Abstract
The intramolecular α-arylation of aldehydes via organo-SOMO catalysis was investigated using density functional theory (B3LYP and M06-2X functionals). The geometries, spin densities, Mulliken charges, and molecular orbitals of the reacting enamine radical cations were analyzed, and the nature of the resulting cyclized radical cation intermediates were characterized. In agreement with experimental observations, the calculated 1,3-disubstituted aromatic system shows ortho selectivity, while the 1,3,4-trisubstituted systems show para, meta (instead of ortho, meta) selectivity. The selectivity change for the trisubstituted rings is attributed to a distortion of the ortho substituents in the ortho, meta cyclization transition structures, causing a destabilization of these isomers and therefore selectivity for the para, meta product.
Introduction
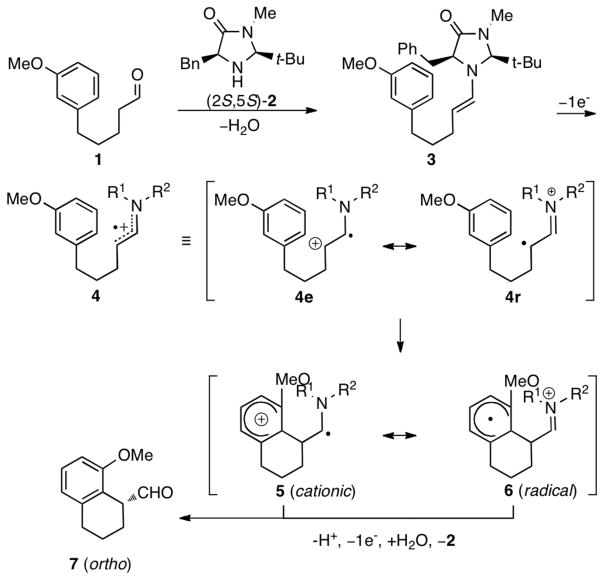
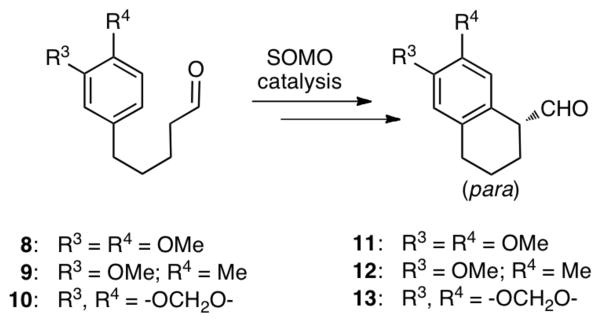
Organo-SOMO catalysis has recently become an important activation mode for the asymmetric α-allylation,1a α-enolation,1b α-vinylation,1c α-carbo-oxidation,1d α-nitroalkylation1e and intramolecular α-arylation1f–g,2 of aldehydes. One of our groups has shown that the reactions proceed via a 3π-electron radical cation species, generated by the one-electron oxidation of a chiral enamine. In a report of the intramolecular α-arylation reactions from the Nicolaou group1f,g and our laboratory,2 the cyclization of enamine radical cation 4 was shown to selectively attack ortho to the methoxy group (Scheme 1).3,4 It was proposed that an intermediate best represented as 5, rather than 6, described earlier,1f–g was involved. The Nicolaou group showed that 1,3,4-trisubstituted aldehydes 8–10, however, react to give para aryl products 11–13 (Scheme 2). We have explored the mechanisms of these reactions, particularly the nature of the intermediates and origins of selectivity using quantum mechanical calculations.5
Scheme 1.
Scheme 2.
