Abstract
Objectives
The soluble epoxide hydrolase (sEH) metabolizes epoxyeicosatrienoic acids (EETs) to their less active dihydroxy derivatives. Because EETs have anti-inflammatory properties, we determined whether or not inhibition of sEH attenuates disease development in the monocrotaline model of pulmonary hypertension in rats.
Methods
sEH inhibition was achieved using 12-(3-adamantan-1-yl-ureido)-dodecanoic acid (25 mg/l) and cis-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid (5 mg/l) administered via drinking water starting 3 days prior to monocrotaline injection (60 mg/kg).
Results
Monocrotaline induced the development of progressive pulmonary hypertension. sEH inhibition increased the plasma ratio of EETs to DHETs and attenuated the monocrotaline-induced increase in pulmonary artery medial wall thickness as well as the degree of vascular muscularization. Moreover, right ventricular pressure was significantly lower in the group treated with sEH inhibitors. Pulmonary sEH protein expression and sEH activity, as well as pulmonary cytochrome P450 epoxygenase activity were all impaired in monocrotaline-treated rats as compared with control animals. sEH inhibitors, however, increased the plasma ratio of EETs to dihydroxy epoxyeicosatrienoic acids. Monocrotaline induced the proliferation of pulmonary endothelial and vascular smooth muscle cells in vivo as determined by 5-Bromo-2′-deoxy-Uridine incorporation, and this effect was significantly blunted in animals treated with sEH inhibitors. Proliferation of cultured pulmonary smooth muscle cell, however, was not affected by EETs or sEH inhibitors suggesting that the in-vivo effects are a consequence of a direct EET-mediated protection against the inflammation induced by monocrotaline.
Conclusion
sEH inhibition reduces pulmonary vascular remodeling and the development of pulmonary hypertension in the monocrotaline model of primary pulmonary hypertension in rats.
Keywords: epoxyeicosatrienoic acids, lipid mediators, monocrotaline
Introduction
Pulmonary hypertension is the common denominator of several diseases of different causes characterized by increased pulmonary artery pressure. Apart from forms of pulmonary hypertension that are secondary to left ventricular failure or chronic thromboembolic disease, the present condition is the consequence of an increase in the resistance of the pulmonary vascular tree. Pulmonary vasoconstriction, vascular obliteration by proliferating cells and vascular remodeling have all been shown to contribute to the development of pulmonary hypertension, and a considerable interaction between these processes exists [1].
Current strategies for the treatment of the disease are aimed at lowering pulmonary vascular resistance and attenuating structural vascular remodeling and cellular proliferation [2]. The role of eicosanoids, metabolites of arachidonic acid, in pulmonary hypertension has been the focus of a large number of pharmacological as well as pathophysiological studies, and while leukotrienes and thromboxane A2 can promote the development of pulmonary hypertension in certain scenarios [3], prostacyclin analogues have become a central part of the therapeutic arsenal in the treatment of the disease [2]. There are, however, additional pulmonary eicosanoids that can potentially affect pulmonary function.
The epoxygenation of arachidonic acid by cytochrome P450 monoxygenases (CYPs) yields epoxyeicosatrienoic acids (EETs). These lipid mediators have recently been implicated in the Euler–Liljestrand mechanism, and the exposure to hypoxia significantly increases the pulmonary expression of CYP epoxygenases [4]. In the systemic circulation, the EETs have been attributed vasoprotective effects as they are vasodilators [5], stimulate angiogenesis [6] and exert anti-inflammatory effects [7]. As a consequence, it is difficult to predict whether EETs are likely to promote or attenuate the progression of non-hypoxic idiopathic pulmonary hypertension.
Directly studying the physiological consequences of authentic EETs is problematic as these eicosanoids are usually generated within cells and the effects elicited by the compounds may differ depending on whether they are endogenously generated or exogenously applied [8]. An attractive alternative to the application of EET is, therefore, the inhibition of their metabolism. EETs are metabolized by cyclooxygenases (COX) and the β-oxidation pathway, but the most important pathway is the conversion by the soluble epoxide hydrolase (sEH) [9]. Pharmacological inhibition of sEH has been shown to increase plasma EET concentrations [9] and most importantly a large number of studies have reported positive, tissue protective effects of the sEH inhibitors, which lower the blood pressure in different models of systemic hypertension [10], prevent cardiac remodeling after aortic banding [11] and suppress the inflammatory response elicited by bacterial lipopolysaccharide [12]. It is, therefore, attractive to speculate that sEH inhibition might be beneficial in a model of pulmonary hypertension in which remodeling and inflammation predominate. Such a model is monocrotaline (MCT)-induced pulmonary hypertension in rats [13]. In these animals, MCT is metabolized in the liver to a pyrrole derivative, which is highly toxic to endothelial cells in the lung, which then undergo apoptosis [13]. The pulmonary hypertension in this model is, therefore, secondary to endothelial cell death and is associated with cellular proliferation, vascular inflammation and remodeling.
In the present study, we therefore determined whether inhibition of the sEH can affect the development of MCT-induced pulmonary hypertension in rats. We used two structurally different inhibitors to ascertain that differences observed are not a consequence of potential side effects of the individual compound.
Methods
Animals and study protocol
Male Wistar–Kyoto rats (~200 g body weight) were obtained from Charles River Laboratories (Sulzfeld, Germany). The experiments were performed in accordance with the National Institutes of Health Guidelines on the Use of Laboratory Animals. Both the University Animal Care Committee and the Federal Authorities for Animal Research of the Regierungspräsidium Darmstadt (Hessen, Germany) approved the study protocol.
Rats were randomized to receive with their drinking water either the solvent or one of the following two sEH inhibitors synthesized in the laboratory of one of the coauthors: cis-4-[4-(3-adamantan-1-yl-ureido)-cyclohexy-loxy]-benzoic acid (cAUCB, final concentration 5 mg/l) [14] or 12-(3-adamantan-1-yl-ureido)-dodecanoic acid (AUDA, final concentration 25 mg/l) [15].
