Abstract
Reactive oxygen species (ROS) have been implicated in the pathogenesis of a wide range of acute and long-term neurodegenerative diseases. This study was undertaken to examine the efficacy of Pogostemon cablin, a well-known herb in Korean traditional medicine, on ROS-induced brain cell injury. Pogostemon cablin effectively protected human neuroglioma cell line A172 against both the necrotic and apoptotic cell death induced by hydrogen peroxide (H2O2). The effect of Pogostemon cablin was dose dependent at concentrations ranging from 0.2 to 5 mg ml−1. Pogostemon cablin significantly prevented depletion of cellular ATP and activation of poly ADP-ribose polymerase induced by H2O2. The preservation of functional integrity of mitochondria upon the treatment of Pogostemon cablin was also confirmed by 3-(4,5-dimethyl-2-thiazyl)-2,5-diphenyl-2-H-tetrazolium bromide assay. Furthermore, Pogostemon cablin significantly prevented H2O2-induced release of cytochrome c into cytosol. Determination of intracellular ROS showed that Pogostemon cablin might exert its role as a powerful scavenger of intracellular ROS. The present study suggests the beneficial effect of Pogostemon cablin on ROS-induced neuroglial cell injury. The action of Pogostemon cablin as a ROS-scavenger might underlie the mechanism.
Keywords: apoptosis – H2O2 – necrosis – reactive oxygen species (ROS)
Introduction
Ischemia or ischemia/reperfusion-induced cell injury is associated with a variety of life threatening conditions such as myocardial infarction, cerebral stroke and renal failure. Tissue injury resulting from ischemia or ischemia/reperfusion is mediated, in part, by the generation of reactive oxygen species (ROS) such as superoxide anion, hydrogen peroxide, hydroxyl radical and singlet oxygen (1). In the brain, they have been implicated in the pathogenesis of a wide range of acute and long-term neurodegenerative diseases including acute cerebral stroke, Parkinson's disease, Huntington's disease, ischemic trauma and seizures (2,3).
Brain cell membranes have a high content of polyunsaturated fatty acids that are particularly susceptible to the peroxidative attack by ROS. In addition, iron, which promotes cytotoxic radical formation, is accumulated in specific brain regions, such as the globus pallidus and substantia nigra. On the other hand, anti-oxidative defense mechanisms are relatively deficient in brain cells. The brain cell contains almost no catalase and has low concentrations of glutathione, glutathione peroxidase and vitamin E (4). The brain is, therefore, particularly vulnerable to the ROS attack.
We previously reported the effect of ROS on cell death and proliferation, which showed that the concentration of ROS would be very critical for cellular response (5). Cytoprotection against ROS is provided by a multilevel defense system, which comprises anti-oxidants (vitamin A, C, E and reduced glutathione) and anti-oxidant enzymes such as superoxide dismutase, catalase and glutathione peroxidase. Various constituents of natural products such as fruits, wine, tea and green vegetables are known to be a great source of effective anti-oxidants (6). Some Oriental herb medicines also have been expected to exert their effects as anti-oxidants, but little information is available yet.
Pogostemon cablin (P. cablin) is a well-known herb in Korean traditional medicine. It has effects on regulating vital energy and eliminating phlegm, and is a typical prescription used in treatments of apoplexy, syncope of Qi, phlegm syncope and syncope with eating and drinking etc. (7–10). It has been proven to be beneficial for patients with cerebral stroke (11). Several studies have been carried out on the composition of P. cablin and the presence of patchouli alcohol, pogostone, eugenol, α-bulnesene, rosmarinic acid, etc. has been revealed (11). Essential oils constitute about 1.5% of P. cablin and among them >50% is patchouli alcohol. In Korea, water extract of P. cablin was used for the treatment of fever caused by heart stroke, poor appetite, nausea, etc. The patchouli oil obtained by steam distillation is an important natural material in the perfumery and food industry and used to repel insect. Volatile composition of P. cablin obtained by steam distillation was extensively studied but non-volatile composition was not. Water extract was used for the treatment of apoplexy for a long time; however, the target site and mechanism responsible for the clinical efficacies are not clear yet. In 2006, Wang et al. (12) reported that two traditional Chinese medicine formulae reduced the oxidative stress induced by psychological stress.
There are many plants used for medicinal treatment in Asia. Generally, volatile essential oils are used in western country. But in Eastern Asia, plants for medication were generally used in water-extract and this prescription has been very effective for an age. From this point, we can expect that aqueous constituents as well as volatile ones might contain pharmacological activity.
Thus, the present study was carried out to determine whether P. cablin exerts beneficial effects against brain cell injury induced by hydrogen peroxide (H2O2), an oxidant on A172 cells, a human neuroglioma cell line.
Methods
P. cablin Extract Preparation
P. cablin was purchased from Gwangmyung Natural Pharmaceutical Co. (Busan, Korea). Forty grams of dried P. cablin was boiled with 1000 ml distilled water at 100°C for 3 h and total extract was evaporated under reduced pressure for 24 h to give 9.5 g.
