Abstract
This study examines the contribution of GABAergic inhibition to the discharge pattern and recovery properties of 110 bat inferior collicular neurons by means of bicuculline application to their recording sites. When stimulated with single pulses, 74 (67%) neurons discharged one or two impulses (phasic responders), 19 (17%) discharged three to ten impulses (phasic bursters) and 17 (16%) discharged impulses throughout the entire stimulus duration (tonic responders). Bicuculline application changed phasic responders into phasic bursters or tonic responders, increased the number of impulses by 10–2000% and shortened the response latency of most neurons. When stimulated with pairs of sound pulses, the recovery cycles of these neurons can be described as: (1) long inhibition (n = 49, 45%); (2) short inhibition (n = 41, 37%); and (3) fast recovery (n = 20, 18%) based upon the 50% recovery time that was either longer than 20 ms, between 10 and 20 ms or shorter than 10 ms. Bicuculline application shortened the 50% recovery time of most neurons by 11–2350% allowing them to respond to pairs of sound pulses at very short interpulse intervals. These data demonstrate that GABAergic inhibition contributes significantly to auditory temporal processing.
Keywords: Bat, Bicuculline, GABAergic, Inferior colliculus, Recovery cycle
Introduction
The recovery cycle of auditory neurons is an important neuronal property which determines a neuron’s ability to respond to pairs of sound pulses presented at short interpulse intervals. This property is particularly important for bats, which emit intense orientation pulses and listen to the returning echoes to extract information about targets. The recovery cycles and temporal resolution capabilities of auditory neurons underlie the bat’s ability to determine the target distance. Past studies of recovery cycles of auditory neurons in bats have been conducted by recording evoked potential or single neuron responses to identical or pulse-echo pairs (Friend et al. 1966; Grinnell 1963; Pollak et al. 1977a,b; Suga 1964, 1970; Suga and Schlegel 1973). These studies showed that recovery cycles of neurons varied with stimulus duration, frequency, and intensity. For example, inferior collicular neurons have longer recovery cycles when stimulated with short duration pulses (1–2 ms) than with longer-duration pulses (3.4–4.0 ms) (Pollak et al. 1977a). Collicular neurons also have shorter recovery cycles when stimulated with two identical pulses than with pulse-echo pairs (Friend et al. 1966; Grinnell 1963; Suga 1964; Suga and Schlegel 1973).
In the ascending auditory pathway, the inferior colliculus is an obligatory station which receives excitatory and inhibitory inputs from all lower auditory nuclei (Adams 1979; Adams and Mugnaini 1984; Casseday and Covey 1992, 1995, 1996; Covey and Casseday 1986, 1995; Massopust and Ordy 1962; Oliver et al. 1994; Pollak and Casseday 1989; Roberts and Ribak 1987a, b; Rockel and Jones 1973; Saint Marie et al. 1989; Schneiderman and Oliver 1989; Zook and Casseday 1987; Zook et al. 1985). The inferior colliculus also receives descending inputs from the auditory cortex through the cortico-collicular pathways (Hefti et al. 1995; Huffman and Henson 1990; Syka et al. 1988). These excitatory and inhibitory inputs contribute importantly to auditory temporal processing in this nucleus (Casseday and Covey 1995). Previous studies have shown that iontophoretic application of GABA to the inferior colliculus inhibited spontaneous or noise-evoked activity and decreased the number of impulses of recorded neurons. In contrast, application of bicuculline, which is an antagonist for GABAA receptors (Bormann 1988; Cooper et al. 1982), increased the number of impulses dramatically, changed the discharge patterns and response latency of recorded neurons (Ebert and Ostwald 1995; Faingold et al. 1989, 1991; Palombi and Caspary 1992; Park and Pollak 1993a,b; Vater et al. 1992; Yang et al. 1992).
To explore the dynamic aspect of auditory temporal processing in the inferior colliculus, this study examines the role of GABAergic inhibition in shaping the ability of the bat’s inferior collicular neurons with different temporal discharge patterns and recovery cycles to encode rapidly repeating pairs of sound pulses. We hypothesize that if the GABAergic inhibition is one of the determinant factors for a neuron’s recovery cycle, then GAGAergic disinhibition should greatly shorten the neuron’s recovery cycle. We report here that iontophoretic application of bicuculline to recording sites not only shortened the recovery cycles and response latency but also changed the discharge pattern and the number of impulses of more than two-thirds of inferior collicular neurons studied.
Materials and methods
A total of 18 (7 females, 11 males) Eptesicus fuscus, [body weight (b.w.) 14–24 g] were used for this study. The procedures for surgery and recording were basically the same as in previous studies (Jen et al. 1987, 1989; Kamada et al. 1992). Briefly, 1 or 2 days before the recording session, the flat head of a 1.8-cm nail was glued onto the exposed skull of each Nembutal-anesthetized (45–50 mg · kg−1 b.w.) bat with acrylic glue and dental cement. Before recording, the bat was administered the neuroleptanalgesic Innovar-vet (0.08 · mg · kg−1 b.w. of fentanyl, 4 mg · kg−1 b.w. of droperidol) to avoid the suppressive effect of Nembutal on higher-order neurons (Aitkin and Prain 1974; Kuwada et al. 1989). The bat was then tied onto an aluminum plate with a plastic band inside a sound-proof room (temperature 28–30 °C) and its head was immobilized by fixing the shank of the nail into a metal rod with a set screw (Suga and Schlegel 1972). The ceiling and inside walls of the room were covered with 3-inch convoluted polyurethane foam to reduce echoes. Additional doses were administered during later phases of recording when necessary. A local anesthetic (Lidocaine) was applied to the open wound area. The bat’s head was oriented such that the eye-snout line pointed to 0° in azimuth and 0° in elevation with respect to the frontal auditory space (Jen et al. 1989; Shimozawa et al. 1984). Small holes were bored in the skull above the inferior colliculus for insertion of electrodes to record sound activated neural activities.
The electronic instruments used to generate acoustic stimuli were the same as those used in recent studies (Jen et al. 1987, 1989; Kamada et al. 1992). Briefly, continuous sine waves from an oscillator (KH model 1200) were formed into 3-ms tone pulses (0.5-ms rise-decay times) by a homemade tone-burst generator (electronic switch) driven by a stimulator (Grass S88). The 3-ms tone pulses were amplified after passing through a decade attenuator (HP 350D) before being fed into a small condenser loudspeaker (AKG model CK 50, 1.5 cm diameter). The loudspeaker was calibrated with a 1/4 inch B and K microphone (4135) positioned at the bat’s ear. Output was expressed in dB SPL referred to 20-μPa root mean square. A frequency characteristics curve was plotted for the loudspeaker to determine the maximal available stimulus intensity at each frequency. Two independent sets of instruments were available to produce pulse-echo pairs for stimulation. Downward sweeping frequency modulated (FM) signals were generated by means of ramp signals, when necessary.
Piggy-back multibarrel glass microelectrodes were used to record acoustically evoked single neuron responses and to inject drugs iontophoretically to the recording site (Havey and Caspary 1980). Each multi-barrel electrode was composed of a three-barrel electrode (tip: 10–15 μm) “piggybacked” to a 3 mol · l−1 KCl single-barrel electrode (tip: less than 1 μm; impedance: 5–10 MΩ) whose tip was extended about 10 μm from the tip of the three-barrel electrode. The 3 mol · l−1 KCl single-barrel recording electrode was connected by a silver wire to an amplifier (HP 465A) followed by an electronic filter (KH 3500). One of the barrels of a triple-barrel electrode was filled with bicuculline methiodide (10 mmol · l−1 in 0.16 mol · l−1 NaCl, pH 3.0). The bicuculline was prepared just prior to each experiment and the electrode filled immediately before use. This bicuculline channel was connected via silver-silver chloride wire to a microiontophoresis constant current generator (Medical Systems Neurophore BH-2) which was used to generate and monitor iontophoretic currents. During bicuculline application, a 1-s pulse of 40 nA at 0.5 pulses per second (pps) was applied for 1 min before data acquisition. Application current was then changed to 10 nA during data acquisition. The other two barrels were filled with 1 mol · l−1 NaCl (pH 7.4), one of which was used as the ground and the other as the balanced barrel. The balance electrode was connected to balance module. The retaining current was negative 8–10 nA.
