutrophil migration during acute lung injury that might help interpret conflicting results from previous studies.
Materials and Methods
Mice
Wild type male C57Bl/6 mice were obtained from Jackson Labs (Bar Harbor, ME). Breeder pairs of PI3Kγ gene deficient mice (PI3Kγ−/−, C57Bl/6 background) were provided by Dr. Dianqing Wu at the University of Connecticut. Mice were bred, and deletion of the p110 subunit of PI3Kγ was confirmed by PCR [20]. Wild type littermates (PI3Kγ+/+) served as control animals. All animal experiments were approved by the Animal Care and Use Committee of the University of Virginia. Mice were eight to twelve weeks of age.
Differential blood cell counts
Increased blood cell counts in gene deficient mice with targets that alter cell transmigration have been described [21] and will influence the analysis of migratory activity. To reveal possible differences between the different groups of mice, baseline differential blood counts were performed in PI3Kγ+/+ and PI3Kγ−/− mice using an automatic analyzer (Hemavet 850 FS, CDC Technologies).
Generation of chimeric mice
Chimeric mice were generated by transferring bone marrow (BM) between PI3Kγ+/+ and PI3Kγ−/− mice as described earlier [22]. Briefly, recipient mice were lethally irradiated in two doses of 600 rad each (separated by 4 hours). This regimen results in >90% donor-derived neutrophils at 6 weeks of reconstitution. Bone marrow from donor mice was harvested from both femora and tibiae, and ~5 million cells were injected intravenously into recipient mice. Bone marrow transplantation (BMT) was performed in four groups of mice: 1) BM from PI3Kγ−/− into PI3Kγ+/+ (chimeric mice express PI3Kγ on non-hematopoietic cells only), 2) BM from PI3Kγ+/+ into PI3Kγ−/− (chimeric mice express PI3Kγ on hematopoietic cells only), 3) BM from PI3Kγ−/− into PI3Kγ−/−, and 4) BM from PI3Kγ+/+ into PI3Kγ+/+. Mice in groups 3 and 4 served as negative and positive controls for possible radiation effects. Chimeric mice were used for experiments six weeks after BMT.
Small-molecule PI3Kγ inhibitor
We evaluated the small-molecule PI3Kγ inhibitor AS-605240 (5-quinoxalin-6-ylmethylene-thiazolidine-2,4-dione) (Merck Serono, Geneva, Switzerland) [23] for its efficiency to block PMN transmigration in vitro and in vivo. AS-605240 selectively inhibits PI3Kγ enzymatic activity, PI3Kγ-mediated downstream signaling and chemotaxis [23]. Stock solutions were prepared in 0.5% carboxymethyl cellulose (CMC) and 0.25% Tween20 in saline and used at indicated concentrations.
In vitro transendothelial migration
To test whether inhibition of neutrophil PI3Kγ is important in regulating migration, we conducted in vitro transmigration studies with PMNs and pulmonary endothelial cells (PEC) so that we could treat the cell types separately with AS-605240. PECs were harvested from wild type male C57Bl/6 mice using a positive immunomagnetic selection for CD31 (Mec 13.3) (EasySep ® Biotin Selection Kit, StemCell Technologies, Vancouver, BC, Canada). PECs were cultured in DMEM (D-valine instead of L-valine, Chemikon, Phillipsburg, NJ) with 10% of FCS, 20 mM HEPES, 1% penicillin and streptomycin (Invitrogen), and 50 μg/ml endothelial cell growth supplement (ECGS, Sigma). Purity of pulmonary endothelial cells was confirmed by staining for von Willebrand factor (Abcam, Cambridge, MA) and CD31 and their uptake of fluorescein isothiocyanate–labeled acetylated low-density lipoprotein (Biomedical Technologies Inc., Stoughton, MA). Magnetic immunoseparation yielded in a >90% pure endothelial cell culture. Endothelial cells were plated on fibronectin-coated filters in a Transwell system (6.5mm diameter, 3.0μm pore size, Corning Inc. Corning, NJ) and grown until confluent (72h). Medium was replaced with phenol-free DMEM with 1% FBS two hours before the experiment. Filters without endothelial cells served as negative controls.
PMNs from C57Bl/6 or PI3Kγ−/− mice were isolated from bone marrow using a three layer Percoll gradient (78, 66, and 54%) as described [9]. This technique yielded in a cell purity of > 90%. PMNs, endothelial cells, or both were incubated with AS-605240 at 15μM for 30 minutes. This concentration has been previously shown to significantly reduce MCP-1-induced migration of mouse monocytes [23]. Negative controls were treated with vehicle only (CMC 0.5% and Tween20 0.25% in saline). For the final 15 minutes, PMNs were labeled with calcein AM (5μM; Molecular Probes) and washed twice. Filters were moved to outer wells containing 400μl of phenol-free DMEM with or without CXCL2/3 (MIP-2, 200ng/ml, PeproTech Inc.). 2.5×105 PMNs were plated on filters with or without endothelial cells. Filters were incubated for 2 hours at 37 °C and fluorescence was measured in the bottom wells (excitation 485 nm; emission 530 nm).
In vitro transendothelial and transepithelial migration of human cells
PMNs from healthy donors were isolated by a two layer Percoll gradient (72% and 63%) as previously described [24]. The purity of the resulting cell population was greater than 95%. Human A549 pulmonary epithelial cells (American Type Culture Collection, Manassas, VA) were grown in RPMI containing 10% fetal bovine serum, 1% epithelial cell growth supplement, and 1% of penicillin/streptomycin solution. 100,000 epithelial cells were seeded on the collagen-coated undersurface of inverted Transwell filters and allowed to adhere for 2h at 37°C in a humidified 5% CO2 incubator. Non-adherent cells were removed, filters were moved to wells containing culture medium, and cells were incubated for 72h until a confluent monolayer was formed [25]. PMNs, A549 cells, or both were incubated with AS-605240 at 15μM for 30 minutes, and migratory activity was determined as described above. Negative controls were treated with vehicle only (CMC 0.5% and Tween20 0.25% in saline). In additional experiments, human pulmonary microvascular endothelial cells (HPMEC) (ScienCell Research Laboratories, Carlsbad, CA) were plated on fibronectin-coated filters in a Transwell system, and transmigration of human PMNs was assessed as described above.
Murine model of acute lung injury
Up to four mice were exposed to aerosolized LPS in a custom-built cylindrical chamber (20 × 9 cm) connected to an air nebulizer (MicroAir, Omron Healthcare, Vernon Hills, IL). LPS from Salmonella enteritidis (Sigma Co., St. Louis, MO) was dissolved in 0.9% saline (500μg/ml) and mice inhaled LPS for 30 minutes. As previously shown, this mimics several aspects of acute lung injury including PMN recruitment into all compartments of the lung, increase in vascular permeability [26], release of chemokines and disruption of the pulmonary architecture [27]. Control mice were exposed to saline aerosol.
In vivo inhibition of PI3Kγ
To evaluate PMN migration in vivo, wildtype and PI3Kγ−/− mice were intraperitoneally injected with AS-605240 one hour prior to LPS exposure. The inhibitor was used at a concentration of 50mg/kg as previously suggested [23]. Control mice received vehicle only (CMC 0.5% and Tween20 0.25% in saline).
PMN trafficking in the lung
PMN recruitment into the different compartments of the lung (pulmonary vasculature, interstitium, alveolar airspace) was assessed as described [26]. Briefly, 24 hours after LPS exposure (peak of LPS-induced accumulation of PMNs in the BAL), intravascular PMNs were labeled by an intravenous injection of Alexa 633-labeled GR-1. After 5 minutes, mice were euthanized and non-adherent PMN were removed from the pulmonary vasculature by flushing 10ml of PBS at 25 cmH2O through the spontaneously beating right ventricle.
Bronchoalveolar lavage fluid (BAL) was withdrawn and lungs were removed, minced and digested in the presence of excess unlabeled anti-GR-1 to prevent possible binding of the injected antibody to extravascular PMN. A cell suspension was prepared by passing the digested lungs through a 70 μm cell strainer (BD Falcon, Bedford, MA). Total cells in BAL and lung were counted and percentage of PMNs determined by flow cytometry. In the BAL, PMNs were identified by their typical appearance in the forward/sideward scatter and their expression of CD45 (clone 30-F11), 7/4 (clone 7/4), and GR-1 (clone RB6-8C5). In the lung, the expression of GR-1 was used to distinguish intravascular (CD45+7/4+GR-1+) from interstitial (CD45+7/4+GR-1−) PMNs, which were not reached by the injected antibody. In all experiments, isotype control antibodies were used to set the gates.