Preparation of the solutions was as follows: AUDA (25 mg) was dissolved in ethanol (1 ml) and added to 2-hydroxypropyl-β-cyclodextrin solution (50 ml, 5%). AUDA was encapsulated by ultrasound treatment for 10 min, and the solution was then added to normal water (950 ml), yielding a final concentration of 25 mg/l. cAUCB (5 mg) was dissolved in ethanol (2 ml) and added to water (998 ml) to reach a final concentration of 5 mg/ml. Three days after the initiation of sEH inhibitor treatment, rats were exposed to MCT. MCT (Sigma, Deishofen, Germany) was dissolved in HCl (0.1 mol/l, 20 ml), adjusted to pH 7.4 with NaOH solution (0.1 mol/l) and administered as a single subcutaneous injection (60 mg/kg body weight) as described previously [16]. Control animals received an equal volume of isotonic saline.
Hemodynamics
Hemodynamic parameters were determined 2–4 weeks after MCT injection (n = 6–8 for each group) in general anesthesia achieved by inhalation of a standardized iso-flurane concentration in pure oxygen. After a skin incision, the right jugular vein was prepared and a catheter (Millar, 1.4F; ADInstruments, Spechbach, Germany) was inserted into the vessel and advanced to the right ventricle. Right ventricular mean and systolic pressure were recorded using a data acquisition system (Powerlab; ADInstruments).
Histological techniques
After the induction of terminal anesthesia using isoflurane, the abdominal vessels were cut open, the thoracic cavity was opened, a canula was inserted into the right ventricle and the animals were perfused with phosphate-buffered saline (PBS). The left lungs were dissected and shock frozen in liquid nitrogen. Afterwards, the right lungs were perfused and fixed with 4% paraformaldehyde/PBS solution, paraffin-embedded and sectioned in slices (~4 μm). As an index for right ventricular hypertrophy, the ratio of the right ventricle free wall weight to left ventricle and septum weight (RV/LV+S) was calculated.
Evaluation of in-situ cell proliferation
Animals received an intraperitoneal injection of 5-Bromo-2′-deoxy-Uridine [BrdU/Roche applied science (Mannheim, Germany)/100 mg/kg body weight] dissolved in PBS 24 h before sacrifice and BrdU was visualized using a commercially available kit (Roche) in deparaffined pulmonary sections with the modification that the antigens were recovered by heating in citrate buffer (pH6.1). BrdU was detected using an anti-BrdU primary antibody (Roche applied science) and visualized by an Alexa 546 coupled (1: 300; Invitrogen, Karlsruhe, Germany) secondary antibody. α-Smooth muscle actin was detected by a directly labeled fluorescein isothiocyanate (FITC)-coupled antibody (1: 200; Sigma) and nuclei were counterstained using ToPro 3 iodide (1: 1000). Images of each right lung lobe (image size 900 × 900 μm) were acquired by laser scanning microscopy (LSM 510meta; Carl Zeiss, Microimaging, Jena, Germany), and BrdU positive endothelial and vascular smooth muscle cells were counted for each animal by observers blinded to the study protocol.
Morphometrical analysis of pulmonary arteries
Hematoxylin/eosin and elastica staining was performed according to common histopathological procedures. Briefly, at 400× magnification 100 small pulmonary vessels of each animal (n = 5 for each group) ranging from 10–50 μm in external diameter were counted and noted as muscular, partially muscular or nonmuscular. To assess the degree of muscularization, the amount of α-smooth muscle actin-positive vessel wall area was determined. Nonmuscular arterioles were detected by the endothelial anti-von Willebrand staining. Arteries that contained more than 70% α-actin positive vessel wall area were set as muscular; arteries with less than 4% of α-actin positive vessel area were set as nonmuscular. Arteries that contained α-actin positive vessel area between 4 and 70% were defined as partially muscularized. Percentage of medial wall thickness (% MWT) was calculated by (2 × media thickness/external diameter) × 100. Based on the external diameter of the pulmonary arteries, they were categorized as follows: category I included arteries with an external diameter between 20 and 50 μm, category II included arteries with an external diameter between 51 and 90 μm, category III included arteries with an external diameter between 91 and 150 μm and category IV included pulmonary arteries with an external diameter greater than 151 μm. Within category I and II, 60–80 arteries were measured per animal. Morphometrical analysis of pulmonary vessels was carried out using a computerized morphometric analyzing system (Leica Q Win Standard Analyzing Software and Leica DMLA Microscope; Leica Microsystems Wetzlar GmbH, Wetzlar, Germany). Slides were analyzed by light microscopy by one observer in a blinded manner. Extension of smooth muscle cells into normally nonmuscular arterioles of the alveolar wall and alveolar duct was assessed as previously described [16].
Determination of epoxyeicosatrienoic acid and dihydroxy epoxyeicosatrienoic acids by liquid chromatography/mass spectrometry–mass spectrometry
Blood samples were collected at different time points. After coagulation and centrifugation, the serum was stored at −80°C. At a later time point, samples were spiked with deuterated internal standards (5-HETE-d8, 12-HETE-d8, 15-HETE-d8, 20-HETE-d6, 8,9-EET-d8, 11,12-EET-d8 and 14,15-EET-d8) and extracted twice with ethyl acetate (0.5 ml). The sEH assay was extracted similarly but here one-tenth of organic phase was used and spiked. After evaporation of the ethyl acetate in a vacuum block under a gentle stream of nitrogen, the residues were reconstituted with 50 μl of methanol/water (1: 1, v/v) and analyzed with a Sciex API4000 mass spectrometer (AME Bioscience, Toroed, Norway) operating in multiple reaction monitoring (MRM) mode as described in detail elsewhere [6]. Chromatographic separation was performed on a Gemini C18 column (150 × 2 mm inner diameter, 5 μm particle size, Phenomenex, Aschaffenburg, Germany).