Results and Discussion
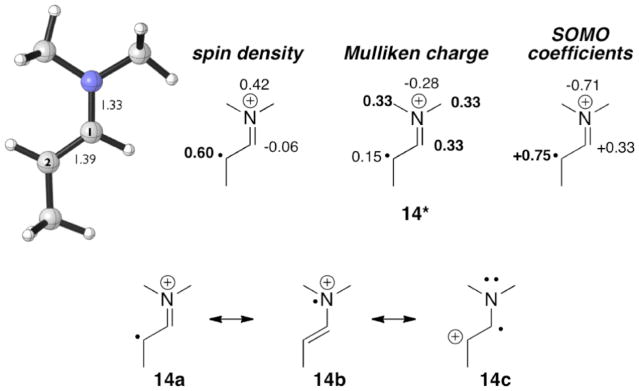
UB3LYP calculations were performed on the simple model radical cation 14*. Bond distances, spin densities, Mulliken charges, and singly occoupied molecular orbital (SOMO) coefficients of model radical cation 14* are shown in Figure 1. The C1–C2 bond distance (1.39 Å) is longer than that of an enamine (1.34 Å), and the N-C1 distance (1.33 Å) is closer to that of an iminium (1.29 Å) than of an enamine (1.40 Å). The majority of the spin is on the carbon β to the nitrogen (C2) and the charge is mainly on the iminium carbons α to the nitrogen, as in ammonium cations.6 A molecular orbital analysis shows that the largest coefficient of the singly occupied π orbital (SOMO) lies on C2. The species is of course a resonance hybrid, 14, but is best characterized as an alkyl radical conjugated to an iminium cation. The spin density is consistent with major contributions from 14a and 14b.
Figure 1.
Model enamine radical cation 14* (UB3LYP/6-31G(d)).
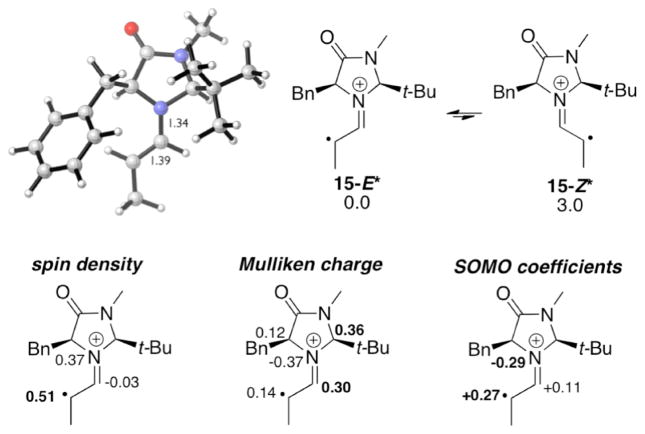
The enamine radical cation of propanal with catalyst 2 is similar (15-E*, Figure 2). The reported enantioselectivity range with this catalyst was 84–98%.1f,g,2 The lowest-energy conformer is expected to direct attack from the less hindered si (“bottom”) face of 15-E*, which is in agreement with the experimentally observed stereoisomer.3 The lowest-energy Z isomer 15-Z*, which would give the opposite enantiomer of the product, is 3.0 kcal/mol higher in energy than 15-E*.
Figure 2.
Model enamine radical cation 15* (UB3LYP/6-31G(d)).
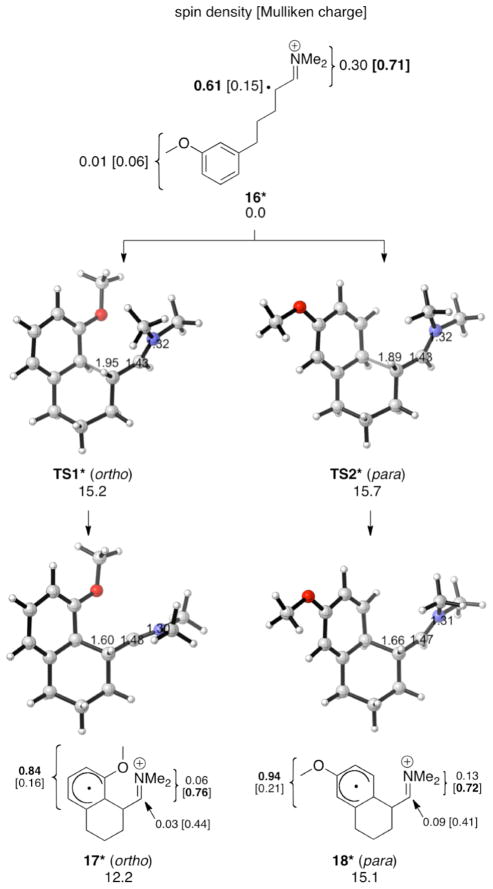
Having established the charge and spin density distribution of the enamine radical cations, we investigated the transition structures for para and ortho attack of achiral radical cation 16* on the anisole ring (Figure 3). In agreement with experimental results, ortho attack (TS1*) is predicted to be favored over para attack (TS2*) by 0.5 kcal/mol. The activation free energy for attack on the unsubstituted benzene is approximately 3 kcal/mol higher (18.3 kcal/mol), in agreement with the failed cyclization of this arene under the same reaction conditions.7
Figure 3.
