Abstract
In honeybee colonies, food collection is performed by a group of mostly sterile females called workers. After an initial nest phase, workers begin foraging for nectar and pollen, but tend to bias their collection towards one or the other. The foraging choice of honeybees is influenced by vitellogenin (vg), an egg-yolk precursor protein that is expressed although workers typically do not lay eggs. The forager reproductive ground plan hypothesis (RGPH) proposes an evolutionary path in which the behavioural bias toward collecting nectar or pollen on foraging trips is influenced by variation in reproductive physiology, such as hormone levels and vg gene expression. Recently, the connections between vg and foraging behaviour were challenged by Oldroyd and Beekman (2008), who concluded from their study that the ovary, and especially vg, played no role in foraging behaviour of bees. We address their challenge directly by manipulating vg expression by RNA interference- (RNAi) mediated gene knockdown in two honeybee genotypes with different foraging behaviour and reproductive physiology. We show that the effect of vg on the food-loading decisions of the workers occurs only in the genotype where timing of foraging onset (by age) is also sensitive to vg levels. In the second genotype, changing vg levels do not affect foraging onset or bias. The effect of vg on workers' age at foraging onset is explained by the well-supported double repressor hypothesis (DHR), which describes a mutually inhibitory relationship between vg and juvenile hormone (JH) — an endocrine factor that influences development, reproduction, and behaviour in many insects. These results support the RGPH and demonstrate how it intersects with an established mechanism of honeybee behavioural control.
Keywords: Apis mellifera, juvenile hormone, reproductive ground plan hypothesis, RNA interference, social foraging, vitellogenin yolk protein
Recent advances in functional genomics have enabled new insights and the direct testing of hypotheses about the molecular regulation of behaviour. The eusocial honeybee has emerged as a model organism for the study of molecular mechanisms that can influence complex social behaviour (Grozinger and Robinson 2007; Marco Antonio et al 2008). Worker bees are functionally sterile females that progress through a series of age-associated tasks, moving from in-nest tasks such as brood care and ending with outside-the-nest foraging (Seeley 1982).
Foraging division of labour is one aspect of the social behaviour of worker honeybees. As foragers, workers collect water, pollen, and nectar that are resources for the colony as a whole. Individual bees bias their collection toward either the carbohydrate (nectar) or protein source (pollen). The bias for carbohydrate or protein collection correlates with variation in worker behaviour and physiology. In commercial honeybee stocks, pollen collection bias is associated with early progression from nest tasks to foraging (foraging onset), increased gustatory responsiveness, high peak titres of the yolk precursor protein vitellogenin (vg), and increased ovariole number (a measure of ovary size), while nectar bias correlates with the opposite traits (Pankiw & Page, 2000; Amdam et al. 2006a, Tsuruda et al. 2008). These many trait associations suggest some level of causality, because when the vg titre is experimentally reduced in commercial honeybees, workers shift their food-loading away from pollen, resulting in significantly heavier nectar loads (Nelson et al. 2007).
The association between worker behavioural variation and reproductive physiology was addressed in the ovarian ground plan hypothesis (OGPH) of West-Eberhard (1987, 1996). The OGPH was proposed to account for reproductive division of labor between queens and workers, and later ‘age polyethism’, a correlation between behaviour and chronological age that is seen in the worker caste of many social insects in which young females remain on the nest while older workers leave the nest on foraging trips (West-Eberhard 1987, West-Eberhard 1996). Here, this age-associated behavioural progression is influenced by ovary development and juvenile hormone (JH), traits uncoupled from their ancestral link to reproduction in workers (West-Eberhard 1996). The reproductive ground plan hypothesis (RGPH), in contrast, focuses on behavioural variation within the forager group. It proposes that the observed relationship between foraging behaviour and female physiology, including vg, emerges from an ancestral network of genes and endocrine factors that links central aspects of female food-handling behaviour to reproductive physiology (Amdam et al. 2004; Amdam et al. 2006a). An association between reproductive physiology and foraging behaviour has also been observed in the Eastern honeybee (Apis cerana: Rueppell et al. 2008) and a harvester ant (Pogonomyrmex californicus: Dolezal et al. 2009). Additionally, a similar linkage of foraging behaviour to a reproductive regulatory network may be seen in a relative of the honeybee, the primitively social sweat bee Lasioglossum zephyrum, where protein foraging is required for reproduction. Ingested protein affects secretion of the systemic JH. (Bell 1973). JH and vg protein then act together to facilitate oocyte development (Bell 1973).
