Abstract
Boehmeria nivea extract (BNE) is widely used in southern Taiwan as a folk medicine for hepato-protection and hepatitis treatment. In previous studies, we demonstrated that BNE could reduce the supernatant hepatitis B virus (HBV) DNA in HBV-producing HepG2 2.2.15 cells. In the present study, we established an animal model of HBV viremia and used it to validate the efficacy of BNE in vivo. In this animal model, serum HBV DNA and HBsAg were elevated in accordance with tumor growth. To evaluate the anti-HBV activity of BNE, HBV-viremia mice were built up after one subcutaneous inoculation of HepG2 2.2.15 tumor cells in severe combined immunodeficiency mice over 13 days. The levels of serum HBV DNA were elevated around 105–106 copies per milliliter. Both oral and intraperitoneal administration of BNE were effective at inhibiting the production of HBsAg and HBV DNA, whereas tumor growth was not affected by all test articles. Intraperitoneal administration of BNE appeared to have greater potential to inhibit serum HBV DNA levels compared with oral administration under the same dosage. Notably, reduced natural killer cell activity was also observed after high dosage of BNE administration, and this correlated with reduced serum HBV DNA. In conclusion, BNE exhibited potential anti-HBV activity in an animal model of HBV viremia.
Keywords: HBsAg, HBV DNA, SCID, NK cells, folk medicine
Introduction
Hepatitis B is caused by hepatitis B virus (HBV) infection that is common in Asia, Africa and the Middle East. Approximately 2–5% of HBV-infected people have the potential to develop a chronic infection, and HBV carriers have elevated risk for the development of cirrhosis and hepatocellular carcinoma (1). HBV is an enveloped DNA virus of the hepadnaviridae family that contains ∼3.2 kb partially double-stranded circular genome. HBV is replicated through core protein encapsidation of the viral pre-genomic RNA (pgRNA) that is subsequently converted to double stranded (dsDNA) by viral reverse transcriptase/polymerase to form a nucleocapsid. The nucleocapsid is then enveloped by the viral surface protein (2). Currently, interferon-α (acting as an immunomodulator) and the nucleoside analogs lamivudine (3TC), adefovir and entecavir (acting as viral reverse transcriptase/polymerase inhibitors) are drugs approved for treatment of chronic hepatitis B (3,4). Despite the synergistic effect of combination therapy of these drugs for the improvement of INF-α unresponsiveness or drug resistance in HBV-infected patients, the drug-resistant HBV variants remain a major problem in hepatitis B management (5–7). Therefore, sustained and diversified strategies for viral suppression are crucial to reduce hepatic inflammation, progression of liver fibrosis, and hepatoma (8).
Approaches for the treatment of chronic HBV infection have been in development for the past decade, and when combined with a number of anti-viral drugs these treatments may have potential to improve the effectiveness of anti-HBV therapy. Chinese medicinal herbs have been used for centuries to treat liver disease (9). Recently, the flavonoid ellagic acid from woody dicotyledonous plants has been found to block hepatitis B e antigen (HBeAg) secretion either in an HBV-infected cell line or in HBeAg transgenic mice (10). In addition, sesquiterpene lactones from Senecio species suppress the expression of hepatitis B s antigen (HBsAg) and HBeAg (11), and an ethanol extract of Polygonum cuspidatum inhibits HBV production in an HBV-producing cell line (12). An extract of the genus Phyllanthus has a positive effect on the clearance of HBsAg in clinical trials conducted on chronic HBV infections, and the extract has a synergistic effect when administered with IFN-α (9). Our previous studies demonstrated that crude root extract of Boehmeria nivea had anti-HBV activity via the inhibition of HBV production in HepG2 2.2.15 cells (13). This B. nivea extract (BNE) suppressed the production of infectious virus but not intracellular HBV DNA replication, and its anti-HBV mechanism appears to differ from that of the nucleoside analogs. Thus, it is conceivable that BNE might work synergistically with other anti-viral compounds for the treatment of HBV infection.
HBV replicates through an RNA intermediate by its reverse transcriptase/polymerase. The viral reverse transcriptase/polymerase lacks a proofreading function, however, thus leading to the formation of drug-resistant mutants that in turn cause serious problems with current management of hepatitis B infection (14). Therefore, effective treatment strategies with different pharmacological modes are urgently needed to lessen the enormous burden of viral hepatitis on health care worldwide.
