Abstract
A new spontaneous mouse mutation named fierce (frc) is deleted for the nuclear receptor Nr2e1 gene (also known as Tlx, mouse homolog of Drosophila tailless). The fierce mutation is genetically and phenotypically similar to Nr2e1 targeted mutations previously studied on segregating genetic backgrounds. However, we have characterized the fierce brain, eye, and behavioural phenotypes on three defined genetic backgrounds (C57BL/6J, 129P3/JEms, and B6129F1). The data revealed many novel and background-dependent phenotypic characteristics. Whereas abnormalities in brain development, hypoplasia of cerebrum and olfactory lobes, were consistent on all three backgrounds, our novel finding of enlarged ventricles in 100% and overt hydrocephalus in up to 30% of fierce mice were unique to the C57BL/6J background. Developmental eye abnormalities were also background-dependent with B6129F1-frc mice having less severe thinning of optic layers and less affected electroretinogram responses. Impaired regression of hyaloid vessels was observed in all backgrounds. Furthermore, retinal vessels were deficient in size and number in 129P3/JEms-frc and B6129F1-frc mice but almost entirely absent in C57BL/6J-frc mice. We present the first standardized behavioural tests conducted on Nr2e1 mutant mice and show that C57BL/6J-frc and B6129F1-frc mice have deficits in sensorimotor assays and are hyperaggressive in both sexes and backgrounds. However, C57BL/6J-frc mice were significantly more aggressive than B6129F1-frc mice. Overall, this extensive characterization of the fierce mutation is essential to its application for the study of behavioural, and brain and eye developmental disorders. In addition, the background-dependent differences revealed will enable the identification of important genetic modifiers.
Keywords: Aggressive behaviour, Sensorimotor, Eye, Brain
1. Introduction
The study of heritable behaviour has been impeded by an evolving code of human ethics and a relative lack of animal models for behaviour [3]. Recently, behavioural studies using mouse model systems have emerged based on the powerful mouse genetics of single gene mutations. Using mouse models, numerous genes have been reported to contribute to reproductive, maternal, ingestive, and social behaviours [2,29,31]. In addition, genes involved in serotonin metabolism [6,25], adrenergic alpha2C receptor, neuronal nitric oxide synthase [30], and enkephalins [23] have been implicated in aggressive behaviour in mice. These models have provided interesting insights into portions of behaviour that are influenced genetically.
To interpret the effects of a genetic lesion accurately, genetic background must be considered [6,13,33]. This may be particularly relevant for variable phenotypes such as behaviour studied in highly inbred strains recognized as having identifiable characteristics [6,24]. Firstly, studying behavioural mutants on controlled backgrounds using inbred or F1 animals allows mutants to be compared with otherwise genetically identical littermates. This strategy also allows for reproducibility in time and place. Secondly, the use of multiple backgrounds highlights genetic variability and more closely parallels the differences between human individuals. This is especially true of F1 hybrid animals that provide the genetic variability of a heterozygous state at all loci on a controlled, reproducible, and non-segregating background. In addition, phenotypic differences between strains can lead to the identification of genetic modifiers in mice, and ultimately, in humans. Thus, the applicability of mouse genetics is enhanced by phenotypic assessment on multiple, defined backgrounds.
Two targeted disruption mutations in mice have been described for the gene Nr2e1 (nuclear receptor subfamily 2, group E, member 1), the mouse homologue of the Drosophila nuclear receptor tailless (also known as Tlx). These mutant mice have an extensive developmental defect of the anterior brain, aggressive behaviour [27], and severe impairment of retinal and optic nerve development [40]. However, a detailed characterization of the extent of aggressive behaviours has not been performed and this, along with brain and eye phenotypes, have only been described on mixed and segregating 129 and C57BL/6 genetic backgrounds. Furthermore, sensorimotor evaluation has not been performed on these mice—an evaluation of which is essential to the interpretation of other behaviours [5,29].
Here we report on a new mouse mutation named ‘fierce’ (frc) that includes a spontaneous deletion of Nr2e1. The frc mutation has been studied on three commonly used genetic backgrounds, C57BL/6J, 129P3/JEms, and B6129F1, to comprehensively examine behaviour, brain, and eye phenotypes [9,34]. Whereas many of the elements of the fierce phenotype are consistent on all three genetic backgrounds indicating their fundamental importance, we have identified some elements that are background-dependent, thus highlighting the genetic heterogeneity and potential for the identification of modifier genes in aggressive behaviour and brain development. In addition, we conducted a panel of sensorimotor, aggression, and mating tests on C57BL/6J and the hybrid B6129F1 mouse backgrounds. Such detailed behavioural characterization increases the value of this important mouse model permitting further inquiry not only into the consistent phenotypes of brain development and aggression but also into traits exposed on genetically distinct mouse backgrounds.
2. Methods
2.1. Mouse backgrounds
The frc mutation was not deliberately induced and thus, is categorized as a spontaneous mutation. It was recovered during a targeted mutagenesis experiment in which a null allele of the zinc finger protein, autosomal (Zfa) was derived using 129E14TG2a embryonic stem cells (from 129P2/OlaHsd mice [18]). Chimeras made by injecting the ES cells into C57BL/6J (JAX®, 000664) embryos were crossed to C57BL/6J mice. A single germline hybrid animal was used to initiate the backcrossing to generate C57BL/6J and 129P3/JEms (JAX®, 002357) congenic strains. Note: 129P3/JEms is the closest available relative to 129P2/OlaHsd [34]. Segregation of the Zfa and frc mutations occurred independently during the backcrossing to both C57BL/6J and 129P3/JEms resulting in the generation of four congenic strains; C57BL/6J-frc, C57BL/6J-ZfatmlEms, 129P3/JEms-frc, 129P3/JEms-ZfatmlEms. Here we report only on the characterization of the fierce mutants.
Animals for experimental analysis were generated by crossing heterozygotes such that controls and mutants are littermates. In accordance with Mendelian inheritance, 25% of the offspring are homozygous mutants. For the C57BL/6J background, animals studied were incipient congenics ranging from generation N6 to N7 (98.4–99.2% C57BL/6J), for 129P3/JEms they were N6 (98.4% 129P3/JEms), and for B6129F1 they were the offspring of C57BL/6J females (N6–N11, 98.4–99.9% C57BL/6J) mated to 129P3/JEms males (N5–N8, 96.9–99.6% 129P3/JEms). Some early experiments were conducted on C57BL/6J animals at N3 (87.5% C57BL/6J: body weights, fat pads, testosterone, and brain morphology) and on 129P3/JEms animals at N4 (93.8% 129P3/JEMS: brain morphology). We observe no changes in the reported phenotypes in these animals, now congenic at >N10 (>99.9% pure). To date we have not identified a heterozygous phenotype in any of the backgrounds studied, indicating that the frc mutation is recessive. Thus, for this study, controls were defined as wild-type or heterozygous mice.
2.2. Southern and northern blots
A Southern blot of BglII-digested mouse DNA (from B6129F1 of N6 parents) was hybridized as previously described [35] with a probe for zinc finger autosomal (Zfa) (1.4 kb SspI genomic fragment from pEMS229), and then Nr2e1 (1078 bp reverse transcription-polymerase chain reaction (RT-PCR) product from embryonic brain including almost all the coding region using oEMS294 TTGCAAAGTGTGTGGTGACC and oEMS295 TCTTGTAATCGGCACATTGC, subsequently cloned as pEMS741). A northern blot of total RNA from adult liver, brain, and testis (from B6129F1 at N6) was hybridized with the same Nr2e1 probe and sequentially with a human GAPDH probe (pHcGAP 1.25 kb PstI cDNA fragment [38]).
