Abstract
We examined seasonal dispersal patterns and timing of new infections of Sin Nombre virus (SNV), as determined by recent acquisition of antibodies (seroconversion), in deer mice (Peromyscus maniculatus) at two Montana rangeland study sites over three years, 2004–2007. One study site was located in grassland habitat, and the other was located in shrub-steppe. In Montana, both of these habitats are commonly associated with peridomestic environments (in and around buildings). Peridomestic environments are where most reported human cases of hantavirus pulmonary syndrome (HPS) likely originate. Furthermore, deer mice dispersing from sylvan habitats colonize peridomestic environments. Thus, a thorough understanding of deer mouse dispersal is needed to help predict when humans are most at risk for exposure to SNV. We trapped mice at each study site twice a month, accumulating 85,200 trap nights of effort and capturing 6,185 individual deer mice a total of 22,654 times. We documented 980 dispersing individuals over 3 yr. We found positive correlations between the number of dispersing mice and number captured at each site, but there were no statistically significant seasonal differences in the number of dispersing mice. However, we did find a spring/summer bias in mice that seroconverted and dispersed, suggesting that recently infected deer mice are most likely to enter settings where humans may be exposed to SNV during spring and summer.
Keywords: Deer mouse, dispersal, hantavirus, Peromyscus maniculatus, population dynamics, Sin Nombre virus
Introduction
Sin Nombre virus (SNV; family Bunyaviridae, genus Hantavirus) was identified as a human health threat in North America in 1993 when it was determined to be the agent responsible for an outbreak of hantavirus pulmonary syndrome (HPS) in the southwestern United States (Nichol et al., 1993). Hantavirus pulmonary syndrome is a potentially fatal zoonotic disease that affects the pulmonary system of humans. Since the initial outbreak, >480 laboratory-confirmed human cases have been reported in the United States with a case fatality of 35% (CDC, 2007). Thirty states have reported HPS, with the majority of cases occurring in the western United States. Human exposures are generally restricted to rural areas and occur most frequently in spring or early summer (CDC, 2002; Douglass et al., 2005).
The principal host of SNV is the deer mouse (Peromyscus maniculatus; Childs et al., 1994). Deer mice occupy a wide array of natural habitats, including coniferous and deciduous forests (Garman et al., 1994; Douglass et al., 2001), grasslands, and shrub-steppe (Douglass et al., 2001) in rural areas in the western United States. These habitat types may often include man-made structures such as houses, cabins, barns, sheds, and outhouses. Peridomestic settings (in and around buildings) create a contact link between humans and deer mice. Glass et al. (1997) documented rodent activity (primarily deer mice) frequently occurring in and around rural buildings. These rural buildings provide useful microenvironments for deer mice by creating nesting sites and, often, food sources. Deer mice use outbuildings for both nesting and resource gathering (Kuenzi et al., 2001; Douglass et al., 2003).
Rodents shed hantaviruses in their feces, urine, and saliva (Glass et al., 1988; Safronetz et al., 2005). As infected deer mice use buildings, they may be shedding virus in their excreta or in deposits of saliva left behind when they gnaw on materials within buildings. Kuenzi et al. (2001) reported that the prevalence of antibodies against SNV was higher in deer mice within peridomestic settings than in those from sylvan (natural) settings. Peridomestic environments are also where most reported human cases of hantavirus pulmonary syndrome (HPS) originate (Armstrong et al., 1995). The ultimate source of mice in peridomestic settings is through dispersal of individuals from surrounding sylvan habitats. Infected, dispersing individuals can potentially transport infectious virus from sylvan to peridomestic settings. Thus, a thorough understanding of seasonal dispersal patterns of sylvan deer mice is needed to better understand SNV–deer mouse ecology and human risk of contracting HPS within adjacent peridomestic settings.
Our study was designed to examine seasonal dispersal patterns of sylvan deer mice at two study sites located in Montana rangeland habitats. We also examined timing of SNV infection in these deer mouse populations. By identifying both periods of high deer mouse dispersal and timing of SNV infection, we can potentially identify periods of elevated human risk of exposure to SNV.
Materials and Methods
Study sites
We established two study sites in western Montana, one located near Cascade (46°59.3′N, 111°35.3′W), and the other located near Polson (47°38.4′N, 114°20.7′W). Elevations at the Cascade and Polson study sites are approximately 1408 and 823 m, respectively. The Cascade study site is predominately grassland, and the Polson site is shrub-steppe dominated by big sagebrush (Artemisia tridentata), with sparse juniper (Juniperus scopulorum) and ponderosa pine (Pinus ponderosa) trees distributed throughout. Each study site has a pair of small-mammal live-trapping grids (100 traps, 1 ha in area) created for ongoing longitudinal hantavirus studies initiated in 1994 (Douglass et al., 1996). The two trapping grids at each study site are approximately 550 m (Cascade) and 840 m (Polson) apart.