A major obstacle to the development of anti-HBV drugs is the lack of an efficient in vitro cell model or an easy-to-use conventional animal model that is promising for natural viral infection and replication (15). Availability of laboratory animal models of HBV infection is imperative for the development of effective methods to treat these diseases. One of the few animal models for HBV infection is the chimpanzee, which is extensively used to evaluate the safety and immunogenicity of HBV vaccines (16). However, the limited availability and the high cost of these primates severely restrict their use for such purposes. Investigators have attempted to establish conventional animal models to mimic human HBV replication in rats (17), nude mice (18) and transgenic mice (19). Other animal models, such as woodchuck hepatitis virus in woodchuck and duck hepatitis virus in duck, have also been developed to assess anti-viral drugs (20). However, testing of anti-viral agents, including nucleoside analogs, in these non-human HBV models may produce aberrant results as a consequence of virus-specific differential susceptibility of the viral polymerase to these agents (21). Thus, establishment of a rapid, convenient and less expensive animal model would greatly facilitate this area of research. In this study, we established an HBV-viremia animal model and studied the efficacy of BNE in vivo.
Subjects and Methods
Experimental Animals and Cell Culture
Male severe combined immunodeficiency (SCID) mice 4–6 weeks of age, C.B17/icr-scid, were purchased from the experimental animal center of National Taiwan University (Taipei, Taiwan, R.O.C.). The human hepatoma HepG2 cell line was purchased from American Type Culture Center (ATCC, USA), and the HepG2 2.2.15 cell line was kindly given by Dr Ho, MS (Academia Sinica, Taipei, Taiwan, R.O.C.). These hepatoma cells were maintained in minimum essential medium (Gibco, USA) containing 10% fetal bovine serum (Hyclone, USA), 1.5 g l−1 sodium bicarbonate, 0.1 mM non-essential amino acids, 1.0 mM sodium pyruvate, and 100 U ml−1 penicillin G (Gibco, USA) and 100 μg ml−1 streptomycin (Gibco, USA). A final concentration of 200 μg ml−1 G418 (Gibco, USA) was included in the medium for the maintenance of HepG2 2.2.15 cells. YAC-1 cells (ATCC, USA) for the natural killer (NK) cell activity assay were cultured in RPMI 1640 medium containing 2 mM l-glutamine, 1.5 g l−1 sodium bicarbonate, 10 mM HEPES and 10% fetal bovine serum.
Anti-HBV Drugs and BNE
To prepare the B. nivea (L.) Gaudich plant extract (i.e. BNE) utilized in our experiments, the roots of the plants were collected and dried (13). Briefly, 100 g of the dried roots was cut into pieces ∼0.5 cm in length before boiling in 1 l of 20% ethanol under boiling reflux for 3 h. The decoction was filtered through a 0.22 μm filter and lyophilized into powder. 3TC was purchased from GlaxoSmithKline (Holland).
Establishment of an HBV-Viremia Animal Model
To establish HBV-viremia animals, the 20 experimental SCID mice were divided into three groups: HepG2 group (n = 8), HepG2 2.2.15 group (n = 8) and one vehicle control group (n = 4). The SCID mice were inoculated with 107 HepG2 or HepG2 2.2.15 cells in 100 μl phosphate-buffered saline (PBS) into the flank subcutaneously. The control group was inoculated likewise with PBS. Tumors were measured using a caliper, and tumor weight was calculated with the equation: tumor weight (in mg) = length (in mm) × width (in mm2)/2 (22). On post-inoculation Day 0 and Day 14, blood was collected by retro-orbital sampling. On Day 28, the mice were sacrificed and blood was collected directly from the heart. Serum HBsAg and HBV DNA were analyzed as described subsequently.
Determination of Serum Alanine Aminotransferase (ALT) and Serum HBsAg
A blood sample was withdrawn from tumor-bearing animals at the indicated times (Days 0, 14 and 28), and liver function was evaluated based on the levels of ALT. ALT activity was determined with the DTSC II Module detection system (Johnson-Johnson Co., USA) using the Vitros ALT/SGPT kit (Johnson-Johnson) as per the user manual. HBsAg levels in mouse serum were determined semi-quantitatively by enzyme-linked immunosorbent assay (ELISA; General Biologicals Corp., Taiwan, R.O.C.) according to the user manual. The cutoff value was calculated by the formula: cutoff value =ODnagative control + 0.025. An OD450 value higher than the cutoff value implies an HBV-positive result.