2.3. Growth experiments
Body weight of C57BL/6J mice (N3) was recorded from embryos at e12.5, newborns, and then weekly from 1 to 10 weeks of age from females (ten frc, 27 controls), and males (nine frc, 24 controls). Body weight was also recorded from frc and control 3-week-old mice from all three backgrounds at N6 (C57BL/6J n = 86, 129P3/JEms n = 88, B6129F1 n = 143). Total fat was analyzed from 3-month-old C57BL/6J mice at N3 (four frc, eight controls). Fat pads were removed and weighed from gonadal, inguinal, mesenteric, and peritoneal regions [37]. Total fat was the sum of all fat pads from each animal.
2.4. Brain morphology
Mice were injected with a lethal dose of tribromoethanol (Avertin), flushed with saline, and perfused with Bouin's fixative. Brains of 24 C57BL/6J at N3 (two frc and four controls from each of four time points: 1 week, 1, 3, and 6 months), ten adult 129P3/JEms at N4 and 28 B6129F1 at N6 (seven adults, ten 1-week old, 11 newborns) mice were processed for serial sectioning and stained with hematoxylin and eosin (H and E), or Luxol fast blue/Cresyl violet (LFB-CV). Sections from the 3-month-old C57BL/6J mice were also stained for glial fibrillary acidic protein (GFAP) using an avidin–biotin-labeled immunoperoxidase and a GFAP antibody (Sigma, St. Louis, MO). Brains of B6129F1 embryos at N6–N10 (13 at e16.5, nine at e14.5, and 16 at e12.5) were harvested immediately from CO2-euthanized mothers and stained with H and E.
2.5. Retinal histology
Eyes were removed immediately from CO2-euthanized mice, placed in Telly's fixative, and processed routinely for H and E sections from 18 mice, 11–12 weeks old (three frc and three littermate controls from all three backgrounds at N6–N7). In addition, H and E sections were examined from three frc and three littermate controls at e16.5 from B6129F1 (N10 and N8).
2.6. Retinal morphometry
The thickness of the retinal layers was measured in one eye from 22 mice 11–12 weeks old at N6–N7 (12 controls: C57BL/6J, two males, two females; 129P3/JEms, two females; B6129F1, four females, two males. Ten fierce: C57BL/6J, one male, one female; 129P3/JEms, two females; B6129F1, two females, four males). The plane of section studied included the optic nerve and six measurements were made from a standardized location in each eye; three temporal and three nasal of the nerve. (1) The full thickness of the retina from internal limiting membrane to apex of retinal pigment epithelium. (2) The inner nuclear layer from innermost to outermost nucleus. (3) The photoreceptor nuclear layer from innermost nucleus to external limiting membrane.
2.7. Electroretinograms
Electroretinograms (ERGs) were performed on 7–12 week old control and frc mice from all three backgrounds (C57BL/6J and 129P3/JEms at N6, B6129F1 at N7–N10) as previously described [15]. Briefly, prior to dark adaptation of 60 min, the eyes were dilated with 1% topical atropine. Once dark adapted, mice were anesthetized typically with a mixture of 0.46 mg xylazine and 0.72 mg ketamine injected subdermally. Reference and ground were standard EEG needle electrodes placed subdermally over the mandibles of the anesthetized mouse, while the active electrode was a platinum wire (Grass E2, Quincy, MA) placed so it was gently touching the superior cornea. Several intensities were used to stimulate the evoked response using the Grass photostimulator and standard neutral density filters.
2.8. Behavioural testing
2.8.1. Behaviour tests
Twenty-four control and three fierce males, and 20 control and five fierce females of the C57BL/6J background (N6) and 11 control and 11 fierce males, and six control and six fierce females of the B6129F1 background (from N6–N10 mothers and N6–N8 fathers) were evaluated for behaviour. Within each background, mice were either littermates or close relatives. All animals were coded with randomized experimental numbers although the ‘hard to handle’ phenotype made it difficult to conduct a completely blind study. Mice were 80±16 days of age at the onset of testing, and were housed individually in polycarbonate cages (28×17×12 cm) with ad libitum access to both tap water and food, except where noted (Agway Prolab 2000, Syracuse, NY). A 16:8 h light:dark cycle was maintained for the duration of the study (lights on at 07:00 h Eastern Standard Time), with ambient temperature kept at 21±2 °C and relative humidity 50±5% throughout the behavioural study. All animals completed the full panel of tests.
2.8.2. Sensorimotor tests
The following standardized sensorimotor tests were performed on three separate trials as previously described [22] and were completed prior to the onset of aggression and mating tests. Olfactory ability was assessed with the Find the Hidden Cookie test conducted immediately after a 12 h (C57BL/6J) or 6 h (B6129F1) period of food deprivation. A chocolate chip cookie was hidden beneath 3–6 cm of wood shavings in an aquarium. Each mouse was released into the aquarium and the time required to locate the cookie was recorded for a maximum of 10 min. Vision and depth perception were assessed with the Visual Placing test: mice lowered towards the edge of a table by their tail were assigned a positive score if they extend their forepaws prior to touching the table on >2 of three trials. Gait, locomotion, agility, and motor coordination were assessed with the combination of tests: Turning in a Blind Alley, and Turning on an Inclined Screen. In the former, the mouse was placed facing the back wall of an alley (3-cm wide, 35-cm long, with walls 15-cm high). The amount of time for the mouse to turn around and face the open end of the alley was recorded. In the latter, the mouse was placed at the center of a wire mesh screen (35 cm2) that was tilted at a 45° angle approximately 75 cm above a large pillow. The mouse was placed on the screen facing downward, and the time taken by the mouse to turn to face upward was recorded. In both tests, the time to turn was recorded in a 2-min period. Exploratory behaviour was assessed with the Elevated Plus Maze test: mice were placed in the center of an elevated plus maze with two open arms (67×5.5 cm) and two closed arms (67×5.5 cm with 15-cm high black-tinted plexiglass walls and a 65-cm removable roof) placed 75 cm above the floor on a tripod. The number of visits to each arm and the time spent in each arm, as well as in the central area were recorded for 5 min [5]. Hearing/auditory function was assessed by the Click and Tone-Pip Stimuli test [19].
2.8.3. Aggression tests
Aggression and mating tests were performed as previously described [22]. Male and female experimental mice in their home cages were presented with a control same-sex adult mouse in the Aggression Against Intruder in Home Cage test. Male experimental mice were presented with a control adult male in a neutral cage in the Aggression in a Neutral Arena test. In both tests, the duration of each hostile encounter, and the number of aggressive encounters during a 15-min test was recorded. The test was immediately aborted if a mouse became injured. If injured, mice were removed and had their wounds treated and dressed daily until healed. A unique pairing was made for each test.
2.8.4. Mating tests
Males of the C57BL/6J background were allowed to acclimate to a home cage for 5 days prior to testing. Control females were primed with 20 μg of 17-β-estradiol 48 h prior to testing, and 500-μg progesterone 5 h prior to testing. Females that displayed lordosis were selected using stud males, and then individually introduced to the mating arena with the experimental male. Latency to begin mounting behaviour, the number of mounts, and the number of aggressive encounters towards females were recorded as previously described [30].
2.9. Testosterone and corticosterone RIA
Coritcosterone levels were measured on all mice used for behaviour testing. Blood samples were collected via retro-orbital bleeding into iced heparinized tubes from lightly anesthetized mice 2 days before and 2 days after all behavioural testing. Plasma was stored at −80 °C so that all samples could be processed in a single batch. Corticosterone concentrations were assayed in duplicate by RIA 125I kits (ICN Biomedicals, Costa Mesa, CA). Testosterone levels were measured on C57BL/6J mice at N3 (males: eight frc and 16 controls; females: five frc and ten controls). Blood collection and serum assay were performed as previously described [1].
2.10. Data analysis
Statistical evaluation of mean differences between fierce and control mice were performed by t-tests, or, for measurements lacking equal variance, by a Mann–Whitney Rank Sum Test on Ranks using the Sigma Stat software package (Jandel Scientific, San Rafael, CA) or the JMP statistical software (SAS, Cary, NC). Males and females were analyzed separately. Mean differences were considered statistically significant when P<0.05. Differences not found to be significant were not interpreted if the tests had less than 80% power. Analyses of retinal thickness between all three backgrounds were done with an ANOVA or, for measurements lacking equal variance, with Kruskal–Wallis ANOVA using the JMP. Data are reported as mean values±1 S.E. of the mean.