Culture of A172 Cells
A172 cells were purchased from the American Type Culture Collection (Rockville, MD, USA) and maintained by serial passages in 75 cm2 culture flasks (Costar, Cambridge, MA, USA). Cells were grown in Dulbeccos’ modified Eagles medium (DMEM, Gibco-BRL, Grand Island, NY, USA) containing 10% heat-inactivated fetal bovine serum (Gibco-BRL, Grand Island, NY, USA) at 37°C in humidified 95% air/5% CO2 incubator. When the cultures reached confluence, subculture was prepared using a 0.02% EDTA–0.05% trypsin solution.
HPLC Measurement
The HPLC system consisted of a Waters 1525 pump, Waters 2487 detector, Waters 717 autosampler (Waters, Milford, MA, USA) and a Waters Symmetry C-18 column (4.6 × 150 mm2, 5 μm). The mobile phase consisted of acetonitrile (mobile phase A) and water containing 1% acetic acid (mobile phase B). The mobile phase flow rate was 1 ml min−1 and the split ratio to the HPLC was 3 : 7 and the detector wavelength was 320 nm.
Rosmarinic acid from Aldrich (St Louis, MO, USA) was used as standard. A total of 8.4 mg of rosmarinic acid was solubilized in 10.0 ml methanol. The methanol stock solution of rosmarinic acid was diluted to different concentrations. Aliquot (10 μl) of each diluted solution was analyzed by HPLC and standard curve was derived by plotting the peak area ratios of rosmarinic acid as a function of concentration.
One milligram of dried extract was dissolved into 1.0 ml of distilled water and then filtered using 0.45 μm syringe filter. Ten microliters was used for HPLC analysis and concentration was calculated from the following equation:
Fluorescence Microscopic Analysis of Cell Injury
Cells were grown on microscopic coverglass in 12-well culture plates and all the experiments were carried out 2 days after seeding. After washout of the culture media, cells were exposed to the indicated concentration of H2O2 in Hank's balanced salt solution (HBSS) for 3 h at 37°C. After exposure, each cell group was stained with 2 μl mixture of 270 μM acridine orange and 254 μM ethidium bromide (1 : 1), and observed under reflected fluorescence microscopy. Injured cells appeared as orange color, whereas intact cells appeared as green.
Trypan Blue Exclusion Assay
Measurement of cell viability by trypan blue exclusion experiment is based on the fact that injured cells lose the ability to exclude the dye (13). After exposure to chemicals, cells were detached and harvested using 0.05% trypsin–0.53 mM EDTA, incubated with 4% trypan blue solution and were counted by using a hemocytometer under light microscopy. Cells failed to exclude the dye were considered non-viable.
Assay of LDH Release
After exposure of cells to P. cablin on 12-well culture plates, media bathing the cells were collected separately, and cells on the plates were lyzed by sonication in 0.2% Triton X-100. Lactate dehydrogenase (LDH) activity in the cellular extracts and incubation media were determined by using an LDH assay kit. LDH release was calculated as the percentage of LDH activity in incubation media over the total activity.
Analysis of Apoptotic Cell Death
Cells were exposed to 0.1 mM H2O2 for 3 h and, after washout of floating dead cells by necrotic injury, returned to H2O2-free culture media containing 10% fetal bovine serum. After further incubation for 18 h, cells were assayed for apoptosis by analysis of DNA fragmentation and TUNEL staining. For analysis of DNA fragmentation, low molecular weight genomic DNA was extracted as follows. Cells (2 × 106) were scraped from the culture dish, washed in phosphate-buffered saline and lyzed in 0.5 ml of extraction buffer (10 mM Tris, pH 7.4, 0.5% Triton X-100 and 10 mM EDTA) for 20 min on ice. The lysates were then centrifuged at 15 000g for 10 min at 4°C. DNA in supernatants were extracted with phenol–chloroform, precipitated in ethanol, resuspended in Tris/EDTA, pH 8.0, containing 20 μg ml−1 RNase A and incubated for 1 h at 37°C to digest RNA. Recovered DNA fragments were separated by electrophoresis in 2% agarose gel and visualized by staining with ethidium bromide.
For in situ detection of apoptotic cells, TUNEL assay was performed using ApoTag peroxidase in situ apoptosis detection kit (Intergen, Purchase, NY, USA). In brief, cells cultured on coverglasses were exposed to chemicals and fixed in 1% paraformaldehyde for 10 min. The fixed cells were incubated with digoxigenin-conjugated dUTP in a TdT-catalyzed reaction for 60 min at 37°C and were then immersed in stop/wash buffer for 10 min at room temperature. The cells were then incubated with anti-digoxigenin antibody conjugated with peroxidase for 30 min. The DNA fragments were stained using 3,3′-diaminobenzidine (DAB) as a substrate.
Measurement of ATP Content
ATP levels were measured on cells with a luciferin–luciferase assay (14). After an exposure to oxidant stress, the cells were solubilized with 500 μl of 0.5% Triton X-100 and acidified with 100 μl of 0.6 M perchloric acid and placed on ice. Cell suspension was then diluted with 10 mM potassium glutamate buffer containing 4 mM MgSO4 (pH 7.4). One hundred microliters of 20 mg ml−1 luciferin–luciferase was added to 10 μl of diluted sample. Light emission was recorded at 20 s with a luminometer (MicroLumat LB96P, Berthold, Germany) and normalized with total protein content.