Determination of HBV DNA by the Quantitative Real-time Polymerase Chain Reaction (PCR)
Blood (500 μl) was withdrawn from test animals at the indicated times (Days 13 and 24). Blood was allowed to clot for 30 min and then centrifuged at 800g for 15 min at room temperature. Serum was collected and stored at −70°C prior to analysis of HBsAg and HBV DNA. Serum HBV DNA was extracted by a QIAamp DNA Blood Mini kit (Qiagen, USA). HBV DNA was quantitated with the ABI 7500 Sequence Detection System using the HBV RealQuant PCR kit (General Biologicals Corp.). Briefly, PCR was performed with initial denaturing steps at 50°C for 2 min and 95°C for 10 min, followed by 45 cycles at 95°C for 15 s and annealing/extending at 58°C for 1 min.
Validation of the Efficacy of BNE in HBV-viremia Animals
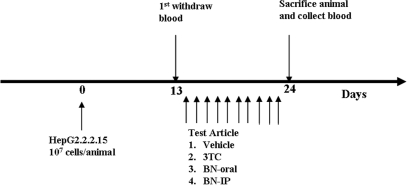
For drug treatments, SCID mice were divided into five groups of eight mice each. Each animal was inoculated with 1 × 107 cells of HepG2 2.2.15 in 100 μl PBS on the flank subcutaneously; animals in the sham group received 100 μl PBS (Fig. 1 shows the experimental protocol). Tumors were allowed to grow, and blood was withdrawn on Day 13 prior to drug treatments. For drug treatments, 3TC (13 mg kg−1 day−1) was given by gavages, and BNE (195 mg kg−1 day−1) was administrated to animals via intraperitoneal and oral routes starting on Day 14 for 10 consecutive days (for both 3TC and BNE). Tumor-bearing animals that were fed distilled water constituted the vehicle control group. All animals were sacrificed on Day 24, and the spleen was taken for NK cell activity analysis and blood was withdrawn to assess ALT, HBsAg and HBV DNA as described as earlier. All animals received human care, and the study protocol followed the guidelines of the Institutional Animal Care and Use Committees of the Development Center for Biotechnology.
Figure 1.
Scheme of the drug treatment protocol used in the HBV-viremia animal experiments.
Assessment of the NK Cell Activity
The spleen was taken from each drug-treated HBV-viremia mouse and then homogenized into a single cell suspension in Hank's Balanced Salt Solution buffer (Gibco, USA) by passing through a 40 μm diameter nylon cell strainer (Becton Dickinson, USA). NK cell activity was measured using the LIVE/DEAD Cell-Mediated Cytotoxicity kit (Molecular Probes, USA). In brief, 1 × 106 YAC-1 cells were pre-labeled with 5 μl 3 mM DiOC18 fluorescent dye for 2 h, and excess dye was removed by washing with PBS. Then 1 × 106 of labeled YAC-1 cells were co-incubated with 2 × 106 splenocytes for 3 h, and 100 μl of 3.75 mM propidium iodine dye was added to the reaction at 4°C for 20 min avoiding the light. NK cell activity was analyzed by flow cytometry and was calculated with the equation: NK activity (%) = viability of treated group (%) – viability of blank group (%).
Statistical Analysis
Statistical analysis was performed using one-way ANOVA to analyze variances, and significant differences were assessed by Dunnett's test for multiple comparisons. The paired t-test was used to assess significant differences before and after treatment in the same individuals. A P-value of <0.05 was considered statistically significant.