Cytospins of BAL
Cytospins of BAL from wildtype and PI3Kγ−/− mice harvested 24 hours after LPS exposure were prepared using a cytocentrifuge (Thermo Shandon). Cytospun cells were stained (Diff-Quick staining; IMEB Inc.), air dried, and coverslipped.
Pulmonary microvascular permeability
Pulmonary microvascular permeability in wildtype and PI3Kγ−/− mice was determined by measuring extravasation of Evans blue dye [28]. Evans blue (20mg/kg; Sigma-Aldrich) was injected intravenously 30 minutes prior to euthanasia. Lungs were perfused with cold PBS through the spontaneously beating right ventricle to remove intravascular dye. Lungs were removed and Evans blue was extracted as described [29]. The absorption of Evans blue was measured at 620 nm and corrected for the presence of heme pigments: A620 (corrected) = A620 − (1.426 × A740 + 0.030) [30]. Extravasated Evans blue was determined in the different animal groups 6 hours after LPS (peak of LPS-induced increase in microvascular permeability) or saline inhalation and calculated against a standard curve (micrograms Evans blue dye per gram lung). In additional experiments, wildtype mice were pretreated with AS-605240 (50mg/kg i.p.) and microvascular permeability was determined.
BAL protein
We measured LPS-induced accumulation of protein in the BAL of wildtype mice as an indicator of epithelial permeability. 6h after LPS, protein in the BAL was determined by a colorimetric method against a standard curve according to the manufacturer’s direction (bicinchoninic acid, BCA, Thermo Scientific, Rockford, IL). Some mice were pretreated with AS-605240 (50mg/kg i.p.).
Statistical analysis
Statistical analysis was performed with JMP Statistical Software (version 7.0, SAS Institute Inc., Cary, NC). Differences between the groups were evaluated by one way analysis of variance (ANOVA) followed by a post hoc Tukey test. Data were presented as mean ± SD and P < 0.05 was considered statistically significant.
Results
Blood counts
To reveal potential PMN count alterations in the PI3Kγ−/− mice, baseline differential blood counts were determined using an automatic analyzer. No differences in PMN counts were detected between wildtype and PI3Kγ−/− mice. However, monocyte counts were elevated in PI3Kγ−/− mice (0.6 ± 0.3 × 103/μl vs. 0.3 ± 0.2 × 103/μl, P < 0.05; Table 1).
Table 1.
Baseline cell counts
| PI3Kγ+/+ | PI3Kγ−/− | P | |
|---|---|---|---|
| Leukocytes | 8.8 ± 3.8 | 11.3 ± 4.9 | 0.22 |
| Neutrophils | 1.6 ± 0.6 | 2.2 ± 0.9 | 0.08 |
| Lymphocytes | 6.8 ± 3.2 | 8.7 ± 4.5 | 0.28 |
| Monocytes | 0.3 ± 0.2 | 0.6 ± 0.3 | <0.05 |
| Neutrophils % | 18.9 ± 4.2 | 19.5 ± 4.9 | 0.76 |
| Lymphocytes % | 76.4 ± 4.1 | 73.9 ± 4.9 | 0.23 |
| Monocytes % | 3.6 ± 1.8 | 5.1 ± 1.9 | 0.10 |
Baseline differential cell counts (103/μl) were performed in PI3Kγ +/+ and PI3Kγ −/− mice using an automatic analyzer. Data are mean ± SD of 8 mice.
PI3Kγ regulates transepithelial PMN transmigration into the lung
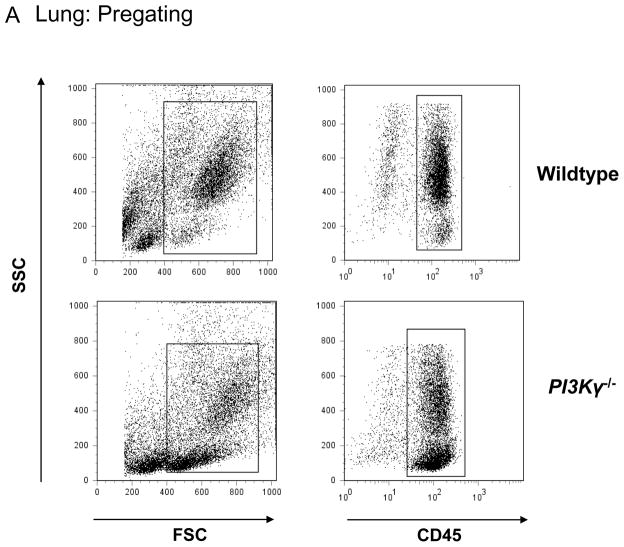
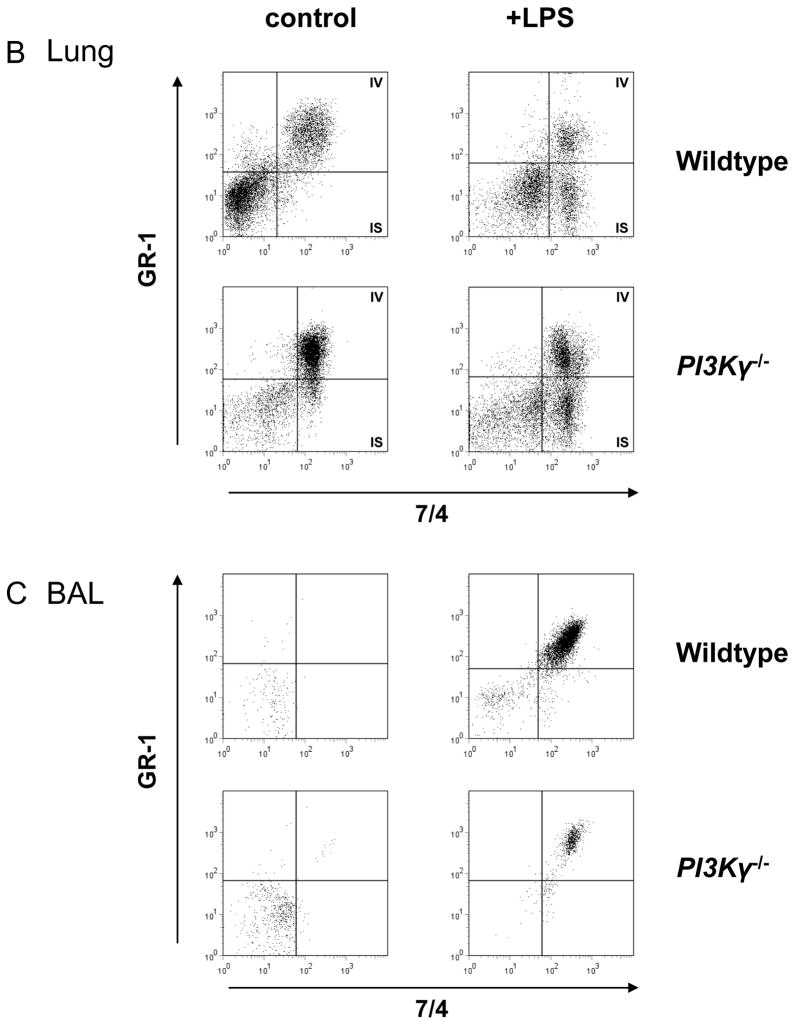
We used a flow cytometry-based method to detect PMNs in the different compartments of the lung of wildtype and PI3Kγ−/− mice. PMNs were identified by their typical appearance in the forward-side scatter and their expression of CD45 and 7/4 (Figure 1A). In the lung, we defined intravascular PMNs by their additional expression of GR-1+ (Figure 1B). In the BAL, all PMNs were identified by their expression of CD45, 7/4, and GR-1 (mAb added after harvesting) (Figure 1C). At baseline (no LPS), all PMNs in the lung were intravascular (Figure 1B left panels, 7/4+ and GR-1+, right upper square). LPS inhalation induced transendothelial migration into the lung interstitium as confirmed by the occurrence of GR-1− PMNs (Figure 1B, right panels, right lower square). In the BAL, no PMNs were detected at baseline (Figure 1C, left panels). Baseline PMN counts in lung interstitium and BAL did not differ between wildtype and PI3Kγ−/− mice, however PI3Kγ−/− mice demonstrated a higher PMN accumulation in the pulmonary microvasculature (Figure 2A).