To determine any potential artifacts due to passing current or low pH values, the balanced barrel was filled with 1 mol · l−1 NaCl (pH 3.0) and the same amount of current used for bicuculline application was passed through the balanced barrel. Stimulus artifacts were considered negligible when the number of impulses of a neuron was affected by less than 10% before and after current application (Ebert and Ostwald 1995). Otherwise, the data were discarded and a new electrode was used for the experiment. Data were also discarded when the impedance of the bicuculline-filled electrode varied more than 20 MΩ before and after the recording, the tip of the three-barrel electrode broke when withdrawn from the recording site, or both tips of the single- and the three-barrel electrode separated from each other.
Acoustic stimuli were delivered at 2 pps from the loudspeaker which was attached to an aluminum arm at a distance of 29 cm from the bat’s head. The loudspeaker was placed 30° contralateral to the recording site. During recording, the best frequency (BF) and minimum threshold (MT) of each isolated collicular neuron were first determined audiovisually by systematically adjusting the intensity and frequency of the stimulus. At the MT, the neuron on average responded with 50% probability to a BF stimulus. After determining the response latency of an isolated neuron with BF pulses delivered at 30 dB above the neuron’s MT, the MT was redetermined with FM pulses sweeping one octave downward across the neuron’s BF. The neuron’s recovery cycles were then studied either with a pair of identical FM or BF pulses of 80 dB SPL or with a pair of FM or BF pulses of 80–60 dB SPL depending upon which type of stimulus was most effective in evoking the neuron’s response. Recovery cycles of collicular neurons were studied with the interpulse interval set at 5, 10, 15, 25, 50, 75 and 100 ms. Because the stimulus duration was 3 ms, there was no overlap at any interval. The neuron’s number of impulses discharged to the first and second (echo) pulse alone were also recorded.
The recording depth was read from the scale of a microdrive (David-Kopf). A common indifferent electrode (silver wire) was placed at the nearby temporal muscles. Recorded action potentials were amplified with conventional techniques and sent to a computer (Gateway 2000, 486) for acquisition of post-stimulus-time (PST) histograms (bin width: 500 μs, sampling period: 300 ms) and dot-raster patterns of the neuron’s responses to 16 stimulus presentations. The PST histograms quantitatively describe the discharge pattern of each neuron in response to different stimuli. The total number of impulses in each PST histogram was used to quantify a neuron’s response under each stimulus condition.
From each PST histogram, the number of impulses discharged to each presented pulse was determined by a special computer program. At longer interpulse intervals, the number of impulses to each presented pulse could be easily determined from the PST histogram. At short interpulse intervals, it was often difficult to distinguish a neuron’s response to the second pulse from that to the first pulse, in particular after bicuculline application to the recording site. In such cases, the number of impulses to the second pulse was determined by calculating the difference between the total number of impulses discharged to the pair pulses and the number of impulses discharged to the first pulse alone.
As in previous studies (Casseday and Covey 1995, 1996; Friend et al. 1966; Grinnell 1963; Suga 1964, 1970; Suga and Schlegel 1973), the recovery cycle of a neuron was studied by plotting a recovery curve which shows the percentage recovery at each interpulse interval. When studied with identical pulses, the percentage recovery of a neuron at each interpulse interval was calculated by dividing the number of impulses discharged to the second pulse by the number of impulses discharged to the first pulse. When stimulated with a pulse-echo pair, the percentage recovery of a neuron at each interpulse interval was calculated by dividing the number of impulses discharged to the echo by the number of impulses discharged to the echo alone. The effect of GABAergic inhibition on a neuron’s recovery cycle was determined by comparing the neuron’s recovery curves obtained before and after the bicuculline application to its recording site. The effect of bicuculline often appeared immediately after application but reached a stabilized state within 5 min. Data acquisition was conducted when the effect of bicuculline was stabilized. Bicuculline application is considered to produce an effective change on a neuron’s recovery cycle when the 50% recovery time and at least three data points of the neuron’s pre- and post-drug recovery curves differ by more than 10%.
Results
A total of 110 inferior collicular neurons were isolated at depths between 281 and 2052 μm. Only 19 were spontaneously active (2–11 impulses s−1). The BFs, MTs and response latency of these 110 neurons were between 16.2 and 86.2 kHz; 1 and 68 dB SPL; and 6 and 19 ms, respectively. These neurons were tonotopically organized along the dorsoventral axis of the inferior colliculus according to their BFs and recording depths. High BF neurons tended to have high MTs and short response latencies. These findings are similar to those obtained from previous studies on the same species of bat (Casseday and Covey 1992; Jen and Schlegel 1982; Jen et al. 1987; Pinheiro et al. 1991; Poon et al. 1990; Wu and Jen 1991).
Recovery cycles were studied with identical 80–80 dB SPL pulse pairs in 62 neurons (FM pulses for 50 neurons and BF pulses for 12 neurons) and with 80–60 dB SPL pulse-echo pairs in 48 neurons (FM pulses for 38 neurons and BF pure tones for 10 neurons) before and after bicuculline application to their recording sites. For convenience, neurons in which recovery cycles were studied with identical pulse pairs are called P-P stimulated neurons and those studied with pulse-echo pairs are called P-e stimulated neurons.
Bicuculline application on neuronal responses
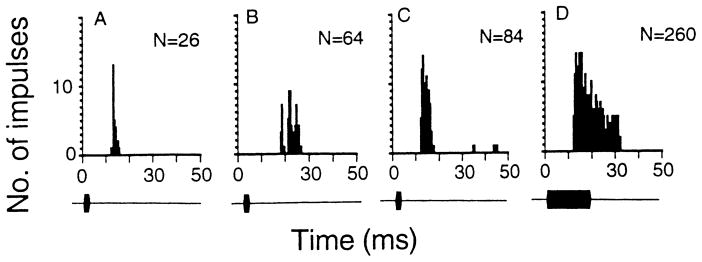
The discharge patterns of all 110 neurons studied can be described as phasic responders (P; n = 74, 67%), phasic bursters (PB; n = 19, 17%) or tonic responders (T; n = 17, 16%) as in previous studies (Jen and Schlegel 1982; Pollak et al. 1978). When stimulated with 3- and 19-ms pulses, phasic responders always discharged 1–2 impulses (Fig. 1A), whereas phasic bursters typically discharged 3–7 impulses (Fig. 1B). In contrast, tonic responders discharged impulses throughout or longer than the duration of the presented pulses (Fig. 1C, D). Table 1 shows the basic auditory response properties of these three types of neurons. It appears that more tonic responders were recorded at a depth of more than 1000 μm and they tended to have higher BFs and MTs than the other two types of neurons.
Fig. 1.
A–D The post-stimulus-time (PST) histograms showing the discharge patterns of three representative inferior collicular neurons: phasic responder (A), phasic burster (B) and tonic responder (C, D). When stimulated with acoustic stimuli of 3 or 19 (not shown) ms, the phasic responder discharged 1–2 impulses (A) and the phasic buster discharged 3–10 impulses (B). In contrast, the tonic responder discharged impulses throughout the entire duration of the acoustic stimulus (C, D). N: number of impulses discharged to 16 stimuli. The BF (kHz) and MT (dB SPL) of these three neurons were 59.9, 31 (A); 38.7, 33 (B) and 64.0, 52 (C, D). The stimulus intensity used to obtain these PST histograms was always 80 dB SPL. Bin width: 500 μs, sampling period: 300 ms. To highlight the discharge pattern, the sampling period beyond 50 ms is not shown
Table 1.