3. Results
3.1. The fierce mutation includes a deletion of the nuclear receptor Nr2e1
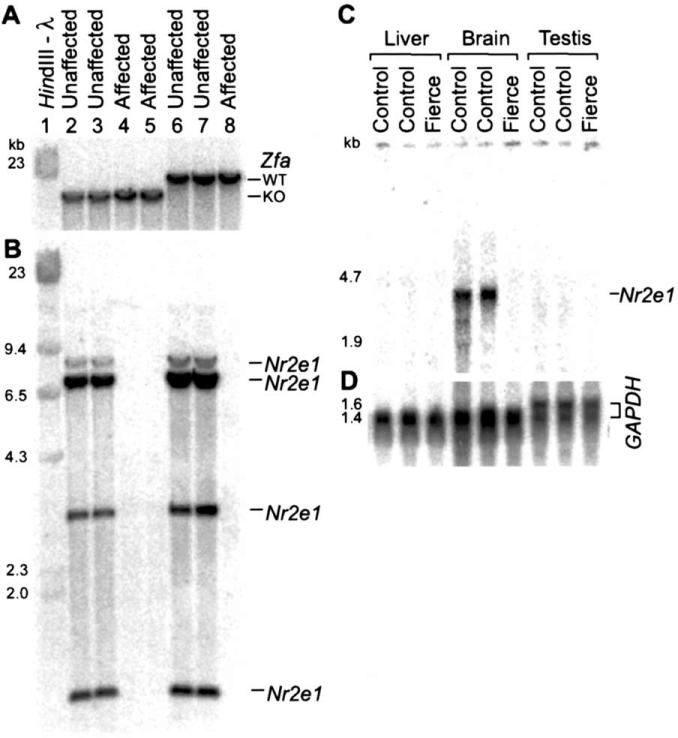
We have developed a mouse mutation named fierce (frc) due to its dramatically violent behaviour. Preliminary characterization also revealed that frc mice had stunted growth, hypoplasia of the forebrain regions, and lack of maternal instinct. The frc mutation is a spontaneous mutation recovered during a targeted mutagenesis experiment of the zinc finger protein, autosomal (Zfa). Although the phenotypic characteristics were originally attributed to Zfa, analyses of subsequent generations showed segregation of the Zfa targeted allele and the frc mutation as defined by the ‘hard to handle’ behaviour. The independence of the fierce phenotype from the Zfa null allele is demonstrated in Fig. 1A where mice unaffected for handling behaviour are homozygous mutant for Zfa and a mouse affected for handling behaviour is wild type at the Zfa locus. The frc and Zfa mutations have been bred apart and are now maintained in separate mouse colonies. Subsequent genetic analyses have confirmed consistently the association between the frc mutation and the handling behaviour. Because the fierce mutation was an unknown spontaneous mutation, we initiated a candidate gene search for the responsible locus. Included in this search was the Nr2e1 gene, since mice targeted for this locus show a reduced body weight, cerebral and olfactory hypoplasia, lack of maternal behaviour, and aggression [27]. As expected, the phenotypic similarities turned out to be more than coincidental; fierce includes a deletion of the coding region of Nr2e1. The Southern blot presented in Fig. 1B shows that mice affected with abnormal handling behaviour are homozygous deleted for Nr2e1, but unaffected mice maintain the Nr2e1 gene. Northern blots confirmed the lack of an Nr2e1 transcript in the brains of frc mice, but strong expression in the brains of control littermates (Fig. 1C and D).
Fig. 1.
(A and B) Southern blot analyses of genomic DNA from mice show that the ‘hard to handle’ behaviour is associated with deletion of Nr2e1, not Zfa. (A) Hybridization with Zfa probe demonstrates unaffected Zfa mutant (KO) animals (lanes 2 and 3) and one affected wild-type (WT) (lane 8). (B) Same Southern hybridized with Nr2e1 probe demonstrates that the gene is deleted in all affected mice and is present in all unaffected mice studied. (C) Northern analysis of adult total RNA shows that fierce brain lacks the Nr2e1 transcript present in control littermates. The Nr2e1 transcript is absent in two control tissues, liver, and testis. Size markers are 28S and 18S RNA. (D) Same northern hybridized with GAPDH as quantitative control.
3.2. Two congenics permitted comparison of three genetic backgrounds
Each of the inbred strains used (C57BL/6J or 129P3/JEms) had advantages and disadvantages for the study of this mutation. C57BL/6J-frc mice were highly aggressive but developed hydrocephalus with a frequency that increased with increasing backcross generation, eventually leading to 30% mutant animals with overt hydrocephalus. Such mice do not make an ideal model for the study of other brain abnormalities. In contrast, the 129P3/JEms-frc mice showed little or no hydrocephalus but had such small litters that it was difficult to produce sufficient animals for behavioural studies. We, therefore, developed B6129F1-frc hybrids by breeding C57BL/6J-frc heterozygous females to 129P3/JEms-frc heterozygous males. This breeding scheme produces C57BL/6J-like sized litters, and the F1 hybrid mutant animals showed little or no hydrocephalus. F2 animals, produced from F1 × F1 crosses, have mixed, segregating backgrounds, and thus, were not studied. Hence, we generated three genetic backgrounds for study from two congenic strains [33].
3.3. Fierce mice have stunted growth parameters
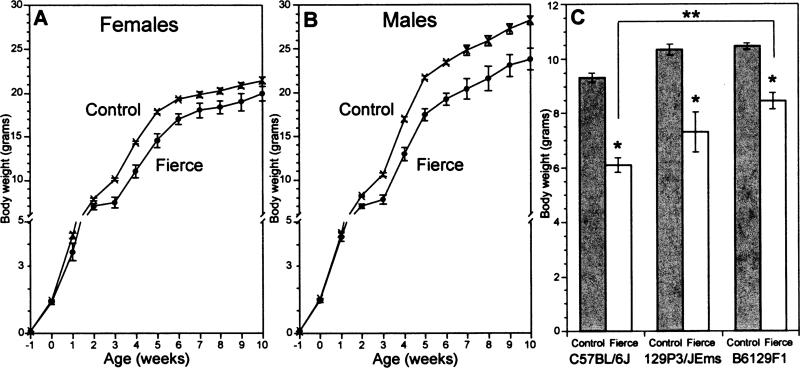
Fierce mice are, on average, smaller than their control littermates just as described for Nr2e1 targeted mutant mice [27]. This observation was true for both male and female fierce mice (Fig. 2A and B). The growth differential is not present in embryos or in newborn pups but develops between postnatal weeks 2 and 3. Having either not gained or, in some cases, lost weight during this period, on average, the mutant animals remain smaller than littermates as adults. In addition, as a percentage of total body weight, the mean total fat pads from frc mice was significantly less than those from controls (for C57BL/6J: frc 3.1±1.0% vs. control 5.7±2.0%). Although some of the difference in weight is attributable to the fact that the frc mutants are generally leaner than their littermates, the mutants also show a trend to be smaller on average from nose to rump. These observations were true for mice from all three backgrounds although the C57BL/6J-frc mice were significantly more affected than the B6129F1-frc mice; mean weight at 3 weeks was 6.0±0.3 g for B6129F1, and 8.2±0.3 g for B6129 (P<0.001, Fig. 2C).
Fig. 2.
Fierce mice show postnatal growth failure. (A) Female and (B) male frc pups born of heterozygous C57BL/6J-frc parents fail to gain weight at the rate of their littermates, beginning at week 2. Age −1 is e12.5 and 0 is birth. (C) All three genetic backgrounds show smaller homozygous mutant mice at 3 weeks of age; data from both sexes are pooled. *, P<0.05. C57BL/6J: 25 frc, 61 controls; 129P3/JEms: 14 frc, 74 controls; B6129F1, 36 frc, 107 controls. **, P<0.001.