Figure 1.
LPS-induced accumulation of PMNs in the different compartments of wildtype and PI3Kγ−/− mice at 24 hrs after LPS. PMNs are identified by their typical appearance in the forward-/sidescatter and their expression of CD45 and 7/4 (A). In the lung (B), only intravascular PMNs are also labeled by GR-1. In the BAL (C), all PMNs are identified by their expression of CD45, GR-1, and 7/4. IV intravascular, IS interstitial, representative plots from one experiment in each group.
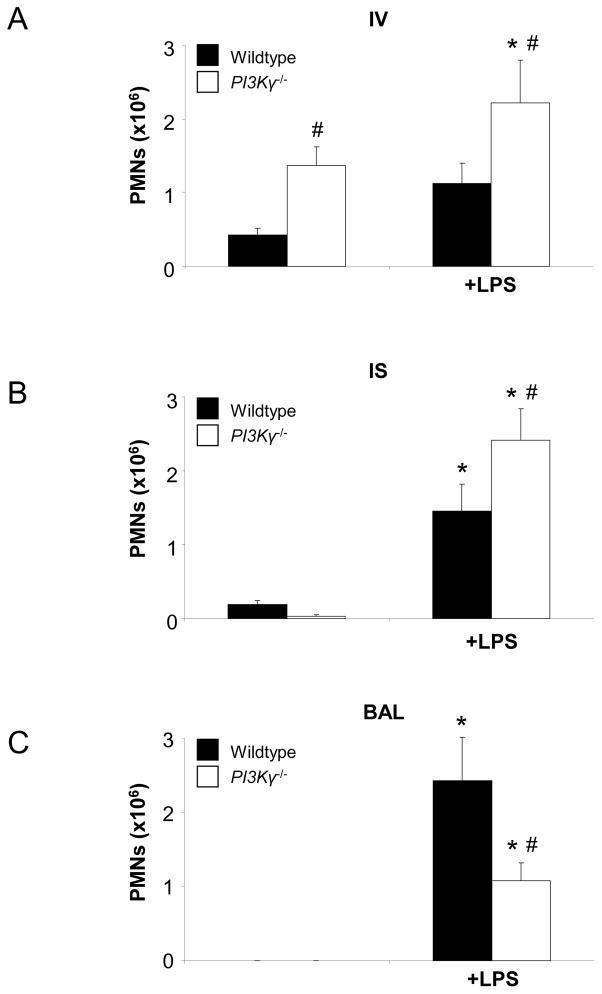
Figure 2.
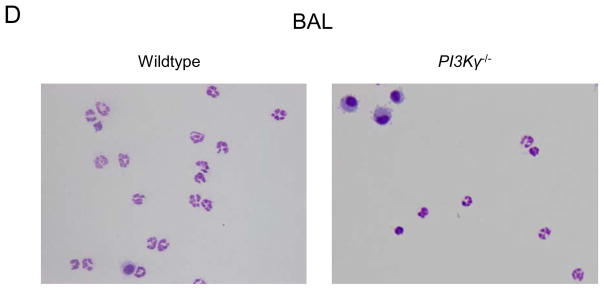
LPS-induced migration of PMNs into the different lung compartments of wildtype (black bars) and PI3Kγ−/− (white bars) mice. Accumulation of PMNs in the vasculature (IV) (A), the lung interstitium (IS) (B), and the bronchoalveolar space (BAL) (C) were analyzed. Cytospins of LPS-exposed BAL in wildtype and PI3Kγ−/− mice are shown to illustrate quantitative data (D). Values are means ± SD of n = 5 experiments. * P < 0.05 versus negative control without LPS, # P < 0.05 versus wildtype mice within the same treatment group (+/− LPS).
LPS inhalation induced significant PMN recruitment into all compartments of the lung of wildtype and PI3Kγ−/− mice (Figures 1 and 2). LPS-induced PMN accumulation in the pulmonary circulation (IV) was significantly higher in PI3Kγ−/− compared to wildtype mice at 24 hrs after LPS (2.2 ± 0.6 ×106 vs. 1.1 ± 0.3×106, P < 0.05, Figure 2A). In addition, PMN migration into the interstitium (IS) was significantly higher in PI3Kγ−/− mice (2.4 ± 0.4 ×106 vs. 1.5 ± 0.4×106, P < 0.05) (Figure 2B). Despite higher PMN counts in the lung tissue (IV + IS), PMN migration into the alveolar space (BAL, Figure 2C) was reduced in PI3Kγ−/− mice (1.1 ± 0.2 ×106 vs. 2.4 ± 0.6×106, P < 0.05), suggesting that in vivo, PI3Kγ−/− is required for transepithelial but not for transendothelial migration in the lung. Reduced PMN counts in the alveolar airspace of PI3Kγ−/− was confirmed by cytospin of BAL (Figure 2D).
PMN trafficking in chimeric mice
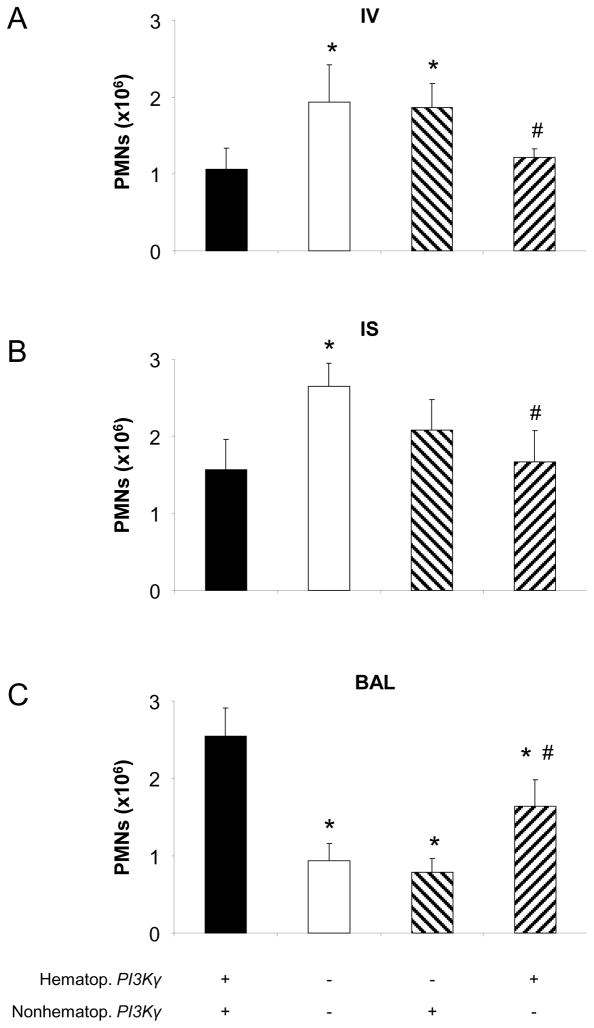
To characterize the role of PI3Kγ on hematopoietic and non-hematopoietic cells, we created chimeric mice by transferring BM between wildtype and PI3Kγ−/− mice. LPS-induced PMN migration in control mice that received BM from mice of the same genotype was similar to wildtype (positive control group) or PI3Kγ−/− (negative control group) mice, respectively (Figure 3). In mice that expressed PI3Kγ on non-hematopoietic cells only, transepithelial migration into the BAL was significantly reduced (0.8 ± 0.2 ×106 vs. 2.4 ± 0.5×106, P < 0.05, Figure 3C). The reduction was to a level similar to mice of the negative control group (BM of PI3Kγ−/− into PI3Kγ−/− mice; 0.8 ± 0.2 ×106 vs. 0.9 ± 0.2 ×106, NS). Consistent with a defect in transepithelial migration, intravascular and interstitial PMN counts were elevated in these mice (Figures 3A and B). It is possible that neutrophils get “backed up” in the intravascular and interstitial compartment when their transepithelial migration is impaired in PI3Kγ−/− mice. When PI3Kγ−/− mice were reconstituted with BM from wildtype mice, transepithelial migration was only partially restored (1.6 ± 0.3 ×106 vs. 2.4 ± 0.5×106 in mice that express PI3Kγ on all cells, P < 0.05, Figure 3C). Intravascular and interstitial PMN counts in PI3Kγ−/− mice reconstituted with BM from wildtype mice were not different from wildtype mice reconstituted with BM of wildtype mice, but significantly less than in mice of the negative control group or in PI3Kγ−/− mice. This finding supports the hypothesis that PI3Kγ on non-hematopoietic cells is involved in transepithelial migration of PMNs.