Cytochrome P450 monoxygenase P450 epoxygenase and soluble epoxide hydrolase activity assay
Frozen lung tissue was mortared in liquid nitrogen and homogenized in 5 vol. of ice-cold Tris/HCl buffer (pH 7.4, 50 mmol/l) containing sucrose (250 mmol/l), potassium chloride (150 mmol/l), ethylenediaminetetraacetic acid (EDTA; 2 mmol/l), dithiothreitol (2 mmol/l; DTT), flavin adenine dinucleotide (FAD) and flavin mononucleotide (FMN; 0.5 μmol/l), and phenylmethylsulfanylfluoride (PMSF; 0.25 mmol/l) using an Ultra turrax (IKA Staufen, Germany) and a glass Potter homogenizer. Subsequently, the samples were subjected to differential centrifugation (10 min, 1000 g; 20 min, 10 000 g; 90 min, 100 000 g); the 100 000 g pellet representing the microsomal fraction was used for the cytochrome P450 expoxygenase assay, whereas the 100 000 g supernatant (cytosolic fraction) was used for the sEH activity assay. For storage, the 100 000 g pellet was resuspended and homogenized in Tris/HCl buffer (50 mmol/l, pH 7.7) containing glycerol (20% v/v), EDTA (5 mmol/l), DTT (1 mmol/l) and FAD and FMN (0.5 μmol/l). The protein content was determined by the standard Bradford method.
For the cytochrome P450 epoxygenase activity assay, which was performed as described previously [17], 20 μg of microsomes was incubated at 37° C for 10 min in reaction buffer containing potassium phosphate buffer (100 mmol/l, pH 7.2) and arachidonic acid (20 μmol/l). The reaction was started by the addition of nicotinamide adenine dinucleotide phosphate (NADPH; 1 mmol/l final concentration) and terminated after 20 min by rapid cool down on ice and immediate extraction. All CYP-dependently formed EETs and dihydroxy epoxyeicosatrienoic acids (DHETs) as determined by liquid chromatography/mass spectrometry–mass spectrometry (LC/MS-MS; see above) were added up for each animal and EET formation was expressed in mmol/mg per min.
The sEH activity was assayed using a previously described method [18] with minor modifications. Reactions were performed at 37° C for 20 min in a total volume of 100 μl of potassium phosphate buffer (100 mmol/l, K2HPO4/KH2PO4, pH 7.2), containing 5 μg of protein. Reactions (37° C) were started by the addition of 14,15-EET (10 μmol/l) and were stopped by putting the reaction on ice and adding ethyl acetate. Experiments were performed in the absence and presence of the solvent [1% dimethyl sulfoxide (DMSO)] and sEH inhibitor 1-adamantyl-3-cyclohexylurea (ACU; 10 μmol/l).
Immunoblotting
For western blot analysis, cytosolic lung protein (100 000 g supernatant, 20 μg) was boiled in Laemmli buffer, separated on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE; 8%) and transferred onto nitrocellulose membrane as described [10]. Proteins were detected using antibodies against sEH (provided by one of the coauthors) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH/Santa Cruz Biotechnology, Santa Cruz, California, USA). Proteins were detected using appropriate secondary antibodies labeled with infrared dyes and visualized using the Odyssey infrared imaging system (Li-COR Biosciences, Bad Homburg, Germany) system. Densitometry was carried out using the integrated odyssey software.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide pulmonary arterial smooth muscle cells proliferation assay
Rat pulmonary artery smooth muscle cells (PASMCs) were seeded in multiwell plates after isolating them from rat pulmonary arteries. After 1 day of serum starving [only 0.1% bovine serum albumin (BSA) in standard cell culture medium], cell culture medium was replaced by a 0.5% fetal calf serum (FCS) containing medium. Afterwards, 11,12-methyl-ester-EET (1 μmol/l; Cayman Chemicals Company, Ann Arbor, Michigan, USA), ACU (1 μmol/l) and solvent (DMSO) were added separately or together (combined) to the cell culture medium (0.5% FCS) of subconfluently grown PASMC and stimulated by platelet-derived growth factor-BB (PDGF-BB; 30 ng/ml). After 2 days of incubation period (37°C, 5% CO2), multiwell plates were washed and (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma) was added. After a further incubation period (2 h, 37°C, 5% CO2), cell cultural medium was supplemented with acidified isopropanol for dissolving precipitated formazan. Absorbance was analyzed by a spectrophotometer (550 nm wavelength; Wallac Victor; EG&G Wallac, Freiburg, Germany).
Data and statistical analysis
All values are mean ±SEM. Statistical analysis was performed using analysis of variance (ANOVA) for repeated measurements; ANOVA followed by Bonferroni-corrected Fisher’s LSD test, respectively, or, wherever appropriate, using an unpaired t-test. A P value less than 0.05 was considered statistically significant.
Results
Soluble epoxide hydrolase inhibition attenuates the development of pulmonary hypertension
The effect of sEH inhibition on the development of pulmonary hypertension was determined 2–4 weeks after the injection of MCT. The treatment group received the sEH inhibitor AUDA, which was administered with drinking water throughout the whole experiment starting 3 days prior to MCT injection, whereas the control groups were treated with the vehicle.
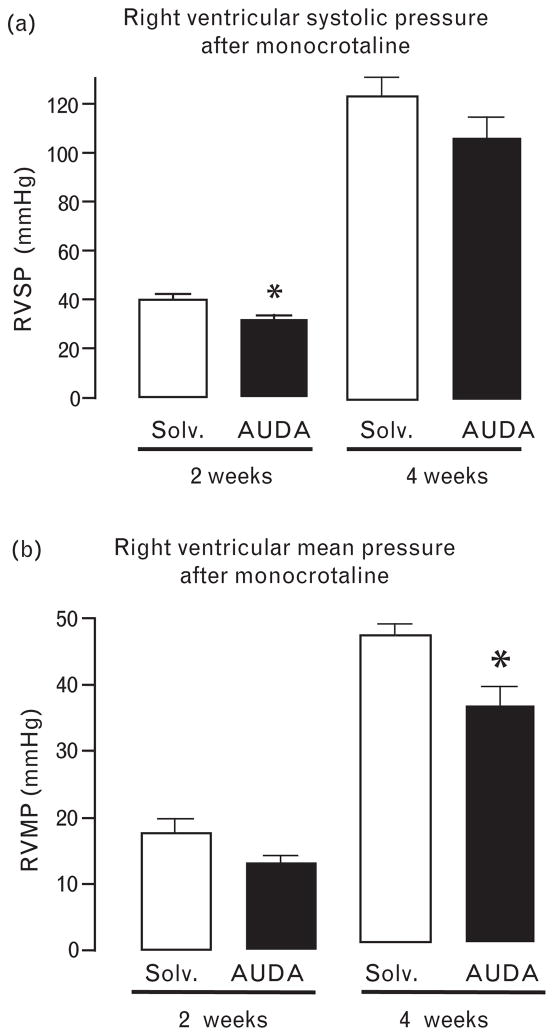
Two weeks after MCT injection, right ventricular systolic pressure was significantly lower in the sEH inhibitor-treated than in the vehicle-treated animals (Fig. 1a). However, 4 weeks after MCT administration, severe pulmonary hypertension was evident and comparable in the AUDA-treated and the vehicle-treated groups. Nevertheless, right ventricular mean pressure was still significantly lower, and there was still a trend (P <0.1) toward a reduced systolic pressure in the animals treated with AUDA (Fig. 1b). Nevertheless, right ventricular hypertrophy determined as the ratio of right to left ventricular weight was not affected by sEH inhibition (no MCT: 0.27 ± 0.15, MCT +vehicle: 0.4 ± 0.04, MCT + sEH-inhibition 0.4 ± 0.03, n = 5). These observations suggest that sEH inhibition is most effective in the early phase after pulmonary vascular injury. Indeed, MCT-induced mortality, which occurs about 6 weeks after injection, was not affected by sEH inhibition (data not shown). As mortality was also not affected by cAUCB, additional experiments with pressure measurements were not carried out in this group.