We established dispersal trapping arrays between the two longitudinal trapping grids at each study site. These arrays consisted of ten evenly spaced parallel lines of traps (50-m line spacing at Cascade and 76-m line spacing at Polson) set perpendicular to the paired longitudinal grids (Fig. 1). The line spacing differed between the two study sites because the distances between paired grids at Polson and Cascade were different, and we wanted to evenly fit ten trap lines between the grids at each site. The dispersal trapping arrays at Cascade and Polson covered areas of approximately 14 and 25 ha. Each of the ten lines in an array contained 25 Sherman nonfolding, aluminum live traps (8 × 9 × 23 cm, H.B. Sherman Trap Co., Tallahassee, Florida, USA) with 15-m spacing (along each line) between traps. Individual trap stations were marked with colored surveying flags and were assigned Universal Transverse Mercator (UTM) coordinates using a satellite global positioning system (GPS) unit.
Figure 1.
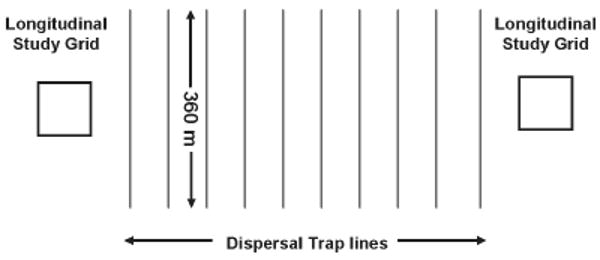
Arrangement of trapping grids and dispersal trap lines. Twenty-five live traps were placed at 15-m intervals along each dispersal trap line. Each 1-ha grid contained 100 evenly spaced traps. Trap lines were spaced at 50- and 76-m intervals at Cascade and Polson, respectively. Area covered by dispersal trap lines was approximately 14 ha at Cascade and 25 ha at Polson, Montana, USA, June 2004 through October 2005.
Rodent collection
Sampling was conducted twice monthly at Cascade (June 2004–October 2007) and twice monthly during the snow-free months at Polson (June 2004–October 2005 and April–October 2005 and April–October 2006 and April–October 2007). The paired longitudinal grids were operated during the first monthly sampling session, and the dispersal trapping arrays were operated during the second monthly sampling session. The second monthly sampling session took place 7–10 days after the first sampling session, allowing sufficient time for movements of animals to occur between sampling events. Capture data were standardized to the number of individuals/1,000 trap night effort.
Trapping was conducted during each sampling session for three consecutive nights. Traps were baited with peanut butter and oatmeal, and insulated with polyester bedding. Small-mammal blood collection and handling procedures followed Mills et al. (1995). Each morning, traps were checked, and animals were processed and released at the site of capture. Rodents were ear tagged using individually numbered metal tags (National Band and Tag Co., Newport, Kentucky, USA), and their capture location, species, sex, body mass, reproductive condition, and presence of scars or wounds were recorded. Variables describing reproductive condition included testes, scrotal or abdominal, for males, and vagina, nonperforate or perforate, pubic symphysis, closed or open (by palpation, open suggests imminent or just past parturition), and nipples, normal or enlarged, for females. Deer mice were aged based on the following weight categories: >17 g = adult, 14–17 g = subadult, and <14 g = juvenile (Fairbairn, 1977; Sullivan, 1977).
Blood samples were taken from captured rodents, from 2005 onward, that were anesthetized using Isoflurane (Abbott Laboratories, North Chicago, Illinois, USA). Approximately 0.2 ml of blood were collected from the suborbital sinus by insertion of a heparinized microcapillary tube (Biven and Smith, 1984). Individuals were bled only once during each trapping session, but they were bled again upon recapture in subsequent sessions. Blood samples were immediately frozen on dry ice until they could be analyzed. Blood samples were tested in the laboratory for antibodies reactive with SNV recombinant nucleocapsid protein by an enzyme-linked immunosorbent assay (ELISA; Feldmann et al., 1993). To prevent human-facilitated infection of mice, processing tools and handlers' hands were disinfected between handling of individual mice. Soiled traps were washed using an antiviral detergent (Betco 256, Toledo, Ohio, USA) prior to reuse, and a clean trap was placed at the trap site after each capture.
Definition of dispersing individuals
Our definition of dispersal (Lonner et al., 2008) was partially derived from data from studies on natural populations of white-footed mice (Peromyscus leucopus), which estimated individual home-range size to be approximately 75 m in diameter (Stickel, 1969; Krohne et al., 1984). We considered dispersing individuals as mice that moved a minimum of 75 m and did not return to within a 75-m radius of their initial capture location.