JH has broad effects on insect behaviour and physiology and is often a positive regulator of vg gene expression (Bell 1973; Flatt et al. 2005). In many insects, JH acts as a gonadotropin and can influence ovarian development both by stimulating vg production in the fat body and vg uptake by the ovary (Bell & Barth 1971; Nijhout & Riddiford 1979; Wyatt & Davey 1996). In honeybees, however, this relationship is reversed. When vg expression is high JH synthesis is low, and if the vg protein level is suppressed in workers an increase in JH titre can be elicited (Guidugli et al. 2005). Reciprocally, application of JH mimics or analogs can reduce vg synthesis (Pinto et al. 2000). However, the occurrence of a small peak of JH prior to vg synthesis in worker bees is suggestive of a potential role for JH in the induction of vg production (Jassim et al. 2000)
The inverse relationship between vg and JH titres is consistent with nest bees (nurses) having high vg titres and low JH levels (Engels & Fahrenhorst 1974) as well as with vg titres dropping and JH levels rising prior to foraging (Fluri et al. 1982; Robinson 1987; Huang et al. 1991; Jassim et al. 2000). The novel function hypothesis of Huang and Robinson (1992) suggests that foraging behaviour is activated by JH. They hypothesize that JH has lost its gonadotropic function on the ovary in highly eusocial insects, and instead has evolved a novel function as a behavioural pacemaker through action on the brain (Huang and Robinson, 1992). This view is in contrast to the split function hypothesis of JH suggested by West-Eberhard (1996) which argues that both the gonadotropic and behavioural pacing functions of JH can be ancestral and linked. Here the gonadotropic action of JH is dependent on a critical period in development. In workers, poor nutrition during that critical window decouples the effects of JH on the ovary from its effects on behavioural maturation (West-Eberhard 1996).
A third hypothesis, the double repressor hypothesis (DRH) also proposes a role for JH in the regulation of behavioural maturation. However, the DRH proposes vg precedes JH in regulation of behavioural development. High vg titres have an inhibitory influence on foraging behaviour and JH synthesis, and it is a drop in the vg titre that activates the behavioural shift to foraging and releases an increase in JH titre (Amdam & Omholt 2003). According to this hypothesis, JH is a systemic integrator with many effects on physiology including further suppression of vg synthesis. Together, these changes reinforce the transition to foraging behaviour and lock the bee into the forager behavioural state. The DRH is supported directly by the finding that reduction of vg expression by RNA-interference (RNAi) can release increased JH titres in European (Guidugli et al. 2005) as well as Africanized (Marco-Antonio et al. 2007) stocks of honeybees. Additionally vg suppression triggers early foraging onset in commercial stocks of both European and Africanized honey bees, demonstrating that vg is causally linked to the release of foraging behaviour in workers (Nelson et al. 2007; Marco-Antonio et al. 2008).
The RGPH and the DRH both address the role of vg in the regulation of the social foraging behaviour of honeybees. The RGPH is an evolutionary framework explaining relationships between foraging bias and female reproductive physiology that includes vg and JH levels, while the DRH is a mechanistic framework describing how foraging onset can be controlled by interplay between vg and JH. Yet, while the role of vg in foraging onset outlined in the DRH is broadly accepted (Smith et al. 2008; Hewes 2008; Woyciechowski & Morón 2009) the RGPH remains controversial (Toth & Robinson 2007; Oldroyd & Beekman 2008).
In a test of the RGPH, Oldroyd & Beekman (2008) predicted that anarchistic worker bees would collect more pollen than commercial stocks based on the assumption that the anarchistic bees were the more reproductive genotype. Anarchistic bees lay eggs in the presence of a functional queen, a phenotype that is not uncommon in honeybees (Page & Erickson 1988). Because the anarchistic bees failed to show bias towards collecting pollen, the authors rejected the RGPH and the association between vg and behaviour as an explanation for foraging division of labour. Later, Amdam et al. (2009) and Tsuruda et al. (2008) pointed out considerable differences in the methodology of Oldroyd and Beekman (2008), and suggested that anarchistic bees are selected to resist pheromonal suppression of oviposition, a trait not known to be associated with social foraging. The controversy that surrounds the RGPH should be resolved to achieve a better understanding of social behaviour.
Here, we address the challenge to the RGPH. We ask, again, whether an association between foraging bias and female reproductive physiology is present in honeybees. We refine our analysis by testing the relationship between vg expression and foraging bias in the context of the relationship between vg and foraging onset that is explained by the DRH. To test this, we downregulate vg in two selected strains of honeybee that differ in vg titres, foraging onset, and foraging bias, traits that are part of a larger suite of genetic, biochemical, and behavioural traits shown repeatedly to associate with foraging behavioural biases (Page et al. 2006). These strains were selected by Page and Fondrk (1995) for high and low quantities of stored pollen.
Workers of the high pollen-hoarding strain are characterized by early peak titres of vg, high sensitivity of the relationship between vg and JH (as measured by significant increase in JH in response to vg downregulation; Amdam et al. 2007), relatively high JH titres at emergence (Schulz et al. 2004), early foraging onset, and bias toward pollen collection as foragers (Pankiw & Page 2001). Low strain bees, on the other hand, show relatively constant vg titres, low sensitivity of the relationship between vg and JH (no increase in JH in response to vg downregulation; Amdam et al. 2007), lower titres of JH at emergence (Schulz et al. 2004), late foraging onset, and bias toward nectar collection as foragers. These phenotypes appear to broadly represent the tails of distributions of traits and trait associations of commercial stocks (for a more detailed discussion, see Amdam & Page, this issue, pp. xx-xx). In the experiments reported here, we exploit the lack of vg sensitivity in the low pollen-hoarding strain, somewhat analogous to a “null mutation”, to decouple effects of vg alone from those of the active vg/JH feedback relationship. Thereby, we are first to use RNAi-mediate gene knockdown in combination with alternative trait architectures produced by artificial selection to dissect the behaviour of a social insect.