Measurement of Poly ADP-ribose Polymerase (PARP) Activity
Cells were treated with H2O2 for 10 min in a buffer containing 28 mM NaCl, 28 mM KCl, 2 mM MgCl2, 56 mM HEPES (pH 7.5), 0.01% digitonin and 125 mM NAD (containing 0.25 μCi [3H]NAD). Permeabilized cells were incubated for 5 min at 37°C, and the protein ribosylated with [3H]NAD was precipitated with 200 μl of 50% (w/v) trichloroacetic acid. After washing twice with trichloroacetic acid, the protein pellet was solubilized with 200 μl of 2% (w/v) sodium dodecyl sulfate in 0.1 M NaOH and incubated at 37°C for overnight, and the radioactivity was determined by scintillation counting.
Assay of MTT Reduction
Intact mitochondria reduce 3-(4,5-dimethyl-2-thiazyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) to formazan (15). Therefore, the ability of cells to form formazan from MTT is a good indicator of mitochondrial function. A total 5 μl of MTT was added to each well (final concentration of 62.5 μg ml−1). After exposure to chemicals, the supernatant was removed and the formed formazan crystals in viable cells were solubilized in DMSO. The concentration of formazan was determined by reading the absorbance at 570 nm using a spectrophotometer.
Detection of Cytochrome c Release
After exposure to chemicals, cells were washed twice with ice-cold phosphate-buffered saline at 4°C and resuspended in 1 ml of extraction buffer (50 mM PIPES, pH 7.0, 50 mM KCl, 5 mM MgCl2, 5 mM ethylene glycol tetraacetic acid (EGTA), 1 mM phenylmethylsulfonyl fluoride, 10 μg ml−1 leupeptin and 10 μg ml−1 pepstatin A). Cells were lyzed by five cycles of freezing in liquid nitrogen and thawing at 37°C. After verifying >90% of cells were lyzed by microscopic examination, the lysates were centrifuged at 100 000g for 1 h at 4°C. The resulting supernatant mainly consisted of cytosolic fraction was separated from the pellet containing the cellular membrane and organelles, and was analyzed for cytochrome c by western blots using anti-cytochrome c antibody.
Measurement of ROS
Intracellular level of ROS was determined by measuring 2,7-dichlorofluorescein (DCF) fluorescence (16). The non-fluorescent ester dye 2,7-dichlorofluorescein diacetate (DCFH-DA) penetrates into the cells and is hydrolyzed to DCFH by the intracellular esterases. The probe is rapidly oxidized to the highly fluorescent compound DCF in the presence of cellular peroxidase and ROS, such as H2O2. Cells were pre-incubated at 37°C for 10 min in a fluorescent cuvette containing 3 ml of glucose-free buffer with 20 μM DCFH-DA (from a stock solution of 20 mM DCFH-DA in ethanol). After pre-incubation, cells were treated with H2O2 and incubated up to 60 min. During the incubation process, fluorescent intensity was monitored on a spectrophotometer with excitation wavelength at 485 nm and emission wavelength at 530 nm.
Chemicals
Acridine orange and 2,7-dichlorofluorescein diacetate (DCFH-DA) were purchased from Molecular Probes (Eugene, OR, USA). Other chemicals were obtained from Sigma Chemical Co. (St Louis, MO, USA).
Data analysis
Data are presented as means ± SE When necessary, data were analyzed by analysis of variance (ANOVA) followed by Duncan's multiple comparison test. A value of P < 0.05 was considered statistically significant.
Results
Contents of P. cablin Extract
To check whether the effective contents in P. cablin were present after extraction, we carried out HPLC analysis. As described in introduction, rosmarinic acid is one of the effective ingredients in P. cablin so we used commercial rosmarinic acid as standard. Extraction was carried out after boiling for 30 min, 1, 2 and 4 h each and then the extracts were applied to HPLC analysis. As shown in Table 1, rosmarinic acid was detected in extracts of P. cablin and the concentration of rosmarinic acid was increased as the extraction time became longer. It implies that the effective constituents in P. cablin could be present in extracts after even >4 h boiling.
Table 1.
Concentration of rosmarinic acid in extract of P. cablin
| Extraction time | Peak area | Concentration (μg/ml−1) |
|---|---|---|
| 30 min | 6747 | 0.78 |
| 1 h | 9471 | 0.98 |
| 2 h | 9942 | 1.01 |
| 4 h | 17499 | 1.56 |
Effects of P. cablin on Oxidant-induced Cell Injury
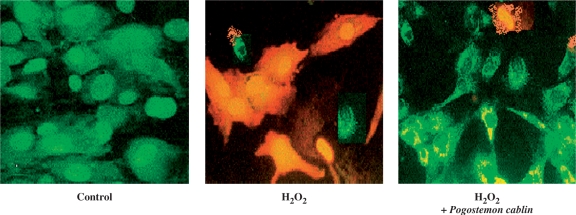
The fluorescence microscopic analysis in Fig. 1 visualizes the typical image of A172 cells injured by 0.5 mM H2O2. Injured cells were stained with acridine orange. While non-viable cells fluoresced orange or red, viable cells fluoresced green. Preserved contour of the nuclei indicated that the cell injury was necrotic rather than apoptotic. Protective effect of P. cablin against H2O2-induced injury is shown in the right panel of the Fig. 1.