Intramolecular α-arylation of 16* (UB3LYP/6-31G(d) ΔG values for aqueous solution at 268 K; optimizations in CPCM water).
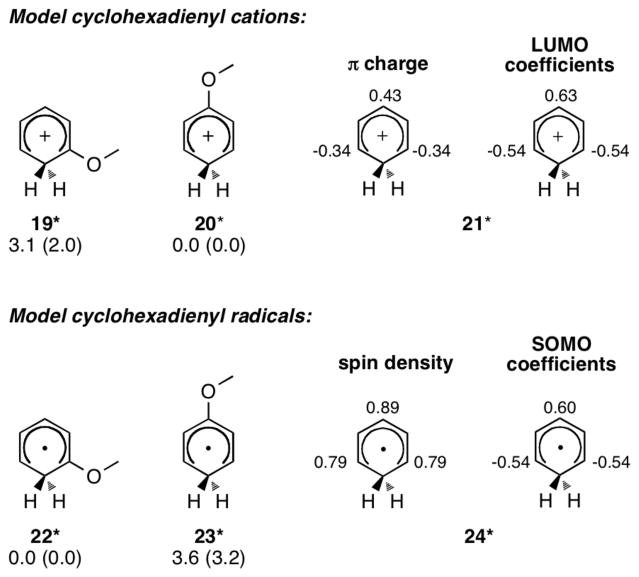
The endergonicities (17* and 18*) suggest that the cyclization step is easily reversible. Given that 17* should accumulate in higher concentrations than 18*, the potential subsequent steps were explored. Deprotonation of the more abundant and faster formed 17* leads to the favored ortho product. Oxidation of 17*, as suggested in ref. 2, was calculated to be at least as feasible as oxidation of the enamine that gives 16*.8 Radical trapping of 17*, followed by deprotonation/aromatization and iminium hydrolysis, or trapping of the iminium by water,9 followed by oxidation and subsequent deprotonation/aromatization and hydrolysis, are potential fates of the cyclized radical cations. Both of these trapping mechanisms are expected to occur readily with little rate dependence on ortho/para selectivity, so the greater stabilization of the cyclohexadienyl radical by the methoxy at the 1- rather than 3-position provides the basic origin of selectivity. Exactly the opposite would happen were the intermediate to have primarily cyclohexadienyl cation character. CBS-QB3 calculations of model 1- and 3-methoxy cyclohexadienyl cations (19* and 20*, respectively, Figure 4) show that the 3-methoxy cation (20*) is 3.1 kcal/mol more stable than the 1-methoxy cation (19*). This can be explained by the stabilization of the positive charge of 21* at C3 by the methoxy group and a larger LUMO coefficient at this position. On the contrary, the 1-methoxy cyclohexadienyl radical (22*) is 3.6 kcal/mol more stable than the 3-methoxy radical (23*). Both the spin density and SOMO coefficient of 24* are larger at C3 than at C1. Thus the explanation for the relative stabilities of cyclohexadienyl radicals requires more than a simple spin density or molecular orbital analysis.10
Figure 4.
Model ortho- and para-methoxy cyclohexadienyl cations and radicals. CBS-QB3 ΔH. B3LYP/6-31G(d) values in parentheses.