Identification of dispersing individuals by season
Seasons were defined as: spring (trap sessions during March–May), summer (June–August), autumn (September–November), and winter (December–February). Within each season, the distances moved in meters from one capture event to the next were calculated for individual mice recaptured at different trap locations. These series of individual movements were then examined to identify dispersers (see previous definition). Seasons were considered independently when defining dispersal movements. Thus, only movements occurring within seasons were analyzed; movements occurring between seasons were omitted from our analyses. Consequently, an individual could be classified as a disperser for multiple seasons but only once within a season. Dispersers for each season were further categorized by sex and age.
Identification of seroconverting deer mice
Individual deer mice were classified as being seroconverters if they changed from SNV antibody-negative (antibody titer <400 by ELISA) to antibody-positive (antibody titer >400) within a season. In our analysis, we used deer mice that had seroconverted during the time that they also dispersed.
Data analysis
All analyses were conducted using SPSS 15.0 (SPSS Inc., Chicago, Illinois, USA). To initially assess whether the number of dispersing individuals/1,000 trap nights of effort differed between Cascade and Polson, we used a general linear model (GLM). Differences among the sites were observed (see Results section). Further analyses examining relationships among the number of individuals captured and the number of dispersing individuals, and the number dispersing among years and seasons, were conducted separately for Polson and Cascade. The relationship between the number of individuals captured at each site and the number dispersing was assessed using linear regression. Finally, GLM was used to examine if the number of dispersing individuals differed among years at each site and if the number dispersing differed among seasons.
Because the number and proportion of dispersing mice that were seroconverting did not differ between sites (see Results), we combined sites and used a two-way GLM to examine the ways in which the number and percentage of dispersing individuals that were seroconverting differed among years and seasons.
Data were examined for normality and equality of variances using Shapiro-Wilk's and Levene's tests for normality and equal variances, respectively. When these assumptions were violated, data were normalized by log transformation. Percentage data were arcsine transformed prior to analysis.
Results
From June 2004 through October 2007, we captured 6,185 deer mice (1,506 at Cascade and 4,679 at Polson) during 85,200 trap nights (53,400 at Cascade and 31,800 at Polson). Of the 6,185 individuals captured, 3,138 were males and 3,047 were females. We recorded 980 dispersers (338 at Cascade and 650 at Polson; Tables 1 and 2). Of the 338 dispersers documented at Cascade, 186 (55.0%) were adult males, 99 (29.3%) were adult females, 25 (7.4%) were subadult males, and 28 (8.3%) were subadult females. At Polson, 335 (51.6%) were adult males, 244 (37.6%) were adult females, 31 (4.7%) were subadult males, and 40 (6.1%) were subadult females.
Table 1.
Trap effort (number trap nights) and number of dispersing deer mice captured by season at a study site near Cascade, Montana, USA, August 2004–October 2007.
| Season | Trap nights | Individual micea | Dispersers | Dispersers/1,000 trap nights |
|---|---|---|---|---|
| Winter 2004 | 1,350 | 69 | 4 | 3.0 |
| Winter 2005 | 4,050 | 160 | 12 | 3.0 |
| Winter 2006 | 4050 | 207 | 35 | 8.6 |
| Winter 2007 | 4,050 | 64 | 14 | 3.5 |
| Spring 2005 | 4,050 | 127 | 34 | 8.4 |
| Spring 2006 | 4,050 | 127 | 23 | 5.7 |
| Spring 2007 | 4,050 | 110 | 40 | 9.9 |
| Summer 2004 | 750 | 37 | 5 | 6.7 |
| Summer 2005 | 4,050 | 162 | 38 | 9.4 |
| Summer 2006 | 4,050 | 179 | 21 | 5.2 |
| Summer 2007 | 4,050 | 73 | 8 | 2.0 |
| Autumn 2004 | 4,050 | 119 | 19 | 4.7 |
| Autumn 2005 | 4,050 | 201 | 32 | 7.9 |
| Autumn 2006 | 4,050 | 234 | 38 | 9.4 |
| Autumn 2007 | 2,700 | 166 | 15 | 5.6 |
| Total | 53,400 | 338 | ||
| Average | 6.2 |
Total individuals not reported because some were captured during multiple seasons.
Table 2.
Trap effort (number of trap nights) and number of dispersing deer mice captured by season at a study site near Polson, Montana, USA, August–October 2004 and April–October 2005–2007.
| Season | Trap nights | Individual micea | Dispersers | Dispersers/1,000 trap nights |
|---|---|---|---|---|
| Spring 2005 | 2,700 | 554 | 66 | 24.4 |
| Spring 2006 | 2,700 | 542 | 76 | 28.1 |
| Spring 2007 | 2,700 | 527 | 45 | 16.7 |
| Summer 2004 | 750 | 145 | 11 | 14.7 |
| Summer 2005 | 4,050 | 765 | 83 | 20.5 |
| Summer 2006 | 4,050 | 896 | 88 | 21.7 |
| Summer 2007 | 4,050 | 754 | 77 | 19.0 |
| Autumn 2004 | 2,700 | 489 | 43 | 15.9 |
| Autumn 2005 | 2,700 | 771 | 58 | 21.5 |
| Autumn 2006 | 2,700 | 715 | 37 | 13.7 |
| Autumn 2007 | 2,700 | 583 | 66 | 24.4 |
| Total | 31,800 | 650 | ||
| Average | 20.1 |
Total individuals not reported because some were captured during multiple seasons.