Figure 1.
Protection by P. cablin (1 mg ml−1) against H2O2 (0.5 mM)-induced A172 cell injury visualized by acridine orange fluorescence image. Injured cells fluoresced orange, whereas intact cells fluoresced green.
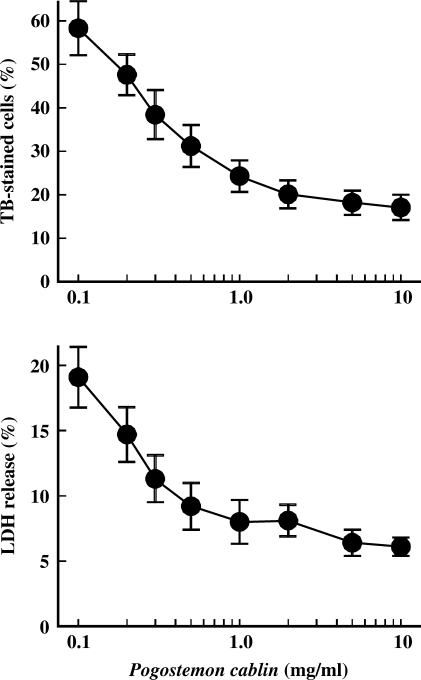
In Fig. 2, P. cablin protected cell injury induced by H2O2 in a dose-dependent manner. Trypan blue exclusion ability and LDH release were adopted as indexes of cell injury. Measurement of cell viability by trypan blue exclusion ability was based on the fact that injured cells lose the ability to exclude the dye (13). LDH is an intracellular enzyme and its release is a useful marker of membrane damage, a characteristic of necrotic cell death. When cells were treated with 0.5 mM H2O2 for 3 h, 58.4% of cells lost their ability to exclude the dye and 19.6% of the LDH activity was found in incubation media. P. cablin treatment decreased both indexes of cell injury in a concentration-dependent manner. At the concentration of 1 mg ml−1, the levels of trypan blue stain and LDH release were decreased 58.2 and 63.4%, respectively.
Figure 2.
Concentration-dependent protection by P. cablin against H2O2-induced cell injury. Cells were treated with 0.5 mM H2O2 for 3 h at 37°C in the presence of indicated concentrations of P. cablin, and cell viability was determined by trypan blue exclusion ability and LDH release. Each point represents mean ± SE of five experiments.
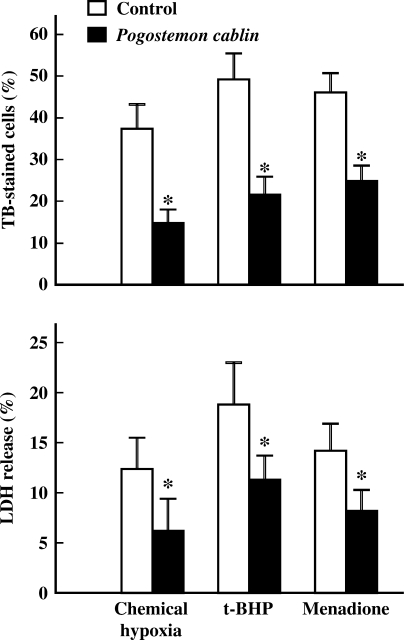
In Fig. 3, the protective effects of P. cablin upon other types of oxidant-induced cell injuries were examined. Cells were exposed to chemical hypoxia or chemical oxidants, t-butyl hydroperoxide (t-BHP) and menadione. Chemical hypoxia was induced by combination of glucose deprivation and the treatment of antimycin A (20 μM), mitochondrial electron transport inhibitor. All these chemicals induced cell injury or death as determined by trypan blue exclusion and LDH release assays. In the presence of P. cablin (1 mg ml−1), cellular injury was significantly decreased (Fig. 3). This result suggests that P. cablin might have protective effect against various types of oxidant-induced injuries.
Figure 3.
Effect of P. cablin on cell injuries induced by chemical hypoxia, t-BHP and menadione. Cells were exposed to chemical hypoxia or treated with t-BHP (0.5 mM) or menadione (2 mM) for 3 h at 37°C in the absence and presence of P. cablin (1 mg ml−1), and cell viability was determined by trypan blue exclusion and LDH release assay. Chemical hypoxia was induced using a combination of glucose deprivation and mitochondrial electron transport inhibitor antimycin A (20 μM). Each point represents mean ± SE of five experiments. *P < 0.01 versus control.