Recording depth, best frequency (BF), minimum threshold (MT), and response latency (Lat) of 110 inferior collicular neurons with different discharge patterns
| Discharge pattern | No. | Depth (μm) | BF (kHz) | MT (dB SPL) | Lat (ms) |
|---|---|---|---|---|---|
| Phasic responder | 74 | 1240 ± 442 | 39.0 ± 15.5 | 34.0 ± 14.3 | 11.7 ± 3.2 |
| 332–2052 | 16.2–86.4 | 1–68 | 6.0–19.0 | ||
| 66% > 1000 | 39% > 40 | 32% > 40 | 30% < 10 | ||
| Phasic burster | 19 | 1055 ± 429 | 33.2 ± 10.9 | 31.5 ± 14.4 | 13.4 ± 3.2 |
| 281–1693 | 19.7–61.0 | 5–55 | 9.0–19.0 | ||
| 58% > 1000 | 21% > 40 | 26% > 40 | 16% < 10 | ||
| Tonic responder | 17 | 1397 ± 424 | 46.5 ± 14.1 | 38.6 ± 14.6 | 11.7 ± 3.4 |
| 441–1932 | 17.9–66.5 | 13–66 | 6.5–18.0 | ||
| 82% > 1000 | 76% > 40 | 47% > 40 | 29% < 10 |
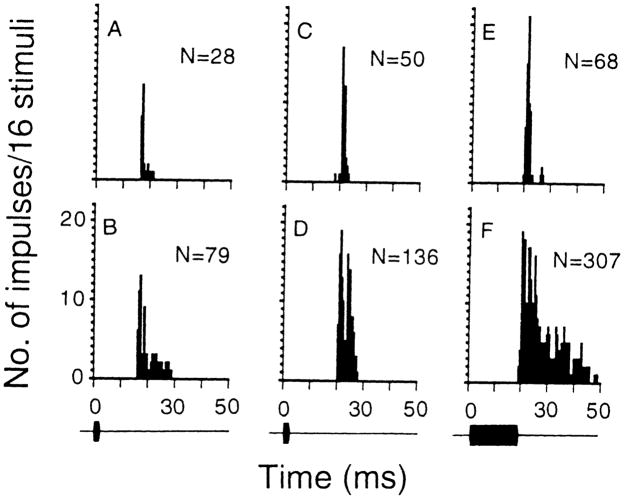
When stimulated with 3-ms single pulses, bicuculline application to the recording sites of these neurons increased the number of sound-evoked impulses of 108 neurons by 10–2000% (65 neurons increased by more than 100%) and decreased the number of impulses of two tonic responders by 25 and 57%, respectively. Bicuculline application shortened the response latency of 49 (45%) neurons by 0.5–3.5 ms (phasic responders: n = 36, 1.2 ± 0.9 ms; phasic bursters: n = 5, 0.6 ± 0.2 ms; tonic responders: n = 8, 0.8 ± 0.4 ms). The application also lengthened the response latency of 16 (15%) neurons by 0.5–3.5 ms (phasic responders: n = 11, 1.2 ± 0.9 ms; phasic bursters: n = 3, 1.5 ± 0.9 ms; tonic responders: n = 2, 0.8 ± 0.4 ms). Whereas bicuculline application generally increased the response duration of all collicular neurons studied, the application did not change the response pattern of phasic bursters and tonic responders. However, the application changed 55 (74%) phasic responders into phasic bursters (Fig. 2A, B) and 5 (7%) into tonic responders (Fig. 2C–F).
Fig. 2.
A–F PST histograms showing the discharge patterns of two inferior collicular neurons obtained with 80-dB SPL sounds before and after bicuculline application to their recording sites. The discharge patterns of A–D were obtained with 3-ms sounds and those in E, F were obtained with 19-ms sounds. The discharge pattern of one collicular neuron (BF: 43.5 kHz, MT: 22 dB SPL) changed from phasic responder (A) into phasic burster (B) after bicuculline application. The discharge pattern of another neuron (BF: 35.1 kHz, MT: 47 dB SPL) changed from phasic responder (C, E) into tonic responder (D, F) after bicuculline application such that this neuron discharged impulses with a duration longer than the presented pulses. N: total number of impulses in the histogram. Bin width: 500 μs, sampling period: 300 ms. To highlight the change in discharge pattern, the sampling period beyond 50 ms is not shown
Recovery cycles before and after bicuculline application
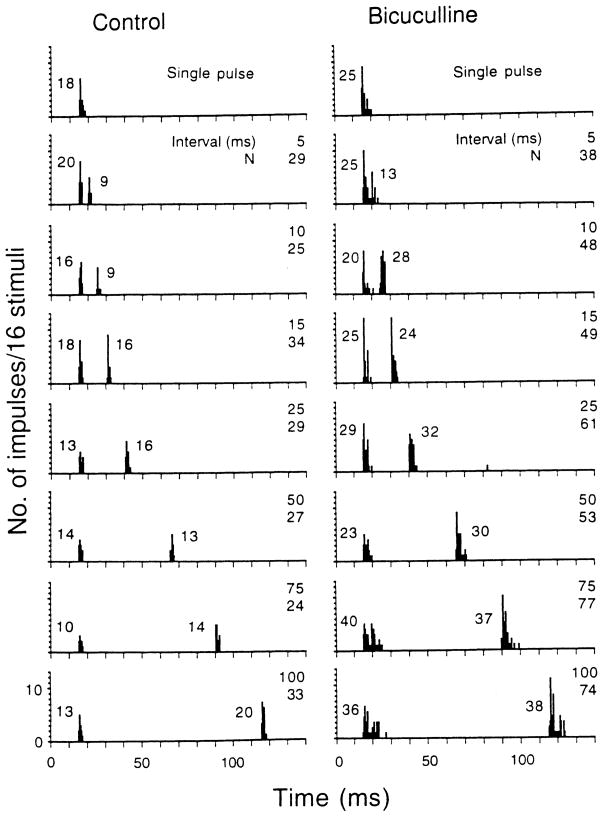
Figure 3. shows the responses of a representative collicular neuron to single pulses and to paired identical pulses at seven different interpulse intervals. This neuron discharged phasically to each presented pulse regardless of interpulse interval. Bicuculline application increased the neuron’s number of impulses and response duration to each presented pulse at all interpulse intervals tested (right panel) such that the neuron’s discharge pattern changed from a phasic responder into a phasic burster. The neuron’s number of impulses discharged to each presented pulse varied with interpulse intervals to different degrees both before and after bicuculline application.
Fig. 3.
