Abstract
Background and Aim
The mechanism of action of bisacodyl in the unprepared human colon is unclear. Our aim was to evaluate the effect of oral bisacodyl on the overall and regional colonic transit in humans. \
Methods
In a double-blind, randomized, placebo-controlled study of 25 healthy participants, effects of oral bisacodyl (5mg p.o. per day) and placebo on colonic transit were compared. Validated scintigraphy using 111In-charcoal delivered to the ileocolonic region in a delayed-release capsule was used to measure colonic transit. The primary transit endpoints, ascending colon emptying (AC) t1/2 and geometric center (GC) of colon isotope at 24 hours (overall transit), were compared (Wilcoxon rank sum test).
Results
There were significant treatment effects on AC t1/2, with the bisacodyl group demonstrating accelerated emptying [median 6.5 h, interquartile range (IQR) 5.0 – 8.0 h] relative to the placebo group [11.0 h (7.0 – 17.1)], P=0.03]. Numerical differences in colonic GC 24 hours [bisacodyl median 3.0 (2.2 – 3.8), placebo 4.0 (3.1 – 4.6)] were not significant (P=0.19). There were no significant differences observed in GC 4 hours.
Conclusion
Oral 5mg bisacodyl accelerates AC in the unprepared colon in healthy adults; this action may contribute to the drug’s efficacy in constipation.
Keywords: bisacodyl, ascending colon emptying, overall colonic transit
INTRODUCTION
Bisacodyl is a diphenyl methane laxative that has been used for the treatment of constipation since its introduction into the market in 1952 and, more recently, in facilitating bowel evacuation before investigational procedures (e.g. colonoscopy, barium enema) or surgery. Its action appears to be mainly in the large intestine, and it is usually effective within 6 to 12 hours following administration by mouth and within 15 to 60 minutes following rectal administration.
The Physician’s Desk Reference1 indicates that, for constipation, bisacodyl is given in usual doses of 5 to 10 mg daily as enteric-coated tablets administered at night or 10 mg as a suppository or enema administered in the morning. Doses of 10 to 20 mg are given by mouth for complete bowel evacuation, followed by 10 mg as a suppository the next morning.
After both oral and rectal administration in humans, bisacodyl is hydrolyzed by intestinal and bacterial enzymes to a deacetylated active metabolite which is responsible for the laxative action. Absorption from the gastrointestinal tract is minimal with enteric-coated tablets or suppositories; the small amount that is absorbed is excreted in the urine as the glucuronide. Bisacodyl is mainly excreted in the feces. In fact, Roth et al.2 have shown that only 16% of the orally administered formulation is absorbed, the laxative effect started 7.7±1.7 hours after intake, and there was no apparent relationship between laxative effect and plasma level. Rectal suppository administration of bisacodyl resulted in undetectable or low plasma levels, despite prompt laxative effect after 20±10 minutes. Flig et al.3 confirmed these findings and concluded that the laxative effect of the suppository results from a local effect in the rectum. The rapidity of this action of bisacodyl suggests that an effect on small intestinal secretion is unlikely to be the only mechanism that leads to laxation.
Thus, bisacodyl’s laxative action is based on two modes of action: stimulation of myoelectrical and motor activity in the colon4–7 and stimulation of intestinal secretion.2 However, the reported effects on motor functions focused on the prepared or cleansed distal colon, except for a study which evaluated the transit of a single metallic marker through the entire colon7 and a study using radiopaque markers which was compromised by a low number of radiographs.8 The mechanism of action of oral bisacodyl in the unprepared colon, particularly the proximal or ascending colon, is unclear.
The aim of this study was to compare the effects of bisacodyl, 5 mg p.o., and placebo on overall colonic transit and the emptying time of the ascending colon.
METHODS
Trial Design and Participants
We conducted a randomized, double-blind, placebo-controlled study of the effects of bisacodyl, 5 mg p.o., on colonic transit in 25 healthy male and non-pregnant, non-breastfeeding female participants, aged 18–52 years old. The study was approved by Mayo Clinic Institutional Review Board, and all participants signed informed consent. Participants were allowed to continue low, stable doses of thyroid replacement, estrogen replacement, and birth control pills or depot estrogen injection.