Results
Tumor Formation Caused by HepG2 and HepG2 2.2.15 Cells in SCID Mice
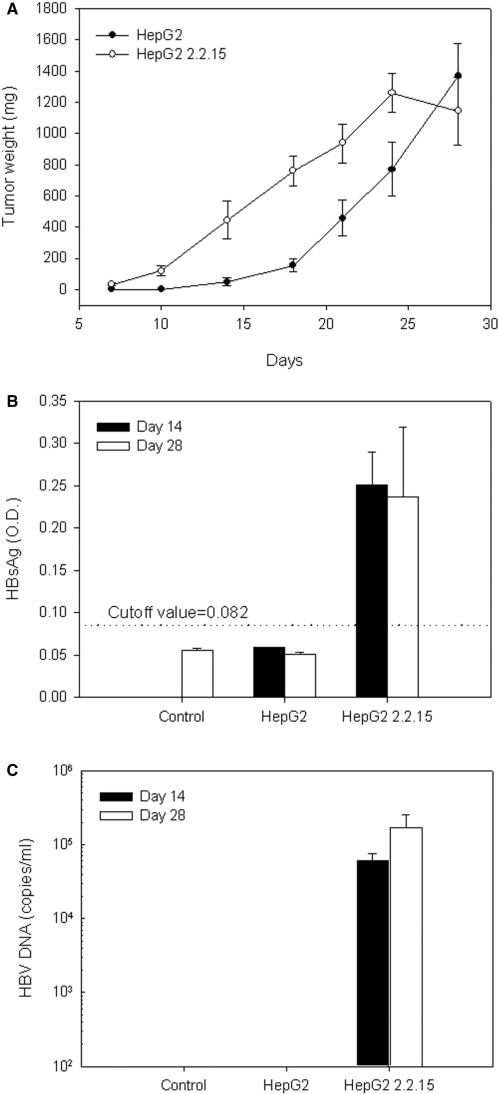
Human hepatoma cells, HepG2 and HepG2 2.2.15, were implanted subcutaneously into the flank of SCID mice, and tumor size was measured and calculated in terms of mass (Fig. 2A). Both hepatoma cells produced tumors of ∼1130–1250 mg within 28 days. The tumor-take rate of each cell line was 100%. HepG2 2.2.15–derived tumors grew at a faster rate than HepG2-derived tumors.
Figure 2.
Tumor weight, serum HBsAg level and HBV DNA level in the HBV-viremia animal model. HepG2 or HepG2 2.2.15 cells (107) were inoculated subcutaneously into the flank of SCID mice. (A) Tumor size was measured every 4 days as shown. Blood was withdrawn from animals on post-inoculation Days 14 and 28. (B) Serum HBsAg and (C) serum HBV DNA were measured by ELISA and quantitative real-time PCR, respectively. Data represent mean ± SE.
Secretion of Serum HBsAg in HepG2 2.2.15 Tumor-bearing Mice
On Days 14 and 28 after tumor cell inoculation, sera from tumor-bearing mice were collected and analyzed for HBV DNA and the HBV-related maker, HBsAg. HepG2 2.2.15 tumor-bearing mice had high levels of serum HBsAg, whereas the HepG2 tumor-bearing mice did not (HepG2 is a hepatocellular carcinoma without HBV infection; Fig. 2B).
Production of HBV DNA in Serum from HepG2 2.2.15 Tumor-bearing Mice
To investigate whether the HepG2 2.2.15 cells inoculated into the SCID mice produced HBV particles in circulating blood, serum was analyzed for HBV DNA using quantitative real-time PCR. In the HepG2 2.2.15 group, the level of serum HBV DNA was 6 × 104 copies ml−1 on Day 14 and increased to 1.4 × 105 copies ml−1 on Day 28 (Fig. 2C). These data indicate that HBV virema was produced in SCID animals upon injection of HBV-producing HepG2 2.2.15 cells. Additionally, serum HBV DNA level was proportional to the size of the HepG2 2.2.15 tumor, thereby simulating the increase in virus load in chronic hepatitis B patients. These results suggest that our HepG2 2.2.15 SCID mouse system constitutes an animal model of HBV-viremia.
Variation in Tumor Weight and Body Weight Among the Experimental Groups
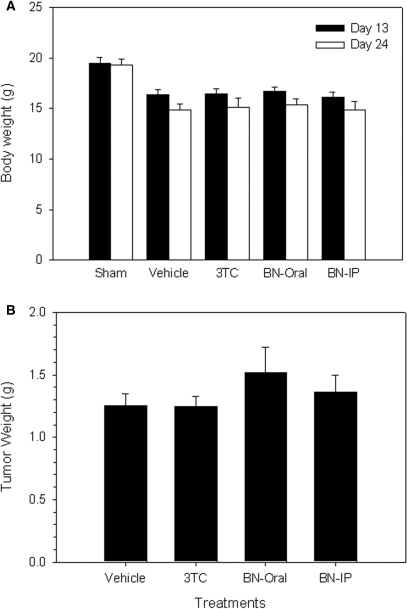
We used our HBV-viremia model to evaluate the effects of anti-HBV drugs (see below). In the absence of drugs, all HepG2 2.2.15 tumor-bearing mice had lower body weight compared with the sham group (injected with PBS but no cells) (Fig. 3A). The loss of body weight caused by HepG2 2.2.15 tumor was associated with tumor formation. Thus, body weight loss was associated with tumor formation.
Figure 3.
Body weight and implanted tumor weight of HBV-viremia mice. HepG2 2.2.15 cells (107) were inoculated subcutaneously into the flank of SCID mice. (A) Body weight was measured on post-inoculation Days 13 and 24. (B) HepG2 2.2.15 tumors were excised and weighed after animals were sacrificed on Day 28. Data represent mean ± SE.