Figure 3.
Hematopoietic and non-hematopoietic cell PI3Kγ participation in LPS-induced PMN trafficking in the lung. Accumulation of PMNs in the vasculature (IV) (A), the lung interstitium (IS) (B), and the bronchoalveolar space (BAL) (C) were analyzed in chimeric mice (hatched bars). Values are means ± SD of n = 5 experiments. * P < 0.05 versus positive control (bone marrow transfer between wildtype mice, black bars), # P < 0.05 versus negative control (bone marrow transfer between PI3Kγ−/− mice, white bars)
AS-605240 inhibits in vitro transmigration
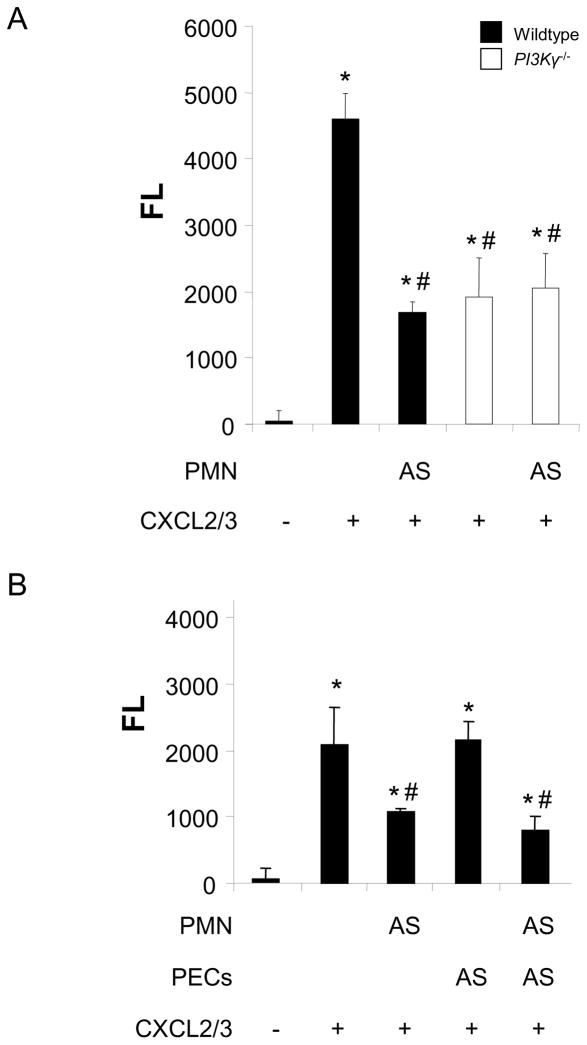
To evaluate its efficiency to inhibit chemokine-induced PMN migration in vitro, we incubated PMNs from wild type C57Bl/6 or PI3Kγ−/− mice with the small-molecule PI3Kγ inhibitor AS-605240 (15μM), and allowed them to migrate through a Transwell filter. Migratory activity of PI3Kγ−/− PMNs was significantly reduced compared with wildtype PMNs. AS-605240 reduced CXCL2/3-stimulated migration of wildtype but not of PI3Kγ−/− PMNs by more than 60% (P < 0.05 versus untreated control) (Figure 4A), confirming a specific effect of AS-605240 on this subtype of PI3K.
Figure 4.
The effect of the PI3Kγ inhibitor AS-605240 (AS) on chemokine-induced transmigration was evaluated in vitro. Transmigration across a Transwell filter alone (A) or a confluent layer of cultured murine PECs (B), human A549 cells (C), or human pulmonary microvascular endothelial cells (D) in a Transwell filter was significantly reduced when PMNs were pretreated with AS-605240. No effect was seen when PECs or human pulmonary microvascular endothelial cells were pretreated with the PI3Kγ inhibitor. However, blocking PI3Kγ in A549 cells reduced migration significantly. Data are mean ± SD from 3 separate experiments (each in duplicates). * P < 0.05 versus negative control without chemokine, # P < 0.05 versus positive control without inhibitor.
Next, we sought to determine the effect of AS-605240 on pulmonary endothelial cells versus PMNs. Pulmonary endothelial cells (PECs) were grown to confluence, and CXCL2/3-induced transendothelial PMN migration was measured. PMNs, PECs, or both cell types were pretreated with the PI3Kγ inhibitor as indicated.
CXCL2/3-stimulated migration through the endothelial layer was significantly reduced when PMNs were pretreated with AS-605240 (more than 50% reduction, P< 0.05 versus untreated control, Figure 4B). No effect was observed when PECs were pretreated with the PI3Kγ inhibitor. When both PMNs and PECs were pretreated simultaneously, migration was similar to wells in which only PMNs were pretreated, indicating that PI3Kγ in PMNs but not in endothelial cells is required for chemokine-induced endothelial transmigration (Figure 4B).
Our in vivo experiments implicated a distinct role of PI3Kγ for the transepithelial migration. We therefore hypothesized that blocking PI3Kγ in A549 cells would reduce transepithelial PMN migration in vitro. CXCL2/3-induced transepithelial migration was significantly reduced when PMNs were pretreated with AS-605240 similar to the transendothelial migration (46% reduction, P< 0.05 versus untreated control, Figure 4C). When PI3Kγ was blocked in A549 cells alone, PMN migration was reduced by 26% (P< 0.05 versus untreated control). This was in contrast to our findings with endothelial cells where blocking PI3Kγ did not affect migration and supports our hypothesis that epithelial PI3Kγ is involved in PMN trafficking in the lung. Blocking PI3Kγ in A549 cells and PMNs did not further decrease migration, indicating that PI3Kγ on PMNs limits PI3Kγ-dependent trafficking in our system. This is in line with our in vivo findings (Figure 3C).
To reveal potential species differences with respect to PI3K-dependent transmigration of PMNs, we repeated the in vitro transmigration assays with human pulmonary microvascular endothelial cells (HPMEC). In analogy to our findings with murine cells, inhibition of PI3K in HPMEC did not affect PMN migration (Figure 4D), suggesting that both species are comparable.
Effects of AS-605240 on in vivo transmigration
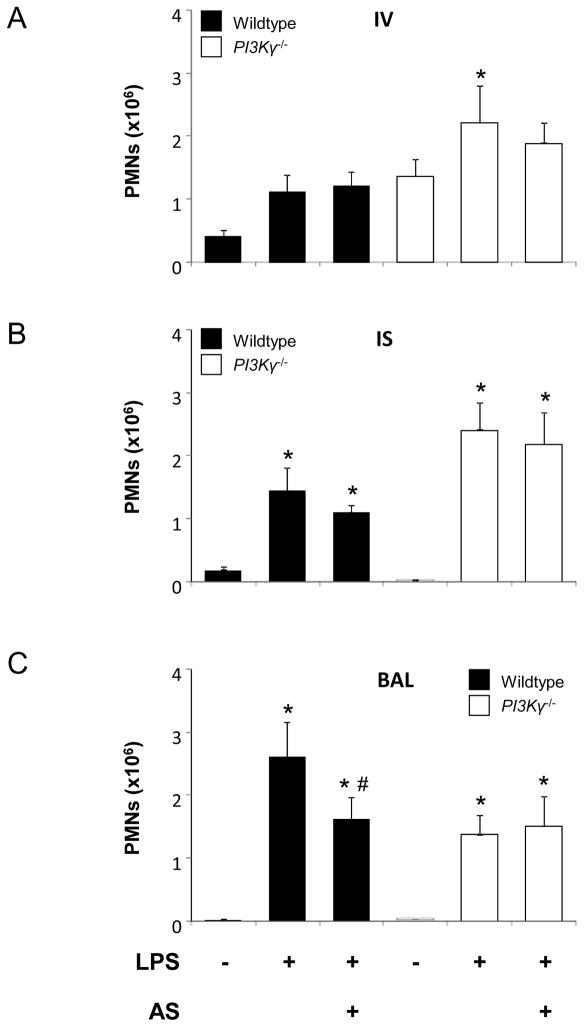
Next, we sought to determine the effects of AS-605240 (50 mg/kg) on LPS-induced PMN migration in vivo. Wildtype and PI3Kγ−/− mice received AS-605240 30 minutes prior to LPS exposure. After 24 hours, accumulation of PMNs in the different compartments of the lung was determined by flow cytometry. In wildtype mice, LPS-induced influx of PMNs into the BAL was significantly reduced by the pretreatment with AS-605240 (1.6 ± 0.3 ×106 vs. 2.6 ± 0.6×106, P < 0.05) (Figure 5). The inhibitor did not reduce recruitment of PMNs to the pulmonary vasculature or transendothelial migration into the interstitium. In addition, the inhibitor exhibited no effects on LPS-induced PMN migration in PI3Kγ−/− mice, supporting its specificity for PI3Kγ.