Fig. 1.
Impact of the soluble epoxide hydrolase (sEH) inhibitor 12-(3-adamantan-1-yl-ureido)-dodecanoic acid (AUDA) or solvent (Solv) on right ventricular systolic (a, RVSP) and right ventricular mean (b, RVMP) pressure 2–4 weeks after monocrotaline (MCT) injection. n = 5 and 6, *P < 0.05 AUDA vs. solvent.
Soluble epoxide hydrolase inhibition prevents pulmonary vascular remodeling
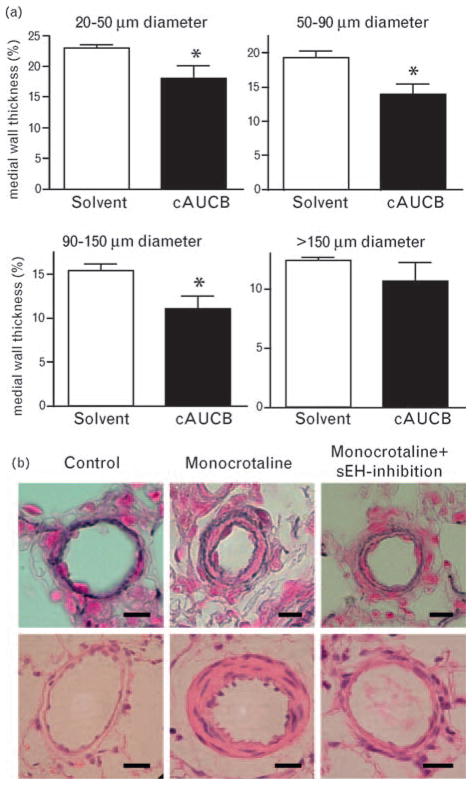
Pulmonary vascular remodeling was determined 21 days after MCT injection. At that time point, MWT and the degree of muscularization were significantly increased in rats receiving MCT (Fig. 2). sEH inhibition (in this case using a second inhibitor; cAUCB) was effective in blocking pulmonary vascular remodeling as recorded after 21 days; MWT in all three of the vessel sizes (i.e. 20–50, 50–90 and 90–150 μm diameter arteries) responsible for pulmonary resistance was significantly lower in animals receiving MCT together with the sEH inhibitor cAUCB, compared with MCT-treated rats that received only solvent (Fig. 2). Accordingly, the number of fully muscularized pulmonary arteries detected after 21 days was significantly lower in the group treated with cAUCB than with solvent (P = 0.03; MCT + solvent: 21 ± 1.52%, MCT + cAUCB 11.75 ± 3.27%, P < 0.03, n = 6).
Fig. 2.
Effect of soluble epoxide hydrolase (sEH) inhibition on the medial wall thickness of pulmonary arteries. Effect of sEH inhibitor cis-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid (cAUCB) treatment or solvent treatment on medial wall thickness of pulmonary arteries sized 20–50; 51–90, 91–150 and greater than 150 μm 21 days after monocrotaline (MCT) injection. n = 6, *P < 0.05 cAUCB vs. solvent (a) and representative photomicrographs of pulmonary arterial vessels of Elastica van Gieson (upper panel) and hematoxyline eosine (lower panel) stained sections (b). Upper panel: bar = 10 μm; lower panel: bar = 20 μm.
Soluble epoxide hydrolase inhibition increases the availability of epoxyeicosatrienoic acids
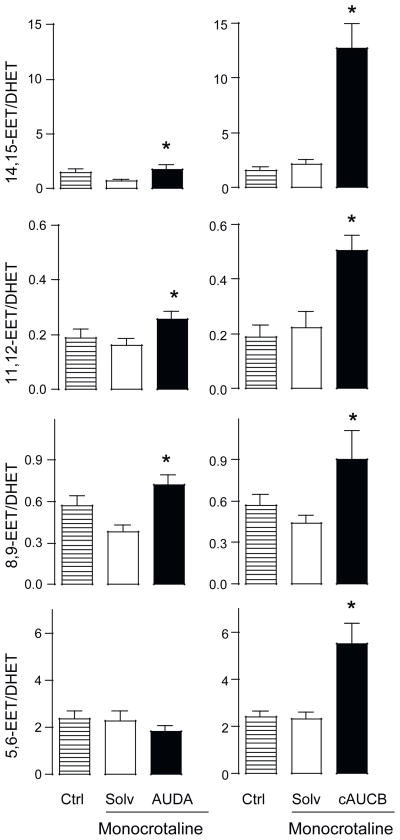
To determine the effectiveness of sEH inhibition, the ratio of EETs to their corresponding dihydroxy derivatives (DHETs) was determined. No difference in the EET: DHET ratio was recorded in both control rats (not receiving any treatment) and rats treated with MCT (Fig. 3). Inhibition of sEH increased the concentration of EETs and the EET: DHET ratio, the latter effect was more pronounced in animals treated with cAUCB than those treated with AUDA, reflecting the higher potency of cAUCB. As expected, the increase in the EET: DHET ratio was most pronounced for 14,15-EET and 11,12-EET, which are predominantly metabolized by sEH. Interestingly, cAUCB but not AUDA also increased the ratio of 5,6-EET: DHET.