Serum HBsAg and HBV DNA in the HBV-viremia SCID mice were elevated by Day 13, thus allowing us to evaluate the anti-HBV efficacy of BNE. HBV-viremia mice were treated with oral 3TC, oral BNE and intraperitoneal BNE administration for 10 consecutive days. Tumor growth in all HBV-viremia animals was not affected by drug administration (Fig. 3B), suggesting that 3TC and BNE do not have anti-tumor activity in this animal model.
Suppression of Serum HBsAg by 3TC and BNE
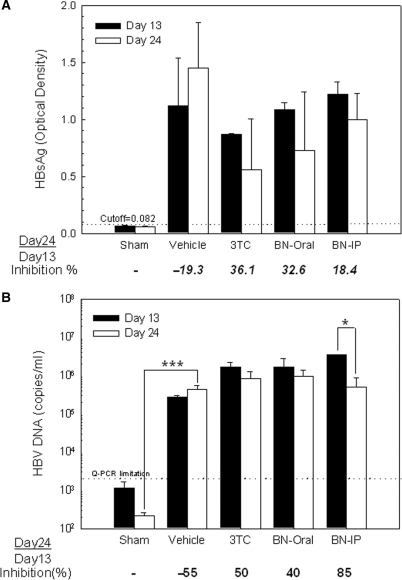
Blood was withdrawn from animals when tumor size reached ∼400 mg on Day 13 and after the 10 consecutive days of drug treatment that ended on Day 24 (Fig. 1). After the 10-day treatment with 3TC (by gavage), the level of HBsAg decreased by 36.1% (Fig. 4A). When animals were fed BNE by gavage or were injected intraperitoneally with the same amount of BNE (195 mg kg−1), serum HBsAg level decreased by 32.6% and 18.4%, respectively. In contrast, serum HBsAg increased by 19.3% in the vehicle-only control group fed distilled water. Thus, although the suppression of HBsAg level was not significant in all treatments, a trend of serum HBsAg suppression was observed.
Figure 4.
Effects of BNE on serum HBsAg level and serum HBV DNA level in HBV-viremia mice. HepG2 2.2.15 cells (107) were subcutaneously inoculated into the flank of SCID mice. On post-inoculation Days 13 and 24, blood was withdrawn from drug-treated HBV-viremia animals, and (A) serum HBsAg level was measured by ELISA, and (B) serum HBV DNA level was determined by quantitative real-time PCR. Data represent mean ± SE *P < 0.05 by paired t-test. ***P < 0.001 by one-way ANOVA and Dunnett's test.
Suppression of Serum HBV DNA by 3TC and BNE
Quantitative real-time PCR showed that no serum HBV DNA was detected in the sham group (Fig. 4B). However, a 55% increase in HBV DNA level was observed in the vehicle group (2.78 × 105 copies ml−1 on Day 13 to 4.31 × 105 copies ml−1 on Day 24), in which only distilled water was given by gavage during the 10-day period. In the 3TC group, the serum HBV DNA level was suppressed by ∼50% from Day 13 to Day 24 (1.65 × 106 copies ml−1 to 8.25 × 105 copies ml−1). For the BNE group, BNE administered intraperitoneally suppressed the serum HBV DNA level by 85% (3.47 × 106 copies ml−1 to 5.12 × 105 copies ml−1) compared with only 40% for the gavage route (1.64 × 106 copies ml−1 to 9.81 × 105 copies ml−1).
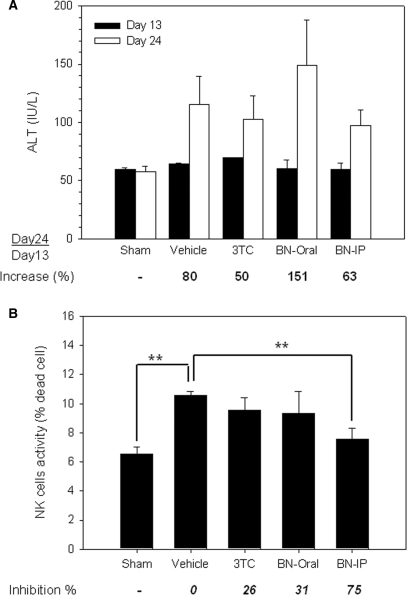
Assessment of ALT Activity in the Experimental Groups
To evaluate the biochemical and immunological effects by BNE, we measured the levels of serum ALT. There was no significant difference in the serum ALT level of the sham group between Days 13 and 24 (Fig. 5A). In contrast, the serum ALT level was elevated in all HBV-viremia animals, suggesting that these animals responded biochemically to the implanted tumors. However, this elevated ALT activity was not suppressed by 3TC or BNE administration (Fig. 5A).