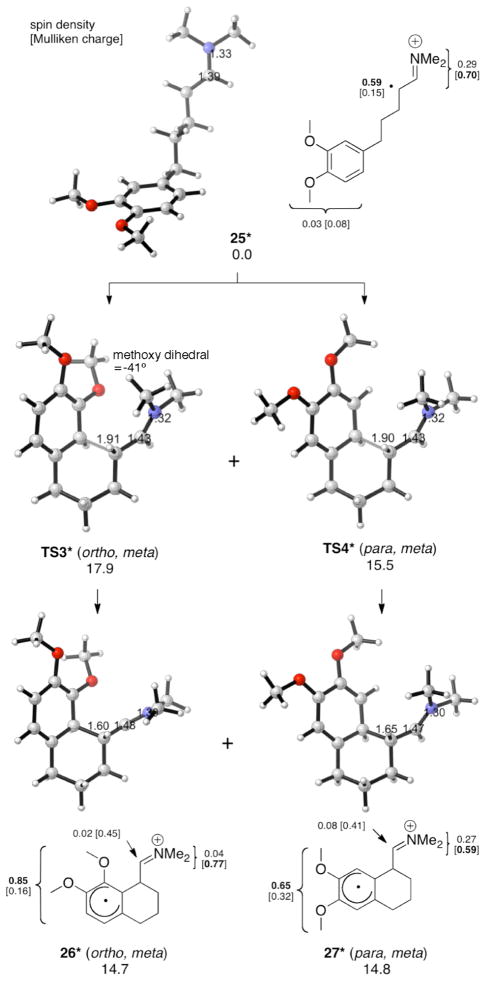
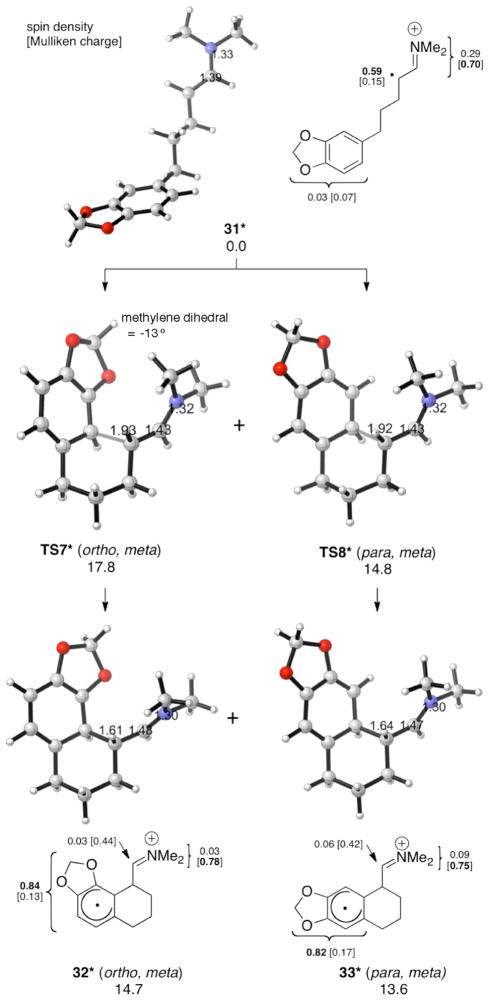
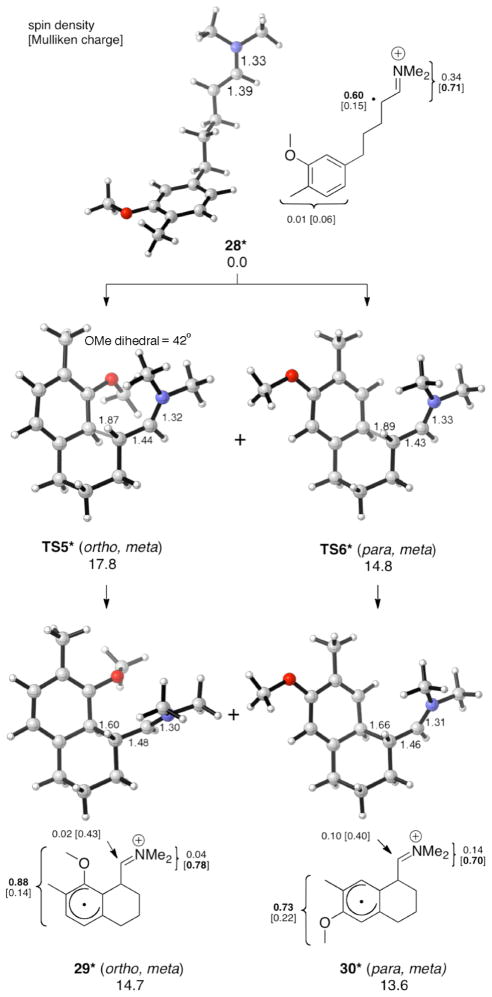
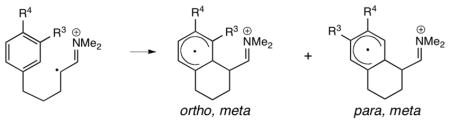
We next investigated the cyclization of aldehydes 8–10 via the model dimethyl enamine radical cations (Figures 5–7). Our calculations show that para, meta cyclization is favored over ortho, meta cyclization by 2.5–3.4 kcal/mol, in agreement with experiment. The intermediates, 26*–27*, 29*–30*, and 32*–33*, have the same predominantly cyclohexadienyl radical character as described for 17* and 18*. The para, meta cyclization barriers TS4*, TS6* and TS8* are similar to that of TS1* (approximately 15–16 kcal/mol), while the ortho, meta cyclization barriers TS3*, TS5* and TS7* are larger than TS1* (approximately 18 kcal/mol). These results suggest that the meta substituent (R4) causes the ortho, meta transition states to be destabilized. Given that the activation energies of TS3*, TS5* and TS7* are similar to that of the unsubstituted system, it is possible that the ortho, meta cyclizations do not occur under the reaction conditions (−30 °C). M06-2X has been found to be reliable for weak dispersion interactions and has had success with organic reactions involving radicals,11 so we applied this method by applying single point calculations to the B3LYP optimized geometries. The results are summarized with the B3LYP energies in Table 1.