Methods
dsRNA preparation for vg downregulation
Double stranded RNA (dsRNA) toward vg was prepared as described by the RNAi protocol of Amdam et al. (2003; 2006b). In brief, we used cDNA clone AP4a5 as template (GenBank accession #: AJ517411). Primers were fused to T7 promoter sequence (underlined):
Fw: 5′-TAATACGACTCACTATAGGGCGAACGACTCGACCAACGACTT-3′
Re: 5′-TAATACGACTCACTATAGGGCGAAACGAAAGGAACGGTCAATTCC-3′
PCR product was purified using the QIAquick PCR purification kit (Qiagen, Valencia, California, United States), and RNA was prepared with the Promega RiboMax T7 system (Promega, Madison, Wisconsin, United States). RNA was extracted by TRIzol LS reagent (GIBCO-BRL, San Diego, California, United States), resuspended in nuclease-free water, heated at 96°C for 2 min in an Eppendorf Thermomixer (Brinkmann Instruments, Westbury, New York, United States), and left to cool at room temperature for 20 min. dsRNA products were diluted with nuclease-free H2O (Qiagen) to the final concentration of 5 μg/μl (Amdam et al. 2003; Amdam et al. 2006b; Seehuus et al. 2006; Nelson et al. 2007).
Bees
Queens from two high and two low strain source colonies were caged overnight to allow collection of same-aged bees. Frames were pulled from colonies after 20 days, and worker bees emerged in an incubator at 32°C. Newly emerged workers were randomly assigned to one of three treatments: noREF, a non-handled reference group; injC, a control group injected with vehicle (nuclease-free H2O); and vgRNAi, the dsRNA-injected vg knockdown group, after experimental designs and protocols established before, e.g. (Guidugli et al. 2005; Nelson et al. 2007). Bees to be used in the age of first foraging experiment (below) were individually tagged, while bees used in the foraging preference study were marked with paint (Testors Enamel; Testor Corporation, Rockford, Illinois, United States) to indicate treatment identity. Injections were performed between the fifth and sixth tergite using Hamilton syringes with G30 disposable needles (BD, Palo Alto, California, United States). Injection volume was 2 μl.
Efficacy of this vg RNAi procedure was confirmed previously in honeybees of diverse commercial stocks as well as in the pollen-hoarding strains (Supplement; Amdam et al. 2003; Amdam et al. 2007; Nelson et al. 2007; Marco Antonio et al. 2008). Verification of vg knockdown for this study is presented in the Supplement, confirming it to be highly effective (p < 0.0001 for each strain; Fig. S1).
Age of first foraging
Injections took place over three days for each of two colonies. Treated bees (n = 200 bees per treatment group, per strain, per colony) were introduced into nucleus hives containing four frames of honey, pollen, and brood, and background populations of bees of commercial stock, as before (Nelson et al. 2007). For each colony, a glass-walled observation hive was prepared on the fourth day of the experiment (i.e., following the last day of injections). The colony entrances of the observations hives were monitored daily for two 30 min periods between 6:00 AM and 10:00 AM. Individual tag numbers of returning foragers were recorded using standard procedures (Amdam et al. 2007; Nelson et al. 2007) to determine when workers first started foraging (age of first foraging). Following Nelson et al. (2007), only those bees observed on at least 2 days were included in analysis. Colony level effects were not measured because the introduced, treated bees represented only a tiny portion of the population in each instance.
Foraging preference
Injections took place over three days for each colony. Treated bees (n = 200 bees per treatment, per genotype, per colony) were marked to indicate treatment group (noREF, injC, and vgRNAi, see above) and placed into each of two nucleus colonies with a background honeybee population of commercial stock. The experimental bees were allowed to mature inside the hive for 10-16 days. Thereafter, returning foragers were collected over a five-day period (Nelson et al. 2007). Pollen loads were removed from one corbicula for each bee and weighed. We expelled liquid from foragers' honey stomachs into pre-weighed capillary tubes and measured nectar load weight with a digital balance (Pankiw & Page 2001; Nelson et al. 2007). Sucrose concentration was measured using a digital refractometer (Misco, Cleveland, Ohio, United States). With this method we were able to distinguish nectar loads from water loads, but were unable to take multiple measurements from a forager.