Figure 5.
Effects of BNE on the activities of serum ALT and NK cells in HBV-viremia mice. HepG2 2.2.15 cells (107) were subcutaneously inoculated into the flank of SCID mice. (A) On post-inoculation Days 13 and 24, blood was withdrawn from test animals, and the activity of serum ALT was measured. (B) On Day 24, drug-treated HBV-viremia animals were sacrificed and the spleen was homogenized into a single-cell suspension; the activity of NK cells was subsequently assayed as described in Subjects and methods section. Data represent mean ± SE **P < 0.01 by one-way ANOVA and Dunnett's test.
Inhibition of NK Cell Activity by BNE
To investigate the effect of BNE on innate immunity, animals were sacrificed and the spleens were taken for NK cell activity analysis. NK activity was increased by 60% in HBV-viremia animals (the vehicle group) compared to the sham group (no tumor) (Fig. 5B). This elevated NK activity—set as 100% with respect to the difference between the sham group and the vehicle group (distilled water)—was inhibited by 26% by 3TC. NK cell activity also was inhibited by 31% in animals treated orally with BNE. Dramatically, NK activity was inhibited by 75% in animals injected intraperitoneally with BNE (195 mg kg−1—the same dose given to the gavage group), suggesting that intraperitoneally administered BNE is more effective than by gavage. These data suggest that serum HBV DNA level is inhibited by BNE, which in turn results in a decrease in NK activity.
Discussion
Extensive efforts have been put forth in the development of an HBV animal model to mimic the natural infection (15). However, these HBV animal models can only explain a part of the mechanism of anti-HBV drugs; moreover, the maintenance of animals in these models, such as HBV transgenic mice (23–25) and the Peking duck model (26), is very expensive. In this study, we implanted the HBV-producing cell line, HepG2 2.2.15, in SCID mice to simulate the phenomenon of HBV viremia, because viruses generated from HepG2 2.2.15 cells have been reported to be infectious (27). HBV particles and HBsAg were detected in the circulation of HepG2 2.2.15 tumor-bearing mice, and the serum HBV DNA level correlated with tumor growth. Therefore, this HBV-viremia model might provide information not only on the absorption, distribution, and metabolism of anti-HBV drugs but also their efficacy, and these aspects may be applicable to the treatment of HBV in humans.
Clinical evaluation of the levels of HBsAg, HBeAg and HBV DNA in serum represents the different stages of HBV infection (28). Although high levels of serum HBsAg represent the acute phase of HBV infection, HBV DNA is one of the most important factors for diagnosis of different stages of hepatitis B (29). In this study, elevated serum HBsAg and HBV DNA were detected in the vehicle group because of HBV production from HepG2 2.2.15 tumors, and the elevated HBV DNA in serum was sufficient to allow us to evaluate the anti-HBV activities of 3TC and BNE. Serum HBsAg and HBV DNA levels were suppressed by BNE, but tumor growth was unaffected. BNE caused a decrease in both serum HBsAg and HBV DNA levels. Furthermore, the intraperitoneal administration of BNE was more effective than by gavage. These findings are consistent with our previous in vitro study, in which HBV DNA level was reduced to a greater extent than HBsAg level (13). 3TC was also effective at suppressing HBV replication (i.e. ∼50% reduction in serum HBV DNA level) in HBV-viremia animals, and this efficacy may improve with prolonged therapy.
Although SCID mice lack adaptive immunity (functional T and B cells), they retain innate immunity in C.B17/icr species (e.g. functional granulocytes and NK cells). Thus, liver damage that might be caused by adaptive immunity in normal mice might not be observed in SCID mice (30). Apparent suppression of NK cell activity was observed after intraperitoneal administration of BNE. NK cells play a critical role in host innate defense against viruses and may be partly responsible for liver injury resulting from anti-viral responses. However, the exact role of NK cells in liver injury remains unclear (31). In this model, the observed increase in ALT level could not be conclusively attributed to the mouse liver cells or tumor cells. Nonetheless, histological examination of the liver of all mice revealed no apparent damage (data not shown), suggesting that the elevated serum ALT activity probably was a result of expression from the implanted tumor cells. Taken together, the data support the anti-HBV effects of BNE and the chemical composition of BNE is subject to for further investigation.