Exclusion criteria included the use of medications within 48 hours of dose initiation, structural or metabolic diseases/conditions that affect the gastrointestinal system, and functional gastrointestinal disorders. Participants with previous abdominal surgery other than appendectomy, cholecystectomy, hysterectomy, or hernia repair, or other known illnesses such as diabetes, cardiovascular or lung disease were excluded. For screening, the shortened version of the Bowel Disease Questionnaire9 was used to exclude subjects with dyspepsia, irritable bowel syndrome, or significant gastrointestinal symptoms. Of the 16 questions, participants had to have 3 or less positive responses (ranked as mild to worst) to be eligible to participate. Subjects who had participated in another clinical study within the prior 30 days were ineligible.
Colonic Transit Measurements
To evaluate colonic transit, an adaptation of our established scintigraphic methods was used.10–12 Briefly, 111In adsorbed on activated charcoal particles were delivered to the colon by a methacrylate-coated, delayed-release capsule.12 All participants ingested the capsule containing 111In charcoal at 6:00 a.m. after an overnight fast (last meal prior to 9:00 p.m. on the previous day). When the capsule had been demonstrated to have emptied from the stomach (with images obtained every 30 minutes while the patient remained fasting), all participants received the study medication and standard breakfast (218 kcal egg meal) at time 0 and two standardized meals at 4 hours (530 kcal chicken meal) and 8 hours (750 kcal steak dinner) after the medication was administered.
A variable region of interest program was used to measure transit, as in previous studies.10–12 Time 0 was the time of ingestion of the study medication. We obtained abdominal images every 30 minutes for the first 10 hours after administration of the study medication, with scans being increased to every 15 minutes for the first 2 hours after meals. A final scan was obtained 24 hours after ingestion of the study medication. The performance characteristics of this test are summarized elsewhere.13
Transit Data Analysis
The geometric center (GC) is the weighted average of counts in the different colonic regions [ascending colon (AC), transverse colon (TC), descending colon (DC), rectosigmoid (RS)] and stool, respectively 1 to 5. Thus, at any time, the proportion of colonic counts in each colonic region is multiplied by its weighting factor as follows: (%AC × 1 + %TC × 2 + %DC × 3 + %RS × 4 + %stool × 5)/100 = GC. Thus, a higher GC reflects a faster colonic transit.
AC emptying was summarized by the t1/2 calculated by linear interpolation of values on the AC emptying curve. The demonstration of either acceleration or delay of transit (using the same assessment parameters) appears to predict the efficacy of the respective agents on bowel dysfunction in prior studies with prucalopride, tegaserod, alosetron, and recombinant human neurotrophin 3.14–17 The primary efficacy parameters were AC t1/2 and GC at 24 hours. Secondary endpoint was GC at 4 hours.
Statistical Analysis
Data are expressed as the median and interquartile range (IQR), with full range and outliers shown on box and whisker plots. Wilcoxon Rank Sum Test was used to compare transit profiles in response to bisacodyl and placebo (SigmaStat, SPSS, Chicago, IL, USA). P value <0.05 was deemed significant.
Sample Size Assessment
The primary assessment measures (GC at 4 and 24 hours and AC t1/2) have coefficients of variation [(CV) %] of 44%, 40%, and 51%, respectively, based on data for healthy volunteers studied using the previous methods in the same laboratory (Table 1).14–17 The table indicates the ‘effect sizes’ (difference in mean values as a percentage of the overall groups mean value) that could be detected with 80% power at an α level of 0.05 using a simple two-sample z-test between the two groups (N=12 per group).
Table 1.
Statistical power atatement
| Colonic Transit Response Variable | CV (%) in Healthy Subjects | Effect Size (%) Detectable with 80% Power (n = 12/group) |
|---|---|---|
| Geometric Center (at 4 hours) | 44% | 50% |
| Geometric Center (at 24 hours) | 40% | 46% |
| Ascending Colon T1/2 | 51% | 58% |
CV, coefficients of variation.
RESULTS
Participants
All participants completed the study. As a result of randomization, 13 subjects received the placebo and 12 subjects received bisacodyl. Mean age of the bisacodyl group was 34.1±3.8 years old, and that of placebo group was 30.9±2.0 years old. The female to male ratio of the bisacodyl group was 7/6 and that of placebo group was 7/5. The mean body mass index of the bisacodyl group was 26.8±1.2 kg/m2 and that of the placebo group was 26.4±1.3 kg/m2. There was no significant difference in these parameters between the two groups.