Fig. 3.
Effect of monocrotaline and soluble epoxide hydrolase (sEH) inhibition on the epoxyeicosatrienioc acid (EET) to dihydroxyeicosatrienoic acid (DHET) plasma level ratio. Cumulative EET/DHET plasma concentration ratio in control rats (Ctrl) and rats after monocrotaline-injection receiving solvent (Solv) or the sEH inhibitor 12-(3-adamantan-1-yl-ureido)-dodecanoic acid (AUDA) or cis-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid (cAUCB). *P < 0.05 monocrotaline + Solvent (Solv) vs. monocrotaline +sEH-inhibition (AUDA or cAUCB), n = 6.
Monocrotaline reduces the pulmonary epoxyeicosatrienoic acid formation and soluble epoxide hydrolase expression
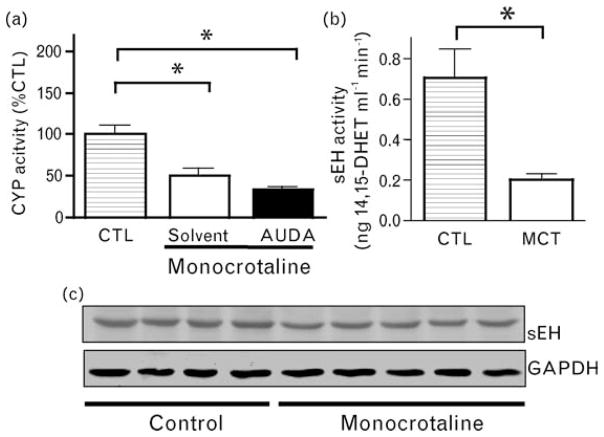
Because EET: DHET plasma levels reflect the balance between their production by tissues in the whole organism, including (but not exclusively) the lungs and their renal clearance, we measured EET formation and sEH expression directly in pulmonary tissue. Two weeks after MCT injection, pulmonary CYP activity was reduced by approximately 50%, an effect that was not sensitive to sEH inhibition (Fig. 4a). Interestingly, there was a pronounced decrease in pulmonary sEH activity (Fig. 4b), and this was associated with a significant (−32 ± 4%, P < 0.05) decrease in sEH protein levels (Fig. 4c).
Fig. 4.
Effect of monocrotaline on pulmonary cytochrome P450 epoxygenase activity and soluble epoxide hydrolase (sEH) expression and activity. (a) Pulmonary cytochrome P450 epoxygenase activity as determined by the formation of 14,15-epoxyeicosatrienoic acid (EET) from arachidonate in pulmonary tissue harvested 2 weeks after the injection of solvent (Ctrl) or monocrotaline and subject solvent treatment (solvent) or 12-(3-adamantan-1-yl-ureido)-dodecanoic acid (AUDA) treatment (AUDA). *P < 0.05 Control vs. AUDA + monocrotaline and solvent + monocrotaline. n = 5. (b) Pulmonary sEH activity as determined by the conversion of 14,15-EET to dihydroxyeicosatrienoic acid (DHET) in pulmonary tissue obtained 2 weeks after the injection of monocrotaline (MCT) or solvent (Ctrl). *P < 0.05 MCT vs. control, n = 5. (c) Pulmonary sEH expression (upper lane) and 3-phosphate dehydrogenase (GAPDH as cytosolic housekeeping gene, lower lane) as determined by western blot analysis 3 weeks after the injection of solvent (control) or monocrotaline.
Inhibition of soluble epoxide hydrolase attenuates the proliferation of pulmonary vascular cells in response to monocrotaline
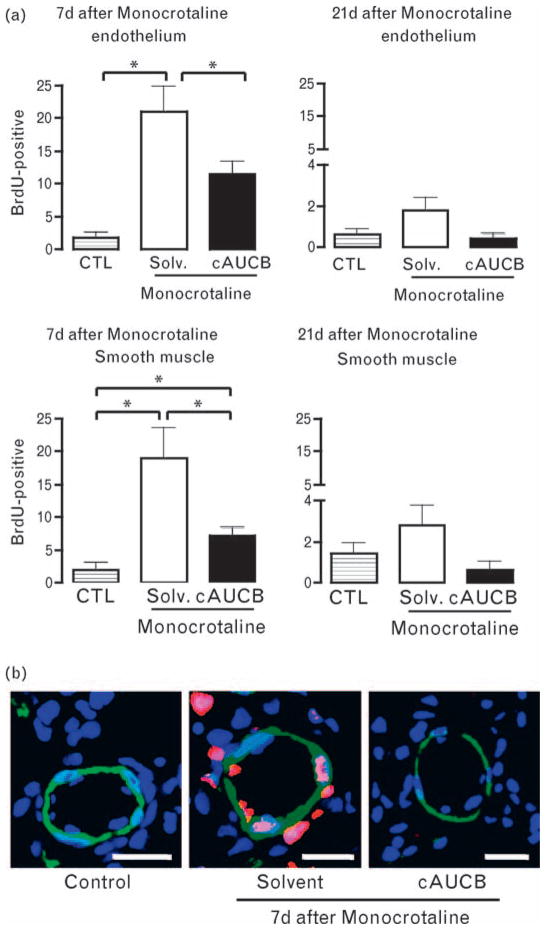
MCT induced a significant increase in the proliferation of pulmonary vascular smooth muscle and endothelial cells as determined by the BrdU incorporation assay. This effect was most prominent 7 days after the administration of the compound (Fig. 5). Inhibition of sEH significantly attenuated the proliferation of both cell types but failed to normalize the response completely. Twenty-one days after injection, MCT-induced proliferation was reduced to almost control level and no significant differences were observed between the groups. In order to identify a possible direct effect of EETs on cell proliferation, we studied the effect of 11,12-EETs and sEH inhibition on proliferation in response to PDGF, serotonin and serum in cultured rat pulmonary artery SMC. However, in this assay, EETs and sEH inhibition had no effect on stimulation (1 μmol/l 11,12-EETs: 93 ± 2% of control group, 1 μmol/l ACU 96 ± 1% of control group, 1 μmol/l 11,12-EET ± 1 μmol/l ACU: 95 ± 2% of control group).