Statistics
Age of first foraging was analyzed using the Kaplan-Meier survival analysis (Amdam et al. 2007). The Kaplan-Meier test examines the time when a specified event occurs: in this case the initiation of foraging. Planned pair-wise comparisons between knockdown and injected control groups as well as between injected control and non-handled reference groups were performed using the Cox-Mantel test (Nelson et al. 2007). Foraging loads were analyzed using ANOVA as the sample sets conformed to Bartlett's assumption test of homogeneity of variance. Planned, pair-wise comparisons were analyzed with a student's t-test. Correlative patterns were tested using multiple regression models. For studies of putative effects between treatment groups, both Factorial ANOVA and the nonparametric Kruskal-Wallis test were used (Amdam et al. 2007; Nelson et al. 2007). The two analyses were largely in agreement. Analyses were conducted with Statistica 6.0 (StatSoft, Inc. Tulsa, Oklahoma, United States).
Results
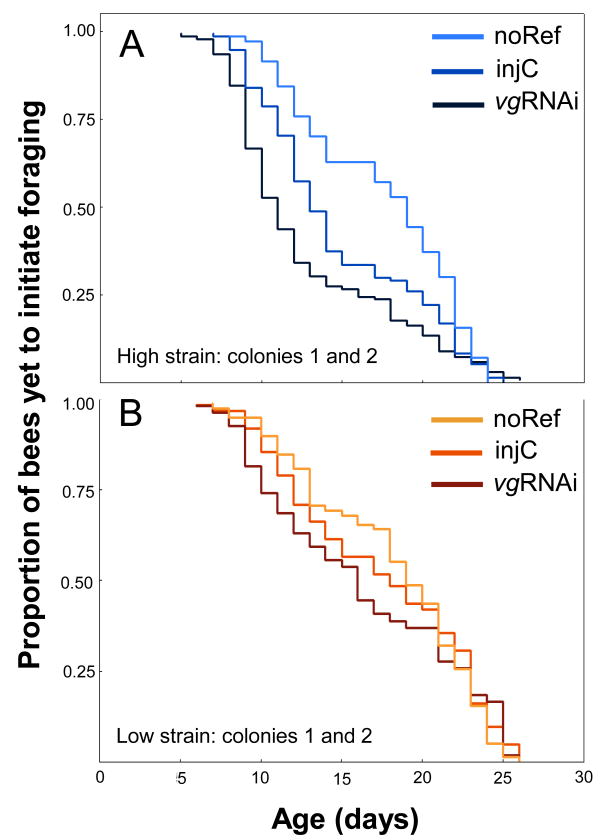
We found that high strain vg knockdowns (vgRNAi, n = 359) initiated foraging earlier in life than controls (injC, n = 355, Kaplan-Meier, p < 0.0001; Cox-Mantel, p = 0.012; Fig. 1A), while low strain workers did not (vgRNAi n = 340, injC n = 354, Kaplan-Meier, p = 0.324; Fig. 1B). This finding is consistent with the central role of feedback between vg and JH in the release of foraging behaviour outlined by the DRH. High sensitivity of the feedback relationship, as exemplified by high strain bees (Amdam et al. 2007), would fuel a speedy and reliable foraging onset in response to vg RNAi. Likewise, the reduced sensitivity of the vg-to-JH interaction that is seen in the low strain would translate into a loss-of-response phenotype to vg downregulation.
Figure 1.
Age of pollen-hoarding strain bees at foraging onset. In A) the high strain, vg knockdown (vgRNAi) caused workers to forage earlier in life than injected controls (injC). Relative to the reference (noREF), we also confirmed a handling effect (Cox-Mantel injC vs. noREF, p = 0.009) described in wild-type bees (Nelson et al. 2007). In B) the low strain, downregulation of vg gene activity did not affect foraging onset. An effect of handling could not be detected in the low strain, also in agreement with low strain bees having a reduced JH response (Amdam et al. 2007) and with JH taking part in the insect stress response (Amdam et al. 2007; Corona et al. 2007; Amdam et al. 2008),. The panels show merged data from two colonies. Results were consistent between the replicates.
