The selective separation of alkaline salts from aqueous media is of fundamental importance in chemistry. It is, for instance, critical to the production of commodity materials (e.g., bromine and potassium) from high-salt sources, such as the Dead Sea and the Great Salt Lake[1] and, on a very different scale, to the regulation of taste[2] and the maintenance of osmotic balance in cells.[3] Materials that could allow for such separations are thus of potential interest in a wide range of applications.[4] Polymeric systems are particularly attractive in this regard because they are generally easy to isolate from solutions or mixtures.
Recently, we reported that copolymers of a polymerizable derivative 2 (Scheme 1) of octamethylcalix[4]pyrrole (1)[5] and methyl methacrylate (MMA) are effective at extracting tetrabutylammonium chloride or fluoride from aqueous media.[6] Unfortunately, these polymeric materials displayed relatively low affinities for the corresponding salts containing cations less soluble in organic media (e.g., Na and K). We envisioned that by appending recognition groups capable of binding such ions to modified calixpyrrole-containing polymers, their affinities toward common salts would be improved. For example, crown ethers (e.g., 3) are well-known for their ability to complex alkali cations, particularly potassium.[7] This has led to their use in phase-transfer catalysis[8] and as extractants for picrate anion salts under organic–aqueous interfacial conditions.[9] In fact, polymeric systems containing crown ethers have been used to extract the potassium salts of relatively hydrophobic anions.[9] However, neither these latter systems nor any of which we are aware possess the capability of extracting “hard” potassium salts, such as KF or KCl, from aqueous media.
Scheme 1.
Structures of compounds examined for their abilities to extract KF from water.
Herein, we report the synthesis, characterization, and extraction properties of mixed MMA copolymers containing pendant calix[4]pyrrole subunits known to bind halide anions in a 1:1 ratio in organic media[5] and benzo-[15]crown-5 subunits capable of forming 2:1 sandwich complexes with potassium cations.[7] It was thus expected that strong, potentially mutually enhancing, interactions would enable these polymeric materials to extract potassium halide salts, such as KCl and KF, from aqueous solutions.