Fig. 5.
Effect of soluble epoxide hydrolase (sEH) inhibition of the monocrotaline-induced proliferation of pulmonary vascular cells. Rats received a single injection of 5-Bromo-2′-deoxy-Uridine (BrdU) 7 and 21 days after the injection of monocrotaline or solvent. The subsequent day, animals were sacrificed and BrdU-positive proliferating vascular cells were identified by immunohistochemistry and confocal microscopy. (a) Statistical analysis and (b) exemplary images of BrdU-positive endothelial cells (intraluminal) and smooth muscle cells (colocalization of BrdU and α-actin) in control animals (Ctrl), animals receiving monocrotaline and solvent (Solv) or monocrotaline and the sEH-inhibitor cis-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid (cAUCB). n = 6 animals, *P < 0.05. Immunofluorescence for topro-III-iodine as nuclear stain (blue encoded), α-smooth muscle cell-actin (green) and BrdU (red). Bar = 20 μm.
Discussion
In this study, we demonstrate that inhibition of the sEH attenuates pulmonary vascular remodeling induced by the application of MCT to rats. sEH inhibitors reduced pulmonary artery smooth muscle proliferation, MWT, attenuated the degree of wall muscularization and right ventricular pressure in an animal model of primary pulmonary hypertension. In cell culture, however, sEH inhibition had no direct effect on pulmonary artery smooth muscle proliferation, suggesting that the protective effects of the sEH inhibitors studied are most likely related to indirect actions.
EETs exert a plethora of beneficial effects, including the protection of endothelial cells against apoptosis [19,20] as well as the inhibition of inflammation [7]. Despite the wealth of data demonstrating physiological actions of EETs in the systemic vasculature, their impact on lung physiology and pathophysiology is uncertain. This is particularly relevant as CYP-derived EETs are reported to augment hypoxic pulmonary vasoconstriction and aggravate hypoxia-induced pulmonary remodeling [4]. MCT is frequently used to induce primary pulmonary hypertension in rats. The reaction to its administration is characterized by an initial inflammatory mononuclear infiltration, pulmonary artery endothelial cell (PAEC) damage and subsequent endothelial cell apoptosis [21,22]. The resolution of MCT-induced damage involves proliferation of PAEC and PASMCs and subsequent vascular remodeling, which result in pulmonary hypertension [23], and finally, in right ventricular failure.
In the present study, we observed that sEH inhibition was effective in reducing the smooth muscle cell proliferation after MCT treatment in vivo. The proliferation of cultured cells in response to agonists such as PDGF, serum or serotonin, however, was affected neither by EETs nor by sEH inhibition. This finding is, however, not necessarily unexpected because controversy also exists regarding the impact of EETs on smooth muscle proliferation in the systemic circulation [24,25]. It could, therefore, be speculated that either EETs interfere with a specific, so far undefined, proliferative stimulus in vivo or the antiproliferative effects of EETs in vivo are indirect in nature. Indeed, EETs prevent endothelial cell apoptosis through pathways involving the phosphoinositide 3-kinase as well as the Akt and ERK kinases [19,20]. Moreover, the anti-inflammatory actions of the EETs in endothelial cells have been attributed to the inhibition of the IκB-kinase [7]. MCT induces an initial inflammatory response accompanied by mononuclear infiltration and NFκB activation [21]. Indeed, the inhibition of NFκB reduces both pulmonary arterial pressure and right ventricle remodeling after MCT administration [26]. Thus, it is tempting to speculate that the initial inflammatory response to MCT is attenuated by sEH inhibition.
In addition to attenuating smooth muscle cell proliferation, sEH inhibition also attenuated the proliferation of endothelial cells in response to MCT. Endothelial cell proliferation in the model studied is generally agreed to be a reaction to the endothelial cell apoptosis [22], which supports our hypothesis that the inhibition of the sEH predominately affects the early stages of disease development. This is supported by our observation that the protective effects of the sEH inhibitors studied became less obvious the longer the remodeling process had time to take place. Given that a large portion in the development of pulmonary hypertension is the self-preserving modeling process, such a model may also help understand why sEH inhibition did not affect right ventricular remodeling at a late stage in the disease and mortality.
In the context of a potential deleterious effect of sEH inhibition in hypoxic-pulmonary vasoconstriction, the present observations indicate that the in-vivo application of sEH inhibitors is safe. The molecular mechanisms underlying the increase in pulmonary resistance differ considerably between hypoxic and primary pulmonary hypertension. In the former situation, a direct vasoconstrictor mechanism predominates [2], which is slowly consolidated by structural remodeling and more importantly, EETs have been shown to induce vasoconstriction in isolated pulmonary artery [27]. The MCT model, in contrast, is characterized by vascular obstruction and cellular proliferation. Moreover, different sets of transcription factors are activated by the two situations and chronic hypoxia-induced smooth muscle cell proliferation appears to involve the hypoxia inducible factor-1α pathway [26].
An unexpected finding was that cAUCB, but not AUDA also increased the ratio of 5,6-EET/DHET, though it is assumed that 5,6-EET is not a sEH but rather a COX substrate [28,29]. Given that cAUCB does not inhibit COX, a direct inhibitory effect of high EET level on COX activity is conceivable. Indeed, evidence for such a cross talk has been presented for different models [12,30]. sEH does not exclusively metabolize EETs. Thus, other substrates may also be of relevance in the model studied. Particularly, the aliphatic epoxides leukotoxin (LTX) and isoleukotoxin (ILTX) should be considered. The toxicity of both compounds increases dramatically after metabolism by sEH, which may suggest that sEH inhibition actually reduces the formation of these toxic products [31]. Indeed, the diol derivatives of LTX and ILTX have been implicated in the acute respiratory distress syndrome (ARDS) [32]. Although the pathomechanisms of ARDS and MCT-induced pulmonary hypertension differ in key aspects, inflammation plays a crucial role in both. The attenuated production of the LTX and ILTX diols by sEH inhibition might also, at least partially, contribute to the protective effects of sEH inhibition in the MCT model.
sEH inhibition is also reported to reduce left ventricular remodeling in the pressure overload model of aortic banding in mice [11]. Although we expected to see a reduction in cardiac remodeling in the present study, the cardiac hypertrophy observed following MCT treatment was completely unaffected by sEH inhibition. At this point, it is only possible to speculate about the underlying differences; it is worth commenting that pressure overload in the left ventricle is usually associated with a marked activation of the renin–angiotensin system and sEH inhibitors attenuate many of the effects of angiotensin II [10].