Effect of Bisacodyl on Overall Colonic Transit
The colonic GC at 4 hours of the placebo group was 1.0 (IQR; 1.0–1.2), and that of bisacodyl group was 1.2 (IQR; 1.0–1.2). There was no significant difference between the two groups (Figure 1). The colonic GC at 24 hours of placebo group was 3.0 (IQR; 2.2 – 3.8), and that of bisacodyl group was 4.0 [(IQR; 3.1–4.6), P = 0.19, Figure 1].
Figure 1.
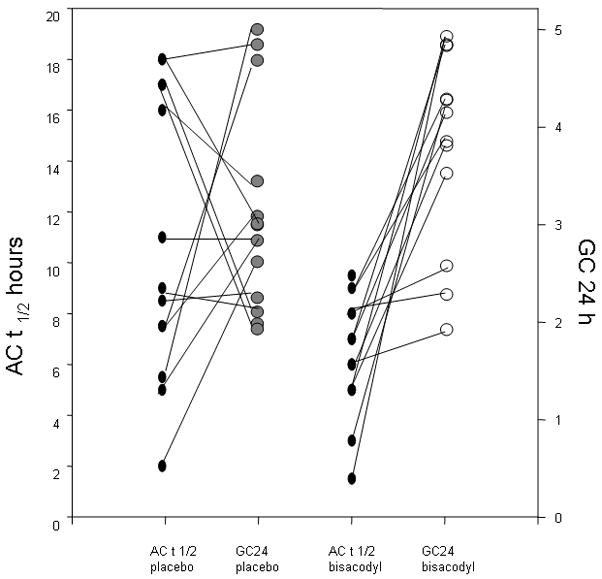
Effect of bisacodyl on ascending colon emptying t1/2 and on overall colonic transit at 24 hours. The bisacodyl group had significantly accelerated ascending colon emptying compared to the placebo group (p=0.03). Note that, in response to placebo, the progression from ascending colon to overall transit at 24 hours is highly variable; in contrast, there is a consistent progression for ascending colon to overall colonic transit in the bisacodyl group in all but three participants. Thus, although overall colonic geometric center was not significant in bisacodyl vs. placebo (p=0.19), 9 of the 12 participants appear to show substantial progression from ascending to overall colonic transit.
Effect of Bisacodyl on Ascending Colon Emptying
Figure 2 shows examples of sequential scintiscans in participants randomized to bisacodyl or placebo. The AC t1/2 was 11.0 (IQR; 7.0 – 17.1) hours in the placebo group, and that of the bisacodyl group was 6.5 [(IQR; 5.0 – 8.0), Figure 1]. The bisacodyl group had significantly accelerated AC emptying compared to the placebo group (P = 0.03). Figure 1 also shows the progression for each participant from ascending colon emptying t1/2 (where lower numbers reflect faster emptying) to overall colonic geometric center at 24 hours (where higher numbers reflect faster transit). The figure shows erratic progression with placebo. In contrast, for all except 3 participants receiving bisacodyl, there appears to be a relatively fast ascending colon emptying t1/2, but there is relatively little effect on overall colonic transit.
Figure 2.
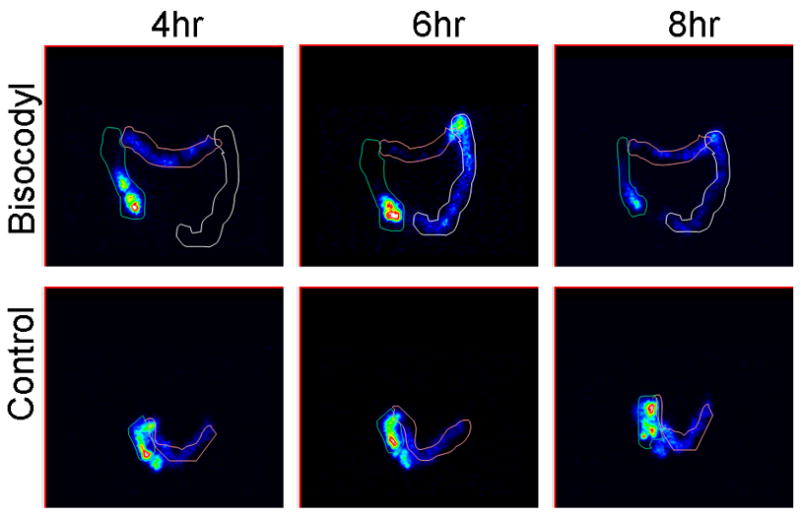
Colonic transit scintiscans at 4, 6 and 8 hours in patients receiving bisacodyl or placebo. Intensity of image reflects the concentration of counts in each region. Variable regions of interest are drawn around isotope in ascending, transverse, and descending regions. Note that bisacodyl accelerates ascending colon emptying relative to placebo.