PST histograms showing the discharge pattern of an inferior collicular neuron obtained with single pulses (top histogram of both columns) and with an identical pulse pair (BF pulses of 80–80 dB SPL, 3 ms duration with 0.5-ms rise-decay times) delivered at different interpulse intervals before (control, left column) and after (bicuculline, right column) iontophoretic application of bicuculline to the recording site. All stimuli were delivered at 30° contralateral to the neuron’s recording site. In the top histogram of both columns, the number of impulses discharged to 16 single pulses is shown. In the remaining histograms, the number of impulses elicited by the first and the second pulse in 16 presentations are shown. In the right upper corner of each histogram, the interpulse interval (ms) is shown by the upper number and the total number of impulses discharged to both presented pulses is shown by the lower number (N). When the neuron’s discharge to the first and second pulses was inseparable, the neuron’s discharge to the second pulse was calculated as the difference between the neuron’s discharge to both pulses (i.e., N) and its discharge to the first pulse alone (shown at the top of each column). The BF (kHz) and MT(dB SPL) of this neuron was 39.5, 25. Bin width: 500 μs, sampling period: 300 ms. To save space, the sampling period beyond 140 ms is not shown
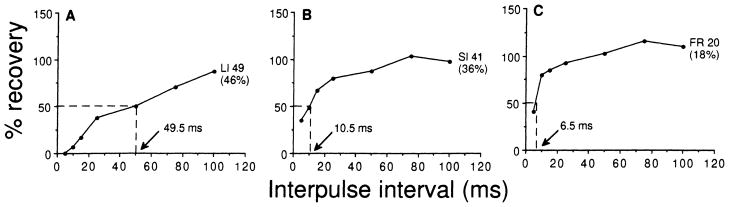
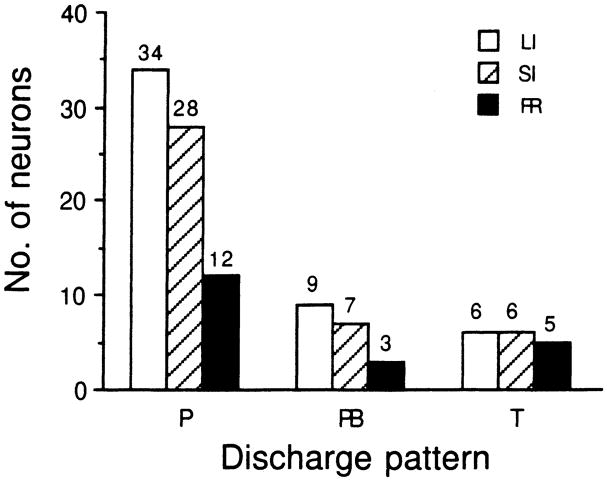
As described in Materials and methods, recovery cycles obtained before and after bicuculline application were plotted according to the number of impulses discharged to each presented pulse at each interpulse interval. The recovery cycle is expressed as percentage recovery at each interpulse interval. The 50% recovery times ranged from 2 to 98 ms among all recovery cycles plotted for the 110 collicular neurons studied before bicuculline application (i.e., pre-drug recovery cycles). Based upon the 50% recovery time, we classify the recovery cycles as long inhibition (LI) with the 50% recovery time longer than 20 ms (Fig. 4A), short inhibition (SI) with the 50% recovery time between 10 and 20 ms (Fig. 4B), and fast recovery (FR) with the 50% recovery time shorter than 10 ms (Fig. 4C). Figure 5 shows the histogram distribution of these three different types of recovery cycles according to their discharge patterns. Although all three types of recovery cycles can be obtained from neurons with each specific discharge pattern, most neurons (82%) had LI or SI recovery cycles regardless of discharge pattern (Fig. 5).
Fig. 4.
A–C Three types of recovery cycles obtained from the inferior collicular neurons of Eptesicus fuscus. Long inhibition recovery cycle (LI) has a 50% recovery time longer than 20 ms (A). Short inhibition recovery cycle (SI) has a 50% recovery time between 10 and 20 ms (B). Fast recovery cycle (FR) has a 50% recovery time shorter than 10 ms (C). The number and percentage of neurons of each type is shown at each curve. The 50% recovery time for each representative curve is shown by the arrow. Ordinates and abscissas represent percentage recovery and interpulse interval in ms
Fig. 5.
Bar histograms showing the distribution of different types of recovery cycles of inferior collicular neurons according to their discharge patterns before the application of bicuculline to their recording sites. The number of neurons of each type of recovery cycle is shown atop each bar. P: phasic responder, PB: phasic burster, T: tonic responder, LI (unfilled bars): long inhibition recovery cycle, SI (hatched bars): short inhibition recovery cycle, FR (filled bars): fast recovery cycle
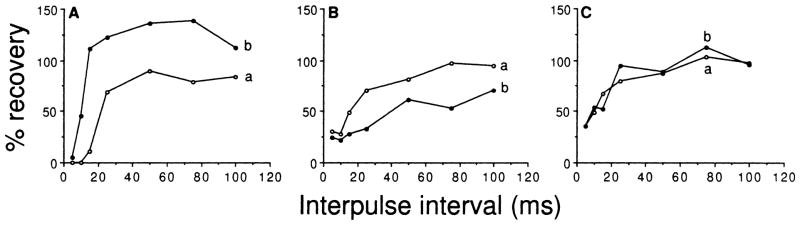
Bicuculline application to the recording sites of these neurons shortened (n = 79; Fig. 6, Ab vs Aa), lengthened (n = 8; Fig. 6, Bb vs Ba), or had no effect (n = 23; Fig. 6, Ca vs Cb) on the recovery cycles of studied neurons. Shortening or lengthening the recovery cycle of a neuron after bicuculline application often changed the recovery cycle from one type into another. For example, bicuculline application changed the recovery cycle of the neuron shown in Fig. 3 from a SI type into a FR type. Table 2 shows the distribution of each type of recovery cycle before and after bicuculline application. Whereas all three types of recovery cycles were obtained from both P-P- and P-e-stimulated neurons, bicuculline application greatly decreased the number of neurons with a LI recovery cycle and increased the number of neurons with a FR cycle regardless of the type of stimuli (i.e., P-P or P-e) used. Bicuculline application proportionally affected more neurons with LI or SI recovery cycles than neurons with fast recovery cycles.
Fig. 6.
A–C Recovery cycles of three representative inferior collicular neurons (A, B, C) determined before (curve a) and after (curve b) iontophoretic application of bicuculline to their recording sites. Bicuculline application either shortened (Ab), lengthened (Bb) or did not affect (Cb) their recovery cycles
Table 2.
The effect of iontophoretic application of bicuculline on the recovery cycles of 110 inferior collicular neurons determined either with identical (n = 62) or pulse-echo (n = 48) pair stimuli. P-P or P-e stimulated neurons: neurons in which recovery cycles were studied with identical or pulse-echo pairs stimuli (LI long inhibition, SI short inhibition, FR fast recovery)
| Predrug | No. | Bicuculline application |
% change | ||
|---|---|---|---|---|---|
| LI | SI | FR | |||
| 62 P-P stimulated neurons | |||||
| LI | 23 | 6 | 9 | 8 | 74% |
| SI | 26 | 4 | 13 | 9 | 50% |
| FR | 13 | 0 | 2 | 11 | 15% |
| 48 P-e stimulated neurons | |||||
| LI | 26 | 13 | 9 | 4 | 50% |
| SI | 15 | 2 | 8 | 5 | 47% |
| FR | 7 | 1 | 1 | 5 | 29% |
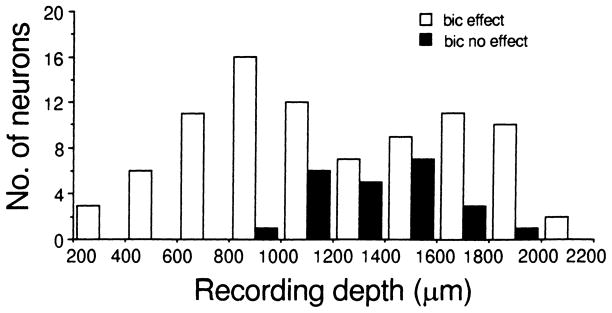
Figure 7. shows the distribution of the recording depth of collicular neurons according to whether or not their recovery cycles were affected by bicuculline application. Although all 87 neurons (BF: 16.2–86.2; mean: 38.2 ± 14.9 kHz) whose recovery cycles were affected by bicuculline application were recorded along the entire dorso-ventral axis of the inferior colliculus, 64 (74%) were recorded at depths less than 1600 μm. In contrast, 16 (70%) of the 23 neurons (BF: 27.4–84.2; mean: 43.0 ± 15.8 kHz) whose recovery cycles were not affected by bicuculline application were recorded at depths greater than 1200 μm.
Fig. 7.
Bar histogram showing the distribution of two types of inferior collicular neurons according to their recording depths (abscissa). Unfilled bars: inferior collicular neurons whose recovery cycles were affected by bicuculline application (bic effect). Solid bars: inferior collicular neurons whose recovery cycles were not affected by bicuculline application (bic no effect)
According to the pre-drug discharge pattern, we compared the pre-drug and post-drug 50% recovery time of the neurons whose recovery cycles were affected by bicuculline application. Table 3 shows the average 50% recovery time of all neurons with different discharge patterns before and after bicuculline application. In spite of different discharge patterns, there is no significant difference in the 50% recovery time among these neurons obtained either before or after bicuculline application. However, bicuculline application significantly shortened the 50% recovery time of phasic responders (P < 0.01) and phasic bursters (P < 0.05) but not that of the tonic responders (P > 0.05, t-test, see Table 3).
Table 3.