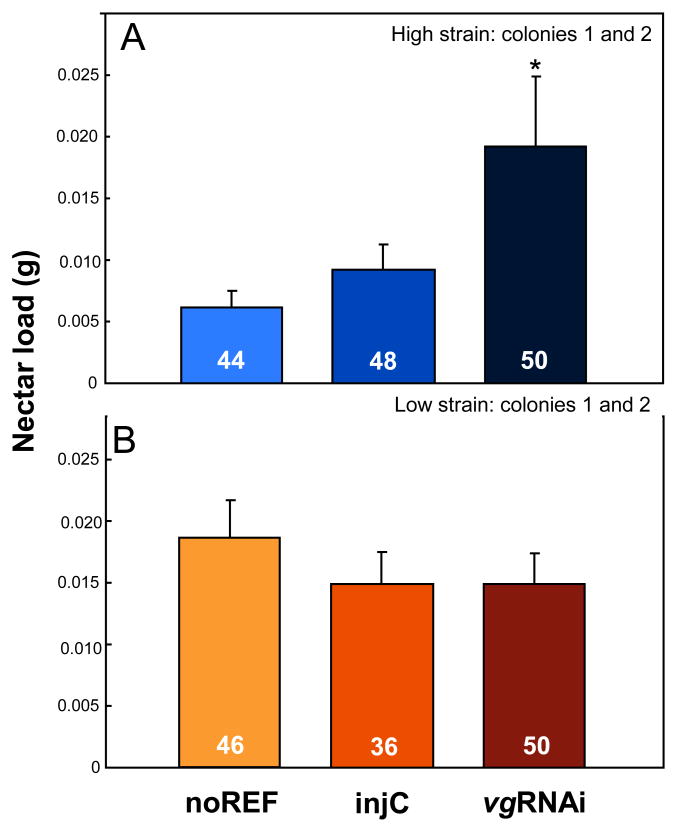
Next, we established that high strain vg knockdowns (n = 50) collected significantly larger nectar loads than controls (n = 49, ANOVA, p = 0.003; Fig. 2A). In contrast, the foraging bias of low strain bees did not change (vgRNAi n = 44, injC n = 46, ANOVA, p = 0.570; Fig. 2B). Foraging loads were within the range normally collected by honeybees (up to 60-mg nectar and 30-mg pollen; Hunt et al. 1995; Pankiw & Page 2001), and thus vg knockdown did not change the maximum load sizes of workers (Nelson et al. 2007). Our data suggest that honeybee foraging bias can change as a function of vg expression level, but only in workers where variation in vg also can influence the onset of foraging behaviour. Thereby, the effect of vg on foraging bias, as proposed by the RGPH, is conditional on an operational antagonistic relationship between vg and JH; e.g. on sufficient sensitivity of the honeybee endocrine system to changes in the vg expression level. This new insight is intuitive, similar functional integration of endocrine responses with physiology and behaviour underlies the female reproductive cycle, upon which the RGPH is built (Amdam et al. 2004).
Figure 2.
Foraging preference in pollen-hoarding strain bees. A) Suppression of vg gene activity by RNAi significantly affected the foraging bias of high strain workers that collected heavier nectar loads than controls (*planned comparison students t-test, p = 0.024). Knockdown of vg did not affect the nectar loading of low strain bees. No handling effect was observed in either strain, which is consistent with the view that JH, or laboratory-induced stress, per se does not influence foraging bias (Amdam et al. 2008).. Bars are means ± standard errors of data from two colonies. The effect of RNAi was consistent between the replicates.
Discussion
Our results demonstrate that the behavioural effect of changed vg levels in honeybees is tied to the physiological sensitivity of the response. vg knockdown high pollen-hoarding strain bees foraged earlier in life and collected more nectar than did the controls, while the low strain bees were behaviourally insensitive to vg downregulation. The results of vg knockdown on foraging onset are consistent with established strain differences in the endocrine feedback sensitivity between vg and JH. Thus, the effect (or lack thereof) of vg RNAi on foraging onset can be explained by the model provided by the DRH. Our results for foraging bias show for the first time that the effect of vg on nectar versus pollen loading is conditional on the sensitivity of a larger network, and that at least a part of this network overlaps with the endocrine apparatus described by the DRH, mechanistically linking the RGPH and DRH.
Contrary to the findings and suggestions of Oldroyd and Beekman (2008) our results along with those of Nelson et al. (2007) show a direct causal relationship between reproductive physiology and foraging bias. vg gene activity is central to the regulation of social foraging behaviour in honeybees. Genetic variation within and between honeybee populations contributes to variation in social foraging behaviour in part by affecting the network connecting vg and JH. Foraging onset and foraging bias are linked in individuals with genotypes that are sensitive to changes in vg titre, as observed by JH increase in response to vg knockdown (Guidugli et al. 2005). vg may not influence JH or behaviour in individuals with genotypes that are insensitive to vg levels. This new insight provides the foundation for an integrative understanding of the origins and current regulation of social foraging in honeybees (see Amdam and Page, this issue pp. xx-xx).
The factors regulating a honeybee's sensitivity to her vg titre are currently unknown, and future studies of the molecular regulation of social foraging behaviour must address this gap in our knowledge. Recent studies, however, suggest that vg may affect JH and worker foraging behaviour by suppressing insulin/insulin-like signalling (IIS) (Corona et al. 2007; Page & Amdam 2007, Ament et al. 2008). In higher eukaryotes, IIS is a conserved regulatory pathway of diverse processes such as growth, metabolism and reproduction that has effects on sensory perception (Stranahan et al. 2008), behaviour (Wu et al. 2005), endocrine (Bruning et al., 2000), and reproductive physiology (Flatt et al. 2005), and is required for JH synthesis in Drosophila (Tu et al. 2005). The honeybee insulin-like peptide 1 (ilp1) and insulin receptor (InR1, InR2) genes are often transcribed at high levels in foragers, that have low vg levels and high JH titres, and topical application of JH analogue can increase ilp 1 expression in workers (Corona et al. 2007; Ament et al. 2008). Furthermore, workers bias foraging behaviour toward pollen (Y. Wang, K.E. Ihle, N. Mutti and G.V. Amdam, unpublished data) when insulin receptor substrate (IRS) expression is experimentally reduced. This finding is consistent with high vg levels reducing IIS and biasing behaviour toward pollen collection. However, the mechanism by which IIS intersects with vg and JH expression is unknown so it is unclear if IIS can influence vg-to-JH feedback sensitivity, the association that links vg and foraging behaviour.