Acknowledgment
We thank Dr Chang DTM for polishing the English and giving us comments on this article.
References
- 1.Nakamoto Y, Guidotti LG, Kuhlen CV, Fowler P, Chisari FV. Immune pathogenesis of hepatocellular carcinoma. J Exp Med. 1998;188:341–50. doi: 10.1084/jem.188.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ganem D, Varmus HE. The molecular biology of the hepatitis B viruses. Annu Rev Biochem. 1987;56:651–93. doi: 10.1146/annurev.bi.56.070187.003251. [DOI] [PubMed] [Google Scholar]
- 3.Ocama P, Opio CK, Lee WM. Hepatitis B virus infection: current status. Am J Med. 2005;118:1413. doi: 10.1016/j.amjmed.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 4.Mailliard ME, Gollan JL. Emerging therapeutics for chronic hepatitis B. Annu Rev Med. 2006;57:155–66. doi: 10.1146/annurev.med.57.121304.131422. [DOI] [PubMed] [Google Scholar]
- 5.Tenney DJ, Levine SM, Rose RE, Walsh AW, Weinheimer SP, Discotto L, et al. Clinical emergence of entecavir-resistant hepatitis B virus requires additional substitutions in virus already resistant to lamivudine. Antimicrob Agents Chemother. 2004;48:3498–507. doi: 10.1128/AAC.48.9.3498-3507.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee SK, Wong CK, Poon PM, Ip PS, Che CT, Fung KP, et al. In vitro immunomodulatory activities of a newly concocted traditional Chinese medicine formula: VI-28. Phytother Res. 2006;20:883–8. doi: 10.1002/ptr.1955. [DOI] [PubMed] [Google Scholar]
- 7.Fischer KP, Gutfreund KS, Tyrrell DL. Lamivudine resistance in hepatitis B: mechanisms and clinical implications. Drug Resist Updat. 2001;4:118–28. doi: 10.1054/drup.2001.0190. [DOI] [PubMed] [Google Scholar]
- 8.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35–50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Lin H, McIntosh H. Genus Phyllanthus for chronic hepatitis B virus infection: a systematic review. J Viral Hepat. 2001;8:358–66. doi: 10.1046/j.1365-2893.2001.00307.x. [DOI] [PubMed] [Google Scholar]
- 10.Kang EH, Kown TY, Oh GT, Park WF, Park SI, Park SK, et al. The flavonoid ellagic acid from a medicinal herb inhibits host immune tolerance induced by the hepatitis B virus-e antigen. Antiviral Res. 2006;72:100–6. doi: 10.1016/j.antiviral.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Li H, Zhou C, Zhou L, Chen Z, Yang L, Bai H, et al. In vitro antiviral activity of three enantiomeric sesquiterpene lactones from Senecio species against hepatitis B virus. Antivir Chem Chemother. 2005;16:277–82. doi: 10.1177/095632020501600407. [DOI] [PubMed] [Google Scholar]
- 12.Chang JS, Liu HW, Wang KC, Chen MC, Chiang LC, Hua YC, et al. Ethanol extract of Polygonum cuspidatum inhibits hepatitis B virus in a stable HBV-producing cell line. Antiviral Res. 2005;66:29–34. doi: 10.1016/j.antiviral.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Huang KL, Lai YK, Lin CC, Chang JM. Inhibition of hepatitis B virus production by Boehmeria nivea root extract in HepG2 2.2.15 cells. World J Gastroenterol. 2006;12:5721–5. doi: 10.3748/wjg.v12.i35.5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Locarnini S. Molecular virology and the development of resistant mutants: implications for therapy. Semin Liver Dis. 2005;25(Suppl 1):9–19. doi: 10.1055/s-2005-915645. [DOI] [PubMed] [Google Scholar]
- 15.Guha C, Mohan S, Roy-Chowdhury N, Roy-Chowdhury J. Cell culture and animal models of viral hepatitis. Part I: hepatitis B. Lab Anim. 2004;33:37–46. doi: 10.1038/laban0704-37. [DOI] [PubMed] [Google Scholar]