The effect of iontophoretic application of bicuculline on 50% recovery time of 87 inferior collicular neurons with different discharge patterns. Ordinary ANOVA was used to determine the significant difference in 50% recovery time among different discharge patterns. Paired t-test was used to determine the significant difference in 50% recovery time between predrug and bicuculline application
| Discharge pattern | No. | 50% recovery time |
t-test P | |
|---|---|---|---|---|
| Predrug | Bicuculline | |||
| Phasic responder | 62 | 32.4 ± 26.6 | 15.3 ± 26.5 | <0.0001*** |
| Phasic burster | 14 | 25.4 ± 19.1 | 13.9 ± 8.1 | 0.0453* |
| Tonic responder | 11 | 25.2 ± 17.3 | 19.3 ± 19.9 | 0.0521 |
| ANOVA test P | 0.4812 | 0.6981 | ||
significant difference (P < 0.05);
highly significant difference (P < 0.001)
We also compared the 50% recovery time before and after bicuculline application according to the pre-drug recovery cycles. Bicuculline application only significantly shortened the 50% recovery time of 41 collicular neurons with a long inhibition recovery cycle (Table 4). Most of these neurons (91%) were either phasic responders (71%) or phasic bursters (20%).
Table 4.
The effect of iontophoretic application of bicuculline on 50% recovery time of 87 inferior collicular neurons with different types of recovery cycles. (LI long inhibition, SI short inhibition, FR fast recovery). Paired t-test was used to determine the significant difference in 50% recovery time between predrug and bicuculline application
| Type of Recovery cycle | No. | 50% recovery time |
t-test P | |
|---|---|---|---|---|
| Predrug | Bicuculline | |||
| LI | 41 | 48.6 ± 21.7 | 21.2 ± 18.7 | <0.0001*** |
| SI | 33 | 15.4 ± 3.2 | 14.5 ± 7.4 | 0.3419 |
| FR | 13 | 6.2 ± 2.3 | 7.4 ± 5.3 | 0.9443 |
highly significant difference (P < 0.001)
Facilitated responses by the second pulse
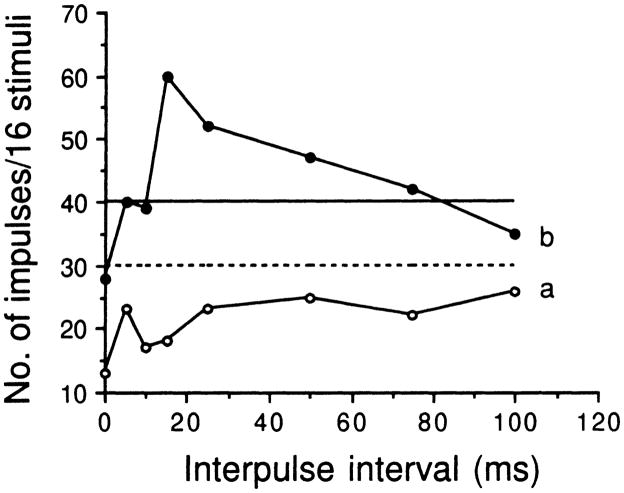
To determine if a neuron’s response is facilitated by the second pulse at a certain interpulse interval (pulse-echo delay), we plotted the total number of impulses of these neurons discharged to paired pulses (P-P or P-e) at each interpulse interval relative to the summation of the number of impulses discharged to the first and second pulses alone. Among all 110 neurons studied, none showed any facilitative response to the presented pair of pulses at any interpulse interval tested before bicuculline application (Fig. 8a). After bicuculline application, responses of 3 neurons were facilitated at a certain inter-pulse interval (Fig. 8b) such that the total number of impulses of each neuron at most interpulse intervals was always larger than the sum of the number of impulses discharged to the first and the second pulses alone. Furthermore, the total number of impulses discharged at the most sensitive interpulse interval was at least 50% larger than the minimal number of impulses obtained at other interpulse intervals.
Fig. 8.
Variation in the number of impulses of an inferior collicular neuron discharged to paired identical pulses (80–80 dB SPL) obtained at different interpulse intervals (shown by abscissa) before (a: unfilled circle curve) and after (b: filled circle curve) iontophoretic application of bicuculline to its recording site. The dashed and solid horizontal lines represent the summation of the number of impulses discharged in response to the pulse and echo alone before and after bicuculline application to its recording site. The BF and MT of this neuron were 32.0 kHz and 21 dB SPL
Discussion
Using the big brown bat, Eptesicus fuscus, as a mammalian model auditory system, we demonstrated in this study that bicuculline application to the recording site affected the number of impulses, response duration, discharge pattern and the recovery cycle of most inferior collicular neurons studied (Figs. 2, 3, 6). These data demonstrate that GABAergic inhibition contributes significantly to the auditory temporal processing. Specifically, bicuculline application affects the discharge pattern and shortens the recovery cycle of most collicular neurons allowing them to respond to pairs of sound pulses at very short interpulse interval.
Previous studies of responses of evoked potentials and inferior collicular neurons showed that recovery cycles of inferior collicular neurons varied with the interval, intensity, frequency, and duration of two sound pulses presented at different interpulse intervals (Friend et al. 1966; Grinnell 1963; Pollak et al. 1977a; Suga 1964; Suga and Schlegel 1973). While some neurons require long interpulse intervals for recovery, others recover within an interpulse interval as short as 0.5 ms. According to the interpulse interval at which a neuron shows detectable responses to the second pulse, recovery cycles of inferior collicular neurons have been described as short suppression, delayed inhibition, temporary recovery, and undelayed inhibition (Friend et al. 1966), or poor recovery, independent-like recovery, selective recovery, and pulse-enhanced recovery (Pollak et al. 1977a). Although the 50% recovery time for each type of recovery cycle was not described, these studies indicate that responses of inferior collicular neurons to the second pulse can be either facilitated or suppressed. It has been suggested that the wide spectrum of recovery cycles may be due to a long-lasting and complex temporal pattern of excitatory and inhibitory inputs (Casseday and Covey 1995). It has also been proposed that the wide spectrum of recovery cycles of inferior collicular neurons might provide a mechanism for discriminating between echoes from different distances (Friend et al. 1966).
In the present study, the recovery cycles of the 110 collicular neurons studied were described as long inhibition, short inhibition and fast recovery according to their 50% recovery time. Although 50% recovery times ranged from 2 to 98 ms (Fig. 4), the 50% recovery time of most neurons (n = 89, 81%) was below 50 ms. This is comparable to the range 0.7–50.2 ms for 48 collicular neurons reported in an earlier study of the same species of bat (Casseday and Covey 1995). The 50% recovery time in this study was also used as an indication of an inhibitory period following the responses to the first presented sound.
The present study shows that application of bicuculline increased the number of impulses and response duration in all but 2 neurons and changed 55 (74%) phasic responders into phasic bursters and 5 (7%) into tonic responders. Bicuculline application also shortened or lengthened the response latency of most neurons studied. Similar observations have been reported in earlier studies (Faingold et al. 1991; Johnson 1993; Park and Pollak 1993a,b). These findings probably represent the result of different temporal sequences of excitatory-inhibitory interactions in the neurons. For example, it has been suggested that phasic discharge patterns are due to GABAergic inhibition following the neuron’s excitatory responses to sound stimulation (Casseday and Covey 1995, 1996; Johnson 1993; Nelson and Erulkar 1963; Suga 1964). Shortening of response latency upon bicuculline application is due to the removal of GABAergic inhibition that precedes the neuron’s excitatory response to the sound stimulation (Casseday and Covey 1996).
Bicuculline application significantly affects the 50% recovery time of phasic responders or phasic bursters (Table 3) and neurons with the LI recovery cycle (Table 4). The LI recovery cycle is comparable to the undelayed inhibition recovery cycle or poor recovery in other studies (Friend et al. 1966; Pollak et al. 1977a). Our data showed that most of collicular neurons (91%) with LI recovery cycle, which were significantly shortened by bicuculline application, were either phasic responders (71%) or phasic bursters (20%). Whether or not collicular neurons with phasic discharge patterns and long recovery cycles receive more predominant GABAergic inputs than other neurons remains to be explored.