There was a significantly greater number of dispersing individuals/1,000 trap nights at Polson than at Cascade (F1,24=60.416, P<0.001). Therefore, analyses examining relationships between the number of individuals captured and the number of dispersing individuals, and the number of dispersing individuals between years and seasons, were conducted separately for Polson and Cascade.
Dispersing versus abundance
At both Cascade and Polson, there was a statistically significant positive correlation between the number of dispersing individuals and the number captured per season (Fig. 2; r2=0.438, P=0.01, n=15 and r2=0.583, P=0.01, n=11, respectively).
Figure 2.
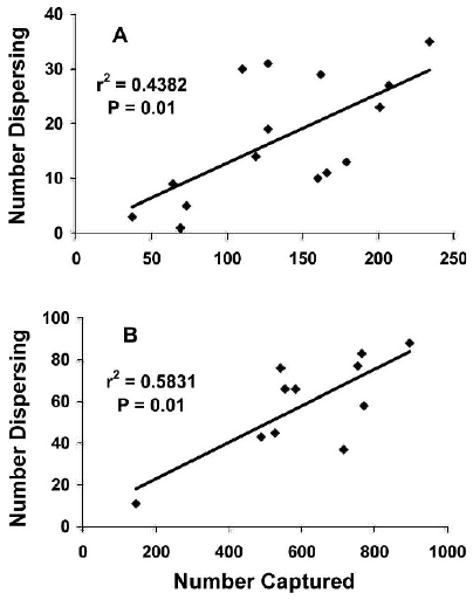
Regression of number of dispersing deer mice captured on total number of deer mice captured by season at (A) Cascade and (B) Polson study sites, Montana, USA.
Seasonal dispersal comparisons
At Cascade, the number of dispersers/1,000 trap nights, varied both seasonally and annually, but showed no consistent trend. Peak numbers of dispersers/1,000 trap nights occurred during a different season each year. The peak dispersal occurred during the summer of 2005, winter of 2006, and fall of 2007 (Fig. 3). There was no statistically significant difference in the number of dispersing individuals/1,000 trap nights among years (F3,15=0.816, P=0.51) or among seasons (F3,15=1.157, P=0.37).
Figure 3.
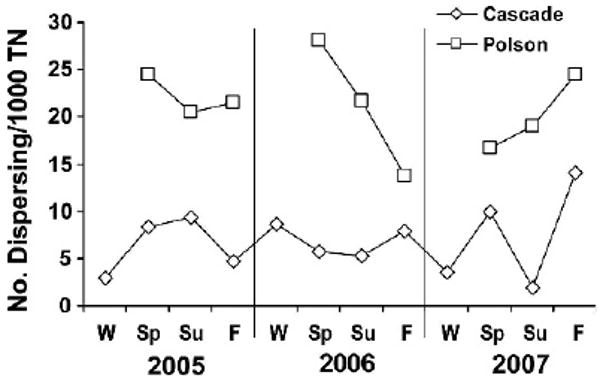
Number of dispersing deer mice/1,000 trap nights (TN) of effort by year and season for Cascade and Polson, Montana, USA. W=winter; Sp=spring; Su=summer; F=fall.
Similarly, there was variability in the number of dispersers by season and year but no consistent trend at Polson. Peaks of dispersing individuals occurred during the spring of 2005 and 2006, while the peak in 2007 occurred during the fall (Fig. 3). There was no statistically significant difference in the number of dispersing individuals/1,000 trap nights among years (F3,11=0.823, P=0.52) or among seasons (F2,11=0.856, P=0.46).
Seasonality of seroconversion and dispersal
We found 36 deer mice that seroconverted during the season they dispersed. The number and proportion of dispersing individuals that were seroconverting did not differ between Polson and Cascade (F1,19=2.032, P =0.17 and F1,19=0.147, P=0.71, respectively). Therefore, we combined sites to determine the ways in which the number and percentage of dispersers that were seroconverting differed between years and seasons. Both the number and proportion of dispersers that seroconverted while dispersing were significantly higher in spring and summer (Table 3 and Fig. 4).
Table 3.