3.4. Fierce mice have abnormal brain development
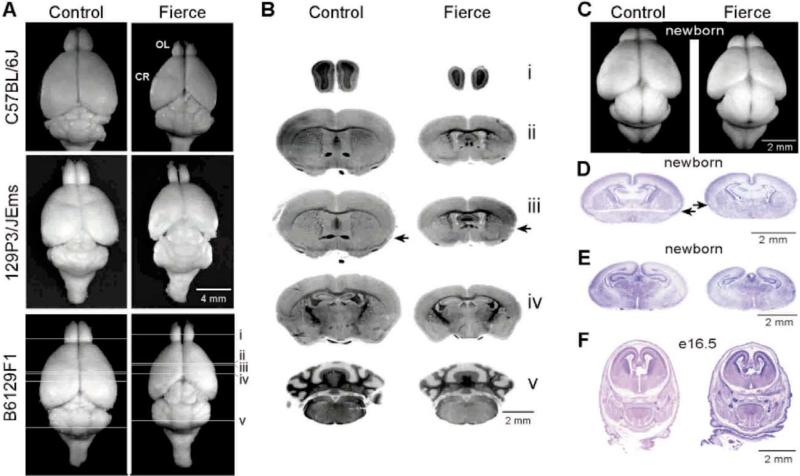
Histopathological examination of the brains from frc mice identified hypoplasia and distortion of the anterior aspects of the brain in mice from all three backgrounds. In a mouse with a mild reduction in size of the whole animal, it is not normal to see reduction in brain size [32,36]. In fact, whereas the cerebellar regions in frc mice were not different from controls, the cerebrum and olfactory regions of the fierce brain were disproportionately reduced (Fig. 3A). The fierce cerebrum appeared flattened and did not overhang the rostral colliculi. Despite gross hypoplasia, no nuclei or tract was completely absent in homozygous fierce brains. The rostral commissure was almost always thinner in frc mice. In the severe example shown, this commissure was small throughout its course and was particularly thin and wispy as it crossed the midline (Fig. 3B and D). Overall, the developmental brain abnormalities in adult mice homozygous for frc were similar to those described for mice homozygous for the targeted null allele of Nr2e1 [27].
Fig. 3.
Fierce mice have affected brains as adults, newborns and at embryonic day 16.5. (A) Whole brains of frc adult mice are differentially smaller in the anterior regions and have hypoplastic cerebrum (CR) and olfactory lobes (OL) on all three backgrounds. (B, D and E) Coronal sections from B6129F1-frc mice stained with Luxol fast blue (myelin)/Cresyl violet (neurons) show gross hypoplasia and distortion of the anterior aspects of the brain. The rostral commissure (indicated by an arrow) is malformed; in some animals, it is dramatically thin and wispy as it crosses the midline. (C) Whole brain of newborn B6129F1-frc mouse also shows hypoplastic cerebrum and olfactory lobes. (F) Coronal sections from B6129F1 day 16.5 embryos stained with H and E show early evidence of morphological changes.
We extended our studies to determine whether the brain phenotype was due to degenerative or developmental abnormalities. As an assessment of neural degeneration, immunohistochemical staining for GFAP was performed on sections from 3-month-old mice. The brains of all mice stained with no evidence of reactive gliosis. A histological comparison of LFB-CV sections from frc brains at different time points revealed no obvious difference between 3 and 6 months of age. The distinctive morphology evident in adult frc mice was also present in frc brains at 1 month and 1 week of age, although less prominent in the lesser developed brain. Examination of newborn and embryonic B6129F1-frc brains revealed that the pathological changes were observed consistently in newborns (Fig. 3C, D and E), and hypoplasia of the anterior brain was evident even in embryos at e16.5 (Fig. 3F) but not at e14.5 or e12.5. This observation is consistent with the expression of Nr2e1 in the developing brain, which peaks at e13 [28]. Taken together, the lack of GFAP staining and the patterning of pathological changes that parallels development suggest that frc causes a developmental abnormality and not degeneration of a normally developed brain.
A novel observation in the C57BL/6J-frc mice was an increased risk of hydrocephalus with as many as 30% of frc mice displaying overt hydrocephalus and all frc mice showing enlarged ventricles. In contrast, few, if any, of the frc mice had enlarged ventricles on the 129P3/JEms or B6129F1 backgrounds.
3.5. Fierce mice have impaired ocular development
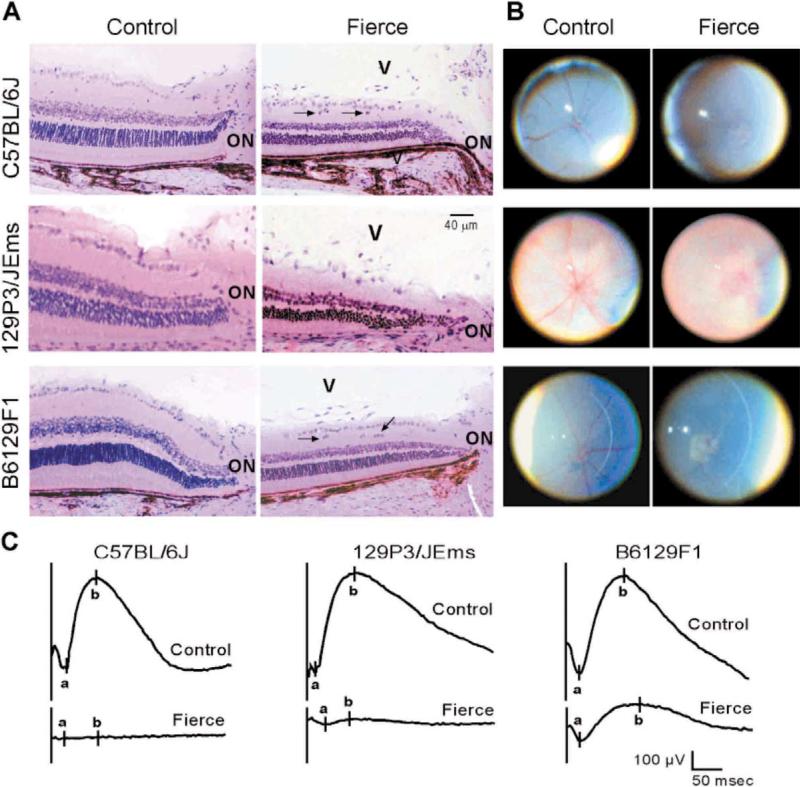
The retinas of all frc mice studied showed abnormalities compared with littermate controls. The frc retinas showed either a poorly defined or absent nerve fiber layer and thinner inner nuclear and photoreceptor layers than the controls (three cells thick vs. eight to ten cells). In addition, the ganglion cell layer showed incomplete differentiation, with cell nuclei remaining in the inner plexiform layer resembling the developing retina characteristic of a day 14–15 embryo (Fig. 4A). Furthermore, the optic nerves were smaller in diameter and the architecture was disrupted with collapse of the pial septa due to the absence of optic nerve axons. Since the nerve fibres of the optic nerve arise from the retinal ganglion cells, the two observations are likely to be related [16]. These ophthalmic changes are consistent with those described for an Nr2e1 targeted mutant mouse [40]. However, a novel observation from the studies of retinas from B6129F1-frc day 16.5 embryos showed that, although the retinal thickness looked normal, the inner retinal layer showed retarded separation into bipolar and ganglion cell layers. This observation likely explains the misplaced cells in the inner plexiform layer of adult frc mice and suggests that the eye phenotype is, at least partly, developmental.
Fig. 4.
Fierce mice have abnormal ocular development. Representative examples are shown from all three backgrounds. (A) Retinas stained with H and E show thinner inner nuclear and photoreceptor layers in 11–12 week old frc mice. Arrows indicate displaced retinal ganglion cells. Optic nerves (ON) and inappropriate hyaloid vessels (V) are labeled. (B) Fundus photos show diminished vascularization of the retina in 8-week-old frc mice compared to controls. (C) Electroretinograms from 8-week-old mice show impaired maximal response from a 10-μs single flash stimulation (intensity, 73 foot-lamberts-sec) vs. littermate control.