This work contributes to a growing body of literature that elucidates how genetic differences within a colony can contribute to division of labour, an adaptive trait believed to be central to the ecological success of animal societies (Robinson & Page 1989; Giray & Robinson 1994; Chapman et al. 2007). Many studies have identified genetic effects on the performance of worker tasks, such as pollen versus nectar collection (Robinson & Page 1989; Hunt et al. 1995), guarding (Breed et al. 1990), and removing corpses from the nest (Robinson & Page 1988), as well as to the rate of behavioural maturation and plasticity (Page et al. 1992; Giray et al. 1994; Chapman et al. 2007; Rüppell 2009). A current challenge is to understand how genotypic differences contribute to the observed variation in behaviour between workers. Our data suggest that some plasticity in foraging behaviour is dependent on an individual's sensitivity to an internal signal: falling titres of vg, giving new insight into how genotype can affect the physiological mechanisms that underlie division of labour.
Supplementary Material
Acknowledgments
We thank S. Pratt, T. Flatt and Ø. Halskau for suggestions, P Mehta and Y. Wang for assistance with real time RT-PCR. REP was supported by U.S. Department of Agriculture (NRI-CSREES 2003-01620) and the National Institute on Aging (NIA P01 AG22500); GVA by the Norwegian Research Council (175413, 180504, 185306), U.S. National Science Foundation (0615502), and the PEW Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amdam GV, Omholt SW. The hive bee to forager transition in honeybee colonies: the double repressor hypothesis. Journal of Theoretical Biology. 2003;223:451–464. doi: 10.1016/s0022-5193(03)00121-8. [DOI] [PubMed] [Google Scholar]
- Amdam GV, Simões ZLP, Guidugli KR, Norberg K, Omholt SW. Disruption of vitellogenin gene function in adult honeybees by intra-abdominal injection of double-stranded RNA. BMC Biotechnology. 2003;3:1–8. doi: 10.1186/1472-6750-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam GV, Norberg K, Fondrk MK, Page RE. Reproductive ground plan may mediate colony-level selection effects on individual foraging behavior in honeybees. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:11350–11355. doi: 10.1073/pnas.0403073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam GV, Csondes A, Fondrk MK, Page RE. Complex social behavior derived from maternal reproductive traits. Nature. 2006a;439:76–78. doi: 10.1038/nature04340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam GV, Norberg K, Page RE, Erber J, Scheiner R. Downregulation of vitellogenin gene activity increases the gustatory responsiveness of honeybee workers (Apis mellifera) Behavioural Brain Research. 2006b;169:201–205. doi: 10.1016/j.bbr.2006.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam GV, Nilsen KA, Norberg K, Fondrk MK, Hartfelder K. Variation in endocrine signaling underlies variation in social life history. American Naturalist. 2007;170:37–46. doi: 10.1086/518183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam GV, Ihle KE, Page RE. Regulation of worker honeybee (Apis mellifera) life histories by vitellogenin. In: Fahrbach S, editor. Hormones, Brain and Behavior. San Diego, CA: Elsevier; Pages: 2008. pp. 1003–1025. [Google Scholar]
- Amdam GV, Page RE. Oldroyd and Beekman do not test ground plan hypothesis that explains origins of social behavior. PloS Biology. 2009 comment on. [Google Scholar]; Oldroyd BP, Beekman M. Effects of selection for honeybee worker reproduction on foraging traits. PLoS Biology. 2008;6:e56. doi: 10.1371/journal.pbio.0060056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ament S, Corona AM, Pollock HS, Robinson GE. Insulin signaling is involved in the regulation of worker division of labor in honeybee colonies. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:4226–4231. doi: 10.1073/pnas.0800630105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell WJ, Barth RH. Initiation of yolk deposition by juvenile hormone. Nature-New Biology. 1971;230:220. doi: 10.1038/newbio230220a0. [DOI] [PubMed] [Google Scholar]
- Bell WJ. Factors controling vitellogenesis in a primitatively social bee Lasioglossum zephyrim (Hymenoptera: Halictidae) Insectes Sociaux. 1973;29:253–260. [Google Scholar]
- Breed MD, Robinson GE, Page RE. Divsion-of-labor during honey-bee colony defense. Behavioral Ecology and Sociobiology. 1990;27:395–401. [Google Scholar]
- Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- Chapman NC, Oldroyd BP, Hughes WOH. Differential responses of honeybee (Apis mellifera) patrilines to changes in stimuli for the generalist tasks of nursing and foraging. Behavioral Ecology and Sociobiology. 2007;61:1185–1194. [Google Scholar]