DISCUSSION
This double-blind, randomized, placebo-controlled study has demonstrated that low-dose oral bisacodyl accelerates emptying of the unprepared ascending colon in healthy subjects.
Bisacodyl has been used as a first-line laxative for many years, and clinical experience suggests that it is effective, yet only a few controlled studies are available to examine its mechanism of action.18,19 Previous studies showed that bisacodyl was a hydragogue, that is, it induced intestinal water secretion.20 Bisacodyl stimulates other mechanisms that may result in laxation and secretion, including stimulation of nitric oxide synthase,21 mucin secretion22 and increasing mucosal permeability. 23 Other agents that cause intestinal secretion, such as lubiprostone and linaclotide, also accelerate colonic transit.24,25
There is also evidence that bisacodyl acts locally in the large bowel by directly enhancing motility, reducing overall colonic transit7,26,27 and increasing the water content of the stool.28 However, there are few studies to evaluate its mechanisms under the more physiological condition in the unprepared colon. Ewe et al.7 demonstrated impressive acceleration of overall colonic transit of radiopaque markers [7±8 (SD) hours for bisacodyl versus 31±14 hours for controls). Bisacodyl, 10 mg per day for three days, also accelerated overall colonic transit.29 However, it is unclear from prior studies whether this was due to effects on proximal or distal colonic transit.
In general, noninvasive techniques are required to evaluate regional colonic transit in greater detail. Radiopaque marker techniques, widely used for colonic transit assessments by individual images at 4 or 5 days after marker ingestion, would require high radiation doses to assess AC emptying. Furthermore, larger non-digestible markers may be less physiological when compared with the 1–2 mm or smaller markers used in scintigraphy.30
Significantly accelerated emptying of the unprepared proximal colon with 5 mg bisacodyl p.o. is likely to be relevant to the mechanism of the drug’s benefit in inducing laxation. Thus, the significant acceleration of AC emptying may reduce the time for fluid absorption in a colonic region that is known for its high capacity for fluid absorption.31 Acceleration of AC emptying may change stool consistency, complementing the secretory effects of the medication. Ratnaike et al.32 demonstrated that bisacodyl impairs fluid absorption by activating adenylate cyclase in enterocytes, increasing cyclic AMP, and causing active secretion of Cl− and HCO3−, passive efflux of Na+, K+ and water and inhibition of Na+ and Cl− absorption into the enterocyte. It is also reasonable to hypothesize that the effects of bisacodyl on small intestinal and colonic secretion of water and electrolytes contribute to accelerated emptying of the AC or colonic transit, as observed with other secretory agents activating chloride channels (lubiprostone)33 or the guanylate cyclase–C receptor that induces chloride secretion through the cystic fibrosis transmembrane regulator.24
Another example is carcinoid diarrhea that causes both secretory and motor diarrhea.33 The effects of locally delivered bisacodyl on motor functions have been demonstrated in the left or distal colon. Bisacodyl may act locally in the AC to directly enhance proximal colonic motility. Kamm et al.6 infused 5.5 mg bisacodyl into the splenic flexure and documented the induction of high amplitude propagated contractions in the distal colon. Bassotti et al.27 investigated the effect of a 10 mg bisacodyl solution and found that about 90% of patients with slow transit constipation showed motor response characterized by one or more high amplitude propagated contractions. However, motor effects in the proximal colon were not previously documented.