General linear model (GLM) of the number and proportion of dispersing deer mice/1,000 trap nights that seroconverted, study sites combined, Montana, USA, August 2004–October 2007.
| GLM | No. seroconverting | % seroconverting | ||||
|---|---|---|---|---|---|---|
| F | df | P | F | df | P | |
| Year | 0.048 | 2 | 0.953 | 0.043 | 2 | 0.958 |
| Season | 7.522 | 3 | 0.008 | 6.002 | 3 | 0.016 |
| Year × season | 0.302 | 6 | 0.921 | 0.493 | 6 | 0.799 |
| Error | 9 | 9 | ||||
Figure 4.

Seasonal patterns of deer mice that both seroconverted and dispersed from Cascade and Polson Montana study sites combined. (A) Number per 1,000 trap nights (TN) and (B) percentage of dispersing deer mice that seroconverted by season. Bars with similar letters are not significantly different.
Discussion
Predictions of periods of elevated human risk of exposure to SNV have proven difficult for researchers. This difficulty is created by a high degree of ecologic variability in populations of the reservoir host, the deer mouse. Deer mice are a very ecologically diverse species. Their range covers most of North America, extending northward to tree line in Alaska and northern Canada and southward into central Mexico (Burt and Grossenheider, 1976). Deer mice inhabit a wide variety of habitats, including boreal, temperate, and tropical forests (Baker, 1968). In addition to occupying such a large geographic area and wide variety of habitats, deer mouse populations constantly fluctuate in abundance, population demographics, and reproductive success. All of these factors influence dispersal (Lidicker, 1975; Krebs, 1996).
A thorough understanding of a species influenced by so many ecologic factors takes years of research on a variety of parameters. After more than 14 yr since the initial outbreak of HPS in the United States, researchers still lack a complete understanding of the SNV–deer mouse system. An understanding of deer mouse dispersal is important because it may be the link between deer mouse ecology and human exposure to SNV.
Previous research has indicated that dispersal plays a key role in the demography of mouse populations existing in habitat mosaics (Krohne and Hoch, 1999) such as the sylvan-peridomestic interface. Although typical mouse movements have been reported to be less than 200 m on average (Swilling and Wooten, 2002; Lonner et al., 2008), individuals have the ability to move much larger distances. Thomas (2005) recorded a movement of greater than 3,000 m by a subadult male deer mouse in the southeastern Yukon, and Bowman et al. (1999) reported an individual deer mouse movement of 1,768 m. A deer mouse tagged on our grids was later captured in a ranch building over 5,400 m from its original capture (Lonner, unpubl.). During this study, we found individuals moving greater than 600 m, demonstrating the movement capabilities of dispersing mice, especially males, who made up the majority of dispersers in this study, and who are more likely to have SNV antibodies (Calisher et al., 2007; Lonner et al., 2008). It has been suggested that long range movement of infected mice is important in maintaining and introducing viral infection within and between populations and into new areas (Root et al., 1999; Calisher et al., 2001).
Previous research has shown that rodents infected with other hantaviruses shed the greatest amount of virus during the early stages of infection (Lee et al., 1981; Yanagihara et al., 1985; Hutchinson et al., 1998; Bernshtein et al., 1999). Douglass et al. (2007) investigated the timing of SNV infection (as determined by recent acquisition of antibody) in Montana deer mice. The majority of mice acquired antibody during the breeding season, beginning with low numbers of individuals acquiring antibody during the late winter, then steadily increasing through the spring. Adult males were the group most likely to become infected and seroconvert during the spring (Douglass et al., 2007; see also Calisher et al., 2007; Lehmer et al., 2007; Lonner et al., 2008). Therefore, adult males dispersing during the spring, especially those that recently seroconverted, are the most likely suspects for transmitting virus to other deer mice, including those in peridomestic populations, which are more likely to have contact with humans. The periods that mice, especially seroconverting mice, are dispersing may correlate to periods of increased risk for human exposure to SNV.
Our data and that of other investigators suggest that individual movement of deer mice varies seasonally. Fairbairn (1977) found high numbers of deer mice disappearing from the population in the spring. In females, this loss was attributed to mortality associated with breeding, while the loss of males was attributed to individuals dispersing. With further research, Fairbairn (1978) measured rates of immigration and emigration (dispersal) of deer mice from two control areas and showed that rates of dispersal were positively correlated with population densities. Fairbairn (1978) also found that higher rates of dispersal occurred during spring and fall and nonbreeding males were the primary spring dispersers, while breeding males and juveniles dispersed during the fall, and various-aged mice of both sexes dispersed during the winter. Further evidence of spring movement by deer mice was demonstrated by King (1983), who showed that dispersal-like movements of a seminatural population increased during the spring and summer when maturing individuals began entering the population. Dispersal of deer mice is attributed to social influences (competition) or availability of essential resources.