To determine the effects of background on this phenotype, we measured the thickness of retinal layers and optic nerve in male and female frc and control mice from all three backgrounds. In all three backgrounds, frc mice showed thinner layers than control mice. This was significant for all six layers in C57BL/6J mice (P<0.003) but in only two layers for the 129P3/JEms mice (photoreceptor temporal P<0.04, full nasal P<0.02) and four layers for B6129F1 mice (bipolar temporal P<0.02, photoreceptor temporal P<0.002, bipolar nasal P = 0.05, and photoreceptor nasal P<0.006). The C57BL/6J mice had a trend for all retinal layers to be thicker than in the other two backgrounds. This reached statistical significance for the bipolar nasal layer in fierce mice (P<0.03) and for four of the layers in control mice (full temporal P<0.007, photoreceptor temporal P<0.03, full nasal P<0.008, bipolar nasal P<0.03).
Impaired regression of the hyaloid vascular system of the eye was a novel observation in frc mice on all three backgrounds. These blood vessels, present in the developing mouse, normally start to involute between post-natal day 5–10 and disappear completely by 1 month of age [20]. However, in adult frc mice on all three backgrounds, large and small vascular channels were present in the vitreous, close to the optic nerve (Fig. 4A) and the peripheral retina. It appeared that some vessels were coming directly out of the retina into the vitreous rather than via central vessels. Since the embryonic hyaloid vascular system never develops vessels of this diameter, it seems likely that these are hyaloid vessels that have failed to undergo apoptosis and have, perhaps, enlarged. There was no evidence for a retinal origin of these vessels. In contrast to the extraneous vessels in the vitreous, frc mice lacked a normal pattern of retinal vessels. Examination of the fundus of the eyes revealed that retinal vessels were small in size and few in number in all 129P3/JEms-frc and B6129F1-frc mice studied but were mostly absent in C57BL/6J-frc mice (Fig. 4B).
The electroretinograms (ERGs) of adult frc mice were consistently abnormal on all three backgrounds (Fig. 4C). The ERG signal was non-detectable with single flash methods in C57BL/6J-frc mice, supporting the phenotype of the Nr2e1 targeted mutant [40]. An identical profile was found with frc mice from the 129P3/JEms background. However, background-dependent variation was apparent for B6129F1-frc mice whose ERGs demonstrated a b-wave amplitude of 207 V (~25% of normal) in all six animals studied.
3.6. Auditory function
No deficiencies in auditory-evoked brainstem response were detected in 4-month-old C57BL/6J-frc male mice using click and tone-pip stimuli [21]. Both heterozygotes and homozygotes were compared with standard hearing for C57BL/6J [41] and no differences were detected (data not shown).
3.7. Fierce mice lack maternal behaviour, are ‘hard to handle’ and violently aggressive
Female frc mice lack maternal behaviour, often choosing to abandon their nest rather than nurse their pups. Furthermore, both male and female frc mice display a ‘hard to handle’ phenotype, responding to being held by the tail by vocalizing, struggling, jumping and biting. These behaviours are identical to those described for the targeted mutant mouse [27]. In addition, these behaviours are consistently expressed in mice from all three backgrounds; the lack of maternal behaviour is less evident on the 129P3/JEms and B6129F1 backgrounds. We also observed that the B6129F1-frc mothers gave birth to the same number of pups as controls but that the lack of maternal behaviour caused a reduction in the number of pups surviving to wean (average # pups weaned: 9.2±1.5 vs. 5.8±0.7, P<0.009 for control vs. frc, respectively). In general, C57BL/6J-frc female mice had decreased fertility, presumably due to the presence of hydrocephaly. However, among successful matings, female frc mice had the same number of pups as controls, none of which survived to wean (average # pups weaned: 5.8±1.3 vs. 0, P<0.02 for control vs. frc, respectively).
More extraordinary than the ‘hard to handle’ phenotype, was the violent (i.e. drawing blood) aggressive behaviour displayed by frc mice starting at about 5 weeks of age. Despite their smaller stature, frc males routinely and repeatedly attacked their siblings, occasionally leading to death if the frc males were not separated. Male violence was also directed toward mating partners. In a retrospective analysis of initial breeding experiments designed to test fertility in Zfa C57BL/6J mutants, females in every mating were either wounded or killed. These devastating results have precluded the use of frc males in any subsequent mating experiments. Although aggression to this degree is occasionally seen in certain rare inbred strains, we have never seen it in C57BL/6J, 129P3/JEms, or B6129F1 backgrounds or in control littermates from these frc colonies. We, therefore, conducted standardized testing of sensorimotor skills and aggressive behaviour.
3.8. Fierce mice show deficits in standardized sensorimotor tests
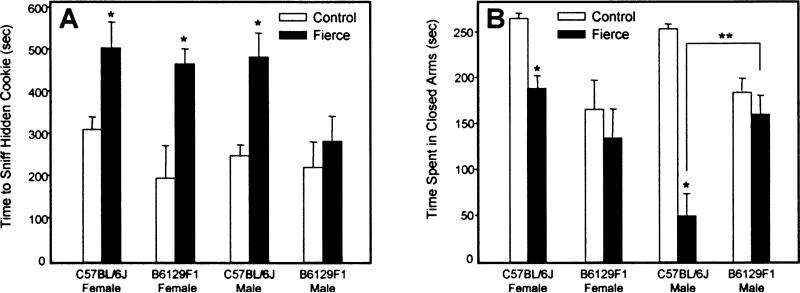
The lower productivity of the 129P3/JEms strain rendered it impractical to obtain quantities of age-matched frc mice and so sensorimotor and behaviour tests were only conducted on the C57BL/6J and B6129F1 backgrounds (Table 1). Female frc mice from both backgrounds showed diminished but functional ability to find the hidden cookie but took longer than controls to sniff and nibble it. Likewise, male C57BL/6J-frc mice took longer than controls to sniff the cookie (Fig. 5A). Male C57BL/6J-frc mice also showed deficits in visual placing compared with controls. When lowered to the edge of a table, frc mice extended their paws less often, scoring an average of 0.22±0.2 compared with 0.72±0.1 for controls (P = 0.04).
Table 1.
Sensorimotor tests reveal differences between fierce and control mice; mean values ± (S.E.M.)