The current study revealed that oral bisacodyl, 5 mg, did not significantly accelerate overall colonic transit, which is not consistent with previous studies.27,34 Two possible explanations are that, in previous studies,27,34 bisacodyl was administered directly to the distal colon and doses of bisacodyl were higher than the 5 mg used in this study. It is conceivable that oral bisacodyl, 5 mg, did not reach sufficient levels through the colon to affect overall colonic transit. A medication delivery system that releases the bisacodyl to the colon in a single bolus without dilution during its transit through the small intestine may accelerate overall or distal colonic transit.
We considered the possibility of a type II error due to the relatively small number of subjects (n=12) treated with bisacodyl. However, the observed pooled CV for GC at 24 hours was 31%, which was lower than the 40% used to estimate the required sample for the study. We believe that there may be inter-individual differences in efficacy (as illustrated in 3 participants in Figure 1) in stimulation of overall colonic transit. Further studies would be needed to explore the reason(s) for the lack of efficacy in nonresponders.
There are some potential limitations in this study. First, we did not evaluate the plasma level of the active metabolite of bisacodyl, deacetylbisacodyl. However, the effects of bisacodyl are mediated through a direct action in the gastrointestinal mucosa, and previous study18 showed that there was no apparent relationship between the plasma level of the metabolite and the laxative effect, suggesting that systemic absorption was not required for the laxative action. Second, we did not evaluate intestinal secretion in this study. Further mechanistic studies of effects of bisacodyl on colon motility, mucosal permeability and secretion will enhance our understanding of the pharmacological effects of oral bisacodyl in the human gastrointestinal tract.
In summary, bisacodyl has been previously shown to be efficacious in the treatment of chronic idiopathic constipation.35 Our study shows that accelerating AC emptying is likely to be an important mechanism of oral bisacodyl’s action in healthy subjects. The current data provide further support for the potential use of bisacodyl for the occasional treatment of patients with idiopathic constipation.
Acknowledgments
This study was funded in part by grant DK54681 to Dr. Camilleri from National Institutes of Health.
Abbreviations used in this paper
- GC
geometric center
- AC
ascending colon
- TC
transverse colon
- DC
descending colon
- RS
rectosigmoid
- AC t1/2
ascending colon emptying time
- IQR
interquartile range
- CV
coefficient of variation
Footnotes
STATEMENT OF INTERESTS
Authors Personal Interests None.
References
- 1.Physicians’ Desk Reference. MICROMEDEX(R) Healthcare Series Integrated Index.
- 2.Roth W, Beschke K. Pharmacokinetics and laxative effect of bisacodyl following administration of various dosage forms [German] Arzneimittelforschung. 1988;38:570–4. [PubMed] [Google Scholar]
- 3.Flig E, Hermann TW, Zabel M. Is bisacodyl absorbed at all from suppositories in man? Int J Pharm. 2000;196:11–20. doi: 10.1016/s0378-5173(99)00385-3. [DOI] [PubMed] [Google Scholar]
- 4.Taylor I, Duthie HL, Smallwood R, Brown BH, Linkens D. The effect of stimulation on the myoelectrical activity of the rectosigmoid in man. Gut. 1974;15:599–607. doi: 10.1136/gut.15.8.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schang JC, Hemond M, Hebert M, Pilote M. Changes in colonic myoelectric spiking activity during stimulation by bisacodyl. Can J Physiol Pharmacol. 1986;64:39–43. doi: 10.1139/y86-005. [DOI] [PubMed] [Google Scholar]