Effect of P. cablin on Apoptotic Cell Death
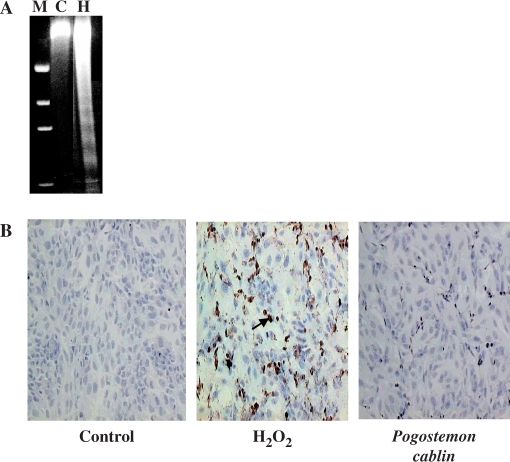
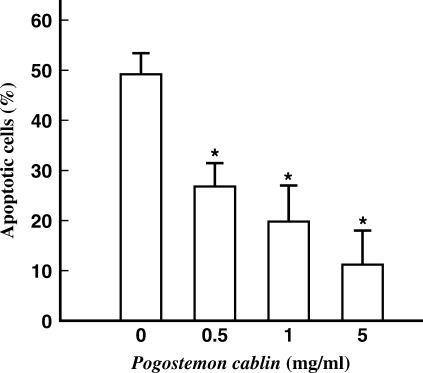
The protective effect of P. cablin against oxidative stress-induced apoptosis was examined with human neuroglioma cells. DNA fragmentation is one of the most characteristic biochemical features of apoptosis. A172 cells were first exposed to 0.1 mM H2O2 for 3 h and cell viability was checked with trypan blue exclusion assay. Less than 15% of cells resulted in necrotic cell death (data not shown). After removing the floating dead cells, cells were returned to H2O2-free culture media. After further incubation for 18 h, cells were analyzed for apoptosis by DNA fragmentation and TUNEL assay. Treatment of cells with 0.1 mM H2O2 induced DNA fragmentation as shown by typical laddering pattern in the agarose gel electrophoresis (Fig. 4A). Apoptotic cells were visualized by TUNEL staining in the micrographs shown in Fig. 4B. Nearly half (47.1%) of cells in H2O2-treated cells were TUNEL-positive (mid panel of Fig. 4B). In the presence of P. cablin, the numbers of TUNEL-positive cells were significantly reduced (right panel of Fig. 4B). The dosage-dependent effects of P. cablin on H2O2-induced apoptosis are summarized in Fig. 5.
Figure 4.
Effect of P. cablin on apoptotic cell death. Cells were first exposed to 0.1 mM H2O2 for 3 h in the absence and presence of P. cablin (1 mg ml−1), and were then returned to H2O2-free culture media. After incubation for 18 h, cells were assayed for apoptosis by DNA fragmentation (A) and TUNEL assay (B). Arrow indicates TUNEL-positive cells. M, molecular marker; C, control; H, H2O2.
Figure 5.
Effect of P. cablin on apoptotic cell death. Cells were first exposed to 0.1 mM H2O2 for 3 h in the absence and presence of P. cablin (1 mg ml−1), and were then returned to H2O2-free culture media. After further incubation for 18 h, cells were assayed for apoptosis by TUNEL staining. Each point represents mean ± SE of four experiments. *P < 0.01 versus the value in the absence of P. cablin.
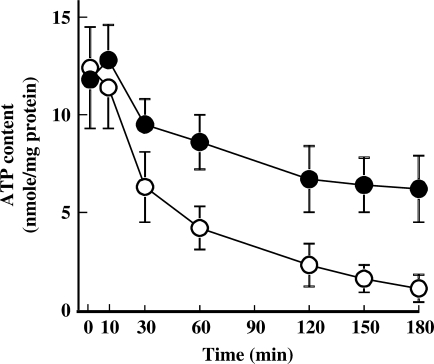
Effect of P. cablin on H2O2-induced ATP Depletion
In general, ROS-induced tissue injury is accompanied by decrease in intracellular ATP concentration (1). To examine whether P. cablin could prevent H2O2-induced ATP depletion, time-dependent changes of intracellular ATP content of H2O2-treated cells in the absence or presence of P. cablin (1 mg ml−1) were checked (Fig. 6). ATP concentration in H2O2-treated cells decreased to <10% of its control value within 3 h. However, in the presence of P. cablin, H2O2-induced ATP depletion was significantly attenuated.
Figure 6.
Effect of P. cablin on H2O2-induced depletion of cellular ATP. Cells were treated with 0.5 mM H2O2 for indicated time periods in the absence (open circle) and presence (closed circle) of P. cablin (1 mg ml−1). Each point represents mean ± SE of four experiments.
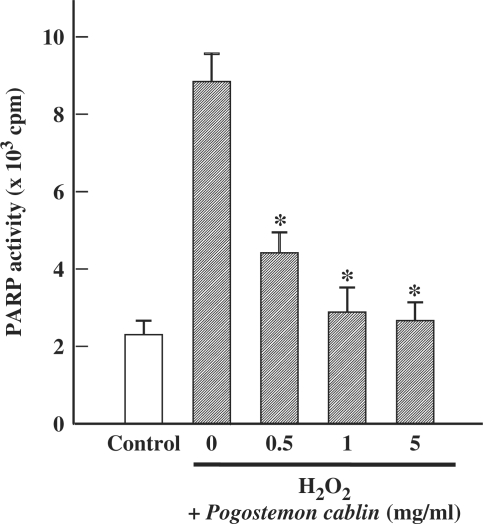
Effect of P. cablin on H2O2-induced PARP Activation
It has been reported that activation of PARP is implicated in H2O2-induced ATP depletion and neurotoxicity (17). As shown in Fig. 7, exposure of cells to H2O2 resulted in a large increase in PARP activity. However, this increase in PARP activity was significantly inhibited by P. cablin.