Research conducted primarily on manipulated populations of deer mice has suggested that higher rates of dispersal, especially among male deer mice, occur during the spring (Sullivan, 1977). Our data, based on natural populations of deer mice surrounding peridomestic environments, suggest no consistent seasonal differences in numbers of dispersing individuals. This lack of a consistent seasonal dispersal pattern has been found by others (Fairbairn, 1978; Krohne et al., 1984; Adler and Tamarin, 1985), and it suggests that levels of dispersal in mouse populations may be influenced more by current population densities than by seasonality. However, when observing animals that both seroconvert and disperse within seasons, we found a strong spring or late spring–early summer bias. In previous work over a broad area of Montana, we found that seroconversions occurred during most months of the year (the exceptions being October–December; Douglass et al., 2007), though there was an increase in March through June. Similarly, in this study, we found a spring peak in seroconversion in dispersing mice. If these recently infected dispersing deer mice move into peridomestic settings, then humans may be at increased risk of contracting HPS during spring. Fewer than 30 HPS cases have been reported in Montana, but most of those occurred between March and June.
In summary, when combined with the results of previous research, our data indicate that during the spring, especially in years of high population densities, there could be a peak in the number of recently infected deer mice (Douglass et al., 2007), which may be more likely to shed infectious virus (Lee et al., 1981; Yanagihara et al., 1985; Hutchinson et al., 1998; Bernshtein et al., 1999). When these recently infected mice are also dispersing, they may be transporting and shedding SNV into areas of high risk for human exposure, such as rural buildings. This combination of findings describes a potential period of increased risk of human exposure to SNV during the spring to early summer. It is likely not a coincidence that data collected by the Centers for Disease Control and Prevention report that elevated numbers of human cases of HPS occur annually during the spring and early summer (CDC, 2007).
Acknowledgments
We thank Dave Cameron, Dana Ranch Inc., and the Confederated Salish and Kootenai Tribes at Polson for unlimited access to their properties. K. Hughes, B. Mires, A. Leary, A. Alvarado, T. Spear, J. Wilson, F. Mazzini, F. Arneson, K. Bagamian, J. Lumsden, and A. Skypala provided valuable assistance in the field. M. Minnick and B. Steele provided significant logistic support for field work and data analysis. S. Carver also provided statistical advice. Financial support was provided by the National Institutes of Health (NIH) grant P20RR16455-05,06,07,08 from the INBRE–BRIN program of the National Center for Research Resources, and the US Centers for Disease Control and Prevention, through cooperative agreement US3/CCU813599.
Literature Cited
- Adler GH, Tamarin RH. Dispersal of white-footed mice Peromyscus leucopus in low density island and mainland populations. Canadian Field Naturalist. 1985;99:331–336. [Google Scholar]
- Armstrong LR, Zaki SR, Goldoft MJ, Todd RL, Khan AS, Khabbaz RF, Ksiazek TG, Peters CJ. Hantavirus pulmonary syndrome associated with entering or cleaning rarely used rodent infested structures. Journal of Infectious Diseases. 1995;172:1166. doi: 10.1093/infdis/172.4.1166. [DOI] [PubMed] [Google Scholar]
- Baker RH. Habits and distribution. In: King JA, editor. Biology of Peromyscus (rodentia) The American Society of Mammalogists; Lawrence, Kansas: 1968. pp. 98–126. [Google Scholar]
- Bernshtein A, Apekina N, Mikhailova T, Myasnikov Y, Khlyap L, Korotkov YS, Gavrilovskaya IN. Dynamics of Puumala hantavirus infection in naturally infected bank voles (Clethrionomys glareolus) Archives of Virology. 1999;144:2415–2428. doi: 10.1007/s007050050654. [DOI] [PubMed] [Google Scholar]
- Biven WS, Smith GD. Techniques of experimentation. In: Fox JG, Cohen BJ, Loew FM, editors. Laboratory animal medicine. Academic Press; Orlando, Florida: 1984. pp. 564–594. [Google Scholar]
- Bowman J, Edwards M, Sheppard L, Forbes G. Record distance for non-homing deer mouse. Peromyscus maniculatus. Canadian Field Naturalist. 1999;113:292–293. [Google Scholar]