Figure 5.
Intramolecular α-arylation of 25* (UB3LYP/6-31G(d) ΔG values for aqueous solution at 268 K; optimizations in CPCM water).
Figure 7.
Intramolecular α-arylation of 31* (UB3LYP/6-31G(d) ΔG values for aqueous solution at 268 K; optimizations in CPCM water).
Table 1.
Intramolecular α-arylation of aryl rings
 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| UB3LYP/6-31G(d)a | M06-2X/6-31+G(d)a,b | ||||||||
| entry | uncyclized radical cation | ΔG‡ortho, (meta) | ΔG‡para, (meta) | ΔGrxnortho, (meta) | ΔGrxnpara, (meta) | ΔG‡ortho, (meta) | ΔG‡para, (meta) | ΔGrxnortho, (meta) | ΔGrxnpara, (meta) |
| 1 | R3 = OMe, R4 = H, 16* | 15.2 TS1* |
15.7 TS2* |
12.2 17* |
15.1 18* |
12.1 TS1* |
13.6 TS2* |
6.1 17* |
10.5 18* |
| 2 | R3 = R4 = OMe, 25* | 17.9 TS3* |
15.5 TS4* |
14.7 26* |
14.8 27* |
14.7 TS3* |
12.4 TS4* |
9.0 26* |
8.9 27* |
| 3 | R3 = OMe, R4 = Me, 28* | 18.8 TS5* |
15.4 TS6* |
15.8 29* |
14.9 30* |
14.6 TS5* |
13.3 TS6* |
9.9 29* |
10.2 30* |
| 4 | R3, R4 = –OCH2O–, 31* | 17.8 TS7* |
14.8 TS8* |
14.8 32* |
13.6 33* |
14.7 TS7* |
12.4 TS8* |
9.2 32* |
8.2 33* |
| 5 | R3 = R4 = H, 34* | 18.3, TS9* | 16.0, 35* | 16.0, TS9* | 10.7, 36* | ||||
UB3LYP/6-31G(d) thermal corrections at 268 K have been applied.
Reported values represent single point calculations of the UB3LYP/6-31G(d) optimized geometries.
Compared to the B3LYP activation free energies, the M06-2X barriers are approximately 2–3 kcal/mol lower. The M06-2X reaction free energies are less endergonic than the B3LYP values by 4–7 kcal/mol. The calculated M06-2X selectivities are in good agreement with experimental observations and B3LYP predictions. For cyclization of monomethoxy radical cation 25*, the difference between TS1* and TS2* is 1.5 kcal/mol at the M06-2X level, compared to 0.5 kcal/mol using B3LYP. The ortho cyclized radical cation 17* is 4.4 kcal/mol more stable than the para isomer 18* using M06-2X. Of the 1,3,4-trisubstituted systems, 25* and 31* show a 2.3 kcal/mol selectivity for para, meta cyclization at the M06-2X level (TS4* versus TS3* and TS8* versus TS7*), while 28* shows a smaller selectivity of 1.3 kcal/mol (TS6* versus TS5*). M06-2X calculations show little difference in the relative stabilities of para, meta cyclized radical cations 27* and 30* with respect to the ortho, meta isomers (26* and 29*, respectively). The para, meta radical cation 33* is more stable than the ortho, meta isomer 32* by 1.0 kcal/mol. Given the lack of stability difference between the ortho, meta and para, meta cyclized radical cations, and given the consistently high ortho, meta arylation activation barriers which are within 1.4 kcal/mol of the barrier for the unsubstituted aldehyde (which is completely unreactive), we conclude that the selectivity of 1,3,4-trisubstituted aldehydes 8–10 is controlled by the activation free energies.