Figure 7.
Effect of Pogostemon cablin on H2O2-induced activation of PARP activity. Cells were treated for 3 h with 0.5 mM H2O2 and indicated concentrations of P. cablin. Each point represents mean ± SE of four experiments. *P < 0.01 versus H2O2 alone.
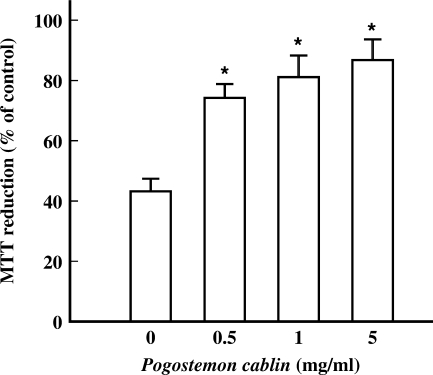
Effect of P. cablin on H2O2-induced Mitochondrial Dysfunction
In addition to PARP activation, deterioration of mitochondrial function is a major determinant to deplete intracellular ATP. Exposure of cells to 0.5 mM H2O2 produced a significant loss of mitochondrial function as evidenced by the decrease in MTT reduction level to 42.3% of that of control cells (Fig. 8). In the presence of P. cablin, the decease in MTT reduction was significantly prevented. These data suggest that P. cablin prevents mitochondrial dysfunction induced by H2O2.
Figure 8.
Effect of P. cablin on H2O2-induced deterioration of mitochondrial function. Cells were treated for 3 h with 0.5 mM H2O2 and indicated concentrations of P. cablin. MTT reduction ability was assayed as an index of mitochondrial function. Each point represents mean ± SE of six experiments. *P < 0.01 versus the value in the absence of P. cablin.
Effect of P. cablin on H2O2-induced Cytochrome c Release
It is well established that release of cytochrome c from mitochondrial inner membrane into cytosol is a key event to relate mitochondrial damage with apoptotic cell death (18). As shown in Fig. 9, treatment of cells with H2O2 induced release of cytochrome c and this effect was remarkably prevented by P. cablin.
Figure 9.
Effect of P. cablin on H2O2-induced cytochrome c release. Cells were treated for 3 h with 0.1 or 0.5 mM H2O2 in the absence and presence of P. cablin (1 mg ml−1). Levels of cytochrome c were analyzed by western blot with the cytosolic fractions isolated from the treated cells.
Effect of P. cablin on Intracellular ROS
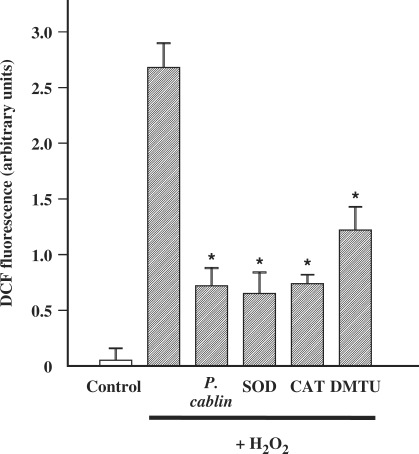
There is increasing evidence that tissue injury resulting from ischemia/reperfusion or chemical oxidant is mediated by intracellular generation of ROS (1). As shown in Fig. 10, H2O2 induced a significant increase of intracellular ROS. In the presence of P. cablin, intracellular ROS was remarkably reduced. The effectiveness of P. cablin as intracellular ROS reducer at the concentration of 1 mg ml−1 was comparable to that of well-known ROS scavengers such as superoxide dismutase (200 U ml−1), catalase (200 U ml−1) and dimethylthiourea (10 mM). This result suggests that P. cablin might exert its effect as an effective ROS scavenger.
Figure 10.
Effect of P. cablin and various ROS scavengers on H2O2-induced formation of ROS in A172 cells. H2O2 (0.5 mM)-induced increase in ROS formation for 60 min was determined by DCF fluorescence assay in the absence and presence of P. cablin (1 mg ml−1), superoxide dismutase (SOD, 200 U ml−1), catalase (CAT, 200 U ml−1) and dimethylthiourea (DMTU, 10 mM). Each point represents mean ± SE of four experiments. *P < 0.01 versus H2O2 alone.