The present study shows that recovery cycles were shortened in 79 neurons (Fig. 6, Ab vs Aa) and length-ened in 8 neurons (Fig. 6, Bb vs Ba) upon bicuculline application. Shortening of the recovery cycle of a neuron upon bicuculline application is likely due to removal of the neuron’s GABAergic inhibitory period after excitation thus allowing the neuron to respond to more rapidly occurring sound pulses. Local inhibitory circuits or intrinsic membrane properties may be responsible for the 8 neurons whose recovery cycles were lengthened by bicuculline application. For example, these collicular neurons may receive convergent inhibitory inputs from both GABAergic and glycinergic neurons from lower auditory nuclei and the glycinergic neuron also receives a collateral GABAergic input in the form of axon-axonal synapse. In such a case, bicuculline application would remove GABAergic inhibition to both the recorded collicular neuron and the glycinergic neuron resulting in activation of glycinergic inhibition to the recorded neuron. Although future work needs to be performed to confirm this possibility, inferior collicular neurons which receive convergent GABAergic and glycinergic inhibitory inputs have been demonstrated in bats (Casseday et al. 1994; Fubara et al. 1996; Johnson 1993; Park and Pollak 1993a,b; Vater et al. 1992).
Bicuculline application did not produce any noticeable effect on the recovery cycles of 23 neurons (Fig. 6C). There are several possible explanations for this observation. Firstly, GABAergic inputs may contribute very little to the recovery properties of these neurons. Secondly, these neurons may receive GABAergic inhibitory inputs from neurons with tonic activity which do not respond to sound stimuli and only modulate the overall excitability of inferior collicular neurons. Thirdly, these neurons may receive predominant glycinergic rather than GABAergic inhibitory inputs.
We found that 74% of the collicular neurons in which recovery cycles were affected by bicuculline application were recorded at depths less than 1600 μm, while 70% of the neurons whose recovery cycles were not affected by bicuculline application were recorded at depths more than 1200 μm (Fig. 7). Although it may be due to sampling bias, this finding would appear to be consistent with a recent study of the distribution of GABA and glycine receptors in the inferior colliculus of this species of bat. Neurons with GABAA receptors are mostly distributed in the dorsomedial region of the inferior colliculus but are sparsely distributed in the ventrolateral region which contains mostly glycinergic neurons (Fubara et al. 1996).
During hunting a bat systematically increases the repetition rate of emitted pulses as it approaches the insect (Griffin 1958; Simmons et al. 1979). Presumably this increasing rate of pulse emission allows the bat to extract as much information as possible about the localized insect. To successfully intercept the insect, the bat’s auditory neurons have to be able to process an increasing number of echoes as it approaches the insect. The recovery cycles of the auditory neurons are the most important factors determining the ability of auditory neurons to follow rapidly occurring echoes. The fact that bicuculline application affects the recovery cycles of most collicular neurons studied (Fig. 6A, B) clearly demonstrates the important contribution of GABAergic inhibition to the neuron’s ability to respond to rapidly occurring pulses. Our recent studies have demonstrated that auditory cortical neurons can enhance or reduce the intensity coding, frequency tuning and auditory spatial sensitivity of inferior collicular neurons through cortico-collicular pathways. Furthermore, this corticofugal regulation of acoustic signal processing can be mimicked by GABA or bicuculline application to recorded collicular neurons (Jen et al. 1996; Sun et al. 1996). If a bat can regulate the recovery cycle of collicular neurons through corticofugal pathways by means of GABAergic inhibition, then it can actively increase the spectrum of recovery cycles of collicular neurons and adjust the dynamic aspect of temporal processing in the inferior colliculus. Further work needs to be done to explore this possibility.
Neurons which show facilitative responses to pairs of pulses at a certain interpulse interval are called delay-sensitive neurons and they are mostly reported in the auditory cortex (Dear et al. 1993a,b; Feng et al. 1978; Fitzpatrick et al. 1993; Paschal and Wong 1994; Tsuzuki and Suga 1988; Wong et al. 1992) or medial geniculate body (Olsen and Suga 1991; Wenstrup and Grose 1995). Whereas delay-sensitive neurons were recorded in the inferior colliculus of the mustached bat (Mittmann and Wenstrup 1995; Yan and Suga 1996; Wenstrup and Grose 1995), delay-sensitive neurons were recorded in the intercollicularis (Feng et al. 1978) or intertectal nucleus (Dear and Suga 1995) of the big brown bat. In the present study, delay-sensitive neurons were not recorded before bicuculline application. Only three delay-sensitive neurons were observed after bicuculline application (Fig. 8). We do not know if the paucity of delay-sensitive neurons in the inferior colliculus of this bat species is due to sampling bias or to species differences.
In summary, the present study demonstrates that GABAergic disinhibition upon bicuculline application affects the discharge pattern, number of impulses, response latency and recovery cycle of most inferior collicular neurons studied. These findings support the previous claims that auditory signal processing in the inferior colliculus is heavily influenced by GABAergic inhibition (Fubara et al. 1996; Casseday et al. 1994; Casseday and Covey 1995, 1996).
Acknowledgments
We thank Dr. G Summers and two anonymous reviewers for their critical comments on an earlier version of this manuscript. This work is supported in part by the National Institute of Deaf and Other Communication Disorders, National Institutes of Health (5 RO1 DC 00247-08), a Summer Research Fellowship, and a supplemental grant from the Research Council of the University of Missouri-Columbia (UMC 96-039). The experiments were conducted complying with NIH publication No. 85-23, “Principles of Laboratory Animal Care” and with the approval of the Institutional Animal Care and Use Committee (#1438) of the University of Missouri-Columbia.