Copolymers 4–6 (Scheme 1) were prepared from MMA, calix[4]pyrrole 2, and the benzo-[15]crown-5 derivative 7[9] using conventional free-radical polymerization techniques.[10] In general, azoisobutyronitrile (1 mol%) was added to THF solutions of these monomers in various ratios. After heating at 70°C for 17 h, the solutions were independently poured into excess methanol, which caused the polymer to precipitate. After collection by filtration, the resulting materials were characterized by NMR spectroscopy (CD2Cl2) and gel permeation chromatography.[6] The molecular weights (33–90 kDa) and polydispersities (PDI = 2.1–2.5) of these copolymers were typical of those obtained from free-radical polymerizations.
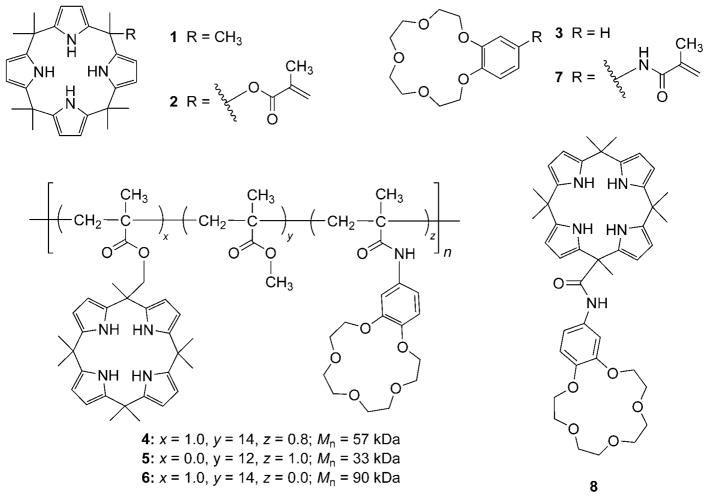
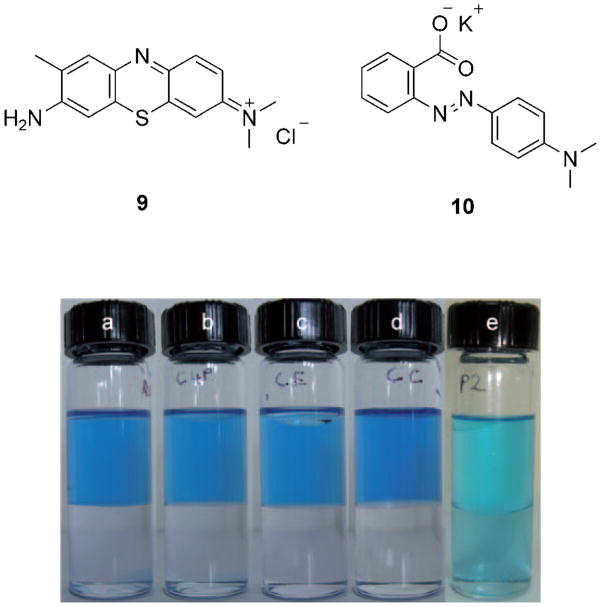
Initial qualitative evidence that copolymer 4, which contains both calix[4]pyrrole and crown ether subunits, could extract chloride salts into organic media came from a visual test involving 9, a water-soluble dye that contains a chloride counteranion. Treatment of an aqueous solution of 9 (25.5 μM) with a CH2Cl2 solution of copolymer 4 (effective concentration of the calix[4]pyrrole and crown ether repeat units was 1.56 and 1.22 mM, respectively) resulted in a colored organic phase (Figure 1). As controls, solutions of the dye were also exposed to CH2Cl2 solutions of 1 (1.56 mM), 3 (1.22 mM), or a mixture of 1 and 3 (1.56 and 1.22 mM, respectively); however, no transfer of color was observed. These results were quantified using UV/Vis spectroscopy. As shown in Figure 2, analysis of the aqueous phases of these extraction experiments confirmed that 4 was able to extract 9 into the organic phase more effectively (greater than 54%) than 1, 3, or their mixture. Similar qualitative and quantitative results were observed for aqueous solutions of 10, a water-soluble dye that contains a potassium countercation. In this case, copolymer 4 proved more effective as an extractant (greater than 30%) relative to either 1, 3, or the same mixture of 1 and 3 used above (see the Supporting Information).
Figure 1.
Aqueous solutions of 9 (top layers): a) After treatment with CH2Cl2 (bottom layer). b) After treatment with a CH2Cl2 solution of 1 (bottom layer). c) After treatment with a CH2Cl2 solution of 3 (bottom layer). d) After treatment with a CH2Cl2 solution of 1 and 3 (bottom layer). e) After treatment with a CH2Cl2 solution of polymer 4 (bottom layer). See text for details.
Figure 2.
UV/Vis spectra of aqueous solutions of 9 (initial concentration = 25.5 μM) after exposure to an equal volume of a CH2Cl2 solution of polymer 4 (effective concentration of the calix[4]pyrrole and crown ether repeat units was 1.56 and 1.22 mM, respectively), 1 (1.56 mM), 3 (1.22 mM), or a mixture of 1 and 3 (1.56 and 1.22 mM, respectively).