Figure 5.
Effect of the PI3Kγ inhibitor AS-605240 (AS) on LPS-induced PMN migration into the different compartments of the lung. Accumulation of PMNs in the vasculature (IV) (A), the lung interstitium (IS) (B), and the bronchoalveolar space (BAL) (C) of wildtype (black bars) and PI3Kγ−/− (white bars) mice were analyzed. Mice were pretreated 30 minutes prior to LPS exposure. AS-605240 significantly inhibited PMN migration in wildtype mice. No effect was seen in PI3Kγ−/− mice. Data are means ± SD of n = 5 experiments. * P < 0.05 versus negative control without LPS, # P < 0.05 versus LPS without PI3Kγ inhibitor.
Microvascular permeability and BAL protein
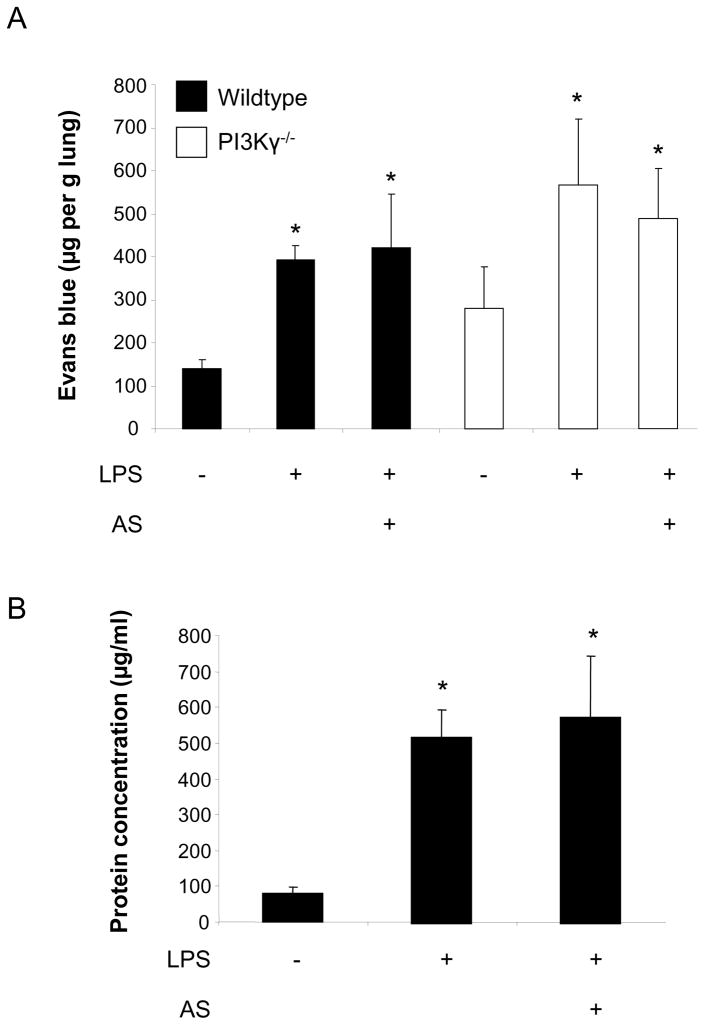
Disturbance of endothelial integrity and efflux of protein-rich fluid into the lung tissue is one of the critical events in the early development of ARDS that accompanies PMN infiltration. We therefore determined the role of PI3Kγ in LPS-induced microvascular permeability assessed by the extravasation of Evans blue and protein accumulation in the alveolar space as indicators of endothelial and epithelial permeability, respectively. LPS induced a significant increase in microvascular permeability in wildtype (394 ± 33 vs. 151 ± 11 μg/g lung, P < 0.05) and PI3Kγ−/− mice (568 ± 153 vs. 279 ± 97 μg/g lung, P < 0.05) (Figure 6A). Although both baseline and LPS-induced microvascular permeability tended to be higher in PI3Kγ−/− mice, differences were not significant. Pretreatment with AS-605240 did not prevent LPS-induced microvascular permeability in wildtype or PI3Kγ−/− mice. In addition, LPS-induced protein efflux into the alveolar space was not affected by inhibition of PI3Kγ (Figure 6B). This suggests a distinct role of PI3Kγ for cell trafficking in our model.
Figure 6.
LPS inhalation induced a significant increase in microvascular permeability in wildtype (394 ± 33 vs. 151 ± 11 μg/g lung, P < 0.05, black bars) and PI3Kγ−/− mice (568 ± 153 vs. 279 ± 97 μg/g lung, P < 0.05, white bars) as assessed by the extravasation of Evans blue (A). Pretreatment with AS-605240 did not reduce LPS-induced microvascular permeability in wildtype mice. In addition, inhibition of PI3Kγ did not affect LPS-induced protein efflux into the BAL (B). Data are mean ± SD from 4 experiments, * P < 0.05 versus negative control within the same group (wildtype or PI3Kγ−/− mice, resp.).
Discussion
The present study was designed to characterize the role of PI3Kγ in the distinct steps of PMN trafficking in the lung. In a murine model of ALI/ARDS, PI3Kγ was required for the transepithelial migration of PMNs from the lung interstitium into the alveolar airspace while adhesion to and migration through the pulmonary endothelium remained unaffected in the absence of PI3Kγ. Transmigration was mainly dependent on PI3Kγ on bone marrow-derived cells although PI3Kγ on non-hematopoietic cells contributed to the transepithelial migration of PMNs. The small molecule PI3Kγ inhibitor AS-605240 reduced PMN migration in vitro and PMN infiltration into the lung in vivo.
The key role of PI3Kγ in migration of leukocytes to inflamed tissue has led to several experimental studies that sought to identify the effects of PI3Kγ-involving pathways in acute lung injury, a disease that is largely characterized by the infiltration of inflammatory cells. Consistent with the hypothesis that inhibition of PI3Kγ attenuates ALI, Puri et al. found that LPS-induced PMN migration into the BAL was almost completely abolished in PI3Kγ−/− mice [19]. PI3Kγ−/− PMNs in the lungs exhibited diminished activation of nuclear factor-κB and expression of proinflammatory chemokines interleukin-1β and TNF-α [31]. Similar results were found in a model of ventilator-induced lung injury where blocking PI3K with a non-selective PI3K inhibitor reduced nuclear translocation of nuclear factor-κB and the release of interleukin-6 and macrophage inflammatory protein-2 in the lung [15]. In a similar model, PI3Kγ−/− mice exhibited less lung damage as assessed by respiratory mechanics and the formation of hyaline membranes [14]. In that study, effects of the PI3Kγ pathway were independent of the release of chemotactic chemokines, but the authors observed an increased apoptotic activity in pulmonary cells of PI3Kγ−/− mice while cell necrosis was reduced in these mice.
The role of the PI3K pathway in mediating apoptosis is well established [32]. However, the role of apoptosis in the pathophysiology of inflammatory diseases remains controversial. Increased apoptosis particular in lymphoid tissue contributes to immune suppression and organ failure that occurs during sepsis [33]. On the other hand, apoptosis, in contrast to necrosis, generally does not produce inflammation and tissue damage [34]. In lung injury, cell necrosis rather than apoptosis is associated with an inflammatory response and inversely correlates with lung function [35]. In addition, PI3Kγ-dependent pathways seem notably important for the integrity of the alveolar epithelium [36], consistent with our finding that PI3Kγ was mediating the epithelial but not endothelial barrier function in the lung. Bonnans et al. identified an endogenous PI3K inhibitory pathway that is initiated by the production of presqualene diphosphate (PSDP) [37]. In acid-induced lung injury, PSDP is suppressed and PI3Kγ activity increased. Consequently, pretreatment with a PSDP analog reduced acid-induced PMN infiltration and lung tissue damage.