- Burt WH, Grossenheider RP. A field guide to the mammals of North America, north of Mexico. Houghton Mifflin; Boston, Massachusetts: 1976. p. 289. [Google Scholar]
- Calisher CH, Mills JN, Sweeney WP, Choate JR, Sharp DE, Canestorp KM, Beaty BJ. Do unusual site-specific population dynamics of rodent reservoirs provide clues to the natural history of hantaviruses? Journal of Wildlife Diseases. 2001;37:280–288. doi: 10.7589/0090-3558-37.2.280. [DOI] [PubMed] [Google Scholar]
- Calisher CH, Wagoner KD, Amman BR, Root JJ, Douglass RJ, Kuenzi AJ, Abbott KD, Parmenter CA, Yates TL, Ksiazek TG, Beaty BJ, Mills JN. Demographic factors associated with prevalence of antibody to Sin Nombre virus in deer mice in the western United States. Journal of Wildlife Diseases. 2007;43:1–11. doi: 10.7589/0090-3558-43.1.1. [DOI] [PubMed] [Google Scholar]
- CDC. Hantavirus pulmonary syndrome—United States: Updated recommendations for risk reduction. Morbidity and Mortality Weekly Report. 2002;51(RR-9):1–12. [PubMed] [Google Scholar]
- CDC. National Center for Infectious Diseases Special Pathogens Branch; 2007. [April 2007]. Case information: Hantavirus pulmonary syndrome case count and descriptive statistics as of March 2007. http://www.cdc.gov/ncidod/diseases/hanta/hps/noframes/caseinfo.htm. [Google Scholar]
- Childs JE, Ksiazek TG, Spiropoulou CF, Krebs JW, Morzunov S, Maupin GO, Rollin PE, Sarisky J, Enscore RE. Serologic and genetic identification of Peromyscus maniculatus as the primary rodent reservoir for a new hantavirus in the southwestern United States. Journal of Infectious Diseases. 1994;169:1271–1280. doi: 10.1093/infdis/169.6.1271. [DOI] [PubMed] [Google Scholar]
- Douglass RJ, Van Horn RC, Coffin KW, Zanto SN. Hantavirus in Montana deer mouse populations: Preliminary results. Journal of Wildlife Diseases. 1996;32:527–530. doi: 10.7589/0090-3558-32.3.527. [DOI] [PubMed] [Google Scholar]
- Douglass RJ, Wilson T, Semmens WJ, Zanto SN, Bond CW, Van Horn RC, Mills JN. Longitudinal studies of Sin Nombre virus in deer mouse–dominated ecosystems of Montana. American Journal of Tropical Medicine and Hygiene. 2001;65:33–41. doi: 10.4269/ajtmh.2001.65.33. [DOI] [PubMed] [Google Scholar]
- Douglass RJ, Kuenzi AJ, Williams C, Douglass SJ, Mills JN. Removing deer mice from buildings and the risk for human exposure to Sin Nombre virus. Emerging Infectious Diseases. 2003;9:390–392. doi: 10.3201/eid0903.020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglass RJ, Calisher CH, Bradley K. State by state incidences of hantavirus pulmonary syndrome in the United States, 1993– 2004. Vector-Borne and Zoonotic Diseases. 2005;5:89–92. doi: 10.1089/vbz.2005.5.189. [DOI] [PubMed] [Google Scholar]
- Douglass RJ, Calisher CH, Wagoner KD, Mills JN. Sin Nombre virus infection of deer mice in Montana: Characteristics of newly infected mice, incidence and temporal pattern of infection. Journal of Wildlife Diseases. 2007;43:12–22. doi: 10.7589/0090-3558-43.1.12. [DOI] [PubMed] [Google Scholar]
- Fairbairn DJ. The spring decline in deer mice: Death or dispersal? Canadian Journal of Zoology. 1977;55:84–92. [Google Scholar]
- Fairbairn DJ. Dispersal of deer mice, Peromyscus maniculatus, proximal causes and effects on fitness. Oecologia. 1978;32:171–193. doi: 10.1007/BF00366070. [DOI] [PubMed] [Google Scholar]
- Feldmann H, Sanchez A, Morzunov S, Spiropoulou CF, Rollin PE, Ksiazek TG, Peters CJ, Nichol ST. Utilization of autopsy RNA for the synthesis of the nucleocapsid antigen of a newly recognized virus associated with hantavirus pulmonary syndrome. Virus Research. 1993;30:351–367. doi: 10.1016/0168-1702(93)90101-r. [DOI] [PubMed] [Google Scholar]
- Garman SL, O'Connell AF, Connery JH. Habitat use and distribution of the mice Peromyscus leucopus and P. maniculatus on Mount Desert Island, Maine. Canadian Field Naturalist. 1994;108:67–71. [Google Scholar]
- Glass GE, Johnson J, Hodenbach G, Disalvo C, Peters CJ, Childs JE, Mills JN. Experimental evaluation of rodent exclusion methods to reduce hantavirus transmission to humans in rural housing. American Journal of Tropical Medicine and Hygiene. 1997;56:359–364. doi: 10.4269/ajtmh.1997.56.359. [DOI] [PubMed] [Google Scholar]