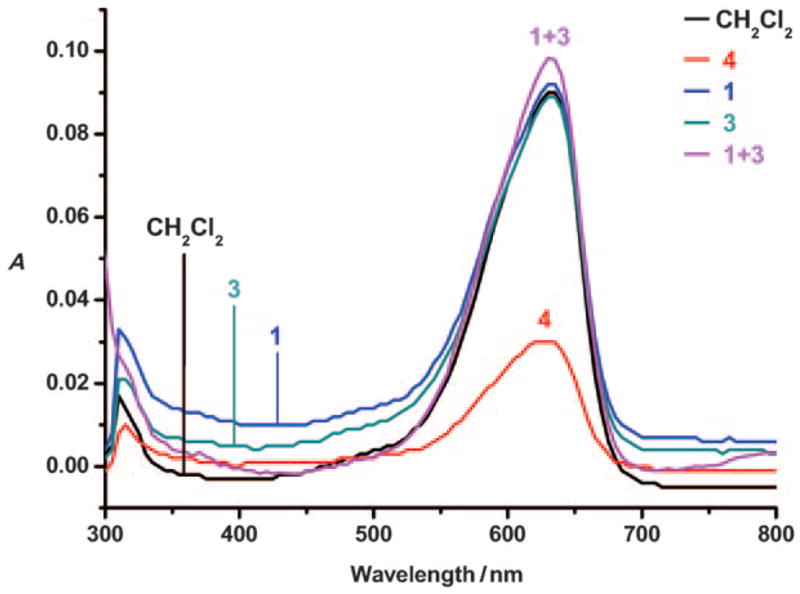
Encouraged by these initial results, we next sought to address the question of whether copolymer 4 could extract a salt consisting of two hard ions, namely potassium fluoride. In parallel, the extraction properties of 5 and 6 were examined. These systems contain either calixpyrrole or crown ether recognition subunits, respectively, and were designed to assess the relative importance of each individual ion recognition unit on the overall extraction properties of 4. As shown in Figure 3, addition of a 3.4M D2O solution of KF to a CD2Cl2 solution of 4 (effective concentration of the calix[4]pyrrole and crown ether repeat units 6.25 and 4.86 mM, respectively) resulted in the appearance of a signal at δ = −121.7 ppm in the 19F NMR spectrum of the organic phase. A similar signal, but of reduced intensity, was seen in the case of 5, whereas very little signal was observed in the case of 6.
Figure 3.
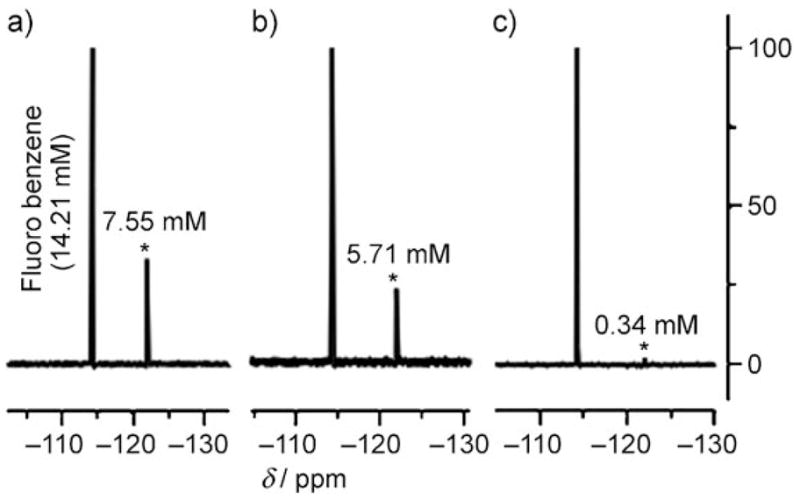
19F NMR spectra of CD2Cl2 solutions of copolymers a) 4 (effective [calix[4]pyrrole] = 6.25 mM), b) 5 ([calix[4]pyrrole] = 6.50 mM), and c) 6 (no calix[4]pyrrole) after adding D2O solutions of KF (3.4 M), shaking the tubes vigorously, and then separating the phases with the aid of centrifugation (10 min). * denotes KF.