- 6.Kamm MA, Lennard-Jones JE, Thompson DG, Sobnack R, Garvie NW, Granowska M. Dynamic scanning defines a colonic defect in severe idiopathic constipation. Gut. 1988;29:1085–92. doi: 10.1136/gut.29.8.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ewe K, Ueberschaer B, Press AG, Kurreck C, Klump M. Effect of lactose, lactulose and bisacodyl on gastrointestinal transit studied by metal detector. Aliment Pharmacol Ther. 1995;9:69–73. doi: 10.1111/j.1365-2036.1995.tb00354.x. [DOI] [PubMed] [Google Scholar]
- 8.Park JM, Choi MG, Choi H, et al. Measurement of colonic transit using a delayed-release capsule containing radio-opaque markers. Scand J Gastroenterol. 2008;43:545–50. doi: 10.1080/00365520701850204. [DOI] [PubMed] [Google Scholar]
- 9.Talley NJ, Phillips SF, Wiltgen CM, Zinsmeister AR, Melton LJ., 3rd Assessment of functional gastrointestinal disease: the bowel disease questionnaire. Mayo Clin Proc. 1990;65:1456–79. doi: 10.1016/s0025-6196(12)62169-7. [DOI] [PubMed] [Google Scholar]
- 10.Proano M, Camilleri M, Phillips SF, Brown ML, Thomforde GM. Transit of solids through the human colon: regional quantification in the unprepared bowel. Am J Physiol. 1990;258:G856–62. doi: 10.1152/ajpgi.1990.258.6.G856. [DOI] [PubMed] [Google Scholar]
- 11.Camilleri M, Zinsmeister AR. Towards a relatively inexpensive, noninvasive, accurate test for colonic motility disorders. Gastroenterology. 1992;103:36–42. doi: 10.1016/0016-5085(92)91092-i. [DOI] [PubMed] [Google Scholar]
- 12.Burton DD, Camilleri M, Mullan BP, Forstrom LA, Hung JC. Colonic transit scintigraphy labeled activated charcoal compared with ion exchange pellets. J Nucl Med. 1997;38:1807–10. [PubMed] [Google Scholar]
- 13.Cremonini F, Mullan BP, Camilleri M, et al. Performance characteristics of scintigraphic transit measurements for studies of experimental therapies. Aliment Pharmacol Ther. 2002;16:1781–90. doi: 10.1046/j.1365-2036.2002.01344.x. [DOI] [PubMed] [Google Scholar]
- 14.Bouras EP, Camilleri M, Burton DD, Thomforde G, McKinzie S, Zinsmeister AR. Prucalopride accelerates gastrointestinal and colonic transit in patients with constipation without a rectal evacuation disorder. Gastroenterology. 2001;120:354–60. doi: 10.1053/gast.2001.21166. [DOI] [PubMed] [Google Scholar]
- 15.Coulie B, Szarka LA, Camilleri M, et al. Recombinant human neurotrophic factors accelerate colonic transit and relieve constipation in humans. Gastroenterology. 2000;119:41–50. doi: 10.1053/gast.2000.8553. [DOI] [PubMed] [Google Scholar]
- 16.Camilleri M, Northcutt AR, Kong S, et al. Efficacy and safety of alosetron in women with irritable bowel syndrome: A randomised, placebo-controlled trial. Lancet. 2000;355:1035–40. doi: 10.1016/S0140-6736(00)02033-X. [DOI] [PubMed] [Google Scholar]
- 17.Mueller-Lissner S, Fumagalli I, Bardhan KD, et al. Tegaserod, a 5-HT4 receptor partial agonist, relieves key symptoms of irritable bowel syndrome. Gastroenterology. 2000;118:A175. doi: 10.1046/j.1365-2036.2001.01094.x. [DOI] [PubMed] [Google Scholar]
- 18.Kienzle Horn S, Vix JM, Schuijt C, Peil H, Jordan CC, Kamm MA. Efficacy and safety of bisacodyl in the acute treatment of constipation: a double-blind, randomized, placebo-controlled study. Aliment Pharmacol Ther. 2006;23:1479–88. doi: 10.1111/j.1365-2036.2006.02903.x. [DOI] [PubMed] [Google Scholar]
- 19.Zingg U, Miskovic D, Pasternak I, Meyer P, Hamel CT, Metzger U. Effect of bisacodyl on postoperative bowel motility in elective colorectal surgery: a prospective, randomized trial. Int J Colorectal Dis. 2008;23:1175–83. doi: 10.1007/s00384-008-0536-7. [DOI] [PubMed] [Google Scholar]
- 20.Forth W, Nell G, Rummel W, Andres H. The hydragogue and laxative effect of the sulfuric acid ester and the free diphenol of 4,4′-dihydroxydiphenyl-(pyridyl-2)-methane. Naunyn Schmiedebergs Arch Pharmacol. 1972;274:46–53. doi: 10.1007/BF00501005. [DOI] [PubMed] [Google Scholar]