- Glass GE, Childs JE, Korch G, Leduc JW. Association of intraspecific wounding with hantaviral infection in wild rats (Rattus norvegicus) Epidemiology and Infection. 1988;101:459–472. doi: 10.1017/s0950268800054418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson KL, Rollin PE, Peters CJ. Pathogenesis of a North American hantavirus, Black Creek Canal virus, in experimentally infected Sigmodon hispidus. American Journal of Tropical Medicine and Hygiene. 1998;59:58–65. doi: 10.4269/ajtmh.1998.59.58. [DOI] [PubMed] [Google Scholar]
- King JA. Seasonal dispersal in a semi natural population of Peromyscus maniculatus. Canadian Journal of Zoology. 1983;61:11. [Google Scholar]
- Krebs CJ. Population cycles revisited. Journal of Mammalogy. 1996;77:8–24. [Google Scholar]
- Krohne D, Hoch G. Demography of Peromyscus leucopus population on habitat patches: The role of dispersal. Canadian Journal of Zoology. 1999;77:1247–1253. [Google Scholar]
- Krohne D, Dubbs B, Baccus R. An analysis of dispersal in an un-manipulated population of Peromyscus leucopus. American Midland Naturalist. 1984;112:146–156. [Google Scholar]
- Kuenzi AJ, Douglass RJ, White D, Bond CW, Mills JN. Antibody to Sin Nombre virus in rodents associated with peridomestic habitats in west central Montana. American Journal of Tropical Medicine and Hygiene. 2001;64:137–146. doi: 10.4269/ajtmh.2001.64.137. [DOI] [PubMed] [Google Scholar]
- Lee HW, Lee PW, Beak LJ, Song CK, Seong IW. Intraspecific transmission of Hantaan virus, etiologic agent of Korean hemorrhagic fever, in the rodent Apodemus agrarius. American Journal of Tropical Medicine and Hygiene. 1981;30:1106–1112. doi: 10.4269/ajtmh.1981.30.1106. [DOI] [PubMed] [Google Scholar]
- Lehmer E, Clay C, Willson E, St Jeor S, Dearing MD. Differential resource allocation in deer mice exposed to Sin Nombre virus. Physiological and Biochemical Zoology. 2007;80:514–521. doi: 10.1086/520128. [DOI] [PubMed] [Google Scholar]
- Lidicker WZ. The role of dispersal in the demography of small mammals. In: Golley FB, Petrusewicz K, Ryszkowski L, editors. Small mammals. Cambridge University Press; Cambridge, UK: 1975. pp. 103–128. [Google Scholar]
- Lonner BN, Douglass RJ, Kuenzi AJ, Hughes K. Seroprevalence against Sin Nombre virus in resident and dispersing deer mice. Vector-Borne and Zoonotic Diseases. 2008;8:433–441. doi: 10.1089/vbz.2007.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills JN, Yates TL, Childs JE, Parmenter RR, Ksiazek TG, Rollin PE, Peters CJ. Guidelines for working with rodents potentially infected with hantavirus. Journal of Mammalogy. 1995;76:716–722. [Google Scholar]
- Nichol ST, Spiropoulou CF, Morzunov S, Rollin PE, Ksiazek TG, Feldmann H, Sanchez A, Childs J, Zaki S, Peters CJ. Genetic identification of a hantavirus associated with an outbreak of acute respiratory illness. Science. 1993;262:914–917. doi: 10.1126/science.8235615. [DOI] [PubMed] [Google Scholar]
- Root JJ, Calisher CH, Beaty BJ. Relationships of deer mouse movement, vegetative structure and prevalence of infection with Sin Nombre virus. Journal of Wildlife Diseases. 1999;35:311–318. doi: 10.7589/0090-3558-35.2.311. [DOI] [PubMed] [Google Scholar]
- Safronetz D, Lindsay R, Dibernado A, Hjelle B, Xiao R, Artsob H, Drebot MA. A preliminary study of the patterns of Sin Nombre viral infection and shedding in naturally infected deer mice (Peromyscus maniculatus) Vector-Borne and Zoonotic Diseases. 2005;5:127–132. doi: 10.1089/vbz.2005.5.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickel LF. Home range and travels. In: King JA, editor. The biology of Peromyscus. The American Society of Mammalogists; Lawrence, Kansas: 1969. pp. 373–407. [Google Scholar]
- Sullivan TP. Demography and dispersal of island and mainland populations of the deer mouse Peromyscus maniculatus. Ecology. 1977;58:964–978. [Google Scholar]
- Swilling WR, Wooten MC. Subadult dispersal in monogamous species: The Alabama beach mouse (Peromyscus polionotus ammobates) Journal of Mammalogy. 2002;83:252–259. [Google Scholar]
- Thomas JS. Long distance movement of a dispersing deer mouse. Peromyscus maniculatus in the boreal forest. Canadian Field Naturalist. 2005;119:451–452. [Google Scholar]
- Yanagihara R, Amyx HL, Gajdusek DC. Experimental infection with Puumala virus, the etiologic agent of nephropathia epidemica in bank voles (Clethrionomys glareolus) Journal of Virology. 1985;55:34–38. doi: 10.1128/jvi.55.1.34-38.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