- Corona M, Velarde RA, Remolina S, Moran-Lauter A, Wang Y, Hughes KA, Robinson GE. Vitellogenin, juvenile hormone, insulin signaling, and queen honeybee longevity. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:7128–7133. doi: 10.1073/pnas.0701909104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolezal AG, Brent CS, Gadau J, Holldobler B, Amdam GV. Endocrine physiology of the division of labour in Pogonomyrmex californicus founding queens. Animal Behaviour. 2009;77:1005–1010. [Google Scholar]
- Engels W, Fahrenhorst H. Alters-und kastenspezifische Veränderungen der Haemolymph-Protein-Spektren beiApis mellificia. Wilhelm Roux Archiv. 1974;174:285–196. doi: 10.1007/BF00573233. [DOI] [PubMed] [Google Scholar]
- Flatt T, Tu MP, Tatar M. Hormonal pleiotropy and the juvenile hormone regulation of Drosophila development and life history. Bioessays. 2005;27:999–1010. doi: 10.1002/bies.20290. [DOI] [PubMed] [Google Scholar]
- Fluri P, Lüscher M, Wille H, Gerig L. Changes in weight of the pharyngeal gland and haemolymph titres of juvenile hormone, protein and vitellogenin in worker honeybees. Journal of Insect Physiology. 1982;28:61–68. [Google Scholar]
- Giray T, Robinson GE. Effects of intracolony variability in behaviora-development on plasticity of division-of-labor in honey-bee colonies. Behavioral Ecology and Sociobiology. 1994;35:13–20. [Google Scholar]
- Grozinger CM, Robinson GE. Endocrine modulation of a pheromone-responsive gene in the honey bee brain. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology. 2007;193:461–470. doi: 10.1007/s00359-006-0202-x. [DOI] [PubMed] [Google Scholar]
- Guidugli KR, Nascimento AM, Amdam GV, Barchuk AR, Omholt SW, Simões ZLP, Hartfelder K. Vitellogenin regulates hormonal dynamics in the worker caste of a eusocial insect. FEBS Letters. 2005;579:4961–4965. doi: 10.1016/j.febslet.2005.07.085. [DOI] [PubMed] [Google Scholar]
- Hewes RS. The buzz on fly neuronal remodeling. Trends in Endocrinology and Metabolism. 2008;19:317–323. doi: 10.1016/j.tem.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Huang ZY, Robinson GE, Tobe SS, Yagi KJ, Strambi C, Strambi A, Stay B. Hormonal-regulation of behavioral development in the honey-bee is based on changes in the rate of juvenile hormone biosynthesis. Journal of Insect Physiology. 1991;37:733–741. [Google Scholar]
- Huang ZY, Robinson GE. Honeybee colony integration - worker worker interactions mediate hormonally regulated plasticity in division-of-labor. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:11726–11729. doi: 10.1073/pnas.89.24.11726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt GJ, Page RE, Fondrk MK, Dullum CJ. Major quantitative trait loci affecting honey bee foraging behavior. Genetics. 1995;141:1537–1545. doi: 10.1093/genetics/141.4.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jassim O, Huang ZY, Robinson GE. Juvenile hormone profiles of worker honey bees, Apis mellifera, during normal and accelerated behavioural development. Journal of Insect Physiology. 2000;46:243–249. doi: 10.1016/s0022-1910(99)00176-6. [DOI] [PubMed] [Google Scholar]
- Marco Antonio DS, Guidugli-Lazzarini KR, Nascimento AM, Simões ZLP, Hartfelder K. RNAi-mediated silencing of vitellogenin gene function turns honeybee (Apis mellifera) workers into extremely precocious foragers. Naturwissenschaften. 2008;95:953–961. doi: 10.1007/s00114-008-0413-9. [DOI] [PubMed] [Google Scholar]
- Nelson CM, Ihle KE, Amdam GV, Fondrk MK, Page RE. The gene vitellogenin has multiple coordinating effects on social organization. PLoS Biology. 2007;5:673–677. doi: 10.1371/journal.pbio.0050062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijhout MM, Riddiford LM. Juvenile-hormone and ovarian growth in Manduca-sexta. International Journal of Invertebrate Reproduction. 1979;1:209–219. [Google Scholar]
- Oldroyd BP, Beekman M. Effects of selection for honeybee worker reproduction on foraging traits. PLoS Biology. 2008;6:e56. doi: 10.1371/journal.pbio.0060056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page RE, Erickson EH. Reproduction by worker honey bees. Behavioral Ecology and Sociobiology. 1988;23:117–126. [Google Scholar]
- Page RE, Robinson GE, Britton DS, Fondrk MK. Genotypic variability for rates of behavioral-development in worker honeybees (Apis-mellifera L) Behavioral Ecology. 1992;3:173–180. [Google Scholar]
- Page RE, Fondrk MK. The effects of colony-level selection on the social organization of honeybee (Apis mellifera L.) colonies: colony-level components of pollen hoarding. Behavioral Ecology and Sociobiology. 1995;36:135–144. [Google Scholar]