However, beneficial effects of PI3Kγ inhibition in acute lung injury did not remain indisputable. Not inhibition, but activation of PI3K-dependent pathways were found to promote lung epithelial repair in vitro induced by Fas-induced apoptosis [38] or mechanical injury [39]. In Escherichia coli-induced sepsis, pulmonary PMN accumulation and microvascular permeability was pronounced in PI3Kγ−/− mice and associated with increased expression of CD47 and β3-integrins [16]. Consistent with our findings, the authors observed increased PMN counts in the lung interstitium by using morphometric analyses and suggested that upregulation of the CD47-associated β3-integrin complex led to increased adhesion of PMNs within the extracellular matrix and accumulation of PMNs in the lung interstitium. Transepithelial migration into the BAL was not determined in that study. In endotoxemic mice, non-specific PI3K inhibition led to a state of hypercoagulation, increased release of cytokines and, most notably, increased mortality [40]. In addition, anti-inflammatory effects of lipoic acid or glucan phosphate, both stimulating the PI3K pathway, were abolished when PI3K signaling was blocked [41], indicating that the PI3K pathway is a physiologic inhibitor of inflammation in endotoxemia and sepsis. In a model of S. pneumoniae-induced lung inflammation, bacterial clearance was significantly reduced when PI3K signaling was inhibited, most likely due to a defect in respiratory burst and insufficient production of reactive oxygen species [17]. In addition, PI3Kγ−/− mice failed to sufficiently recruit monocytes into the lung while PMN trafficking remained unaffected, confirming cell-specific effects of PI3K signaling observed by others [42].
The activation of multiple PI3K-dependent pathways with opposing effects might be one explanation for the apparent discrepancies seen in lung injury in different studies [43,40,14,16,17]. It is also important to mention that so far, the use of non-selective PI3K inhibitors such as wortmannin or LY294002 hampered the validation of the PI3Kγ pathway as a therapeutic target.
Signaling of endothelial PI3K is known to mediate cell migration, vascular permeability and angiogenesis [44,45] and has therefore been implicated as a promising target in various malignant diseases [46]. However, involvement of endothelial PI3Kγ in inflammatory responses has been controversial. PI3Kγ was not required for TNF-induced upregulation of nuclear factor-κB in human umbilical vein endothelial cells (HUVECs) [47]. Others, however, demonstrated PI3Kγ-dependent NF-κB binding to the ICAM-1 promoter in pulmonary microvascular endothelial cells that was required for static adhesion of PMNs [48]. Chemokine-induced leukocyte adhesion was reduced in PI3Kγ−/− mice and in lethally irradiated wildtype mice that had been reconstituted with bone marrow from PI3Kγ−/− mice [49]. Interestingly, impairment of adhesion was not as severe when PI3Kγ−/− deletion was confined to bone marrow-derived cells (50% vs. 80% reduction), underlining a contribution of nonleukocyte PI3Kγ. In our model, PI3Kγ-deficiency led to a three-fold increase in intravascular PMNs in the lungs. Several reasons may have contributed to this discrepancy: 1.) Smith et al. tested the role of PI3Kγ in P-selectin-dependent adhesion. In our model, adhesion to the pulmonary microcirculation is P-selectin-independent (unpublished observation). 2.) Cell trafficking in the systemic circulation differs substantially from the pulmonary microcirculation. Adhesion in the small pulmonary capillaries occurs largely independent of adhesion molecules and chemokines. 3.) Smith et al. found that PI3Kγ was essential to keep leukocytes attached to postcapillary venules within a period of 60 seconds. In the lung, PMNs reside for a much longer time before they are released back to the circulation or migrate into the lung (1–2 hours) [26]. Short time effects have not been investigated in the present study. 4.) It is important to recognize that accumulation of PMNs in the pulmonary circulation is directly related to the migratory activity of these cells. Reduced migration into the alveolar space will increase numbers of PMNs in upstream compartments, i.e. interstitium and intravascular space.
In addition, endothelial but not leukocyte PI3Kγ mediated TNFα-induced PMN adhesion to cremaster muscle venules, and nonleukocyte PI3Kγ contributed to LPS-induced migration of PMNs into the BAL [19]. E-selectin-mediated adhesion of PNMs to cremaster muscle venules was almost completely abolished when PI3Kγ was absent on endothelial cells [19]. Others confirmed a role for PI3Kγ in chemokine-induced PMN transmigration but did not observe PI3Kγ-dependent adhesion and rolling [50]. In the present study, compartmentalization of PMN trafficking in the lung revealed that adhesion to and transmigration through the pulmonary endothelium did not require PI3Kγ. Consistent with these findings, we found no effects when pulmonary endothelial cells were treated with AS-605240 in vitro. Although migratory activity was reduced when PI3Kγ was blocked in PMNs, the inhibitory effect of AS-605240 on neutrophil migration through an endothelial monolayer was comparable to that seen without monolayer. These studies indicate that pulmonary endothelial cells do not significantly contribute to PI3Kγ-mediatied PMN migration. In contrast, blocking PI3Kγ in human pulmonary epithelial cells significantly reduced PMN migration in an in vitro transmigration system (figure 4C). This supports our hypothesis of a distinct role of epithelial PI3Kγ in pulmonary leukocyte trafficking.
In addition, PI3Kγ−/− mice had significantly higher PMN counts in the intravascular space than wildtype mice (figures 1B and 2A). This increased availability of intravascular neutrophils may have contributed to increased migration of PMNs through the endothelial barrier in PI3Kγ−/− mice. However, transepithelial migration into the alveolar airspace was significantly reduced when PI3Kγ was absent. The defect was prominent in PI3Kγ−/− mice and remained when PI3Kγ function on leukocytes was restored. The mechanisms that link nonleukocyte PI3Kγ-signaling to the recruitment of inflammatory cells are not fully understood, but PI3K-dependent activation of adhesion molecules appears to be involved. In HUVECs, cytokine-induced expression of ICAM-1 and VCAM-1 involves PI3K-signaling [51]. Others, however, demonstrated that PI3K rather suppressed the expression of adhesion molecules on endothelial cells [52]. ICAM-1 is a critical mediator in LPS-induced lung injury [7]. It is worth mentioning that ICAM-1 on alveolar and bronchial epithelium significantly contributes to inflammatory leukocyte recruitment to the lung [53]. PI3K deletion may reduce epithelial ICAM-1 expression and result in disturbed transepithelial migration that has been observed in our study. Additional mechanisms of nonleukocyte PI3K-signaling in inflammation include activation of heat shock protein 70 [54] and release of reactive oxidant species [48].
In summary, our study reveals a differentiated role of PI3Kγ signaling in LPS-induced PMN trafficking in the lung. Our findings point to a specific role of PI3Kγ for the transepithelial migration into the alveolar space that involves PI3Kγ on non-hematopoietic cells. A small molecule PI3Kγ inhibitor effectively reduced PMN transmigration but did not reduce LPS-induced microvascular permeability. Further investigations are required to determine its therapeutic potential in acute lung injury.
Acknowledgments
This study was supported by the German Research Foundation (grant RE 1683/3-1 to J. Reutershan), by NIH grant HL73361 to K. Ley.