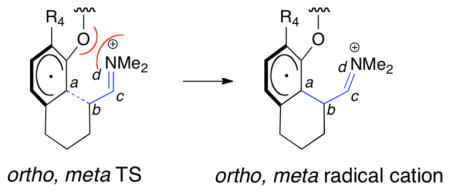
The destabilization of ortho, meta transition states TS3*, TS5*, and TS7* compared to para, meta isomers TS4*, TS6*, and TS8* can be explained by the conformation of the meta substituent R3. It has been established that the methoxy groups of anisole prefer a planar conformation.12 The same conformational preference holds true for ortho (22*) and para (23*) methoxy cyclohexadienyl radicals. In ortho, meta transition states TS3* and TS5*, the ortho methoxy group cannot be planar due to steric hindrance from the meta R4 group, raising the energies compared to the para, meta transition states. The meta oxygen of ortho, meta transition state TS7* is also nonplanar because the parent 1,3-benzodioxole prefers a non-planar conformation in which the methylene moiety is puckered with respect to the 5-membered ring.13 This is due to the anomeric effect—stabilizing n → σ* interactions between the oxygen lone pairs and C–O σ* orbitals of the puckered conformation. Electrostatic repulsion may exist between the oxygen lone pairs and the iminium moiety of the catalyst. This repulsion is relieved in cyclized radical cations 26*, 29* and 32* as the iminium group bends away from the cyclohexadienyl radical. (Compare dihedral angles a-b-c-d, Table 2).
Table 2.
Comparison of dihedral angles (a-b-c-d) in ortho, meta TSs versus ortho, meta cyclized radical cations.
 | |||
|---|---|---|---|
| entry | R3, R4 | TS dihedral angle (°) | Cyclized radical cation dihedral angle (°) |
| 1 | R3 = R4 = OMe | 95, TS3* | 128, 26* |
| 2 | R3 = OMe, R4 = Me | 95, TS5* | 119, 29 * |
| 3 | R3, R4 = –OCH2O– | 98, TS7* | 131, 32* |
In conclusion, the selectivity of the α-arylation reactions is attributed to the activation energies and relative stabilities of the isomeric transition states. In the case of the monomethoxy aryl system 1*, both ortho and para arylation barriers are possible under the reaction conditions. These reactions are endergonic, and the transition state energies are related to the relative stabilities of the resulting cyclized radical cation intermediates. For the more highly substituted 8*–10*, R3 is distorted from planarity, which raises the energy of the ortho, meta transition states with respect to the para, meta isomers. The ortho, meta activation energies are similar to those of unreactive aldehydes. Thus, ortho, meta cyclization does not occur under the reaction conditions, resulting in the experimentally observed para, meta selectivity. A detailed study of the origins of the relative stabilities of cyclohexadienyl radicals is underway and will be reported in due course.
Supplementary Material
Figure 6.
Intramolecular α-arylation of 28* (UB3LYP/6-31G(d) ΔG values for aqueous solution at 268 K; optimizations in CPCM water).
Acknowledgments
We are grateful to the National Institute of General Medical Sciences, National Institutes of Health (GM 36700, K.N.H. and R01 GM 078201-01-01, D.W.C.M.); Ronald S. Gabriel, M.D./Scrubs Unlimited SRF (O.G.); Novartis/ACS (J.M.U.); and Alexander von Humboldt foundation for a Feodor Lynen Fellowship (F.S.) for support of this work. Computations were performed on the NSF TeraGrid resources provided by NCSA (CHE0400414) and the UCLA Academic Technology Services (ATS) Hoffman2 and IDRE clusters.
Footnotes
Supporting information available: Cartesian coordinates and energies of all reported structures and model systems; full authorship of reference 5. This material is available free of charge via the Internet at http://pubs.acs.org.
Contributor Information
K. N. Houk, Email: houk@chem.ucla.edu.
David W. C. MacMillan, Email: dmacmill@princeton.edu.