To quantify the amount of fluorine present in the organic phases of the aforementioned extraction experiments, fluorobenzene (final concentration: 14.21 mM) was added to each sample as an internal standard (δ = −114.3 ppm). On the basis of comparative integrations (i.e., comparing total fluoride content in the CD2Cl2 layer relative to this standard), copolymer 4 was found to be capable of extracting KF more efficiently (7.55 ± 0.04 mM) then polymer 5 (5.71 ± 0.03 mM) under conditions where the effective concentration of the calix[4]pyrrole repeat units in both polymers were essentially the same (6.25 mM versus 6.50 mM for 4 and 5, respectively). In addition, both of these polymers were found to extract significantly more fluoride into the organic phase than 6 ([F] = 0.34 ± 0.03 mM in the CD2Cl2 layer), a copolymer that does not contain any calix[4]pyrrole subunits, as noted above. As control experiments, extractions were also performed in an analogous manner using 1, 3, MMA homopolymer,[6] an equimolar mixture of calixpyrrole 1 and crown ether 3, and the calixpyrrole crown ether pseudo dimer 8, which was envisioned as a small-molecule analogue of 4.[11] No quantifiable fluorine signal was observed in the organic phase when any of these control systems were used as extractants.[12]
Flame emission spectroscopy (FES) was used to confirm the coextraction of potassium in the above experiments.[13] The organic phase obtained after extracting KF with polymer 4 afforded an emission intensity (EI) of 0.401 (at 766.5 nm, i.e., the emission wavelength of the excited potassium ion produced by the flame source) after dilution with a known amount of ethyl acetate. By way of comparison, the organic phases produced EI values of 0.277 and 0.038, respectively, when polymers 5 and 6 were used as extractants under otherwise identical conditions. Based on quantification with a series of standards,[14] extracted potassium concentrations of 6.84, 4.73, and 0.65 ± 0.05 mM were calculated for CD2Cl2 solutions of polymers 4, 5, and 6, respectively (at effective crown ether concentrations of 5.60, 0.00, and 5.00 mM, respectively). These values are in good agreement with those obtained from the 19F NMR spectroscopy data. A summary of the KF extraction data is presented in Table 1.
Table 1.
Summary of KF extraction efficiencies.a
| Cmpdb | calix/crownc | eff. [%] (total)d,e | eff. [%] (calix)d,f | eff. [%] (total)e,g | eff. [%] (crown)g,h |
|---|---|---|---|---|---|
| 4 | 1.0:0.8 | 67 | 121 | 61 | 137 |
| 5 | 1.0:0.0 | 88 | 88 | 73 | n.d.i |
| 6 | 0.0:1.0 | 6 | n.d.i | 12 | 12 |
| 8 | 1.0:1.0 | 0 | 0 | 0 | 0 |
Extraction efficiencies (eff.) are reported as the percent (%) of extractant populated with KF upon exposure to a saturated aqueous solution of KF. See text for additional details.
See Scheme 1 for the structures of the compounds studied.
Relative molar ratios of calixpyrrole (calix) to crown ether (crown) units in the extractant.
Calculated from total fluoride extracted.
Based on the total number of ion receptors (calixpyrrole plus crown ether) in the extractant.
Based on the total number of calixpyrrole units in the extractant.
Calculated from total potassium extracted.
Based on the total number of crown ether units in the extractant.
n.d. = not determined.
Next, the ability of polymers 4, 5, and 6 to extract KCl from aqueous media was evaluated using conditions analogous to those employed for the KF studies described above. In this case, after exposing the polymers to 3.4M solutions of KCl in D2O, FES was again used to determine the relative amounts of potassium extracted. Polymer 4 proved to be the most effective extractant, displaying an EI value of 0.761, which corresponded to a potassium concentration of 12.97 ± 0.08 mM in the organic phase, with the exact quantification being based on a series of standards.[14] Polymers 5 and 6 displayed lower EI values, namely 0.507 and 0.081, respectively, which corresponded to potassium concentrations of 8.64 and 1.38 ± 0.08 mM. The higher overall extraction values for KCl compared to KF is consistent with the relative aqueous solvation energies (ΔGh) of chloride and fluoride anions (ΔGh = −340 kJmol−1 for Cl− vs. −465 kJmol−1 for F−).[15] Specifically, the more hydrophobic anion (Cl−) was extracted more effectively than its more hydrophilic analogue (F−).[16,17]
Finally, an effort was made to determine if potassium salts could be selectively extracted in the presence of their sodium analogues. Towards this end, a 0.5 mL H2O solution of KCl (134.1 mM) and NaNO3 (1.47 M) was treated with polymer 4 (in 0.75 mL CH2Cl2) and analyzed using FES. (The two aforementiond inorganic salts were combined deliberately to form NaCl in situ.) The EI of the signal corresponding to potassium (0.734) was over an order of magnitude greater than the signal corresponding to sodium (0.043). On this basis it was concluded that polymer 4 extracts potassium chloride much more effectively than it does sodium chloride.[18] This finding, which is in accord with the relative hydration energies of K+ and Na+ (ΔGh = −295 kJmol−1 for K+ and −365 kJmol−1 for Na+),[15] suggests that these materials may ultimately enable the selective separation of potassium halide salts from complex aqueous mixtures. This could be especially valuable in specialty medical applications, such as the control of hyperkalemia, where potassium ion exchange resins (e.g., sodium polystyrene sulfonate; kayexalate) have seen widespread use, despite being subject to inherent chemical and clinical limitations.[19]
In conclusion, we have prepared the first well-defined and homogenous polymeric systems capable of extracting potassium fluoride and chloride salts from aqueous media. These polymers contain pendant calixpyrrole and crown ether subunits, key features that permit the concurrent complexation of both halide and potassium ions. This, in turn, allows the system as a whole to overcome the relatively high hydration energies of KF and KCl and enables their extraction from aqueous media with efficiencies that exceed those expected on the basis of the effective concentration of the individual receptors (crown ether and calixpyrrole). To our knowledge this has not hitherto proved possible with any other simple polymeric material. Ongoing efforts are focused on fine-tuning the choice of receptors and investigating the effect of polymer molecular weight and microstructure on the overall extraction performance of these materials.