- Page RE, Scheiner R, Erber J, Amdam GV. The Development and Evolution of Division of Labor and Foraging Specialization in a Social Insect (Apis mellifera L.) Current Topics in Developmental biology. 2006;74:254. doi: 10.1016/S0070-2153(06)74008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page RE, Amdam GV. The making of a social insect: developmental architectures of social design. BioEssays. 2007;29:334–343. doi: 10.1002/bies.20549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankiw T, Page RE. Response thresholds to sucrose predict foraging division of labor in honeybees. Behavioral Ecology and Sociobiology. 2000;47:265–267. [Google Scholar]
- Pankiw T, Page RE. Genotype and colony environment affect honeybee (Apis mellifera L.) development and foraging behavior. Behavioral Ecology and Sociobiology. 2001;51:87–94. [Google Scholar]
- Pinto LZ, Bitondi MMG, Simões ZLP. Inhibition of vitellogenin synthesis in Apis mellifera workers by a juvenile hormone analogue, pyriproxyfen. Journal of Insect Physiology. 2000;46:153–160. doi: 10.1016/s0022-1910(99)00111-0. [DOI] [PubMed] [Google Scholar]
- Robinson GE. Regulation of honeybee age polyethism by juvenile hormone. Behavioral Ecology and Sociobiology. 1987;20:329–338. [Google Scholar]
- Robinson GE, Page RE. Genetic determination of guarding and undertaking in honeybee colonies. Nature. 1988;333:356–358. [Google Scholar]
- Robinson GE, Page RE. Genetic determination of nectar foraging, pollen foraging, and nest-site scouting in honey bee colonies. Behavioral Ecology Sociobiology. 1989;24:317–323. [Google Scholar]
- Rueppell O, Hunggims E, Tingek S. Association between larger ovaries and pollen foraging in queenless Apis cerana workers supports the reproductive ground-plan hypothesis of social evolution. Journal of Insect Behavior. 2008;21:317–321. [Google Scholar]
- Rueppell O. Characterization of Quantitative Trait Loci for the Age of First Foraging in Honey Bee Workers. Behavior Genetics. 2009;39:541–553. doi: 10.1007/s10519-009-9278-8. [DOI] [PubMed] [Google Scholar]
- Schulz DJ, Pankiw T, Fondrk MK, Robinson GE, Page RE. Comparisons of juvenile hormone hemolymph and octopamine brain titers in honey bees (Hymenoptera : Apidae) selected for high and low pollen hoarding. Annals of the Entomological Society of America. 2004;97:1313–1319. [Google Scholar]
- Seehuus SC, Norberg K, Gimsa U, Krekling T, Amdam GV. Reproductive protein protects sterile honeybee workers from oxidative stress. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:962–967. doi: 10.1073/pnas.0502681103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley TD. Adaptive significance of the age polyethism schedule in honeybee colonies. Behavioral Ecology and Sociobiology. 1982;11:287–293. [Google Scholar]
- Smith CR, Toth AL, Suarez AV, Robinson GE. Genetic and genomic analyses of the division of labour in insect societies. Nature Reviews Genetics. 2008;9:735–748. doi: 10.1038/nrg2429. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nature Neuroscience. 2008;11:309–317. doi: 10.1038/nn2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth AL, Robinson GE. Evo-devo and the evolution of social behavior. Trends in Genetics. 2007;23:334–341. doi: 10.1016/j.tig.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Tsuruda J, Amdam GV, Page RE. Sensory response system of social behavior tied to female reproductive traits. PLoS ONE. 2008;3:e3397. doi: 10.1371/journal.pone.0003397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu MP, Yin CM, Tatar M. Mutations in insulin signaling pathway alter juvenile hormone synthesis in Drosophila melanogaster. General and Comparative Endocrinology. 2005;142:347–356. doi: 10.1016/j.ygcen.2005.02.009. [DOI] [PubMed] [Google Scholar]
- West-Eberhard MJ. Flexible Strategy and Social Evolution. In: Itô Y, Brown JL, Kikkawa J, editors. Animal Societies: Theories and Fact. Tokyo: Japan: Sci Soc Press; 1987. pp. 35–51. [Google Scholar]
- West-Eberhard MJ. Wasp societies as microcosms for the study of development and evolution. In: Turillazzi S, West-Eberhard MJ, editors. Natural History and Evolution of Paper Wasp. New York: Oxford University Press; 1996. pp. 290–317. [Google Scholar]
- Woyciechowski M, Moron D. Life expectancy and onset of foraging in the honeybee (Apis mellifera) Insectes Sociaux. 2009;56:193–201. [Google Scholar]
- Wu Q, Zhao Z, Shen P. Regulation of aversion to noxious food by Drosophila neuropeptide Y- and insulin-like systems. Nature Neuroscience. 2005;8:1350–1355. doi: 10.1038/nn1540. [DOI] [PubMed] [Google Scholar]
- Wyatt GR, Davey KG. Cellular and molecular actions of juvenile hormone.2. Roles of juvenile hormone in adult insects. Advances in Insect Physiology. 1996;26:1–155. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.