| Genotypes | C57BL/6J male | P-value | C57BL/6J female | P-value | B6129F1 male | P-value | B6129F1 female | P-value | |
|---|---|---|---|---|---|---|---|---|---|
| Time to sniff hidden cookie (s) | Control | 254.7 (25.7) | 0.005 | 316.7 (27.5) | 0.006 | 227.0 (58.9) | 0.47 | 201.2 (77.2) | 0.008 |
| Fierce | 488.9 (72.6) | 510.2 (56.3) | 288.8 (59.4) | 472.1 (34.9) | |||||
| Time to nibble hidden cookie (s) | Control | 463.1 (22.5) | 0.1 | 479.7 (24.0) | 0.004 | 290.6 (65.1) | 0.42 | 225.5 (71.3) | 0.002 |
| Fierce | 596.3 (63.6) | 600.0 (49.2) | 365.6 (63.6) | 536.1 (32.1) | |||||
| Visual placing | Control | 0.72 (0.1) | 0.04 | 0.73 (0.1) | 0.33 | 0.55 (0.3) | 1 | 2.08 (0.3) | 0.63 |
| Fierce | 0.22 (0.2) | 0.66 (0.1) | 0.55 (0.1) | 1.75 (0.3) | |||||
| Turning in a blind alley (s) | Control | 13.9 (1.2) | 0.82 | 12.3 (1.3) | 0.003 | 8.5 (0.9) | 0.24 | 18.8 (8.7) | 0.13 |
| Fierce | 13.0 (3.4) | 20.3 (2.6) | 10.8 (1.7) | 17.0 (5.3) | |||||
| Turning on an inclined screen (s) | Control | 10.6 (1.1) | 0.02 | 7.5 (3.2) | 0.14 | 6.3 (0.8) | 0.02 | 6.5 (0.9) | 0.008 |
| Fierce | 37.0 (17.1) | 12.2 (3.0) | 20.5 (6.4) | 18.9 (4.9) | |||||
| EPM: number of visits to open arms | Control | 0.3 (0.1) | 0.007 | 1.1 (0.3) | 0.58 | 2.2 (0.6) | 0.65 | 1.0 (1.5) | 0.1 |
| Fierce | 6.0 (3.1) | 0.7 (0.3) | 1.8 (0.5) | 2.1 (1.2) | |||||
| EPM: time in open arms (s) | Control | 0.68 (0.03) | 0.007 | 8.9 (4.6) | 0.55 | 19.1 (6.0) | 0.12 | 7.7 (4.1) | 0.1 |
| Fierce | 103.0 (55.0) | 10.7 (8.7) | 65.3 (19.7) | 55.4 (40.8) | |||||
| EPM: number of visits to closed arms | Control | 6.5 (0.6) | 0.8 | 5.8 (0.7) | 0.28 | 8.9 (0.7) | 0.001 | 6.3 (1.1) | 1.0 |
| Fierce | 4.0 (2.8) | 4.0 (1.2) | 4.7 (0.8) | 6.4 (1.4) | |||||
| EPM: time in closed arms (s) | Control | 259.0 (5.0) | 0.001 | 270.0 (4.3) | 0.001 | 188.5 (14.7) | 0.33 | 170.9 (26.9) | 0.5 |
| Fierce | 52.0 (22.6)a | 192.7 (13.7) | 164.3 (19.7)a | 145.5 (27.3) |
EPM, elevated plus maze. Note: comparisons with P-values > 0.05 lack sufficient power to exclude the null hypothesis.
P < 0.02 for male fierce mice, C57BL/6J versus B6129F1.
Fig. 5.
Sensorimotor deficits in fierce mice. *, P-value for frc vs. controls; **, P-value for inter-strain comparisons. (A) Time to sniff the hidden cookie. *, P<0.005 for male C57BL/6J; P<0.008 for female mice. (B) Time spent in closed arms, *, P<0.001; **, P<0.02.
All four groups of frc mice showed some degree of deficiency in motor tests (Table 1). Male frc mice on both backgrounds took three times longer than controls to turn on an inclined screen, as did female B6129F1-frc mice. Female C57BL/6J-frc mice took longer than controls to turn in an alley.
Differences in exploratory behaviour in frc mice were noted in the elevated plus maze test (Table 1; Fig. 5B). Male C57BL/6J-frc mice showed a preference for the unprotected open arms spending more time and more visits than controls. Moreover, male frc mice on both backgrounds avoided the closed arms compared with controls. B6129F1-frc males made fewer visits to closed arms than controls while C57BL/6J-frc males spent less time in closed arms than controls (Fig. 5B). This observation was also true for female C57BL/6J-frc mice. In inter-background comparisons, the time spent in closed arms by male frc mice was less in C57BL/6J mice than in B6129F1.
3.9. Aggressive behaviour is background-dependent in standardized tests
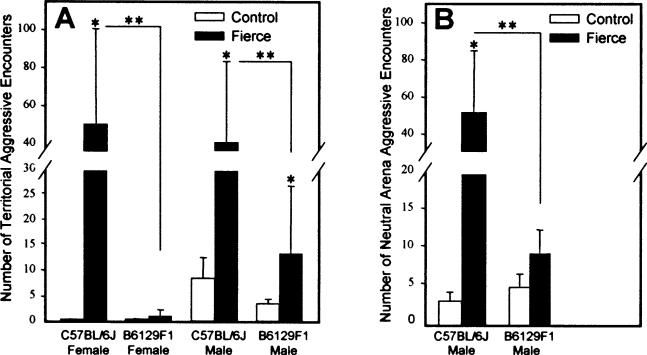
Male C57BL/6J-frc and B6129F1-frc mice were more aggressive than controls in home cage tests (C57BL/6J P<0.02, B6129F1 P<0.001, Fig. 6A). Male C57BL/6J-frc mice were also more aggressive than controls in neutral arena tests (P<0.01, Fig. 6B). In inter-background comparisons, frc males from the C57BL/6J background were more aggressive than those from B6129F1, attacking their intruders more often in both the home cage (P<0.002) and neutral arena (P<0.03) encounters. Importantly, this inter-background difference was not seen in control mice.
Fig. 6.
Aggressive behaviour in frc mice is more prominent in C57BL/6J mice. *, P-value for frc vs. controls; **, P-value for comparisons between backgrounds. (A) Home cage aggressive encounters C57BL/6J: *, P = 0.02 for males; *, P<0.001 for females; B6129F1: *, P<0.001 for males. **, P<0.002 for males; **, P<0.001 for females. (B) Neutral arena aggressive encounters, *, P<0.01; **, P<0.03.
Standard tests for female aggression are based on nest defense [12]. However, for these mutants, where a lack of maternal care is observed, the assessment of aggression during nest defense seemed inappropriate. Instead, an intruder paradigm equivalent to that used for male mice was employed with female intruders and residents. Aggressive behaviour was prominent in the female C57BL/6J-frc mice who engaged in 96% more aggressive attacks than controls in their home cage test (P<0.001, Fig. 6A). These females initiated all attacks, and persisted despite obvious displays of submission observed in the control mice. And, as observed for the males, inter-background differences were significant as female C57BL/6J-frc mice attacked intruders more often than did B6129F1-frc mice (P<0.001). This inter-background difference was not seen in the control mice.
3.10. Fierce mice show abnormal behaviour in mating tests
Latency to initiate mounting behaviour towards hormonally primed females was significantly longer in frc mice than in controls (1800±0.0 vs. 473±21.5 s, P<0.05). The total number of mounts was significantly less in the frc males than in the controls (0 vs. 21.5±4.1, P<0.05). During mating tests, frc mice engaged in ten-fold more aggressive encounters towards females than controls (10.7±8.3 vs. 1.1±0.6).
3.11. Corticosterone and testosterone concentrations are not abnormal in fierce mice
Concentrations of corticosterone from C57BL/6J and B6129F1 mice did not differ between backgrounds or between frc mice and sex-matched littermate controls. Testosterone concentrations from C57BL/6J mice did not differ between male frc and control mice (>80% power to detect a difference of 2.5 ng/ml at P = 0.05). No testosterone was detected above 0.1 ng/ml for female mice. All hormone concentrations fell within the standard range (Table 2).
Table 2.
Hormonal concentrations did not differ between fierce and control mice; mean values + (S.E.M.)
| Genotype | Sex | C57BL/6J | B6129F1 | |
|---|---|---|---|---|
| Testosterone (ng/ml) | Control | F | <0.1 | |
| Fierce | F | <0.1 | ||
| Control | M | 2.80 (0.96) | ||
| Fierce | M | 2.89 (1.63) | ||
| Corticosterone (ng/ml) | Control | F | 61.7 (4.4) | 190.4 (61.6) |
| Fierce | F | 72.0 (12.6) | 257.9 (28.4) | |
| Control | M | 25.8 (1.7) | 89.4 (20.8) | |
| Fierce | M | 47.6 (19.6) | 121.8 (19.3) |
4. Discussion
We have identified a novel spontaneous mouse mutation named ‘fierce’ that includes a deletion of Nr2e1. From phenotypic comparisons and molecular analyses, we conclude that the frc mutation is very similar to that introduced in Nr2e1 targeted mutant mice [27,40]. Further characterization of frc mice has identified novel phenotypes for this lesion that have not been reported for Nr2e1 targeted mutants. These include decreased body fat, increased incidence of hydrocephaly on the C57BL/6J background, failed regression of the hyaloid vasculature, absence of normal patterns of retinal vessels and retarded development in the embryonic retina. These distinct traits are most likely to be observed because of the extent of phenotypic characterization, the effects of genetic background, or both. Although we cannot exclude the possibility that the frc mutation includes genetic alterations beyond Nr2e1, preliminary data suggest the deletion is small (<50 kb) (unpublished data).