References
- 1.Beeson TD, Mastracchio A, Hong JB, Ashton K, MacMillan DWC. Science. 2007;316:582.Jang HY, Hong JB, MacMillan DWC. J Am Chem Soc. 2007;129:7004. doi: 10.1021/ja0719428.Kim H, MacMillan DWC. J Am Chem Soc. 2008;130:398. doi: 10.1021/ja077212h.Graham TH, Jones CM, Jui NT, MacMillan DWC. J Am Chem Soc. 2008;130:16494. doi: 10.1021/ja8075633.Wilson JE, Casarez AD, MacMillan DWC. J Am Chem Soc. 2009;131:11332. doi: 10.1021/ja904504j.Nicolaou KC, Reingruber R, Sarlah D, Bräse S. J Am Chem Soc. 2009;131:2086. doi: 10.1021/ja809405c.Correction: J Am Chem Soc. 2009;131:6640.
- 2.Conrad JC, Kong J, Laforteza BN, MacMillan DWC. J Am Chem Soc. 2009;131:11640. doi: 10.1021/ja9026902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The authors of ref. 1f used (2R,5R)-2 as the catalyst. Because our calculations and experiments were performed with the opposite enantiomer, this paper reports the study of catalyst (2S,5S)-2.
- 4.For ortho-selective radical additions to aromatics, see: Tiecco M, Testaferri L. In: Reactive Intermediates. Abramovitch RA, editor. Vol. 3. New York: Plenum Press; 1983. p. 61.Guadarrama-Morales O, Mendéz F, Miranda LD. Tetrahedron Lett. 2007;48:4515.Muchowski JM, Cho IS, Jaime-Figueroa S, Artis RD. J Org Chem. 1994;59:2456.For a study on the stability of cyclohexadienyl radicals, see: Birch AJ, Hinde AL, Radom L. J Am Chem Soc. 1980;102:4074.
- 5.With the exceptions of 14*, 15-E*, 15-Z* and 19*–24*, which were optimized in the gas phase, all geometries were optimized in water (CPCM model) using density functional theory, UB3LYP/6-31G(d), as implemented in the Gaussian03 suite of programs (Frisch, M. J.; et al. Gaussian 03, revision D.01; Gaussian, Inc.: Pittsburgh, PA, 2004). Model cations and radicals 19*–24* were optimized using both CBS-QB3 (Gaussian 03) and UB3LYP/6-31G(d). All stationary points were verified by vibrational frequency analysis. Single point calculations were also performed using M06-2X/6-31+G(d) as implemented in Gaussian09. All computed structures are designated with an asterisk (*). The resulting energies are reported in all figures and schemes in kcal/mol.
- 6.(a) Olah GA, Prakash GKS, Rasul G. Chem Eur J. 2009;15:8443. doi: 10.1002/chem.200900335. [DOI] [PubMed] [Google Scholar]; (b) No KT, Grant JA, Jhon MS, Scheraga HA. J Phys Chem. 1990;94:4740. [Google Scholar]; (c) Würthwein E-U, Sen KD, Pople JA, Schleyer Pv-R. Inorg Chem. 1983;22:496. [Google Scholar]
- 7.Unpublished results.
- 8.See the Supporting Information.
- 9.For an example of water-assisted reactivity of radical cations, see: Heinemeann C, Demuth M. J Am Chem Soc. 1999;121:4894.
- 10.This study is underway and will be reported in due course.
- 11.For examples, see: Zhao Y, Truhlar DG. J Phys Chem A. 2008;112:1095. doi: 10.1021/jp7109127.Hohenstein EG, Chill ST, Sherrill CD. J Chem Theory Comput. 2008;4:1996. doi: 10.1021/ct800308k.Valdes H, Pluhácková K, Pitonák M, Rezác J, Hobza P. Phys Chem Chem Phys. 2008;10:2747. doi: 10.1039/b719294k.
- 12.The preferred planar methoxy conformation holds true for anisole. For example, see: Emsley JW, Foord EK, Lindon JC. J Chem Soc, Perkin Trans. 1998;2:1211.
- 13.(a) Sakurai S, Meinander N, Morris K, Laane J. J Am Chem Soc. 1999;121:5056. [Google Scholar]; (b) Moon S, Kwon Y, Lee J, Choo J. J Phys Chem A. 2001;105:3221. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.