References
- 1.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 2.Abraham E, Carmody A, Shenkar R, Arcaroli J. Neutrophils as early immunologic effectors in hemorrhage- or endotoxemia-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1137–L1145. doi: 10.1152/ajplung.2000.279.6.L1137. [DOI] [PubMed] [Google Scholar]
- 3.Reutershan J, Ley K. Bench-to-bedside review: Acute respiratory distress syndrome - how neutrophils migrate into the lung. Crit Care. 2004;8:453–461. doi: 10.1186/cc2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 5.Rubenfeld GD, Herridge MS. Epidemiology and Outcomes of Acute Lung Injury. Chest. 2007;131:554–562. doi: 10.1378/chest.06-1976. [DOI] [PubMed] [Google Scholar]
- 6.Pallister I, Dent C, Topley N. Increased neutrophil migratory activity after major trauma: a factor in the etiology of acute respiratory distress syndrome? Crit Care Med. 2002;30:1717–1721. doi: 10.1097/00003246-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Basit A, Reutershan J, Morris MA, Solga M, Rose CE, Jr, Ley K. ICAM-1 and LFA-1 play critical roles in LPS-induced neutrophil recruitment into the alveolar space. Am J Physiol Lung Cell Mol Physiol. 2006;291:L200–L207. doi: 10.1152/ajplung.00346.2005. [DOI] [PubMed] [Google Scholar]
- 8.Andonegui G, Bonder CS, Green F, Mullaly SC, Zbytnuik L, Raharjo E, Kubes P. Endothelium-derived Toll-like receptor-4 is the key molecule in LPS-induced neutrophil sequestration into lungs. J Clin Invest. 2003;111:1011–1020. doi: 10.1172/JCI16510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reutershan J, Morris MA, Burcin TL, Smith DF, Chang D, Saprito MS, Ley K. Critical role of endothelial CXCR2 in LPS-induced neutrophil migration into the lung. J Clin Invest. 2006;116:695–702. doi: 10.1172/JCI27009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 11.Deane JA, Fruman DA. Phosphoinositide 3-kinase: diverse roles in immune cell activation. Annu Rev Immunol. 2004;22:563–598. doi: 10.1146/annurev.immunol.22.012703.104721. [DOI] [PubMed] [Google Scholar]
- 12.Hirsch E, Katanaev VL, Garlanda C, Azzolino O, Pirola L, Silengo L, Sozzani S, Mantovani A, Altruda F, Wymann MP. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science. 2000;287:1049–1053. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- 13.Ruckle T, Schwarz MK, Rommel C. PI3Kgamma inhibition: towards an ‘aspirin of the 21st century’? Nat Rev Drug Discov. 2006;5:903–918. doi: 10.1038/nrd2145. [DOI] [PubMed] [Google Scholar]
- 14.Lionetti V, Lisi A, Patrucco E, De GP, Milazzo MG, Ceci S, Wymann M, Lena A, Gremigni V, Fanelli V, Hirsch E, Ranieri VM. Lack of phosphoinositide 3-kinase-gamma attenuates ventilator-induced lung injury. Crit Care Med. 2006;34:134–141. doi: 10.1097/01.ccm.0000190909.70601.2c. [DOI] [PubMed] [Google Scholar]
- 15.Uhlig U, Fehrenbach H, Lachmann RA, Goldmann T, Lachmann B, Vollmer E, Uhlig S. Phosphoinositide 3-OH kinase inhibition prevents ventilation-induced lung cell activation. Am J Respir Crit Care Med. 2004;169:201–208. doi: 10.1164/rccm.200303-343OC. [DOI] [PubMed] [Google Scholar]
- 16.Ong E, Gao XP, Predescu D, Broman M, Malik AB. Role of phosphatidylinositol 3-kinase-gamma in mediating lung neutrophil sequestration and vascular injury induced by E. coli sepsis. Am J Physiol Lung Cell Mol Physiol. 2005;289:L1094–L1103. doi: 10.1152/ajplung.00179.2005. [DOI] [PubMed] [Google Scholar]
- 17.Maus UA, Backi M, Winter C, Srivastava M, Schwarz MK, Ruckle T, Paton JC, Briles D, Mack M, Welte T, Maus R, Bohle RM, Seeger W, Rommel C, Hirsch E, Lohmeyer J, Preissner KT. Importance of phosphoinositide 3-kinase gamma in the host defense against pneumococcal infection. Am J Respir Crit Care Med. 2007;175:958–966. doi: 10.1164/rccm.200610-1533OC. [DOI] [PubMed] [Google Scholar]
- 18.Williams DL, Li C, Ha T, Ozment-Skelton T, Kalbfleisch JH, Preiszner J, Brooks L, Breuel K, Schweitzer JB. Modulation of the phosphoinositide 3-kinase pathway alters innate resistance to polymicrobial sepsis. J Immunol. 2004;172:449–456. doi: 10.4049/jimmunol.172.1.449. [DOI] [PubMed] [Google Scholar]
- 19.Puri KD, Doggett TA, Huang CY, Douangpanya J, Hayflick JS, Turner M, Penninger J, Diacovo TG. The role of endothelial PI3K{gamma} activity in neutrophil trafficking. Blood. 2005;106:150–157. doi: 10.1182/blood-2005-01-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Z, Jiang H, Xie W, Zhang Z, Smrcka AV, Wu D. Roles of PLC-beta2 and -beta3 and PI3Kgamma in chemoattractant-mediated signal transduction. Science. 2000;287:1046–1049. doi: 10.1126/science.287.5455.1046. [DOI] [PubMed] [Google Scholar]
- 21.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Forlow SB, Schurr JR, Kolls JK, Bagby GJ, Schwarzenberger PO, Ley K. Increased granulopoiesis through interleukin-17 and granulocyte colony-stimulating factor in leukocyte adhesion molecule-deficient mice. Blood. 2001;98:3309–3314. doi: 10.1182/blood.v98.12.3309. [DOI] [PubMed] [Google Scholar]
- 23.Camps M, Ruckle T, Ji H, Ardissone V, Rintelen F, Shaw J, Ferrandi C, Chabert C, Gillieron C, Francon B, Martin T, Gretener D, Perrin D, Leroy D, Vitte PA, Hirsch E, Wymann MP, Cirillo R, Schwarz MK, Rommel C. Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med. 2005;11:936–943. doi: 10.1038/nm1284. [DOI] [PubMed] [Google Scholar]
- 24.Leavell KJ, Peterson MW, Gross TJ. Human neutrophil elastase abolishes interleukin-8 chemotactic activity. J Leukoc Biol. 1997;61:361–366. doi: 10.1002/jlb.61.3.361. [DOI] [PubMed] [Google Scholar]
- 25.Weppler A, Rowter D, Hermanns I, Kirkpatrick CJ, Issekutz AC. Modulation of endotoxin-induced neutrophil transendothelial migration by alveolar epithelium in a defined bilayer model. Exp Lung Res. 2006;32:455–482. doi: 10.1080/01902140601059463. [DOI] [PubMed] [Google Scholar]
- 26.Reutershan J, Basit A, Galkina EV, Ley K. Sequential recruitment of neutrophils into lung and bronchoalveolar lavage fluid in LPS-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2005;289:L807–L815. doi: 10.1152/ajplung.00477.2004. [DOI] [PubMed] [Google Scholar]
- 27.Reutershan J, Chang D, Hayes JK, Ley K. Protective Effects of Isoflurane Pretreatment in Endotoxin-induced Lung Injury. Anesthesiology. 2006;104:511–517. doi: 10.1097/00000542-200603000-00019. [DOI] [PubMed] [Google Scholar]
- 28.Green TP, Johnson DE, Marchessault RP, Gatto CW. Transvascular flux and tissue accrual of Evans blue: effects of endotoxin and histamine. J Lab Clin Med. 1988;111:173–183. [PubMed] [Google Scholar]
- 29.Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H, Pearse D, Tuder RM, Garcia JGN. Protective Effects of Sphingosine 1-Phosphate in Murine Endotoxin-induced Inflammatory Lung Injury. Am J Respir Crit Care Med. 2004;169:1245–1251. doi: 10.1164/rccm.200309-1258OC. [DOI] [PubMed] [Google Scholar]
- 30.Wang LF, Patel M, Razavi HM, Weicker S, Joseph MG, McCormack DG, Mehta S. Role of Inducible Nitric Oxide Synthase in Pulmonary Microvascular Protein Leak in Murine Sepsis. Am J Respir Crit Care Med. 2002;165:1634–1639. doi: 10.1164/rccm.2110017. [DOI] [PubMed] [Google Scholar]