- 16.Prince AM, Brotman B. Perspectives on hepatitis B studies with chimpanzees. Ilar J. 2001;42:85–8. doi: 10.1093/ilar.42.2.85. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi H, Fujimoto J, Hanada S, Isselbacher KJ. Acute hepatitis in rats expressing human hepatitis B virus transgenes. Proc Natl Acad Sci USA. 1995;92:1470–4. doi: 10.1073/pnas.92.5.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhai WR, Vajta G, Acs G, Paronetto F. A nude mouse model for the in vivo production of hepatitis B virus. Gastroenterology. 1990;98:470–7. doi: 10.1016/0016-5085(90)90840-w. [DOI] [PubMed] [Google Scholar]
- 19.Sato H, Goto W, Yamamura J, Kurokawa M, Kageyama S, Takahara T, et al. Therapeutic basis of glycyrrhizin on chronic hepatitis B. Antiviral Res. 1996;30:171–7. doi: 10.1016/0166-3542(96)00942-4. [DOI] [PubMed] [Google Scholar]
- 20.Dandri M, Volz TK, Lutgehetmann M, Petersen J. Animal models for the study of HBV replication and its variants. J Clin Virol. 2005;34(Suppl 1):S54–62. doi: 10.1016/s1386-6532(05)80011-3. [DOI] [PubMed] [Google Scholar]
- 21.Condreay LD, Jansen RW, Powdrill TF, Johnson LC, Selleseth DW, Paff MT, et al. Evaluation of the potent anti-hepatitis B virus agent (-) cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine in a novel in vivo model. Antimicrob Agents Chemother. 1994;38:616–9. doi: 10.1128/aac.38.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Euhus DM, Hudd C, LaRegina MC, Johnson FE. Tumor measurement in the nude mouse. J Surg Oncol. 1986;31:229–34. doi: 10.1002/jso.2930310402. [DOI] [PubMed] [Google Scholar]
- 23.Sitia G, Iannacone M, Muller S, Bianchi ME, Guidotti LG. Treatment with HMGB1 inhibitors diminishes CTL-induced liver disease in HBV transgenic mice. J Leukoc Biol. 2007;81:100–7. doi: 10.1189/jlb.0306173. [DOI] [PubMed] [Google Scholar]
- 24.Morrey JD, Bailey KW, Korba BE, Sidwell RW. Utilization of transgenic mice replicating high levels of hepatitis B virus for antiviral evaluation of lamivudine. Antiviral Res. 1999;42:97–108. doi: 10.1016/s0166-3542(99)00009-1. [DOI] [PubMed] [Google Scholar]
- 25.Morrey JD, Korba BE, Sidwell RW. Transgenic mice as a chemotherapeutic model for hepatitis B virus infection. Antivir Ther. 1998;3:59–68. [PubMed] [Google Scholar]
- 26.Jilbert AR, Botten JA, Miller DS, Bertram EM, Hall PM, Kotlarski J, et al. Characterization of age- and dose-related outcomes of duck hepatitis B virus infection. Virology. 1998;244:273–82. doi: 10.1006/viro.1998.9095. [DOI] [PubMed] [Google Scholar]
- 27.Liu N, Zhu B, Huang Z, Zhu Y, Chen Q, Guo X, et al. [The inhibitive effects of the ethanol extract from Radix et Rhizoma Rhei on the secretion of HBsAg and HBeAg] Zhong Yao Cai. 2004;27:419–21. [PubMed] [Google Scholar]
- 28.Ganem D, Prince AM. Hepatitis B virus infection–natural history and clinical consequences. N Engl J Med. 2004;350:1118–29. doi: 10.1056/NEJMra031087. [DOI] [PubMed] [Google Scholar]
- 29.Mommeja-Marin H, Mondou E, Blum MR, Rousseau F. Serum HBV DNA as a marker of efficacy during therapy for chronic HBV infection: analysis and review of the literature. Hepatology. 2003;37:1309–19. doi: 10.1053/jhep.2003.50208. [DOI] [PubMed] [Google Scholar]
- 30.Sun Y, Chen HY, Xin SJ. Effect of IL-18 on peripheral blood mononuclear cells of chronic hepatitis B and hepatitis B virus DNA released by HepG2.2.15 cell lines. Hepatobiliary Pancreat Dis Int. 2004;3:230–4. [PubMed] [Google Scholar]
- 31.Sjolin H, Tomasello E, Mousavi-Jazi M, Bartolazzi A, Karre K, Vivier E, et al. Pivotal role of KARAP/DAP12 adaptor molecule in the natural killer cell-mediated resistance to murine cytomegalovirus infection. J Exp Med. 2002;195:825–34. doi: 10.1084/jem.20011427. [DOI] [PMC free article] [PubMed] [Google Scholar]