One important finding was that the activity and expression of the enzymes studied, that is, CYP epoxygenases and the sEH declined during the course of MCT-induced pulmonary hypertension. The plasma concentrations of EET, however, remained unaffected by the developing disease. These observations suggest that the lung is not the predominant site of EET production and conversion. Considering that EETs may act locally at the sites of production in an autocrine or paracrine manner or even intracellularly, it is possible that the lack of effect of the sEH inhibitors studied on disease progression is a consequence of the downregulation of the above-mentioned enzymes. As little is known regarding the factors that control the expression of CYP enzymes and sEH in the lung, the mechanisms underlying this observation remain to be elucidated. Moreover, due to technical limitations (antibodies), we are unable to identify the impact of pulmonary hypertension on the expression of specific CYP enzymes in the rat lung. It is, however, possible to rule out that the effects observed reflect only changes in the matrix composition as the levels of cytosolic housekeeping proteins, such as GAPDH, were unaffected by the developing disease.
To conclude, in the present study, we identified sEH inhibition as a possible strategy to delay but not prevent pulmonary vasculature remodeling in a model of primary pulmonary hypertension. Our observations suggest that the CYP epoxygenase–sEH system is of particular importance in early stages of MCT-induced pulmonary hypertension. It will be interesting to determine whether or not the present observations can be extended to other models of primary pulmonary hypertension or even to the similar situation in humans.
Acknowledgments
We thank Katalin Wandzioch and Sina Bätz for excellent technical assistance.
The present study was supported by the Deutsche For-schungsgemeinschaft (BR1839/4-1 & FG501, FI830/2-3) and by Exzellenzcluster 147 ‘Cardio-Pulmonary Systems’, by the Medical Faculty of the Goethe-University, by the LOEWE Lipid Signaling Forschungszentrum Frankfurt (LiFF) and the NIEHS (grant R37 ER02710).
Abbreviations
- AUDA
12-(3-adamantan-1-yl-ureido)-dodecanoic acid
- cAUCB
cis-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid
- COX
cyclooxygenase
- CYP
cytochrome P450 monoxygenase
- DHET
dihydroxy epoxyeicosatrienoic acid
- EET
epoxyeicosatrienoic acid
- MCT
monocrotaline
- PASMC
pulmonary arterial smooth muscle cells
- sEH
soluble epoxide hydrolase
Footnotes
There are no conflicts of interest.
References
- 1.Simonneau G, Galie N, Rubin LJ, Langleben D, Seeger W, Domenighetti G, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2004;43:5S–12S. doi: 10.1016/j.jacc.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 2.Ghofrani HA, Voswinckel R, Reichenberger F, Weissmann N, Schermuly RT, Seeger W, Grimminger F. Hypoxia- and nonhypoxia-related pulmonary hypertension – established and new therapies. Cardiovasc Res. 2006;72:30–40. doi: 10.1016/j.cardiores.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 3.Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med. 2004;351:1655–1665. doi: 10.1056/NEJMra035488. [DOI] [PubMed] [Google Scholar]
- 4.Pokreisz P, Fleming I, Kiss L, Barbosa-Sicard E, Fisslthaler B, Falck JR, et al. Cytochrome P450 epoxygenase gene function in hypoxic pulmonary vasoconstriction and pulmonary vascular remodeling. Hypertension. 2006;47:762–770. doi: 10.1161/01.HYP.0000208299.62535.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, Busse R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature. 1999;401:493–497. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- 6.Michaelis UR, Fisslthaler B, Barbosa-Sicard E, Falck JR, Fleming I, Busse R. Cytochrome P450 epoxygenases 2C8 and 2C9 are implicated in hypoxia-induced endothelial cell migration and angiogenesis. J Cell Sci. 2005;118:5489–5498. doi: 10.1242/jcs.02674. [DOI] [PubMed] [Google Scholar]
- 7.Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, et al. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleming I. Cytochrome p450 and vascular homeostasis. Circ Res. 2001;89:753–762. doi: 10.1161/hh2101.099268. [DOI] [PubMed] [Google Scholar]
- 9.Inceoglu B, Schmelzer KR, Morisseau C, Jinks SL, Hammock BD. Soluble epoxide hydrolase inhibition reveals novel biological functions of epoxyeicosatrienoic acids (EETs) Prostaglandins Other Lipid Mediat. 2007;82:42–49. doi: 10.1016/j.prostaglandins.2006.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jung O, Brandes RP, Kim IH, Schweda F, Schmidt R, Hammock BD, et al. Soluble epoxide hydrolase is a main effector of angiotensin II-induced hypertension. Hypertension. 2005;45:759–765. doi: 10.1161/01.HYP.0000153792.29478.1d. [DOI] [PubMed] [Google Scholar]
- 11.Xu D, Li N, He Y, Timofeyev V, Lu L, Tsai HJ, et al. Prevention and reversal of cardiac hypertrophy by soluble epoxide hydrolase inhibitors. Proc Natl Acad Sci U S A. 2006;103:18733–18738. doi: 10.1073/pnas.0609158103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmelzer KR, Inceoglu B, Kubala L, Kim IH, Jinks SL, Eiserich JP, Hammock BD. Enhancement of antinociception by coadministration of nonsteroidal anti-inflammatory drugs and soluble epoxide hydrolase inhibitors. Proc Natl Acad Sci U S A. 2006;103:13646–13651. doi: 10.1073/pnas.0605908103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schultze AE, Roth RA. Chronic pulmonary hypertension – the monocrotaline model and involvement of the hemostatic system. J Toxicol Environ Health B Crit Rev. 1998;1:271–346. doi: 10.1080/10937409809524557. [DOI] [PubMed] [Google Scholar]