The importance of phenotyping genetic lesions on multiple backgrounds is three-fold. Firstly, the mere isolation of the frc mutation proffers the warning that genetic purification through backcrossing is imperative to associate genetic lesions with phenotypic outcome. Without the appropriate degree of backcrossing, the frc ‘hard to handle’ phenotype would have been inappropriately attributed to the targeted Zfa allele. Secondly, the identification of traits consistent on all backgrounds studied is suggestive of the fundamental functions of the gene that are likely to be consistent across species. Thirdly, through genetic purification, background-specific modulations of frc traits have been revealed. Such variable phenotypes in mice are important for the identification of potential genetic modifiers and as predictors of syndromes in humans.
A prominent background-dependent phenotype was overt hydrocephalus seen only in frc mice on the C57BL/6J background. In contrast, all other developmental brain abnormalities, as well as stunted growth, were consistent across all three backgrounds studied. These observations support the known predisposition of C57BL/6J inbred mice to hydrocephalus of 1–3% [7,8] and the known expression of Nr2e1 in brain development [28].
Background-dependent phenotypes were also observed among the ophthalmic abnormalities. On both inbred backgrounds studied, C57BL/6J and 129P3/JEms, the frc mutation resulted in more pronounced optic lesions than that found in the hybrid background, B6129F1. Furthermore, ERG testing showed the most diminished transmission in frc mice from the C57BL/6J and 129P3/JEms backgrounds. Visual placing tests of the C57BL/6J versus B6129F1 backgrounds paralleled these ERG results. Since the retina develops as an outgrowth of the anterior brain and Nr2e1 is expressed in the anterior brain and optic cup during development, the developmental defects in frc retina, optic nerves, and optic vessels are not surprising [17,28,40]. The role of Nr2e1 as a nuclear receptor important in eye development is well known [17]. However, the mechanism of action of Nr2e1 is not understood and our novel observation of failed regression of the hyaloid vasculature and absence of normal pattern of retinal vessels in frc mice adds new perspective to the role of Nr2e1 in eye development.
Background-dependence was not a prominent feature in sensorimotor tests. In both backgrounds, frc mice showed normal auditory function consistent with the fact that the ear develops from the hindbrain, a structure that normally lacks Nr2e1 expression [28]. In both backgrounds, frc mice showed functional, albeit reduced, olfactory abilities although we cannot exclude the possibility that differences in motivation may con tribute to the mice's ability to find a hidden cookie. Mating and suckling are olfactory-dependent activities [26]; although the frc mice can do both, neither is entirely normal. Furthermore, olfactory abilities are critical for maternal location of pups [5]. Thus, the olfactory deficit may play a role, albeit minor, when compared to the central nervous system defects, in these behaviours. Although the frc mice are blind or have reduced vision, this impairment is not likely to contribute significantly to a lack of maternal behaviour as other blind mice are known to raise their young appropriately [11]. The avoidance of the protection of closed arms in the ‘elevated plus maze test’ by frc mice from both backgrounds could reflect either a reduced anxiety behaviour or simply, a lack of avoidance of the open arms due to vision defects. Behavioural abnormalities must also be considered when interpreting deficits in motor coordination, as the increased time taken by frc mice to turn on an inclined screen and in a blind alley could not be explained by any obvious abnormalities in gait or posture.
Standardized aggression tests revealed significant differences between backgrounds. Although frc mice from both backgrounds were more aggressive than controls in all comparisons (and reached significance in most), frc mice from the C57BL/6J background were always more aggressive than those from the B6129F1 background. The striking difference between these two backgrounds illustrates the critical importance of background on phenotype assessment and offers a means of identifying genetic modifiers of aggression. The aggression and differences in aggression between backgrounds are not the result of reduced or differing vision. Other strains of blind mice show that impaired vision and aggression are not linked [10]. However, the association between aggression and reduced olfaction is more complex, making a role for olfaction difficult to exclude completely. Aggression in female mice is rare. Although some targeted mutant strains have been characterized as more aggressive than their wild-type littermates, the increased aggression is often only observed in adults and only in the males [4,30]. Rarely is offensive aggressive behaviour observed outside of maternal nest defense in females from the same mutant strain [12]. Although it may appear that frc female behaviour has been masculinized, we detected no increase in testosterone in either sex and females become pregnant and have same number of pups as wildtype mice. Thus, the major cause for the aggression in frc mice is most likely the direct effect of the mutation on brain regions known to mediate aggression as demonstrated by septal region lesions in ‘rage’ mice and rats [14,39].
In conclusion, the frc mouse model provides a tool not only for studies in brain development, metabolism, violent aggression, and maternal behaviour, but also for identifying genes important in hydrocephaly, ocular development, and sex-limited modulation of aggression in females. This mutant will also serve as a tool to explore unusual events that may complicate targeting experiments. Finally, the detailed phenotypic characterization between backgrounds reveals similarities to elucidate fundamental roles for Nr2e1 and differences that may reveal genetic modifiers of aggression, thus demonstrating a complex genetic syndrome.
Acknowledgements
The authors are grateful to Ed Silverman for animal care at Johns Hopkins University, to Violette Renard and Sue Yang for help with behavioural testing, and to Tracey Weir for manuscript preparation. This work was funded by the following grants from the National Institute of Health: Mental Health grant MH/HD57465 awarded to EMS, Mental Health grant MH57760 awarded to RJN, National Eye Institute grant EY07758 awarded to BC and NLH, and National Institute on Deafness and other Communication Disorders grant DC62108 awarded to QYZ.