Abbreviations
- BF
best frequency
- FM
frequency modulated
- MT
minimum threshold
- PST
post-stimulus time
- SPL
sound pressure level
- pps
pulses per second
- SI
short inhibition
- LI
long inhibition
- FR
fast recovery
References
- Adams JC. Ascending projections to the inferior colliculus. J Comp Neurol. 1979;183:519–538. doi: 10.1002/cne.901830305. [DOI] [PubMed] [Google Scholar]
- Adams JC, Mugnaini E. Dorsal nucleus of the lateral lemniscus: a nucleus of GABAergic projection neurons. Brain Res Bull. 1984;13:585–590. doi: 10.1016/0361-9230(84)90041-8. [DOI] [PubMed] [Google Scholar]
- Aitkin LM, Prain SM. Medial geniculate body: unit responses in awake cats. J Neurophysiol. 1974;37:512–521. doi: 10.1152/jn.1974.37.3.512. [DOI] [PubMed] [Google Scholar]
- Bormann J. Electrophysiology of GABAA and GABAB receptor subtypes. Trend Neurosci. 1988;11:112–116. doi: 10.1016/0166-2236(88)90156-7. [DOI] [PubMed] [Google Scholar]
- Casseday JH, Covey E. Frequency tuning properties of neurons in the inferior colliculus of an FM bat. J Comp Neurol. 1992;319:34–50. doi: 10.1002/cne.903190106. [DOI] [PubMed] [Google Scholar]
- Casseday JH, Covey E. Mechanisms for analysis of auditory temporal patterns in the brainstem of echolocating bats. In: Covey E, Hawkins HL, Port RF, editors. Neural representation of temporal patterns. Plenum; New York: 1995. pp. 25–51. [Google Scholar]
- Casseday JH, Covey E. A neuroethological theory of the operation of the inferior colliculus. Brain Behav Evol. 1996;47:311–336. doi: 10.1159/000113249. [DOI] [PubMed] [Google Scholar]
- Casseday JH, Ehrlich D, Covey E. Neural tuning for sound duration: role of inhibitory mechanisms in the inferior colliculus. Science. 1994;264:847–850. doi: 10.1126/science.8171341. [DOI] [PubMed] [Google Scholar]
- Cooper JR, Bloom FE, Roth RH. The biomedical basis of neuropharmacology. Oxford University Press; New York: 1982. [Google Scholar]
- Covey E, Casseday JH. Connectional basis for frequency representation in the nuclei of the lateral lemniscus of the bat, Eptesicus fuscus. J Neurosci. 1986;6:2926–2940. doi: 10.1523/JNEUROSCI.06-10-02926.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey E, Casseday JH. The lower brainstem auditory pathways. In: Papper AN, Fay RR, editors. Springer handbook of auditory research, vol 5. Hearing by bats. Springer; Berlin Heidelberg New York: 1995. pp. 235–295. [Google Scholar]
- Dear SP, Suga N. Delay-tuned neurons in the midbrain of the big brown bat. J Neurophysiol. 1995;73:1084–1100. doi: 10.1152/jn.1995.73.3.1084. [DOI] [PubMed] [Google Scholar]
- Dear SP, Fritz J, Haresign T, Ferragamo M, Simmons JA. Tonotopic and functional organization in the auditory cortex of the big brown bat, Eptesicus fuscus. J Neurophysiol. 1993a;70:1988–2009. doi: 10.1152/jn.1993.70.5.1988. [DOI] [PubMed] [Google Scholar]
- Dear SP, Simmons JA, Fritz J. A possible neuronal basis for representation of acoustic scenes in auditory cortex of the big brown bat. Nature. 1993b;364:620–623. doi: 10.1038/364620a0. [DOI] [PubMed] [Google Scholar]
- Ebert U, Ostwald J. GABA alters the discharge pattern of chopper neurons in the ventral cochlear nucleus. Hearing Res. 1995;91:160–166. doi: 10.1016/0378-5955(96)83100-5. [DOI] [PubMed] [Google Scholar]
- Faingold CL, Gehlbach GA, Caspary DM. On the role of GABA as an inhibitory neurotransmitter in inferior colliculus neurons: iontophoretic studies. Brain Res. 1989;500:302–312. doi: 10.1016/0006-8993(89)90326-0. [DOI] [PubMed] [Google Scholar]
- Faingold CL, Boersma-Anderson CA, Caspary DM. Involvement of GABA in acoustically-evoked inhibition in inferior colliculus neurons. Hearing Res. 1991;52:201–216. doi: 10.1016/0378-5955(91)90200-s. [DOI] [PubMed] [Google Scholar]
- Feng AS, Simmons JA, Kick SA. Echo detection and target-ranging neurons in the auditory system of the bat, Eptesicus fuscus. Science. 1978;202:645–648. doi: 10.1126/science.705350. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick DC, Kanwal JS, Butman JA, Suga N. Combination-sensitive neurons in the primary auditory cortex of the mustached bat. J Neurosci. 1993;13:931–940. doi: 10.1523/JNEUROSCI.13-03-00931.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend JH, Suga N, Suthers RA. Neural responses in the inferior colliculus of echolocating bats to artificial orientation sounds and echoes. J Cell Physiol. 1966;67:319–332. doi: 10.1002/jcp.1040670212. [DOI] [PubMed] [Google Scholar]
- Fubara BM, Casseday JH, Covey E, Schwartz-Bloom RD. Distribution of GABAA, GABAB and glycine receptors in the central auditory system of the big brown bat, Eptesicus fuscus. J Comp Neurol. 1996;369:83–92. doi: 10.1002/(SICI)1096-9861(19960520)369:1<83::AID-CNE6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Griffin DR. Listening in the dark. Yale Univ Press; New Haven, CT: 1958. (Reprinted by Dover Publications, New York, 1974) [Google Scholar]
- Grinnell AD. The neurophysiology of audition in bats: temporal parameters. J Physiol (Lond) 1963;167:67–96. doi: 10.1113/jphysiol.1963.sp007133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havey DC, Caspary DM. A simple technique for constructing ‘piggy-back’ multibarrel microelectrodes. Electro-encephalogr Clin Neurophysiol. 1980;48:249–251. doi: 10.1016/0013-4694(80)90313-2. [DOI] [PubMed] [Google Scholar]
- Hefti BJ, Diehl JJ, Winer JA. Parallel auditory cortical projections to the cat inferior colliculus. Neurosci Abstr. 1995;21:674. [PubMed] [Google Scholar]
- Huffman RF, Henson OW., Jr The descending auditory pathways and acoustico-motor systems: connections with the inferior colliculus. Brain Res Rev. 1990;15:295–323. doi: 10.1016/0165-0173(90)90005-9. [DOI] [PubMed] [Google Scholar]
- Jen PHS, Schlegel P. Auditory physiological properties of the neurons in the inferior colliculus of the big brown bat, Eptesicus fuscus. J Comp Physiol. 1982;147:351–363. [Google Scholar]
- Jen PHS, Sun XD, Chen DM, Teng HB. Auditory space representation in the inferior colliculus of the FM bat, Eptesicus fuscus. Brain Res. 1987;419:7–18. doi: 10.1016/0006-8993(87)90563-4. [DOI] [PubMed] [Google Scholar]
- Jen PHS, Sun XD, Lin PJ. Frequency and space representation in the primary auditory cortex of the FM bat, Eptesicus fuscus. J Comp Physiol. 1989;165:1–14. doi: 10.1007/BF00613794. [DOI] [PubMed] [Google Scholar]
- Jen PHS, Sun XD, Chen QC. Corticofugal control of central auditory sensitivity. Neurosci Abstr. 1996;22:404. doi: 10.1016/0304-3940(96)12788-9. [DOI] [PubMed] [Google Scholar]
- Johnson BR. PhD Dissertation. Duke University; Durham, NC: 1993. GABAergic and glycinergic inhibition in the central nucleus of the inferior colliculus of the big brown bat. [Google Scholar]
- Kamada T, Wu M, Jen PHS. Auditory response properties and spatial response areas of single neurons in the pontine nuclei of the big brown bat, Eptesicus fuscus. Brain Res. 1992;575:187–198. doi: 10.1016/0006-8993(92)90079-o. [DOI] [PubMed] [Google Scholar]
- Kuwada S, Batra R, Stanford TR. Monaural and binaural response properties of neurons in the inferior colliculus of the rabbit: effects of sodium pentobarbital. J Neurophysiol. 1989;61:269–282. doi: 10.1152/jn.1989.61.2.269. [DOI] [PubMed] [Google Scholar]
- Massopust LC, Jr, Ordy JM. Auditory organization of the inferior colliculus in the cat. Exp Neurol. 1962;6:465–477. doi: 10.1016/0014-4886(62)90072-9. [DOI] [PubMed] [Google Scholar]
- Mittmann DH, Wenstrup JJ. Combination-sensitive neurons in the inferior colliculus. Hearing Res. 1995;90:185–191. doi: 10.1016/0378-5955(95)00164-x. [DOI] [PubMed] [Google Scholar]
- Nelson PG, Erulkar SD. Synaptic mechanisms of excitation and inhibition in the central auditory pathway. J Neurophysiol. 1963;26:908–923. doi: 10.1152/jn.1963.26.6.908. [DOI] [PubMed] [Google Scholar]