Discussion
All aerobic cells generate ROS including superoxide, H2O2 and hydroxyl radicals, enzymatically or non-enzymatically. The mitochondrial electron transport chain is the principal site of cellular production of ROS (19,20). ROS can also be generated during the process of phagocytosis, ischemia/reperfusion and metabolism of many drugs and other xenobiotic chemicals (1). At the same time, the abundant anti-oxidant defense systems of most cells, both enzymatically and non-enzymatically, prevent ROS from causing cell injury. Nevertheless, there are a number of conditions in which the rate of formation of ROS is increased and/or anti-oxidant defenses of cells are weakened. Therefore, the targeted development of new anti-oxidant drugs has been required.
The data in the present study clearly suggested that P. cablin might act as an effective cytoprotectant against neuroglial cell injury induced by ROS. It was effective to protect apoptotic cell death as well as necrotic cell death by various types of oxidants.
Several lines of evidence suggest that the effect of P. cablin to preserve mitochondrial function during oxidative stress might be a key event responsible for its protective effect against cell death. Furthermore, it significantly attenuated H2O2-induced ATP depletion. The effect of P. cablin to prevent PARP activation as shown in Fig. 7 could provide another clue to explain the mechanism of ATP preservation.
Since PARP catalyzes the transfer of ADP-ribose from NAD to protein with the concomitant release of nicotinamide, the activation of this enzyme results in depletion of NAD and a consequent reduction of ATP, which may be involved in the pathogenesis of oxidant-induced injury. PARP has been demonstrated to be involved in the cell death caused by H2O2 in neuronal cells (15,21) and various non-neuronal cell systems (22,23). However, the role of PARP in oxidant-induced glial cell death has not been established. Although, present study clearly demonstrated that PARP is activated by H2O2 and P. cablin prevents the activation of PARP stimulated by H2O2, it is unclear whether ATP depletion resulting from PARP activation is a mediator of H2O2-induced cell death. While ATP depletion has been reported to induce the oxidant-induced cell death (24), other studies have revealed that the oxidant-induced injury is disassociated with ATP depletion (25,26).
It is interesting to note that P. cablin prevents H2O2-induced cytochrome c release (Fig. 9). Onset of ischemia leads to depletion of oxygen in the tissue resulting in impaired mitochondrial oxidative phosphorylation within seconds. ATP concentration almost amounts to about 10% of normal within 5 min. This failure of energy production results in MPT and subsequent release of cytochrome c. Released cytochrome c activates caspase cascades, which are critical for the execution phase of apoptosis, and generates ROS. Generated ROS initiates another cycle of apoptosis described earlier and propagates ischemic cell death (18,27). P. cablin-induced down-regulation of ROS blocks the propagation of injury. From this result, we can postulate that P. cablin exerts its pharmacologic effect through ROS scavenging.
The conclusive results from Fig. 10 clearly show that P. cablin would be an effective scavenger of ROS. The overall protective effects observed in this study might be associated with the role of P. cablin as a ROS-scavenger. It was not measured in this study, which component of P. cablin is responsible for the ROS-scavenging effect. It may be a natural component(s) included in P. cablin or a newly formed compound(s) through extraction process. Further analytical study should be needed to elucidate this.
The standardization of herbal medicine has been managed through the government agency. The Korea Food and Drug Administration (KFDA) guides this by the Korean Herbal Pharmacopoeia (28), which suggests criteria for oriental medicine. The extraction method used in this study was same as the method in clinical application.
In conclusion, the present study provides clear evidence for the anti-oxidant effect of P. cablin on neuroglial cell line. ROS scavenging activity of P. cablin might underlie the mechanism. However, the exact component and mechanism responsible for the cytoprotection against ROS should be further identified.
References
- 1.Farber JL, Kyle ME, Coleman JB. Mechanisms of cell injury by activated oxygen species. Lab Invest. 1990;62:670–9. [PubMed] [Google Scholar]
- 2.Bondy SC. The relation of oxidative stress and hyperexcitation to neurological disease. Proc Soc Exp Biol Med. 1995;208:337–45. doi: 10.3181/00379727-208-43862. [DOI] [PubMed] [Google Scholar]
- 3.Sendtner M, Thoenen H. Neurodegenerative disease. Oxidative stress and motorneuron disease. Curr Biol. 1994;4:1036–9. doi: 10.1016/s0960-9822(00)00237-2. [DOI] [PubMed] [Google Scholar]
- 4.Olanow CW. A radical hypothesis for neurodegeneration. TINS. 1993;16:439–44. doi: 10.1016/0166-2236(93)90070-3. [DOI] [PubMed] [Google Scholar]