References
- 1.Beamer WJ, Eicher EM, Maltais LJ, Southard JL. Inherited primary hypothyroidism in mice. Science. 1981;212:61–3. doi: 10.1126/science.7209519. [DOI] [PubMed] [Google Scholar]
- 2.Boissinot S, Boursot P. Discordant phylogeographic patterns between the Y chromosome and mitochondrial DNA in the house mouse: selection on the Y chromosome? Genetics. 1997;146:1019–34. doi: 10.1093/genetics/146.3.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown SD, Peters J. Combining mutagenesis and genomics in the mouse—closing the phenotype gap. Trends Genet. 1996;12:433–5. doi: 10.1016/0168-9525(96)30094-2. [DOI] [PubMed] [Google Scholar]
- 4.Cases O, Seif I, Grimsby J, Gaspar P, Chen K, Pournin S, Müller U, Aguet M, Babinet C, Shih JC, De Maeyer E. Aggressive behaviour and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science. 1995;268:1763–6. doi: 10.1126/science.7792602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crawley JN. Behavioural phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioural tests. Brain Res. 1999;835:18–26. doi: 10.1016/s0006-8993(98)01258-x. [DOI] [PubMed] [Google Scholar]
- 6.Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioural phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology. 1997;132:107–24. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- 7.Dagg CP. Teratogenesis. In: Green EL, editor. Biology of the laboratory mouse. McGraw-Hill; New York: 1966. pp. 309–28. [Google Scholar]
- 8.Dagg CP, Schlager G, Doerr A. Polygenic control of the teratogenicity of 5-fluorouracil in mice. Genetics. 1966;53:1101–17. doi: 10.1093/genetics/53.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Festing MFW, Simpson EM, Davisson MT, Mobraaten LE. Revised nomenclature for strain 129 mice. Mammalian Genome. 1999;10:836. doi: 10.1007/s003359901099. [DOI] [PubMed] [Google Scholar]
- 10.Festing MW. Mouse genome informatics: inbred strains of mice. The Jackson Laboratory; 2000. p. 2000. [Google Scholar]
- 11.Fox RR, Witham BA, editors. Handbook on genetically standardized JAX mice. The Jackson Laboratory; Bar Harbor: 1997. p. 143. [Google Scholar]
- 12.Gammie SC, Nelson RJ. Maternal aggression is reduced in neuronal nitric oxide synthase-deficient mice. J Neurosci. 1999;19:8027–35. doi: 10.1523/JNEUROSCI.19-18-08027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerlai R. Gene-targeting studies of mammalian behaviour: is it the mutation of the background genotype? Trends Neurosci. 1996;19:177–81. doi: 10.1016/s0166-2236(96)20020-7. [DOI] [PubMed] [Google Scholar]
- 14.Goodlett CR, Engellenner WJ, Burright RG, Donovick PJ. Influence of environmental rearing history and postsurgical environmental change on the septal rage syndrome in mice. Physiol Behav. 1982;28:1077–81. doi: 10.1016/0031-9384(82)90178-0. [DOI] [PubMed] [Google Scholar]
- 15.Heckenlively JR, Winston JV, Roderick TH. Screening for mouse retinal degeneration. I. Correlation of indirect ophthalmoscopy, electroretinograms, and histology. Doc Ophthalmol. 1989;71:229–39. doi: 10.1007/BF00170972. [DOI] [PubMed] [Google Scholar]
- 16.Hogan MJ, Alvaragdo JA, Weddell JE. Histology of the human eye. Saunders; Philadelphia: 1971. pp. 523–6. [Google Scholar]
- 17.Hollemann T, Bellefroid E, Pieler T. The Xenopus homologue of the Drosophila gene tailless has a function in early eye development. Development. 1998;125:2425–32. doi: 10.1242/dev.125.13.2425. [DOI] [PubMed] [Google Scholar]
- 18.Hooper M, Hardy K, Handyside A, Hunter S, Monk M. HPRT-deficient (Lesch–Nyhan) mouse embryos derived from germline colonization by cultured cells. Nature. 1987;326:292–5. doi: 10.1038/326292a0. [DOI] [PubMed] [Google Scholar]
- 19.Ikeda A, Zheng QY, Rosenstiel P, Maddatu T, Zuberi AR, Roopenian DC, North MA, Naggert JK, Johnson KR, Nishina PM. Genetic modification of hearing in tubby mice: evidence for the existence of a major gene (moth1) which protects tubby mice from hearing loss. Hum Mol Genet. 1999;8:1761–7. doi: 10.1093/hmg/8.9.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito M, Yoshioka M. Regression of the hyaloid vessels and pupillary membrane of the mouse. Anat Embryol (Berl) 1999;200:403–11. doi: 10.1007/s004290050289. [DOI] [PubMed] [Google Scholar]
- 21.Kirsch JP, Money MK, Webster DB. Mice heterozygous for the deafness gene have normal auditory thresholds. Hear Res. 1993;67:51–4. doi: 10.1016/0378-5955(93)90231-o. [DOI] [PubMed] [Google Scholar]
- 22.Klein SL, Kriegsfeld LJ, Hairston JE, Rau V, Nelson RJ, Yarowsky PJ. Characterization of sensorimotor performance, reproductive and aggressive behaviours in segmental trisomic 16 (Ts65Dn) mice. Physiol Behav. 1996;60:1159–64. doi: 10.1016/0031-9384(96)00218-1. [DOI] [PubMed] [Google Scholar]
- 23.König M, Zimmer AM, Steiner H, Holmes PV, Crawley JN, Brownstein MJ, Zimmer A. Pain responses, anxiety and aggression in mice deficient in pre-proenkephalin. Nature. 1996;383:535–8. doi: 10.1038/383535a0. [DOI] [PubMed] [Google Scholar]
- 24.Lyon MF. In: Genetic variants and strains of the laboratory mouse. Lyon MF, Searle AG, editors. Oxford University Press; Oxford: 1989. p. 876. [Google Scholar]
- 25.Maxson SC. Potential genetic models of aggression and violence in males. In: Driscoll P, editor. Genetically defined animal models of neurobehavioural dysfunctions. Birkhäuser; Boston: 1992. pp. 174–88. [Google Scholar]
- 26.Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–86. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- 27.Monaghan AP, Bock D, Gass P, Schwäger A, Wolfer D, Lipp H-P, Schütz G. Defective limbic system in mice lacking the tailless gene. Nature. 1997;390:515–7. doi: 10.1038/37364. [DOI] [PubMed] [Google Scholar]
- 28.Monaghan AP, Grau E, Bock D, Schütz G. The mouse homolog of the orphan nuclear receptor tailless is expressed in the developing forebrain. Development. 1995;121:839–53. doi: 10.1242/dev.121.3.839. [DOI] [PubMed] [Google Scholar]
- 29.Nelson RJ. The use of genetic ‘knockout’ mice in behavioural endocrinology research. Horm Behav. 1997;31:188–96. doi: 10.1006/hbeh.1997.1381. [DOI] [PubMed] [Google Scholar]
- 30.Nelson RJ, Demas GE, Huang PL, Fishman MC, Dawson VL, Dawson TM, Snyder SH. Behavioural abnormalities in male mice lacking neuronal nitric oxide synthetase. Nature. 1995;378:383–6. doi: 10.1038/378383a0. [DOI] [PubMed] [Google Scholar]
- 31.Nelson RJ, Young KA. Behaviour in mice with targeted disruption of single genes. Neurosci Biobehav Rev. 1998;22:453–62. doi: 10.1016/s0149-7634(97)00053-5. [DOI] [PubMed] [Google Scholar]
- 32.Roderick TH, Wimer RE, Wimer CC, Schwartzkroin PA. Genetic and phenotypic variation in weight of brain and spinal cord between inbred strains of mice. Brain Res. 1973;64:345–53. doi: 10.1016/0006-8993(73)90188-1. [DOI] [PubMed] [Google Scholar]
- 33.Silva A, Simpson EM, Takahashi JS, Lipp H-P, Nakanishi S, Wehner JM, Giese KP, Tully T, Abel T, Chapman PF, Fox K, Grant S, Itohara S, Lathe R, Mayford M, McNamara JO, Morris RJ, Picciotto M, Roder J, Shin H-S, Slesinger PA, Storm DR, Stryker MP, Tonegawa S, Wang Y, Wolfer DP. Mutant mice and neuroscience: recommendations concerning genetic background. Neuron. 1997;19:755–9. doi: 10.1016/s0896-6273(00)80958-7. [DOI] [PubMed] [Google Scholar]
- 34.Simpson EM, Linder CC, Sargent EE, Davisson MT, Mobraaten LE, Sharp JJ. Genetic variation among 129 substrains and its importance for targeted mutagenesis in mice. Nat Genet. 1997;16:19–27. doi: 10.1038/ng0597-19. [DOI] [PubMed] [Google Scholar]
- 35.Simpson EM, Page DC. An interstitial deletion in mouse Y chromosomal DNA created a transcribed Zfy fusion gene. Genomics. 1991;11:601–8. doi: 10.1016/0888-7543(91)90067-o. [DOI] [PubMed] [Google Scholar]
- 36.Storer JB. Relation of lifespan to brain weight, body weight, and metabolic rate among inbred mouse strains. Exp Gerontol. 1967;2:173–82. [Google Scholar]
- 37.Taylor BA, Phillips SJ. Detection of obesity QTLs on mouse chromosomes 1 and 7 by selective DNA pooling. Genomics. 1996;34:389–98. doi: 10.1006/geno.1996.0302. [DOI] [PubMed] [Google Scholar]
- 38.Tso JY, Sun XH, Kao TH, Reece KS, Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Res. 1985;13:2485–502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turner BH. Neural structures involved in the rage syndrome of the rat. J Comp Physiol Psychol. 1970;71:103–13. doi: 10.1037/h0029113. [DOI] [PubMed] [Google Scholar]
- 40.Yu RT, Chiang MY, Tanabe T, Kobayashi M, Yasuda K, Evans RM, Umesono K. The orphan nuclear receptor Tlx regulates Pax2 and is essential for vision. Proc Natl Acad Sci USA. 2000;97:2621–5. doi: 10.1073/pnas.050566897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear Res. 1999;130:94–107. doi: 10.1016/s0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]