Supplementary Material
Footnotes
This work was supported by the National Science Foundation (CHE-0645563), the National Institutes of Health (GM 58907), and the INEST Group.
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.200803970.
Contributor Information
Ahmet Akar, Email: akara@itu.edu.tr.
Christopher W. Bielawski, Email: bielawski@cm.utexas.edu.
Jonathan L. Sessler, Email: sessler@mail.utexas.edu.
References
- 1.a) Mansour AR, Takrouri KJ. Chem Eng Commun. 2007;194:803. [Google Scholar]; b) Krumgalz BS, Millero FJ. Mar Chem. 1989;27:219. [Google Scholar]; c) Carpenter A. Circ—Okla Geol Surv. 1978;79:78. [Google Scholar]; d) Marcus Y. Geochim Cosmochim Acta. 1977;41:1739. [Google Scholar]
- 2.a) Park KH, Hernandez L, Cai SQ, Wang Y, Sesti F. J Biol Chem. 2005;280:21893. doi: 10.1074/jbc.M502732200. [DOI] [PubMed] [Google Scholar]; b) Bobkov YV, Kolesnikov SS. Neurosci Lett. 1999;264:25. doi: 10.1016/s0304-3940(99)00170-6. [DOI] [PubMed] [Google Scholar]
- 3.a) Sweeney G, Klip A. Cell Mol Biol. 2001;47:363. [PubMed] [Google Scholar]; b) Morris S, Edwards T. Comp Biochem Physiol C. 1995;112:129. [Google Scholar]
- 4.Camiolo S, Gale PA. Chem Commun. 2000:1129.Schmidtchen FP. Org Lett. 2002;4:431. doi: 10.1021/ol017137u.Schofield K. Energy Fuels. 2003;17:191.Eller LR, Stepien M, Fowler CJ, Lee JT, Sessler JL, Moyer BA. J Am Chem Soc. 2007;129:11020. doi: 10.1021/ja074568k.Correction: Eller LR, Stpieri M, Fowler CJ, Lee JT, Sessler JL, Moyer BA. J Am Chem Soc. 2007;129:14523. doi: 10.1021/ja074568k.Moyer BA, Delmau LH, Fowler CJ, Ruas A, Bostick DA, Sessler JL, Katayev E, Pantos GD, Llinares JM, Hossain A, Kang SO, Bowman-James K. In: Advances in Inorganic Chemistry: Including Bioinorganic Studies. Eldik R, Bowman-James K, editors. Vol. 59. Academic Press; New York: 2007. p. 175.Shishkanova TV, Sykora D, Sessler JL, Kral V. Anal Chim Acta. 2007;587:247. doi: 10.1016/j.aca.2007.01.044.Kral V, Sessler JL, Shishkanova TV, Gale PA, Volf R. J Am Chem Soc. 1999;121:8771.
- 5.Octamethylcalix[4]pyrrole (1) is a commercially available receptor that recognizes fluoride and chloride anions in organic media (Ka ≥ 104 and 350M−1 for the tetrabutylammonium salts of F− and Cl−, respectively, in CD2Cl2): Gale PA, Sessler JL, Kral V. Chem Commun. 1998:1.