- Oliver DL, Winer JA, Beckius GE, Saint Marie RL. Morphology of GABAergic neurons in the inferior colliculus of the cat. J Comp Neurol. 1994;340:27–42. doi: 10.1002/cne.903400104. [DOI] [PubMed] [Google Scholar]
- Olsen JF, Suga N. Combination-sensitive neurons in the medial geniculate body of the mustached bat: encoding of target range information. J Neurophysiol. 1991;65:1275–1296. doi: 10.1152/jn.1991.65.6.1275. [DOI] [PubMed] [Google Scholar]
- Palombi PS, Caspary DM. GABAA receptor antagonist bicuculline alters response properties of posteroventral cochlear nucleus neurons. J Neurophysiol. 1992;67:738–746. doi: 10.1152/jn.1992.67.3.738. [DOI] [PubMed] [Google Scholar]
- Park TJ, Pollak GD. GABA shapes sensitivity to interaural intensity disparities in the mustache bat’s inferior colliculus: implications for encoding sound location. J Neurosci. 1993a;13:2050–2067. doi: 10.1523/JNEUROSCI.13-05-02050.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TJ, Pollak GD. GABA shapes a topographic organization of response latency in the mustache bat’s inferior colliculus. J Neurosci. 1993b;13:5172–5187. doi: 10.1523/JNEUROSCI.13-12-05172.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschal WG, Wong D. Frequency organization of delay-sensitive neurons in the auditory cortex of the FM bat, Myotis lucifugus. J Neurophysiol. 1994;72:366–379. doi: 10.1152/jn.1994.72.1.366. [DOI] [PubMed] [Google Scholar]
- Pinheiro AD, Wu M, Jen PHS. Encoding repetition rate and duration in the inferior colliculus of the big brown bat, Eptesicus fuscus. J Comp Physiol. 1991;169:69–85. doi: 10.1007/BF00198174. [DOI] [PubMed] [Google Scholar]
- Pollak GD, Casseday JH. The neural basis of echolocation in bats. Springer; Berlin Heidelberg New York: 1989. [Google Scholar]
- Pollak GD, Bodenhamer R, Marsh DS, Souther A. Recovery cycles of single neurons in the inferior colliculus of unanesthetized bats obtained with frequency-modulated and constant-frequency sounds. J Comp Physiol. 1977a;120:215–250. [Google Scholar]
- Pollak GD, Marsh DS, Bodenhamer R, Souther A. Characteristics of phasic on neurons in inferior colliculus of unanesthetized bats with observations relating to mechanism for echo ranging. J Neurophysiol. 1977b;40:926–942. doi: 10.1152/jn.1977.40.4.926. [DOI] [PubMed] [Google Scholar]
- Pollak GD, Marsh DS, Bodenhamer R, Souther A. A single-unit analysis of inferior colliculus in unanesthetized bats: response patterns and spike-count functions generated by constant-frequency and frequency-modulated sounds. J Neurophysiol. 1978;41:677–691. doi: 10.1152/jn.1978.41.3.677. [DOI] [PubMed] [Google Scholar]
- Poon PW, Sun XD, Kamada T, Jen PHS. Frequency and space representation in the inferior colliculus of the FM bat, Eptesicus fuscus. Exp Brain Res. 1990;79:83–91. doi: 10.1007/BF00228875. [DOI] [PubMed] [Google Scholar]
- Roberts RC, Ribak CE. GABAergic neurons and axon terminals in the brainstem auditory nuclei of the gerbil. J Comp Neurol. 1987a;258:267–280. doi: 10.1002/cne.902580207. [DOI] [PubMed] [Google Scholar]
- Roberts RC, Ribak CE. An electron microscopic study of GABAergic neurons and terminals in the central nucleus of the inferior colliculus of the cat. J Neurocytol. 1987b;16:333–345. doi: 10.1007/BF01611345. [DOI] [PubMed] [Google Scholar]
- Rockel AJ, Jones EG. The neuronal organization of the inferior colliculus of the adult cat. I. The central nucleus. J Comp Neurol. 1973;147:11–60. doi: 10.1002/cne.901470103. [DOI] [PubMed] [Google Scholar]
- Saint Marie RL, Morest DK, Brandon CJ. The form and distribution of GABAergic synapses on the principle cell types of the ventral cochlear nucleus of the cat. Hearing Res. 1989;42:97–112. doi: 10.1016/0378-5955(89)90120-2. [DOI] [PubMed] [Google Scholar]
- Schneiderman A, Oliver DL. EM autoradiographic study of the projections from the dorsal nucleus of the lateral lemniscus: a possible source of inhibitory inputs to the inferior colliculus. J Comp Neurol. 1989;286:28–47. doi: 10.1002/cne.902860103. [DOI] [PubMed] [Google Scholar]
- Shimozawa T, Sun XD, Jen PHS. Auditory space representation in the superior colliculus of the big brown bat, Eptesicus fuscus. Brain Res. 1984;311:289–296. doi: 10.1016/0006-8993(84)90091-x. [DOI] [PubMed] [Google Scholar]
- Simmons JA, Fenton MB, O’Farell MJ. Echolocation and pursuit of prey by bats. Science. 1979;203:16–21. doi: 10.1126/science.758674. [DOI] [PubMed] [Google Scholar]
- Suga N. Recovery cycles and responses to frequency modulated tone pulses in auditory neurons of echolocating bats. J Physiol (Lond) 1964;175:50–80. doi: 10.1113/jphysiol.1964.sp007503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga N. Echo-ranging neurons in the inferior colliculus of bats. Science. 1970;170:449–452. doi: 10.1126/science.170.3956.449. [DOI] [PubMed] [Google Scholar]
- Suga N, Schlegel P. Neural attenuation of responses to emitted sound in echolocating bats. Science. 1972;177:82–84. doi: 10.1126/science.177.4043.82. [DOI] [PubMed] [Google Scholar]
- Suga N, Schlegel P. Coding and processing in the auditory system of FM signal producing bats. J Acoust Soc Am. 1973;54:174–190. doi: 10.1121/1.1913561. [DOI] [PubMed] [Google Scholar]
- Sun XD, Chen QC, Jen PHS. Corticofugal control of central auditory sensitivity in the big brown bat, Eptesicus fuscus. Neurosci Lett. 1996;212:131–134. doi: 10.1016/0304-3940(96)12788-9. [DOI] [PubMed] [Google Scholar]
- Syka J, Popelar J, Druga R, Vlkova A. Descending central auditory pathway structure and function. In: Syka J, Masterton RB, editors. Auditory pathway: structure and function. Plenum Press; New York: 1988. [Google Scholar]
- Tsuzuki K, Suga N. Combination-sensitive neurons in the ventroanterior area of the auditory cortex of the mustached bat. J Neurophysiol. 1988;60:1908–1923. doi: 10.1152/jn.1988.60.6.1908. [DOI] [PubMed] [Google Scholar]
- Vater M, Habbicht H, Kossl M, Grothe B. The functional role of GABA and glycine in monaural and binaural processing in the inferior colliculus of horseshoe bats. J Comp Physiol. 1992;171:541–553. doi: 10.1007/BF00194587. [DOI] [PubMed] [Google Scholar]
- Wenstrup JJ, Grose CD. Inputs to combination-sensitive neurons in the medial geniculate body of the mustached bat: the missing fundamental. J Neurosci. 1995;15:4693–4711. doi: 10.1523/JNEUROSCI.15-06-04693.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong D, Maekawa M, Tanaka H. The effect of pulse repetition rate on the delay sensitivity of neurons in the auditory cortex of the FM bat, Myotis lucifugus. J Comp Physiol. 1992;170:393–402. doi: 10.1007/BF00191456. [DOI] [PubMed] [Google Scholar]
- Wu M, Jen PHS. Encoding of stimulus intensity by inferior collicular neurons of the big brown bat, Eptesicus fuscus. Chin J Physiol. 1991;34:145–155. [PubMed] [Google Scholar]
- Yan J, Suga N. The midbrain creates and the thalamus sharpens echo-delay tuning for the cortical representation of target-distance information in the mustached bat. Hearing Res. 1996;93:102–110. doi: 10.1016/0378-5955(95)00209-x. [DOI] [PubMed] [Google Scholar]
- Yang L, Pollak GD, Resler C. GABAergic circuits sharpen tuning curves and modify response properties in the mustache bat inferior colliculus. J Neurophysiol. 1992;68:1760–1774. doi: 10.1152/jn.1992.68.5.1760. [DOI] [PubMed] [Google Scholar]
- Zook JM, Casseday JH. Convergence of ascending pathways at the inferior colliculus of the mustache bat, Pteronotus parnellii. J Comp Neurol. 1987;261:347–361. doi: 10.1002/cne.902610303. [DOI] [PubMed] [Google Scholar]
- Zook JM, Winer JA, Pollak GD, Bodenhamer RD. Topology of the central nucleus of the mustache bat’s inferior colliculus: correlation of single unit properties and neuronal architecture. J Comp Neurol. 1985;231:530–54. doi: 10.1002/cne.902310410. [DOI] [PubMed] [Google Scholar]