- 31.Yum HK, Arcaroli J, Kupfner J, Shenkar R, Penninger JM, Sasaki T, Yang KY, Park JS, Abraham E. Involvement of phosphoinositide 3-kinases in neutrophil activation and the development of acute lung injury. J Immunol. 2001;167:6601–6608. doi: 10.4049/jimmunol.167.11.6601. [DOI] [PubMed] [Google Scholar]
- 32.Yang KY, Arcaroli J, Kupfner J, Pitts TM, Park JS, Strasshiem D, Perng RP, Abraham E. Involvement of phosphatidylinositol 3-kinase gamma in neutrophil apoptosis. Cell Signal. 2003;15:225–233. doi: 10.1016/s0898-6568(02)00063-3. [DOI] [PubMed] [Google Scholar]
- 33.Oberholzer C, Oberholzer A, Clare-Salzler M, Moldawer LL. Apoptosis in sepsis: a new target for therapeutic exploration. FASEB J. 2001;15:879–892. doi: 10.1096/fj.00-058rev. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz LM, Osborne BA. Programmed cell death, apoptosis and killer genes. Immunol Today. 1993;14:582–590. doi: 10.1016/0167-5699(93)90197-S. [DOI] [PubMed] [Google Scholar]
- 35.Fischer S, de PM, Liu M, Maclean AA, Cardella JA, Imai Y, Suga M, Keshavjee S. Interleukin 10 gene transfection of donor lungs ameliorates posttransplant cell death by a switch from cellular necrosis to apoptosis. J Thorac Cardiovasc Surg. 2003;126:1174–1180. doi: 10.1016/s0022-5223(03)00114-4. [DOI] [PubMed] [Google Scholar]
- 36.Xu D, Guthrie JR, Mabry S, Sack TM, Truog WE. Mitochondrial aldehyde dehydrogenase attenuates hyperoxia-induced cell death through activation of ERK/MAPK and PI3K-Akt pathways in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;291:L966–L975. doi: 10.1152/ajplung.00045.2006. [DOI] [PubMed] [Google Scholar]
- 37.Bonnans C, Fukunaga K, Keledjian R, Petasis NA, Levy BD. Regulation of phosphatidylinositol 3-kinase by polyisoprenyl phosphates in neutrophil-mediated tissue injury. J Exp Med. 2006;203:857–863. doi: 10.1084/jem.20052143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bao S, Wang Y, Sweeney P, Chaudhuri A, Doseff AI, Marsh CB, Knoell DL. Keratinocyte growth factor induces Akt kinase activity and inhibits Fas-mediated apoptosis in A549 lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;288:L36–L42. doi: 10.1152/ajplung.00309.2003. [DOI] [PubMed] [Google Scholar]
- 39.Lai JP, Dalton JT, Knoell DL. Phosphatase and tensin homologue deleted on chromosome ten (PTEN) as a molecular target in lung epithelial wound repair. Br J Pharmacol. 2007;152:1172–1184. doi: 10.1038/sj.bjp.0707501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schabbauer G, Tencati M, Pedersen B, Pawlinski R, Mackman N. PI3K-Akt pathway suppresses coagulation and inflammation in endotoxemic mice. Arterioscler Thromb Vasc Biol. 2004;24:1963–1969. doi: 10.1161/01.ATV.0000143096.15099.ce. [DOI] [PubMed] [Google Scholar]
- 41.Zhang WJ, Wei H, Hagen T, Frei B. Alpha-lipoic acid attenuates LPS-induced inflammatory responses by activating the phosphoinositide 3-kinase/Akt signaling pathway. Proc Natl Acad Sci U S A. 2007;104:4077–4082. doi: 10.1073/pnas.0700305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wrann CD, Tabriz NA, Barkhausen T, Klos A, van GM, Pape HC, Kendoff DO, Guo R, Ward PA, Krettek C, Riedemann NC. The phosphatidylinositol 3-kinase signaling pathway exerts protective effects during sepsis by controlling C5a-mediated activation of innate immune functions. J Immunol. 2007;178:5940–5948. doi: 10.4049/jimmunol.178.9.5940. [DOI] [PubMed] [Google Scholar]
- 43.Miyahara T, Hamanaka K, Weber DS, Drake DA, Anghelescu M, Parker JC. Phosphoinositide 3-kinase, Src, and Akt modulate acute ventilation-induced vascular permeability increases in mouse lungs. Am J Physiol Lung Cell Mol Physiol. 2007;293:L11–L21. doi: 10.1152/ajplung.00279.2005. [DOI] [PubMed] [Google Scholar]
- 44.Shiojima I, Walsh K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circ Res. 2002;90:1243–1250. doi: 10.1161/01.res.0000022200.71892.9f. [DOI] [PubMed] [Google Scholar]
- 45.Ispanovic E, Haas TL. JNK and PI3K differentially regulate MMP-2 and MT1-MMP mRNA and protein in response to actin cytoskeleton reorganization in endothelial cells. Am J Physiol Cell Physiol. 2006;291:C579–C588. doi: 10.1152/ajpcell.00300.2005. [DOI] [PubMed] [Google Scholar]
- 46.Maffucci T, Piccolo E, Cumashi A, Iezzi M, Riley AM, Saiardi A, Godage HY, Rossi C, Broggini M, Iacobelli S, Potter BV, Innocenti P, Falasca M. Inhibition of the phosphatidylinositol 3-kinase/Akt pathway by inositol pentakisphosphate results in antiangiogenic and antitumor effects. Cancer Res. 2005;65:8339–8349. doi: 10.1158/0008-5472.CAN-05-0121. [DOI] [PubMed] [Google Scholar]
- 47.Madge LA, Pober JS. A phosphatidylinositol 3-kinase/Akt pathway, activated by tumor necrosis factor or interleukin-1, inhibits apoptosis but does not activate NFkappaB in human endothelial cells. J Biol Chem. 2000;275:15458–15465. doi: 10.1074/jbc.M001237200. [DOI] [PubMed] [Google Scholar]
- 48.Frey RS, Gao X, Javaid K, Siddiqui SS, Rahman A, Malik AB. Phosphatidylinositol 3-kinase gamma signaling through protein kinase Czeta induces NADPH oxidase-mediated oxidant generation and NF-kappaB activation in endothelial cells. J Biol Chem. 2006;281:16128–16138. doi: 10.1074/jbc.M508810200. [DOI] [PubMed] [Google Scholar]
- 49.Smith DF, Deem TL, Bruce AC, Reutershan J, Wu D, Ley K. Leukocyte phosphoinositide-3 kinase {gamma} is required for chemokine-induced, sustained adhesion under flow in vivo. J Leukoc Biol. 2006;80:1491–1499. doi: 10.1189/jlb.0306227. [DOI] [PubMed] [Google Scholar]
- 50.Liu L, Puri KD, Penninger JM, Kubes P. Leukocyte PI3Kgamma and PI3Kdelta have temporally distinct roles for leukocyte recruitment in vivo. Blood. 2007;110:1191–1198. doi: 10.1182/blood-2006-11-060103. [DOI] [PubMed] [Google Scholar]
- 51.Min JK, Kim YM, Kim SW, Kwon MC, Kong YY, Hwang IK, Won MH, Rho J, Kwon YG. TNF-related activation-induced cytokine enhances leukocyte adhesiveness: induction of ICAM-1 and VCAM-1 via TNF receptor-associated factor and protein kinase C-dependent NF-kappaB activation in endothelial cells. J Immunol. 2005;175:531–540. doi: 10.4049/jimmunol.175.1.531. [DOI] [PubMed] [Google Scholar]
- 52.Wang JG, Mahmud SA, Nguyen J, Slungaard A. Thiocyanate-dependent induction of endothelial cell adhesion molecule expression by phagocyte peroxidases: a novel HOSCN-specific oxidant mechanism to amplify inflammation. J Immunol. 2006;177:8714–8722. doi: 10.4049/jimmunol.177.12.8714. [DOI] [PubMed] [Google Scholar]
- 53.Floreani AA, Wyatt TA, Stoner J, Sanderson SD, Thompson EG, Allen-Gipson D, Heires AJ. Smoke and C5a induce airway epithelial intercellular adhesion molecule-1 and cell adhesion. Am J Respir Cell Mol Biol. 2003;29:472–482. doi: 10.1165/rcmb.2002-0143OC. [DOI] [PubMed] [Google Scholar]
- 54.Rafiee P, Theriot ME, Nelson VM, Heidemann J, Kanaa Y, Horowitz SA, Rogaczewski A, Johnson CP, Ali I, Shaker R, Binion DG. Human esophageal microvascular endothelial cells respond to acidic pH stress by PI3K/AKT and p38 MAPK-regulated induction of Hsp70 and Hsp27. Am J Physiol Cell Physiol. 2006;291:C931–C945. doi: 10.1152/ajpcell.00474.2005. [DOI] [PubMed] [Google Scholar]