- 14.Hwang SH, Tsai HJ, Liu JY, Morisseau C, Hammock BD. Orally bioavailable potent soluble epoxide hydrolase inhibitors. J Med Chem. 2007;50:3825–3840. doi: 10.1021/jm070270t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morisseau C, Goodrow MH, Newman JW, Wheelock CE, Dowdy DL, Hammock BD. Structural refinement of inhibitors of urea-based soluble epoxide hydrolases. Biochem Pharmacol. 2002;63:1599–1608. doi: 10.1016/s0006-2952(02)00952-8. [DOI] [PubMed] [Google Scholar]
- 16.Schermuly RT, Kreisselmeier KP, Ghofrani HA, Samidurai A, Pullamsetti S, Weissmann N, et al. Antiremodeling effects of iloprost and the dual-selective phosphodiesterase 3/4 inhibitor tolafentrine in chronic experimental pulmonary hypertension. Circ Res. 2004;94:1101–1108. doi: 10.1161/01.RES.0000126050.41296.8E. [DOI] [PubMed] [Google Scholar]
- 17.Barbosa-Sicard E, Markovic M, Honeck H, Christ B, Muller DN, Schunck WH. Eicosapentaenoic acid metabolism by cytochrome P450 enzymes of the CYP2C subfamily. Biochem Biophys Res Commun. 2005;329:1275–1281. doi: 10.1016/j.bbrc.2005.02.103. [DOI] [PubMed] [Google Scholar]
- 18.Muller DN, Schmidt C, Barbosa-Sicard E, Wellner M, Gross V, Hercule H, et al. Mouse Cyp4a isoforms: enzymatic properties, gender- and strain-specific expression, and role in renal 20-hydroxyeicosatetraenoic acid formation. Biochem J. 2007;403:109–118. doi: 10.1042/BJ20061328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhanasekaran A, Al-Saghir R, Lopez B, Zhu D, Gutterman DD, Jacobs ER, Medhora M. Protective effects of epoxyeicosatrienoic acids on human endothelial cells from the pulmonary and coronary vasculature. Am J Physiol Heart Circ Physiol. 2006;291:H517–H531. doi: 10.1152/ajpheart.00953.2005. [DOI] [PubMed] [Google Scholar]
- 20.Yang S, Lin L, Chen JX, Lee CR, Seubert JM, Wang Y, et al. Cytochrome P-450 epoxygenases protect endothelial cells from apoptosis induced by tumor necrosis factor-alpha via MAPK and PI3K/Akt signaling pathways. Am J Physiol Heart Circ Physiol. 2007;293:H142–H151. doi: 10.1152/ajpheart.00783.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dorfmuller P, Perros F, Balabanian K, Humbert M. Inflammation in pulmonary arterial hypertension. Eur Respir J. 2003;22:358–363. doi: 10.1183/09031936.03.00038903. [DOI] [PubMed] [Google Scholar]
- 22.Thomas HC, Lame MW, Dunston SK, Segall HJ, Wilson DW. Monocrotaline pyrrole induces apoptosis in pulmonary artery endothelial cells. Toxicol Appl Pharmacol. 1998;151:236–244. doi: 10.1006/taap.1998.8458. [DOI] [PubMed] [Google Scholar]
- 23.McMurtry MS, Moudgil R, Hashimoto K, Bonnet S, Michelakis ED, Archer SL. Overexpression of human bone morphogenetic protein receptor 2 does not ameliorate monocrotaline pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;292:L872–L878. doi: 10.1152/ajplung.00309.2006. [DOI] [PubMed] [Google Scholar]
- 24.Fang X, Moore SA, Stoll LL, Rich G, Kaduce TL, Weintraub NL, Spector AA. 14,15-Epoxyeicosatrienoic acid inhibits prostaglandin E2 production in vascular smooth muscle cells. Am J Physiol. 1998;275:H2113–H2121. doi: 10.1152/ajpheart.1998.275.6.H2113. [DOI] [PubMed] [Google Scholar]
- 25.Sun J, Sui X, Bradbury JA, Zeldin DC, Conte MS, Liao JK. Inhibition of vascular smooth muscle cell migration by cytochrome p450 epoxygenase-derived eicosanoids. Circ Res. 2002;90:1020–1027. doi: 10.1161/01.res.0000017727.35930.33. [DOI] [PubMed] [Google Scholar]
- 26.Sawada H, Mitani Y, Maruyama J, Jiang BH, Ikeyama Y, Dida FA, et al. A nuclear factor-kappaB inhibitor pyrrolidine dithiocarbamate ameliorates pulmonary hypertension in rats. Chest. 2007;132:1265–1274. doi: 10.1378/chest.06-2243. [DOI] [PubMed] [Google Scholar]
- 27.Zhu D, Bousamra M, Zeldin DC, Falck JR, Townsley M, Harder DR, et al. Epoxyeicosatrienoic acids constrict isolated pressurized rabbit pulmonary arteries. Am J Physiol Lung Cell Mol Physiol. 2000;278:L335–L343. doi: 10.1152/ajplung.2000.278.2.L335. [DOI] [PubMed] [Google Scholar]
- 28.Carroll MA, Balazy M, Margiotta P, Falck JR, McGiff JC. Renal vasodilator activity of 5,6-epoxyeicosatrienoic acid depends upon conversion by cyclooxygenase and release of prostaglandins. J Biol Chem. 1993;268:12260–12266. [PubMed] [Google Scholar]
- 29.Zeldin DC. Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem. 2001;276:36059–36062. doi: 10.1074/jbc.R100030200. [DOI] [PubMed] [Google Scholar]
- 30.Schmelzer KR, Kubala L, Newman JW, Kim IH, Eiserich JP, Hammock BD. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc Natl Acad Sci U S A. 2005;102:9772–9777. doi: 10.1073/pnas.0503279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishizaki T, Shigemori K, Nakai T, Miyabo S, Ozawa T, Chang SW, Voelkel NF. Leukotoxin, 9,10-epoxy-12-octadecenoate causes edematous lung injury via activation of vascular nitric oxide synthase. Am J Physiol. 1995;269:L65–L70. doi: 10.1152/ajplung.1995.269.1.L65. [DOI] [PubMed] [Google Scholar]
- 32.Moghaddam MF, Grant DF, Cheek JM, Greene JF, Williamson KC, Hammock BD. Bioactivation of leukotoxins to their toxic diols by epoxide hydrolase. Nat Med. 1997;3:562–566. doi: 10.1038/nm0597-562. [DOI] [PMC free article] [PubMed] [Google Scholar]