- 6.Aydogan A, Coady DJ, Lynch VM, Akar A, Marquez M, Bielawski CW, Sessler JL. Chem Commun. 2008:1455. doi: 10.1039/b718021g. [DOI] [PubMed] [Google Scholar]
- 7.Pedersen CJ. J Am Chem Soc. 1967;89:7017. [Google Scholar]
- 8.a) Albanese D, Landini D, Maia A, Penso M. J Mol Catal A. 1999;150:113. [Google Scholar]; b) Makosza M, Fedorynski M. Catal Rev Sci Eng. 2003;45:321. [Google Scholar]; c) Albanese D. Catal Rev Sci Eng. 2003;45:369. [Google Scholar]
- 9.This particular crown ether was chosen because it was found to be more effective in potassium cation extraction than the corresponding crown-6 system when incorporated in a polymer; see: Yagi K, Sanchez MC. In: Polymer Science and Technology. Mathias LJ, Carraher CE, editors. Vol. 24. Plenum; New York: 1984. p. 345.
- 10.A copolymer of 2 and 7 (i.e., no MMA) was also prepared but was found to afford an insoluble white preciptate upon exposure to aqueous solutions of KF or KCl. This result is thought to reflect the formation of strong complexes within the polymer matrix involving either the chloride or fluoride anion and the calix[4]pyrrole subunit, or the potassium cation and the crown ether subunit, perhaps in a cooperative fashion. However, the lack of solubility precluded further analysis of this material. Empirically, the presence of methacrylate units in the backbones of these polymeric extractants (i.e., 4–6) appears necessary to retain solubility in dichloromethane.
- 11.Several other mixed calixpyrrole crown ether pseudo dimers, containing different covalent linkages, different size crowns, etc., were also prepared; none of these proved effective as KF extractants under the conditions described above (see the Supporting Information).
- 12.a) Mammen M, Choi S-K, Whitesides GM. Angew Chem. 1998;110:2908. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 1998;37:2754. [Google Scholar]; b) Kim IB, Dunkhorst A, Gilbert J, Bunz UHF. Macromolecules. 2005;38:4560. [Google Scholar]
- 13.Jackson KW, Lu SJ. Anal Chem. 1998;70:363. [Google Scholar]
- 14.Standard solutions for FES analyses were prepared by dissolving potassium tetrakis(2-thienyl)borate in an ethyl acetate/CH2Cl2 (9:1 v/v) solution in concentrations of 0.5, 1.0, and 2.0 ppm; this produced 0.330, 0.630, and 1.180 emission intensities, respectively. After 0.5 mL aliquots of the extracts were diluted to 20 mL with CH2Cl2, 1 mL portions of the resulting solutions were then diluted to 10 mL with ethyl acetate and analyzed using FES.
- 15.Hydration energies were taken from Table 1 of Marcus Y. J Chem Soc Faraday Trans. 1991;87:2995.
- 16.Further support for this conclusion was obtained from 1H NMR spectroscopic analysis of the pyrrolic NH signal as a function of KF and KCl concentration under the extraction conditions described in the text. As detailed in the Supporting Information, the NH signals shifted downfield and decreased in intensity with increasing concentrations of the potassium salts, KCl and KF. In accord with expectations, KCl gave rise to greater downfield shifts in the NH peak shift (δ NH = 7.39 ppm for [KCl] ≥ 1.04 mM) than KF (δ = 7.35 ppm for [KF] ≥ 1.27 mM).
- 17.The temperature of ultimate mass loss exhibited by polymers 4–6 was seen to increase (to over 400°C by thermogravimetric analysis) after extraction with KCl or KF (see Supporting Information). The increased thermal stabilites are consistent with the notion that these polymers contained ionic species.
- 18.NaCl and NaF extractions were also carried out using the same conditions employed for the corresponding potassium salts. However, no evidence of efficient extraction was observed in the case of any of the polymeric or control systems considered herein.
- 19.a) Putcha N, Allon M. Semin Dial. 2007;20:431. doi: 10.1111/j.1525-139X.2007.00312.x. [DOI] [PubMed] [Google Scholar]; b) Palmer BF. N Engl J Med. 2004;351:585. doi: 10.1056/NEJMra035279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.