- 5.Kim BY, Han MJ, Chung AS. Effects of reactive oxygen species on proliferation of Chinese hamster lung fibroblast (V79) cells. Free Radic Biol Med. 2001;30:686–98. doi: 10.1016/s0891-5849(00)00514-1. [DOI] [PubMed] [Google Scholar]
- 6.Kanter M. Free radicals, exercise and antioxidant supplementation. Proc Nutr Soc. 1998;57:9–13. doi: 10.1079/pns19980004. [DOI] [PubMed] [Google Scholar]
- 7.Hwang DY. Zeung Mak Bang Yak Hap Pyun. Seoul: Nam San Dang; 1978. pp. 138–40. [Google Scholar]
- 8.Yun GY. Catagraphology of Oriental Medicine. Seoul: Ko Mun Company; 1980. 40, 41, 108, 144, 155, 173. [Google Scholar]
- 9.Ku BH. Cardiology of Oriental Medicine. Seoul: Seo Won Dang; 1985. pp. 305–11. [Google Scholar]
- 10.Kim JJ. Summary of Treatment. Vol. 1. Seoul: Laboratory of Oriental Medicine; 1974. pp. 448–449. 453, Vol. 2, 431. [Google Scholar]
- 11.Hsu HC, Yang WC, Tsai WJ, Chen CC, Huang HY, Tsai YC. Alpha-bulnesene, a novel PAF receptor antagonist isolated from Pogostemon cablin. Biochem Biophys Res Commun. 2006;345:1033–8. doi: 10.1016/j.bbrc.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Muxin G, Nishida H, Shirakawa C, Sato S, Konishi T. Psychological stress-induced oxidative stress as a model of sub-healthy condition and the effect of TCM. Evid Based Complement Alternat Med. 2007;4:195–202. doi: 10.1093/ecam/nel080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bjorkerud S, Bondjers G. Endothelial integrity and viability in the aorta of the normal rabbit and rat as evaluated with dye exclusion tests and interference contrast microscopy. Atherosclerosis. 1972;15:285–300. doi: 10.1016/0021-9150(72)90019-6. [DOI] [PubMed] [Google Scholar]
- 14.Lyman GE, DeVincenzo JP. Determination of picogram amounts of ATP using the luciferin-luciferase enzyme system. Anal Biochem. 1967;21:435–43. doi: 10.1016/0003-2697(67)90318-1. [DOI] [PubMed] [Google Scholar]
- 15.Morgan DM. Tetrazolium (MTT) assay for cellular viability and activity. Methods Mol Biol. 1998;79:179–83. doi: 10.1385/0-89603-448-8:179. [DOI] [PubMed] [Google Scholar]
- 16.LeBel CP, Ischiropoulos H, Bondy SC. Evaluation of the probe 2',7'-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol. 1992;5:227–31. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- 17.Cookson MR, Ince PG, Shaw PJ. Peroxynitrite and hydrogen peroxide induced cell death in the NSC34 neuroblastoma x spinal cord cell line: role of poly (ADP-ribose) polymerase. J Neurochem. 1998;70:501–8. doi: 10.1046/j.1471-4159.1998.70020501.x. [DOI] [PubMed] [Google Scholar]
- 18.Kroemer G, Dallaporta B, Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol. 1998;60:619–42. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- 19.Boveris A, Oshino N, Chance B. The cellular production of hydrogen peroxide. Biochem J. 1972;128:617–30. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turrens JF, Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem J. 1980;191:421–7. doi: 10.1042/bj1910421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mailly F, Marin P, Israel M, Glowinski J, Premont J. Increase in external glutamate and NMDA receptor activation contribute to H2O2-induced neuronal apoptosis. J Neurochem. 1999;73:1181–8. doi: 10.1046/j.1471-4159.1999.0731181.x. [DOI] [PubMed] [Google Scholar]
- 22.Chatterjee PP, Cuzzocrea S, Thiemermann C. Inhibitors of poly (ADP-ribose) synthetase protect rat proximal tubular cells against oxidant stress. Kidney Int. 1999;56:973–84. doi: 10.1046/j.1523-1755.1999.00644.x. [DOI] [PubMed] [Google Scholar]
- 23.Schraufstatter IU, Hyslop PA, Hinshaw DB, Spragg RG, Sklar LA, Cochrane CG. Hydrogen peroxide-induced injury of cells and its prevention by inhibitors of poly(ADP-ribose) polymerase. Proc Natl Acad Sci USA. 1986;83:4908–12. doi: 10.1073/pnas.83.13.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thies RL, Autor AP. Reactive oxygen injury to cultured pulmonary artery endothelial cells: mediation by poly(ADP-ribose)polymerase activation causing NAD depletion and altered energy balance. Arch Biochem Biophys. 1991;286:353–63. doi: 10.1016/0003-9861(91)90051-j. [DOI] [PubMed] [Google Scholar]
- 25.Andreoli SP, Mallett CP. Disassociation of oxidant-induced ATP depletion and DNA damage from early cytotoxicity in LLC-PK1 cells. Am J Physiol. 1997;272:F729–F735. doi: 10.1152/ajprenal.1997.272.6.F729. [DOI] [PubMed] [Google Scholar]
- 26.Frenkel K, Gleichauf C. Hydrogen peroxide formation by cells treated with a tumor promoter. Free Radic Res Commun. 1991;12:783–94. doi: 10.3109/10715769109145860. [DOI] [PubMed] [Google Scholar]
- 27.Sims NR, Anderson MF. Mitochondrial contributions to tissue damage in stroke. Neurochem Int. 2002;40:511–26. doi: 10.1016/s0197-0186(01)00122-x. [DOI] [PubMed] [Google Scholar]
- 28.Seoul: Korea Food and Drug Administration; 2003. Korea Food and Drug Administration. The Korean Herbal Pharmacopoeia 2002: Korea Food and Drug Administration. [Google Scholar]