- 21.Gaginella TS, Mascolo N, Izzo AA, Autore G, Capasso F. Nitric oxide as a mediator of bisacodyl and phenolphthalein laxative action. J Pharmacol Exp Ther. 1994;270:1239–45. [PubMed] [Google Scholar]
- 22.Farack UM, Gruber E, Loeschke K. The influence of bisacodyl and deacetylbisacodyl on mucus secretion, mucus synthesis and electrolyte movements in the rat colon in vivo. Eur J Parmacol. 1985;117:215–22. doi: 10.1016/0014-2999(85)90606-5. [DOI] [PubMed] [Google Scholar]
- 23.Farack UM, Nell G. Mechanism of action of diphenolic laxatives: the role of adenylate cyclase and mucosal permeability. Digestion. 1984;30:191–4. doi: 10.1159/000199105. [DOI] [PubMed] [Google Scholar]
- 24.Andersen V, Camilleri M, Busciglio IA, et al. Effect of 5 days linaclotide on transit and bowel function in females with constipation-predominant irritable bowel syndrome. Gastroenterology. 2007;133:761–8. doi: 10.1053/j.gastro.2007.06.067. [DOI] [PubMed] [Google Scholar]
- 25.Sweetser S, Busciglio IA, Camilleri M, et al. Effect of a chloride channel activator, lubiprostone, on colonic sensory and motor functions in healthy subjects. Am J Physiol Gastrointest Liver Physiol. 2009;296:G295–301. doi: 10.1152/ajpgi.90558.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herve S, Savoye G, Behbahani A, Leroi AM, Denis P, Ducrotte P. Results of 24h manometic recording of colonic motor activity with endoluminal instillation of bisacodyl in patients with severe chronic slow transit constipation. Neurogastroenterol Motil. 2004;16:397–402. doi: 10.1111/j.1365-2982.2004.00535.x. [DOI] [PubMed] [Google Scholar]
- 27.Bassotti G, Chiarioni G, Germani U, Battaglia E, Vantini I, Morelli QA. Endoluminal instillation of bisacodyl in patients with severe (slow transit type) constipation is useful to test residual colonic propulsive activity. Digestion. 1999;60:69–73. doi: 10.1159/000007591. [DOI] [PubMed] [Google Scholar]
- 28.Ewe K. Effect of bisacodyl on intestinal electrolyte and water net transport and transit. Digestion. 1987;37:247–53. doi: 10.1159/000199508. [DOI] [PubMed] [Google Scholar]
- 29.Stivland T, Camilleri M, Vassallo M, et al. Scintigraphic measurement of regional gut transit in idiopathic constipation. Gastroenterology. 1991;101:107–15. doi: 10.1016/0016-5085(91)90466-x. [DOI] [PubMed] [Google Scholar]
- 30.Debongniie JC, Phillips SF. Capacity of the human colon to absorb fluid. Gastroenterology. 1978;74:698–703. [PubMed] [Google Scholar]
- 31.Ratnaike RN, Jones TE. Mechanisms of drug-induced diarrhea in the elderly. Drug Aging. 1998;13:245–53. doi: 10.2165/00002512-199813030-00007. [DOI] [PubMed] [Google Scholar]
- 32.Camilleri M, Bharucha AE, Ueno R, et al. Effect of a selective chloride channel activatior, lubiprostone, on gastrointestinal transit, gastric sensory and motor functions in healthy volunteers. Am J Physiol Gastrointest Liver Physiol. 2006;290:G942–7. doi: 10.1152/ajpgi.00264.2005. [DOI] [PubMed] [Google Scholar]
- 33.von der Ohe MR, Camilleri M, Kvis LK, Thomforde GM. Motor dysfunction of the small bowel and colon in patients with carcinoid syndrome and diarrhea. N Engl Med. 1993;329:1073–8. doi: 10.1056/NEJM199310073291503. [DOI] [PubMed] [Google Scholar]
- 34.Schryver AMP, Samsom M, Smout AIPM. Effect of a meal and bisacodyl on colonic motility in healthy volunteers and patients with slow-transit constipation. Dig Dis Sci. 2003;48:1206–12. doi: 10.1023/a:1024178303076. [DOI] [PubMed] [Google Scholar]
- 35.Ramkumar D, Rao SSC. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol. 2005;100:936–71. doi: 10.1111/j.1572-0241.2005.40925.x. [DOI] [PubMed] [Google Scholar]


